B14
Furan
Hector D. Garcia, Ph.D., and John T. James, Ph.D.
Johnson Space Center Toxicology Group
Medical Operations Branch
Houston, Texas
PHYSICAL AND CHEMICAL PROPERTIES
Furan is a volatile, clear, colorless liquid that turns brown upon standing. It is soluble in alcohol and ether and insoluble in water (Windholz 1976; Sax and Lewis 1989). It forms resins on evaporation or when in contact with mineral acids but is stable to alkalies (Sax and Lewis 1989).
|
Formula: |
C4H4O |
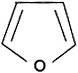 |
|
CAS no.: |
110-00-9 |
|
|
Synonyms: |
1,4-Epoxy-1,3-butadiene, furfuran, oxole, tetrole, divinylene oxide, oxacyclopentadiene, NCI-C56202 |
|
|
Molecular weight: |
68.07 |
|
|
Boiling point: |
31.36°C |
|
|
Melting point: |
–85.65°C |
|
|
Saturated vapor concentration: |
650,000 ppm at 20°C |
|
|
Lower explosive limit: |
2.3% |
|
|
Upper explosive limit: |
14.3% |
|
|
Density: |
0.9371; vapor density, 2.35 |
|
|
Conversion factors: |
1 ppm = 2.78 mg/m3 1 mg/m3 = 0.359 ppm |
|
OCCURRENCE AND USE
Furan occurs in oils obtained by the distillation of rosin-containing pine wood (Windholz 1976). Commercial production of furan is performed by the
decarbonylation of furfural or by the oxidation of butadiene (NTP 1993). Furan is widely used industrially, primarily as a solvent for resins, in the formation of lacquers, a binder in foundry applications, and a chemical intermediate for organic syntheses. It is also found in the vapor phase of cigarette smoke at a concentration of about 8.4 µg/40 mL puff (Egle and Gochberg 1979).
Furan has been identified as a major component of certain processed foods and drinks. It has been found in the volatile fraction from roasted ground coffee, in the vapor from baked white bread, and in head-space samples from canned meat (Merritt et al. 1963; Mulders et al. 1972; Persson and von Sydow 1973).
Derivatives of furan, such as tetrahydrofuran and 2-methylfuran, have been detected occasionally in spacecraft atmospheres (James 1991). Furan has not been detected in the atmosphere of American spacecraft but has been detected occasionally at low concentrations (0.12 mg/m3) in the Russian space-station Mir core module.
TOXICOKINETICS AND METABOLISM
Absorption
Anesthetized dogs exposed for 1 to 2 min to furan vapors at concentrations of 400 to 600 mg/m3 were found to have total respiratory-tract retentions between 90% and 95% and lower tract retentions of 87% to 93% (Egle and Gochberg 1979). The fraction of inhaled furan retained was not affected by tidal volume changes but was directly related to concentrations inhaled (Egle and Gochberg 1979).
Administration of furan by oral gavage leads to absorption from the gastrointestinal tract into the portal vein and to the liver. In rats, it has been shown that the rate of furan metabolism in hepatocytes greatly exceeds the ability of furan to be delivered by the blood flow to the liver (Kedderis and Held 1996); hence, little unmetabolized furan should reach the general circulation after passing through the liver. It appears, however, that some furan might bypass the liver. In CIIT studies of mice receiving a single gavage dose of furan at 50 mg/kg, histopathology showed that the subcapsular parenchyma of the visceral surface of the left and caudate hepatic lobes had necrosis with some inflammatory cell infiltrate primarily at 12 hafter exposure, suggesting that some of the furan diffused through the stomach wall and into the liver subsequent to gavage (Wilson et al. 1992). Some unmetabolized furan reaches the lungs, either by diffusion or through the general circulation, because unmetabolized furan is found in the expired air of rats given furan by oral gavage (see below).
Distribution
The tissue distribution of radioactivity 24 h after oral gavage of [2, 5-14C-furan] to male Fischer 344 (F344) rats at 8 mg/kg has been reported (Burka et al. 1991). The nmol eq/g of tissue were as follows: liver, 307; kidney, 60; large intestine, 25; small intestine, 13; stomach, 6; blood, 6; and lung, 4. The labeling distributed in the tissues represented approximately 15% of the total dose administered. The label in liver included no unmetabolized furan, and none of the label was bound to DNA; most of the label was associated with protein and could not be extracted from it. Over the next 7 d, the radioactivity in liver, kidney, and blood declined to near the detection limits. Repeated administration of the same dose of furan for 8 d resulted in the accumulation of labeling to 1100 nmol eq/g of liver tissue, 320 nmol eq/g of kidney, and 37 nmol eq/g of blood.
Elimination
During the first 24 h after an 8-mg/kg oral gavage in male rats, approximately 80 % of the radioactivity derived from furan was eliminated in the expired air, urine, and feces (Burka et al. 1991). The radioactivity in expired air consisted of unchanged furan (14 %) and carbon dioxide (26%). The urine contained 20% of the radioactivity administered, and HPLC analysis suggested at least 10 metabolites were present. Approximately 22% of the single dose was eliminated in the feces. The fraction eliminated in the feces was unchanged during 8 d of administration, but the fraction found in the urine increased to 33% of the total dose after the eighth dose.
Metabolism
There is convincing evidence that, in F344 rats, furan is bioactivated in vitro and in vivo to cis-2-butene-1,4-dial by cytochrome P-450 2E1-catalyzed oxidation (Burka et al. 1991; Carfagna et al. 1993; Chen et al. 1996). Cytochrome P-450 2E1 is present not only in hepatocytes but also in nasal, lung, and other tissues. Thus, the route of exposure might influence the site of toxic lesions.
Kedderis and colleagues at the Chemical Industry Institute of Toxicology (CIIT) used computational models to study interspecies differences in the rate of metabolism of furan by estimating the effect of body size and blood-flow rates on the delivery of furan to the liver (and hence, the rate of production of
active metabolites by hepatocytes of the liver). Using a physiologically based pharmacokinetic (PBPK) model, Kedderis and Held (1996) showed that, although furan is rapidly metabolized by isolated hepatocytes from humans and rodents, the rate of metabolism of inhaled furan in humans is 10-fold lower than that in mice and 3-fold lower than that in rats. That result was based on a simulated exposure to furan at a concentration of 10 ppm for 4 h. The lower rate in humans is due to the comparative kinetics of hepatic blood flows in rodents and humans using a model in which inhaled furan vapor is metered into the bloodstream via the breathing rate and distributed throughout the organism at rates that are a function of body size (Kedderis and Held 1996).
In rats, furan is bioactivated by a cytochrome-P-450-dependent pathway to one or more metabolites that uncouple oxidative phosphorylation, deplete cellular ATP, and induce DNA double-strand breaks before cell death (Mugford and Kedderis 1997). Errors in repair of the DNA breaks have been hypothesized to result in mutations and contribute to the neoplastic effects of furan (Mugford and Kedderis 1997).
TOXICITY SUMMARY
The toxicity of furan in animals has been studied primarily by using the oral exposure route. A few studies examined the toxic effects of intraperitoneal (ip) injection of furan, but only two reports investigated the inhalation toxicity of furan vapors in animals. No studies were found on the effects of furan in humans by any route of exposure.
Acute and Short-Term Exposures
General Toxicity
Since the mid-1920s, various investigators have shown that furan is quite toxic to many organ systems by various routes of exposure. Furan can be absorbed through the skin, and its vapors are narcotic (anesthetic and convulsant) (Koch and Cahan 1925; Sax and Lewis 1989). Because its narcotic properties were recognized first, early investigators had hoped to use furan as an anesthetic but found that, although it has some anesthetic effects, it is extremely toxic (Koch and Cahan 1925).
Furan injected ip has been found to cause acute pulmonary edema (Egle and Gochberg 1979) and liver and kidney damage in mice (Wiley et al. 1984). Early studies (Koch and Cahan 1925) reported that furan has a corrosive effect
on mucous membranes of the gastrointestinal tract when given orally in capsules (0.25 mL/kg) to dogs and rabbits. Oral exposure induced a copious flow of bloody saliva, watery fluid from the nose, marked hyperemia of lungs and dilation of blood vessels, and gastrointestinal-tract hemorrhages (Koch and Cahan 1925). Other symptoms of oral exposure include cherry-red blood, soft swollen kidneys, and liver resembling the first stages of chloroform poisoning (Koch and Cahan 1925).
Lethality
Since the mid-1920s, studies have reported on the acute lethality of furan in animals. With intravenous injection (1.5 mL) in dogs, furan caused symptoms and postmortem changes similar to acute cyanide poisoning (Koch and Cahan 1925). Convulsions were followed rapidly by death. Intraperitoneal injection of 0.2 mL furan into a rat stopped respiration within 30 s, although the heart continued to beat for 3 min (Koch and Cahan 1925). Effects noted by the investigator included hyperemic liver and intestines, cherry-red blood, and dilation of the blood.
Furan vapor is highly toxic to Sprague-Dawley rats and has a steep dose-response curve with a 1-h LC0 of 2850 ppm and a 1-h LC50 of 3464 ppm (Terrill et al. 1989). The symptoms of intoxication by inhalation are an increase in respiratory rate, fall of blood pressure, convulsions, complete anesthesia, and death from asphyxia (Koch and Cahan 1925; Terrill et al. 1989). Although no concentrations were given, an early report indicated that rats and rabbits inhaling furan from a saturated cotton wad collapsed after a short struggle and died shortly thereafter (Koch and Cahan 1925). Similarly, dogs anesthetized with ether died suddenly when furan was substituted for ether after induction of light anesthesia (Koch and Cahan 1925). Another early paper reported that a lethal concentration (presumably by inhalation for an unspecified exposure time) for cats was 136 mg/m3 (Johnston 1931).
Two LC50 studies have been published for furan. The 1-h LC50 for furan inhaled by mice was reported to be 0.12 µg/mL (43 ppm) (Egle and Gochberg 1979). The exposure methods used in the study, however, would be unacceptable today. The mice in the study were exposed in 5.2-L sealed glass desiccators into which furan vapor was injected from a 100-cc glass syringe. Calculations of the volume of air breathed in 1 h by four mice (4 × 40 mL/min × 60 min = 9.61) suggest that hypoxia might have contributed to the observed deaths of mice in this early study. The authors stated, ''Behaviorally, in all mouse inhalation experiments, those mice that did not survive the hour became hyperactive for periods of 10 to 15 minutes, then their breathing appeared
labored, and they died soon after . . . . Examination of the lungs revealed inflammation and fluid accumulation." The relative contributions of furan exposure and oxygen deprivation to those effects cannot be determined from that study. Therefore, it was not used in determining an AC for prevention of lethality.
Ten years after Egle and Gochberg's report, Terrill et al. (1989) reported an 80-fold higher 1-h LC50 (3500 ppm) for Sprague-Dawley rats exposed to analytical concentrations of 1000, 2850, or 4050 ppm in a dynamic Hinnerstype chamber; lethality was observed only at the highest exposure concentration. Signs of toxicity during exposure included respiratory distress, increased secretory responses, and, at the highest concentration, death, which in many instances, was delayed until the end of the first week or the beginning of the second week after exposure. No exposure-related lesions were found in gross postmortem evaluations of 14-d survivors (Terrill et al. 1989).
Nephrotoxicity and Hepatotoxicity
Severe kidney and liver toxicity were seen in animals following high doses of furan. In experiments designed to examine the ability of two chemicals to inhibit the liver and kidney toxicity of furan, furan was injected ip into eight mice at a dose of 0.2 mL/kg (187 mg/kg). When the animals were sacrificed 24 h after injection, they were found to have severe coagulation necrosis of tubular cells of the kidney cortex, atrophy of the glomeruli of the kidneys, and massive coagulation necrosis of centrilobular parenchymal cells of the liver (Masuda et al. 1984). Similar results were found 24 h after ip injection of furan into mice at 5.1 mmol/kg (347 mg/kg) (Wiley et al. 1984).
In 16-d gavage studies conducted by the National Toxicology Program (NTP 1993), mottled and enlarged livers were observed at necropsy in male rats receiving furan at 20, 40, or 80 mg/kg and in females receiving 40, 80, or 160 mg/kg. In similarly exposed mice, no furan-related lesions were observed at necropsy.
Subchronic and Chronic Exposures
Nephrotoxicity and Hepatotoxicity in Gavaged Rodents
In 13-w gavage studies conducted by the NTP, rats exposed to furan in corn oil for 5 d/w at 0, 4, 8, 15, 30, or 60 mg/kg had dose related increases in the incidence and severity of toxic lesions of the liver, including bile-duct hyper-
plasia, cholangiofibrosis, cytomegaly and degeneration of hepatocytes, and nodular hyperplasia of hepatocytes (NTP 1993). Kidney lesions were present in rats receiving 30 or 60 mg/kg. Toxic liver lesions (cytomegaly, degeneration, and necrosis of hepatocytes) were also present in all groups of mice exposed to furan at the same doses as the rats.
Dose-related increases in the incidence of numerous non-neoplastic lesions were noted in rats of both sexes exposed by gavage for 5 d/w with furan in corn oil at 0, 2, 4, or 8 mg/kg in the NTP 2-y bioassay. These lesions included multiocular cysts, fibrosis, metaplasia, hyperplasia, and cytomegaly of the biliary tract and chronic inflammation, proliferation and hepatocellular cytomegaly, cytoplasmic vacuolization, degeneration, nodular hyperplasia, and necrosis in the liver (NTP 1993). In mice, non-neoplastic hepatocellular lesions included hepatocyte cytomegaly, degeneration, necrosis, multifocal hyperplasia, and cytoplasmic vacuolization and biliary-tract dilatation, fibrosis, hyperplasia, and inflammation (NTP 1993).
More recent studies at the National Institute of Environmental Health Sciences (NIEHS) and CIIT have examined the effects of lower doses of furan. A lowest-observed-adverse-effect level (LOAEL) of 4 mg/kg and a no-observed-adverse-effect level (NOAEL) of 2 mg/kg for histological changes and hepatocyte proliferation were found in male mice exposed by gavage for 5 d/w for 3, 6, and 13 w with furan at 0, 0.5, 2, 4, 8 or 15 mg/kg (R. Maronpot, National Toxicology Program, NIEHS, personal commun., 1994).
Carcinogenicity
No studies have been reported on the carcinogenicity of inhaled furan vapors; however, furan has been shown to be a potent, multi-target carcinogen by the oral route. Furan has been tested by gavage in a 2-y bioassay by the NTP (1993). Groups of 70 rats of each sex were administered furan in corn oil by gavage at 0, 2, 4, or 8 mg/kg for 5 d/w for 2y. Groups of 50 mice of each sex received doses of furan at 0, 8, or 15 mg/kg, for 5 d/w for 2 y. Exposure was found to induce a variety of neoplastic lesions in both rats and mice (Table 14-1).
Rats were found to be the more sensitive species for induction of neoplasias, the most prevalent neoplasm being cholangiocarcinomas. Interim sacrifices revealed high (but less than 100%) incidences of cholangiocarcinomas in rats given 2 mg/kg for 9 or 15 mo (Table 14-2 and Figure 14-1).
Exposure of male rats to furan at 30 mg/kg for 13 w and observation for the remainder of 2 y yielded an overall incidence of cholangiocarcinoma at 100% (NTP 1993). As shown in Table 14-3, furan also induced hepatocellular
TABLE 14-1 Neoplastic Lesions in 2-y Study of 50 Rats and 50 Mice
|
|
|
|
Doses, mg/kg |
||||
|
Species |
Sex |
Neoplasm |
0 |
2 |
4 |
8 |
15 |
|
Rat |
Male |
Monocytic leukemia |
8 |
11 |
17 |
25 |
— |
|
|
|
Cholangiocarcinoma |
0 |
43 |
48 |
49 |
— |
|
|
|
Hepatocellular adenoma/carcinoma |
1 |
5 |
22 |
35 |
— |
|
Rat |
Female |
Monocytic leukeumia |
8 |
9 |
17 |
21 |
— |
|
|
|
Cholangiocarcinoma |
0 |
49 |
50 |
48 |
— |
|
|
|
Hepatocellular adenoma/carcinoma |
0 |
2 |
4 |
7 |
— |
|
Mice |
Male |
Hepatocellular adenoma/carcinoma |
26 |
— |
— |
44 |
50 |
|
|
Female |
Hepatocellular adenoma/carcinoma |
7 |
— |
— |
34 |
50 |
TABLE 14-2 Incidences of Cholangiocarcinomas in Rats in the NTP Bioassay
|
|
9 months |
15 months |
24 months |
|||
|
Dose, mg/kg |
Male |
Female |
Male |
Female |
Male |
Female |
|
0 |
0/10 |
0/10 |
0/10 |
0/10 |
0/50 |
0/50 |
|
2 |
5/10 |
4/10 |
7/10 |
9/10 |
43/50 |
49/50 |
|
4 |
7/10 |
9/10 |
9/10 |
9/10 |
48/50 |
50/50 |
|
8 |
10/10 |
10/10 |
6/10 |
7/10 |
49/50 |
48/50 |
adenomas and hepatocellular carcinomas in male and female mice and rats and mononuclear-cell leukemias in male and female rats. The NTP (1993) concluded that there was clear evidence of carcinogenic activity of furan in male and female F344/N rats and male and female B6C3F1 mice.
Genotoxicity
Although furan is not mutagenic in the Salmonella-microsome preincubation test, it is a mutagen in the L5178Y TK+/- mouse lymphoma-cell forward-
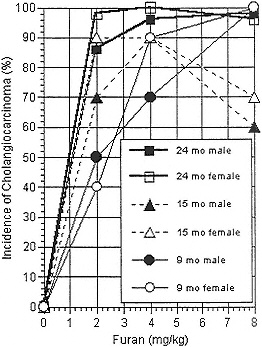
FIGURE 14-1 Results of NTP bioassay of furan in F344 rats.
mutation assay without the need for metabolic activation by rat liver S9 mix (McGregor et al. 1988). It is also genotoxic in the phage T7 inactivation test (Ronto et al. 1989). Furan does not induce sex-linked recessive lethal mutations in germ cells of male Drosophila melanogaster (NTP 1993). Furan does induce chromosomal aberrations and sister chromatid exchanges in cultured Chinese hamster ovary cells in vitro, with and without metabolic activation, and induces forward mutations in cultured mouse L5178Y lymphoma cells (NTP 1993). It induces chromosomal aberrations, but not sister chromatid exchanges, in bone-marrow cells of mice injected ip (NTP 1993). Furan produces chromosomal aberrations in cultured Chinese hamster ovary cells after a 3-h exposure only in the presence of complete S9 mixture; removal of NADP from the S9 abolished furan's clastogenicity (Stich et al. 1981).
Comparison of activated oncogenes from furan-induced B6C3F1 mouse-liver tumors with oncogenes from spontaneously developing mouse-liver tumors suggests that furan causes an increased incidence in mouse-liver tumors at least in part by induction of novel weakly activating point mutations in H-ras genes
(Reynolds et al. 1987). Slower than normal electrophoretic mobilities were observed for some of the mutated c-H-ras proteins (e.g., p21) from furaninduced liver-tumor DNA transfectants, whereas faster than normal mobilities were observed for mutated c-H-ras proteins from spontaneously developed liver-tumor DNA transfectants (Reynolds et al. 1987). It should be noted that the B6C3F1 strain of mice used in the Reynolds et al. study have a background incidence of hepatocellular carcinomas of 50%. In that case and on the basis of the data alone, furan appears to induce mouse-liver tumors by a direct genotoxic mechanism involving activation of oncogenes.
Reproductive Toxicity
No data were found on the reproductive toxicity of furan.
Developmental Toxicity
No data were found on the developmental toxicity of furan.
Interactions with Other Chemicals
No data were found on the interaction of furan with other chemicals.
TABLE 14-3 Toxicity Summary
|
Dose or Concentration |
Exposure Duration |
Species |
Effects |
Reference |
|
|
|
|
Inhalation |
|
|
43 ppm |
1 h |
Mouse |
LC50 |
Egle and Gochberg 1979 |
|
3,464 ppm |
1 h |
Rat |
LC50 |
Terrill et al. 1989 |
|
|
|
|
Oral (Capsules) |
|
|
0.25 cc/kg |
Once |
Dog, rabbit |
Copious flow of bloody saliva; bright cherry-red blood; hyperemic liver, kidneys, lungs; lethal to 4 of 5 dogs and 2 of 2 rabbits |
Koch and Cahan 1925 |
|
|
|
|
Oral (Gavage) |
|
|
2 mg/kg |
5 d/w, 13 W |
Mouse, male |
NOAEL for histological changes and hepatocyte proliferation |
R. Maronpot, personal commun., 1994 |
|
4 mg/kg |
5 d/w, 13 W |
Mouse, male |
LOAEL for histological changes and hepatocyte proliferation |
R. Maronpot, personal commun., 1994 |
|
20, 40, 80 mg/kg |
16 d |
Rat, male |
Mottled and enlarged livers |
NTP 1993 |
|
40, 80, 160 mg/kg |
16 d |
Rat, female |
Mottled and enlarged livers |
NTP 1993 |
|
20, 40, 80 mg/kg |
16 d |
Mouse |
NOAEL for lesions at necropsy |
NTP 1993 |
|
2, 4, 8 mg/kg |
24 mo |
Rat |
Various neoplasms (see Table 14-1) |
NTP 1993 |
|
4 mg/kg |
13 w |
Rat |
Cholangiofibrosis, biliary-tract hyperplasia, and Kupffer cell pigmentation in 4 of 10 males; biliary-tract hyperplasia in 7 of 10 females |
NTP 1993 |
|
Dose or Concentration |
Exposure Duration |
Species |
Effects |
Reference |
|
8 mg/kg |
13 w |
Rat |
Cholangiofibrosis in 7 of 10 females, 7 of 10 males; biliarytract hyperplasia in 9 of 10 males, 10 of 10 females; hepatocyte degeneration in 7 of 10 males; Kupffer cell pigmentation in 6 of 10 males and 8 of 10 females |
NTP 1993 |
|
8 mg/kg |
24 mo |
Rat |
Urinary bladder pappilomas in 3 of 50 females |
NTP 1993 |
|
8, 15 mg/kg |
24 mo |
Mouse |
Hepatocellular adenomas or carcinomas (see Table 14-1) |
NTP 1993 |
|
15, 30, 60 mg/kg |
13 w |
Rat |
Cholangiofibrosis and biliary tract hyperplasia; hepatocyte cytomegaly, degeneration, necrosis, and Kupffer cell pigmentation |
NTP 1993 |
|
60 mg/kg |
13 w |
Rat |
Renal tubule dilation and necrosis; thymus atrophy; testicular atrophy; ovarian atrophy |
NTP 1993 |
|
|
|
|
Injection |
|
|
187 mg/kg |
Single ip injection |
Mouse |
Severe kidney necrosis; massive liver necrosis |
Masuda et al. 1984 |
|
347 mg/kg |
Single ip injection |
Mouse |
Liver necrosis and kidney toxicity |
Wiley et al. 1984 |
|
937 mg/kg |
Single ip injection |
Rat |
Respiration stopped in 0.5 min; heart stopped in 3 min; hyperemic liver and intestines, cherry red blood, dilated blood vessels |
Koch and Cahan 1925 |
No exposure limits have been set by other organizations.
TABLE 14-4 Spacecraft Maximum Allowable Concentrations
|
Duration |
Concentration, ppm |
Concentration, mg/m3 |
Target Toxicity |
|
1 h |
4 |
1.4 |
Hepatotoxicity |
|
24 h |
0.4 |
1 |
Hepatotoxicity |
|
7 da |
0.025 |
0.07 |
Carcinogenicity |
|
30 d |
0.025 |
0.07 |
Carcinogenicity |
|
180 d |
0.025 |
0.07 |
Carcinogenicity |
|
a Previous 7-d SMAC = 0.11 mg/m3. |
|||
RATIONALE FOR ACCEPTABLE CONCENTRATIONS
To the extent possible, the guidelines from the National Research Council (NRC 1992) were used to develop the rationale for furan SMACs (Tables 14-4 and 14-5). Setting long-term exposure standards for furan inhalation is a challenge because of the serious deficiencies in the data base and the implications that it could be highly carcinogenic based on liver tumors induced in rodents given furan by oral gavage. The data base is deficient because there are no inhalation exposures except for two acute exposures that report very different LC50 values. Although some workers seem to have potential exposure to the vapor, there are no epidemiological studies. The data base is strongest in the areas of metabolism and molecular mechanisms involved in cancer induction by furan. The weight of evidence, which is summarized below, suggests that furan will not induce cancer if its toxic metabolites are kept below concentrations that cause cytotoxicity. Furan has been reported in several types of foods at relatively high concentrations; however, the quantification of furan in the food was not sufficiently complete to assist the risk assessment.
Ordinary Human Exposures
By way of background to the risk assessment, it is essential to note that humans are exposed to significant quantities of furan when they consume certain types of food or drink. Merritt et al. (1963) used gas chromatogra-
phy-mass spectrometry (GC-MS) to show that the volatile compounds released from dry roasted ground coffee in room-temperature vacuum distillation consisted of 2.3% furan and 4.0% 2-methyl furan (mole percentages). Mulders et al. (1972) used GC-MS analysis of vapor from freshly baked white bread to show that furan was a major component of the 15 compounds identified. Persson and von Sydow (1973), using GC-MS analyses, found furan from 0.75 to 3.9 ppm in the headspace of canned meat. The variations were caused by changes in the formulations used in the canning process and the time held at the process temperature of 121°C. Furan was the second or third most-concentrated compound in the headspace sample of the canned beef. All three studies were designed to characterize the chemical basis for the odors from the foods, so the results cannot be used to quantify human exposures to furan; however, furan is clearly a component of processed foods consumed by many people.
Acute Lethality and Hepatotoxicity
The 1-h LC50 value of 3500 ppm (9700 mg/m3) of Terrill et al. (1989) was used to derive an acceptable concentration (AC) for hepatotoxicity. That was done to avoid setting an AC based on lethality. Data from oral exposures indicate that hepatotoxicity is the most likely effect at lower exposures. To extrapolate from the LC50 to a nonhepatotoxic concentration, the dose of furan retained by rats during the 1-h exposure was estimated and compared with the oral NOAEL as follows:
Dose = R × LC50 × Vh = 0.9 × 9700 mg/m3 × 0.01 m3/h = 90 mg.
The Vh was calculated from the minute volume of 0.16 L/min (Crosfill and Widdicombe 1961) for 250-g rats, and the respiratory retention, indicated by "R," was estimated from studies on dogs (Egle and Gochberg 1979).
The single oral doses of furan that are considered "severely toxic" to the livers of male rats are those above 100 mg/kg (Wilson et al. 1992). The stated age of the rats dosed with furan was 10 w to 1 y, so the weight range was approximately 350 to 450 g; hence, the 100 mg/kg dose averaged about 40 mg per rat. That seems consistent with the calculation above showing that the LC50 dose was about 90 mg per rat. Studies with the same strain of rat show that 8 mg/kg (about 3 mg/400 g of rat) given orally is a high NOAEL based on increased liver enzymes in serum (Wilson et al. 1992). Based on the comparison of the LC50 and the oral NOAEL, the factor needed to extrapolate from the
LC50 to an inhalation NOAEL for hepatotoxicity is estimated to be 90 mg ÷ 3 mg = 30. The NRC Committee on Toxicology discussed factors of 20 to 50 for extrapolation of an LC50 to a NOAEL for sublethal effects (Paulson 1998), and the value of 30 for furan is within that expected range. The 1-h AC to avoid hepatotoxicity was estimated as follows:
1-h AC = 9700 mg/m3 × 1/30 × 1/3 × 1/10 = 11 mg/m3 = 4 ppm.
In addition to the factor of 30 for extrapolation of the LC50 to a NOAEL, factors of 3 and 10 were used. The factor of 3 was applied for species extrapolation from rats to humans. The species factor was less than the usual factor of 10 because pharmacokinetic data indicate that, on a milligram-per-kilogram body-weight basis, humans have a lower rate of metabolism of inhaled furan vapors than do rats when exposed to 10 ppm (Kedderis and Held 1996). The species extrapolation factor was not reduced to 1 because it was uncertain whether human liver would be more susceptible than rat liver to furan toxicity. A factor of 10 was applied because of the inadequate data on the sublethal effects of inhaled furan vapors, the lack of data on effects in humans by any route of exposure, and the need to be more consistent with the very low AC values calculated for exposure durations of 7 d, 30 d, and 180 d (see below). The NRC does not normally recommend the use of a factor for lack of data; however, the nature of the data base for the toxicity of furan suggests the need for such a factor in this case.
AC for a 24-h Exposure
There is a 160-fold difference between the 1-h AC to protect against hepatotoxicity and the 7-d AC to protect against liver cytotoxicity and cholangiocarcinomas (see below). The 24-h AC should be suitably placed between the 1-h value and the 7-d value. A 24-h AC of 1 mg/m 3 (0.4 ppm) seems reasonable in this circumstance. That value is 11 times less than the 1-h AC and 14 times greater than the 7-d AC of 0.07 mg/m3 (0.025 ppm). This AC is set to protect against hepatotoxicity.
Carcinogenicity
Inspection of Table 14-1 shows that furan is a potent carcinogen when given by gavage to either rats or mice. The most prevalent neoplasms are the
cholangiocarcinomas in rats; however, the incidence of tumors is so high that rational application of the linearized multistage model is impossible. In assessing the risk of furan-induced cancers, we began by making the assumption that an exposure concentration that adequately controls the risk of cholangiocarcinomas will also control leukemias and hepatocellular adenomas and carcinomas. Several recent investigations have elucidated the mechanisms of furan carcinogenesis, and those findings can be used to estimate safe concentrations for bolus oral ingestion.
The weight of evidence is that furan, or an active metabolite of furan, affects DNA indirectly through a mechanism involving cytotoxicity and does not react directly with the DNA in target cells. The evidence is as follows:
-
Furan was negative in in vitro genotoxicity assays using four strains of Salmonella typhimurium with and without S9 fraction (Mortelmans et al. 1986).
-
Radiolabeled furan given once orally to male F344 rats at 8 mg/kg did not produce metabolites that covalently bound to DNA. That result was shown after the DNA fraction was digested to remove DNA associated proteins (Burka et al. 1991). It does not preclude mechanisms involving noncovalent reactions with DNA.
-
Furan did not cause unscheduled DNA synthesis in hepatocytes isolated from male F344 rats or male B6C3F1 mice given a single gavage dose (5-100 mg/kg in rats and 10-200 mg/kg in mice) (Wilson et al. 1992). This type of evaluation has not been reported for bile-duct cells, which seem to be the most sensitive target for furan.
-
Cytotoxicity to the liver, as indicated by the release of liver-associated enzymes into plasma, was induced by a single dose of furan at one of the higher concentrations that induced cancer in the 2-y bioassay (Wilson et al. 1992). Note that the radiolabel from furan accumulates in male rats from about 300 nmol eq/g of liver after a single administration of 8 mg/kg to 1100 nmol eq/g of liver after eight administrations (Burka et al. 1991). Taken together, the data suggest that all doses used in the 2-y bioassay were cytotoxic to the liver. That possibility seems to have been confirmed by Fransson-Steen et al. (1997) in mice given 3 w (15 doses) of exposure at or below the NTP bioassay doses of 8 and 15 mg/kg/d, 5 d/w. Female mice showed increased liver-associated plasma enzymes if given 8 or 15 mg/kg/d, 5 d/w, but the increase was not statistically significant when they were given 4 mg/kg/d, 5 d/w for 3 w.
-
Attempts have been made to understand the sequence of events that could lead to indirect effects on DNA. Furan-treated hepatocytes in vitro and mitochondria isolated from furan-exposed rats show 95% loss of ATP due to uncoupling of oxidative phosphorylation (Mugford et al. 1997). That depletes the energy needed to maintain calcium ''pumps" in cellular membranes, cal-
-
cium increases within the cell, and cytotoxic enzymes are activated, including endonucleases. Preliminary results show that the endonuclease inhibitor aurintricarboxylic acid reduced the formation of DNA double-strand breaks because of endonuclease activity (Mugford and Kedderis 1997). Although this study does not prove the absence of a direct mechanism of DNA interaction, the weight of evidence favors an indirect mechanism.
Two pieces of evidence, considered at face value, suggest that furan or its proximate metabolite can react directly with DNA. They are considered below:
-
Liver tumor cells from furan-exposed and unexposed rodents have differing patterns of mutations leading to oncogene activation, suggesting that furan (or a metabolite) can directly activate proto-oncogenes (Reynolds et al. 1987; Butterworth et al. 1994). An alternative explanation is that error-prone repair of the double-strand DNA lesions resulting from endonuclease induction leads to the mutations (Mugford and Kedderis 1997). Another interpretation is that increased rates of cell proliferation (secondary to necrosis and apoptosis) amplify background mutation rates, leading to the observation of rare mutations (Ames and Gold 1990).
-
Furan is positive in many in vivo chromosomal-aberration tests and in mutagenicity tests in some eukaryotic cells, suggesting that it or its metabolite can react directly with DNA (summarized in the Genotoxicity section). Evidence cited above for the cytotoxicity mechanism involving double-strand DNA breaks can explain these observations without the need for a direct interaction with DNA.
If one accepts the postulate that furan is an indirect carcinogen, as the weight of evidence above suggests, then a failure to complete that indirect process effectively eliminates the potential for furan to cause cancer. To complete the indirect process, the reactive metabolite(s) of furan must be in sufficient concentration in the target tissue to uncouple oxidative phosphorylation and substantially reduce ATP production. That will occur only if the concentration of metabolite(s) at the target site is above some threshold, and this threshold will be reached only if the amount of furan administered is above the threshold to deliver that amount of metabolite(s) to the site. To complete the risk assessment, that threshold must be estimated.
Estimation of a NOAEL for Cancer Risk in Gavaged Rats
There are no long-term exposures that demonstrate a NOAEL for injury to bile-duct cells by any route of administration. The 9-mo oral gavage study
demonstrated a LOAEL (5/10 incidence) for cholangiocarcinomas of 2 mg/kg/d. The 13-w study showed that 4 mg/kg/d, 5 d/w was a LOAEL (4/10) for biliary hyperplasia. To estimate a NOAEL, one can assume that this lesion is a precursor to the cholangiocarcinomas found in rats given furan by oral gavage. In male rats, the data were as follows (NTP 1993):
|
Dose |
Biliary hyperplasia 13 w exposure |
Cholangiocarcinomas 9 mo exposure |
|
Control |
0 |
0/10 |
|
2 mg/kg |
— |
5/10 |
|
4 mg/kg |
4 |
7/10 |
|
8 mg/kg |
9 |
10/10 |
|
15 mg/kg |
10 |
— |
The 1% benchmark dose rate (BD01), using a probit model and the 13-w data, was found to be 1.63 mg/kg/d (central estimate), with a 95% lower confidence limit of 0.09 mg/kg/d. The 95% lower confidence on the BD01, 0.09 mg/kg/d, was taken to be a NOAEL in rats by oral gavage.
Alternatively, the NRC SMACs subcommittee noted that 2 mg/kg/d, 5 d/w for 9 mo seemed to be a LOAEL for a severe effect (cancer). Its recommendation was to reduce that value by a safety factor of 30 rather than the usual 10 because of the seriousness of the effect, the high incidence (50%) even in the lowest-dose group, and the lack of lifetime observation for tumors after 9 mo of exposure. Thus, using that approach, the NOAEL was estimated to be 0.07 mg/kg/d. The benchmark approach and the safety-factor approach gave comparable estimates for the NOAEL, so the estimates were averaged to 0.08 mg/kg/d. Because exposures were for 5 d/w, the weekly dose was 0.4 mg/kg/w.
Extrapolation from Rat NOAEL to Human Inhalation ACs
Assuming that a human weighs 70 kg, that 90% of inhaled furan is absorbed, and that a human inhales 20 m3/d (140 m3/W), the equivalent human inhalation NOAEL was found as follows:
NOAELinhaled = (0.4 mg/kg/w × 1/3 × 70 kg)/(140 m3/w × 0.9)
7-d, 30-d, and 180-d AC = 0.07 mg/m3 (human) = 0.025 ppm
The species extrapolation factor was reduced from the usual 10 to 3 because the dose of furan of 10 ppm absorbed by inhalation was estimated on a milligram-per-killogram basis, to be 3-fold less in humans than in rats (Kedderis and Held 1996); a species extrapolation factor of 3 was retained because the relative susceptibility of human liver tissue and rat liver tissue is unknown. Furthermore, when protecting against an end point, such as cytotoxicity, leading to cancer, equating a bolus oral dose, which is rapidly absorbed, to an inhalation dose spread over 24 h provides a wide margin of safety. The "threshold" NOAEL estimated above is applicable to 7-d, 30-d, 180-d ACs. ACs for carcinogenicity are not set for 1-h or 24-h exposures.
Is the Respiratory System a Target by Inhalation?
One must consider whether the respiratory system could be a target for inhaled furan. Some of the descriptions of the acute inhalation exposures suggest that possibility, certain furan analogues (e.g., 3-methylfuran) specifically target the respiratory system (Boyd et al. 1978), and the respiratory system has the enzymes necessary to oxidize furan to cis-2-butene-1, 4-dial. There were no furan-induced respiratory-system neoplasms in male rats in the 2-y bioassay (NTP 1993), and approximately one-seventh of a single 8-mg/kg oral dose, which was the highest dose used in rats in the bioassay, is exhaled as unchanged furan (Burka et al. 1991). Hence, the respiratory system of the rats received substantial exposure to furan during the oral gavage study, but there were no furan-associated neoplasms or other lesions in the respiratory system. That is sufficient evidence that the respiratory system would not be a target of inhaled furan, particularly at the 0.07 mg/m3 (0.025 ppm) AC proposed above.
Spaceflight Effects
Lift-off, microgravity, and re-entry are not anticipated to have any effect on the carcinogenicity, toxicity, or lethality of furan.
RECOMMENDATIONS
Substantial research efforts are under way in several laboratories to understand the mechanisms associated with certain carcinogens that do not directly bind DNA. Some of that work is focused on the mechanisms of furan carcino-
genesis in rodents. It is expected that these efforts will answer many of the questions that must be answered to improve the risk analysis. Both acute and chronic inhalation studies are needed to improve the descriptive toxicity database. These studies should include assessment of sublethal end points, tissue-specific metabolic activation, and DNA lesions in target tissues. Humantissue studies in vitro would provide a valuable adjunct to improve species extrapolations.
TABLE 14-5 Acceptable Concentrations
|
End Point, Exposure Data, Reference |
|
Uncertainty Factors |
Acceptable Concentrations, ppm |
|||||||
|
Species |
NOAEL |
Time |
Species |
Spaceflight |
1 h |
24 h |
7 d |
30 d |
180 d |
|
|
Hepatotoxicity |
Rat |
30 |
(see text) |
3 |
1 |
4a |
0.4b |
NSc |
NS |
NS |
|
LC50 = 9700 mg/m3(Terrill et al. 1989) |
|
|||||||||
|
Biliary hyperplasia |
Rat |
BD01 |
1 |
3 |
1 |
NS |
NS |
0.025d |
0.025 |
0.025 |
|
Dose response, 13 w (NTP 1993) |
|
|||||||||
|
Cholangiocarcinomas |
Rat |
30 |
1 |
3 |
1 |
NS |
NS |
0.025d |
0.025 |
0.025 |
|
LOAEL, 2 mg/kg, 9 mo (NTP 1993) |
|
|||||||||
|
SMACs |
|
|
|
|
|
4 |
0.4 |
0.025 |
0.025 |
0.025 |
|
a An additional uncertainty factor of 10 was applied due to weakness in the inhalation data base. b Set between 1-h and 7-d values (see text). c NS, not set. d NOAELs from benchmark and safety-factor approaches were averaged to give 0.08 mg/kg/d or 0.4 mg/kg/w (for rats exposed by gavage, see text). |
||||||||||
REFERENCES
Ames, B.N., and L.S. Gold.1990. Too many rodent carcinogens: Mitogenesis increases mutagenesis. Science 249:970-071.
Boyd, M.R., C.N. Statham, R.B. Franklin, and J.R. Mitchell. 1978. Pulmonary bronciolar alkylation and necrosis by 3-methyl furan, a naturally occurring potential atmospheric contaminant. Nature 272:270.
Burka, L.T., K.D. Washburn, and R.D. Irwin. 1991. Disposition of [14C]-furan in the male F-344 rat. J. Toxicol. Environ. Health 34:245-257.
Butterworth, B.E., C.S. Sprankle, S.M. Goldsworthy, D.M. Wilson, and T.L. Goldsworthy. 1994. Expression of myc, fos, and Ha-ras in the livers of furan-treated F344 rats and B6C3F1 mice. Mol. Carcinogen. 9:24-32.
Carfagna, M.A., S.D. Held, and G.L. Kedderis. 1993. Furan-induced cytolethality in isolated rat hepatocytes: Correspondence with in vivo dosimetry. Toxicol. Appl. Pharmacol. 123:265-273.
Chen, L.-J., S.S. Hecht, and L.A. Peterson. 1996. Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem. Res. Toxicol. 8:903-906.
Crosfill, M.L., and J.G. Widdicombe. 1961. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J. Physiol. 158:1.
Egle, J.L.J., and B.J. Gochberg. 1979. Respiratory retention and acute toxicity of furan. Am. Ind. Hyg. Assoc. J. 40:310-314.
Fransson-Steen, R., T.L. Goldsworthy, G. Kedderis, and R.R. Maronpot. 1977. Furaninduced liver cell proliferation and apoptosis in female B6C3F1 mice. Toxicology. 118:195-204.
James, J.T. 1991. Memorandum to SA/Director, Space and Life Sciences: "Toxicological Assessment of the Noxious Odors Produced by the Orbiter Refrigerator/Freezer (ORF) During the STS-40 Mission", . NASA Johnson Space Center. Houston, Texas.
Johnston, J.F.A. 1931. On the anaesthetic action of furan. J. Pharmacol. Exp. Ther. 43:85.
Kedderis, G., and S. Held. 1996. Prediction of furan pharmacokinetics from hepatocyte studies: Comparison of bioactivation and hepatic dosimetry in rats, mice and humans. Toxicol. Appl. Pharmacol. 140:124-130.
Koch, E.M., and M.H. Cahan. 1925. Physiological action of furane. J. Pharmacol. Exp. Ther. 26:281.
Masuda, Y., N. Nakayama, A. Yamaguchi, and M. Murohashi. 1984. The effects of diethyldithiocarbamate and carbon disulfide on acute nephrotoxicity induced by furan, bromobenzene and cephaloridine. Japan. J. Pharmacol. 34:221-229.
McGregor, D.B., A. Brown, P. Cattanach, I. Edwards, D. McBride, C. Riach, and W. J. Caspary. 1988. Responses of the L5178Y tk+/tk-mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ. Mol. Mutagen. 12:85-154.
Merritt, C., M.L. Bazinet, J.H. Sullivan, and D.H. Robertson. 1963. Mass spectrometric determination of the volatile components from ground coffee. J.Argric. Food Chem. 11:152-155.
Mortelmans, K., S. Haworth, T. Lawlor, W. Speck, B. Tainer, and E. Zeigler. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mutagen. 8(Suppl):1-119.
Mugford, C.A., M.A. Carfagna, and G.L. Kedderis. 1997. Furan-mediated uncoupling of hepatic oxidative phosphorylation in Fischer-344 rats: An early event in cell death. Toxicol. Appl. Pharmacol. 144:1-11.
Mugford, C.A., and G.L. Kedderis. 1997. Cytotoxic rodent carcinogens: Investigation of the role of cytolethality in furan-mediated DNA damage. CIIT Activities 17:1-6.
Mulders, E.J., H. Maarse, and C. Weurman. 1972. The odour of white bread. Z. Lebensm.. Unters. Forsch 150:168-174.
NRC. 1992. Guidelines for Developing Spacecraft Maximum Allowable Concentrations for Space Station Contaminants. Washington, DC: National Academy Press.
NTP. 1993. Toxicology and carcinogenesis studies of furan (CAS No. 110-00-9) in F344/N rats and B6C3F1 mice (gavage studies). NTP TR 402. U.S. Department of Health and Human Services, National Toxicology Program, Research Triangle Park, NC.
Paulson, L.R. 1998. Minutes of meeting held 4-5 May, 1998 in League City, TX. National Research Council Committee on Toxicology, Subcommittee on SMACs, Washington, DC.
Persson, T., and E. von Sydow. 1973. Aroma of canned beef: Gas chromatographic and mass spectrometric analysis of the volatiles. J. Food Sci. 38:377-385.
Reynolds, S., S. Stowers, R. Patterson, R. Maronpot, S. Aaronson, and M. Anderson. 1987. Activated oncogenes in B6C3F1 mouse liver tumors: Implications for risk assessment. Science 237:1309-1316.
Ronto, G., A. Fekete, P. Grof, C. Bilger, J.-P. Buisson, A. Tromelin, and P. Demerseman. 1989. Genotoxicity testing: Phage T7 inactiviation test of various furan and arenofuran derivatives. Mutagenesis 4:471-475.
Sax, N.I., and R.J.S. Lewis, Eds. 1989. Dangerous Properties of Industrial Materials, 7th Ed. New York: Van Nostrand Reinhold.
Stich, H.F., M.P. Rosin, C.H. Wu, and W.D. Powrie. 1981. Clastogenicity of furans found in food. Cancer Lett. 13:89-95.
Terrill, J.B., W.E. Van Horn, D. Robinson, and D. Thomas. 1989. Acute inhalation toxicity of furan, 2-methyl furan, furfuryl alcohol, and furfural in the rat. Am. Ind. Hyg. Assoc. J. 50:A359-A361.
Wiley, R.A., G.J. Traiger, S. Baraban, and L.M. Gammal. 1984. Toxicity-distribution relationships among 3-alkylfurans in mouse liver and kidney. Toxicol. Appl. Pharmacol. 74:1-9.
Wilson, D.M., T.L. Goldsworthy, J.A. Popp, and B.E. Butterworth. 1992. Evaluation of genotoxicity, pathological lesions, and cell proliferation in livers of rats and mice treated with furan. Environ. Mol. Mutagen. 19:209-222.
Windholz, M., ed. 1976. The Merck Index, 9th Ed. Merck & Co., Rahway, N.J.























