B4
Isoprene
John T. James, Ph.D.
Johnson Space Center Toxicology Group
Medical Operations Branch
Houston, Texas
PHYSICAL AND CHEMICAL PROPERTIES
Isoprene is a colorless, highly volatile liquid or gas with a weak aromatic odor (IARC 1994).
|
Formula: |
H2C=C(CH3)-CH=CH2 |
|
Synonyms: |
2-Methyl-1,3-butadiene (IUPAC), isopentadiene |
|
CAS no.: |
78-79-5 |
|
Molecular weight: |
68.12 |
|
Boiling point: |
34°C |
|
Melting point: |
–146°C |
|
Vapor pressure at 20°C: |
60.7 kPa |
|
Conversion factors at 25°C: |
1 ppm = 2.79 mg/m3 |
|
|
1 mg/m3 = 0.358 ppm |
OCCURRENCE AND USE
Isoprene occurs widely in nature and is an important industrial chemical. Isoprene is produced by plants during the photosynthesis process and constitutes a major portion of the nonmethane hydrocarbons released by the biosphere. The concentration of isoprene inside a forest canopy might be 10 times higher than outside the canopy (Khalil and Rasmussen 1992). Isoprene is present in tobacco and wood smoke and can be considered the building block of many natural products including natural rubber, terpenes, vitamins A and K, and the steroid sex hormones (IARC 1994). Isoprene is produced endo-
geneously in humans at a rate of about 0.15 to 0.35 µmol/kg•h (Hartmann and Kessler 1990; Filser et al. 1996), and the blood concentration ranges from 10 to 70 nmol/L (Cailleux et al. 1992; Filser et al. 1996). Similarly, isoprene is endogenously produced in rats at a rate of 1.9 µmol/kg•h and in mice at a rate of 0.4 µmol/kg•h (Peter et al. 1987). Ambient air concentrations in cities have been reported as high as 0.04 mg/m3 (0.014 ppm) (Lonneman et al. 1979), although most reports indicate a much lower concentration (IARC 1994).
Industrially, isoprene is used to make isoprene rubber, which is used in vehicle tires; styrene block polymers, which are useful in adhesives; and butyl rubber, which is useful in lining hoses and tires to limit gas escape (IARC 1994). Isoprene is commercially important in the synthesis of selected terpenes that are used in flavorings and in fragrances (IARC 1994).
Isoprene has been found in about one-third of of the space-shuttle air samples taken with either the grab sample containers (GSCs) or the solid sorbent air sampler (SSAS). Typical concentrations are below 0.1 mg/m3 (0.036 ppm) (James et al. 1994). Seven air samples obtained with the SSAS during Mir 17 showed a concentration range of 0.17 to 0.35 mg/m3 (0.061 to 0.12 ppm), whereas 12 samples taken with GSCs during Mir 18 showed concentrations below 0.11 mg/m3 (0.039 ppm) (J.T. James, T.F. Limero, S.W. Beck, L. Yang, M.P. Martin, M.L. Matney, P.A. Covington, and J.F. Boyd, Johnson Space Center, Houston, Tex., unpublished data, 1995).
TOXICOKINETICS AND METABOLISM
The absorption, distribution, metabolism, and excretion of isoprene has been studied by inhalation exposure and in vitro methods in several species; however, metabolism studies in humans are limited in scope. The differences in susceptibility between rats and mice (see toxicity section) are not clearly explained by known differences in the toxicokinetics of isoprene. Because of structural similarities, the metabolism of isoprene has been often compared to that of 1,3-butadiene; however, that comparison will be avoided here.
Absorption
In human subjects, the pulmonary retention of isoprene from cigarette smoke deeply inhaled by mouth in a single breath was found to be 99% (Dalhamn et al. 1968). The concentration of isoprene in the smoke was not apparent, so it is difficult to relate these data to experimental data on rodents or other species. In anesthesized dogs, percentage retention ranged from 65% to 75% when
evaluated over a ventilatory range of 6 to 30 breaths/min, tidal volumes of 110 to 220 mL, and exposure concentrations of 360 to 720 mg/m3 (130 to 260 ppm) (Egle and Gochberg 1975). At an exposure concentration of 960 mg/m3 (344 ppm), the apparent retention was only 40%.
The retention and metabolism of 14C-isoprene was studied in rats exposed for 6 h to concentrations that ranged from 8 to 8200 ppm (Dahl et al. 1987). As the exposure concentrations increased, the percentage of unchanged isoprene exhaled increased from 75% to 96% and the percentage inhaled and metabolized decreased from 25% to 4%. In mice exposed for 6 h to 14C-isoprene at concentrations from 18 to 2000 ppm, the percentage retained decreased from 19% to 6% at the highest concentration (Bond et al. 1991). Rats metabolized a greater fraction of the inhaled dose than did mice at all exposure concentrations.
Distribution
The tissue distribution of inhaled isoprene and its tentatively identified metabolites has been reported in detail in rats exposed to 1480 ppm for 6 h (Dahl et al. 1987). Isoprene and its metabolites were found in nasal tissue, lung, liver, kidney, fat, and blood. Highest amounts were evident in the liver and especially the body fat; several metabolites were also demonstrated in nasal and lung tissue (Dahl et al. 1987). Rats exposed to near-lethal concentrations of isoprene for 3 to 4 h showed 5-10-fold higher concentrations of isoprene in fat tissue than in brain, liver, kidney or spleen (Shugaev 1968).
Metabolism
Metabolic studies have been reported in several species, particularly in rats and mice; however, no studies of the metabolism of isoprene in humans or human tissue could be found. Studies in animals consist of toxicodynamic studies in whole animals and in vitro studies of liver microsomes. Studies of metabolism in nonhepatic tissues were lacking.
The metabolism of isoprene in liver microsomes from rats, mice, rabbits, and hamsters occurs by oxidation of either of the double bonds to form monoepoxides, which in turn can be hydrolized to diols; diepoxides can be formed by oxidation of a second double bond as shown in Figure 4-1 (Gervasi and Longo 1990; Wistuba et al. 1994). The major pathway in all species tested is through the epoxide formed on the methylated double bond. Oxygen and a NADPH-generating system were necessary, and the metabolism could be
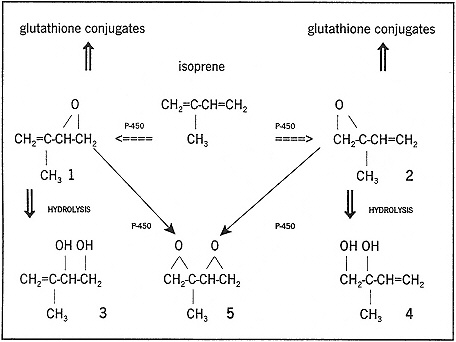
FIGURE 4-1 Metabolism of isoprene in microsomes from several species (Gervasi and Longo 1990; Wistuba et al. 1994).
inhibited by several known inhibitors of the cytochrome-P-450 system (Gervasi and Longo 1990).
Isoprene itself was not mutagenic in Salmonella typhimurium strains TA98 and TA100, even with metabolic activation, nor were either of the epoxides; however, the diepoxide was mutagenic (Gervasi and Longo 1990).
In male Wistar rats and B6C3F1 mice exposed to concentrations from 5 to about 4000 ppm, the rate of isoprene metabolism was proportional to concentrations below 300 ppm, but saturation was apparent above 1000 and 2000 ppm in rats and mice, respectively (Peter et al. 1987). At moderate concentrations, the half-life of isoprene was 7 min in rats and 4 min in mice, and the fraction exhaled in unchanged form was 15% and 25%, respectively. The maximum velocity for metabolism of isoprene was 3 times higher in mice than in rats (Peter et al. 1987).
There are important species differences in the metabolism of inhaled isoprene in Fischer 344 (F344) rats and B6C3F1 mice (Dahl et al. 1987; Bond et al. 1991). B6C3F1 mice appear to be more sensitive to inhaled isoprene than
rats. The basis for that observation is that rat respiration changes little during high-concentration exposures, whereas the minute volume in mice decreases about 20% during exposures at 2000 ppm (Bond et al. 1991).
As shown in Figure 4-1, the epoxidation of isoprene occurs with preferential oxygen attack at the di-substituted carbon-carbon double bond. In B6C3F1 mouse-liver microsomes, there was no more than slight enantiomer selectivity in either product 1 or 2. In F344 rat-liver microsomes, product 2 was formed with only slight enantiomer selectivity; however, product 1 was formed with a strong selectivity for the (S)-enantiomer (Wistuba et al. 1994). A similar species dependence has been noted for other small aliphatic alkenes.
Isoprene forms hemoglobin adducts in rodents, and the adducts might be a useful marker of repeated exposure to isoprene (Sun et al. 1989). After male B6C3F1 mice and Sprague-Dawley rats were injected intraperitoneally (ip) with 14C-isoprene at 0.3 to 3000 µmol/kg of body weight, the amount of hemoglobin adduct formed after 24 h was proportional to the dose up to 500 µmol/kg (Sun et al. 1989). Repeated administration of 500 µmol/kg for 3 d resulted in linear accumulation of hemoglobin adducts that persisted for approximately 24 or 65 d in mice and rats, respectively. The efficiency of adduct formation, corrected for exhaled isoprene, was twice as high in mice as in rats (Sun et al. 1989).
Excretion
In both F344 rats and B6C3F1 mice, most isoprene metabolites (52-81%) are excreted in the urine, with lesser amounts in the feces and breath (as carbon dioxide) (Bond et al. 1991). As mentioned above, much of an inhaled dose of isoprene is exhaled unchanged from rats and mice. The proportion of metabolites excreted via several routes has been compared in rats and mice exposed for 6 h to concentrations ranging from 8 to 2000 ppm (Bond et al. 1991). The major quantitative differences were the percentage of total metabolites in the feces (mice 6-37%, rats 2-3%) and the percentage of total metabolites present in the bodies 64 h after exposure (mice 3-9% and rats 8-18%).
Comparative Toxicokinetics
In view of the large differences in tumorigenic responses of rats and mice (see below) and the need to extrapolate rodent data to human risks, it is necessary to compare the toxicokinetics of isoprene in these three species. Filser et al. (1996) measured partition coefficients using an in vitro head-space technique and blood from rats, mice, and humans. The tissue/air partition coeffi-
cients were determined for rat tissue. In addition, the uptake of isoprene in intact mice, rats, and humans was measured. They found that a two-compartment model provided a best fit to the data. On the basis of data taken from humans breathing into a respirometer, the endogenous production of isoprene was estimated to be 24 µmol/h for a 70-kg person. Of the endogenous production, 90% underwent metabolism, and the remaining 10% was exhaled. Filser et al. (1996) estimated that 3.4 mg/24 h was exhaled, an estimate that agrees well with earlier estimates of 0.4 to 9.4 mg/24 h and 2 to 4 mg/24 h from Conkle et al. (1975) and Gelmont et al. (1981), respectively. At a concentration of 40 ppm or lower, the rate of isoprene metabolism was 14-fold slower in mice than in humans and 8-fold slower in rats than in humans. Filser et al. (1996) suggest that it will be necessary to measure blood concentrations of isoprene and its metabolites in humans exposed to isoprene to determine whether the toxicokinetic model is correct. The modeling did not address formation of the diepoxide, the putative active metabolite of isoprene. As a result, the model and ensuing risk assessment must be updated when the data become available.
TOXICITY SUMMARY
A reasonably complete toxicity data base is available for short-term and chronic exposures of mice and rats to isoprene by inhalation. Mice exhibit a broad spectrum of lesions, both neoplastic and non-neoplastic, that often does not show an expected dose-response relationship. The data base consistently shows the much greater susceptibility of mice over rats. Data from acute-and human-exposure studies, including epidemiological studies, are sparse.
Acute Exposures
Some data are available on the effects of acute exposure of mice to very high concentrations of isoprene. Citing an earlier study, von Oettingen (1940) stated that 100,000 to 120,000 mg/m3 (36,000 to 43,000 ppm) caused deep narcosis in mice and that 140,000 mg/m3 (50,000 ppm) was fatal. Likewise, Gostinskii (1965) reported that 109,000 mg/m3 (39,000 ppm) resulted in collapse of 50% of the white mice exposed for 2 h. The LC50 (lethal concentration for 50% of the animals) for males and females combined was 144,000 mg/m3 (51,500 ppm) (range 138,000 to 149,000 mg/m3); females were somewhat more resistant than males. Exposed mice that recovered from narcosis were able to do better than control mice in a swin test administered 24 h later. White mice exposed for 40 min to a mean concentration of 2200 mg/m3 (790 ppm) exhibited a reduced ability of their central nervous system to ''summate subthreshold impulses," and
at half that concentration, there was an increase in motor activity (Gostinskii 1965). Also, exposures of rabbits to concentrations of 1500 to 8000 mg/m3 (540 to 2900 ppm) "hastened the development of the reflex muscular contraction and weakened the strength of the reflex" during exposures (Gostinskii 1965). In another investigation, the 2-h LC50 for mice was 160,000 mg/m3 (57,000 ppm), and the 4-h LC50 in rats was 180,000 mg/m3 (64,00 ppm) (Shugaev 1968). Mamedov (1979) reported increases in the thymus mitotic index and peripheral lymphocyte count in rats exposed at only 0.8 mg/m3 (0.3 ppm) for 4 h, but data and statistical analysis to support that observation were not provided.
In one of the few human studies, Gostinskii (1965) reported that 10 subjects could perceive the odor of isoprene at 10 mg/m3 (3.6 ppm) within a minute. Slight irritation of the mucosa of the nose, pharynx, or larynx was reported when concentrations were raised to 160 mg/m3 (57 ppm). Increases in the respiratory rate of rabbits (presumably a measure of irritation) were noted at 190 mg/m3 (68 ppm).
Short-Term Exposures
The species difference in susceptibility was evident in exposures of B6C3F1 mice and F344 rats exposed 6 h/d, 5 d/w for 2 w to isoprene at concentrations of 0, 440, 880, 1750, 3500, or 7000 ppm (Melnick et al. 1990). Animals were evaluated by observation of clinical signs, measurement of body weights, hematology, clinical chemistry, urinalysis (rats only), gross pathology, and histopathology. Histopathology was performed on the liver, 6 points in the respiratory system, heart, brain, thymus, spleen, kidney, and testis. No changes could be detected in any of the rats exposed to isoprene; however, the mice showed changes as summarized in Table 4-1. A NOAEL was not found in mice for red-blood-cell (RBC) changes, decreased relative liver weight, vacuolization of hepatocytes, and epithelial hyperplasia of the forestomach (Melnick et al. 1990).
Male Wistar rats exposed 4 h/d for 30 d to isoprene vapor at a concentration of 0.36 ppm or 0.035 ppm showed variations in thymus cellularity and mitotic index (Mamadov 1979). The changes were not large, and there was not a consistent dose-response relationship. If the thymus is a target organ of isoprene in the rat, then it is surprising that rats exposed to much higher concentrations (Melnick et al. 1990) showed no evidence of injury. Furthermore, endogenous isoprene production in rats has been estimated to be equivalent to inhalation exposures of 0.5 ppm (Melnick et al. 1994); hence, it is unclear how exposures as low as those used by Mamedov (1979) could cause adverse effects.
TABLE 4-1 Incidence of Lesions in Mice Exposed to Isoprene for 2 W in the Melnick et al. (1990) Study
|
|
Exposure Concentration, ppm |
|||||
|
Lesion |
0 |
438 |
875 |
1750 |
3500 |
7000 |
|
Males |
|
|||||
|
Thymic atrophy |
0/10 |
nea |
ne |
ne |
0/10 |
7/9 |
|
Testes atrophy |
0/10 |
ne |
ne |
ne |
0/10 |
9/10 |
|
Liver vacuolized cytoplasm |
0/10 |
8/10 |
9/10 |
10/10 |
10/10 |
10/10 |
|
Olfactory epithelial degeneration |
0/10 |
ne |
0/10 |
3/10 |
6/10 |
9/10 |
|
Forestomach epithelial hyperplasia |
0/10 |
3/10 |
5/10 |
10/10 |
8/10 |
9/10 |
|
Females |
|
|||||
|
Forestomach epithelial hyperplasia |
1/10 |
8/10 |
7/10 |
10/10 |
9/10 |
9/10 |
|
a Not evaluated. |
||||||
Subchronic and Chronic Exposures
The data base consists of Russian epidemiological studies, a variety of animal studies of noncancer end points, a 26-w inhalation carcinogenesis study conducted in rats and mice with an unusually long 26-w recovery period, and a chronic oncogenicity study in mice exposed by inhalation up to 80 w. Despite the apparent high quality of the two latter studies, their conclusions regarding neurotoxicity in mice were found to be difficult to reconcile. The genotoxicity and carcinogenesis data, along with the data showing endogenous production of isoprene, indicate clearly that isoprene is a threshold-type carcinogen. A threshold-type carcinogen does not increase the incidence of tumors if the exposure concentration is below the "threshold" concentration, no matter how long the exposure.
Epidemiological Data
Three Russian epidemiological studies reported effects in workers associated with isoprene exposure in the isoprene rubber industry. Workers exposed to
isoprene, dimethyldioxane, formaldehyde, and methanol (unknown concentrations) appeared to have a reduced immune response to typhoid vaccination compared with controls (Nikul'tseva 1967). The confounding of exposures and the unknown concentrations prevent those data from being useful for setting limits. Similarly, isoprene rubber workers were reported to have excess fatigue, as measured by a photogenic reflex test, at the end of their work shift because of exposure to many organic compounds, including isoprene. Isoprene concentrations were above the Russian maximum allowable concentration (MAC) 16% of the time; however, the experiment was not well controlled in that some of the 300 workers were exposed to excess heat (up to 30 to 35°C), and only 15 control subjects (plant managers) were used (Pigolev 1971). In another study of isoprene rubber workers, Mitin (1969) studied 630 workers for 4 y with 150 control subjects. The exposed workers were divided into three groups based on the length of work service. The author concluded that upper-respiratory-tract injury increased with increasing work time, nose bleeds increased with longer work times, and loss of olfactory sensitivity increased. Those findings cannot be used to set limits for isoprene because other chemicals were present (formaldehyde and dimethyldioxane) at poorly defined concentrations in the production plant, and exposed groups were not controlled for age and smoking habits.
Animal Data on Noncancer End Points
Subchronic and chronic exposures of rats, mice, and rabbits have been reported for isoprene. Rats, mice, and rabbits were exposed for 4-5 mo, 4 h/d to isoprene vapor at a target concentration of 5000 mg/m3 (1800 ppm) (Gostinskii 1965). Actual concentrations varied between 2200 and 4900 mg/m3 (790 to 1750 ppm) based on a conductometric analytical method. There were no weight-gain differences as a result of isoprene exposure in any species. Rabbits showed an increase in leukocyte counts, a decrease in erythrocyte count (not quite statistically significant), and histological evidence of irritation in the bronchi, hepatocyte degeneration, myocardium changes, and thyroid irritation (Gostinskii 1965). Mice exposed to isoprene showed a 50% decrease in swim time and increases in the weights of the lungs, brain, and kidney. Rats had histopathological changes similar to those seen in rabbits. Those findings led the author to recommend an exposure limit for workers of 40 mg/m3 (Gostinskii 1965).
Mamedov (1979) reported a decrease in thymus cellularity, weight, and mitotic index during a 4-mo exposure of rats to isoprene at 0.042 ppm. Apparently, exposures at 0.0039 ppm did not elicit a significant difference from controls. That result is not consistent with results reported in 2-w exposures of
F344 rats to concentrations as high as 7000 ppm; however, a decrease in thymus weight was reported in B6C3F1 mice exposed at 875 ppm and above (Melnick et al. 1990).
The long-term effects of isoprene exposure have been assessed in F344 rats and B6C3F1 mice exposed 6 h/d, 5 d/w for 13 w (males and females) or 26 w (males only) to concentrations ranging from 70 to 7000 ppm (Melnick et al. 1994). After exposure, the animals were evaluated by hematology, clinical chemistry, urinalysis (rats only), sperm motility and vaginal cytology, limb grip strength (mice only), and histopathology. No discernable effects were apparent in rats exposed for 13 w, and the change in rats after 26 w of exposure was limited to interstitial-cell hyperplasia in the testis of the 7000-ppm group. After a 26-w recovery of rats exposed for 26 w, rats in the 700-ppm and higher groups showed an increased incidence of interstitial-cell adenomas, with a very weak dose-response relationship (Melnick et al. 1994).
Mice exhibited a broad range of histological changes in the 13-w study as shown in Table 4-2. A similar pattern of non-neoplastic lesions was reported by Placke et al. (1996) in the same strain of mice, which were exposed up to 80 w to isoprene. One exception was that Placke and co-workers reported myeloid hyperplasia of the bone marrow without significant changes in RBC
TABLE 4-2 Lesions in Mice Exposed to Isoprene for 13 W in the Melnick et al. (1994) Study
|
|
Exposure Concentration, ppm |
|||||
|
Lesion |
0 |
70 |
220 |
700 |
2200 |
7000 |
|
Males |
|
|||||
|
Forestomach epithelial hyperplasia |
0a |
0 |
0 |
9b |
8b |
9b |
|
Olfactory epithelial degeneration |
0 |
0 |
0 |
0 |
0 |
10b |
|
Liver cytoplasmic vacuolization |
0 |
0 |
0 |
0 |
0 |
10b |
|
Testis atrophy |
0 |
0 |
0 |
0 |
0 |
2 |
|
Females |
|
|||||
|
Forestomach epithelial hyperplasia |
0 |
0 |
0 |
10b |
9b |
10b |
|
a Incidence in 10 mice. b Significantly different from controls (p < 0.01). |
||||||
measures, whereas a mild anemia was reported in males and females exposed for 24 d with a no-observed-adverse-effect level (NOAEL) at 70 ppm (Melnick et al. 1994). Histopathological lesions showed a NOAEL at 220 ppm. Hindlimb grip strength was substantially lower in mice exposed at 220 ppm and above for 26 w; however, 4 w into recovery, the grip strengths had returned to control values. The NOAEL for neoplastic and non-neoplastic lesions in mice exposed for 26 w and recovered for 26 w was 220 ppm except for nasal-turbinate epithelial degeneration and spinal-cord degeneration. The incidences of the lesions are given in Table 4-3.
The dose-response relationship for olfactory epithelial degeneration in "recovered" mice suggests that this lesion is directly related to isoprene exposures; however, the dose-response curve for spinal-cord degeneration in recovered mice is difficult to understand in terms of isoprene exposures. The situation is confounded because loss of hind-limb grip strength was present in mice exposed at only 220 ppm immediately after the 26-w exposures were completed; however, the grip strengths apparently returned to control values during the first few weeks of the recovery period, even as spinal-cord degeneration was developing.
A similar uncoupling of functional and morphological evidence of neurotoxicity has been recently reported in rats given ip injections of acrylamide (Crofton et al., 1996). Male Long-Evans hooded rats given acrylamide (20 mg/kg/d) for 30 d showed a 50% loss of grip strength, which was recovered to near normal 30-60 d after the exposures ended. Rats given a slightly lower dose (15 mg/kg/d) for 30 d did not show axonal degeneration at the end of exposure; however, 28 d after dosing ended, moderate degeneration (1-5% of the fibers degenerated) was found in the sciatic nerve and spinal cord. The authors attributed that degeneration to a "delay between initiation of fiber breakdown and the histological degeneration at the level of the sciatic nerve."
TABLE 4-3 Incidences of Lesions in Mice Exposed for 26 W in the Melnick et al. (1994) Study
|
Lesion |
26-w Recovery |
0 |
70 |
220 |
700 |
2200 |
7000 |
|
Olfactory |
Yes |
1/30 |
2/30 |
5/29a |
11/30a |
25/30a |
28/28a |
|
epithelial degeneration |
No |
0/10 |
1/10 |
0/10 |
1/10 |
1/10 |
10/10a |
|
Spinal-cord |
Yes |
4/30 |
20/30a |
19/29a |
28/30a |
17/29a |
13/28a |
|
degeneration |
No |
0/10 |
0/10 |
0/10 |
0/10 |
1/10 |
10/10a |
|
a Incidence was statistically greater than in controls. |
|||||||
Unfortunately, very recent data on B6C3F1 mice exposed up to 80 w to isoprene at relatively high concentrations did not confirm the findings of neurotoxicity, even after considerable recovery times (Placke et al. 1996). Mice exposed at 2200 ppm (8 h/d, 5 d/w) for 40 or 80 w with recovery periods of 56 w or 16 w, respectively, exhibited no apparent effects on motor function and showed no exposure-related lesions in the spinal cord (Placke et al. 1996).
Carcinogenicity
The IARC evaluation of the carcinogenesis data on isoprene concluded that there was inadequate evidence in humans and sufficient evidence in experimental animals that isoprene is carcinogenic (IARC 1994). The compound was placed in category 2B, meaning that isoprene is possibly carcinogenic to humans. There is weak evidence of carcinogenicity in rats and convincing evidence in mice after 26 w of exposure and a 26-w recovery period. The pertinent data are in Table 4-4 (Melnick et al. 1994).
The IARC noted that the exposure duration of only 26 w was too short to evaluate carcinogenicity adequately in rats; however, interstitial-cell tumors were also noted to have a high spontaneous incidence in F344 rats (IARC 1994). The mouse data clearly show the carcinogenic potential of isoprene, although the dose-response relationship appears flat for liver and harderiangland tumors induced at concentrations above 220 ppm.
The weight of evidence supports the hypothesis that isoprene induces cancer by mechanisms that are threshold in nature. The evidence consists of genotoxicity data, reports that isoprene is endogenously produced, and tumor-incidence data (Table 4-4). The genotoxicity data consist of observations suggesting that isoprene exhibits activity most consistent with aneugenic activity rather than clastogenic activity. Specifically, isoprene induces an increase in micronuclei in polychromatic erythrocytes without altering the frequency of gene mutations (Tice et al. 1988). This observation can be readily explained by postulating that isoprene is an aneugen, which exerts its effect by interfering with microtubule proteins in the spindle formed during cell division so that a whole chromosome is left behind in the cytoplasm to form a micronucleus. A threshold occurs because many microtubles would have to be disrupted before chromosomal separation is disrupted. On the other hand, a leveling off would be expected at higher concentrations as the cells become incapable of division because spindle function is nearly eliminated. The data in Table 4-4 show the kind of leveling off that would be expected from an aneugen. Finally, the endogenous production of isoprene (Hartmann and Kessler 1990) is more consistent with a carcinogenic mechanism based on a threshold, presumably one above the endoge-
TABLE 4-4 Neoplasms Induced in Mice by Exposure to Isoprene for 26 W Followed by 26 W of Recovery
|
|
|
Exposure Concentration, ppm |
|||||
|
Species |
Tumor |
0 |
70 |
220 |
700 |
2200 |
7000 |
|
Male rat |
Testis interstitial cell adenoma |
3/30 |
3/30 |
4/30 |
7/30 |
8/30 |
9/30 |
|
Male mouse |
Liver adenoma or carcinoma |
7/30 |
3/30 |
7/29 |
15/30a |
18/30 |
17/28 |
|
|
Lung adenoma or carcinoma |
2/30 |
2/30 |
1/29 |
5/30 |
10/30a |
9/28 |
|
|
Forestomach papilloma or carcinoma |
0/30 |
0/30 |
0/29 |
1/30 |
4/30 |
6/28a |
|
|
Harderian-gland adenoma |
2/30 |
6/30 |
4/29 |
14/30a |
13/30 |
12/28 |
|
a Statistically significant at and above this concentration. |
|||||||
nous production rate, than a model that postulates finite risk even with a single molecular "hit."
A recently reported chronic inhalation exposure of male B6C3F1 mice to isoprene strongly supports the hypothesis that isoprene is a threshold-type carcinogen. Groups of 50 male mice were exposed to isoprene for 20, 40 or 80 w (4 or 8 h/d, 5 d/w) at concentrations of 0, 10, 70, 140, 280, 700, and 2200 ppm (Placke et al. 1996). Only selected combinations of the exposure characteristics were tested. Groups of 50 female mice were exposed at 0, 10 or 70 ppm, 8 h/d for 80 w. An increased incidence of tumors was found in various sites; the lowest-observed-adverse-effect levels (LOAELs) were dependent on the site and sex of the group of mice. Exposures at 10 ppm for 80 w were NOAELs in both sexes, but 70 ppm appeared to be a LOAEL for certain tumors (harderian gland in both sexes and possibly pituitary adenomas in females). Placke et al. (1996) found that the traditional multistage risk model does not adequately predict the changes in tumor incidence when concentrations and cumulative exposure times are halved or doubled. For example, the authors concluded that "a threshold effect level and strong nonlinearities with respect to concentration appeared to exist for tumor development." Statistical analysis of the tumor incidence data to test the applicability of the equivalent-dose-metric hypothesis indicated that that concept was not applicable for isoprene carcinogenesis in mice (Cox et al. 1996)
Genotoxicity
The data base of genotoxicity on isoprene includes results of mutagenicity testing in Salmonella, demonstration of mutations in the ras proto-oncogene from isoprene-induced harderian-gland tumors in mice, and cytogenetic studies in inhalation-exposed mice.
On the basis of results from several laboratories, isoprene does not appear to be mutagenic in Salmonella tester strains, even after activation by rat-liver microsomes (de Meester et al. 1981; Gervasi et al. 1985; Kushi et al. 1985; Mortelmans et al. 1986). In testing of isoprene metabolites, the main metabolite of isoprene (compound 2, Figure 4-1) was not mutagenic or alkylating (Gervasi and Longo 1990). Likewise, the minor metabolite (compound 1) was not mutagenic or alkylating; however, the diepoxide (compound 5) was found to be both mutagenic and alkylating (Gervasi and Longo 1990).
Hong et al. (1995) have reported in an abstract that the harderian-gland tumors induced in B6C3F1 mice by isoprene exposures at 2200 and 7000 ppm for 26 w, with a 26-w recovery (Melnick et al. 1994), showed mutations in K- and H-ras proto-oncogenes. These mutations were detected at a high frequency
(100%) in the isoprene-induced tumors but were not detected in tumors that appeared in controls. The predominant mutations were A to T transversions at K-ras codon 61 (15/30) and C to A transversions at H-ras codon 61 (8/30). The authors concluded that activation of ras proto-oncogenes contributes to induction of harderian-gland tumors by isoprene (Hong et al. 1995).
The results of cytogenetic studies of inhaled isoprene in mice, completed before any carcinogenesis studies had been started, led to the prediction that isoprene would induce tumors at multiple sites in mice (Tice et al. 1988). Male B6C3F1 mice were exposed to isoprene at concentrations of 0, 438, 1750, and 7000 ppm, 6 h/d for 12 d. Isoprene induced an increase in sister chromatid exchanges (SCE) in bone-marrow cells and in the level of micronucleated polychromatic erythrocytes (PCE) (Tice et al. 1988). The exposure did not alter the frequency of chromosomal aberrations or mitotic index in bonemarrow cells. Shelby and Witt (1995) point out that it is unusual for a compound to be positive in a micronucleus test and negative in a chromosomal aberration test; however, the data used to make their observation (Tice et al., 1988) were obtained before application of the centromere fluorescent-probe technique, which facilitates detection of whole chromosomes in micronuclei caused by aneugens. Recent comparisons of aneugens and clastogens and their ability to produce micronuclei without chromosomal aberrations (Elhajouji et al. 1995) suggest that isoprene could be more of an aneugen than clastogen. Carcinogenic activity caused by an aneugen could have a low-concentration threshold for cancer induction and might also show a plateau at high concentrations where spindle destruction occurs.
Developmental Toxicity
An inhalation developmental study has been reported in Swiss (CD-1) mice and Sprague-Dawley rats exposed to isoprene at 0, 280, 1400, or 7000 ppm for 6 h/d, 7 d/w on gestational days 6-17 (mice) or 6-19 (rats) (Mast et al. 1989). In rats, there were no effects on body-weight gains, reproductive indices, or fetal malformations; however, an increased incidence of reduced vertebral ossifications in the 7000-ppm group suggests that this concentration might be the threshold for developmental toxicity. In mice, the body weights and uterine weights were reduced at 7000 ppm. Fetal weights were subnormal in females from the 280-ppm group and in males from the 1400-ppm group. An increase in supernumerary ribs in mouse fetuses from the 7000-ppm group was reported. The authors concluded that 1400 ppm is a NOAEL for rat maternal and developmental toxicity and mouse maternal toxicity. The NOAEL for mouse developmental toxicity cannot be assigned from the results (Mast et al. 1990).
Reproductive Toxicity
Indirect evidence in rats includes results from exposures up to 2200 ppm for 26 w in which no abnormalities were found (Melnick et al. 1994). At 7000 ppm, interstitial-cell hyperplasia of testis and adenoma were seen in rats after recovery, and testicular atrophy was found in mice after recovery (Melnick et al. 1994). Testicular atrophy was also noted in mice exposed for only 2 w to isoprene at 7000 ppm (Melnick et al. 1990). A functional reproductive study has not been reported.
Interactions with Other Chemicals
No reports of interactions of isoprene with other chemicals were found. A summary of the toxicity data on isoprene is presented in Table 4-5.
TABLE 4-5 Toxicity Summary
|
Concentration, ppm |
Exposure Duration |
Species |
Effects |
Reference |
|
Unknown |
Industrial, 1 y |
Human (n = 148) |
Immunological response to vaccination was different from control response |
Nikul'tseva 1967 |
|
Unknown + other compounds |
4 y |
Human (n = 630) |
Inflammation and degeneration of nasal mucosa |
Mitin 1969 |
|
Up to 26 + other hydrocarb. |
8h |
Human (n = 300) |
20% slower photogenic reflex response at end of work day; cardiovascular changes |
Pigolev 1971 |
|
57 |
Few min |
Human (n = 10) |
Slight mucosal irritation |
Gostinskii 1965 |
|
0.035 or 0.36 |
4 h/d, 30d |
Rat (M) |
Variations in thymus cellularity and mitotic index. |
Mamedov 1979 |
|
0.042 |
4 h/d, 4 mo |
Rat (M) |
Reversible variations in thymus weight, cellularity, and mitotic index |
Mamedov 1979 |
|
0.3 or 0.8 |
4 h |
Rat(M) |
Increased thymus mitotic index and peripheral lymphocyte count (1 d post-exposure) |
Mamedov 1979 |
|
10 |
8 h/d, 5 d/w, 80w |
Mouse (M, F) |
NOAEL for increased incidence of neoplasms |
Placke et al. 1996. |
|
70 |
8 h/d, 5 d/w, 80 w |
Mouse (M, F) |
LOAEL for harderian-gland tumors, NOAEL for lung, liver, and forestomach tumors |
Placke et al. 1996 |
|
70 |
6 h/d, 5 d/w, 26 w, and recovery |
Mouse (M) Mouse (M) |
20 of 30 with spinal-cord degeneration, 4 of 30 in controls NOAEL for neoplastic lesions |
Melnick et al. 1994 |
|
220 |
6 h/d, 5 d/w, 26 w, and recovery |
Mouse (M) |
Olfactory epithelial degeneration, reversible decrease in hind-limb grip strength |
Melnick et al. 1994 |
|
220 |
6 h/d, 24 d |
Mouse (F) Mouse (M) |
Decreased RBC count, hemoglobin, hematocrit Decreased RBC volume |
Melnick et al. 1994 |
|
Concentration, ppm |
Exposure Duration |
Species |
Effects |
Reference |
|
438 |
6 h/d, 5 d/w, 2 w |
Mouse (M) Mouse (F) |
Vacuolized cytoplasm in hepatocytes, Forestomach epithelial hyperplasia |
Melnick et al. 1990 |
|
540 |
40 min |
Rabbit |
Hastened and weakened flexor reflex contractions |
Gostinskii 1965 |
|
700 |
6 h/d, 5 d/w, 26 w, and recovery |
Mouse (M) |
Liver tumors, forestomach epithelial, hyperplasia, harderian-gland adenoma |
Melnick et al. 1994 |
|
700 |
6 h/d, 5 d/w, 13 w |
Mouse (M, F) |
Forestomach epithelial hyperplasia |
Melnick et al. 1994 |
|
790 |
40 min |
Mouse |
Decreased ability of CNS to summate subthreshold impulses |
Gostinskii 1965 |
|
790 to 1750 |
4 h/d, 4-5 mo |
Rabbit |
Increased leukocyte count |
Gostinskii 1965 |
|
|
|
Rat |
Decreased RBC count, bronchial irritation, hepatocyte degeneration, myocardial changes, thyroid irritation |
|
|
|
|
Mouse |
50% decrease in swim time |
|
|
1670 |
6 h/d, 15 d |
Rat |
No toxic signs, no gross organ damage |
Gage 1970 |
|
6000 |
6 h/d, 6 d |
Rat |
No toxic signs, lung slightly congested, other normal |
Gage 1970 |
|
7000 |
6 h/d, 26 w |
Rat (M) |
Interstitial cell hyperplasia of testis (no recovery) and adenoma (after 26-w recovery) |
Melnick et al. 1994 |
|
|
|
Rat (F) |
NOAEL |
|
|
36-43,000 |
Unknown |
Mouse |
Deep narcosis |
von Oettingen 1940 |
|
39,000 |
2 h + ? |
Mouse |
Collapse in 50 |
Gostinskii 1965 |
|
51,000 |
2 h + ? |
Mouse |
LC 50 |
Gostinskii 1965 |
|
57,000 |
2 h |
Mouse |
LC50 |
Shugaev 1968 |
|
64,000 |
4 h |
Rat |
LC50 |
Shugaev 1968 |
RATIONALE FOR ACCEPTABLE CONCENTRATIONS
Table 4-6 presents exposure limits for isoprene set by other organizations or countries and Table 4-7 presents the SMACs established by NASA.
In many respects, the toxicity data base on isoprene is useful in setting SMACs; however, there are several observations that are perplexing and confound the process of setting SMACs. The most obvious question is why mice are approximately 100-fold more susceptible than rats. Rats show virtually no effects (with the possible exception of testicular adenoma), even after long-term exposures at 7000 ppm, whereas exposures as low as 70 ppm are needed before most adverse effects disappear in mice. There are no data to suggest which species is the best model for humans; hence, the most sensitive species, mice, will be used to set limits (NRC 1992). There are several reports from Russian literature, but the results are difficult to reconcile with more recent U.S. studies and with the levels of endogenous isoprene present in rodents. Finally, the carcinogenesis data are perplexing in that there seems to be a threshold effect rather than an increasing incidence of tumors with increasing doses; therefore, the linearized multistage model, which is ordinarily used to estimate cancer risks, will not be applied.
TABLE 4-6 Exposure Limit Set by Other Organizations or Countries
|
Organization or Country |
Exposure Limit, ppm |
Reference |
|
Russia |
14 (STEL) |
IARC 1994 |
|
STEL, short-term exposure limit. |
||
TABLE 4-7 Spacecraft Maximum Allowable Concentrations
|
Duration |
Concentration, ppm |
Concentration, mg/m3 |
Target Toxicity |
|
1 h |
50 |
140 |
Mucosal irritation |
|
24 h |
25 |
70 |
Mucosal irritation |
|
7 da |
2 |
6 |
Anemia, mucosal irritation |
|
30 d |
2 |
6 |
Anemia, mucosal irritation |
|
180 d |
1 |
3 |
Anemia, respiratory-system injury, neurotoxicity |
|
a Previous 7-d SMAC = 200 ppm (560 mg/m3). |
|||
Perspective Provided by Comparison to 1,3-Butadiene
It is tempting to evoke analogies between isoprene and 1,3-butadiene to explain some of the important aspects of isoprene toxicity. On the other hand, one must remain aware of important differences in the metabolism, genotoxicity, carcinogenicity, and neurotoxicity of the compounds. In addition, isoprene is endogeneously produced in humans and rodents, but butadiene is not endogenously produced. Both compounds are metabolized to mutagenic compounds; however, metabolic saturation of activation pathways might limit the ability of isoprene to achieve the same levels of mutagenic epoxides as butadiene does at lower concentrations (Melnick et al, 1994). Butadiene appears to be a much more potent genotoxin in mice than isoprene (Tice 1988; Shelby 1990). Both induce cancer in multiple organs; however, isoprene does not produce an increased incidence of lymphomas in mice as butadiene does (Melnick et al., 1994). Furthermore, repeated exposure to isoprene is neurotoxic to mice (Melnick et al. 1994), but butadiene is not neurotoxic in similar experiments.
Short-Term Limits (1-h or 24-h Exposure)
Acute Neurotoxicity
The reports of lethality or deep narcosis as a result of exposures near or above 40,000 ppm are not useful for setting short-term limits. Gostinskii (1965) reported that mice exposed for only 40 min to isoprene at 2200 mg/m3 (790 ppm) had changes in their ability to summate subthreshold impulses, and rabbits, after a 40-min exposure at 4100 mg/m3 (1500 ppm), had changed flexor reflexes. No details of the test method, control method, or statistical analyses were given, so these reported findings are not suitable for setting limits for acute neurotoxicity.
Mucosal Irritation
In the Gostinskii report, 10 human subjects experienced slight mucosal irritation when exposed to isoprene at 160 mg/m3 (57 ppm) for an unspecified period of time. That suggests that a 1-h AC for exposure to isoprene should be 50 ppm to produce no more than slight irritation; however, a lower concentration might be necessary for a 24-h exposure. A factor of 2 was applied to give a 24-h AC of 25 ppm to prevent all but minimal irritation.
Lesions of the Forestomach
Additional data come from the 2-w exposure of rats and mice to concentrations ranging from 440 to 7000 ppm (Melnick et al. 1990). The exposures were 6 h/d for 10 d for a total of 60 h. No exposure-related changes were found in rats; however, mice showed forestomach epithelial degeneration. In contrast, mice exposed to isoprene for 13 w at 220 ppm did not show either of these types of lesions (the 700-ppm group did, however), which suggests a thresholdtype effect with a NOAEL at 220 ppm. The AC for a 24-h exposure was calculated as follows:
|
24-h AC = 220 ppm × 1/10 = 22 ppm. |
|
|
NOAEL |
Species |
These data are not suitable for estimating a 1-h limit. Neither type of lesion (liver or forestomach) should be increased in risk because of spaceflight factors.
Long-Term Exposure Limits (7- to 180-d Exposures)
Mucosal Irritation
The NOAEL for mucosal irritation can be conservatively estimated to be 10 fold lower than the 57-ppm short-term LOAEL in humans reported by Gostinskii (1965), or 6 ppm. Time factors were not applied, because mucosal irritation generally is experienced only in the first few minutes of exposure until adaptation occurs, and the 10-fold factor applied to the LOAEL is conservative.
Neurotoxicity
Physiological and histopathological data in mice exposed to isoprene for 26 w suggest that the nervous system is a target organ (Melnick et al. 1994). At the end of 26 w of exposure, hind-limb grip strength was reduced in mice exposed at 220 ppm or more when compared with controls; however, within 4 w the hind-limb grip strength in exposed mice recovered to control values. That finding is perplexing in view of the appearance of spinal-cord degeneration (Table 4-3). The degeneration was not observed at the end of the 26-w
exposure except in the 7000-ppm group, whereas it was observed in all exposure groups (including the 70-ppm group) at the end of the 26-w recovery period. In addition, the dose response of the spinal-cord degeneration does not suggest a direct relationship with isoprene exposure. For example, less than half of the 7000-ppm group showed the lesion, whereas two-thirds of the 70-ppm group showed the lesion. Data on acrylamide-exposed rats tend to support the significance of this lesion (Crofton et al. 1996); however, similar lesions were not found in the same strain of mouse exposed to much higher concentrations of isoprene (Placke et al. 1996).
Because there was no logical approach to gleaning a dose-response relationship from the incidence of spinal-cord degeneration (Table 4-3), the lowest concentration of 70 ppm was taken as a NOAEL in mice, because functional deficits were not found at this concentration (Melnick et al. 1994) and because spinal-cord lesions were not reported by Placke et al. (1996) at much higher concentrations. To convert that NOAEL to a NOAEL in humans, a factor of 10 for potential species differences was applied. The human NOAEL was estimated to be 7 ppm or 2 mg/m3. This result is from mice exposed for a total of 780 h (30 h/w × 26 w), which is slightly longer than 30 d of continuous exposure, so 7 ppm was the AC for 30 d of exposure. There are no data showing that the neurotoxicity would not be more severe with prolonged exposure, so the default approach using Haber's rule was applied for the 180-d AC.
180-d AC = 7 ppm × (30/180) = 1 ppm.
According to the NRC guidelines, the 7-d AC for neurotoxicity should not be increased from the 30-d AC without evidence that the effect would be reduced with shorter exposure. There was no such evidence, so the 7-d AC was set at 7 ppm.
Spaceflight is not known to cause nervous-system effects that could interact with isoprene-induced neurotoxicity to increase the risk of an effect.
Hematological Changes
Chronic exposure of mice to isoprene up to 2200 ppm resulted in chronic myeloid hyperplasia of the bone marrow without any effect on RBC measures (Placke et al. 1996); however, Melnick et al. (1994) described an anemia in mice resulting from isoprene exposures that was initially (3 d) normocytic but converted to macrocytic anemia later (24 d). These anemias were evident not only after 3 or 24 d but also after 13 w or 26 w in exposed mice. On the basis
of the 24-d data, the 70-ppm group was not adversely affected, and the anemia did not appear to become worse with prolonged exposures. The ACs to protect against hematological effects were calculated as follows:
7-d, 30-d, 180-d AC = 70 ppm × 1/10 × 1/3 = 2.3 ppm.
The ACs are independent of time of exposure; however, a spaceflight factor of 3 was applied because of the loss of RBC mass in crew members during spaceflight.
Liver Effects and Tumors
Cytoplasmic vacuolization of hepatocytes was reported in male mice exposed for 2 w to isoprene concentrations as low as 440 ppm (Table 4-1); however, the same investigators reported no such effects in the same strain of male mice exposed at 700 ppm for 13 w (Table 4-2). These effects were not mentioned in male mice exposed for 26 w and recovered for 26 w; however, liver tumors were significantly increased in exposed mice when compared with control mice at exposures of 700 ppm or more (Table 4-4). The NOAEL for such tumors was 220 ppm. The hepatocyte vacuolization was considered an adaptive rather than adverse effect. Liver tumors were no more sensitive as a neoplastic end point than harderian-gland tumors, and the incidence of liver tumors in unexposed mice was so high (11/50) that a separate risk assessment on hepatocellular tumors was not attempted. The discussion below on harderian-gland tumors would apply to liver tumors as well.
The NOAELs for harderian-gland tumors were 220 ppm for 26 w of exposure (Melnick et al. 1994) and 70 ppm for 80 w of exposure (Placke et al. 1996). Because the weight of evidence suggests that isoprene is a threshold-type carcinogen, the ACs for cancer will be estimated on that basis. The mice in the Melnick study were exposed for 30 h/w × 26 w = 780 h, which is approximately equivalent to 30 d of continuous exposure. The mice in the Placke study were exposed for 40 h/w × 80 w = 3200 h, which is roughly 180 d of continuous exposure. Applying a species factor of 10 gives the following estimates:
7 and 30-d AC = 22 ppm.
180-AC = 7 ppm.
Isoprene exposures at those concentrations are not expected to present any risk
of inducing cancer in crew members. Spaceflight would not be expected to increase the risk of isoprene-induced cancer.
Respiratory-System Injury
Olfactory degeneration and lung tumors have been associated with isoprene exposures in male mice (Tables 4-1, 4-2, 4-3, and 4-4). Again, the data are perplexing because the lowest concentration that induced olfactory degeneration for 2-w exposures was between 1750 and 3500 ppm (Table 1-1), whereas for 13-w and 26-w (no recovery) exposures, the lowest effect level was 7000 ppm (Tables 4-2 and 4-3). The incidence of lung tumors was significantly above controls in the mice exposed for 26 w (with recovery) at concentrations of 2200 ppm and 7000 ppm (Table 4-4). The 2-w data, because of the short cummulative exposure time of only 60 h, were not used to set ACs. From the longer-term data, it is apparent that 70 ppm is a NOAEL for respiratory-system injury (olfactory epithelial degeneration, Table 4-3). Using a species factor of 10 for all ACs and a time factor for the 180-d AC, the ACs were as follows:
7-d AC = 70 ppm × 1/10 = 7 ppm.
30-d AC = 70 ppm × 1/10 =7 ppm.
180-d AC =70 ppm × 1/10 × 780 h/4320 h = 1.3 ppm.
The risk of respiratory-system injury should not increase as a result of changes associated with spaceflight.
Forestomach Epithelial Hyperplasia
Although the 2-w exposures (Table 4-1) suggest hyperplasia at concentrations of isoprene as low as 440 ppm, later data (Tables 4-2 and 4-3) suggest that the effect does not occur until mice are exposed at 700 ppm for 13 or 26 w (26 w recovery). Apparently, 220 ppm is a NOAEL, and the effect is independent of time of exposure (threshold-type effect). A significant increase in fore-stomach tumors was noted in mice exposed for 26 w at 7000 ppm; however, setting the AC to protect against hyperplasia will also protect against tumors. Using a species factor of 10 and no time factors, the ACs were as follows:
7-d, 30-d, 180-d ACs = 220 ppm × 1/10 = 22 ppm.
The risk of forestomach lesions should not be affected by spaceflight.
Testicular Injury
Exposures of mice to isoprene for 2 w or 26 w resulted in testicular atrophy at 7000 ppm but not at 3500 ppm (Table 4-1; Melnick et al. 1994). Male rats exposed for 26 w with a 26-w recovery showed a gradually increasing incidence of testicular tumors (Table 4-4) and interstitial-cell hyperplasia after 26-w exposures at 7000 ppm without recovery (Melnick et al. 1994). Apparently, 220 ppm is a NOAEL for these effects; hence, with a species factor of 10 and no time factor, the ACs were as follows:
7-d, 30-d, 180-d ACs = 220 ppm × 1/10 = 22 ppm.
The study from which this calculation was made was not the usual 2-y bioassay; therefore, no-effect concentrations estimated from the findings are subject to the caveat that a longer study could have resulted in lower no-effect concentrations. The 80-w study reported by Placke et al. (1996) reported adverse effects on testes of mice; however, details on the incidence were not provided. For those reasons, the AC analysis is not presented in Table 4-8. The risk of this injury would not be increased as a result of spaceflight.
Endogenous Production of Isoprene
For systemic effects such as neurotoxicity, it would be illogical to set an acceptable exposure concentration for isoprene that would lead to an uptake rate much below the endogenous production rate. According to an abstract, the endogenous production rate for isoprene in humans is 0.15 µmol/(kg•h) or 10 µg/(kg•h) (Hartmann and Kessler 1990). For a 70-kg person, that is equivalent to 0.71 mg/h. This is about half the rate of 24 µmol/h (1.6 mg/h), which was estimated by Filser et al. (1996). The latter rate will be used in the calculations below because it is more reliably reported.
The uptake of a pollutant from air is the product of the uptake percentage, the concentration in the air, and the hourly ventilation. Assuming an uptake of 50% and a ventilation rate of 0.6 M3/h (15 M3/d), the airborne concentration to match endogenous production would need to be 1.6/(0.5 × 0.6) = 5 mg/M3, which is equivalent to 2 ppm. This lower concentration bound for isoprene-induced systemic effects is comparable to the lowest longer-term ACs for isoprene-induced toxic effects (Table 4-8). This comparison suggests that the limits set for isoprene are fully protective.
TABLE 4-8 Acceptable Concentrations
|
End Point, Exposure Data, Reference |
|
Uncertainty Factors |
Acceptable Concentrations, ppm |
|||||||
|
Species |
NOAEL |
Time |
Species |
Spaceflight |
1 h |
24 h |
7 d |
30 d |
180 d |
|
|
Mucosal irritation |
Human |
—a |
1-2 |
1 |
1 |
50 |
25 |
— |
— |
— |
|
Slight irritation at 57 ppm; LOAEL, 57 ppm (Gostinskii 1965) |
10 |
1 |
1 |
1 |
— |
— |
6 |
6 |
6 |
|
|
Neurotoxicity |
Mice |
— |
1 HRb |
10 |
1 |
— |
— |
7 |
7 |
1 |
|
NOAEL, 70 ppm for functional deficit, 26-w exposure (Melnick et al. 1994) |
|
|
|
|
|
|
|
|
|
|
|
Hematological changes |
Mice |
— |
1 |
10 |
3 |
— |
— |
2 |
2 |
2 |
|
NOAEL, 70 ppm for anemia, 26-w exposure (Melnick et al. 1994) |
|
|
|
|
|
|
|
|
|
|
|
Cancer |
|
|||||||||
|
NOAEL, 220 ppm 780-h exposure (Melnick et al. 1994) |
Mice |
— |
1 |
10 |
1 |
— |
— |
22 |
22 |
— |
|
NOAEL, 70 ppm; 3200-h exposure (Placke et al. 1996) |
Mice |
— |
1 |
10 |
1 |
— |
— |
— |
— |
7 |
|
Respiratory system injury |
Mice |
— |
1 HR |
10 |
1 |
— |
— |
7 |
7 |
1 |
|
NOAEL, 70 ppm; olfactory degeneration tumors, 26-w exposure (Melnick et al. 1994) |
|
|
|
|
|
|
|
|
|
|
RECOMMENDATIONS
The isoprene exposure limits proposed may be extremely conservative because they were based on the most susceptible species, mice. Rats are approximately 100-fold less susceptible than mice. Human metabolic and tissue studies are needed to determine which of the rodent species is the best model. In particular, these studies should address the formation of the diepoxide in both human tissues and in test subjects. Better data are needed on the effects of acute exposures to moderate concentrations of isoprene.
REFERENCES
Bond, J.A., W.E. Bechtold, L.S. Birnbaum, A.R. Dahl, M.A. Medinsky, J.D. Sun, and R.F. Henderson. 1991. Disposition of inhaled isoprene in B6C3F1 Mice. Toxicol. Appl. Pharmacol. 107:494-503.
Cailleux, A., M. Cogny, and P. Allain. 1992. Blood isoprene concentrations in human and in some animal species. Biochem. Med. Metab. Biol. 47:157-160.
Conkle, J.P., B.J. Camp, and B.E. Welch. 1975. Trace composition of human respiratory gases. Arch. Environ. Health 30:290-295.
Cox, L.A., Jr., M.G. Bird, and L. Griffis. 1996. Isoprene cancer risk and the time pattern of dose administration. Toxicology 113:263-272.
Crofton, K.M., S. Padilla, H.A. Tilson, D.C. Anthony, J.H. Raymer, and R.C. MacPhail. 1996. The impact of dose rate on the neurotoxicity of acrylamide: The interaction of administered dose, target tissue concentrations, tissue damage, and functional effects. Toxicol. Appl. Pharmacol. 139:163-176.
Dahl, A.R., L.S. Birnbaum, J.A. Bond, P.G. Gervasi, and R.F. Henderson. 1987. The fate of isoprene inhaled by rats: Comparison to butadiene. Toxicol. Appl. Pharmacol. 89:237-248.
Dalhamn, T., M.-L. Edfors, and R. Rylander. 1968. Retention of cigarette smoke components in human lungs. Arch. Environ. Health 17:746-748.
Egle, J.L., and B.J. Gochberg. 1975. Retention of inhaled isoprene and methanol in the dog. Am. Ind. Hyg. Assoc. J. 36(5):369-373.
Elhajouji, A., P.V. Hummelen, and M. Kirsch-Volders. 1995. Indications for a threshold of chemically induced aneuploidy in vitro in human lymphocytes. Environ. Mol. Mutagen. 26:292-304.
Filser, J.G., G.A. Csanady, B. Denk, M. Hartmann, A. Kauffman, W. Kessler, P.E. Kreuzer, C. Putz, J.H. Shen, and P. Stei. 1996. Toxicokinetics of isoprene in rodents and humans. Toxicology 113:278-287.
Gage, J.C. 1970. The subacute inhalation toxicity of 109 industrial chemicals. Br. J. Ind. Med. 27:1-18.
Gelmont, D., R.A. Stein, and J.F. Mead. 1981. Isoprene-the main hydrocarbon in
human breath. Biochem. Biophys. Res. Commun. 99:1456-60.
Gervasi, P.G., and V. Longo. 1990. Metabolism and mutagenicity of isoprene. Environ. Health Perspect. 86:85-87.
Gervasi, P.G., L. Citti, M. Del Monte, V. Longo, and D. Benetti. 1985. Mutagenicity and chemical reactivity of epoxidic intermediates of the isoprene metabolism and other structurally related compounds. Mutat. Res. 156:77-82.
Gostinskii, V.D. 1965. Toxicity of isoprene and maximal safe concentration of the vapor in air. Fed. Proc. Transl. Suppl. 24(6):1123-1126.
Hartmann, M., and W. Kessler. 1990. Pharmacokinetics and endogenous production of isoprene in humans. Naunyn-Schmiedeberg's Arch. Pharmacol. 341:R13.
Hong, H.L., T.R. Devereux, G.A. Boorman, and R.C. Sills. 1995. ras oncogene mutations in harderian gland neoplasms of B6C3F1 mice exposed to isoprene for 6 months [abstract]. FASEB J. 9(3):A132.
IARC. 1994. Isoprene. Pp. 215-232 in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Industrial Chemicals. Vol. 60. Lyon, France: IARC Press.
James, J.T., T.F. Limero, H.J. Leano, J.F. Boyd, and P.A. Covington. 1994. Volatile organic contaminants found in the habitable environment of the space shuttle: STS-26 to STS-55. Aviat. Space Environ. Med. 65:851-857.
Khalil, M.A.K., and R.A. Rasmussen. 1992. Forest hydrocarbon emission: Relationships between fluxes and ambient concentrations. J. Air Waste Manage. Assoc. 42:810-813.
Kushi, A., D. Yoshida, and S. Mizusaki. 1985. Mutagenicity of gaseous nitrogen oxides and olefins on Salmonella TA102 and TA104. Mutat. Res. 147:263-264.
Lonneman W.A., G.R. Namie, and J.J. Bufalini. 1979. Hydrocarbons in Houston Air. Atmospheric Chemistry and Physics Division Environmental Sciences Research Laboratory, Research Triangle Park, NC.
Mamedov, A.M. 1979. Response of lymphoid tissue to isoprene inhalation and some relevant integral indices [in Russian]. Gig. Tr. Prof. Zabol. (6):35-37.
Mast, T.J., J.J. Evanoff, K.H. Stoney, R.B. Westerberg, R.L. Rommereim, and R.J. Weigel. 1989. Inhalation Developmental Toxicology Studies: Teratology Study of Isoprene in Mice and Rats. National Institute of Environmental Health Sciences Final Report No. NIH-Y01-ES-70153. 27 pp.
Mast, T.J., R.L. Rommereim, R.J. Weigel, K.H. Stoney, B.A. Schwetz, and R.E. Morrissey. 1990. Inhalation developmental toxicity of isoprene in mice and rats. Toxicologist 10(1):42.
deMeester, C., M. Mercier, and F. Poncelet. 1981. Mutagenic activity of butadiene, hexachlorobutadine and isoprene. Pp. 195-203 in Industrial and Environmental Xenobiotics, I. Gut, M. Cikrt, and G.L. Plaa, eds. Berlin: Springer.
Melnick, R.L., J.H. Roycroft, B.J. Chou, H.A. Ragan, and R.A. Miller. 1990. Inhalation toxicology of isoprene in F344 rats and B6C3F1 mice following two-week exposures. Environ. Health Perspect. 86:93-98.
Melnick, R.L., R.C. Sills, J.H. Roycroft, B.J. Chou, H.A. Ragan, and R.A. Miller. 1994.
Isoprene, an endogenous hydrocarbon and industrial chemical, induces multiple organ neoplasia in rodents after 26 weeks of inhalation exposure
. Cancer Res. 54:5333-5339.Mitin, Y.V. 1969. Changes in the upper respiratory tract in isoprene rubber production workers. Zhurnal Ushnykh Nosovykh i Gorlovykh Boleznei. 29:79-83.
Mortelmans, K., S. Haworth, T. Lawlor, W. Speck, B. Tainer, & E. Zeiger. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. Mutagen. 8(7):1-119.
Nikul'tseva, A.A. 1967. The effect of isoprene rubber products on some indices of antityphoid immunity in workers [in Russian]. Gig. Tr. Prof. Zabol. 11(12):41-44.
NRC. 1992. Guidelines for Developing Spacecraft Maximum Allowable Concentrations for Space Station Contaminants. Washington, DC: National Academy Press.
Peter, H., H-J. Weigand, J. Filser, H.M. Bolt, and R.J. Laib. 1990. Inhalation pharmacokinetics of isoprene in rats and mice. Environ. Health Perspect. 86:89-92.
Peter, H., H.J. Wiegand, H.M. Bolt, H. Greim, G. Walter, M. Berg, and J.G. Filser. 1987. Pharmacokinetics of isoprene in mice and rats. Toxicol. Lett. 36:9-14.
Pigolev, S.A. 1971. Physiological changes in machine operators in the isoprene rubber industry [in Russian]. Gig. Tr. Prof. Zabol. 15(2):49-50.
Placke, M.E., L. Griffis, M. Bird, J. Bus, R.L. Persing, L.A. Cox, Jr. 1996. Chronic inhalation oncogenicity study of isoprene in B6C3F 1 mice. Toxicology 113:253-262.
Shelby, M.D. 1990. Results of NTP-sponsored mouse cytogenetic studies on 1,3-butadiene, isoprene, and chloroprene. Environ. Health Perspect. 86:71-73.
Shelby, M.D., and K.L. Witt. 1995. Comparison of results from mouse bone marrow chromosome aberration and micronucleus tests. Environ. Mol. Mutagen. 25:302-313.
Shugaev, B.B. 1968. Distribution in the organism and toxicity of aliphatic hydrocarbons [in Russian]. Farmakol. Toksikol. 31:360-363.
Sun, J.D., A.R. Dahl, J.A. Bond, L.S. Birnbaum, and R.F. Henderson. 1989. Characterization of hemoglobin adduct formation in mice and rats after administration of [14C] butadiene or [14C] isoprene. Toxicol. Appl. Pharmacol. 100:86-95.
Tice, R.R., R. Boucher, C.A. Luke, D.E. Paquette, R.L. Melnick, and M.D. Shelby. 1988. Chloroprene and isoprene: Cytogenetic studies in mice. Mutagenesis 3(2):141-146.
Von Oettingen, W.F. 1940. Toxicity and potential dangers of aliphatic and aromatic hydrocarbons: A critical review of the literature. Pp. 26-28 in Public Health Bulletin No. 255. U.S. Public Health Service.
Wistuba, D., K. Weigand, and H. Peter. 1994. Stereoselectivity of in vitro isoprene metabolism. Chem. Res. Toxicol. 7:336-343.






























