6
Drug Formularies
The efficacy of drug formularies in health care systems is in question. Formularies were present in hospitals before managed care became prevalent, and were intended to help reduce prescription drug costs and promote proper prescribing. In practice, however, costs have not been reduced and antibiotic resistance has risen. The following presentation summaries examine how managed care systems govern formulary management decisions, how pharmaceutical companies interact with the managed care system, and how formulary decisions could effectively address, in part, the problem of antibiotic resistance in emerging infections with respect to discovery and development of new agents.
REGULATORY ISSUES
Presented by Laurie Burke, R.Ph., M.P.H.
Chief of Managed Care Outcome and Labeling, Drug Marketing, Advertising, and Communication Division, Center for Drug Evaluation and Research, U.S. Food and Drug Administration
An important component of managed care systems in combating infectious diseases is the implementation of good regulatory policies, which is the responsibility of the U.S. Food and Drug Administration (FDA). Among FDA concerns is the proper dissemination of drug product information to health care practitioners and individuals responsible for making health care decisions. Another of its primary missions is to ensure that safe and effective drugs are available to the public as soon as possible. Because of its regulatory responsibility, FDA is concerned about how the changing health care environment will affect
society's health care needs. This is a major concern for FDA given that many changes are taking place in health care, such as a move toward managed care systems, a shift to more restrictive drug formularies, a move to outcomes-based research, increased availability of information, a restructuring of the pharmaceutical industry, and the rise of drug-resistant pathogens.
In an effort to address the challenges posed by the changing health care environment, FDA has implemented several initiatives. For instance, FDA is exploring how it can provide information and expertise useful for the evaluation of drug formularies and drug benefits programs. It is collaborating with other agencies, such as the Health Care Financing Administration (HCFA) and the Agency for Health Care Policy and Research, to sponsor a workshop intended to assist managed care organizations and HCFA with evaluating drug benefit designs. It is also implementing sections of the FDA Modernization Act of 1997 that deal both with the efficient and swift dissemination of information on new uses (offlabel information) of drugs and devices (Section 401) and with the dissemination of economic information in drug promotion (Section 114).
In addition, FDA recently published draft guidance on medical product promotion in the managed care environment. The agency acted because of evidence that suggested that medical product manufacturers could avoid regulatory oversight of promotional activities by allowing pharmacy benefit management companies and other health care organizations to disseminate volatile information on their behalf. Although not a legally binding document, the guidance represents FDA's expectations with respect to existing laws. Another draft guidance recently published by FDA has focused on policies related to advertising directly to consumers, which has resulted in a sharp increase in advertising on television. It is hoped that continued revision of draft guidance and policies will continue to have positive effects on the changing health care environment.
RELATIONSHIP BETWEEN MANAGED CARE FORMULARIES AND TREATMENT OUTCOMES
Presented by Susan Horn, Ph.D.
Senior Scientist, Institute for Clinical Outcomes Research, Vice President of Research for International Severity Information Systems, and Professor of Medical Informatics, University of Utah School of Medicine
Although formulary management is an important component in health care practice, its role in cost control and its effect on quality of care have not been well understood. In an effort to analyze the relationship between managed care formularies and treatment outcomes, the Institute for Clinical Outcomes Research (ICOR) conducted a 12-month study that analyzed the consequences of the cost-containment practices of health maintenance organizations (HMOs) and resulting outcomes. Six HMOs participated in a program that studied almost
13,000 patients with five different diseases: arthritis, asthma, ulcers, hypertension, and otitis media. The findings, published in the American Journal of Managed Care in March 1996, revealed that during a 12-month period, patients with these conditions had more than 99,000 office visits, incurred almost 500 emergency department visits and more than 1,000 hospitalizations, and used more than 240,000 different prescriptions (Horn et al., 1996). The 12-month study also examined practical cost-containment practices believed to have no negative repercussions on the quality of health care. These included greater control of second-opinion requirements, increased rigidity of gatekeepers and case managers, implementation of drug and physician office visit copayment levels, greater use of generic drugs, and greater limitation on formularies (defined as the percentage of FDA-approved drugs for a specific condition included in a provider's formulary and not requiring physician approval before generic prescription drugs are dispensed).
The ICOR study also controlled for patient variables to determine rates of health care utilization and treatment outcomes. The study confirmed that severely and acutely ill patients tend to consume more medical services. Findings also showed that visits to many different health care providers over the course of a year are associated with increased rates of utilization of health care services, along with increased rates of drug use, hospitalizations, and emergency hospital visits. After controlling for severity of illness and other confounding variables, the study found that for every condition (except otitis media), increased formulary restrictions were associated with increased numbers of physician office visits, emergency department visits, hospitalizations, and prescriptions and an increased cost of prescriptions over a 12-month period.
The methods used in the ICOR study to measure severity of illness include patient factors and their conditions, as well as the patient's physiologic symptoms and psychosocial characteristics. By controlling for differences in severity of illness among patients, data can be analyzed to determine which management strategies, interventions, and medications are most appropriate to provide better treatment outcomes. These methods are being implemented in many places—managed care settings, hospitals, long-term-care facilities, and ambulatory-care settings—in an effort to determine how to improve outcomes on the basis of patients' needs. This methodology also encourages health care systems to practice beyond their standard operating procedures and allows patient conditions to be identified more readily. The traditional method of using diagnostic codes, on the other hand, provides only a vague description of symptoms. If managed care systems use a more accurate picture of a patient's condition, physicians in these settings can become more knowledgeable about a patient's ailment and, therefore, can be better prepared to treat the disease.
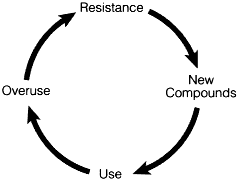
Figure 6-1 Drawbacks of the antibiotic era: the accelerating cycle of antibiotic resistance. Source: Modified from the work produced by John McGowan, Professor, Rollins School of Public Health, Emory University, Atlanta, Georgia.
BACTERIAL RESISTANCE, THE ANTIBIOTIC FORMULARY, AND OTHER MANAGEMENT STRATEGIES
Presented by Jerome J. Schentag, Pharm. D.
Professor of Pharmacy, State University of New York at Buffalo and Director, Clinical Pharmacokinetics Laboratory, Millard Fillmore Health System
It has become difficult to target the causes of antibiotic resistance in the current health care environment. There is reason for concern because the cycle of antibiotic resistance is not only complex but it also seems to be intensifying (Figure 6-1).
Several principal causes of endemic antimicrobial resistance have been proposed. Resistance patterns are believed to be the consequence of the many bacterial genetic pressures and antibiotics that humans have introduced into the environment. Subsequently, the importance of antibiotic selective pressure is increasingly a concern. The organisms that infect patients may develop resistance on the basis of the dose of the prescribed drug relative to the organism's susceptibility, a problem generally overlooked and understudied. In one study, researchers analyzing the relationship between dose and resistance for methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus faecium (VREF) in a large population of the Millard Fillmore Health System in Buffalo, New York, found that the manifestations of these infections resulted in an increasing incidence of serious infections. The use of suboptimal doses produced resistance in organisms from 93 percent of patients, whereas the use of optimal doses produced resistance in organisms from only 8 percent of patients.
Through extensive epidemiological and time course studies, researchers determined the endemic microbial resistance pattern in the Millard Fillmore Health System study. The linkage between these resistance patterns was exposure to an antibiotic at a dosage that was insufficient to exceed the minimum inhibitory concentration (MIC; or the lowest concentration of antibiotic to which an organism is susceptible) for the target organism for at least 80 percent of the dosing interval. For S. pneumoniae, antibiotic selective pressure was linked to the community use of oral cephalosporins, such as cefaclor. Findings also indicated that MRSA was linked to the use of cefazolin, whereas VREF was linked to the use of cephalosporins followed by the use of oral vancomycin. Researchers determined that this resistance pattern began in the early stages of hospitalization when at-risk patients received multiple doses of cephalosporins and other antibiotics. In addition, it was determined that the majority of these organisms originated within the infected patients and were not a result of cross transmission (Schentag et al., 1998).
In another study, patients with serious infections like nosocomial pneumonia required antibiotic therapy. Research demonstrated that the minimum effective antimicrobial dose consisted of an area under the inhibitory concentration titer (AUIC; an average level in serum over 24 hours in relation to the MIC) of 125. However, there was considerable variability in the actual AUICs for patients when antibiotics were given at the recommended dosages. When more than one antibiotic was used to treat a condition, higher doses of one drug were shown to reduce susceptibility to the other drug being used, as was the case with piperacillin and ceftazidime (Figure 6-2).
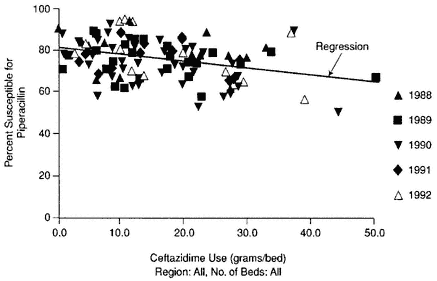
Figure 6-2 Percentage of Enterobacter cloacae isolates susceptible by antibiotic co-use. Source: Resistance Web, available at http://resistanceweb.mfhs.edu.
Microbial resistance has also been exacerbated by the use of broadspectrum antibiotic therapy. When hospitals adopted formularies in the mid-1980s to control costs, antibiotic resistance among isolates from patients surged dramatically (Rifenburg et al., 1996). The resulting monopolistic use of antibiotics has favored situations in which the extensive use of certain antibiotics for the treatment of a broad array of conditions has created a situation favorable to the emergence of resistant bacterial strains (Schentag et al., 1998). The current epidemic of resistant gram-positive strains, for example, can be traced back to the initiation of the formulary system in hospitals. Because infection control measures are not designed to address either the endogenous production of a pool of resistant organisms or the increase in the inoculum arising from the use of broad-spectrum suppressive antibiotic therapy, it is critical that antibiotic selective pressure be managed if there is to be any hope of successfully suppressing the endemic resistance patterns.
Several management strategies have already been implemented to Counter some of the less desirable effects of formularies as well as microbial resistance patterns. Emphasis on dosing so that concentrations in serum exceed the MIC, for instance, is an important component of the antibiotic use management strategy. This technique proposes that the concentration in serum determined from the AUIC be greater than 125, or that the concentration in serum be greater than the MIC for 80 percent of isolates tested. When the dose does not exceed this level, the probability of resistance has been shown to increase dramatically. Additional antiresistance strategies have included cycling of both the prophylactic antibiotics and the antibiotics used for treatment. Switching of medications during the course of therapy, such as a change from intravenous cephalosporin combination regimens to oral ciprofloxacin after the third day of treatment, has shown promising results as an antiresistance strategy. By exposing the patient to a variety of drugs, it becomes more difficult for bacteria to develop resistance. In addition, this practice has also been shown to be cost-effective (Paladino et al., 1991).
As stated earlier, cost reductions have been a major focus in the health care industry. Hospitals have implemented several administrative changes to realize greater cost savings. These have included shifting to managed care options, capitation, and HMOs and terminating private practice and fee-for-service systems. During the mid-1990s, benchmarking techniques were used by hospitals to locate institutions that successfully controlled their expenditures on antibiotics. Between 1993 and 1996, a benchmarking survey of more than 140 institutions nationwide and in Canada revealed that hospitals with average numbers of beds (between 200 and 400 beds) witnessed an increase in total antibiotic expenditure of over $300 (current dollars) per occupied bed (OB). This represents a 4 to 5 percent annual increase in antibiotic expenditures per OB. Only 9 percent of all the institutions surveyed experienced significant decreases (more than $500 per OB) in total antibiotic expenditures, although changes in the antibiotics included in the formulary had been made in all of these institutions to try to decrease antibiotic costs (Rifenburg et al., 1996).
Although adoption of formularies has influenced the increase in the rates of antibiotic resistance in hospitals, there is no evidence that embracing this practice has produced significant cost savings, a primary objective of formulary implementation. In fact, total antibiotic expenditures have experienced little fluctuation, despite replacement of the drugs included in the formulary, because alterations in the drugs in a formulary have resulted in cost shifting between categories rather than an overall decrease in costs. An attempt to restrict the use of one particular type of antibiotic generally increases the rate of use of other, more expensive antibiotics or less expensive antibiotics used in multiple combinations. The resulting net effect of these changes has led to an increase in total antibiotic expenditures. The antibiotic formulary system appears to be floundering; it is controlling neither costs nor resistance. On the contrary, this system seems to be making both situations worse (Rifenburg et al., 1996).
In addition to tracking costs, benchmarking studies can be used to analyze the susceptibility patterns of indicator organisms and the antibiotic regimens prescribed to combat these pathogens. One particular study conducted by the State University of New York (SUNY) at Buffalo specifically looked for the resistance and antibiotic expenditure patterns that developed as a result of antibiotic use. The targeted combination of antibiotics and indicator organisms included the percentages of ceftazidime-resistant Enterobacter cloacae, piperacillin-and penicillin-(all penicillins) resistant Escherichia coli, fluoroquinoloneresistant Pseudomonas aeruginosa, vancomycin (intravenous and oral)-resistant Enterococcus faecium, and methicillin-and cephalosporin-resistant Staphylococcus aureus (Ballow and Schentag, 1992).
Rapid communication of medical information is a critical component in the management of antimicrobial resistance. The SUNY study compiled antibiotic resistance profiles for a large selection of indicator organisms. By making these data available on the Internet (http://resistanceweb.mfhs.edu), other institutions were able to quickly access information that could assist them with the development of management strategies that combat antibiotic resistance. Sharing of other types of information is also critical to becoming better prepared to address antimicrobial resistance. Again, the Internet has become an excellent tool to relay this information. Through this mechanism, hospitals can report critical health care information, such as antimicrobial management practices, antibiotic expenditures, antibiograms and other resistance trends, as well as overall expenditures. In addition, because the Internet is interactive, hospitals and pharmacies can enter their own data and track antibiotic use versus resistance in their own institutions.
Besides using the Internet, medical researchers have developed a computer software to help track resistance to antimicrobial agents. This software produces an integrated medical record obtained from patient pharmacy orders and admission history, laboratory results, and financial information. The system, manipulated in a prospective manner for the adoption of patient management strategies, can be useful in identifying when an intervention is necessary (Figure 6-3). Specifically, this can be accomplished by using the indices to identify
patients who are at high risk of therapeutic failure or who are infected with isolates that will acquire resistance in the early stages of therapy before remedial treatment fails.
For example, some studies demonstrated that calculations of the AUIC could be used to target prospective regimens by improving the chances of curing nosocomial pneumonia and other serious infections. An effective method has consisted of organizing a clinical intervention team in which antimicrobial regimens are optimized during the early stages of therapy to lower the chances of costly events, such as acquired bacterial resistance. Besides identifying when an intervention is needed, preliminary data indicate that (3-day) interventions have helped control antibiotic costs (Table 6-1). In essence, this iterative computerized system may become an attractive solution for implementation by managed care organizations; the antibiotic cost savings realized from interventions may cover most expenses associated with the adoption of new strategies.
Antibiotic resistance, especially endemic resistance, is firmly established in today's society. Current practices of antibiotic use have greatly contributed to this problem. Dispensation of new antibiotics alone will not counter antimicrobial resistance; thus, pharmaceutical companies cannot solely be responsible for effectively resolving this problem. A crucial step will involve changing the ways in which hospitals conduct business, such as by using the combined strategies of infection control and antibiotic management. Both of these measures will be critical if societies want to avoid a return to the conditions present in the pre-antibiotic era. [Editor's Note: For additional information on antimicrobial resistance, see the previous workshop report by the Institute of Medicihe's Forum on Emerging Infections: Antimicrobial Resistance: Issues and Options. (IOM, 1998).]
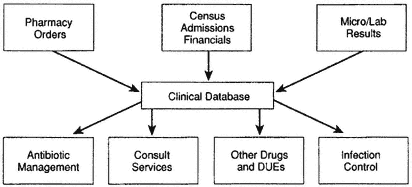
Figure 6-3 Computer-assisted outcomes management. Note: DUEs = drug usage evaluations. Source: Schentag et al., 1996.
TABLE 6-1 Summary of Cost Analysis for Two Treatment Groups, Including Drug and Ancillary Expenses
|
|
Average Cost in Dollars (range) |
|
|
Intervention |
Intravenous Treatment Group |
Ciprofloxacin Group |
|
Days 1–3 (per day) |
61 (10–138) |
62 (7–165) |
|
Day 4+ (per day) |
46* (6–170) |
8 (6–40) |
|
Total per patient |
646 |
353 |
|
Savings per patient |
|
293 |
|
* Does not include the costs of treatment continuation with oral antibiotics (e.g., dicloxacillin, cephalexin, co-trimoxazole). Inclusion of these costs increases the total cost but reduces the average cost per day to $36. SOURCE: Paladino et al., 1991. |
||
ECONOMICS OF RESTRICTIONS ON PHARMACEUTICALS
Presented by Douglas L. Cocks, Ph.D.
Senior Research Scientist for Health Economics, Health Services and Policy Research, Eli Lilly and Company
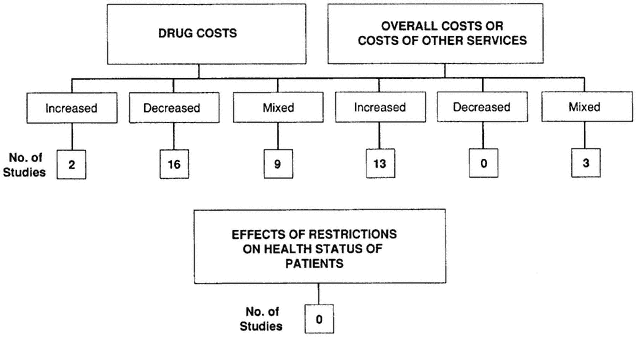
Historically, most formularies were established as a mechanism for reducing costs by limiting access to certain pharmaceuticals. Results from 30 studies that analyzed the effects of formulary restrictions indicated otherwise, especially when overall costs were considered (Figure 6-4).
It is important to acknowledge, however, that this investigation was not a true meta-analysis. These 30 studies not only varied considerably in quality but also involved many different health care settings and a wide range of providers and payers, including hospitals, managed care organizations, Medicaid, and nursing homes.* Although the findings suggest that formularies can control drug costs in some cases, these results further indicated that use of formularies was associated with increases in the costs of other health care services as well as overall costs. Nevertheless, all of these studies were deficient in one major area: none analyzed the effects of formulary restrictions on the health outcomes of patients. Research needs to concentrate on this area.
SUMMARY OF CHALLENGES AND OPPORTUNITIES
Jonathan R. Davis, Ph.D., Editor
The changing health care environment has produced more restrictive drug formularies, a development originally intended to help reduce prescription drug costs while still maintaining quality health care. Some data suggest that these goals have not materialized and that formulary policies concerning antibiotics may be contributing to the rise in the rates of antibiotic resistance. The original goals of formularies need to be reevaluated. Although further studies are needed to address the increasing costs associated with formularies, some cost-containment practices may be possible without imposing negative repercussions on the quality of health care. These include greater control of second-opinion requirements, increased rigidity of gatekeepers and case managers, implementation of drug and physician office visit copayment levels, greater use of generic drugs, and greater limitations on formularies.
Ensuring Availability of Current Information on New Drugs
The Forum members wanted to highlight several initiatives that were discussed at the workshop and that could be implemented to adequately address the changing health care environment. As the regulatory agency of the government, FDA ensures that proper drug development information is disseminated to health care practitioners and individuals responsible for health care decision making and that safe and effective drugs are available to the public in the least amount of time. FDA can thus continue to administer several initiatives to try to make sure that there is a positive transition to a new health care environment. Not only can it provide information and expertise useful for the evaluation of drug formularies and drugs benefits programs, but it can also collaborate with other agencies in sponsoring workshops specifically intended to assist managed care organizations and other health care organizations with evaluating the designs of drug benefits programs. In addition, FDA is uniquely positioned to implement health care policies in an effort to efficiently and swiftly disseminate information on new uses of drugs and medical devices, as well as provide guidance on medical product promotion in the managed care environment and guidance on broadcast advertising policies. A continued revision of guidance and policy is likely to sustain the positive effect that FDA has on health care activities in the changing health care environment.
Managing Antibiotic Selective Pressure
Microbial resistance is exacerbated by the use of broad-spectrum antibiotic therapy, a practice often encouraged by drug formularies. The extensive use of
certain antibiotics for the treatment of a broad spectrum of medical conditions has created a situation favorable to a surge in the number of resistant strains. Furthermore, restricting the use of a particular antibiotic generally results in two outcomes: (1) increased use of other, more expensive antibiotics or (2) increased use of less expensive antibiotics in combinations. The net effect is an increase in total expenditures for antibiotics.
Because it is unlikely that pharmaceutical companies will discover and develop very narrow spectrum agents in the foreseeable future (since this activity is not economically feasible for them), it will become critically important to develop and implement strategies so that antibiotic selective pressure is effectively managed to suppress the endemic resistance patterns.
Management strategies can be implemented or continued to be enforced to counter some of the less desirable effects of formularies as well as microbial resistance patterns. Important antiresistance strategies include an emphasis on dosing on the basis of the concentrations achievable in the serum to decrease the probability of resistance and cycling of both prophylactic antibiotics and those being used in the treatment of a patient's condition. In addition, studies presented at the workshop suggested that switching of medications during the course of therapy, besides being cost-effective, has been shown to be a promising strategy for fighting infections. By using this technique, by which patients are exposed to a variety of drugs, it becomes more difficult for bacteria to develop resistance. Although workshop participants outlined several strategies, they suggested that additional research be conducted to avoid public health risks from managed care formulary decisions.
Need for New Management Strategies for Cost Reduction
Databases are needed to help determine which management strategies, interventions, and medications are most appropriate for providing better treatment outcomes on the basis of the patient's needs. Such databases could be used to identify the effects of drug formulary restrictions on the health outcomes of patients.
Other management strategies that would not compromise the quality of care need to be considered. Workshop participants clearly made the point that antibiotic formularies in managed care organizations are expected to continue to be decided mostly on the basis of procurement costs until data are validated. Because it is becoming increasingly difficult to target antibiotic resistance in the current health care environment, cutting corners in this area will likely exacerbate the problem. Instead, sensible management tactics in managed care could concentrate on the standardization of treatment for costly medical conditions as a way of reducing costs in health care practice. These could include treatments for such conditions as complete hip replacements, heart failure treatments, diabetes, and asthma. However, workshop discussions highlighted the need for more studies to address the effects of formulary restrictions on the health outcomes of patients. This is a major gap on which research efforts need to concentrate.
Achieving Quality Care and Cost Containment
In some cases, the level of use of antibiotics for patients who need them is being reduced as a result of increased pressures to reduce costs. Not only does this breed patient dissatisfaction, but also, most importantly, this activity may compromise the quality of health care and thereby increase incidences of infection. New strategies are required to realize cost savings from lower levels of antibiotic use as a result of formulary management decisions, such as conducting benchmarking studies and developing an iterative computerized system to help track antimicrobial resistance.
Health care policies that provide efficient and swift dissemination of information on new uses of drugs, and that provide guidance on the promotion of medical products in the managed care environment should be implemented. Additionally, workshop participants proposed that, after controlling for severity of illness, minimization of costs and services can be attained while simultaneously controlling costs. This line of argument leads to discussions about the use of databases as a tool for achieving such results by taking into account several patient factors. Databases can help determine which management strategies, interventions, and medications are most appropriate for the provision of better treatment outcomes on the basis of the patient's needs. Although the traditional method of using diagnostic codes provides only a vague description of symptoms, the database methodology encourages health care systems (including managed care settings, hospitals, long-term care facilities, and ambulatory-care settings) to provide services outside their traditional roles and allow patients' conditions to be identified more readily. By obtaining a more accurate picture of a patient's condition, physicians can better ascertain the patient's ailment and thus be in a better position to treat the symptoms. In turn, this may translate into cost savings, in addition to an increased quality of care and faster care.
Summary
Discussions of the preliminary data on drug formularies suggested that formulary restrictions fail to control the overall costs of health care or to improve the quality of care. The drugs available in formularies vary widely among providers. With respect to infectious diseases, however, it appears that formulary restrictions—particularly the monopolistic use of a few broad-spectrum drugs—may actually contribute to the emergence of antibiotic resistance. Better data are still needed on these issues, for in the absence of these data, providers may likely continue to base formulary decisions primarily on cost instead of on the desire to improve the quality of care and to limit the spread of antibiotic resistance.
The panelists thought that it would be desirable to allow physicians to prescribe a greater variety of antibiotics, especially narrow-spectrum drugs, and when trying to treat infections caused by specific microorganisms to adjust the dosage and duration of therapy to match the needs of particular patients. Several
examples of evidence-based, computer-aided systems are available to assist with this kind of therapeutic decision making. In the longer term, it would be desirable to develop technology that bridges the gap between diagnosis and treatment by rapidly identifying the infectious agent but also pointing the way to a highly targeted therapeutic agent. However, few orphan drugs that have been developed to treat rare emerging infectious diseases were identified, and the realities of the pharmaceutical industry (and of clinical trials with small populations of patients) militate against the development of narrow-spectrum, low-volume drugs, particularly when the restrictions created by the use of drug formularies are considered. FDA is developing guidelines for off-label use, product marketing information, direct advertising to the consumer, and on the role of pharmaceutical benefits management companies; the effects of these regulations on drug development and drug formularies remain to be determined.