1
Introduction
The problem forming the subject of this report is the long-term disposition of radioactive high-level waste (HLW1) at the Idaho National Engineering and Environmental Laboratory (INEEL). This report assesses alternative ways to convert this waste into forms that are suitable for interim storage and eventual transport to and disposal in geologic repositories or other appropriate facilities. This chapter summarizes the past activities at INEEL that generated this waste, current waste inventories, current waste management practices and facilities, and future plans. This background material defines the starting point for all treatment options, which are comprised of the technical process steps considered in greater detail in the chapters that follow.
WASTE GENERATED FROM PAST REPROCESSING PRACTICES
INEEL has, among its other missions, reprocessed a variety of nuclear fuels, primarily for recovery of the fissile isotope uranium-235 (235U). These fuels generally used highly enriched uranium (i.e., a blend of 235U and uranium-238 with the isotopic abundance of 235 U in excess of 20 percent by weight) and a variety of cladding materials (aluminum, zirconium, stainless steel, and graphite). In reprocessing, these fuels and their cladding were dissolved in acidic solutions (nitric acid, hydrofluoric acid, and sulfuric acid were used). This permitted species of interest (e.g., 235U, as well as, from time to time, neptunium-237, krypton-85, barium-140, and fission product xenon) to be separated and recovered (Knecht et al., 1997).
The liquid acidic wastes from these activities were stored in subsurface tanks constructed of stainless steel and surrounded by concrete containers called vaults. In time, this liquid waste was convened to a solid form in calciners and stored in bins on site. Various calcination facilities were developed and tested in the 1950s, culminating in the Waste Calcining Facility (WCF), a fluidized bed unit constructed during 1958-1961 and operational on radioactive feed during 1963-1981 (Knecht et al., 1997). The New Waste Calciner Facility (NWCF), another fluidized bed unit with increased capacity and reduced maintenance requirements, began operation in 1982.
Both the liquid reprocessing waste and the solids resulting from its calcination are classified as HLW, as that term is defined by both the U.S. Department of Energy (DOE) and the U.S. Nuclear Regulatory Commission (USNRC), because they come from the "first-cycle
|
1 |
A complete list of acronyms used in this report appears in Appendix F. |
raffinate" produced in nuclear fuel reprocessing.2 This raffinate contains radioactive fission products and actinides.
From 1953 to 1992, these reprocessing activities and associated waste management practices were done at the INEEL's Idaho Chemical Processing Plant (ICPP).3 Reprocessing activities ceased in 1992. Further details of reprocessing campaigns, fuel types, equipment upgrades, and chemical additives are found in Knecht et al. (1997). The INEEL HLW practices differed from those at other DOE sites in two important ways:
-
The acidic raffinates were not neutralized with sodium hydroxide, which would have increased their volume and possibly precluded their calcination.
-
Instead, these raffinates, with added chemicals (such as calcium, whose addition neutralized excess acid and precipitated fluoride as CaF2), were calcined directly to a solid form (Knecht et al., 1997).
SOLID WASTE IN BINS
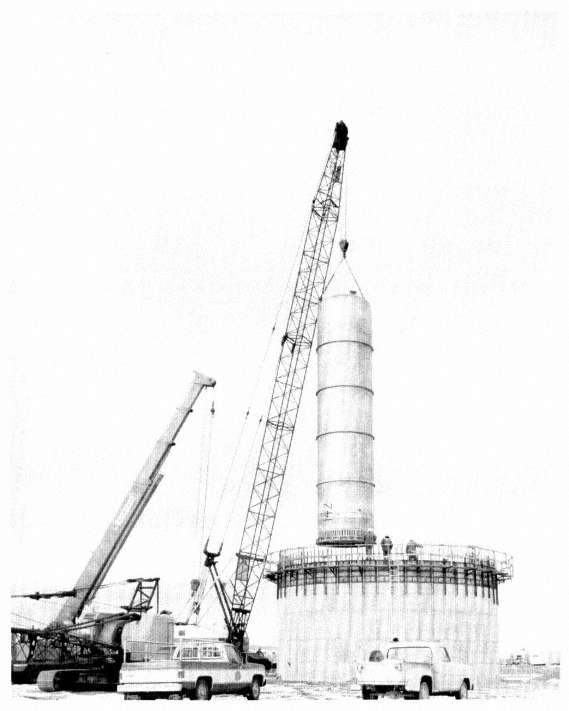
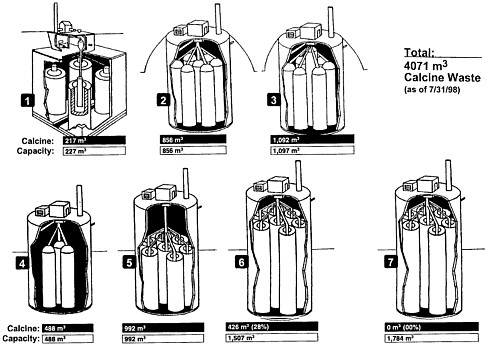
As noted above, the calciners operated to generate solid calcine that is piped directly into bins. The solid waste bins are partially buried stainless steel bins grouped inside concrete vaults (see Figure 1.1), and they are designed to last 500 years (Palmer, 1998; Dirk, 19944; Schindler, 1974).5 Three to seven bins built adjacent to each other and within the same vault form a "bin set," and the piping from the calciner distributes calcine into each bin of a "bin set" (see Figure 1.2). As of August 1998, five of the seven bin sets are filled, one is partially full, and one is empty. The 1998 inventory of calcine was approximately 4,000 m3 (Palmer, 1998).
Calcines have composition dependent upon the nuclear fuel, cladding materials, and chemicals added in reprocessing and calcination. Alumina calcines, comprising approximately 800 m3 of the 1995 inventory (Brewer et al., 1995), are predominantly alumina (Al2O3) from dissolution of aluminum-clad spent fuel. Zirconia-based calcines, comprising approximately 3,000 m3 of the 1995 inventory (Brewer et al., 1995), contain zirconia (ZrO2) from the re-processing of zirconium-based fuels (Knecht et al., 1997) and other constituents such as calcium fluoride (CaF2) and alumina (Al2O3). The fluoride content of zirconia-based calcines comes from the hydrofluoric acid that was used to dissolve the fuel, and the aluminum and calcium content comes from the addition of compounds containing these elements in order to complex the fluoride. Fission products are less than 1 percent by weight but account for most of the radioactive curie content (Garcia, 1997). In addition to radioactive species, hazardous chemical constituents (e.g., compounds containing mercury), added during reprocessing, waste management, and calcination operations, may be present in the calcine. Further details on calcine characterization are found in Garcia (1997).
The NWCF has been shut down periodically for maintenance and recently has been targeted to operate in the future (i.e., beyond June 1, 2000) only if upgrades are performed on the emissions monitoring system, as required by the recently promulgated Maximum Achievable Control Technology (MACT) rule of the Environmental Protection Agency (EPA).6 During past reprocessing campaigns, radioactive species (e.g., ruthenium isotopes 103Ru and 106Ru, which were produced in fuel as fission products and which were volatile in oxide forms) have been present in off-gas emissions (Knecht et al., 1997). Hazardous chemical effluents include mercury (Hg) compounds, carbon monoxide (CO), and NOx species, the last of which appears as an orange plume from the stack. Hg and CO emissions are regulated under MACT rules. The NOx species can interfere with the sampling and removal of Hg and organic effluents (Kimmel, 1999a).
LIQUID WASTE IN TANKS
In February 1998, the last of the liquid HLW generated from past reprocessing practices was calcined. An inventory of liquid waste remains, however, because other waste streams, such as from site decontamination and decommissioning activities have generated sodium-bearing waste (SBW). This waste is mixed transuranic waste, rather than HLW, but, because of its radioactivity and large volume, the site managers found it convenient to store this waste in the underground tanks and thus manage it as if it were HLW. As of August 1998, the amount of this liquid waste was 5,000 m3 (1.3 million gallons), distributed in approximately half of the 11 underground tanks (Palmer, 1998). The radioactivity of the inventory of SBW in the tanks is approximately an order of magnitude less than the radioactivity of the inventory of solid HLW calcine (Olson, 1998b; DOE, 1997b: Table 2.12). Since February 1998, some SBW has been calcined using aluminum nitrate as an additive, and the resulting calcine was added to the HLW calcine in the bins. This process creates additional HLW through mixing, and the possibility that this process may be continued caused the committee to regard SBW treatment as part of the task of this study.
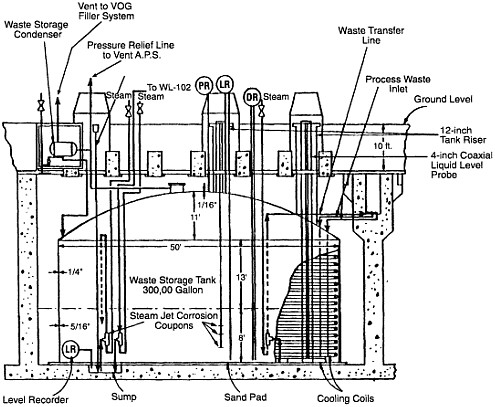
The underground tanks do not have secondary containment liners that meet current regulatory requirements of the Resource Conservation and Recovery Act (RCRA). Thus, because of the way they were constructed (see Figure 1.3), it cannot be assumed that the tanks will continue to hold the liquids in the event of seismic disruptions or a breach of the steel tank wall. Although detection of a leak is possible (Figure 1.4), the tanks cannot serve as a permanent RCRA-approved storage location for the liquid waste. Moreover, the actinide constituents are in sufficient concentration to enable this waste to be classified as transuranic waste, for which shallow subsurface disposal (as in the tanks) is inappropriate. A current regulatory milestone, from a ''Notice of Noncompliance Consent Order," is to cease the use of all tanks by the year 2012 (Wichmann, 1998a). This would entail removal and/or solidification of all liquid waste contents.
OTHER HLW: SPENT NUCLEAR FUEL INVENTORIES
The HLW under consideration in this report is the inventory of solid calcine and liquid SBW (the latter of which is not classified as HLW but which is managed as HLW by
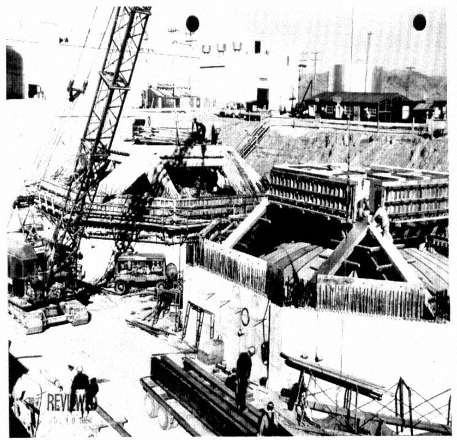
Figure 1.3 The 1951 construction of two 300,000-gallon stainless steel liquid waste tanks, WM-180 and WM-181. Monolithic, reinforced concrete vaults poured in place in an octagonal shape surround these tanks. Four other tanks are surrounded by poured-in-place concrete vaults of square shape. Octagonal concrete vaults of "pillar and panel" construction surround the remaining 5 of the 11 steel tanks. SOURCE: INEEL photograph #51-3374.
DOE, with the rationale that its characteristics are similar to the HLW liquid raffinate that was calcined in the past). Site inventories of spent nuclear fuel (SNF) that have not been reprocessed as described previously are also classified as HLW but are not included in the scope of this study. Therefore, the term HLW as used in this report will denote only the HLW calcine and SBW.
However, the management and ultimate disposition of INEEL HLW is linked to DOE disposal plans for other waste streams, principally SNF. The SNF inventories that can be compared and contrasted to the INEEL HLW calcine and SBW include (a) DOE SNF stored on the INEEL site and generated from on-site DOE reactors that operated in the past in reactor development programs and defense-related missions, (b) Navy SNF also stored on the INEEL site and generated from reactors in the Navy nuclear program, and (c) SNF planned for storage at the INEEL site and generated from other, off-site sources. As used in this report, these DOE SNF inventories are loosely labeled as "defense" in nature and are discussed only insofar as current DOE plans destine the HLW calcine and DOE SNF for co-disposal in the geologic repository under development for commercial SNF from nuclear power plants.
SNF is stored on site in wet and dry storage (specifically, in six wet pools, one dry storage pad, one dry storage vault, and one below-grade dry storage caisson) (Kimmel, 1999a). The present SNF inventory at INEEL is 232 MTHM,7 at a total mass of 1,874 metric tons, within a volume of 558 m3, and with an inventory of approximately 50 million (5 × 107) Ci (Kimmel, 1999a). This inventory of fuel comes from on-site DOE reactors that have operated in the past and from off-site reactors of both Naval and non-Naval origin, and continues to grow as new shipments of fuel in the latter two categories are received. The inventory of SNF from off-site non-Naval sources (i.e., other DOE sites, universities, and foreign research reactors) is estimated to grow, over the next 20 years, to total about 53 MTHM, representing a mass of 145 metric tons, a volume of 34.5 m3, and a curie inventory8 of 10 million (1 × 107) Ci, by the year 2020 (Kimmel, 1999a). The SNF inventory from off-site Naval sources, currently comprising 15 MTHM of the total site inventory, will grow to 65 MTHM by 2035, to a total mass of 4,400 metric tons, a total volume of 900 m3, and a total radioactivity of approximately 300 million (3 × 108) Ci9 (Elmer Naples, private communication with Thomas Kiess, February 1999).
These numbers indicate that the current SNF inventory is largely composed of contributions from on-site DOE and off-site Naval reactors. These SNF figures are provided here only for perspective in a comparison to similar quantities estimated for the calcine. The total HLW calcine radioactivity is approximately 40 million Ci (Garcia, 1997: corrected Table 4), with a total volume of approximately 4,000 m3, which, using 1.4 g/cm3 as ail average density (Garcia, 1997), represents approximately 6,000 metric tons. This HLW calcine comes from the processing at INEEL of 44 metric tons of uranium at "beginning of life" (Kimmel, 1999a). Therefore, this calcine inventory is comparable to (i.e., within an order of magnitude of) the current and furore INEEL SNF inventories, as measured by radioactivity, volume, or mass. Although the HLW calcine is comparable to site SNF inventories by these measures, other features, such as the content of fissile and/or long-lived radionuclides, may differ in ways that would provide a technical rationale for different management and disposition strategies.
OTHER INVENTORIES OF RADIOACTIVE WASTE
As with other large DOE sites, the INEEL also contains buried transuranic and low-level wastes. The radioactivity, volume, and mass of these wastes, and the environmental and safety risks that they pose, would provide additional perspective on relative risks than is provided here by the comparison of the INEEL HLW to the SNF inventory alone. The committee has insufficient information to devise this comparison of all site inventories of radioactive wastes, but simply notes the utility of such a comparison for informed judgment concerning which problems pose the greatest risk and which, of all possible site remediations, provide the greatest reductions in risk.
FUTURE PLANS AND CONTEXT FOR THIS STUDY
A "Settlement Agreement" reached in 1995 resulted in a court order signed by then-Governor of Idaho Philip Batt that provided milestones for DOE activities pertaining to SNF and HLW (and mixed waste, both transuranic and otherwise). To paraphrase the terms of this agreement (see Appendix G), by the year 2035 the INEEL SNF and HLW are to be in forms suitable for out-of-state transport and ultimate disposal, as a condition of the continued receipt of Naval spent fuel into Idaho. These terms are subject to change if a future Environmental Impact Statement (EIS) were to show merit in renegotiating the 2035 milestone (Lodge, 1995).
An EIS is part of the process mandated by the National Environmental Policy Act (NEPA) legislation begun in 1969 that applies to federal facilities. An EIS would examine a range of alternative strategies for addressing a remediation problem that has potential environmental impacts, and analyze these options using several criteria (e.g., public health and environmental consequences, as applied to projected operations and hypothetical accident scenarios and expressed in risk terms). The EIS is a source of information, along with other measures such as assessments of worker safety, for decisionmakers to consider in selecting among various remediation options. The DOE-EM program, created in 1989 to clean up the sites and wastes of the former nuclear weapons complex, routinely conducts EIS evaluations prior to the construction of major facilities.
The DOE-EM is developing an EIS that addresses technical alternatives for the conversion of INEEL's HLW and SBW into waste forms suitable for off-site, out-of-state transport. The EIS evaluations would provide input into a decision as to which of many treatment options to pursue for the inventories of solid HLW and liquid mixed transuranic waste, prior to the selection of one option and the subsequent commitment of resources. To meet a 2035 deadline, a decision in the near future would allow sufficient time for process development and for construction and operation of large facilities to process the current waste into different forms.
PROCESS OPTIONS
The purpose of treating the INEEL HLW would be to convert the present calcine and sodium-bearing liquid into material forms suitable for interim on-site storage and for eventual transport and disposal in repositories or other facilities. In current program plans, radioactive constituents of the HLW that have high activity and/or long half-fives are to be disposed of as high-activity waste (HAW) in a geologic repository. The less radioactive and non radioactive constituents, if separated from the HAW component and sufficiently decontaminated to meet regulatory approval as no longer necessary to manage as HLW, could be
disposed of in other, shallow, land facilities, either on-or off-site. Therefore, segregation of high-and low-activity fractions is one of the process steps currently under consideration by the INEEL HLW program. A succession of such steps, if properly designed and integrated into a treatment system, would produce final waste forms with radiological and material properties tailored to meet requirements imposed by long-term disposal conditions.
For processing INEEL calcines and liquid waste into other forms, several technical options exist, as summarized briefly here. One option is to mix waste with appropriate cement-forming additives to make a cement waste form. Another option is to dissolve calcine into aqueous-based solutions; separate radioactive constituents such as 137Cs, 90Sr, and actinides; and convert the remaining large volume of liquid waste into a low-level grout, while solidifying the fraction containing high-level and/or long-lived radioisotopes into a smaller volume in a glass or cement waste form. A third option is to directly convert calcine into a vitrified product, without dissolution or separation of selected constituents. A fourth option is to convert calcine into an alternative waste form, such as a glass/ceramic, either directly or after a separation operation. A "No-Action" or "Minimal Action" option would involve long-term storage of calcine in the bins. Most of these options involve closure of both the tanks and bins.
These processing alternatives are being evaluated by DOE and its contractors. They are summarized in DOE (1998) and Russell et al. (1998), with further detail found in Lopez and Kimmett (1998); Dafoe and Losinski (1998); Russell and Taylor (1998); Landman and Barnes (1998); Lee and Taylor (1998); Dahlmeir et al. (1998); Spaulding (1998a, b); and Fluor Daniel (1997a, b), which build upon earlier systems analysis studies (Palmer et al., 1994; Murphy et al., 1995; Palmer et al., 1998), program plans (LMITCO, 1996), and evaluations of alternatives (Palmer, 1996). These technical alternatives are represented schematically in Figure 1.5. Further discussion in this report focuses on the individual technology components (represented by the boxes in Figure 1.5) and on the overall strategy of waste retrieval and remediation that these alternatives are designed to implement.
ORGANIZATION OF THIS REPORT
At the request of DOE, this committee study of the National Academies 10 was conducted to provide an independent review of these treatment alternatives and to consider other possible courses of action. This report is intended to assist DOE and its contractors by providing input prior to the publication of the final EIS. As noted in the Preface, which summarizes how this study was conducted to address the committee's "Statement of Task,' the committee reviewed the references cited above and other literature that is listed in Appendix A. The committee also held two information-gathering meetings in Idaho Falls, Idaho, to consider the past work and current plans of DOE and its contractors. The committee invited a group of "technical experts" to attend the first of these meetings and to supply written comments in the form of trip reports that are shown in Appendix B. from different bins (Chapter 2);
Chapters 2 through 8 discuss in detail the important technical steps that would be undertaken in one or more of the treatment alternatives. These steps are as follows:
-
retrieval of calcine from bins, and possible blending of calcine from different bins (Chapter 2);
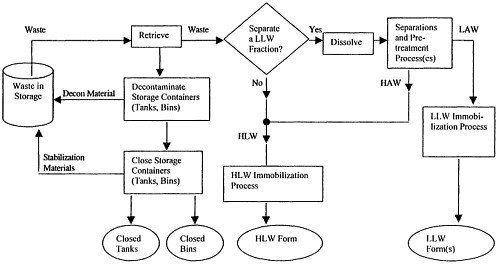
Figure 1.5 This diagram depicts in generic form the technical alternatives under consideration for the INEEL HLW calcine and SBW. The goal of each alternative is the conversion of initial waste products into appropriately immobilized forms. Each treatment alternative involves retrieval of waste and subsequent decontamination and closure of waste storage containers. Some alternatives use dissolution and processing steps to separate a low-level waste form. All alternatives immobilize a HLW form for onsite storage and eventual disposal.
-
dissolution of calcine (Chapter 2);
-
possible separations of selected radioactive species such as 137 Cs, 90Sr, and actinides (Chapter 3);
-
processing of SBW (Chapter 4);
-
vitrification (Chapter 5);
-
cementation (Chapter 6);
-
production of glass/ceramic and other waste forms (Chapter 7); and
-
closure of the tanks and bins (Chapter 8).
These process steps are evaluated in the above chapters to assess what can be done with current technical capability. Chapters 2 through 4 discuss ways to convert the two existing waste forms (i.e., solid calcine particles and liquid SBW) into waste streams that are ready for solidification. Chapters 5 through 7 discuss various solidification methods. Chapter 8 describes the process steps needed to remediate the unrecovered waste from the tanks and bin sets and to provide an in-situ closure of these structures.
Relevant regulations are not only those of the DOE and USNRC for radioactive HLW, but also those of the EPA (principally RCRA) that apply to the hazardous chemical constituents of INEEL HLW and SBW. Chapter 9 briefly discusses these regulatory constraints and considerations of cost, issues that influence a final decision. The full context for this decision includes multiple criteria (e.g., worker risk, public safety, and environmental impact) and multiple and potentially competing objectives (e.g., responsible waste management consistent with INEEL's future plans and missions, local interest in pristine cleanup versus national interest in reducing total program cost), as well as the technical alternatives. These issues pose challenges for program planning and management strategy, as discussed in Chapter 10, and raise the question of what should be done, which is discussed in Chapters 11 and 12 by way of examining the technical options favored by the committee for the calcine and the sodium-bearing waste, respectively.
Chapter 13 summarizes the most important findings, conclusions, and recommendations stated in each of the preceding chapters.