3
Scientific Basis for Natural Attenuation
To evaluate whether natural attenuation can achieve legal standards for groundwater cleanup, the fate of contaminants in the groundwater environment has to be well understood. In what direction do the contaminants move? How far will they spread? Will they degrade to innocuous compounds? While similar questions need to be answered for any proposed remedy for contamination, providing clear answers is especially important for natural attenuation remedies because of the unique public concerns described in Chapter 2.
This chapter describes the common classes of groundwater contaminants, the characteristics of the subsurface environment, and the subsurface processes that can affect contaminants. For each contaminant class, it describes case examples of sites at which natural attenuation has been carefully studied. The chapter then summarizes what is and is not known about natural attenuation of the different contaminant classes and when it is likely to succeed.
Although assessing contaminant fate in the subsurface environment is complex, in many cases science and experience provide a foundation for judging when different combinations of contaminants are likely to degrade or transform in different hydrogeologic settings. In other cases, better scientific understanding is needed before making decisions about natural attenuation. In some cases, natural attenuation works well to minimize risks, because the contaminant’s fate is controlled by a process that destroys the contaminant before it moves far. Biodegradation is the most common example. In other cases, the contaminant can be permanently
immobilized. However, the fate of a contaminant never is controlled by one process alone. Often, several physical, biological, and chemical processes act simultaneously. Which processes are important depends on the contaminant and the hydrogeologic setting.
CONTAMINANTS AND HYDROGEOLOGIC SETTINGS
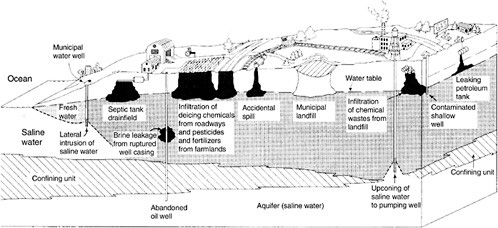
Modern society uses enormous quantities of organic and inorganic chemicals. Through accidental and purposeful releases, some of these chemicals enter the environment and become contaminants. Contaminants enter the subsurface in a variety of forms, including in solid materials, liquids, and vapors. Figure 3-1 illustrates many ways by which wastes can contaminate soil and groundwater. In general, this report focuses on groundwater contaminants released from “point sources,” such as waste pits, landfills, mine wastes, buried containers, and leaking storage tanks. Nonetheless, the principles also apply to contaminants released from nonpoint sources such as agricultural fields, farm animal lots, urban runoff, and polluted rainfall.
Table 3-1 lists the common classes of groundwater contaminants and provides examples in each class, along with common industrial sources or applications. The table is organized by chemical classes, because the various natural attenuation processes tend to affect contaminants within each class in similar ways.
Although the chemicals in Table 3-1 are listed as pure materials, they commonly occur as mixtures. Many products used in industry and commerce are mixtures. For example, gasoline contains hundreds of hydrocarbons, as well as a range of organic and inorganic additives (Rittmann et al., 1994). Solvents and other industrial feedstocks are not 100 percent pure but contain small amounts of other compounds. Landfills usually receive a wide range of chemicals that can leach into groundwater. Wastes generated from cleaning operations contain the cleaning agents, as well as chemicals removed during cleaning. Wastes from nuclear weapons manufacturing sites typically contain mixtures of radionuclides, metals, solvents, and organic chelating agents (DOE, 1990; Rittmann et al., 1994). Complex interactions among contaminants, natural environmental chemicals, and microorganisms—as well as the complex processes affecting contaminant and groundwater movement—typically make understanding the fate of such mixtures in the subsurface a challenge.
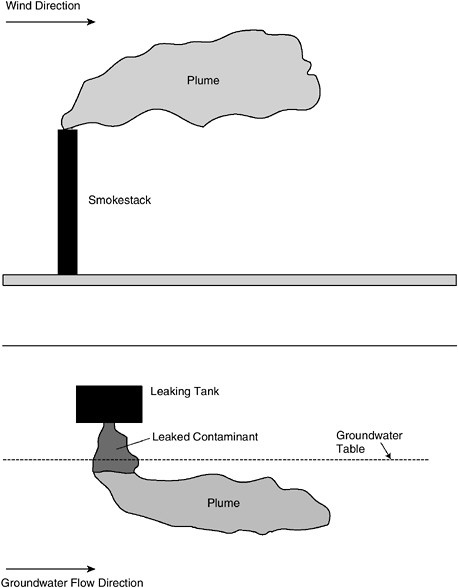
When a contaminant dissolves into the groundwater, it creates a plume, which moves with the groundwater. Figure 3-2 illustrates a groundwater plume formed below a leaking tank and compares it to a visible plume from a smokestack. The contaminants in the plume always move in the same direction, although not necessarily at the same speed, as the
TABLE 3-1 Categories of Subsurface Contaminants, Frequency of Occurrence, and Sources
|
Chemical Class |
Example Compounds |
Occurrence Frequencya |
Examples of Industrial Sources or Applications |
|
Organic |
|
||
|
Hydrocarbons |
|
||
|
Low molecular weight |
BTEX, alkanes |
F |
Crude oil, refined fuels, dyestuffs, solvents |
|
High molecular weight |
Polycyclic aromatic hydrocarbons, nonvolatile aliphatic hydrocarbons |
C |
Creosote, coal tar, crude oil, dyestuffs, lubricating oils |
|
Oxygenated hydrocarbons |
|
||
|
Low molecular weight |
Alcohols, ketones, esters, ethers, phenols, MTBE |
F |
Fuel oxygenates, solvents, paints, pesticides, adhesives, pharmaceuticals, fermentation products, detergents |
|
Halogenated aliphatics |
|
||
|
Highly chlorinated |
Tetrachloroethene, trichloroethene, 1,1,1-trichloroethane, carbon tetrachloride |
F |
Dry cleaning fluids, degreasing solvents |
|
Less chlorinated |
1,1-Dichloroethane, 1,2-dichloroethene, vinyl chloride, methylene chloride |
F |
Solvents, pesticides, landfills, biodegradation by-products, plastics |
|
Halogenated aromatics |
|
||
|
Highly chlorinated |
Pentachlorophenol, PCBs, polychlorinated dioxins, polychlorinated dibenzofurans, chlorinated benzenes |
C |
Wood treatment, insulators, heat exchangers, by-products of chemical synthesis, combustion by-products |
|
Less chlorinated |
Chlorinated benzenes, PCBs |
C |
Solvents, pesticides |
|
Nitroaromatics |
TNT, RDX |
C |
Explosives |
|
Chemical Class |
Example Compounds |
Occurrence Frequencya |
Examples of Industrial Sources or Applications |
|
Inorganic |
|
||
|
Metals |
Cr, Cu, Ni, Pb, Hg, Cd, Zn |
F |
Mining, gasoline additives, batteries, paints, fungicides |
|
Nonmetals |
As, Se |
F |
Mining, pesticides, irrigation drainage |
|
Oxyanions |
Nitrate, (per)chlorate, phosphate |
F |
Fertilizers, paper manufacturing, disinfectants, aerospace |
|
Radionuclides |
Tritium (3H), 238,239,240Pu, 235,238U, 99Tc, 60Co, 137Cs, 90Sr |
I |
Nuclear reactors, weaponry, medicine, food irradiation facilities |
|
NOTE: BTEX = benzene, toluene, ethylbenzene, and xylene; MTBE = methyl tert-butyl ether; PCBs = polychlorinated biphenyls; TNT = trinitrotoluene; RDX = royal Dutch explosive (1,3,5-trinitrohexahydro-s-triazine). a F = very frequent; C = common; I = infrequent. |
|||
groundwater. The core of the plume normally has the highest concentrations of the dissolved contaminant, while the fringes have lower concentrations. Just as a visible plume from a smokestack eventually disappears, a groundwater plume also can become nondetectable due to various subsurface processes, explained later in this chapter.
REMOVAL OF CONTAMINANT SOURCES
At most contaminated sites, the bulk of the contaminant mass is in what remediation professionals call “source zones.” Examples of source zones include landfills, buried tanks that contain residual chemicals, deposits of tars, and mine tailings piles. These types of sources sometimes can be easily located (especially if they are visible like landfills and tailings piles), and complete or partial removal or containment may be possible. However, other common types of sources often are extremely difficult to locate and remove or contain. One example of a source in this category is chemicals that have sorbed to soil particles but have the potential to later dissolve into groundwater that contacts the soil. Another, extremely important example is the class of organic contaminants known as “nonaqueous-phase liquids” (NAPLs). There are two types of NAPLs: those that are more dense than water (dense nonaqueous-phase liquids, or DNAPLs), and those that are less dense than water (light nonaqueous-
phase liquids, or LNAPLs). When released to the ground, these types of fluids move through the subsurface in a pattern that varies significantly from that of the water flow, because NAPLs have different physical properties than water. As shown in Figure 3-3, LNAPLs can accumulate near the water table; DNAPLs can penetrate the water table and form pools along geologic layers; and both types of NAPLs can become entrapped in soil pores. These NAPL accumulations contaminate groundwater that flows by them as they dissolve slowly at concentrations sufficient to pose a public health risk. Common LNAPLs include fuels (gasoline, kerosene, and jet fuel), and common DNAPLs include industrial solvents (trichloroethene, tetrachloroethene, and carbon tetrachloride). Once they have migrated into the subsurface, NAPLs are often difficult or impossible to locate in their entirety.
Normally, the total mass of a contaminant within source zones is very large compared to the mass dissolved in the plume. Therefore, the source usually persists for a very long time. For example, the rate at which contaminants dissolve from a typical NAPL pool is so slow that many decades to centuries often are needed to dissolve the NAPL completely (NRC, 1994).
Given the persistent nature of contaminant sources, removing them would seem like a practical way to speed natural attenuation of the contaminant plume. In many cases, environmental regulators require source removal or containment as part of a natural attenuation remedy. Although requiring source control or removal is good policy for many sites, expert opinions conflict on whether source removal is advisable when using natural attenuation as a remedy, even when such removal is technically feasible.
Goals of source removal would be the following:
-
remove as much contaminant mass as practical, in the hope of reducing the longevity and perhaps concentration of the contaminant plume; and
-
avoid any changes that would reduce the effectiveness of natural attenuation.
In theory, if one can delineate the source essentially completely and succeed in removing most of the mass, then a significant benefit may be achieved. Later, this chapter presents a case study of a polycyclic aromatic hydrocarbon (PAH) plume in which it appears that, after removal of the source, the plume itself attenuated rapidly. However encouraging this example might be, this kind of success may not always be realized. More commonly, the source cannot be delineated completely and/or cannot be removed to any significant degree even if located perfectly. Hence, source
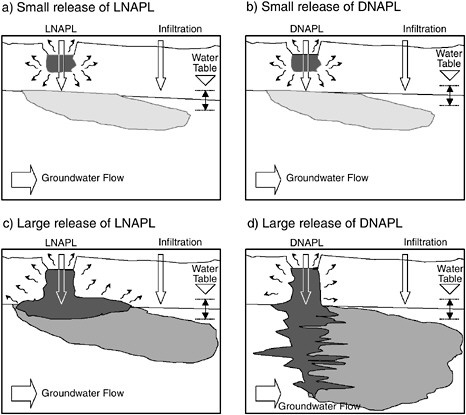
FIGURE 3-3 Examples of sources of groundwater contamination. In (a), the amount of LNAPL released is not large enough to reach the water table in pure form, but components of the LNAPL dissolve in infiltrating water and create the plume of contamination shown beneath the water table. The situation portrayed in (b) is analogous to the scenario in (a), except the contaminant is a DNAPL. As shown in (c), when an LNAPL release is large enough, free-phase LNAPL will pool near the water table. In contrast, as shown in (d), a DNAPL will migrate beneath the water table, because the DNAPL is more dense than water. In all cases, the undissolved LNAPLs and DNAPLs serve as a long-term source feeding the development of contaminant plumes in groundwater.
removal options may be rejected because none are anticipated to remove enough of the source mass to warrant the expense and risks of the removal effort.
In some cases, source removal efforts may directly and adversely affect natural attenuation. Technical guidelines on natural attenuation developed for the Navy present a summary of interactions between
various active remediation technologies, some of which are used in source removal efforts, and natural attenuation. Table 3-2 is adapted and condensed, with some revisions, from the Navy’s summary. As evident in Table 3-2, active technologies that introduce oxygen to the subsurface could have negative effects on the biodegradation of petroleum hydrocarbon or chlorinated solvent plumes. Source control methods that could introduce oxygen include excavation, pumping and treating of groundwater, free-product recovery, in-well stripping, soil vapor extraction, air sparging, bioslurping, cosolvent or surfactant flushing, and thermal treatment. (See NRC, 1997, 1999, for descriptions of these engineered remediation methods.)
An additional potential problem is that removal of the source of one type of contaminant may adversely affect natural attenuation of another type and thus result in minimal or no overall benefit. A good example is the removal of a petroleum hydrocarbon source zone that was serving as a nutrition source for microbes involved in degrading a chlorinated solvent plume. (Details of this type of process are discussed later in this chapter.) Such an action could slow down or completely shut off natural attenuation of the chlorinated solvent.
When natural attenuation is the primary remediation mechanism, source removal has to be undertaken with caution. When negative effects on natural attenuation are not anticipated, and where it is feasible and reasonably efficient, source removal is advisable. However, other than for fuel hydrocarbon NAPLs, removing sufficient contaminant mass to justify the effort can be extremely difficult. Furthermore, source removal efforts may interfere with the present or future efficiency of natural attenuation. For these reasons, source removal may often be unjustified or even undesirable. In such cases, natural attenuation, if effective can serve as a long-term source management method, but the attenuation reactions will have to be sustainable for a long period of time.
Hydrogeologic Settings
Water and contaminants do not flow freely in the subsurface as they would in a river, but instead must travel through the circuitous pore spaces of subsurface materials. In the upper portion of the subsurface, which is known as the “vadose” or “unsaturated” zone, the pore spaces are only partly filled with water (see Figure 3-4).1 Below the vadose zone
TABLE 3-2 Potential Effects of Other Remediation Activities on Natural Attenuation
|
Other Remediation Activities |
Natural Attenuation of Petroleum Hydrocarbons |
Natural Attenuation of Chlorinated Solvents |
||
|
Possible Benefits |
Possible Detriments |
Possible Benefits |
Possible Detriments |
|
|
Excavation and backfilling |
Remove mass; enhance oxygen input |
Alter flow field; enhance spreading |
Mass removal |
Interfere with anaerobic degradation; alter flow field; enhance DNAPL spreading |
|
Capping |
Reduce contaminant flux to groundwater |
Enhance spreading of vapors; reduce oxygen input |
Enhance anaerobic degradation |
Enhance spreading of vapors; reduce fermentative creation of substrates; reduce oxygen input for vinyl chloride biodegradation |
|
Pump and treat (for plume capture) |
Contain plume |
Reduce time available for attenuation reactions |
Contain plume |
Reduce time for natural attenuation; introduce oxygen into plume and source area |
|
Pump and treat (for mass removal) |
Control source; enhance electron acceptor delivery |
Reduce time available for attenuation reactions |
Control source; reduce time for attenuation reactions |
Introduce oxygen; interfere with anaerobic degradation |
|
Free-product recovery |
Decrease source mass |
None |
Reduce source |
Remove electron donor for reductive dehalogenation; introduce oxygen |
|
Other Remediation Activities |
Natural Attenuation of Petroleum Hydrocarbons |
Natural Attenuation of Chlorinated Solvents |
||
|
Possible Benefits |
Possible Detriments |
Possible Benefits |
Possible Detriments |
|
|
In-well stripping and recirculation |
Remove mass; enhance aerobic degradation |
Interfere with anaerobic degradation |
Remove mass; enhance aerobic degradation |
Interfere with anaerobic degradation |
|
Soil vapor extraction |
Remove mass; enhance aerobic degradation |
Interfere with anaerobic degradation |
Remove mass; enhance aerobic degradation |
Interfere with anaerobic degradation; remobilize DNAPL |
|
Air sparging |
Remove mass; enhance aerobic degradation |
Interfere with anaerobic degradation |
Remove mass; enhance aerobic degradation |
Stop anaerobic degradation; remobilize DNAPL |
|
Bioslurping |
Control source; enhance aerobic degradation |
None |
Enhance aerobic degradation |
Interfere with anaerobic degradation |
|
Passive O2 addition |
Enhance aerobic degradation |
Not applicable |
Enhance aerobic degradation |
Interfere with aerobic degradation |
|
Carbon sources addition |
Not applicable |
Not applicable |
Stimulate aerobic cometabolism or anaerobic dechlorination |
Result in incomplete utilization of carbon source; form byproducts |
|
Cosolvent or surfacant flooding |
Remove mass |
Cause spreading of contaminant; result in incomplete removal of cosolvent or surfactant |
Remove mass |
Spread contaminant; result in incomplete removal of cosolvent or surfacant; result in removal of electron donors |
|
Other Remediation Activities |
Natural Attenuation of Petroleum Hydrocarbons |
Natural Attenuation of Chlorinated Solvents |
||
|
Possible Benefits |
Possible Detriments |
Possible Benefits |
Possible Detriments |
|
|
Thermal treatment |
Remove mass |
Sterilize the site for indeterminate time; spread contamination |
Remove mass |
Sterilize the site for indeterminate time; spread contamination |
|
Chemical oxidation |
Remove mass |
Produce explosive vapors; sterilize the site |
Destroy DNAPL mass |
Produce toxic byproducts and explosive vapors; sterilize the site |
|
Phyto-remediation |
Remove mass |
Transfer contaminant across media |
Remove mass |
Transfer contaminant across media |
|
Zero-valent metal walls |
Not applicable |
Not applicable |
Reduce contaminant mass flux |
Add dissolved iron |
|
SOURCE: Adapted and modified from Department of the Navy, 1998. |
||||
is the “phreatic” or “saturated” zone, where the pores are entirely filled with water. The “capillary fringe” consists of the area between these two zones; here, the pores are nearly filled with water. The water table, indicated by the triangle on Figure 3-4, is at the bottom of the capillary fringe, at the start of the zone in which all the pores are filled with water.
Once rain or water from other sources infiltrates below the surface layer of soil, the water and any contaminants that dissolve in it have several possible fates. The water and contaminants may be (1) retained by mineral or organic matter in soil or the underlying vadose zone, (2) intercepted by plant roots, or (3) transmitted to the saturated zone (Domenico and Schwartz, 1990). Water that reaches the saturated zone can move toward surface water bodies (streams, rivers, wetlands, or lakes) or wells, or it can enter closed deep continental groundwater basins. The time before water exits a particular subsurface region (known as the “residence time”) ranges from a few days or weeks when recharge and discharge locations are very close to each other to thousands of years for
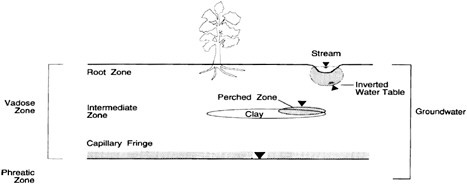
FIGURE 3-4 Conceptual model of the vadose zone. SOURCE: Stephens, 1995. Reprinted, with permission, from Lewis Publishers (1995). © 1995 by Lewis Publishers.
return from a deep continental basin (Freeze and Cherry, 1979; Madsen and Ghiorse, 1993).
The large surface area available on subsurface organic materials strongly influences the physical and chemical conditions of the groundwater (Madsen and Ghiorse, 1993; van Loosdrecht et al., 1990). Rainwater begins as a distillate containing only atmospheric gaseous and particulate materials (for example, iron oxides and salts of nitrate and sulfate) of varying solubility. After coming in contact with soil and deeper subsurface sediments, the water’s chemical composition changes substantially. Components of surface and subsurface solids dissolve, and chemical reactions occur. Some of the reactions are strictly geochemical, but many are brought about by microorganisms (Chapelle, 1993; Domenico and Schwartz, 1990; Stumm and Morgan, 1996).
The chemical composition of a given groundwater sample reflects the history of chemical and microbiological reactions that occurred along the water’s flow path through soil, the vadose zone, and underlying geologic materials. Because of the diversity of flow paths and biogeochemical reactions, groundwater composition varies considerably from one location to another. Nonetheless, some generalizations can be made. In aquifers that are not influenced significantly by human activity, major chemical constituents (those with concentrations higher than 5 mg/liter) typically include calcium, magnesium, silica, sodium, bicarbonate, chloride, and sulfate. Minor constituents (with concentrations between 0.01
and 5 mg/liter) include iron, potassium, boron, fluoride, nitrate, and natural organic humic material (for example, from decayed plants) (Domenico and Schwartz, 1990). Human activities can substantially alter the chemical composition of a groundwater by adding high concentrations of the kinds of contaminants listed in Table 3-1.
Contaminants that enter the groundwater near the surface initially are part of what is called a “local” hydrologic flow system, which responds rapidly to changes in hydrologic conditions, such as rain, pumping, or recharge. Local flow systems are supplied with a constant input of fresh water capable of flushing the aquifer, but, as a result, conditions are not necessarily steady over time. In some cases, plumes of groundwater contamination change direction seasonally. Also, the center of a plume can migrate downward as clean recharge water enters an aquifer above it.
In addition to moving downward due to natural recharge of the groundwater, contaminants can enter deeper systems directly via injection or migration down an open or unfinished well borehole. They also can be drawn down when water is extracted from wells in lower zones. Deep flow systems generally have long residence times and relatively stable flow velocities and geochemical environments (although shallow and heavily pumped portions of deep flow systems may have shorter residence times and oscillating water velocities).
MOVEMENT OF CONTAMINANTS IN THE SUBSURFACE
Whether or not chemical or microbial reactions transform a contaminant, the contaminant always is subject to transport processes—meaning that physical processes cause it to move. All important transport processes for subsurface contaminants can be categorized as advection, dispersion, or “phase transfer” (meaning transfer from one type of physical medium to another, such as from a NAPL to water or from water to air in the soil pores).
Advection
Transport of a solute (a chemical species dissolved in water) occurs when the groundwater moves. This process is called advection or, alternatively, convection or bulk flow. Advection occurs in any moving fluid. Thus, contaminants can advect when they are in air in soil pores or in a moving NAPL, as well as in water.
Advective transport is illustrated simply by considering a solute that does not react chemically or biologically in the subsurface and that moves at the average velocity of the groundwater. Such a chemical is called a “conservative solute” or “tracer.” The vertical line labeled “ideal plug
flow” in Figure 3-5 illustrates this situation. The contaminant moves at exactly the same velocity as the water and does not change from its initial concentration, C0, at the injection point.
The rate at which a dissolved contaminant moves across a vertical plane in the subsurface is the product of the contaminant concentration and the speed of the water. For water, the velocity in the saturated zone is governed by three key factors, each characteristic of specific groundwater flow systems. The factors are hydraulic gradient, conductivity, and porosity:
-
The hydraulic gradient includes gravity and pressure components and is the driving force for water movement. Water always moves in the direction of higher hydraulic head (which can be thought of qualitatively as elevation) to lower head.
-
Hydraulic conductivity is the ability of porous rocks or sediments to transmit fluids and is measured from field tests or samples. Hydraulic conductivity values for common rocks and sediments vary over ten orders of magnitude from almost impermeable crystalline rocks to highly permeable gravels; the hydraulic conductivity values for fractured rocks,
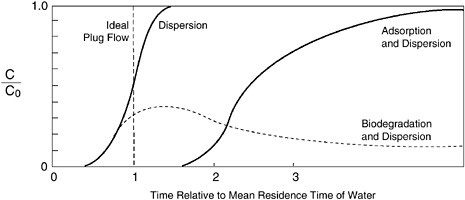
FIGURE 3-5 Effects of dispersion, adsorption, and biodegradation on the concentration of a chemical injected in the subsurface. The vertical “ideal plug flow” line shows that if transport of the chemical were controlled only by the movement of the bulk of the groundwater, the chemical would travel undiluted to the observation well. Dispersion causes the chemical to spread. Adsorption and biodegradation cause the concentration to decrease. SOURCE: Roberts et al., 1980. Reprinted, with permission from Water Environment Federation (1980). © 1980 by Water Environment Federation.
-
sand, and clay are between these extremes. A contaminant plume that is moving with the groundwater will travel faster through sand layers, which have high hydraulic conductivity, than through clays of low hydraulic conductivity, under the same hydraulic head gradient.
-
Porosity is a measure of the volume of open spaces in the subsurfaces relative to the total volume. Like hydraulic conductivity, it depends on the type of geologic material present, and it can be determined from field tests or samples.
The equation for describing the rate of groundwater flow from one location to another is known as Darcy’s equation:
(3-1)
in which KH is the hydraulic conductivity (in units of distance per time) and ∆h/∆X is the change in hydraulic head per unit of distance. To determine the velocity V of a contaminant that travels at the same speed as the groundwater, the Darcy velocity must be divided by the effective porosity ε:
(3-2)
KH and ε can be estimated using various field test methods or laboratory evaluations of cores taken from the subsurface. Uncertainty is inherent in all such measurements, and this uncertainty must be acknowledged by developing a range of possible flow scenarios.
Dispersion
Mixing of substances dissolved in groundwater occurs as the water moves, altering concentrations from those that would occur if advection were the only transport mechanism. This mixing is called dispersion. The mechanisms leading to dispersion in the subsurface include molecular diffusion, different water velocities within individual pores, different water velocities between adjacent pores, and tortuosity of the subsurface flow path. Groundwater scientists quantify the combined mixing effect using a hydrodynamic dispersion coefficient DH. Except at very low water velocities, DH increases linearly with the average speed of groundwater.
The curve labeled “dispersion” in Figure 3-5 illustrates the effects of dispersion for a conservative solute chemical (a dissolved chemical that
does not change due to physical or biological reactions, but instead travels precisely with the water molecules). The solute is detected at the observation well before it would be if advection were the only process affecting its movement. Dispersion causes the solute to spread, rather than moving as an unchanged “plug” (which would occur under the ideal plug flow scenario shown in Figure 3-5).
Phase Transfers
Contaminants can be added to or removed from the groundwater when they transfer between phases. The relevant phases in the subsurface are groundwater, solids, NAPLs, and soil gas (air) in the vadose zone. Phase transfers can increase or decrease the contaminant concentration in groundwater, depending on the mechanism, the contaminant, and the groundwater’s chemical composition. Although the basic concepts of phase transfer are straightforward, quantification of these transfers often is not easy to model and is an ongoing area of research.
The transfer of an organic compound from a NAPL source to the surrounding water increases the contaminant concentration in groundwater. The rate of transfer varies depending on the type of NAPL. Computation of this transfer rate can be complex. The transfer rate depends on chemical properties of the contaminant and the NAPL and on resistance at the interface between the water and the NAPL (Pankow and Cherry, 1996; Peters and Luthy, 1993; Rittmann, 1994). Diffusion of the contaminant within the NAPL itself also can affect the transfer rate for viscous NAPLs (Ortiz et al., 1999).
Sorption slows the movement of contaminants, because the solids temporarily hold back some of the contaminant mass. As Figure 3-5 shows on the curve labeled “adsorption and dispersion,” sorption causes the solute plume to move at a velocity that is lower than that of the water. Because the solids do not move, the sorbed contaminant remains in the subsurface and can be desorbed later and contaminate the water. Equations are available to estimate the effects of sorption on contaminant movement based on measurable properties of the contaminant and the soil, but these equations are very complex for contaminants such as metals and radionuclides for which sorption results from mechanisms other than hydrophobicity (Rittmann et al., 1994).
Volatilization reduces the total mass of the contaminant groundwater system. The potential for volatilization is expressed by the contaminant’s Henry’s law constant (Rittmann et al., 1994). Henry’s law constants are widely available for common volatile contaminants. Because the soil gas often advects and dispersion also occurs in the gas phase, contaminants transferred to the soil gas often migrate away from the location at which
they volatilized. Volatilization itself does not destroy contaminant mass or permanently immobilize it. Volatilized contaminants can biodegrade in some circumstances but also can redissolve in infiltrating groundwater or be transported to the surface, where humans may be exposed to the vapors.
TRANSFORMATION OF CONTAMINANTS IN THE SUBSURFACE
A variety of reactions transform contaminants. The possible reactions are called biogeochemical: all are chemical (prefix chem) and occur in a geological setting (prefix geo), but some are catalyzed by microorganisms (prefix bio). Some biogeochemical reactions can transform a contaminant into a benign form or immobilize it permanently. A contaminant transformed or immobilized in these ways no longer contributes to groundwater pollution. Although other reactions do not directly lead to such positive results, they can control whether or not the transformation or immobilization reactions take place. Often, a suite of chemical reactions (termed a reaction network) leads to contaminant transformation or immobilization. In other instances, the reaction network prevents the contaminants from being transformed or immobilized and may make natural attenuation an ineffective remediation strategy.
TRANSFORMATION BY MICROORGANISMS
Microorganisms can cause major changes in the chemistry of groundwater. Their small size and adaptability, as well as the diversity of nutritional requirements for different microbes, enable them to catalyze a wide range of reactions that often are the basis for natural attenuation (Atlas and Bartha, 1997; Madigan et al., 1997; Schlegel and Jannasch, 1992; Schlesinger, 1991; Tiedje, 1995; Waksman, 1927). Chemical changes brought about by microorganisms can directly or indirectly decrease the concentrations of certain groundwater contaminants.
Microorganisms use enzymes to accelerate the rates of certain chemical reactions. The most important reactions are “reductions” and “oxidations,” together known as “redox” reactions. Box 3-1 explains how these reactions occur. The reactions involve transfer of electrons from one molecule to another. These transfers allow the microorganisms to generate energy and grow.
Microorganisms reproduce by organizing chemical reactions that create daughter cells composed of cellular components (e.g., membranes, proteins, deoxyribonucleic acid [DNA], cell walls) derived from building blocks that they either synthesize or scavenge from the environment. The chemical reactions are made possible by enzymes—protein molecules that
|
BOX 3-1 Redox reactions involve the transfer of electrons from a donor molecule to an acceptor molecule. The electron donor (D) loses n electrons (e−) and is oxidized: D = Dn+ + ne− (1) The electron acceptor (A) gains the n electrons and is reduced: A + ne− = An− (2) The term redox is short-hand for reduction and oxidation. It underscores that reduction of an acceptor and oxidation of a donor always occur together so that all electrons leaving the donor are taken up by the acceptor: D + A = Dn+ + An− (3) When an electron donor is organic and all of the electrons in the outer shells of the carbon atoms are removed, it is mineralized to CO2 and H2O. Redox reactions are very important in groundwater settings. All microbial life is driven by redox reactions, which provide the energy for cells to grow. Microbial redox reactions transform organic molecules to benign products and alter the chemical status of many metals, sometimes leading to their immobilization. |
bring together the chemicals in a way that allows them to react quickly. The reactions are driven to completion by the expenditure of cellular energy in the form of a chemical known as adenosine triphosphate (ATP), which can be thought of as a cellular fuel. Like all living organisms, microorganisms generate ATP by catalyzing redox reactions: they transfer electrons from electron-rich chemicals to electron-poor chemicals. The technical term for the electron-rich chemical is “electron-donor substrate.” The electron-poor chemical is the “electron-acceptor substrate.” As an analogy, human metabolism involves transfer of electrons from chemicals derived from ingested food (the donor substrate) to oxygen (the acceptor substrate) inhaled from the air.
When cells remove electrons from the donor substrate, they do not transfer the electrons directly to the acceptor substrate. Instead, they transfer the electrons to internal electron carriers as shown in Figure 3-6. Although electrons held by the carriers can be used for many purposes, the major purpose is to generate ATP through a process called respiration. In respiration, the electrons are passed from carrier to carrier until
they reach the electron-acceptor substrate. Since this is the last molecule to receive the electrons, it is called the “terminal electron acceptor.” The need for ATP production forces all microorganisms to have one or more electron-donor and electron-acceptor pairs, and these materials largely define the metabolism of individual microorganisms. The amount of energy yielded varies depending on the electron donor and electron acceptor used.
Collectively, microorganisms can use a wide range of electron donors, including both organic and inorganic chemicals. Electron acceptors are more limited. Common electron acceptors include O2, NO3−, NO2−, SO42−, CO2, Fe(III), and Mn(IV). Oxygen has a special status because of its importance in many environments and reactions. Microbial use of oxygen as an electron acceptor is called “aerobic metabolism.” Microbial use of electron-accepting chemicals other than oxygen is called “anaerobic metabolism.”
When biotransformation of a particular contaminant leads directly to energy generation and the growth of more microorganisms, the contaminant is known as a “primary substrate.” However, the reactions that lead to microbial metabolism of contaminants may not be part of cell-building or energy-generating reactions. An important category of such biotransformations is “cometabolism.” Cometabolism is the fortuitous degradation of a contaminant when other materials are available to serve as the microorganisms’ primary substrates. Cometabolic reactions often occur because the enzymes designed for metabolizing primary substrates incidentally transform the cometabolic substrate.
Microbial Transformation of Organic Contaminants
Organic contaminants vary widely in their susceptibility to transformation by microorganisms. Some contaminants are highly biodegradable, while others resist degradation. In general, the more degradable contaminants have simple molecular structures (often similar to the structures of naturally occurring organic chemicals), are water soluble and nontoxic, and can be transformed by aerobic metabolism. In contrast, organic contaminants that resist biodegradation may have complex molecular structures (especially structures not commonly found in nature), low water solubility, or an inability to support microbial growth, or they may be toxic to the organisms.
Microorganisms can completely convert some organic contaminants to carbon dioxide, while they are capable of only partial conversions of others. Complete conversion to carbon dioxide is called “mineralization.” In some cases, the products of partial conversion are more toxic than the original contaminant. Vinyl chloride is an example of a highly
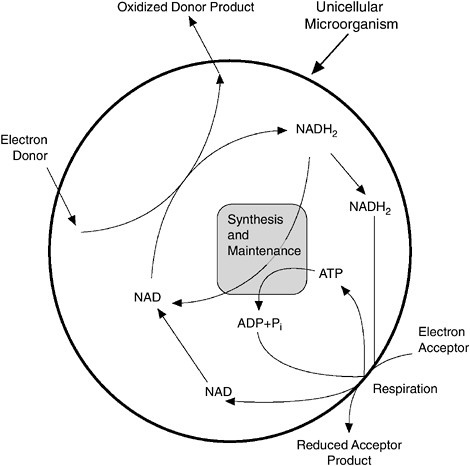
FIGURE 3-6 Microorganisms generate energy to grow and sustain themselves by transferring electrons from an electron-rich donor chemical (analogous to human food) to an electron-poor acceptor chemical (analogous to human use of inhaled oxygen). Electron flow is shown here schematically using arrows. The circle represents the cell wall of a microorganism. Electron flow begins with the electron donor, on the upper left. Microorganisms capture the electrons in an electron carrier, shown here as reduced nicotinamide adenine dinucleotide (NADH2). The energy generated by redox reactions during respiration is captured in high-energy phosphate bonds of adenosine triphosphate (ATP), shown here as being generated from adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP and NADH2 can be used for many purposes, including cell synthesis and maintenance.
toxic chemical that results from incomplete biodegradation of chlorinated solvents.
Table 3-3 indicates the susceptibility of the contaminant classes shown in Table 3-1 to microbial transformation. Table 3-3 shows biodegradation potential in environments with oxygen (aerobic environments) and without oxygen (anaerobic environments). For the organic contaminants, it also indicates whether the contaminants are likely to be completely transformed (mineralized) or only partially degraded.
The discussion below explains how microbial transformations occur for the organic contaminant classes shown in Table 3-3. It describes all of the elements of some metabolic pathways because these illustrate the core concepts of biodegradation. Biodegradation pathways for most contaminants are extremely complex, so pathways for most contaminants are not described in detail. (See Rittmann and McCarty, 2000, for more information about biodegradation pathways.)
Petroleum Hydrocarbons
Petroleum hydrocarbons are a highly varied class of naturally occurring chemicals used as fuels in a variety of commercial and industrial processes. Biodegradation potential varies depending on the type of hydrocarbon.
Benzene, Toluene, Ethylbenzene, and Xylene Benzene, toluene, ethylbenzene, and xylene (BTEX) are components of gasoline. Because of their widespread use and because BTEX storage tanks commonly leaked in the past, BTEX are common groundwater contaminants. A large body of scientific research exists on the biodegradation of BTEX.
BTEX are easily biodegraded to carbon dioxide by aerobic microorganisms. BTEX can biodegrade anaerobically (Beller et al., 1992a, b; Evans et al., 1991a,b; Lovley and Lonergan, 1990; Vogel and Grbic-Galic, 1986). When the volume of BTEX is small enough and/or the supply of oxygen is large enough, microbes can degrade all of the BTEX components within the aerobic zones of a contaminated site (Baedecker et al., 1993; Barker et al., 1987; Lovley, 1997; Morgan et al., 1993; Rice et al., 1995; Salanitro, 1993). When oxygen is depleted in an advancing contaminant plume, anaerobic conditions can develop and lead to the formation of as many as five different downgradient zones, each with a different terminal electron acceptor (Anderson and Lovley, 1997). In these zones, BTEX degradation processes are slower and less reliable than when oxygen is present.
Of the possible electron acceptors, oxygen yields the most energy. Once oxygen is depleted, nitrate is the next most energy-yielding terminal
TABLE 3-3 Overview of Biodegradation Potential for Categories of Environmental Contaminants
|
|
|
|
Susceptibility to Microbiological Transformationa |
|
|
Chemical Class |
Mechanisms of Microbe-Contaminant Interactions |
Type(s) of Contaminant Alteration |
Aerobic |
Anaerobic |
|
Organic |
|
|||
|
Petroleum hydrocarbons |
|
|||
|
BTEX |
Carbon and electron-donor source |
Mineralized to CO2 |
1 |
2 |
|
Low-molecular-weight gasoline, fuel oil |
Carbon and electron-donor source |
Mineralized to CO2 |
1 |
2 |
|
High-molecular-weight oils, PAHs |
Carbon and electron-donor source |
Mineralized to CO2 or partially degraded |
1, 2 |
2, 4 |
|
Creosote |
Carbon and electron-donor source |
Mineralized to CO2 or partially degraded |
1, 2 |
2, 4 |
|
Oxygenated hydrocarbons |
|
|||
|
Low-molecular-weight alcohols, ketones, esters, ethers |
Carbon and electron-donor source |
Mineralized to CO2 |
1, 2 |
2 |
|
MTBE |
Cometabolized; not fully used as carbon and electron-donor source |
Partially degraded |
2-5 |
4, 5 |
|
Halogenated aliphatics |
|
|||
|
Highly chlorinated |
Electron acceptor under anaerobic conditions; cometabolized |
Partially degraded |
2-5 |
2-5 |
|
Less chlorinated |
Electron acceptor under anaerobic conditions; carbon and electron-donor source; cometabolized |
Partially degraded |
2-5 |
2-5 |
|
|
|
|
Susceptibility to Microbiological Transformationa |
|
|
Chemical Classa |
Mechanisms of Microbe-Contaminant Interactions |
Type(s) of Contaminant Alteration |
Aerobic |
Anaerobic |
|
Halogenated aromatics |
|
|||
|
Highly chlorinated |
Electron acceptor under anaerobic conditions; carbon and electron-donor source; cometabolized |
Partially degraded |
2-5 |
2, 3 |
|
Less chlorinated |
Electron acceptor under anaerobic conditions; carbon and electron-donor source |
Partially degraded |
1, 2 |
2 |
|
PCBs |
|
|||
|
Highly chlorinated |
Electron acceptor under anaerobic conditions |
Partially degraded |
4 |
2, 3 |
|
Less chlorinated |
Electron acceptor under anaerobic conditions; carbon and electron-donor source |
Partially degraded or fully mineralized to CO2 |
1, 2 |
2, 4 |
|
Dioxins |
Electron acceptor under anaerobic conditions |
Partially degraded |
4 |
4 |
|
Nitroaromatics (TNT, RDX) |
Carbon and electron-donor source; cometabolized |
Partially degraded; immobilized by precipitation or polymerization |
2 |
2 |
|
Inorganic |
|
|||
|
Metals |
|
|||
|
Cu, Ni, Zn |
Sorbs to extracellular polymers and biomass |
Immobilized by sorption |
2 |
2 |
|
Cd, Pb |
Sorbs to extracellular polymers and biomass |
Immobilized by sorption; methylation possible |
2 |
2 |
|
Fe, Mn |
Electron acceptor under anaerobic conditions; oxidized to form insoluble hydroxides; sorbs to extracellular polymers and biomass |
Mobility (solubilization) increased by reduction; immobilized by precipitation and sorption |
1 |
1 |
|
Cr |
Enzymatically oxidized or reduced to promote detoxification; cometabolized; sorbs to extracellular polymers and biomass |
Immobilized by precipitation |
2 |
2 |
|
Hg |
Enzymatically oxidized or reduced to promote detoxification; sorbs to extracellular polymers and biomass |
Volatilized or immobilized by sorption and precipitation |
2 |
2 |
|
Nonmetals |
|
|||
|
As |
Enzymatically oxidized or reduced; electron acceptor under anaerobic conditions; oxidation of reduced forms linked to microbial growth; sorbs to extracellular polymers and biomass |
Volatilized or immobilized by precipitation and sorption |
2 |
2 |
|
Se |
Enzymatically oxidized or reduced; electron acceptor under anaerobic conditions; cometabolized; sorbs to extracellular polymers and biomass |
Volatilized or immobilized by precipitation of elemental Se or sorption |
1 |
2 |
|
Oxyanions |
|
|||
|
Nitrate |
Electron acceptor under anaerobic conditions |
Converted to nontoxic nitrogen |
4 |
1 |
|
Perchlorate |
Electron acceptor under anaerobic conditions |
Reduced to nontoxic chloride ion |
4 |
2, 5 |
|
Radionuclides |
|
|||
|
U |
Electron acceptor under anaerobic conditions; sorbs to extracellular polymers and biomass |
Immobilized by precipitation |
4 |
2 |
|
Pu |
Cometabolized; sorbs to extracellular polymers and biomass |
Mobility increased by reduction to soluble Pu(III); immobilized by precipitation and sorption |
4 |
2 |
|
|
|
|
Susceptibility to Microbiological Transformationa |
|
|
Chemical Classa |
Mechanisms of Microbe-Contaminant Interactions |
Type(s) of Contaminant Alteration |
Aerobic |
Anaerobic |
|
Tc |
Enzymatically oxidized or reduced; cometabolized; sorbs to extracellular polymers and biomass |
Immobilized by precipitation |
4 |
2 |
|
a The numeric entries for each compound class provide a rating of susceptibility to microbial transformation under aerobic conditions (in the presence of oxygen) and anaerobic conditions (when oxygen is absent): 1 = readily mineralized or transformed; 2 = degraded or transformed under a narrow range of conditions; 3 = metabolized partially when second substrate is present (cometabolized); 4 = resistant; 5 = insufficient information. NOTE: BTEX = benzene, toluene, ethylbenzene, and xylene; MTBE = methyl tert-butyl ether; PCB = polychlorinated biphenyl; RDX = royal Dutch explosive; TNT = trinitrotoluene. |
||||
electron acceptor. If nitrate is abundant in groundwater, zones in which microbes use nitrates as the electron acceptor will develop. A Mn(IV)-reducing zone may develop next if Mn(IV) is present in the subsurface mineral matrix (although the coupling of Mn reduction to BTEX degradation has not been well studied). Upon depletion of the Mn(IV), Fe(III) reduction will prevail if iron oxide minerals are present. In the next zones, sulfate and CO2 will serve as electron acceptors. Table 3-4 summarizes the reliability of different electron acceptors for biodegradation of BTEX compounds.
Many field studies of BTEX biodegradation in the subsurface have been carried out. For example, several lines of evidence indicated that all BTEX components were biodegrading mainly in the Fe(III)-reducing zone of an aquifer in Bemidji, Minnesota, that was contaminated with crude oil (Baedecker et al., 1989; 1993; Lovley et al., 1989). At a petroleum spill site in South Carolina, toluene, but not benzene, was metabolized as it moved through a sulfate-reducing zone (Chapelle et al., 1996). In a recent study of an anaerobic gasoline-contaminated aquifer in Seal Beach, California (Reinhard et al., 1997), researchers injected BTEX components (along with bromide as a tracer) and either sulfate or nitrate into a sandy aquifer. Periodic withdrawal of samples from the injected zones showed that under nitrate-reducing conditions, toluene, ethylbenzene, and m-xylene, (but not benzene) were transformed in less than 10 days. Under sulfate-reducing conditions, toluene, m-xylene, and o-xylene were completely
TABLE 3-4 Reliability of BTEX Biodegradation When Various Terminal Electron Acceptors are Present
transformed in 72 days, while benzene loss was uncertain (Reinhard et al., 1997).
Polycyclic Aromatic Hydrocarbons In contrast to BTEX, PAHs biodegrade very slowly. PAH contamination comes mostly from fossil fuel use and the manufactured-gas industry. Combustible gas manufactured from coke, coal, and oil at some 1,000 to 2,000 U.S. plants served as the major gaseous fuel for urban lighting, cooking, and heating in the United States for nearly 100 years (Harkins et al., 1988; Rhodes, 1966). Groundwater contamination at manufactured gas plants has persisted for decades because of the slow, continuous dissolution of PAHs from subsurface coal tar. These compounds have complex molecular structures and low water solubility, and they tend to sorb strongly to solids in the subsurface. However, because PAHs dissolve slowly, natural attenuation could control the contamination even if biodegradation is slow, as long as it occurs at the same rate as or faster than dissolution.
The fate of PAHs in subsurface systems is governed largely by their hydrophobic nature (the reason for their low solubility and tendency to attach to surfaces). PAH molecules held within NAPLs or adsorbed to surfaces cannot be biodegraded. Consequently, understanding dissolution (Ghoshal et al., 1996) and the sorption processes (Luthy et al., 1994) for PAHs often is the key to understanding biodegradation and natural attenuation potential.
Studies have shown that some microorganisms can metabolize dissolved PAHs composed of up to five benzene rings. Microorganisms generally use oxygenase enzymes to initiate the biodegradation, these reactions require the presence of oxygen. However, microbial degradation of PAHs with lower molecular weights (fewer benzene rings) can occur under nitrate-reducing (McNally et al., 1998; Mihelcic and Luthy, 1988) and sulfate-reducing conditions (Coates et al., 1997; Zhang and Young, 1997).
Oxygenated Hydrocarbons
Although microbiologists have long known that low-molecular-weight alcohols, ketones, esters, and ethers biodegrade readily, one prominent oxygenated hydrocarbon that is notably resistant to biodegradation is methyl tert-butyl ether (MTBE). MTBE often is added to gasoline at up to 15 percent by volume. Recently, it has been found in groundwater near many leaking underground gasoline storage tanks. MTBE has a foul odor, and when it contaminates drinking water supplies it can render the water unusable.
MTBE is generally resistant to biodegradation because of its stable molecular structure and its reactivity with microbial membranes. However, when microorganisms possess one of several possible oxygenase enzymes, these enzymes can fortuitously insert oxygen into the MTBE molecule (Steffan et al., 1997). Oxygen insertion may render MTBE susceptible to further breakdown by enzymes. Researchers have observed slow MTBE biodegradation in one field study (Borden et al., 1997) and in aerobic (Salanitro et al., 1994; Mo et al., 1997) and anaerobic (Mormile et al., 1994) laboratory studies. Recently, Hanson et al. (1999) described a bacterium able to mineralize and grow slowly on MTBE. Nonetheless, other field observations (e.g., Landmeyer et al., 1998, and the MTBE case study described later in this chapter) support the belief that MTBE may be only partially metabolized to tert-butyl alcohol, which is a health hazard. Present knowledge of MTBE biodegradation from both laboratory and field observations is limited. Preliminary reports suggest that MTBE might be biotransformed slowly once it migrates past the BTEX plume. These early findings have not been published in peer-reviewed journals, and the natural attenuation potential is unclear at this time.
Halogenated Aliphatic Compounds
Halogenated aliphatics are effective solvents and degreasers that are widely used in many manufacturing and service industries. For example, trichloroethene (TCE) is used commonly to degrease metal parts, and tetrachloroethene (PCE) is a dry cleaning agent. The halogen atoms (chlorine, bromine, or fluorine) added to organic molecules to produce these chemicals significantly change many properties, including solubility, volatility, density, hydrophobicity, stability, and toxicity. These changes are valuable for commercial products, but also can make the compounds less biodegradable. Most halogenated chemicals are resistant to biodegradation.
The biodegradation potential of many halogenated aliphatics has been extensively researched (see, for example, Semprini, 1997a, b). Table 3-5 summarizes existing knowledge about the susceptibilities of chlorinated aliphatic hydrocarbons to various types of microbial biotransformation.
Researchers first demonstrated the potential for anaerobic biotransformation of halogenated aliphatic hydrocarbons in 1981 (Bouwer et al., 1981). Subsequent studies have shown that these compounds can biotransform under a variety of environmental conditions in the absence of oxygen (Elfantraussi et al., 1998; McCarty, 1993, 1999; McCarty and Semprini, 1994; Semprini, 1997a,b; Wackett et al., 1992). A primary mechanism by which this transformation can occur is “reductive dechlorination,” in which one Cl− ion is released as the molecule accepts two electrons
TABLE 3-5 Known Biotransformation Reactions for Major Chlorinated Aliphatic Hydrocarbons Found in Groundwater
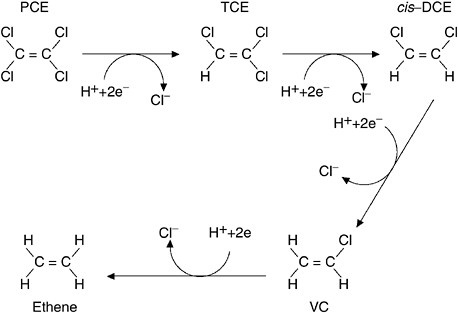
FIGURE 3-7 Reductive dechlorination of PCE. Microorganisms serve as catalysts for the reactions that progressively remove chlorine atoms from PCE, eventually converting it to ethene, which microbes can then convert to methane, carbon dioxide, and hydrogen chloride (which are all harmless). Curved arrows show that at each step of the process, the chlorinated compound receives a hydrogen atom (H+) and two electrons (2e−) as it gives up a chlorine atom. These reactions do not always proceed to completion, and cis-DCE and vinyl chloride (both of which are hazards) can accumulate.
from an electron carrier. As an example, PCE can be reductively dechlorinated to TCE, which in turn can be reduced anaerobically to cis-dichloroethene (DCE), which can be converted to vinyl chloride (VC) and ethene. Figure 3-7 shows this sequential transformation process.2
Biodegradable organic materials must be present as electron donors for reductive dechlorination of chlorinated aliphatic hydrocarbons to occur. In addition, the transformation requires consortia of many microorganisms, as shown in Figure 3-8. First, some of the organisms convert the organic electron donors to sugars, amino acids, and organic acids and
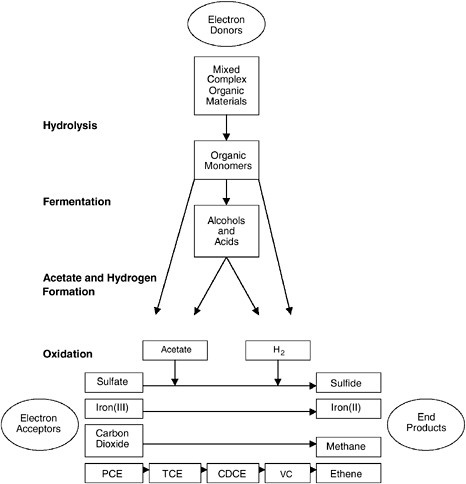
FIGURE 3-8 Steps in the process of biodegradation of PCE by reductive dechlorination. As shown, biodegradable organic matter is required as an electron donor to initiate the process. Different types of microbes are involved at each stage. The bottom step shows that PCE must compete for electrons with sulfate, iron, and carbon dioxide, meaning that a large amount of organic electron donors may be needed to supply enough electrons.
NOTE: CDCE = cis-dichloroethene. SOURCE: After McCarty, 1997.
then ferment these products to alcohols and fatty acids for energy. Second, other microbes oxidize the alcohols and organic acids, producing acetate and molecular hydrogen (H2). Third, another set of microbes oxidizes the acetate and hydrogen as electron donors, using either the contaminant or naturally available chemicals (such as sulfate, Fe(III), or carbon dioxide)
as an electron acceptor. As shown in Figure 3-8, degradation of chlorinated solvents occurs during this last step.
Reductive dechlorination of the contaminants competes with other electron acceptors for the electrons from hydrogen and acetate (Smatlak et al., 1996; Yang and McCarty, 1998). When reductive dechlorination is not highly successful in this competition, it gains only a small share of the available electrons. Then, the microorganisms oxidize a large amount of H2 or acetate to reduce only a small amount of the chlorinated contaminant. Theoretically, a minimum of 0.04 mole of H2 is required to reduce 0.01 mole of PCE (1.7 g) to ethene. This amount of hydrogen can be produced biologically under suitable anaerobic conditions from decomposition of 1.0 to 1.5 g of organic matter. However, because of competition, as little as 1 to 10 percent of the hydrogen intermediate produced may be used for dehalogenation. Thus, if 0.10 mg/liter were present, from 1.0 to 10.0 mg/liter of organic matter might be needed to achieve complete dehalogenation. Such a large amount of organic matter generally is not present in aquifers. An insufficient concentration of electron donors is a primary reason the dechlorination of chlorinated aliphatic hydrocarbons often is incomplete.
In limited cases, aerobic cometabolism of partially halogenated aliphatics is possible when microorganisms are supplied with electron donors such as methane, toluene, or phenol. Wilson and Wilson (1985) first showed that TCE is susceptible to aerobic degradation by feeding natural gas to the microbes in soil samples contaminated with TCE. The processes involved methanotrophs, or organisms that oxidize methane for energy and growth (see Figure 3-9). As shown in Figure 3-9, in the process of degrading methane, the microbes produce an enzyme (methane monooxygenase) that also degrades TCE.
Aerobic cometabolism of chlorinated aliphatic hydrocarbons is subject to many restrictions. First, the reaction requires molecular oxygen, but oxygen may be absent in highly contaminated groundwater (because it is used up quickly by biodegradation reactions). Second, cometabolism requires a primary substrate: methane, toluene, phenol, or some other oxygenase-inducing electron donor must be present. Third, the ratio of the concentration of this primary electron donor to that of the chlorinated aliphatic hydrocarbon must be relatively high to supply electrons for the dechlorination reaction and also to sustain the activity of the organisms (Anderson and McCarty, 1997; Semprini, 1997a,b). Because of these requirements, natural attenuation of halogenated aliphatics by aerobic cometabolism is limited. The process may be important around the fringes of a contaminant plume in aerobic aquifers, where oxygen can diffuse into the plume from the outside and where methane and ethene are present from anaerobic transformations inside the plume. Also, as ground-
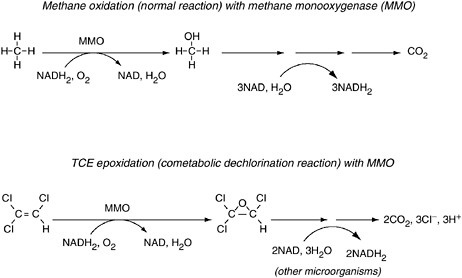
FIGURE 3-9 The top reaction shows how methanotrophs (“methane eaters”) produce the enzyme methane monooxygenase (MMO) in the process of converting methane (CH4) to CO2. The bottom reaction shows how MMO then causes the conversion of TCE to CO2 and HCl. NADH2 serves as the carrier of electrons released from methane and TCE (see Figure 3-6). NOTE: NAD = nicotinamide adenine dinucleotide; NADH2 = reduced nicotinamide adenine dinucleotide.
water emerges from the anaerobic environment of a plume into an aerobic stream or lake, oxygen may cause aerobic cometabolism of chlorinated aliphatics to occur.
One exception to the general rule that chlorinated aliphatic hydrocarbons require special environmental conditions for biodegradation to occur is methylene chloride, known as dichloromethane. Methylene chloride can support the growth of a wide range of microorganisms (both aerobic and anaerobic) under a range of environmental conditions (Freedman and Gossett, 1991; Kohler-Staub et al., 1995; Magli et al., 1998). Methylene chloride therefore is likely to be treated successfully by natural attenuation at a much broader range of sites than other chlorinated aliphatic compounds.
Halogenated Aromatic Compounds
Halogenated aromatic compounds consist of one or more rings of benzene to which halogen atoms (as well as other molecules) are attached.
These compounds are manufactured for a wide range of commercial chemical products, including solvents, pesticides, heat exchanging fluids, and wood treatment chemicals. Halogenated aromatic compounds also are by-products of certain manufacturing processes, such as paper manufacturing, and of incomplete combustion of chlorinated wastes.
Although the benzene ring that is the nucleus of halogenated aromatic compounds is relatively easy for microorganisms to biodegrade, the addition of halogen atoms completely alters the biodegradability of benzene. The number and position of halogen atoms on the benzene ring determine how biodegradable the compound will be. Compounds with many halogen atoms may not be biodegradable at all under aerobic conditions. However, under special environmental conditions, these compounds can be reductively dechlorinated by the same type of microbial dechlorination process that can occur for halogenated aliphatic compounds (Cozza and Woods, 1992; Halden and Dwyer, 1997; McAllister et al., 1996; Mohn and Tiedje, 1992; Safe, 1994). As the reductive dehalogenation process removes halogen atoms from the benzene ring, the molecules become more susceptible to biodegradation by aerobic microbes. When environmental conditions are right, natural attenuation may be able to control halogenated aromatic compounds, but these conditions generally are uncommon.
One partial exception to the general rule that metabolism of halogenated aromatic compounds must proceed first by reductive dehalogenation is the biodegradation of pentachlorophenol (PCP). PCP is a widely used wood preservative that consists of a benzene ring with five chlorine atoms and one hydroxyl group, as shown in Figure 3-10. The presence of the hydroxyl group allows some types of aerobic microbes to completely biodegrade the PCP (McAllister et al., 1996). However, these microbes may not be present or active at many sites contaminated with PCP. Field studies indicated that PCP biodegradation occurs very slowly. Therefore, the degree to which biodegradation can reliably control PCP contamination is unknown.
One prominent category of halogenated aromatic compounds is the polychlorinated biphenyls (PCBs). Prior to being banned in the 1970s due to concern about environmental effects, PCBs were used for a variety of industrial and commercial applications requiring stable, nonflammable chemicals capable of transferring heat. Although PCB use has been banned, these chemicals are still present in the environment, especially in sediment and aquatic systems, and their persistence is due in part to their resistance to biodegradation (Luthy et al., 1997). PCBs consist of up to ten chlorine and hydrogen atoms attached to a structure consisting of two benzene rings attached by a bond between carbon atoms. Chemical synthesis can create various possible combinations—called “congeners”—of
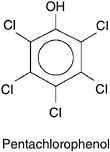
FIGURE 3-10 Pentachlorophenol consists of a central benzene ring with five chlorine atoms and one hydroxide ion.
chlorine and hydrogen atoms in the ten positions. PCBs were marketed as mixtures of congeners called Aroclors (the Monsanto Corporation trade name), characterized according to average chlorine content.
PCBs resist chemical or biological transformation, but biological transformation nonetheless can occur under suitable environmental condition. Highly chlorinated PCBs can undergo a slow process of microbially mediated reductive dehalogenation (Abramowicz, 1990; Bedard and Quensen, 1995; Boyle et al., 1992; Cerniglia, 1992, 1993; Quensen et al., 1988; Safe, 1994; Tiedje et al., 1993). The presence in the environment of congeners containing fewer chlorine atoms than the parent compounds is evidence that reductive dehalogenation reactions occur in nature. Lightly chlorinated PCBs (those containing one to four chlorine atoms) can be aerobically biodegraded at a rate that decreases as the number of chlorine atoms increases (Harkness et al., 1993). PCBs trapped within a NAPL or sorbed onto solids are not accessible to microbial destruction, so the rate of PCB dissolution is an important determinant of the rate of natural attenuation by biodegradation.
Other prominent chlorinated aromatic contaminants include dioxins such as tetrachlorodibenzo-p-dioxin (TCDD). TCDD is a by-product of many industrial processes (such as paper bleaching and pesticide manufacturing) and of incineration. It also was the primary active ingredient in Agent Orange. Although some researchers have observed microbial dechlorination of TCDD (Barkovski and Adriaens, 1996), this chemical’s complex structure and strong sorptive properties render it nearly nonbiodegradable.
Nitroaromatic Compounds
Nitroaromatic organic contaminants are associated uniquely with military activities and include the explosives trinitrotoluene (TNT), royal Dutch explosive (RDX, or hexahydro-1,3,5-trinitro-1,3,5-triazine), and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocene (HMX). Manufacturing, loading, storage, and decommissioning operations have generated large quantities of explosive wastes, some of which were deposited in soils and unlined lagoons and subsequently leached to groundwater.
Despite the number of sites contaminated with explosives, few rigorous field studies have been conducted and published in peer-reviewed journals to determine the transport, fate, and influence of microbial activity on explosives. Further, the field studies carried out to date are inconclusive in establishing the role of biodegradation in the fate of nitroaromatics (Bradley et al., 1994, 1997; Van Denburgh et al., 1993). Laboratory studies clearly show the potential for microorganisms to metabolize nitroaromatic compounds (see, for example, Funk et al., 1995; Kitts et al., 1994; Krumholz et al., 1997; Lewis et al., 1997; Pennington, 1999; Spain, 1995). However, microbes apparently cannot readily use TNT, RDX, or HMX as sources of the carbon and energy needed for their growth. Instead, cometabolic reactions generally prevail (Spain, 1995). Under aerobic and anaerobic conditions, microorganisms routinely reduce the nitro groups on nitroaromatics to amino nitro groups. These changes can increase toxicity of the molecules and cause them to form polymers, and/or strongly sorb onto soils (Funk et al., 1995). Recent reports have shown that aerobically and anaerobically grown bacteria can use TNT and RDX as nutritional nitrogen sources (Binks et al., 1995; Coleman et al., 1998; Esteve-Nunez and Ramos, 1998; French et al., 1998), but metabolite accumulation is common. The possibility of natural attenuation of nitroaromatics cannot be precluded, but the kinds of conditions needed are not clearly understood.
Microbial Transformation of Inorganic Contaminants
Many research reports have documented that microorganisms can transform inorganic contaminants (Babu et al., 1992; Banaszak et al., 1999; Brierley, 1990; Chapatwala et al., 1995; Hinchee et al., 1995; Kalin et al., 1991; Lenhard et al., 1995; Lovley, 1993; McHale and McHale, 1994; Saouter et al., 1995; Summers, 1992; Thompson-Eagle and Frankenberger, 1992; Videla and Characklis, 1992; Whitlock, 1990). However, unlike organic compounds, which microbes can convert completely to CO2, H2O, and other innocuous products, most inorganic contaminants can be changed only to forms with different mobilities. Microbial reactions can
lead to precipitation, volatilization, sorption, or solubilization of inorganic compounds. These outcomes can be the direct result of enzymes produced by the microbes, or they can be the indirect result of microbiological production of materials that alter the geochemical environment.
One nearly universal means by which microorganisms lower concentrations of inorganic contaminants in water is adsorption to the microbe colonies (Diels, 1997; Macaskie and Basnakova, 1998). Adsorption can be caused by electrostatic attraction between the metals and the microbes (Williams et al., 1998) or by highly specific scavenging systems that accumulate metals to high concentration within the cells (Chen and Wilson, 1997). Although sorption to microbial biomass is sure to influence the behavior of inorganic contaminants, microbial biomass probably cannot be harvested from the subsurface, which would be required to prevent later release of the contaminants, so it is not likely to be a major factor in natural attenuation.
Metals
Microbial effects on metals vary substantially depending on the metal involved and the geochemistry of the particular site. The behavior of many toxic metals depends on the microbially mediated cycling of naturally occurring elements, especially iron and manganese. The possible fates of chromium and mercury illustrate the variable effects of microbially mediated reactions on metals.
Chromium Chromium, used for metal plating and other applications, is among the most common groundwater contaminants at Superfund sites (NRC, 1994). As with other metals, the effects of microbial transformation on chromium vary with its chemical form (technically, its oxidation state). In water, the predominant form of chromium is the oxidized form, Cr(VI), present as chromate (CrO42−) and dichromate (Cr2O72−). Cr(VI) (known as hexavalent chromium) is toxic and mobile. Reduced chromium, Cr(III), is less toxic and less mobile because it precipitates at pH 5 and higher. A variety of aerobic and anaerobic microorganisms enzymatically reduce Cr(VI) to Cr(III), but the physiological reason for this ability has not been adequately investigated. Among the hypotheses explaining these reduction reactions are detoxification (to move Cr away from the cells), cometabolism (fortuitous enzymatic reactions), and the use of Cr(VI) as a respiratory electron acceptor. Microbes also may cause indirect reduction of Cr(VI) by producing sulfide, Fe(II), and reduced organic compounds because Cr(VI) reduction occurs spontaneously in the presence of these substances. Regardless of the mechanism involved, natural attenuation that relies on chromium reduction requires
environmental conditions that strongly favor the reduced form of chromium.
Mercury Mercury is sometimes present in soils and sediments at contaminated sites in the form of mercuric ion, Hg(II), elemental mercury, Hg(0), and the biomagnification-prone organic mercury compounds monomethyl- and dimethylmercury (both of which can accumulate at hazardous levels in the food chain). All microbial transformations of mercury are detoxification reactions that microbes use to mobilize mercury away from themselves (Barkay and Olson, 1986). Most reactions are enzymatic, carried out by aerobes and anaerobes, and involve uptake of Hg(II) followed by reduction of Hg(II) to volatile forms (elemental Hg(0) and methyl- and dimethylmercury) or the formation of highly insoluble precipitates with sulfide. In general, natural attenuation based on microbial mercury reduction and volatilization seems implausible because the volatile forms remain mobile, although immobilization as Hg(II) sulfides may be possible if the electron donors needed to sustain the microbial production of enzymes and the sulfate needed for precipitation are present together.
Nonmetals
Arsenic is a relatively common toxic groundwater contaminant, due both to its use in industry and agriculture and to its natural weathering from rocks. Industrial uses of arsenic include semiconductor manufacturing, petroleum refining, wood preservation, and herbicide production. Arsenic can exist in five different valence states: As(–III), As(0), As(II), As(III), and As(V), where the roman numerals indicate the charge on the arsenic atom. Depending on its valence state and the environment in which it exists, arsenic can be present as sulfide minerals (e.g., As2S3), elemental As, arsenite (AsO2–), arsenate (AsO43–), or various organic forms that include methylated arsenates and trimethyl arsine. No form of As is nontoxic, and both anionic forms (arsenite and arsenate) are highly soluble and toxic. The chemical and microbiological reactions of arsenic are complex (Ehrlich, 1996; Frankenberger and Losi, 1995).
Microorganisms can transform arsenic for one of several physiological reasons. Under anaerobic conditions, microbes can use arsenate (As(V)) as a terminal electron acceptor. Under aerobic conditions, oxidation of reduced As (e.g., arsenite) generates energy for microbes. Under anaerobic and aerobic conditions, microbes transform arsenic by methylation, oxidation, or reduction mechanisms that mobilize it away from microbial cells. However, microbial transformation of arsenic is not promising, because this element can exist in many mobile forms.
Selenium, another nonmetal, is used in a number of commercial and industrial processes (including photocopying, steel manufacturing, glass making, and semiconductor manufacturing) and is sometimes present at contaminated sites. Selenium contamination has also resulted from irrigation practices that led to the accumulation of selenium dissolved from soils. Although selenium is an important micronutrient for plants, animals, humans, and some microorganisms (largely because of its role in some key amino acids) when present at very low concentrations, it is toxic at higher concentrations. In natural environments, selenium has four predominant inorganic species: Se(VI) (selenate, SeO42−), Se(IV) (selenite, SeO32−), Se(0) (elemental selenium), and Se(–II) (selenide) (Ehrlich, 1996; Frankenberger and Losi, 1995). Like arsenic, selenium also has many volatile organic forms. Reduced inorganic selenium compounds can be oxidized under aerobic conditions, although the oxidation does not support microbial growth. Oxidized selenium (selenate) can serve as a final electron acceptor for anaerobic microorganisms, resulting in production of selenide and/or elemental Se. Methylation of the various selenium compounds is a detoxification mechanism that mobilizes Se away from microbial cells, but methylselenium is mobile and highly toxic to mammals. Anaerobic microbial reduction of selenate and selenite to insoluble elemental selenium can immobilize and remove Se from aqueous solution. Nonetheless, given the complex chemical and biological processes that influence the fate of selenium and its many mobile forms, microbial reactions are not a promising means for controlling Se contamination.
Oxyanions
Oxyanions are water-soluble, negatively charged chemicals in which a central atom is surrounded by oxygen. Nitrate (NO3−) is one such oxyanion. It can come from natural sources or human sources including nitrogen fertilizers. Although NO3− can occur naturally, it is a serious health concern because it can cause the respiratory stress disease methemoglobinemia in infants and because it can produce cancer-forming nitrosamines.
The major microbial process that destroys nitrate is reduction to nitrogen gas (N2), a process called “denitrification.” Microbes can use nitrate as a terminal electron acceptor when oxygen is not available. The denitrification process is widespread among microorganisms, and it occurs reliably in every anaerobic habitat with abundant carbon and electron sources. Natural attenuation by denitrification is possible, as long as the supply rate of an electron donor is sufficient to sustain the reaction. Many organic compounds, as well as H2 and H2S, can serve as the electron donor.
The oxyanions chlorate (ClO3−) and perchlorate (ClO4−) or their pre-
cursors (chlorine dioxide, hypochlorite, and chlorite) are produced by a variety of paper manufacturing, water disinfection, aerospace, and defense industries. Although not naturally occurring, these highly oxidized forms of chlorine are energetically favorable electron acceptors for microorganisms. Knowledge of chlorate and perchlorate biodegradation reactions is quite limited compared to understanding of denitrification. However, laboratory studies using bacterial cultures and environmental samples (soil, freshwater sediments, and sewage) have shown that microorganisms can reduce perchlorate and chlorate when supplied with common electron donors (such as carbohydrates, carboxylic acids, amino acids, H2, or H2S). Reducing perchlorate and chlorate generates the nontoxic chloride ion (Malmqvist et al., 1991). Microbial transformation of perchlorate or chlorate is plausible if the supply rate of electron donors is adequate.
Radionuclides
Radionuclide contamination of groundwater is common at Department of Energy (DOE) installations that were part of the nuclear weapons production complex. Uranium, one important radionuclide at these sites, can exist in many different forms, of which some are highly soluble and mobile and others are not. Insoluble U(IV)O2 and soluble U(VI)O22+ predominate in nature. Within the past several years, researchers have discovered that U(VI) can serve as a terminal electron acceptor for anaerobic microorganisms (Lovley, 1995). In this process, the organisms convert highly soluble U(VI) to an immobile U(IV) precipitate in the process of metabolizing an organic compound. This type of uranium immobilization may be an effective control strategy. However, complicating factors must be considered. An important complication is the direct chemical oxidation of the immobilized U(IV) by molecular oxygen, which would cause uranium to redissolve. The potential for this reaction to occur must be carefully evaluated.
Plutonium also is susceptible to microbial transformation—but not to the type of transformation that is useful in natural attenuation. Iron-reducing microorganisms reduce insoluble Pu(IV) to the more soluble Pu(III), rendering this contaminant more susceptible to mobilization. In contrast, neptunium, a closely related radionuclide, can be reduced to less mobile forms by sulfate-reducing bacteria. Similarly, a variety of anaerobic microorganisms reduce Tc(VII) to insoluble forms (Tc(IV) and Tc(V) oxides) that can be immobilized on microbial cells and/or other solids (Lloyd and Macaskie, 1997; Lloyd et al., 1999).
The increasing understanding of microbially mediated oxidation-reduction reactions for radionuclides indicates that natural attenuation could control these contaminants under the right conditions. For these
microbial reactions to succeed in controlling radionuclides, the reactions must immobilize the contaminant, and the proper electron donors and acceptors must be present with adequate and sustained supply rates for the immobilized species to be formed and maintained. However, additional fundamental and field research is needed before the importance of biological reactions in controlling radionuclides can be established.
TRANSFORMATION BY CHEMICAL REACTIONS
A variety of geochemical reactions can influence the potential success of natural attenuation in controlling contamination. Types of reactions include acid-base, redox, precipitation and dissolution, chemical sorption, hydrolysis, radioactive decay, and aqueous complexation. Some of these reactions can decrease the hazard posed by contamination, others can increase the hazard, and still others can influence further processes that affect the fate of the contaminants. The discussion below provides brief descriptions of and equations showing each type of reaction. This information is intended for readers with limited knowledge of geochemistry to allow them to better understand the many types of processes that must be considered in assessing natural attenuation potential.
Acid-Base
Acid-base reactions involve the transfer of hydrogen ions (H+). These reactions affect almost every other reaction type in the subsurface and therefore are very important in natural attenuation. Acid-base reactions determine the water’s pH, which in turn affects precipitation, chemical sorption, complexation, hydrolysis, and redox reactions, as well as influencing microbial processes.
The following equation shows the concept of an acid-base reaction:
(3-3)
in which HA is the acid and A− is known as the acid’s conjugate base. The Ka above the equal signs is known as the “acid dissociation equilibrium constant” and indicates the strength of the acid, which is essentially its ability to produce hydrogen ions in solution.3 Acid-base reactions occur very quickly, and are very well understood.
The pH of a solution is a measure of the availability of the H+ ion and is defined as
(3-4)
where the braces indicate H+ activity, which in the dilute solutions likely to be found in groundwater essentially equals the H+ concentration. A low pH (such as 2) indicates that H+ is very available and the solution is acidic. A high pH (such as 12) indicates low availability of H+ and a basic condition. Many chemicals present in the subsurface behave as acids or bases in that they donate or accept the hydrogen ion. They affect the pH of the solution, and the solution’s pH affects their chemical distribution between the acid and base forms.
One important feature of acid-base reactions is to “buffer” (stabilize) the solution’s pH near neutral (pH 7). If acid is added to the water or produced by other reactions, the bases in the solution (i.e., A−) take up H+ to form HA. If base is added to the solution, the acids donate H+ to form the conjugate base (A−) and water. As long as the system contains acids and bases, pH changes are small. The total acid neutralizing capacity of a system is called its alkalinity. Once all the bases are converted to their acid forms, the solution’s buffering capacity for H+ is depleted, and pH can drop dramatically when more acid is added, changing the fate of contaminants.
Redox
Microorganisms cause many of the redox reactions that are important for natural attenuation, but some redox reactions occur without the involvement of microorganisms. Regardless of whether microorganisms catalyze them, the concept of redox reactions is the same. As explained in Box 3-1, these reactions involve transfers of electrons from a donor (also called a reductant) to an acceptor (an oxidant).
Reduced iron, (Fe(II)), is the most important abiotic reductant in the subsurface. For example, dissolved Fe(II) can reduce chromate to insoluble Cr(OH)3. When sorbed to solid materials in the subsurface, Fe(II) can reduce a wide range of organic compounds and metals not reducible by dissolved Fe(II). For example, sorbed Fe(II) can reduce halogenated solvents, including TCE, by reductive dechlorination.
Sulfides (S2−, HS−, and H2S produced from sulfate reduction) also can be important reductants (although they are very toxic). Sulfides reduce iron oxides (which contain oxidized iron in the form of Fe(III)) and precipitate with Fe(II), yielding Fe(II) sulfide solids. These precipitates can scavenge Cu, Zn, Ni, Cd, and As from water, reducing aqueous
concentrations to low levels and functioning as highly effective immobilizing agents. Sulfides also can dechlorinate some chlorinated compounds.
Soluble organic compounds from plant decay and/or microbial activity can participate in a wide range of redox reactions with groundwater contaminants and naturally occurring metals. The most important of these in groundwater are humic substances (soluble remains of decomposed plants and organisms), which have quinone groups that are able to reduce Mn(IV) and Fe(III) oxides, uranyl, chromate, and a variety of organic contaminants.
Precipitation and Dissolution
Cationic (positively charged) dissolved metals can react with anions (negatively charged ions) to form a solid, or precipitate. Precipitation and dissolution reactions are central to the natural attenuation of metals. Precipitation removes dissolved metals from water. When the metal solids form relatively rapidly and are very insoluble, the metal can be immobilized irreversibly, but in other cases the immobilization is not permanent.
The following equation describes the dissolution of a generic metal solid (denoted as CmLn(s)) to a cation (Cu+) and anion (Ll−):
(3-5)
The (s) designation on CmLn(s) indicates a solid. The (aq) designation indicates dissolved substances. Kso, the “equilibrium solubility product,” is a constant related to the solubility of the metal, with a high Kso indicating high solubility.4Kso values are tabulated for many contaminants, but the rates (kinetics) of precipitation are not well understood and can be slow. Mineral surfaces can provide sites for precipitation reactions, and in some cases biological activity plays a role. Given these complexities, predicting the role of precipitation in natural attenuation is a technical challenge.
Carbonate, hydroxide, and sulfide are the most common naturally occurring anions that precipitate with metal contaminants. Generally, these solids precipitate rapidly, and solid formation is complete within days to months. The pH strongly affects the solubility of metal carbonate, oxide, hydroxide, and sulfide precipitates, with higher pH promoting precipitation. Phosphate, sulfate, and silicate also precipitate with metal cations, with phosphate being the most important of these. Metals that
are present as highly charged cations—including U4+, Th4+, Pu4+, and Cr3+—form extremely insoluble precipitates with the hydroxyl ion and a number of other substances in groundwater. In contrast, metals that are present in anionic forms—such as SeO32−, SeO42−, CrO42−, and Cr2O72−—generally do not form low-solubility precipitates. An exception is BaCrO4(s).
An important mechanism that can affect low concentrations of metals is coprecipitation. In this process, the contaminant precipitates along with or is trapped within a solid formed from major ions (such as ions of Ca, Al, or Fe) in the groundwater. For example, Mn2+ and Cd2+ form mixed solids with calcite; Cr3+, V3+, Mn3+, and Co3+ coprecipitate with Fe(III) oxides; Zn2+, Cu2+, Co2+, and Ni2+ form along with Al(III) solids; and Cd2+, Pb2+, and UO22+ form mixed solids with hydroxyapatite. Coprecipitation is important in natural attenuation because major ion precipitation reactions are common and can reduce the solubility of contaminants to concentrations well below those that would occur if the contaminants were the only precipitates. Further, toxic metals will remain immobilized in the major ion solid as long as the solid is stable.
Aqueous Complexation
In addition to forming precipitates, dissolved metal cations can react with anions called “ligands” to form compounds known as “complexes.” The reaction between a dissolved metal cation (Meu+(aq)) and ligand (Ll−(aq)) is of the form
(3-6)
The (aq) designation underscores that in contrast to precipitation reactions, all of the species, including MeL(u−l)(aq), are dissolved. Ligands can be inorganic or organic. Important inorganic ligands in groundwater include SO42−, S2−, CO32−, HCO3−, and OH−. Organic ligands include humic and fulvic acids (from decayed plants and organisms), low-molecular-weight organic acids (e.g., citrate, oxalate, and acetate), proteins, and manmade chemicals (e.g., ethylenediaminetetraacetic acid [EDTA] and nitrilotriacetic acid [NTA]) from commercial processes. The degree of complex formation is determined by the equilibrium stability constant, KMeL, and the concentrations of the ligand and metal, with high values of both promoting the formation of MeL(u−l)(aq).
Although complexation does not remove contaminant mass from the groundwater system, it is important in determining the natural attenuation of dissolved metals in groundwater for three reasons. First, many dissolved metal ions are likely to exist in groundwater primarily as com-
plexes, not as the bare ion (Meu+). Second, metal complexes often prevent the metal from precipitating. Third, metal complexes sorb differently to solids than free metal ions. At some pH values, sorption increases, and movement of the metal ion slows; at other pH values, the reverse is true. Thus, most of the dissolved metal may be part of a complex, and the potential for immobilization of the metal complex may be significantly different from the immobilization potential of the metal alone.
Metal ions vary in their ability to form complexes. Complexation of Mn(II) and Fe(II) is very limited at near neutral pH in groundwater; Fe(III), Al(III), and Cu(II) are likely to be fully complexed by organic matter or OH−; and Ni(II), Co(II), and Pb(II) are moderately likely to form complexes. Complexation is especially important in evaluating natural attenuation of radionuclides (e.g., U, Th, Pu, Np) because many of these elements form highly stable complexes with CO32−, OH−, and organic ligands in groundwater.
Chemical Sorption
Sorption reactions concentrate dissolved chemicals on the surfaces of solids present in the groundwater system. This sorption slows the transport of chemicals in groundwater. Two types of sorption reactions can occur: adsorption and absorption. Adsorption is rapidly reversible, meaning that adsorbed chemicals can quickly redissolve. Adsorption occurs when chemicals in water bond to stable functional groups at the outer surface of the soil solids. In contrast, absorption is slowly reversible, meaning absorbed chemicals do not redissolve as quickly as adsorbed chemicals. In absorption, the dissolved chemicals enter the lattice of the solid. Absorption is most valuable for natural attenuation because absorbed contaminants are less likely to desorb.
Adsorption occurs when the surfaces of mineral and organic materials in groundwater systems contain groups of atoms (known as functional groups) bearing electric charges. These charged functional groups can react with dissolved chemicals by either complexation (as described above) or ion exchange (in which a chemical from the water is attracted to a surface functional group with the opposite charge). The general forms of the complexation reactions for a metal cation (Mm+), a metal complexed with a ligand that gives a net negative charge (MLl−), and a surface group (SOH) are as follows:
(3-7)
and
(3-8)
where KM or KL are reaction equilibrium constants for the metal-exchange and ligand-exchange reactions, respectively. Since H+ is part of each reaction, surface complexation is sensitive to solution pH. Low pH favors sorption of ligands, but high pH favors sorption of metals. Adsorption of metal cations to fixed-charge sites on layer silicates (SX−) is described as an exchange reaction of the form
(3-9)
where KMX is an ion-exchange constant and Cu+ is the exchanging cation. Cation-exchange reactions depend on the concentration of surface-exchanging cations (such as Ca2+ and Na+) on the solid.
All sorption reactions are multicomponent and therefore difficult to predict. The degree of adsorption is controlled by the strength of the adsorption complex (as indicated by KM, KL, or KMX), the concentration of surface functional groups (SOH or SX−), the concentration of the adsorbing species (Mm+ or MLl−), and the concentration of competing species, especially H+ and exchanging cations. Further, sorption reactions sometimes take place in two steps: a rapid surface adsorption reaction followed by slow absorption. Because water enters the small micropores of aquifer sediments where absorption occurs, absorption is limited by the rate of diffusion. Further, the geochemical conditions in these micropores may differ from those of the surface region. The changing geochemical conditions, as well as the slow exchange with the outside of the particles, means that absorbed species (those within micropores) may differ significantly from adsorbed species.
In the past three to four years, scientists have produced convincing evidence (using molecular-scale techniques) that metal sorption on soil minerals and soils can result in the formation of metal hydroxide surface precipitates (Roberts et al., 1999; Thompson et al., 1999a,b). These precipitates form at pH levels typically found in natural subsurface environments (pH ≥ 6.8), on time scales of minutes to hours, and at metal concentrations that are common in contaminated areas. The formation of surface precipitates greatly stabilizes the metals. Recently, such precipitates have been identified in soils that have been contaminated with metals for a long time. It is clear that the formation of such phases can be an important mechanism for immobilizing toxic metals, but understanding and documenting such mechanisms is a complicated process.
The complexity of sorption reactions has two implications for natural
attenuation. First, sorption normally cannot be described with simple mathematical models; detailed models are needed. Second, adsorbed metals may desorb when geochemical conditions, such as pH or exchanging cations, change in such a way that the adsorption reactions act in reverse.
Hydrolysis
Hydrolysis is a chemical reaction in which H2O or OH− substitutes for an electron-withdrawing group, such as chlorine. Under conditions likely to be found in groundwater, 1,1,1-trichloroethane (1,1,1-TCA) is the only major chlorinated solvent that can be chemically hydrolyzed within the one- to two-decade time span of general interest in site remediation. TCA hydrolysis ultimately produces acetic acid (vinegar) and 1,1-DCE:
(3-10)
(3-11)
(3-12)
The rate of hydrolysis depends on the concentration of 1,1,1-TCA and the pH. About 80 percent of 1,1,1-TCA is converted through hydrolysis to acetic acid and about 20 percent to 1,1-DCE. The half-life of 1,1,1-TCA in groundwater is about 12 years at a temperature of 10°C. This half-life is very sensitive to temperature and decreases to about 2.5 years at 20°C (Rittmann et al., 1994). The half-life is also sensitive to pH changes.
Chloroethane, a by-product formed through biological reduction of 1,1-dichloroethane (1,1-DCA), also can be hydrolyzed, with a half-life on the order of months to years (Mabey and Mill, 1978). The product is ethanol, which can easily biodegrade.
Radioactive Decay
All radioactive elements spontaneously decay to what are called “daughter” products. The decay process emits alpha, beta, or gamma radiation that, depending on energy, can be dangerous to living things. However, subsurface solids absorb the emissions; thus, radionuclides that remain in the subsurface are not a risk as long as exposure to the contaminated groundwater does not occur.
Radioactive decay occurs according to the following equation for the decay rate:
(3-13)
in which C is the concentration of the radionuclide and λ is the first-order decay constant for each radionuclide. This leads to the well-known equation indicating that radionuclides decay at an exponential rate:
(3-14)
in which C(0) is the starting concentration, C(t) is the concentration after time t, and t1/2 is the half-life, which equals 0.693/λ. Half-lives—the amount of time required for half of the radionuclide to decay—are known for all radionuclides. Natural attenuation via radioactive decay is possible for radionuclides that have short half-lives. Examples are 3H, 12.5 years; 137Cs, 30 years; 90Sr, also 30 years; and 131I, 8 years. However, some radionuclides have very long half-lives. For examples, the half life of 238U is 4.5 × 109 years. Radioactive decay may form radioactive or nonradioactive daughter products, depending on the particular radionuclide. The daughter products are elementally different from the parent and may behave very differently in the environment.
INTEGRATION OF THE MECHANISMS THAT AFFECT SUBSURFACE CONTAMINANTS
As is clear from this chapter, an enormous variety of processes can affect the potential for natural attenuation of contaminants. These processes include physical mechanisms (advection, dispersion, and phase transfer), microbiological reactions (by a multitude of types of organisms requiring different types of environments to function), and chemical reactions (acid-base, redox, precipitation, dissolution, complexation, sorption, hydrolysis, and radioactive decay). All of the processes relevant for a particular contaminant will occur simultaneously. For example, when a contaminant dissolves from a NAPL or solid, advection and dispersion move it to other locations, while biodegradation and chemical reactions may transform the contaminant as it moves. To understand and evaluate natural attenuation, all the processes have to be combined in a model of the subsurface—a series of equations that represent the environment—to determine which ones are important in controlling the contaminant’s fate. The two most important tools for this integration process are “mass balances” and use of “footprints” of attenuation reactions.
A mass balance is an equation that keeps track of the mass of the
contaminant in a unit volume (which can be thought of as a small cubic section) of the subsurface. The mass balance describes the change in contaminant mass in this section as follows:

(3-15)
The left-hand side of Equation 3-15 represents the net effect of the different processes, shown on the right-hand side. The equation represents separately each of the three transport processes: advection, dispersion, and phase transfer. Separate terms are needed because each transport process behaves differently, and the mathematical representations (which are described in Chapter 4) are distinct. The last term is the sum of all biogeochemical reactions. When more than one reaction affects the contaminant, each should have its own term. Finally, all of the terms in Equation 3-15 must have exactly the same units, such as mass per unit time (for example, milligrams per day).
The second tool for evaluating the many potential natural attenuation reactions is the use of footprints of biogeochemical reactions. Biodegradation or chemical transformation of a contaminant produces or consumes other materials, and these compounds serve as footprints. Examples of footprints from reactions that destroy or immobilize contaminants include the following:
-
the products of reductive dechlorination of TCE and PCE, such as VC, ethene, and Cl−;
-
the depletion of electron acceptors, such as oxygen, nitrate, and sulfate, or the formation of reduced end products, such as methane and Fe(II), from the oxidation of organic contaminants; and
-
the loss of alkalinity from the precipitation of metal hydroxide solids.
All of the biogeochemical reactions that transform groundwater contaminants leave footprints—many of which can be measured—that help to establish the fate of the contaminant.
Chapter 4 describes in detail the methods recommended for integrating the various natural attenuation processes and evaluating footprints in order to assess natural attenuation potential at a site.
CASE STUDIES OF NATURAL ATTENUATION
Enough research has been conducted for some contaminants to indicate that the types of natural attenuation reactions described above can protect human health and the environment in some settings. For some contaminants, this research includes detailed field studies at contaminated sites. The case studies reviewed below provide a sampling of field research on the effectiveness of natural attenuation for contaminants in the classes listed in Table 3-1.
Traverse City Coast Guard Base: Extensive Natural Attenuation of BTEX
In 1969, an estimated 95 m3 (25,000 gallons) of aviation fuel spilled into unsaturated soil and groundwater underlying the U.S. Coast Guard Air Station at Traverse City, Michigan. The NAPL source and the resulting plume of dissolved contaminants went undetected until 1980, when BTEX contamination was discovered in drinking water wells downgradient from the release (see Figure 3-11). Dissolution of aromatic hydrocarbon fuel compounds (especially BTEX) from the NAPL source resulted in 36-40 mg/liter of total alkylbenzenes near the center of the contaminant plume (Wilson et al., 1990).
In 1985, the U.S. Coast Guard installed a pump-and-treat system to control the source of contamination and prevent further off-site migration. Researchers then assessed the site to determine whether microorganisms were metabolizing the BTEX and might be able to clean up the plume once the source was controlled (Hutchins et al., 1991; Wilson et al., 1990). The field geochemistry revealed that the plume’s central core was rich in methane and BTEX, and depleted in oxygen (see Figure 3-12), indicating the presence of an active anaerobic food chain terminating with methane production. This area serves as a zone of anaerobic treatment of BTEX. Elevated concentrations of Fe(II) also were found, indicating the presence of iron-reducing bacteria. That anaerobic bacteria were degrading BTEX was confirmed by documenting the presence of breakdown products (such as cresols and benzoic acids) of anaerobic BTEX biodegradation.
Surrounding the anaerobic treatment zone was an aerobic zone of treatment with measurable oxygen and small quantities of migrating methane and BTEX. The perimeter was surrounded by another zone with high oxygen concentrations and no detectable methane or BTEX. Thus, the high BTEX concentrations in the center of the plume were biodegraded to nondetectable levels at the edges of the plume by populations of anaerobic and aerobic bacteria. Depletion of oxygen and formation of methane
FIGURE 3-11 Schematics showing the plume of BTEX contamination from the U.S. Coast Guard Air Station at Traverse City, Michigan, prior to installation in 1985 of a pump-and-treat system to control the contaminant source. The top diagram shows the migration of the plume off Coast Guard property and through industrial and residential areas before it discharges to Traverse Bay. The lower figure is an enlarged diagram of the portion of the plume located on Coast Guard property. A, B, C, D, P, Q, R, and S represent the locations of monitoring wells. SOURCE: Wilson et al., 1990. Reprinted, with permission, from Taylor and Francis (1990). © 1990 Taylor and Francis.
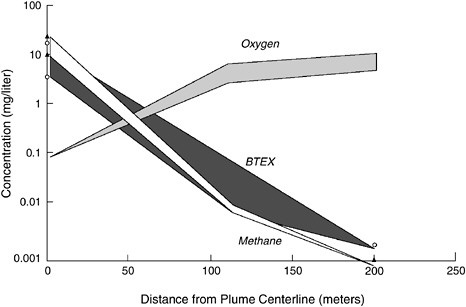
FIGURE 3-12 BTEX, methane, and oxygen concentrations in the plume of contamination at the Traverse City site. The figure shows the ranges of concentrations measured at the sampling wells shown in Figure 3-11. SOURCE: Wilson et al., 1990. Reprinted, with permission from Taylor and Francis (1990). © 1990 Taylor and Francis.
and Fe(II) were footprints of aerobic and anaerobic biodegradation reactions that were responsible for the loss of BTEX. Once the contaminant was controlled, natural attenuation successfully reduced dissolved BTEX concentrations to below harmful levels.
Vandenberg Air Force Base: Persistent MTBE in a Fuel Spill
Leaking fuel-storage facilities at a General Services Administration gas station at Vandenberg Air Force Base in California created a plume of petroleum hydrocarbons and MTBE. The gas station was closed in 1994 after discovery of the fuel leak. Reconciliation of inventory records suggested that a total of 2.16 m3 (572 gallons) of unleaded fuel had spilled (Lee and Ro, 1998). The underground storage tanks and piping were removed in 1995.
Groundwater at the site moves at an estimated average rate of 120 m/ year (400 ft/year), and a relatively large MTBE plume has formed. As shown in Figure 3-13, the MTBE plume is 76-91 m (250-300 ft) wide and
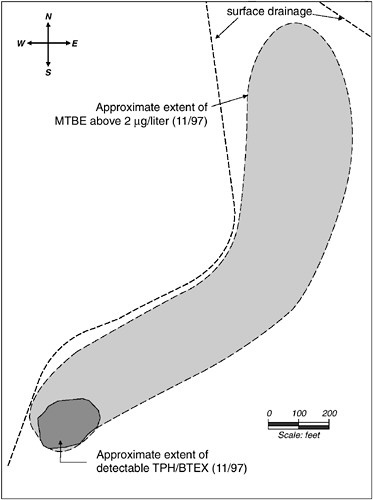
FIGURE 3-13 Approximate extents of MTBE, total petroleum hydrocarbons (TPH), and BTEX contamination arising from gasoline leaks at a former service station at Site 60, Vandenberg Air Force Base, California.
extends approximately 520 m (1,700 ft) beyond the source area.5 In contrast, the BTEX plume apparently stops within 15-30 m (50-100 ft) of the source, presumably due to BTEX biodegradation.
|
5 |
Recent studies suggest that the MTBE plume is migrating within one or more preferred flow channels of medium to fine sand bounded by silts and clays and therefore may be less uniformly distributed than implied by Figure 3-13 (Einarson et al., 1999; Mackay et al., 1998). |
Sample profiling at a number of locations within and upgradient of the plume has revealed weak anaerobic conditions, low organic content (measured as chemical oxygen demand), and high sulfate concentration. Anaerobic biodegradation of MTBE may occur near the source, but much (or all) of the MTBE escapes this zone and then migrates along the weak anaerobic “shadow” created by BTEX degradation. Aerobic transformation of MTBE, if it occurs at all, is limited to very narrow zones of mixing between the plume and surrounding oxygenated water. Thus, although natural attenuation processes at this site appear to have controlled the BTEX plume, they have not controlled the MTBE plume, at least within 520 m of the source (Durrant et al., 1999).
Borden Air Forces Base: Partial Biodegradation of Chlorinated Solvents
Approximately 12 m3 of a contaminant solution were injected into a shallow sand aquifer at the Canadian Air Forces base in Borden, Ontario, for the purpose of studying the fate of chlorinated solvents in the subsurface in a controlled setting. Along with chloride and bromide tracers, the injected solution contained five organic solutes: bromoform, carbon tetrachloride, PCE, 1,2-dichlorobenzene, and hexachloroethane.
The work at this site represents a detailed attempt to understand the fate of chlorinated solvents in the subsurface. Researchers used more than 5,000 closely spaced sampling points to collect nearly 20,000 samples over a three-year period in order to identify the resulting distribution of solutes in time and space and the biogeochemical factors affecting contaminant transport. Data from a chloride tracer indicated an advective velocity of 0.09 m/day (Mackay et al., 1986). Other data indicated that dispersion resulted in spreading of the plume, particularly in the longitudinal direction. The octanol-water partition coefficient (Kow) ranged from 200 to 4,000 m3 water/m3 octanol for the five organic compounds. The organic solutes with the highest Kow values (1,2-dichlorobenzene and hexachloroethane) moved very slowly due to sorption, whereas the others moved faster, although movement of all was slower than the transport of chloride (Mackay et al., 1986).
Researchers also collected data to assess whether the mass of contaminants in solution was decreasing. The mass of bromoform, dichlorobenzene, and hexachloroethane decreased, but the mass of carbon tetrachloride and PCE did not. Although loss of these three compounds provides evidence that natural attenuation was involved, no follow-up microcosm studies with bromoform and dichlorobenzene were conducted to confirm that possibility for these two contaminants. However, microcosm studies indicated that, rather than being destroyed, hexachloro-
ethane was converted to PCE (Criddle et al., 1986). This mechanism is supported by an increase in PCE mass noted field results. This finding demonstrates that loss of a contaminant by a natural attenuation reaction does not necessarily eliminate the hazard. The compound formed in this case was more hazardous than the parent compound.
St. Joseph, Michigan: Extensive Natural Attenuation of a Chlorinated Solvent
Perhaps the most extensively studied case of natural attenuation of a chlorinated solvent is the St. Joseph, Michigan, Superfund site (see photos) (Dolan and McCarty, 1995; Haston et al., 1994; Lendvay, 1998; McCarty and Wilson, 1992; Semprini et al., 1995; Wilson et al., 1994). This site contains concentrations of TCE in groundwater as high as 100 mg/liter.
Concentrations of cis-DCE, VC, and ethene are high at the site, providing an indicator that TCE is biodegrading. A large amount of organic matter leaching from a disposal lagoon is driving the biodegradation of TCE by reductive dechlorination (McCarty and Wilson, 1992). The chemical oxygen demand (COD) created by the organic matter in groundwater near the lagoon is high (400 mg/liter), as shown in Figure 3-14. This chemical oxygen demand is converted nearly completely to methane across the length of the plume, providing a key piece of evidence that reductive dechlorination is occurring (McCarty and Wilson, 1992). Thorough analysis near the source of contamination indicated that 8 to 25 percent of the TCE was converted to ethene, and up to 15 percent of the reduction in COD in this zone was associated with reductive dehalogenation (Semprini et al., 1995). Through more extensive analysis of groundwater further downgradient from the contaminant source, Wilson et al. (1994) found a 24-fold decrease in the TCE concentration across the site. A review of the data at individual sampling points indicated that conversion of TCE to ethene was most complete where methane production and loss of nitrate and sulfate by reduction were highest.
This case study shows several key footprints for a major loss of TCE by reductive dechlorination: (1) cis-DCE, VC, and ethene formation; (2) loss of COD well in excess of that needed for dechlorination; and (3) evidence of anaerobic processes where dechlorination was occurring, as indicated by methane production coinciding with COD loss and by decreases in nitrate and sulfate concentrations. Although extensive dechlorination took place, complete dechlorination of TCE and its intermediates did not occur, as indicated by the TCE, cis-DCE, and VC remaining at the site.

The factory from which the TCE plume arose at the St. Joseph, Michigan, site. The foreground shows the area in which TCE concentrations in groundwater were highest. SOURCE: Courtesy of Perry McCarty, Stanford University.

Collecting groundwater samples at the St. Joseph, Michigan, site. SOURCE: Courtesy of Perry McCarty, Stanford University.
FIGURE 3-14 Concentrations of chlorinated aliphatic hydrocarbons (as represented by chemical oxygen demand) in groundwater at the St. Joseph, Michigan, site. SOURCE: McCarty and Wilson, 1992b.
Edwards Air Force Base: No Natural Attenuation of a Chlorinated Solvent
Edwards Air Force Base is located on the western portion of the Mojave Desert, about 100 km (60 miles) north of Los Angeles. From 1958 through 1967, approximately one 55-gallon drum of TCE was used each month to clean engines for the X-15 rocket plane (McCarty et al., 1998). Disposal of the TCE into the nearby desert created a large plume of groundwater contamination (see Figure 3-15). The plume is about 400 m east of the contamination source, and no other significant contaminant is present.
From pumping tests and hydraulic gradient measurements, researchers estimated the groundwater velocity to be 6.9 cm/day. The TCE plume has traveled 700 m since its origin 40 years ago, indicating a movement rate of about 4.8 cm/day. TCE partition measurements using site aquifer material indicate a retardation coefficient of 1.6. If this retardation coeffi-
FIGURE 3-15 Trichloroethene groundwater plume at Edwards Air Force Base. The concentration of TCE (in micrograms per liter) is shown in each contour. SOURCE: After McCarty et al., 1998.
cient is applied to the plume velocity, and no TCE degradation or other loss process has occurred, a groundwater velocity of 7.7 cm/day is indicated. Since this value is close to the velocity estimated independently from pumping tests, the assumption of no significant TCE degradation or other loss mechanism appears valid.
The dissolved oxygen content of the groundwater is near zero, but nitrate and sulfate concentrations are 26 and 710 mg/liter, respectively. The absence of primary substrates to create reducing conditions and drive dechlorination of TCE explains why the TCE plume moves with the groundwater and without attenuation by biodegradation.
Dover Air Force Base: Natural Attenuation by Sequential Biodegradation Reactions
Groundwater at a portion of Dover Air Force Base, Delaware, known as Area 6 West is contaminated with TCE and 1,1,1-TCA, which were
used for degreasing jet engines at a maintenance facility. In the suspected contaminant source area, TCE and 1,1,1-TCA concentrations are 5 and 31 mg/liter, respectively. The plume is about 2,000 m (6,500 ft) long and 120 m (400 ft) wide and has a narrow and distinct core in which contaminant concentrations are highest (Ellis et al., 1996; Grosso et al., 1999; Klier et al., 1999). TCE has traveled the farthest from the source, about 2,000 m (6,500 ft), and 1,1,1-TCA has traveled about 330 m (1,100 ft).
Several footprints of TCE and 1,1,1-TCA degradation have been detected. Contained within the TCE and TCA plume are shorter cis-1,2-DCE, 1-1 DCA, VC, and ethene plumes—all products of reductive dechlorination. Groundwater in the plume is relatively reducing for about 1,400 m (4,500 ft) of its flow path and contains methane, with concentrations up to 500 (g/liter, and occasionally hydrogen sulfide. The concentration of chlorides, which are released by dechlorination, is high, up to 60 mg/liter, compared with a background of 8 mg/liter. These data suggest that the methanogenic conditions normally accompanying reductive dehalogenation

The X-15 rocket plane, which TCE was used to clean at Edwards Air Force Base. Disposal of TCE in the nearby desert created a large plume of groundwater contamination. SOURCE: Courtesy of Edwards Air Force Base, California.
are present. Extensive microcosm studies have confirmed that bacteria from the contaminated aquifer have capability to carry out reductive dehalogenation, although laboratory work has not confirmed reduction beyond cis-DCE.
The groundwater becomes oxidizing about 1,400 m (4,500 ft) downgradient of the source area. cis-1,2-DCE and VC concentrations decrease more quickly than TCE as the groundwater enters this aerobic zone. These observations suggest that aerobic bacterial processes might be removing cis-1,2-DCE and VC. Aerobic microcosm studies have yielded evidence that cis-1-2-DCE and VC are oxidized directly to CO2.
Despite the presence of these footprints, and unlike the St. Joseph site, extensive groundwater analyses have been unable to identify the source of the electron donor that is driving reductive dechlorination of the solvents. Concentrations of organic carbon, which might serve as an electron donor, are unusually low, and only small amounts of BTEX (another potential donor) have been detected. Researchers have hypothesized that the electron donor is a hydrocarbon such as oil or grease that was co-disposed with the solvents but is sparingly soluble and therefore difficult to detect.

Monitoring groundwater at Dover Air Force Base. SOURCE: Courtesy of Dover Air Force Base staff photographer.
Several types of data at this site provide significant evidence that TCE and 1,1,1-TCA concentrations are decreasing naturally by reductive dechlorination. Evidence includes the presence of daughter products, high chloride concentrations, and the methanogenic conditions that normally accompany reductive dechlorination. Although this evidence is quite convincing, the electron-donor source that is driving the elimination of TCE and 1,1,1-TCA is unknown. Without information about the electron donor, predicting whether natural attenuation will continue to control the contamination is impossible.
The Hudson River: Incomplete Natural Attenuation of PCBs
The sediments of the Hudson River along a 200-mile stretch from Hudson Falls to the Battery (in Manhattan) are contaminated with PCBs as a result of years of discharges from a PCB manufacturing facility. Sediment cores removed from the Hudson River show that the composition of the PCBs has changed over the years from highly chlorinated mixtures to lightly chlorinated ones due to natural biodegradation reactions (Brown et al., 1987). Laboratory tests with microorganisms from the contaminated sediments confirmed that these organisms can remove chlorine atoms from PCBs via the reductive dechlorination process (Bedard and Quensen, 1995; Quensen et al., 1988).
Transformation of the remaining lightly chlorinated PCBs could occur if certain aerobic microorganisms are active. To evaluate whether aerobic metabolism of lightly chlorinated PCBs is feasible, researchers installed large enclosures in the Hudson River and aerated these enclosures to stimulate aerobic organisms. Over a 10-week period, PCB concentrations diminished relative to concentrations of a nonbiodegradable chemical used as a tracer. PCB-related breakdown products (chlorobenzoates) appeared in the sediments (Harkness et al., 1993; NRC, 1993).
Although the potential for anaerobic and aerobic PCB biodegradation exists in Hudson River sediments, at least two additional requirements must be fulfilled before natural attenuation can be considered a sufficient management strategy. First, the anaerobic and aerobic processes must be linked. Movement of deeper anaerobic sediments into shallow aerobic zones can occur as a result of stream-channel and bioturbation processes, but there is no guarantee that sediment transport processes will be precise enough to achieve efficient biodegradation. The PCB components from which some of the chlorine atoms have been removed have to be transported to aerobic surface sediments, while the PCBs that have not partially biodegraded remain behind. In addition, the transport and biodegradation have to occur before PCBs enter the food chain, especially of fish that bioaccumulate PCBs in their tissues. Second, anaerobic and
aerobic reactions must occur at rates that adequately protect the food chain and human health.
A recent study (McNulty, 1997) carefully retrieved, dated, and chemically analyzed sediment samples taken from different depths of the river bottom. In a highly contaminated section of river sediments, significant dechlorination of PCBs had occurred, but the dechlorination rate decreased dramatically after about a year. Even after years to decades, complete dechlorination of PCBs had not occurred. Furthermore, at a moderately contaminated section of sediments, only initial signs of dechlorination had developed. Thus, the observed footprints of PCB dechlorination, although encouraging, are insufficient in themselves to ensure that natural attenuation will be sufficient to decrease contaminant concentrations to meet regulatory standards.
South Glens Falls, New York: Natural Attenuation of PAHs Following Source Removal
The Electric Power Research Institute and Niagara Mohawk Power Company collaborated to assess how removing the contaminant source at a coal tar disposal site would affect natural attenuation of dissolved contaminants from coal tar remaining in groundwater (Taylor et al., 1996). The study area is located in a rural setting near South Glens Falls, New York. The site was used for disposal of tar from a single event that occurred in the early 1960s at a plant that manufactured gas. The site was ideal for study because the contaminant source and hydrogeology are relatively simple. Because the contamination resulted from a single disposal event, there was only one coal tar source, with no unknown residuals, making source characterization relatively straightforward compared to more complex sites. The hydrogeologic setting is an aquifer composed of coarse to fine silty sands, with a confining layer 6 to 9 m (20 to 30 ft) below ground surface preventing extensive downward migration of the contamination. The groundwater velocity is about 12 m (40 ft) per year.
Source removal commenced in May 1991 with installation of a sheet pile enclosure driven to a depth of more than 12 m (40 ft), well below the confining layer 7.6 m (25 ft). Despite the relatively simple hydrologic setting, locating and thoroughly removing the coal tar source material were technically difficult. The enclosed area was about 1,000 m2 (0.25 acre), and approximately 7,200 m3 (9,400 yd3) of tar-contaminated soil and overburden were removed. In October 1991, the sheet pile was removed, and the excavated area was filled with clean native soil with a grain size similar to that of the removed soil.
Figure 3-16 shows an areal view of concentrations of naphthalene—one of the key constituents dissolved from the coal tar—in June 1990 just
before source removal, and a few years later in November 1994. The concentration contours were developed from numerous monitoring wells and multilevel groundwater samplers. By the later time, much of the region between the source removal area and transect B contained less than 10 μg/liter naphthalene, which is below the New York State Department of Environmental Conservation drinking water standard. Phenanthrene—another dissolved constituent from coal tar—has a similar fate. By November 1994, no phenanthrene was detected in any of the monitoring wells.
Four types of evidence indicate the involvement of microorganisms in the attenuation of naphthalene and phenanthrene:
-
depletion of oxygen at the center of the plume, where naphthalene concentrations were highest, and an inverse relationship between oxygen and phenanthrene concentrations throughout the plume;

Excavation operations that successfully removed the source of coal tar waste contamination in South Glens Falls, New York. After the source was delineated, the subsurface was stabilized with sheet pilings, excavated, and filled with clean sandy material. SOURCE: Courtesy of Dr. E. F. Neuhauser, Niagara Mohawk Power Corp.
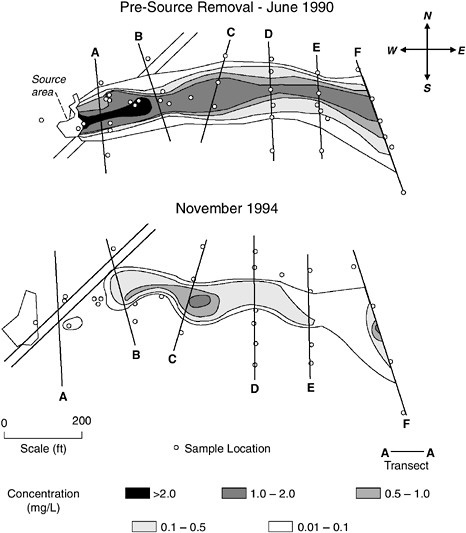
FIGURE 3-16 Change in maximum concentration of naphthalene in a groundwater plume following coal tar source removal. SOURCE: Taylor et al., 1996. Reprinted, with permission from Electric Power Research Institute (1996). © 1996 by Electric Power Research Institute.
-
rapid metabolism of naphthalene and phenanthrene in soil microcosms from inside, but not outside, the contaminant plume (Madsen et al., 1991);
-
protozoan predation of bacteria inside, but not outside, the plume (Madsen et al., 1991); and
-
detection of a unique transient intermediary metabolite (Wilson and Madsen, 1996) and expressed naphthalene biodegradation genes (messenger ribonucleic acid [mRNA]; Wilson et al., 1999) in the contaminated groundwater.
The data from these studies suggest that natural attenuation due to biodegradation was effective in controlling contamination once the source was removed.
Pinal Creek Basin: Multiple Natural Attenuation Processes Affect Metals
Acidic drainage from former mine sites is a common source of metal contamination in groundwater. Large-scale copper mining has occurred in Arizona’s Pinal Creek Basin since the late 1880s, and at many sites the groundwater has become contaminated. A 25-km-long plume extends downgradient from the former location of several unlined mine tailings ponds at the head of the basin. Although the ponds are now drained, the source of the plume probably had a pH of 2 to 3 and iron and sulfate concentrations exceeding 2,000 and 19,000 mg/liter, respectively. U.S. Geological Survey (USGS) researchers have been studying the plume since 1984 (Brown et al., 1997). The acidic part of the plume, extending 12 km from the source and shown in Figure 3-17, contains high concentrations of sulfate, calcium, iron, manganese copper, aluminum, and zinc. The concentrations of several other metals are above the maximum contaminant levels specified by the Safe Drinking Water Act.
Many physical, chemical, and microbiological processes affect the metal contaminants at this site (Stollenwerk, 1994). Studies with a tracer chemical between 1984 and 1993 showed that dilution likely accounts for a 60 percent decrease in contaminant concentration over the first 2 km of the plume. Further, this research indicated that natural carbonate materials in the ground raise the pH to 5-6, which results in the precipitation or sorption of iron, copper, zinc, and other metals onto the solid geologic materials in the aquifer. For example, the concentrations of aqueous copper, cobalt, nickel, and zinc depend strongly on pH. Thus, as the pH increases with distance, the sorption of these metals also increases, resulting in decreased concentrations in groundwater with distance. However, the neutralization reactions eventually deplete the carbonate in the aquifer, so some of the metals continue to spread at a rate of about one-seventh the advective groundwater flow rate. Further, these reactions are reversible, leading to remobilization of metals in the water as the carbonate is depleted and the pH drops.
The plume eventually discharges into Pinal Creek, resulting in a rapid
FIGURE 3-17 Cross section of Pinal Creek Basin showing the pH of the groundwater contaminant plume below the former mine tailings ponds (far left). Groundwater flowing beneath the former ponds has a pH less than 4 (as shown on the dashed line marked 4), but father downgradient the pH increases to 5-6 as carbonate minerals neutralize the acidity. The low-pH region corresponds to the region with high concentrations of dissolved metals. Attenuation of the dissolved metals by precipitation occurs as pH increases. SOURCE: Brown and Harvey, 1996.
increase in pH and dissolved oxygen concentration due to gas exchange with the atmosphere. This increase in pH and oxygen concentration leads to precipitation of manganese oxides in the stream sediments, enhanced by the presence of manganese-oxidizing bacteria, and results in immobilization of about 20 percent of the dissolved manganese. Concentrations of dissolved nickel and cobalt also decrease by sorption onto the manganese oxides (Harvey and Fuller, 1998).
This site illustrates that several natural processes can act to decrease concentrations of metals in groundwater. Neutralization by the dissolution of carbonate solids causes the pH to rise, which enhances precipitation and sorption of heavy metals. Oxidation-reduction reactions in
stream sediments form manganese oxide solids that sorb other metals in the groundwater before these metals reach the stream. Both mechanisms delay the arrival of the heavy-metal plume and its discharge into a nearby stream. However, the size and longevity of the contaminant source may overwhelm the natural attenuation capacity of the aquifer as the carbonate minerals that cause the pH to increase become depleted. Even if the entire source of contamination could be removed, the pH could remain low in carbonate-depleted areas due to reactions involving iron and manganese.
Hanford 216-B-5: Immobilization of Radionuclides
The U.S. government used the Hanford site in south central Washington State to manufacture nuclear materials for weapons beginning in the early 1940s. Nuclear reprocessing at Hanford generated high- and low-level radioactive wastes of many types and compositions, and many of these wastes were disposed on the ground surface or injected directly underground in disposal wells.
One particular well at Hanford, the 216-B-5 reverse well, used for disposal of medium-level radioactive wastes from 1945 to 1947, is representative of the effects of past disposal practices on groundwater quality in the nuclear weapons production complex, now controlled by the Department of Energy. The well was 92 m deep—2 m below the water table—and the lower 18 m were perforated to distribute waste solutions into the surrounding sediments. Approximately 3 × 109 liters (8 × 106 gallons) of low-salt, alkaline waste fluids derived from fuel rod dissolution and waste neutralization processes were disposed of in the well. The primary radioactive contaminants were 90Sr, 106Ru, 137Cs, and 239, 240Pu. Uranium probably was present in the waste stream but was not measured. The geology at this site consists of coarse-textured and highly diverse sediments. The water chemistry is mildly calcareous, with pH near 8.
Between 1947 and 1949, the government installed 11 monitoring wells in groundwater around the 216-B-5 disposal well and used gross counting of radioactivity to monitor the plume (Brown and Rupert, 1950). The concentrations of fission products (gamma and beta emitters, 90Sr, 106Ru, 137Cs) were initially high in groundwater near the injection well, but decreased with time. The predominant gross fission products were anionic radioactive components (e.g., 106RuO4−), which react poorly with Hanford sediment and therefore moved with the groundwater. However, the concentration of fission products in groundwater decreased over the initial two-year observation period as a result of dispersion and radioactive decay of 106Ru (which has a half life of 368 days). Concentrations of gross alpha contamination (e.g., 235, 238U, 239, 240Pu) in groundwater were
lower, but decreased with time. Because the decay of alpha-emitting radioactive contaminants is slow, the decrease was attributed to dispersion and possible chemical reaction. The predominant alpha emitter was hexavalent 238U (i.e., UO22+), which is moderately mobile in the Hanford subsurface as a carbonate complex (e.g., UO2(CO3)22−).
Over the years, a small, stationary contaminant plume developed. The plume consists of radionuclides with longer half-lives (90Sr, 137Cs, and 239, 240Pu). Mass-balance estimates based on concentrations of these contaminants in groundwater suggest that most of this longer-lived radioactivity has remained sorbed. Samples taken at select locations throughout the plume indicate that most of the radionuclides are sorbed to sediment near the injection source, but that limited downgradient migration has occurred, as shown in Figure 3-18 for plutonium.
A combination of adsorption and precipitation reactions appears to be immobilizing the radionuclides. Cesium (Cs+) adsorbs to biotite, vermiculite, and smectite minerals in the Hanford sediments by ion exchange, then slowly diffuses into the interlayer region of these minerals and becomes fixed. Strontium (Sr2+) also adsorbs by ion exchange, but the surface species is exchangeable if solution conditions change. 90Sr may also have precipitated with PO43− or coprecipitated with BiPO4 in the primary waste stream, but confirmatory evidence is absent. Plutonium also appears to be immobilized as hydrous oxide solids (which form from Pu(+IV)).
A risk-based decision analysis (DOE-RL, 1996) concluded that radionuclide contamination from the 216-B-5 site does not represent an unacceptable risk to off-site groundwater users. The combination of sorption and radioactive decay of 90Sr and 137Cs should eliminate these radioisotopes from groundwater long before their discharge to receiving waters (the Columbia River, which is 4-5 km from the site). The 239, 240Pu seems to be immobilized by reactions with sediments near the injection point. DOE proposed to regulators that the stationary plume from this site continue to be monitored to verify long-term radionuclide containment by geochemical reaction and radioactive decay (DOE-RL, 1996).
SUMMARY: APPROPRIATE CIRCUMSTANCES FOR CONSIDERING NATURAL ATTENUATION
Many natural processes can affect the movement and fate of contaminants in groundwater. Biodegradation and some chemical transformations can destroy contaminants. Sorption and precipitation can immobilize contaminants. Advection, dispersion, volatilization, acid-base reactions, and aqueous complexation, while not destroying or immobilizing contaminants, can affect the reactions that contribute to natural attenuation.
All of these processes can help to reduce contaminant concentrations to levels that are below established regulatory criteria.
Table 3-6 summarizes the dominant processes affecting the fate of the different contaminant classes discussed in this chapter. The dominant attenuation processes are those that are likely to be most important in the destruction or immobilization of a contaminant. Several other attenuation processes may occur for a given contaminant, but those listed are the major ones.
The level of understanding of the dominant processes for a given contaminant is divided into three categories: high, moderate, and low. A high level of understanding indicates that comprehensive scientific studies are available and include field evidence. A moderate level of understanding indicates studies confirm that the dominant attenuation process occurs. Low understanding indicates that the attenuation processes may have been observed, but the level of scientific understanding of the processes involved is insufficient to judge whether natural attenuation can achieve regulatory standards for protection of public health and the environment.
The likelihood of success of natural attenuation is a judgment based on the level of understanding of the dominant attenuation processes and the probability that site-specific conditions will result in effective natural attenuation. The likelihood of success is high when the level of understanding is high and the conditions for successful natural attenuation are relatively common. In contrast, if the level of understanding is low, the likelihood of successfully documenting natural attenuation also is low. Further, a low rating is given to poorly understood contaminants because of the chance that the contaminant could transform to another hazardous product. The likelihood that natural attenuation will be sufficiently protective also is low if the dominant attenuation processes require special environmental conditions that are not likely to occur at most sites. Sites rated according to Table 3-6 as having a low likelihood of success might still be candidates for natural attenuation. However, evidence of success will usually require high levels of effort in site characterization, laboratory studies, modeling, and monitoring.
In applying Table 3-6, it is important to keep in mind that natural attenuation processes are always site specific: they depend on the hydrogeology and biogeochemistry of the site in question. Furthermore, mixtures of contaminants behave very differently from single contaminants because of the many interconnecting processes involved. Finally, some processes transform contaminants to forms that are less harmful to humans and the environment, but others form products that are more hazardous or are more mobile in the environment than the parent contaminant. As a consequence, Table 3-6 can serve as a general guide for
TABLE 3-6 Likelihood of Success of Natural Attenuation
|
Chemical Class |
Dominant Attenuation Processes |
Current Level of Understandinga |
Likelihood of Success Given Current Level of Understandingb |
|
Organic |
|
||
|
Hydrocarbons |
|
||
|
BTEX |
Biotransformation |
High |
High |
|
Gasoline, fuel oil |
Biotransformation |
Moderate |
Moderate |
|
Nonvolatile aliphatic compounds |
Biotransformation, immobilization |
Moderate |
Low |
|
Polycyclic aromatic hydrocarbons |
Biotransformation, immobilization |
Moderate |
Low |
|
Creosote |
Biotransformation, immobilization |
Moderate |
Low |
|
Oxygenated hydrocarbons |
|
||
|
Low-molecular-weight alcohols, ketones, esters |
Biotransformation |
High |
High |
|
MTBE |
Biotransformation |
Moderate |
Low |
|
Halogenated aliphatics |
|
||
|
Tetrachloroethene, trichloroethene, carbon tetrachloride |
Biotransformation |
Moderate |
Low |
|
Trichloroethane |
Biotransformation, abiotic transformation |
Moderate |
Low |
|
Methylene chloride |
Biotransformation |
High |
High |
|
Vinyl chloride |
Biotransformation |
Moderate |
Low |
|
Dichloroethene |
Biotransformation |
Moderate |
Low |
|
Halogenated aromatics |
|
||
|
Highly chlorinated |
|
||
|
PCBs, tetrachlorodibenzofuran, pentachlorophenol, multichlorinated benzenes |
Biotransformation, immobilization |
Moderate |
Low |
|
Less chlorinated |
|
||
|
PCBs, dioxins |
Biotransformation |
Moderate |
Low |
|
Monochlorobenzene |
Biotransformation |
Moderate |
Moderate |
|
Nitroaromatics |
|
||
|
TNT, RDX |
Biotransformation, abiotic transformation, immobilization |
Moderate |
Low |
|
Chemical Class |
Dominant Attenuation Processes |
Current Level of Understandinga |
Likelihood of Success Given Current Level of Understandingb |
|
Inorganic |
|
||
|
Metals |
|
||
|
Ni |
Immobilization |
Moderate |
Moderate |
|
Cu, Zn |
Immobilization |
Moderate |
Moderate |
|
Cd |
Immobilization |
Moderate |
Low |
|
Pb |
Immobilization |
Moderate |
Moderate |
|
Cr |
Biotransformation, immobilization |
Moderate |
Low to moderate |
|
Hg |
Biotransformation, immobilization |
Moderate |
Low |
|
Nonmetals |
|
||
|
As |
Biotransformation, immobilization |
Moderate |
Low |
|
Se |
Biotransformation, immobilization |
Moderate |
Low |
|
Oxyanions |
|
||
|
Nitrate |
Biotransformation |
High |
Low |
|
Perchlorate |
Biotransformation |
Moderate |
Low |
|
Radionuclides |
|
||
|
60Co |
Immobilization |
Moderate |
Moderate |
|
137Cs |
Immobilization |
Moderate |
Moderate |
|
3H |
Decay |
High |
Moderate |
|
90Sr |
Immobilization |
High |
Moderate |
|
99Tc |
Biotransformation, immobilization |
Low |
Low |
|
238,239,240Pu |
Immobilization |
Moderate |
Low |
|
235,238U |
Biotransformation, immobilization |
Moderate |
Low |
|
NOTE: Knowledge changes rapidly in the environmental sciences. Some contaminants not rated as having high natural attenuation potential could achieve this status in the future, but this table represents the best understanding of natural attenuation potential at this time. a Levels of understanding: “high” means there is good scientific understanding of the processes involved, and field evidence confirms attenuation processes can protect human health and the environment. “Moderate” means studies confirm that the dominant attenuation process occurs, but the process is not well understood scientifically. “Low” means scientific understanding is inadequate to judge if and when the dominant process will occur and whether it will meet regulatory standards. b “Likelihood of success” relates to the probability that at any given site, natural attenuation of a given contaminant is likely to protect human health and the environment. “High” means scientific knowledge and field evidence are sufficient to expect that natural attenuation will protect human health and the environment at more than 75% of contaminated sites. “Moderate” means natural attenuation can be expected to meet regulatory standards at about half of the sites. “Low” means natural attenuation is expected to be protective at less than 25% of contaminated sites. A “low” rating can also result from a poor level of scientific understanding. |
|||
evaluating natural attenuation potential, but each site must be evaluated individually to determine whether natural attenuation is sufficiently protective of human health and the environment. Chapter 4 describes site evaluation strategies in detail.
CONCLUSIONS
-
Natural attenuation is well established as a remediation approach for only a few types of contaminants, primarily BTEX. For most other contaminant classes, it is not as likely to succeed or not well established. In some cases, the likelihood of success is low because natural attenuation depends on special environmental conditions that may not be present at the site. In other cases, scientific understanding is too limited to judge the potential of natural attenuation as a remedial alternative. Also, at some sites, the possible production of toxic intermediate compounds raises too many regulatory or public concerns about the long-term acceptability of the process.
-
Natural attenuation should never be considered a default or presumptive remedy. Although natural attenuation can be a technically valid means for protecting human health and the environment, its effectiveness must be documented at every site (even those contaminated with BTEX) overseen by environmental regulators.
-
At sites where natural attenuation is shown to be effective, long-term monitoring will be necessary to ensure that key attenuation processes continue to control contamination. To achieve remediation objectives, natural attenuation may have to continue for many years or decades, over which time environmental conditions and natural attenuation processes may change.
-
Natural attenuation of some compounds can form hazardous by-products that in some cases can persist in the environment. Evidence of transformation of a contaminant does not necessarily ensure detoxification.
-
Natural attenuation processes cannot destroy metals but in some cases can immobilize them. The passage of time can enhance or reverse immobilization reactions, depending on the type of reaction, the contaminant, and environmental conditions.
-
In some cases, removing contaminant sources can speed natural attenuation, but in other cases it can interfere with natural attenuation. Removing sources can reduce the mass of contamination that has to be treated by natural processes. However, in some cases it can cut off natural attenuation entirely, if the source is serving as critical fuel for attenuation processes.
REFERENCES
Abramowicz, D. A. 1990. Aerobic and anaerobic biodegradation of PCBs: A review. Critical Reviews in Biotechnology 10(3):241-249.
Anderson, J. E., and P. L. McCarty. 1997. Transformation yields of chlorinated ethenes by a methanotrophic mixed culture expressing particulate methane monooxygenase. Applied and Environmental Microbiology 63(2):687-693.
Anderson, R. T., and D. R. Lovley. 1997. Ecology and biogeochemistry of in situ groundwater bioremediation. Advances in Microbial Ecology 15:289-350.
Atlas, R. M., and R. Bartha. 1997. Microbial Ecology: Fundamentals and Applications, 4th Ed. Menlo Park, Calif.: Benjamin Cummings Publishing Co.
Babu, G. R. V., J. H Wolfram, and K. D. Chapatwala. 1992. Conversion of sodium cyanide to carbon dioxide and ammonia by immobilized cells of Pseudomonas putida. Journal of Industrial Microbiology 9:235-238.
Baedecker, M. J., D. I. Siegel, P. Bennett, and I. M. Cozzarelli. 1989. The fate and effects of crude oil in a shallow aquifer. I. The distribution of chemical species and geochemical facies. Pp. 13-20 in Millar, G. E., and S. E. Rabone (eds.) U.S. Geological Survey Water Resources Division Report 88-4220. Reston, Va.: U.S. Geological Survey.
Baedecker, M. J., J. M. Cozzarelli, D. I. Siegel, P. C. Bennett, and R. P. Eganhouse. 1993. Crude oil in a shallow sand and gravel aquifer. 3. Biogeochemical reactions and mass balance modeling in anoxic ground water. Applied Geochemistry 8:569-586.
Banaszak, J. E., D. T. Reed, and B. E. Rittmann. 1999. Subsurface interactions of actinide species and microorganisms: Implications on bioremediation of actinide-organic mixtures. Journal of Radioanalytical and Nuclear Chemistry 241:385-435.
Barkay, T., and B. H. Olson. 1986. Phenotypic and genotypic adaptation of aerobic heterotrophic sediment bacterial communities to mercury stress. Applied and Environmental Microbiology 52:403-406.
Barker, J. F., G. C. Patrick, and D. Major. 1987. Natural attenuation of aromatic hydrocarbons in a shallow sand aquifer. Ground Water Monitoring Review 7:64-71.
Barkovski, A. L., and P. Adriaens. 1996. Microbial dechlorination of historically present and freshly spiked chlorinated dioxins and diversity of dioxin-dechlorinating populations. Applied and Environmental Microbiology 62:4556-4562.
Bedard, D. L., and J. F. Quensen III. 1995. Microbial reductive dechlorination of polychlorinated biphenyls. Pp. 127-216 in Young, L.Y., and C. E. Cerniglia (eds.) Microbial Transformation and Degradation of Toxic Organic Chemicals. New York: Wiley-Liss, Inc.
Beller, H. R., D. Grbic-Galic, and M. Reinhard. 1992a. Microbial degradation of toluene under sulfate-reducing conditions and the influence of iron on the process. Applied and Environmental Microbiology 58:786-793.
Beller, H. R., M. Reinhard, and D. Grbic-Galic. 1992b. Metabolic by-products of anaerobic toluene degradation by sulfate-reducing enrichment cultures. Applied and Environmental Microbiology 58: 3192-3195.
Binks, P. R., S. Nicklin, and N. C. Bruce. 1995. Degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) by Stenotrophomonas maltophilia PB1. Applied and Environmental Microbiology 61:1318-1322.
Borden, R. C., R. A. Daniel, L. E. LeBrun, and C. W. Davis. 1997. Intrinsic biodegradation of MTBE and BTEX in a gasoline-contaminated aquifer. Water Resources Research 33:1105-1115.
Bouwer, E. J., B. E. Rittmann, and P. L. McCarty. 1981. Anaerobic degradation of halogenated 1- and 2-carbon organic compounds. Environmental Science and Technology 15(5):596-599.
Boyle, A. W., et al. 1992. Bacterial PCB biodegradation. Biodegradation 3(2/3):285-298.
Bradley, P. M., F. H. Chapelle, J. E. Landmeyer, and J. G. Schumacher. 1994. Microbial transformation of nitroaromatics in surface soils and aquifer materials. Applied and Environmental Microbiology 60:2170-2175.
Bradley, P. M., F. H. Chapelle, J. E. Landmeyer, and J. G. Schumacher. 1997. Potential for intrinsic bioremediation of a DNT-contaminated aquifer. Ground Water 35:12-17.
Brierley, C. L. 1990. Bioremediation of metal-contaminated surface and groundwaters. Geomicrobiology 8:201-224.
Brown, J. F., D. L. Bedard, M. J. Brennan, J. C. Carnahan, H. Feng, and R. E. Wagner. 1987. Polychlorinated biphenyl dechlorination in aquatic sediments. Science 236:709-712.
Brown, J.G., and J. W. Harvey. 1996. Hydrologic and geochemical factors affecting metal contaminant transport in Pinal Creek basin near Globe, Arizona. Pp. 1035-1042 in Morganwalp, D.W., and D. A. Aronson, D.A. (eds.) U.S. Geological Survey Toxic Substances Hydrology Program—Proceedings of the Technical Meeting, Colorado Springs, Colo., September 20-24. Geological Survey Water-Resources Investigations Report 94-4015. Vol. 2.
Brown, J. G., R. Brew, and J. W. Harvey. 1997. Research on Acidic Metal Contaminants in Pinal Creek Basin Near Globe, Arizona. FS-005-97. Reston, Va.: U.S. Geological Survey.
Brown, R. E., and H. G. Ruppert. 1950. The Underground Disposal of Liquid Wastes at the Hanford Works, Washington. HW-17088. Richland, Wash.: General Electric Company, Hanford Atomic Products Operation.
Cerniglia, C. E. 1992. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3(2/3):351-368.
Cerniglia, C. E. 1993. Biodegradation of polycyclic aromatic hydrocarbons. Current Opinion in Biotechnology 4(3):331-338.
Chapatwala, K. D., G. R. V. Babu, E. R. Armstead, E. M. White, and J. H. Wolfram. 1995. A kinetic study on the bioremediation of sodium cyanide and acetonitrile by free and immobilized cells of Pseudomonas putida. Applied Biochemistry and Biotechnology 51-52:717-726.
Chapelle, F. H. 1993. Ground-water Microbiology and Geochemistry. New York, N.Y.: John Wiley and Sons.
Chapelle, F. H., P. M. Bradley, D. A. Vroblesky, and D. R. Lovley. 1996. Measuring rates of biodegradation in a petroleum hydrocarbon-contaminated aquifer. Groundwater 34:691-698.
Chen, S., and D. B. Wilson. 1997. Genetic engineering of bacteria and their potential for Hg–2+ bioremediation. Biodegradation 8:97-103.
Coates, J. D., J. Woodward, J. Allen, P. Philip, and D. R. Lovley. 1997. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Applied and Environmental Microbiology 63:3589-3593.
Coleman, N. V., D. R. Nelson, and T. Duxbury. 1998. Aerobic biodegradation of hexachloro-1,3,5-trinitro-1,3,5-triazine (RDX) as a nitrogen source by a Rhodococcus sp., strain DN22. Soil Biology and Biochemistry 30:1159-1167.
Cozza, C. L., and S. L. Woods. 1992. Reductive dechlorination pathways for substituted benzenes: A correlation with electronic properties. Biodegradation 2(4):265-278.
Criddle, C., C. Elliott, P. L. McCarty, and J. F. Barker. 1986. Reduction of Hexachloroethane to Tetrachloroethylene in Groundwater. Journal of Contaminant Hydrology 1(1/2): 133-142
Diels, L. 1997. Heavy metal bioremediation of soil. Pp. 283-295 In Sheehan, J. (ed.) Methods in Biotechnology, 2. Bioremediation protocols. Totowa, N.J.: Humana Press, Inc.
Department of Energy (DOE). 1990. Subsurface Science Program: Program Overview and Research Abstracts. Office of Health and Environmental Research, Office of Energy Research. DOE/ER-0432. Washington, D.C.: DOE.
DOE-RL. 1996. 200-BP-5 Operable Unit Treatability Test Report. DOE/RL-95-59. U.S. Richland, Wash.: Department of Energy, Richland Operations Office.
Department of the Navy. 1998. Technical Guidelines for Evaluating Monitored Natural Attenuation at Naval and Marine Corps Facilities. Draft-Revision 2.
Dolan, M. E., and P. L. McCarty. 1995. Small column microcosm for assessing methane-stimulated vinyl-chloride transformation in aquifer samples. Environmental Science and Technology 29(8):1892-1897.
Domenico, P. A., and F. W. Schwartz. 1990. Physical and Chemical Hydrogeology. New York: John Wiley & Sons.
Durrant, G. C., M. Schirmer, M. D. Einarson, R. D. Wilson, and D. M. Mackay. 1999. Assessment of the dissolution of gasoline containing MTBE at LUST Site 60, Vandenberg Air Force Base, California. In Proceedings of the 1999 Conference on Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Detection, and Remediation, Houston, Tex., November 17-19. Westerville, Ohio: National Ground Water Association.
Ehrlich, H. L. 1996. Geomicrobiology, 3rd Ed. New York: Marcel Dekker.
Einarson, M. D., M. Schirmer, P. Pezeshkpour, D. M. Mackay, and R. D. Wilson. 1999. Comparison of eight innovative site characterization tools used to investigate an MTBE plume at Site 60, Vandenberg Air Force Base, California. In Proceedings of the 1999 Conference on Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Detection and Remediation, Houston, Tex., November 17-19. Westerville, Ohio: National Ground Water Association.
Elfantroussi, S., H. Naveau, and S. N. Agathos. 1998. Anaerobic dechlorinating bacteria. Biotechnology Progress 14(2):167-188.
Ellis, D. E., E. J. Lutz, G. M. Klecka, D. L. Pardiek, J. J. Salvo, M. A. Heitkamp, D. J. Gannon, C. C. Mikula, C. M. Vogel, G. D. Sayles, D. H. Kampbell, J. T. Wilson, and D. T. Maiers. 1996. Remediation Technology Development Forum intrinsic remediation project at Dover Air Force Base, Delaware. In Symposium on Natural Attenuation of Chlorinated Organics in Groundwater. EPA/540/R-96/509. Washington, D.C.: Environmental Protection Agency, Office of Research and Development.
Esteve-Nunez, A., and J. L. Ramos. 1998. Metabolism of 2,4,6,-trinitrotoluene by Pseudomonas sp JLR11. Environmental Science and Technology 32:3802-3808.
Evans, P. J., D. T. Mang, and L. Y. Young. 1991a. Degradation of toluene and m-xylene and transformation of o-xylene by denitrifying enrichment cultures. Applied and Environmental Microbiology 57:450-454.
Evans, P. J., D. T. Mang, K. S. Kim, and L. Y. Young. 1991b. Anaerobic degradation of toluene by a denitrifying bacterium. Applied and Environmental Microbiology 57:1139-1145.
Fetter, C. W. 1999. Contaminant Hydrogeology, 2nd Ed. Englewood Cliffs, N.J.: Prentice Hall.
Frankenberger, W. T., Jr., and M. E. Losi. 1995. Applications of bioremediation in the cleanup of heavy metals and metalloids. Pp. 173-210 in H. D. Skipper and R. F. Turco (eds.) Bioremediation Science and Applications. SSSA, Special Publication Number 43. Madison, Wisc.: SSSA.
Freedman, D. L., and J. M. Gossett. 1991. Biodegradation of Dichloromethane and Its Utilization as a Growth Substrate Under Methanogenic Conditions. Applied and Environmental Microbiology 57:2847-2857.
Freeze, R. A., and J. A. Cherry. 1979. Groundwater. Englewood Cliffs, N.J.: Prentice-Hall.
French, C. E., S. Nicklin, and N. C. Bruce. 1998. Aerobic degradation of 2,4,6-trinitrotoluene by Enterobacter cloacae PB2 and by pentaerythritol tetranitrate reductase. Applied and Environmental Microbiology 64:2864-2868.
Funk, S. B., D. L. Crawford, R. L. Crawford, G. Mead, and W. Davis-Hoover. 1995. Full-scale anaerobic bioremediation of trinitrotoluene (TNT) contaminated soil. Applied Biochemistry and Biotechnology 51-52:625-633.
Ghoshal, S., A. Ramaswami, and R. G. Luthy. 1996. Biodegradation of naphthalene from coal tar and heptamethylnonane in mixed batch systems. Environmental Science and Technology 30:1282-1291.
Grosso, N. R., L. P. Leitzinger, and C. Bartlett. 1999. Site Characterization of Area 6, Dover Air Force Base, in Support of Natural Attenuation and Enhance Bioremediation Projects. EPA/600/R-99/044. Springfield, Va.: National Technical Information Service.
Halden, R. U., and D. F. Dwyer. 1997. Biodegradation of dioxin-related compounds: A review. Bioremediation Journal 1(1):11-25.
Hanson, J. R., C. E. Ackerman, and K. M. Scow. 1999. Biodegradation of methyl tertiarybutyl ether by a bacterial pure culture. Applied and Environmental Microbiology 65:4788-4792.
Harkins, S. M., R. S. Truesdale, R. Hill, Hoffman, and P. S. Winters. 1988. U.S. Production of Manufactured Gases: Assessment of Past Disposal Practices. Research Triangle Institute report. EPA/600/2-88/012. Cincinnati, Ohio: U.S. Environmental Protection Agency.
Harkness, M. R., J. B. McDermott, D. A. Abramowicz, J. J. Salvo, W. P. Flanagan, M. L. Stephens, F. J. Mondello, R. J. May, J. H. Lobos, K. M. Carroll, M. J. Brennan, A. A. Bracco, K. M. Fish, G. L. Warmer, P. R. Wilson, D. K. Dietrich, D. T. Lin, C. B. Morgan, and W. L. Gately. 1993. In situ stimulation of aerobic PCB biodegradation in Hudson River sediments. Science 259:503-507.
Harvey, J. W., and C. Fuller. 1998. Effect of enhanced manganese oxidation in the hyporheic zone on basin-scale geochemical mass balance. Water Resources Research 34(4):623-636.
Haston, Z. C., P. K. Sharma, J. N. Black, and P. L. McCarty. 1994. Enhanced reductive dechlorination of chlorinated ethenes. Pp. 11-14 in Symposium on Bioremediation of Hazardous Wastes: Research, Development, and Field Evaluation. EPA/600/R-94/ 075. Washington, D.C.: U.S. Environmental Protection Agency.
Hinchee, R. E., J. L. Means, and D. R. Burris (eds.). 1995. Bioremediation of Inorganics. Columbus, Ohio: Battelle Press.
Holliger, C., G. Wohlfarth, and G. Diekert. 1998. Reductive dechlorination in the energy metabolism of anaerobic bacteria. Fems Microbiology Reviews 22(5):383-398.
Hutchins, S. R., G. W. Sewell, D. A. Kovacs, and G. A. Smith. 1991. Biodegradation of aromatic hydrocarbons by aquifer microorganisms under denitrifying conditions. Environmental Science and Technology 25:68-76.
Kalin, M., J. Cairns, and R. M. McCready. 1991. Ecological engineering methods for acid mine drainage treatment of coal wastes. Resources Conserv. Recycl. 5:265-276.
Kitts, C. L., D. P. Cunningham, and P. J. Unkeefer. 1994. Isolation of three hexahydro-1,3,5-trinitro-1,3,5-triazine-degrading species of the family Enterobacteriaceae from nitroamine explosive-contaminated soil. Applied and Environmental Microbiology 60:4608-4711.
Klier, N. J., G. M. Kelcka, E. J. Lutz, D. E. Ellis, F. H. Chapelle, and M. E. Witt. 1999. The Groundwater Geochemistry of Area 6, Dover Air Force Base, Dover, Delaware. EPA/ 600/R-99/051. Springfield, Va.: National Technical Information Service.
Kohler-Staub, D., S. Frank, and T. Leisinger. 1995. Dichloromethane as the sole carbon source for Hyphomicrobium sp. strain DM2 under denitrification conditions. Biodegradation 6:229-235.
Krumholz, L. R., J. Li, W. W. Clarkson, G. G. Wilber, and J. M. Suflita. 1997. Transformation of TNT and related amino toluenes in groundwater aquifer slurries under different electron-accepting conditions. Journal of Industrial Microbiology and Biotechnology 18:161-169.
Landmeyer, J. E., F. H. Chapelle, P. M. Bradley, J. F. Pankow, C. D. Church, and P. G. Tratnyek. 1998. Fate of MTBE relative to benzene in a gasoline-contaminated aquifer (1993-1998). Ground Water Monitoring Review 18(4):93-102
Lee and Ro. 1998. Draft Installation Restoration Program (IRP) Remedial Investigation Report for IRP Site 60, Vandenberg Air Force Base, California. Lee and Ro, Inc., 1199 South Fullerton Road, City of Industry, CA 91748. September.
Lendvay, J. M., S. M. Dean, and P. Adrianes. 1998. Temporal and spatial trends in biogeochemical conditions at a groundwater-surface water interface: Implications for natural attenuation. Environmental Science and Technology 32(22):3472-3478.
Lenhard, R. J., R. S. Skeen, and T. M. Brouns. 1995. Contaminants at U.S. DOE sites and their susceptibility to bioremediation. Pp. 157-172 in Skipper, H. D., and R. F. Turco (eds.) Bioremediation Science and Applications. SSSA, Special Publication Number 43. Madison, Wisc.: SSSA.
Lewis, T. A., M. M. Ederer, R. L. Crawford, and D. L. Crawford. 1997. Microbial transformation of 2,4,6-trinitrotoluene. Journal of Industrial Microbiology and Biotechnology 18:89-96.
Lloyd J. R., and L. E. Macaskie. 1997. Microbially-mediated reduction and removal of technetium from solution. Research in Microbiology. 148:530-532.
Lloyd, J. R., J. Ridley, T. Khizniak, N. N. Lyalikova, and L. E. Macaskie. 1999. Reduction of technetium by biocatalyst characterization and use in a flow-through bioreactor. Applied and Environmental Microbiology 65:2691-2696.
Lovley, D. R. 1993. Dissimilatory metal reduction. Annual Review of Microbiology 47:263-290.
Lovley, D. R. 1995. Bioremediation of organic and metal contaminants with dissimilatory metal reduction. Journal of Industrial Microbiology 14:85-93.
Lovley, D. R. 1997. Potential for anaerobic bioremediation of BTEX in petroleum-contaminated aquifers. Journal of Industrial Microbiology and Biotechnology 18:75-81.
Lovley, D. R., and D. J. Lonergan. 1990. Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism, GS-15. Applied and Environmental Microbiology 56:1858-1864.
Lovley, D. R., M. J. Baedecker, D. J. Lonergan, I. M. Cozzarelli, E. J. P. Phillips, and D. I. Siegel. 1989. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339:297-299.
Luthy, R. G., D. A. Dzombak, C. A. Peters, S. B. Roy, A. Ramaswami, D. V. Nakles, and B. R. Nott. 1994. Remediating tar-contaminated soil at manufactured gas plant sites. Environmental Science and Technology 28:266A-276A.
Luthy, R. G., D. A. Dzombak, M. J. R. Shannon, R. Unterman, and J. R. Smith. 1997. Aqueous solubility of PCB congeners from an Aroclor and an Aroclor/hydraulic oil mixture. Water Research 31(3):561-573.
Mabey, W., and T. Mill. 1978. A critical review of hydrolysis of organic compounds in water under environmental conditions. Journal of Physical Chemistry Ref. Data 7(2):383-415.
Macaskie, L. E., and G. Basnakova. 1998. Microbially-enhanced chemosorption of heavy metals: A method for the bioremediation of solutions containing long-lived isotopes of neptunium and plutonium. Environmental Science and Technology 32:184-187.
Mackay, D. M, D. L. Freyberg, P. L. McCarty, P. V. Roberts, and J. A. Cherry. 1986. A natural gradient experiment on solute transport in a sand aquifer: I. approach and overview of plume movement. Water Resources Research 22(13):2017-2030.
Mackay, D.M., M.D. Einarson, R. D. Wilson, and M. Schirmer. 1998. Insights from Detailed Field Screening of a 1,700-foot-long MtBE Plume in California. Waterloo, Canada: University of Waterloo, Department of Earth Sciences.
Madigan, M. T., J. M. Martinko, and J. Parker. 1997. Biology of Microorganisms, 8th Ed. Upper Saddle River, N.J.: Prentice Hall.
Madsen, E. L., and Ghiorse, W. C. 1993. Groundwater microbiology: Subsurface ecosystem processes. Pp. 167-213 in Ford, T. (ed.) Aquatic Microbiology. Boston, Mass.: Blackwell Scientific Publications.
Madsen, E. L., J. L. Sinclair, and W. C. Ghiorse. 1991. In situ biodegradation: Microbiological patterns in a contaminated aquifer. Science 252:830-833.
Magli A., M. Messmer, T. Leisinger. 1998. Metabolism of dichloromethane by the strict anaerobe Dehalobacterium formicoaceticum. Applied and Environmental Microbiology 64:646-650
Malmqvist, A., T. Welander, and L. Gunnarsson. 1991. Anaerobic growth of microorganisms with chlorate as an electron acceptor. Applied and Environmental Microbiology 57:2229-2232.
McAllister, K. A., H. lee, and J. T. Trevors. 1996. Microbial degradation of pentachlorophenol. Biodegradation 7(1):1-40.
McCarty, P. L. 1993. In situ bioremediation of chlorinated solvents. Current Opinion in Biotechnology 4(3):323-330.
McCarty, P. L., and L. Semprini. 1994. Ground-water Treatment for Chlorinated Solvents. Pp. 87-116 in Norris R. D. (ed.) Handbook of Bioremediation. Boca Raton, Fla.: Lewis Publishers.
McCarty, P. L. 1997. Breathing with chlorinated solvents. Science 276:1521-1522.
McCarty, P. L. 1999. Chlorinated organics. Chapter 4 in Anderson, W. C., R. C. Loehr, and B. P. Smith (eds.) Environmental Availability in Soils, Chlorinated Organics, Explosives, Metals. Annapolis, Md.: American Academy of Environmental Engineers.
McCarty, P. L., and J. T. Wilson. 1992. Natural anaerobic treatment of a TCE plume, St. Joseph, Michigan, NPL site. Pp. 47-50 in Bioremediation of Hazardous Wastes. EPA/ 600/R-92/126. Cincinnati, Ohio: U.S. Environmental Protection Agency Center for Environmental Research Information.
McCarty, P. L., M. N. Goltz, G. D. Hopkins, M. E. Dolan, J. P. Allan, B. T. Kawakami, and T. J. Carrothers. 1998. Full-scale evaluation of in situ cometabolic degradation of trichloroethylene in groundwater through toluene injection. Environmental Science and Technology 32:88-100.
McHale, A. P., and S. McHale. 1994. Microbial biosorption of metals: Potential in the treatment of metal pollution. Biotechnological Advances 12:647-652.
McNally, D. L., J. R. Mihelcic, and D. R. Lueking. 1998. Biodegradation of three- and four-ring polycyclic aromatic hydrocarbons under aerobic and denitrifying conditions. Environmental Science and Technology 32:2633-2639.
McNulty, A. K. 1997. In Situ Anaerobic Dechlorination of PCBs in Hudson River Sediments. Master’s thesis. Rensselaer Polytechnic Institute.
Mihelcic, J. R., and R. G. Luthy. 1988. Degradation of polycyclic aromatic hydrocarbon compounds under various redox conditions in soil-water systems. Applied and Environmental Microbiology 54:1182-1187.
Mo, K., C. O. Lora, A. E. Wurken, M. Javanmordian, X. Yang, and C. F. Kulpa. 1997. Biodegradation of methyl t-butyl ether by pure bacterial cultures. Applied Microbiology and Biotechnology 47:69-72.
Mohn, W. W., and J. W. Tiedje. 1992. Microbial reductive dehalogenation. Microbiological Reviews 56(3):482-507.
Morgan, P., S. T. Lewis, and R. J. Watkinson. 1993. Biodegradation of benzene, toluene, ethylbenzene and xylenes in gas-condensate-contaminated groundwater. Environmental Pollution 82:181-190.
Mormile, M. R., S. Liu, and J. M. Suflita. 1994. Anaerobic biodegradation of gasoline oxygenates: Extrapolation of information to multiple sites and redox conditions. Environmental Science and Technology 28:1727-1732.
NRC (National Research Council). 1993. In Situ Bioremediation: When Does It Work? Washington, D.C.: National Academy Press.
NRC. 1994. Alternatives for Ground Water Cleanup. Washington, D.C.: National Academy Press.
NRC. 1997. Innovations in Ground Water and Soil Cleanup: From Concept to Commercialization. Washington, D.C.: National Academy Press.
NRC. 1999. Groundwater and Soil Cleanup: Improving Management of Persistent Contaminants. Washington, D.C.: National Academy Press.
Ortiz, E., M. Kraatz, and R. G. Luthy. 1999. Organic phase resistance to dissolution of polycyclic aromatic hydrocarbon compounds. Environmental Science and Technology 33(2):235-242.
Pankow, J. F., and J. A. Cherry. 1996. Dense Chlorinated Solvents and other DNAPLs in the Groundwater. Portland, Or.: Waterloo Press.
Pennington, J. C. 1999. Explosives. Pp. 85-109 in Anderson, W. C., R. C. Loehr, and B. P. Smith (eds.) Environmental Availability of Chlorinated Organics, Explosives, and Metals in Soils. Annapolis, Md.: American Academy of Environmental Engineers.
Peters, C. P., and R. G. Luthy. 1993. Coal dissolution in water-miscible solvents: Experimental evaluation. Environmental Science and Technology 27:2831-2843..
Quensen, J. F. III, J. M. Tiedje, and S. A. Boyd. 1988. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science 242:752-754.
Reinhard, M., S. Shang, P. K. Kitanidis, E. Orwin, G. O. Hopkins, and C. A. Lebron. 1997. In situ BTEX biotransformation under enhanced nitrate- and sulfate-reducing conditions. Environmental Science and Technology 31:28-36.
Rhodes, E. O. 1966. Chapter 1 in Hoiberg, A.J. (ed.) History of Coal Tar and Light Oil. Huntington: Krieger.
Rice, D. W., R. D. Gorse, J. C. Michaelsen, B. P. Dooher, D. H. MacQueen, S. J. Cullen, W. E. Kastenberg, L. G. Everett, and M. A. Marino. 1995. California leaking underground fuel tank (LUFT) historical case analyses. Report UCRL-AR 122207. Livermore, Calif.: Lawrence Livermore National Laboratory.
Rittmann, B. E., E. Seagren, B. A. Wrenn, A. J. Valocchi, C. Ray, and L. Raskin. 1994. In Situ Bioremediation. Park Ridge, N.J.: Noyes Publications.
Rittmann, B. E., and P. L. McCarty. 2000. Environmental Biotechnology: Principles and Applications. New York: McGraw-Hill Book.
Roberts, D. R., A. M. Scheidegger, and D. L. Sparks. 1999. Kinetics of mixed Ni-Al precipitate formation on a soil clay fraction. Environmental Science and Technology 33(21):3749-3754.
Roberts, P. V., M. Reinhard, and P. L. McCarty. 1980. Organic contaminant behavior during groundwater recharge. Journal Water Pollution Control Federation 52:161-172.
Safe, S. H. 1994. Polychlorinated-biphenyls (PCBs): Environmental impacts; biochemical and toxic responses; and implications for risk assessment. Critical Reviews in Toxicology 24(2):87-149.
Salanitro, J. P. 1993. The role of bioattenuation in the management of aromatic hydrocarbon plumes in aquifers. Ground Water Monitoring Review 13:150-161.
Salanitro, J. P., L. A. Diaz, M. P. Wiliams, and H. L. Wisniewski. 1994. Isolation of a bacterial culture that degrades methyl t-butyl ether. Applied and Environmental Microbiology 60:2593-2596.
Saouter, E., M. Gillman, and T. Barkay. 1995. An evaluation of mer-specified reduction of ionic mercury as a remedial tool of a mercury-contaminated freshwater pond. Journal of Industrial Microbiology 14:343-348.
Schlegel, H. G., and H. W. Jannasch. 1992. Prokaryotes and their habitats. Pp. 75-125 in Balows, A., H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (eds.) The Prokaryotes, 2nd Ed. New York: Springer-Verlag.
Schlesinger, W. H. 1991. Biogeochemistry: An Analysis of Global Change. New York: Academic Press.
Semprini, L. 1997a. In situ transformation of halogenated aliphatic compounds under anaerobic conditions. Pp. 429-450 in Ward, C. H., J. A. Cherry, and M. R. Scalf (eds.) Subsurface Restoration. Chelsea, Mich.: Ann Arbor Press.
Semprini, L. 1997b. Strategies for aerobic co-metabolism of chlorinated solvents. Current Opinion in Biotechnology 8(3):296-308.
Semprini, L., P. K. Kitanidis, D. H. Kampbell, and J. T. Wilson. 1995. Anaerobic transformation of chlorinated aliphatic hydrocarbons in a sand aquifer based on spatial chemical distributions. Water Resources Research 31:1051-1062.
Smatlak, C. R., J. M. Gossett, and S. H. Zinder. 1996. Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environmental Science and Technology 30:2850-2858.
Spain, J. C. 1995. Biodegradation of nitroaromatic compounds. Annual Review of Microbiology 49:523-555.
Steffan, R. J., K. McClay, S. Vainberg, C. W. Condee, and D. Zhang. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane oxidizing bacteria. Applied and Environmental Microbiology 63:4216-4222.
Stephens, D. B. 1995. Vadose Zone Hydrology. Boca Raton, Fla.: Lewis Publishers.
Stollenwerk, K. G. 1994. Geochemical interactions between constituents in acidic groundwater and all uvium in an aquifer near Globe, Arizona. Applied Geochemistry 9(4):353-369.
Stumm, W., and J. J. Morgan. 1996. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd Ed. New York: John Wiley & Sons.
Summers, A. O. 1992. The hard stuff: metals in bioremediation. Current Opinions in Biotechnology 3:271-276.
Taylor, B., D. Mauro, J. Foxwell, J. Ripp, and T. Taylor. 1996. Characterization and Monitoring Before and After Source Removal at a Former Manufactured Gas Plant (MGP) Disposal Site, EPRI TR-105921. Palo Alto, Calif.; Electric Power Research Institute.
Thompson, H. A., G. A. Parks, and G. E. Brown, Jr. 1999a. Ambient-temperature synthesis, evolution, and characterization of cobalt-aluminum hydrotalcite-like solids. Clays and Clay Minerals 47:425-438.
Thompson, H. A., G. A. Parks, and G. E. Brown, Jr. 1999b. Dynamic intereactions of dissolution, surface adsorption, and precipitation in an aging cobalt(II)-clay-water system. Geochimica et Cosmochimica Acta. 63(11-12):1767-1779.
Thompson-Eagle, E. C., and W. T. Frankenberger, Jr. 1992. Bioremediation of soils contaminated with selenium. Advances in Soil Science 17:261-310.
Tiedje, J. M. 1995. Approaches to the comprehensive evaluation of prokaryote diversity of a habitat. Pp. 73-88 In Allsopp, D., R. R. Colwell, and D. l. Hawksworth (eds.) Microbial Diversity and Ecosystem Function. Cambridge, United Kingdom: University Press.
Tiedje, J. M., et al. 1993. Microbial reductive dechlorination of PCBs. Biodegradation 4(4):231-240.
Van Denburgh, A. S., D. F. Goerlitz, and E. M. Godsy. 1993. Depletion of nitrogen-bearing explosive wastes in a shallow ground-water plume near Hawthorne, Nevada. USGS Toxic Substances Hydrology Program, Technical Meeting Colorado Springs, Colo. September 20-24.
van Loosdrecht, M. C. M., J. Lyklema, W. Norde, and A.J.B. Zehnder. 1990. Influence of interfaces on microbial activity. Microbiology Review 54:75-87.
Videla, H. A., and W. G. Characklis. 1992. Biofouling and microbially influenced corrosion. Biodegradation 29:195-212.
Vogel, T. M., and D. Grbic-Galic. 1986. Incorporation of oxygen from water into toluene and benzene during anaerobic fermentative transformation. Applied Environmental Microbiology 5:200-202.
Wackett, L. P., M. S. P. Logan, F. A. Blocki, and C. Bao-li. 1992. A mechanistic perspective on bacterial metabolism of chlorinated methanes. Biodegradation 3(1):19-36.
Waksman, S. A. 1927. Principles of Soil Microbiology. Baltimore, Md.: Williams & Wilkins.
Whitlock, J. L. 1990. Biological detoxification of precious metal processing wastewaters. Geomicrobiology Journal 8:241-249.
Williams, C. J., D. Aderhold, and R. G. J. Edyvean. 1998. Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Research 32:216-224.
Wilson, B. H., J. T. Wilson, D. H. Kampbell, B. E. Bledsoe, and J. M. Armstrong. 1990. Biotransformation of monoaromatic and chlorinated hydrocarbons at an aviation gasoline spill site. Geomicrobiology Journal 8:225-240.
Wilson, J. T., and B. H. Wilson. 1985. Biotransformation of trichloroethylene. Applied and Environmental Microbiology 49(1): 242-243.
Wilson, J. T., J. W. Weaver, and D. H. Kampbell. 1994. Intrinsic bioremediation of TCE in ground water at an NPL Site in St. Joseph, Michigan. In Symposium on Intrinsic Bioremediation of Ground Water. EPA/540/R-94/515. Washington, D.C.: Environmental Protection Agency Office of Research and Development.
Wilson, M. S., and E. L. Madsen. 1996. Field extraction of a unique intermediary metabolite indicative of real time in situ pollutant biodegradation. Environmental Science and Technology 30:2099-2103.
Wilson, M. S., C. Bakermans, and E. L. Madsen. 1999. In situ, real time catabolic gene expression: Extraction and characterization of catabolic mRNA transcripts from groundwater. Applied and Environmental Microbiology 65:80-87.
Yang, Y., and P. L. McCarty. 1998. Competition for hydrogen within a chlorinated solvent dehalogenating mixed culture. Environmental Science and Technology 32(22):3591-3597.
Zhang, X., and L. Y. Young. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic bacteria. Applied and Environmental Microbiology 63:4759-4764.