1
Understanding Nutrient Over-enrichment: An Introduction
Over the last 20 years, there has been agrowing awareness that coastal ecosystems have been experiencing a number of environmental problems that can be attributed to the introduction of excess nutrients. At first glance, many of the diverse problems may seem unrelated and their causes are often not readily apparent. However, there is growing evidence that events such as the deaths of unusually large numbers of sea lions and manatees, unusual patterns of coral reef destruction, widespread fish kills, outbreaks of certain shellfish poisonings, disappearance of seagrasses, and the occurrence of the so-called “dead zone” in the Gulf of Mexico actually have much more in common than originally thought. All of these events reflect both subtle and not-so-subtle changes in the relative and absolute abundance of certain organisms near the very base of the food web. The abundance of these organisms is related, sometimes directly and at other times indirectly, to nutrients flowing into the system from upstream watersheds.
All living things must take in nutrients, respire, synthesize new organic molecules, and eliminate waste. The base of the food web that supports the majority of life on the planet is founded on the ability of photosynthetic organisms to take in nutrients and use the energy of sunlight to produce new organic matter. As takes place in yards, gardens, farms, and forests around the world, photosynthetic organisms in marine environments take in carbon, oxygen, nitrogen, phosphorus, and other elements in varying amounts and use sunlight to produce the simple and complex organic molecules necessary for life.
Each species of photosynthetic marine life uses these elements in specific ratios and concentrations and makes use of specific portions of the light spectrum of the sun, and thus each thrives in slightly differing conditions. If sunlight, nutrients, or any environmental conditions are inadequate to support the growth of these organisms, such conditions are commonly referred to as “limiting.” That is, the lack of sunlight or nutrients or other factor limits the growth of that organism. When the limiting factor is available in adequate amounts, either naturally or by human activity, photosynthetic organisms grow and multiply until some new limiting condition is encountered. Given adequate nutrients, some organisms may multiply until, through their sheer numbers, they shade themselves and cause light limitation.
One fundamental challenge for scientists and the managers responsible for implementing activities to prevent or reduce coastal nutrient over-enrichment is understanding this complex chain of events and impacts. They must develop an understanding of how natural and human modification of the environment influences the functioning of coastal ecosystems, especially how changes in overall quantity and relative abundance of basic life-sustaining parameters affect populations of aquatic organisms and species composition (the relative number and types of species making up a given ecosystem). This is of particular importance when changes in the total or relative abundance of organisms have adverse impacts on the environmental quality of biologically rich coastal waters.
NUTRIENT OVER-ENRICHMENT IN COASTAL WATERS
The coastal regions of the United States are economically vital areas, supporting a diverse range of industries and large population centers. The nation’s coasts—in this report defined to include terrestrial areas located immediately landward of the coastline, the ocean-land interface (including beaches, estuaries, and nearshore marine areas along the coast), and the shallow coastal ocean just offshore of the ocean-land interface—are complex environments characterized by rich biological diversity and natural resources. As society has increasingly populated the coasts, vacationed at the beaches, dammed the rivers feeding the beaches and coasts, harvested fish, disposed of waste, and used these areas for transportation, the deterioration of the coastal environment has become a critical issue.
Although U.S. coastal counties account for only 17 percent of the U.S. landmass, population in these counties exceeds 141 million (U.S. Bureau of the Census 1998). This means that over half of the U.S. population lives in less than one fifth of its total area, and this pattern is expected to continue. For example, 17 of the 20 fastest growing counties are located
along the coast. Nearly 14,000 new housing units are built in coastal counties every week (NOAA 1998). Beaches have become one of the largest vacation destinations in America, with 180 million people visiting the coast every year (Cunningham and Walker 1996). This increase in recreational use, together with the impact of larger year-round populations, demonstrates the high importance individuals place on the environmental quality of coastal areas. But people’s love of the coast puts increasing stress on coastal ecosystems and makes management of coastal areas increasingly challenging. These areas face a variety of major environmental problems, including habitat modification, degraded water resources, toxic contamination, introduction of non-indigenous species, shoreline erosion, and increased vulnerability to storms and tsunamis.
One of the most significant problems, however, is nutrient over-enrichment. Introduction of excess nutrients to these coastal areas leads to a number of impacts. One of the most common is eutrophication—that is, the process of increasing organic enrichment of an ecosystem where the increased supply of organic matter causes changes to that system (Nixon 1995). In coastal ecosystems, eutrophication can lead to excessive, and sometimes toxic, production of algal biomass (including red and brown tides); loss of important nearshore habitat such as seagrass beds (caused by light reduction); changes in marine biodiversity and species distribution; increased sedimentation of organic particles; and depletion of dissolved oxygen (hypoxia and anoxia). Furthermore, these effects can cause adverse impacts farther up the food web. For example, red tides and hypoxia can cause fish kills. Similarly, red tides and blooms of other toxic algae can harm marine mammals: sea lion deaths in California and manatee deaths in Florida have been linked to harmful algal blooms (Reguera et al. 1998; Scholin et al. 2000).
Human activities can greatly accelerate eutrophication by increasing the natural movement of nutrients from inland watersheds to coastal water bodies. The sources of these nutrients include agricultural practices, wastewater treatment plants, urban runoff, and burning of fossil fuels. Human activities on land affect both the quantity and quality (including nutrient content) of freshwater delivered to coastal areas. Because these factors play such a role in eutrophication, any approach to understanding and reducing the impacts of nutrient over-enrichment must consider freshwater inflow and nutrient loading patterns.
The extent and impacts of nutrient over-enrichment in coastal ecosystems are far-reaching: eutrophication-related oxygen-poor waters extend over an area as large as 20,000 km2 on the inner continental shelf of the northern Gulf of Mexico (Rabalais et al. 1999), with significant impacts on important fisheries (Council for Agricultural and Science Technology 1999). Other major ecosystems at risk include Chesapeake Bay, Long
Island Sound, the Neuse-Pamlico Estuary, and San Francisco Bay, as well as portions of the Baltic, North, and Black seas in Europe and estuaries and bays in Australia, Japan, and elsewhere around the globe.
PURPOSE OF THIS STUDY
The problems caused by nutrient over-enrichment are significant and likely to increase as human use of inorganic fertilizers and fossil fuels—the two dominant sources of nutrients—continues to intensify, at least on a global basis. Much remains to be learned about the geographic extent and severity of eutrophication, the relative susceptibility of different coastal ecosystems, and the most effective nutrient control strategies. There is also a great need to better translate scientific knowledge into effective policy and management strategies, which requires an understanding of the complex oceanic, estuarine, and watershed processes that contribute to eutrophication. With this better understanding, more effective techniques can be developed for reducing and preventing nutrient pollution, eutrophication, and associated impacts.
To provide advice to federal, state, and local government agencies charged with addressing the growing problems associated with nutrient over-enrichment, the National Research Council (NRC) created the Committee on the Causes and Management of Coastal Eutrophication (Appendix A). The committee was asked to review the current knowledge of watershed, estuarine, and coastal processes and their roles in eutrophication and to assess past and ongoing efforts to monitor and evaluate water quality on a variety of scales. Based on this review, the committee was then charged (Appendix A) with: (1) recommending ways to help coastal and watershed managers achieve meaningful reductions in the impacts of nutrient over-enrichment in the near-term, and (2) identifying areas where future efforts hold the promise of long-term reductions in nutrient over-enrichment and its effects.
Ongoing federal efforts to address this problem are extensive and complex. The National Oceanic and Atmospheric Administration (NOAA) and the Environmental Protection Agency (EPA) are primarily responsible for research, policymaking, and management related to eutrophication, in part through the tools provided by the Clean Air, Clean Water, and Coastal Zone Management Acts (Box 1-1). However, other significant activities are under way at other agencies. For example, the U.S. Geological Survey (USGS) has important scientific and data-collection responsibilities, particularly with regard to monitoring of the nation’s streams and rivers. The U.S. Department of Agriculture (USDA) has a long history of addressing pollution from agriculture. The National Science Foundation (NSF) funds research into ecological and biological processes related to
|
BOX 1-1 Three major pieces of legislation—the Clean Water Act (Water Pollution Control Act, PL 92-500), Clean Air Act (Air Pollution Prevention and Control Act, PL 91-604), and Coastal Zone Management Act (PL 92-583)—contain elements that directly address the causes and effects of nutrient over-enrichment, and these are described below. Other relevant federal, state, and local programs are described in Appendix C. Clean Water Act The Water Pollution Control Act, or Clean Water Act, is the primary federal law addressing pollution in lakes, rivers, and coastal waters. The goal of the act is “to restore and maintain the chemical, physical, and biological integrity of the nation’s waters.” The federal government, through EPA, sets basic water quality criteria. States are responsible for development of standards from these criteria, and for implementation of these standards. Each state sets effluent limitations for pollutant sources and sets water quality standards for water bodies, but they are required to be at least as stringent as the criteria established by EPA. The Clean Water Act has been up for reauthorization since the end of 1990. A primary recommendation of the National Research Council’s Committee on Watershed Management (NRC 1999a) was to encourage reauthorization of the Act to “allow bottom-up development of watershed agencies that respond to local problems rather than having a rigid institutional structure imposed upon them from the federal level,” a recommendation strongly supported by the Committee on the Causes and Management of Coastal Eutrophication. Clean Air Act The Air Pollution Prevention and Control Act of 1977, commonly called the Clean Air Act, was enacted to maintain and improve air quality in the United States. In recent years, it has become clear that air pollution has a significant impact on water quality through atmospheric deposition of various compounds including toxins and nutrients, and consequently this act has taken on even broader significance. For some waters, including major east coast estuaries, implementation of the Clean Air Act and its amendments and revisions could be an important tool in a national effort to reduce nutrient over-enrichment. EPA estimated the potential benefits that would occur if stationary sources of atmospheric nitrogen oxides met proposed national ambient air quality standards for ozone and particulate matter (EPA 1998a). If the proposed standards were implemented, biologically useable nitrogen compounds deposited from the atmosphere in 12 estuaries studied would drop as much as 17 percent, depending on the location of the estuary and the stringency of the regulations. Estimated “avoided costs” (i.e., costs to implement stormwater or point source controls if atmospheric controls are not implemented) |
|
for these estuaries ranged from $152 million to $248 million, depending on the regulatory alternative (EPA 1998a). As evidence presented in Chapter 5 demonstrates, previously published estimates of atmospheric deposition of nitrogen may be too low; thus, these “avoided costs” may be even greater. Coastal Zone Management Act The Coastal Zone Management Act of 1972 establishes a partnership between federal and state governments for management of the coast. States develop and implement coastal zone management programs with enforceable policies designed to meet national objectives. The federal government provides funds to implement these programs and requires federal agencies to act consistently with federally approved state programs (Millhouser et al. 1998). To obtain federal approval of their coastal zone management programs, states must define a coastal boundary, designate critical areas of concern based on a coastal resources inventory, and adopt enforceable policies covering their most important objectives. Over 99.7 percent, or 153,083 km, of U.S. shoreline is managed by federally approved state coastal zone management programs. The goal of the Coastal Zone Management Act is to protect and conserve the resources of the coastal zone by providing incentives and funding to coastal states (including those around the Great Lakes) to develop and implement management plans for their coastal areas. Unlike the Clean Water Act, participation by states in coastal planning is not compulsory. While the preservation of the coastal zone was the goal of this legislation, the writers recognized that the role of zoning and managing land and nearshore coastal areas was traditionally one of state and local jurisdiction. The act, therefore, provides for and encourages local decisions by allowing federal funding as an incentive for states to participate, based on the specific nature of many of the planning issues. |
nutrient over-enrichment. Together, these many federal efforts have the potential to offer significant resources to help citizens and regional, state, and local managers.
However, nutrient over-enrichment and eutrophication pose an extremely complex and variable problem that occurs at a number of scales. The complexity of sources, fates, and effects of nutrients coupled with associated socioeconomic and political issues mean that solutions will require coordinated local, state, regional, and national efforts and the involvement of an extremely varied range of stakeholders. Because of this complexity, the committee gave considerable thought to the potential audiences for this report and how best to convey the findings and recommendations. Four main audiences were identified:
-
Coastal and watershed managers—these individuals directly or indirectly influence how coastal areas and watersheds are managed, whether by formulating strategies to deal with local or regional problems or through various permitting responsibilities. Their decisions affect significant sectors of local and state economies.
-
Research scientists—these individuals (within academia, industry, and government), conduct research that strives to provide greater understanding and possible solutions to many of the problems faced by resource managers.
-
Federal agencies—these organizations are charged to implement federal policy and advance scientific understanding. Often, they act in support or oversight roles as the federal government works to ensure that local and state entities have the information needed to address environmental problems effectively. They also represent national priorities where they might conflict with local perspectives.
-
Congress and the White House—these entities set policy and delegate specific legal and administrative powers to federal agencies. They also control the fiscal and human resources required to implement programs.
These four audiences vary in their level of technical training and interest. Thus, the report has been divided into three sections. This first section, which includes Chapters 1 and 2, is an introduction to the central issues and an overview of how local actions, supported by state and federal agencies, can lead to nationwide reductions in the adverse effects of nutrient over-enrichment. This overview provides suggestions for how the nation can deal with nutrient over-enrichment effectively, including discussions of source reduction and control, policy design and goal setting, law and regulations, and coordination and communication issues. It is intended for a nontechnical audience. The remainder of the report, Sections II and III, provides a detailed treatment of the topics necessary to understand nutrient over-enrichment and plan actions to combat it. Section II, which includes Chapters 3, 4, 5, and 6, serves as a primer on nutrient enrichment and its impacts, including discussions of sources of nutrients and water body susceptibility. Section III, which includes Chapters 7, 8, and 9, then examines various abatement strategies. Chapter 7 looks in depth at the state of the science related to monitoring and modeling, which are critical for understanding the nature, extent, and impact of nutrient over-enrichment and developing mitigation strategies and goals. Chapters 8 and 9 then address the approaches available for setting and achieving effective water quality goals.
WHY IS NUTRIENT OVER-ENRICHMENT A PROBLEM?
Impacts of Nutrient Over-Enrichment
Coastal waters provide habitat for some of the most productive ecosystems on earth. These resources are in danger from eutrophication and other problems caused by excess inputs of nutrients, especially nitrogen and phosphorus. Because rivers transport the vast majority of nutrients reaching coastal waters, the concentration of land-borne nutrients tends to be high near the mouths of rivers. These areas of mixed fresh and marine water, referred to as estuaries, tend to be relatively slow moving and biologically rich water bodies.
Bricker et al. (1999) concluded that “Nearly all estuarine waters now exhibit some symptoms of eutrophication, though the scale, intensity, and impact may vary widely, the level of nutrient inputs required to produce the symptoms also varies.” This conclusion reflects, in part, the reality that coastal water bodies vary in susceptibility to nutrient loading (Box 1-2). As discussed in Chapter 6, many factors contribute to this variability. However, one of the most important appears to be the ability of the body to exchange water with the open ocean (which results in a reduced residence time for the nutrients to be taken up by local biota). Thus estuaries with low exchange rates with the ocean seem to be particularly vulnerable. Because watershed management offers real possibilities for reducing the nutrient runoff carried in rivers, estuaries can be the greatest benefactors of improved watershed management approaches. Although other marine environments are discussed, estuaries are the major focus of this report.
Nutrient over-enrichment can cause a range of economic and non-economic impacts, including eutrophication and associated anoxia and hypoxia, loss of seagrass beds and corals, loss of fishery resources, changes in ecological structure, loss of biotic diversity, and impairment of aesthetic enjoyment, each of which has associated costs. Impacts resulting from nutrient over-enrichment during a single summer can cost millions of dollars in lost revenue from tourism or harm to the seafood industry. Although the costs for a single high profile problem can be substantial, they may not appear to warrant significant changes in human behavior. However, when the market and non-market costs of multiple events (Box 1-3) are compiled over time and when local costs are aggregated over regions, it becomes clear why many coastal areas are willing to consider expensive options for reducing nutrient loading. For instance, a wastewater treatment plant capable of removing nutrients from wastewater can cost several million dollars and yet will only address a small part of the total nutrient load delivered from a watershed to an estuary.
|
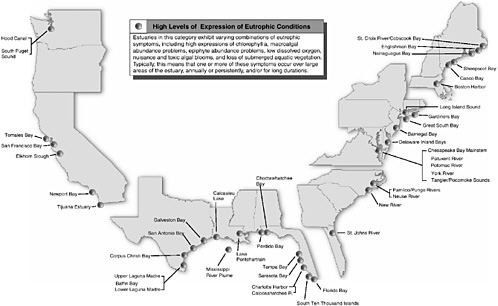
BOX 1-2 Although eutrophication is recognized as a growing problem in many of the nation’s estuaries and coastal areas, ranging from Long Island Sound to the Chesapeake Bay to the Mississippi delta region, the nation’s capability to respond has been limited by lack of knowledge about the extent, severity, and characteristics of eutrophication. To fill this void, NOAA designed the National Estuarine Eutrophication Assessment to gather consistent data nationwide and provide the basis for a national strategy for research, monitoring, and management of this pervasive problem. NOAA began collecting and synthesizing data and information about nutrient related water quality parameters in 1992. More than 300 federal, state, and academic scientists and environmental managers provided information for 138 estuaries and the Mississippi River Plume through a written survey and a series of work-shops. At a national synthesis workshop in 1999, data and information for several water quality parameters were integrated to arrive at an overall assessment of the eutrophic condition of each estuary. Evaluations were made regarding the influence of natural and human related factors in the development of these conditions, estuarine use impairments, and how conditions might change in the next 20 years. Participants made recommendations for management, research, and monitoring to address problems and to prevent worsening conditions. In 1999, NOAA published its synthesis report, The National Estuarine Eutrophication Assessment (Bricker et al. 1999), which provides a comprehensive summary of the assessment results. This report concludes that symptoms of eutrophication are present in many of the nation’s estuaries, with high expression of symptoms in roughly one-third of the estuaries studied (44 of the 139 sites studied; Figure 1-1). Furthermore, the report concludes that left unabated, two out of every three of the estuaries studied will have impaired use by 2020. Problems occur in estuaries along all coasts, but are most prevalent in estuaries along the Gulf of Mexico and mid-Atlantic coasts where human influences are substantial and exchange with the open ocean tends to be slow. |
|
BOX 1-3 The biological and ecological effects of nutrient over-enrichment reduce the value of the nation’s coastal waters to the country. That value derives both from the direct use of the resource for pursuits like recreation or commercial fishing (“use value”) and from the value that individuals place on the existence of a healthy coastal and marine environment, even if they do not directly use the resource (“non-use value”). This value is reduced when eutrophication degrades water quality. The fact that individuals value a coastal environment with high water quality implies that, even if they do not (and perhaps should not) have to pay for water quality improvements, there is some amount they would be willing to pay for those improvements. This “willingness-to-pay” is a measure of what the improvement is worth to them. Economists have devised various methodologies for estimating “willingness to pay,” and empirical studies show that individuals often place a high value on water quality improvements. For example, a study by Boyle et al. (1998) of lakefront property owners in Maine found that a one-meter improvement in water clarity (a measure of the eutrophic state of the lake) would increase the average property prices for lakefront property on selected lakes by $3,545 to $5,604. The average willingness to pay for this improvement was $3,765. Similarly, in a study of water quality in the Albemarle and Pamlico Sounds in North Carolina, Huang et al. (1997) found that on average, a single household would be willing to pay between $82 and $188 annually (depending on the assumptions and methods used) to restore water quality to 1981 levels. Of this, 55 percent is estimated to be derived from non-use value. While these estimates capture only a component of total value (e.g., that associated with lakefront use), they suggest that individuals place a high value on water quality improvements. |
Given the growing magnitude of the problem and the significance of the resources at risk, nutrient over-enrichment represents the greatest pollution threat faced by the coastal marine environment. The impacts of nutrient over-enrichment are discussed in detail in Chapter 4; however, a brief overview of the most significant and common impacts is included here.
Eutrophication
As noted earlier, eutrophication is the process of increasing organic enrichment of an ecosystem where the increased rate of supply of organic matter causes changes to that system (Nixon 1995; see Chapter 4). In moderation, increasing organic matter can sometimes be beneficial, such as when an increased rate of primary production leads to greater fishery production and, ultimately, increased harvests (Nixon 1988; Hansson and
Rudstam 1990; Rosenberg et al. 1990). However, far more often the impacts of high levels of nutrients are negative. Eutrophication can be one of many responses to the introduction of excessive amounts of nutrients. The general process known as eutrophication occurs in both freshwater lakes and in coastal marine ecosystems, with some similarities and some differences in what causes the problem and what impacts result. Marine systems that are most susceptible are those that have limited exchange with the adjacent ocean (e.g., fjords, estuaries, lagoons, and inland seas), but eutrophication can also occur on the continental shelf if nutrient inputs are sufficiently high (see Chapter 6).
Because increased organic content often results from nutrient over-enrichment, the term eutrophication is sometimes loosely used to describe a whole host of environmental problems that can result from nutrient over-enrichment of a system (Rosenberg 1985; Hinga et al. 1995). The distinction in this report between nutrient over-enrichment (cause) and eutrophication (effect) is an important one, as increased primary productivity is only one of many possible responses a coastal ecosystem may have to nutrient over-enrichment. For example, changes in relative abundance of certain nutrients may trigger adverse changes in the relative abundance of some species without triggering an overall increase in net primary productivity. Furthermore, the excessive primary production associated with eutrophication often leads to a secondary set of problems such as dissolved oxygen deficiency, or hypoxia (Box 1-4). Confusing cause and effect can impede mitigation efforts because proposed changes may not bring about desired effects.
What can be considered high in terms of nutrients will vary among systems and in relation to particular uses. The sensitivity of estuaries and coastal systems to accelerated nutrient inputs varies; currently, there is no widely accepted framework for classifying coastal ecosystems by their sensitivity. Mixing, stratification, flushing, dilution, depth, and other physical factors play a role in how sensitive a site is, as do biological factors such as the community structure.
Loss of Seagrasses and the Habitat They Form
Most coastal waters are shallow enough that benthic plant communities contribute significantly to primary production as long as sufficient light penetrates the water column to the seafloor. In good conditions, dense populations of seagrasses and perennial macroalgae can grow and attain rates of net primary production that are as high as the most productive terrestrial ecosystems (Charpy-Roubaud and Sournia 1990).
Benthic organisms such as seagrasses provide important habitat for many species of finfish and shellfish and help stabilize sediment on the
|
BOX 1-4 Each spring in the Gulf of Mexico, the oxygen levels near the bottom become too low to allow most fish and crustaceans to live in a vast region stretching from the Mississippi River westward along the Louisiana and Texas coasts, creating what has come to be called the “Dead Zone.” The cause is complex, but clearly related to nutrient over-enrichment. In essence, the process occurs because nutrients carried in the waters of the Mississippi River lead to rapid growth of phytoplankton in the Gulf, which in turn use up the available oxygen and lead to a condition known as hypoxia. The problem is exacerbated because when the Mississippi’s freshwater enters the sea, it floats over the denser, saltier water, resulting in a two-layered or “stratified” system. This stratification intensifies in the summer, as surface waters warm and the winds that normally mix the water subside, thus preventing the diffusion of oxygen from the surface waters to the lower layer. The low or non-existent oxygen levels drive away fish, shrimp, and crabs, and most bottom-dwellers such as snails, clams, starfish, and worms eventually die. Research indicates that the Dead Zone is caused by a combination of natural and human influences (Rabalais et al. 1991). For instance, the summer stratification is a natural condition. The key driver, however, is excess nutrients. Over the past four decades the amount of nitrogen delivered to the Mississippi River, and carried to the Gulf of Mexico, has tripled and phosphorus loads have doubled (Turner and Rabalais 1991; Justic et al. 1995; Goolsby et al. 1999). Nitrogen loads in the Mississippi Basin come from many sources: industrial discharge, urban runoff, atmospheric deposition, fertilizer runoff, animal wastes, and decomposition of leguminous crops. Over half the nitrogen can be attributed to agriculture, primarily runoff of nitrate from fertilizers (Howarth et al. 1996; Turner and Rabalais 1991). The key source areas are southern Minnesota, Iowa, Illinois, Indiana, and Ohio; streams draining Iowa and Illinois alone contribute on average about 35 percent of the total nitrogen ultimately discharged by the Mississippi River to the Gulf of Mexico (Goolsby et al. 1999). |
estuarine floor. Thus, extensive stands of seagrass not only indicate a healthy ecosystem but play an important role in preserving the environmental quality of estuaries and other coastal settings. These perennial macrophytes are less dependent on water column nutrient levels than phytoplankton and ephemeral macroalgae, and light availability is usually the most important factor controlling their growth (Sand-Jensen and Borum 1991; Dennison et al. 1993; Duarte 1995).
In temperate systems, perennial seagrasses largely obtain their nutrient requirements by using stored nitrogen pools, internal recycling, and nutrient sources in the sediment (Pedersen and Borum 1996). As a result, excess nutrient enrichment rarely stimulates these populations. Instead,
nutrient inputs cause a shift to less desirable phytoplankton or bloom-forming benthic macroalgae. Even in tropical waters, where seagrasses may be more limited by nutrient (phosphorus) availability (Short et al. 1990), fast-growing phytoplankton and macroalgae have more rapid nutrient uptake potential and can replace seagrasses as the dominant primary producers in enriched systems (Duarte 1995; Hein et al. 1995). These fast growing, “nuisance” macroalgae are typically filamentous or sheet-like forms (e.g., Ulva, Cladophora, Chaetomorpha) that can accumulate in extensive thick mats over the seagrass or sediment surface. Massive and persistent macroalgal blooms ultimately displace seagrasses and perennial macroalgae through shading effects (Valiela et al. 1997). In addition to causing the loss of important habitat, these nuisance macroalgae are usually unsightly (Chapter 4).
Harmful Algal Blooms
Harmful algal blooms (HAB) include, but are not restricted to, those events referred to as red or brown tides, and are characterized by the proliferation and occasional dominance of particular species of toxic or harmful algae. As with most phytoplankton blooms, this proliferation results from a combination of physical, chemical, and biological mechanisms and interactions that are, for the most part, poorly understood.
Among the thousands of species of microscopic algae at the base of the marine food web are a few dozen that produce potent toxins or that cause harm to humans and marine mammals, fisheries resources, or coastal ecosystems. These species make their presence known in a variety of ways, ranging from massive blooms of cells that discolor the water (giving rise to the term red or brown tide) to dilute, inconspicuous concentrations of cells noticed only because of the harm caused by their potent toxins. The impacts of these phenomena include mass mortalities of wild and farmed fish and shellfish; human illness or even death from contaminated shellfish or fish; alterations of marine trophic structure through adverse effects on larvae and other life history stages of commercial fisheries species; and death of marine mammals, seabirds, and other animals. HABs and related phenomena such as Pfiesteria outbreaks have attracted intense public and political attention (Box 1-5).
One major category of HAB impact occurs when toxic phytoplankton are filtered from the water as food by shellfish, which then accumulate the algal toxins to levels harmful or lethal to humans or other consumers (Shumway 1990). These poisoning syndromes have been given the names paralytic, diarrhetic, neurotoxic, and amnesic shellfish poisoning (PSP, DSP, NSP, and ASP). Whales, porpoises, seabirds, and other animals can be victims as well, receiving toxins through the food web via contami-
|
BOX 1-5 Prior to 1990, problems attributable to nutrient over-enrichment rarely made national news, but a once little-known species called Pfiesteria piscicida1 gained wide public attention in the 1990s and inadvertently served to increase public understanding of these types of problems. Interest began in May 1991, when a fish kill in the Ablemarle-Pamlico estuarine system in North Carolina was attributed to Pfiesteria piscicida (Burkholder 1997). But wide attention began in earnest in August 1997, when hundreds of dead and dying fish were found in a tributary to Chesapeake Bay, the Pocomoke River near Shelltown, Maryland, prompting state and local officials to close a portion of the river. Subsequent fish kills and observations of Pfiesteria-like organisms led to successive closing of segments of the Manokin and Chicamacomico rivers in Maryland. Soon, Maryland’s Department of Health and Mental Hygiene presented preliminary evidence that adverse human health effects could result from exposure to the toxins released by Pfiesteria piscicida or Pfiesteria-like organisms (Grattan et al. 1998). With the publicity, and despite the fact that the fish most commonly affected by Pfiesteria piscicida are Atlantic menhaden (a fish used primarily as an ingredient in animal feed), the local seafood industry suffered as restaurants and stores stopped selling Chesapeake Bay seafood (Weinraub 1997). In September 1997, the State of Maryland appointed a Citizens Pfiesteria Action Commission, which convened a forum of scientists to provide advice. The final report is referred to as the Cambridge Consensus (Maryland Department of Natural Resources 2000). The scientists discussed questions that had been raised in the scientific community concerning the relationships between Pfiesteria-like dinoflagellates (which included P. piscicida) and nutrients. After thorough analysis, they concluded there was a likely connection between nutrients, toxic outbreaks of Pfiesteria-like dinoflagellates, and fish kills. Also, they determined that it is improbable that toxic contaminants (such as pesticides and trace metals) are primarily responsible for outbreaks of Pfiesteria-like dinoflagellates. The scientists noted that while most evidence comes from North Carolina and environmental conditions vary, their findings apply to the mid-Atlantic region in general. Specifically, they found:
|
Based on their review, the scientists explained that excessive nutrient loading helps create an environment rich in the microbial prey and organic matter that both Pfiesteria and menhaden use as a food supply. By stimulating an increase in Pfiesteria concentrations, nutrient inputs increase the likelihood of a toxic outbreak when adequate numbers of fish are present. However, the presence of excess nutrients appears to be only one of many factors involved in Pfiesteria outbreaks. Stream hydraulics, water temperature, and salinity also seem to play important roles. The work done to understand Pfiesteria piscicida and Pfiesteria-like dinoflagellates has implications for managers concerned with reducing nutrients in marine systems. In the long term, decreases in nutrient loading will reduce eutrophication, thereby improving water quality, and in this context will likely lower the risk of toxic outbreaks of Pfiesteria and harmful algal blooms. However, even drastic decreases in nutrient loading will not completely eliminate the risks of toxic outbreaks of these organisms, which are indigenous species adapted to use toxins to attack fish when presented with the opportunity. While the outbreaks of Pfiesteria piscicida and Pfiesteria-like organisms do represent grounds for concern, the number and distribution have been relatively small compared with other impacts of nutrient over-enrichment. Thus, the Pfiesteria outbreaks to date may be most significant for the attention they have drawn to the larger threat posed by excess nutrient loading of freshwater and coastal systems nationwide. |
nated zooplankton or fish (Geraci et al. 1990). Another type of HAB impact occurs when marine animals are killed by algal species that release toxins and other compounds into the water, or that kill without toxins by physically damaging gills or by creating low oxygen conditions as bloom biomass decays. Farmed fish mortalities from HABs have increased considerably in recent years, and are now a major concern to fish farmers (and their insurance companies).
HABs have become more frequent and longer in duration in recent decades (Figure 1-2). Although not all HABs are caused by nutrient loading, many are at least in part associated with the general change in ecological structure that accompanies eutrophication. The causal mecha-
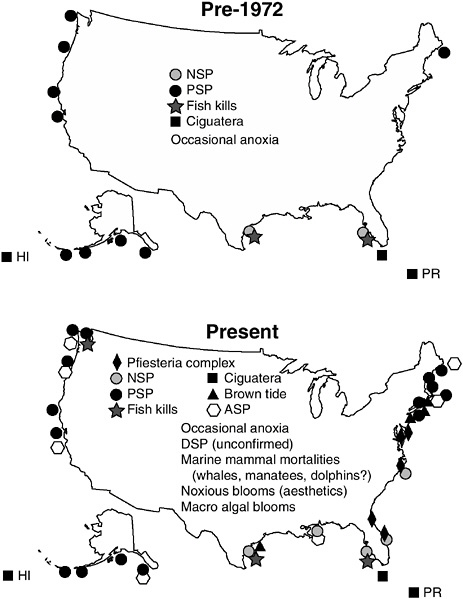
FIGURE 1-2 Expansion of harmful algal bloom (HAB) problems in the United States. These maps depict the HAB outbreaks known before and after 1972. This is not meant to be an exhaustive compilation of all events, but rather an indication of major or recurrent HAB episodes. In addition to the toxic impacts shown, harmful microalgal and macroalgal species have caused whale and other marine mammal mortalities, occasional anoxia, habitat destruction, and a general decline in coastal aesthetics in many coastal areas during the last 20 years. Neurotoxic shellfish poisoning = NSP, paralytic shellfish poisoning = PSP, and amnesic shellfish poisoning = ASP (Anderson 1995).
nisms for such blooms remain poorly known, and some blooms have always occurred and are entirely natural. However, other blooms are tied to nutrient availability, thus leading to more frequent and longer lasting blooms as human-induced nutrient over-enrichment becomes more common in coastal waters. Although reducing the overall availability of nutrients will reduce the likelihood of certain HABs, more research is needed to better understand the role of specific nutrients in the occurrence of various blooms and gain a complete understanding of anthropogenic influences and mitigation options.
Coral Reef Decline
Coral reefs are among the most productive and diverse ecosystems in the world. They grow as a thin veneer of living coral tissue on the outside of the hermatypic (reef-forming) coral skeleton. The world’s major coral reef ecosystems are distributed in nutrient-poor surface waters in the tropics and subtropics. Coral reefs are a paradox because their high gross productivity and biodiversity occur in waters with very low concentrations of dissolved and particulate nutrients. The abundant sunlight characteristic of the earth’s equatorial zones, supported by tight nutrient recycling within the coral-zooxanthellae2 symbiosis (Muscatine and Porter 1977), allows coral reefs to attain high rates of productivity. Thus, the worldwide decline in coral reefs is particularly disturbing. In the 1970s, offshore reefs in the Florida Keys were composed primarily of coral, and some contained more than 70 percent coral cover (Dustan 1977). But now, the “best” reefs have only about 18 percent coral cover. More resilient turf and macroalgae now dominate these reefs, accounting for 48 to 84 percent cover (Chiappone and Sullivan 1997).
Early references to coral reef ecosystems thriving in areas of upwelling or other nutrient sources were incorrect (Hubbard, D. 1997). It is now recognized that high nutrient levels are detrimental to reef health (Kinsey and Davies 1979). That view is supported by observations of phase shifts away from corals and coralline algae toward dominance by algal turf or macroalgae in coastal areas experiencing eutrophication from expanding human activities (Lapointe 1997).
Myriad other direct and indirect effects of coastal nutrient enrichment are known to affect coral reefs. One direct impact associated with elevated nutrients is decreased calcification, which results in dramatic decreases in the growth of reefs as a whole (Kinsey and Davies 1979; Marubini and Davies 1996). Indirect effects of nutrient over-enrichment
include increased phytoplankton biomass (Caperon et al. 1971) that alters the quality and quantity of particulate matter and the optical properties of the water column in a predictable fashion, with subsequent effects on reefs (Yentsch and Phinney 1989). Research has shown that outbreaks of the “Crown-of-Thorns” starfish in the South Pacific, which prey on living coral tissue, are related to the effects of nutrient-rich runoff on starfish larval development (Birkeland 1982). Because sea urchins and other marine herbivores are limited by dietary nitrogen (Mattson 1980), increased nitrogen availability, in particular, increases populations of these organisms. Because some organisms that increase in abundance in response to abnormally high nutrient levels, such as sponges and sea urchins, can damage reef formations (Glynn 1997), nutrient over-enrichment of coastal waters can ultimately lead to the destruction of both the reef framework and also adjacent shorelines due to increased erosion.
Controlling the Right Nutrients
The major nutrients that cause eutrophication and other adverse impacts associated with nutrient over-enrichment are nitrogen and phosphorus. Nitrogen is of paramount importance in both causing and controlling eutrophication in coastal marine ecosystems (Box 1-6). Other elements—particularly silicon and iron—may also be of importance in regulating HAB occurrences in coastal waters and in determining some of the consequences of eutrophication, but their importance with respect to nutrient over-enrichment in coastal waters is secondary to nitrogen.
|
BOX 1-6 The key to controlling eutrophication in freshwater systems is managing phosphorus inputs. Conversely, the key to controlling eutrophication in marine systems is managing nitrogen inputs. This conclusion follows significant debate, and even now some policymakers and the press continue to question the relative role of nitrogen versus phosphorus in coastal eutrophication. But marine scientists recognized the prominent role nitrogen plays in coastal eutrophication decades ago, and the report Managing Wastewater in Coastal Urban Areas (NRC 1993a) clearly concludes that the marine scientific community has reached consensus about the primary importance of nitrogen as the prinicpal cause of nutrient over-enrichment in coastal systems. |
|
Why is the scientific community so clear that the key to controlling eutrophication in coastal systems is managing nitrogen inputs? First, the experimental evidence is much clearer than in the past. Most early studies of nutrient limitation and eutrophication in coastal waters either relied on fairly short-term and small-scale enrichment experiments to infer limitation by nitrogen, or made inferences from pure-culture studies. When applied to the problem of lake-eutrophication in the 1960s and early 1970s, these approaches often led to the erroneous conclusion that nitrogen or carbon rather than phosphorus was limiting in lakes. Later, whole-lake experiments clearly showed that phosphorus and not nitrogen or carbon was the nutrient most regulating eutrophication in lakes (Schindler 1977). Consequently, the scientific community that studied eutrophication in lakes and the water-quality management community that dealt with freshwater systems became skeptical about any results obtained from similar small-scale experiments (NRC 1993a). However, the information produced by more recent estuarine studies is much more reliable. Since 1990, the results of three large-scale enrichment “experiments” have been published from studies carried out in Narragansett Bay, in a portion of the Baltic Sea, and in Laholm Bay in Sweden. Each study was similar in scope and methodology to the pivotal lake experiments of the late 1970s, and all three showed nitrogen limitation in the systems studied (Granéli et al. 1990; Rosenberg et al. 1990; Oviatt et al. 1995; Elmgren and Larsson 1997). Overall, available data from these major studies and from bioassay and nutrient ratio data from many estuaries all give credence to the generalization that nitrogen availability is the primary regulator of eutrophication in most coastal systems. But why should nitrogen usually control eutrophication in coastal marine systems while phosphorus controls eutrophication in lakes? Primary production by phytoplankton is generally thought to be a function of the relative availability of nitrogen and phosphorus in the water. For instance, phytoplankton require approximately 16 moles of nitrogen for every mole of phosphorus they assimilate (the Redfield ratio of nitrogen:phosphorus = 16:1). If the ratio of available nitrogen to available phosphorus is less than 16:1, primary production is nitrogen-limited. If the ratio is higher, production is phosphorus-limited. Lakes receive nutrient inputs from upstream terrestrial ecosystems and from the atmosphere, while estuaries and coastal marine systems receive nutrients from these sources as well as from neighboring oceanic water masses. For estuaries such as those along the northeastern coast of the United States, the ocean-water inputs of nutrients tend to have a nitrogen:phosphorus ratio well below the Redfield ratio due to denitrification on the continental shelves (Nixon et al. 1995, 1996). Thus, given similar nutrient inputs from land, estuaries will tend to be more nitrogen-limited than will lakes. The biogeochemical processes operating in freshwater lakes and coastal estuaries, as well as their watersheds, are complex, and thus whether biological activity at any one location at any given time is nitrogen- or phosphorus-limited is dependent on a number of complex factors. However, despite the complexity of the processes involved, nitrogen-limiting conditions are much more common in estuaries than in lakes, and effective management of these areas and their associated watersheds requires much greater focus on nitrogen. |
Developing an effective strategy for reducing the impacts of nutrient over-enrichment requires an understanding of which nutrients are important, the sources and transport mechanisms for those nutrients, and how human activities have altered the abundance of each (Figure 1-3). Seen broadly, the earth is a closed system and the total amount of nitrogen or phosphorus in all forms is essentially fixed. These elements are constantly recycled, changing forms as they combine with different elements to form a variety of chemical compounds. These varied compounds are stable at different temperatures and pressures, and thus each may be more or less soluble in water, or more or less volatile than its predecessor, and may be used by organisms in different ways. Each element has a specific biogeochemical cycle—it is distributed or moves in a way dictated by its unique chemistry within the air, freshwater lakes and streams, the ocean, and land.
Nitrogen and phosphorus have different chemical properties, and thus each reacts differently to form a different set of compounds, many of which again behave differently. When human activity drastically alters the distribution or relative abundance of the various compounds containing these element forms, they alter the overall biogeochemical cycle of
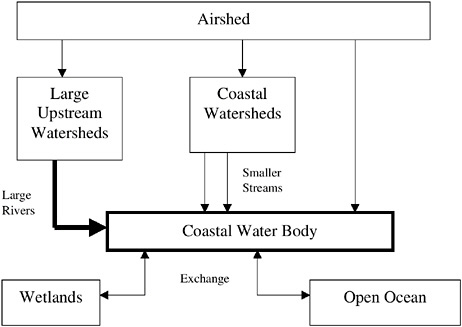
FIGURE 1-3 Schematic showing general sources of nutrients and main routes of transport to coastal waters.
these elements. Thus, one measure of how dramatically human activity is altering the environment is to examine the degree to which this activity is altering the normal geochemical cycle of a number of key elements including nitrogen, phosphorus, and also carbon.
For phosphorus, global fluxes (reflecting the net change from one segment of the geochemical cycle to another) are dominated by the essentially one-way flow of phosphorus carried in eroded soils and wastewater from the land to the oceans, where it is ultimately buried in oceanic sediments (Hedley and Sharpley 1998). The size of this flux is large—estimated at 22 Tg P yr-1 (teragrams of phosphorus per year) (Howarth et al. 1995). It is estimated that the flow of phosphorus prior to increased human agricultural and industrial activity was around 8 Tg P yr-1 (Howarth et al. 1995). Thus, current human activities cause an extra 14 Tg P to flow into the ocean sediment sink each year, or approximately the same as the amount of phosphorus fertilizer (16 Tg P yr-1) applied to agricultural land each year.
The effect of humans on the global cycling of nitrogen is immense, and the rate of change in the pattern of use is extremely rapid (Galloway et al. 1995). The single largest change globally in the nitrogen cycle comes from increased reliance on synthetic inorganic fertilizer, which was invented during World War I and came into widespread use in the late 1950s (Box 1-7).
Inorganic fertilizers account for more than half of the human alteration of the nitrogen cycle (Vitousek et al. 1997). Approximately half of the inorganic nitrogen fertilizer ever used on the planet has been used in the last 15 years. The rate of use increased steadily until the late 1980s, when the collapse of the former Soviet Union led to great disruptions in agriculture and drops in fertilizer use in Russia and much of eastern Europe. This caused a slight decline in global nitrogen fertilizer use for a few years (Matson et al. 1997). By 1995, however, the global use of inorganic nitrogen fertilizer was again growing rapidly, with much of the growth driven by use in China. As of 1996, use was approximately 83 Tg N yr-1.
The increased use of commercial fertilizer over the last 50 years has contributed to a dramatic increase in per acre crop yields. But it has also brought problems (e.g., adverse changes in soil properties and offsite environmental problems caused by runoff). Problems are exacerbated because fertilizers are frequently over applied. Crop absorption of applied nitrogen can be extremely variable, depending on the crop, plant growth, and the method and timing of fertilizer application (NRC 1989). Although some improvements in fertilizer efficiency may have occurred in the last 10 years, the importance of choosing appropriate fertilizer application methods, amounts, and timing remains.
|
BOX 1-7 Unlike phosporus- or potassium-based fertilizers, whose abundance is limited by the extraction of source materials by mining, nitrogen-based fertilizers are largely derived from the direct chemical conversion of inert elemental nitrogen, N2, in the atmosphere to biologically useable forms of nitrogen (typically compounds of nitrogen plus oxygen or hydrogen). Elemental nitrogen is the most abundant gas in the earth’s atmosphere, thus there is an essentially inexhaustible supply of inorganic, nitrogen-based fertilizer. The process used, originally developed to address Germany’s needs for nitrate to produce munitions during World War I, remains the most economical method for the commercial fixation of nitrogen, and with modifications is one of the basic processes of the chemical industry. Over the last decade, ammonia derived from natural gas has emerged as another important source of inorganic fertilizer. When nitrogen-based fertilizer is applied to a field, it can move through a variety of flow paths to downstream aquatic ecosystems. Some fertilizer leaches directly to groundwater and surface waters, varying from 3 to 80 percent of the fertilizer applied, depending on soil characteristics, climate, and crop type (Howarth et al. 1996). On average for North America, about 20 percent leaches directly to surface waters (NRC 1993a). Some fertilizer is volatilized directly to the atmosphere; in the United States, this averages 2 percent of the fertilizer applied, but the value is higher in tropical countries and also in countries that use more ammonium-based fertilizers, such as China (Bouwman et al. 1997). Much of the nitrogen from fertilizer is incorporated into crops and is removed from the field in the crops when they are harvested. An NRC report (NRC 1993a) suggests that on average, 65 percent of the nitrogen applied to croplands in the United States is harvested, although other estimates are somewhat lower (Howarth et al. 1996). Given these paths and rates, about 13 percent of the nitrogen applied builds up in soils or is denitrified back to elemental nitrogen (a gas) and released to the atmosphere. To fully understand nitrogen transport, it is important to trace the eventual fate of the nitrogen harvested in crops. Some nitrogen is consumed directly by humans eating vegetable crops—in North America this constitutes perhaps 10 percent of the amount of nitrogen originally applied to the fields (Bouwman and Booij 1998). Perhaps 10 percent of the nitrogen originally applied to fields is lost during food processing and ends up in landfills or released to surface waters from food-processing plants. The largest part of the nitrogen is fed to animals in feed crops, estimated to be about 45 percent (Bouwman and Booij 1998). Of the nitrogen consumed by animals, much is volatilized from animal wastes to the atmosphere as ammonia. In North America, this volatilization is roughly one-third of the nitrogen fed to animals (Bouwman et al. 1997), or 15 percent of the amount of nitrogen originally placed on the fields. This ammonia is deposited back onto the landscape, often near the source of volatilization, although some of it travels long distances through the atmosphere (Holland et al. 1999). Some of the nitrogen in animals is consumed by humans, an amount roughly equivalent to 10 |
|
percent of the amount of nitrogen fed to the animals, or 4 percent of the nitrogen originally applied to fields. The rest of the nitrogen—over 25 percent of the amount of nitrogen originally applied to the fields—is in animal waste that is accumulating somewhere in the environment. Much of this may be leached to surface waters. Of the nitrogen consumed by humans, either through vegetable crops or meat, some is released through wastewater treatment plants and from septic tanks. In North America, this is an amount equivalent to approximately 5 percent of the amount of nitrogen originally applied to fields (Howarth et al. 1996). The rest is placed as food wastes in landfills or is denitrified to nitrogen in wastewater treatment plants and septic tanks. In conclusion, fertilizer leaching from fields is only a portion of the nitrogen that potentially reaches estuaries and coastal waters. Probably of equal or greater importance in many regions of North America is the nitrogen tied up in ammonia, which is volatilized to the atmosphere or released to surface waters from animals’ wastes and landfills. Since food is often shipped over long distances in the United States, the concentration and subsequent environmental effect of nitrogen over-enrichment can occur well away from the original fertilized cropland. |
Although production of fertilizer is the most significant way human activity mobilizes nitrogen globally, other human-controlled processes, such as combustion of fossil fuels and production of nitrogen-fixing crops in agriculture, convert atmospheric nitrogen into biologically available forms of nitrogen. Overall, human fixation of nitrogen (including production of fertilizer, combustion of fossil fuel, and production of nitrogen-fixing agricultural crops) increased globally some 2- to 3-fold from 1960 to 1990, and continues to grow (Galloway et al. 1995). By the mid 1990s, human activities made new nitrogen available at a rate of some 140 Tg yr-1 (Vitousek et al. 1997), or a rate roughly equivalent to the natural rate of biological nitrogen fixation on all of the land surfaces of the world (Vitousek et al. 1997; Cleveland et al. 1999). Thus, the rate at which humans have altered nitrogen availability globally far exceeds the rate at which humans have altered the global carbon cycle.