3
Pharmacokinetics and Pharmacodynamics in Children versus Adults
Pharmacokinetics and pharmacodynamics are very different in children and adults. For the majority of drugs, in children as well as adults, a relationship exists between pharmacokinetics and pharmacodynamics. The pharmacokinetics of many drugs vary with age (Keams, 1998). For instance, because of the rapid changes in size, body composition, and organ function that occur during the first year of life, clinicians as well as pharmacokineticists and toxicologists are presented with challenges in prescribing safe and effective doses of therapeutic agents (Milsap and Jusko, 1994). Studies with adolescents reveal even more complexity in ding metabolism and differences in drug metabolism between the sexes.
The therapeutic value of understanding differences in pharmacokinetics because of developmental factors thus relies on an ability to understand better the dose versus concentration versus effect profile for a specific drug in patients of various ages (Kearns, 1998). In turn, recognition of differences in pharmacokinetics because of developmental factors can be invaluable for interpretation of data and improving and guiding the design of clinical trials on drug disposition and efficacy. The summaries of the presentations presented below identify new advances in biomedical science that are uniquely applicable to children and that could be applied to the development and testing of drugs and biologics in studies with children. Some of the challenges and successes in pediatric pharmacokinetics for particular studies are also discussed.
DRUG METABOLISM IN CHILDREN AND ADOLESCENTS: INSIGHTS FROM THERAPEUTIC ADVENTURES
Presented by Gregory L. Kearns, Pharm. D., FCP
Marion Merrell Dow/Missouri Chair in Pediatric Pharmacology, and Professor of Pediatrics and Pharmacology, University of Missouri, Kansas City, and Chief, Division of Pediatric Clinical Pharmacology and Experimental Therapeutics, Children's Mercy Hospital and Clinics, Kansas City
Over the past two decades, much information concerning drug metabolism in infants, children, and adolescents has been derived as a ''by-product" of pharmacokinetic investigations designed, in part, to determine whether age-dependent differences in drug disposition (e.g., drug clearance) were evident. For many compounds, developmental differences in drug clearance have, for drugs where the primary biotransformation pathways are known, produced partial developmental "road maps" that have provided information on the patterns of ontogeny for important drug-metabolizing enzymes.
The use of pharmacokinetic data to examine the ontogeny of a drug-metabolizing enzyme is well illustrated by theophylline, a pharmacologic substrate for the P450 cytochrome CYP1A2. In 1981, Nassif et al. reported that the elimination half-lives of theophynine ranged between 9 and 18 hours in term infants 6 to 12 weeks of postnatal age. Furthermore, those investigators found a linear relationship between postnatal age and theophylline half-life, with values declining to approximately 3 to 4 hours by 48 weeks of life. Over a decade later, Krans et al. (1993) demonstrated that the dramatic alterations in theophylline plasma clearance occurring between 30 weeks (i.e., approximately 10 ml/h/kg) and 100 weeks (i.e., approximately 80 ml/h/kg) of postconceptional age was primarily the result of age-dependent differences in the metabolism of theophylline by CYP1A2 were dependent pathways. Further characterization of theophylline biotransformation in humans by Tjia et al. (1996) demonstrated that theophylline was adequate for use as a pharmacologic "probe" for the assessment of CYP1A2 activity given that approximately 80 percent of the formation of 1,3-dimethyluric acid at theophylline concentrations of 100 micromolar (µM) was catalyzed by this P450 isoform. Recently, Tateishi et al. (1999) administered theophylline to 51 pediatric patients ranging in age from 1 month to 14 years of age and examined the urinary ratios of three metabolites: 1-methyluric acid, 3-methylxanthine, and 1,3-dimethyluric acid. Examination of the urinary ratio of 1,3-dimethyluric acid to either 3-methylxanthine or 1-methyluric acid (both of which are generated by CYP1A2) demonstrated that CYP1A2 activity as competent as that of adults was reached by 3 months of postnatal age, a finding that corroborated earlier studies of the pharmacokinetics of the drug in infants (Kraus et al., 1993). Although these data collectively appear to have created a well-
defined pattern of CYP1A2 ontogeny, early studies by Lambert et al. (1986) and a recent investigation by Gotschall et al. (1999a) demonstrated that both puberty and cystic fibrosis, respectively, influence the pattern of CYP1A2 ontogeny (reflected by the use of methylxanthines as probe compounds) and, thus, implicate sexual maturation and disease as potentially important co-variates for the expression of this particular cytochrome P450 during development.
Another example where pharmacokinetic data have shed important insights on the impact of development on drug metabolism resides with CYP3A4; the most abundant cytochrome P450 isoform in the human body which is responsible for catalyzing the biotransformation of well over 20 drugs commonly used in pediatric practice (Leeder and Kearns, 1997). As recently reviewed by de Wildt et al. (1999b), ontogeny appears to have a major impact on the activity of CYP3A4. Like the activity of CYP1A2, CYP3A4 activity appears to be greater in infants and children than in adults. A study of carbamazepine and carbamazepine 10,11-epoxide (a CYP3A4 product) conducted in infants and children 2 weeks to 15 years of age demonstrated an age-dependent decrease in the ratio of the epoxide metabolite to the parent drug in serum (Korinthenberg et al., 1994). These data suggest a higher level of CYP3A4 activity in children and a gradual maturation to adult levels of activity during adolescence; however, variability in the activity of microsomal epoxide hydrolase, which further catalyzes the biotransformation of the 10,11-epoxide to the corresponding trans-dihydrodiol may confound interpretation of the data (Kroetz et al., 1993). Additionally, increased CYP3A4 activity in young children is supported by clinical investigations of cyclosporine which have demonstrated pharmacokinetic differences of a magnitude sufficient to affect both dosing regimen and drug efficacy (Wandstrat et al., 1989). In contrast to other P450 cytochromes such as CYP1A2 and CYP2C9, neither gender nor menstrual cycle appears to alter the activity of CYP3A4, as assessed with either hepatic microsomal samples (Transon et al., 1996) or the in vivo pharmacologic probe midazolam (Kashuba et al., 1998).
With respect to the impact of ontogeny on CYP3A4 activity, the most dramatic differences appear to occur during the first 6 months of life. As recently demonstrated by Lacroix et al. (1997) in an in vitro study (oligonucleotide probes for detection of messenger ribonuclease acid [mRNA], immunoblot analysis for quantitation of CYP3A protein, and biotransformation of the CYP3A substrates dehydroepiandrosterone [DHEA] and midazolam) with human liver microsomes obtained from fetuses, neonates, infants, and children, CYP3A4 expression is transcriptionally activated during the first week of life and is accompanied by a simultaneous decrease in the level of CYP3A7 expression. Additionally, they demonstrated that CYP3A4 activity was extremely low in the fetus and attained 30 to 40 percent of adult activity at 1 month. This investigation demonstrated that adult levels of CYP3A4 were attained sometime between 3 and 12 months of post natal age.
Pharmacokinetic evidence for this pattern of CYP3A4 ontogeny is reflected by studies with midazolam, a pharmacologic probe that enables assessment of both hepatic and intestinal CYP3A4/5 activity, depending upon the route of administration (i.e., intravenous route = hepatic activity; oral route = hepatic route and intestinal activity) (Thummel et al., 1996). As demonstrated by Burtin et al., (1994) (Figure 3-1), the uncorrected (i.e., in liters per hour) plasma clearance of midazolam at birth was directly correlated with birth weight, a surrogate measure for CYP3A4 competence. These data suggest that CYP3A4 activity increases approximately fivefold over the first 3 months of life and corroborate the in vitro findings of Lacroix et al. (1997). This pattern of the development of CYP3A4 activity postnatally can be expected to significantly alter the pharmacokinetics and potentially, the pharmacodynamics of cisapride, a prokinetic agent ding widely used in infants during the first year of life whose biotransformation is dependent upon CYP3A4 activity (Gotschall et al., 1999b). Finally, pharmacokinetic studies of the CYP3A4 substrates nifedipine, lidocaine, cyclosporine, and tacrolimus illustrate the profound and clinically important impact of ontogeny on CYP3A4 activity and potentially, for some drugs that are also substrates for p-glycoprotein, the impact of development on the activity of this drug transporter (de Wildt et al., 1999a).
In addition to CYP1A2 and CYP3A4, there is pharmacokinetic evidence that supports a developmental dependence in the activity of CYP2C9. Biotransformation of phenytoin to the (S)-5-(4-hydroxyphenyl)-5-phenylhydantoin (S-HPPH) by CYP2C9 and subsequent conjugation with glucuronic acid represents the principal metabolic pathway by which the ding is eliminated from the body. Under normal conditions, 95 percent of the HPPH recovered in the urine is the CYP2C9 product S-HPPH (Bajpai et al., 1996). However, as plasma phenytoin concentrations increase from 5 to 60 µM, the contribution of CYP2C19 to overall phenytoin biotransformation is estimated to increase threefold (Bajpai et al., 1996). Nevertheless, changes in phenytoin pharmacokinetics during development provide some insight into the maturation of CYP2C9. Specifically, phenytoin pharmacokinetic data reported by Chiba et al. (1980) three decades ago demonstrated an age dependence in Vmax, which declined from approximately 14.0 mg/kg/day at 6 months of age to 8 mg/kg/day at 16 years of age. These changes were not associated with age-associated differences in the urinary excretion of HPPH. As well, recent pharmacokinetic data for the CYP2C9 substrate ibuprofen collected from 26 patients with cystic fibrosis ranging in age from 5.5 to 29.6 years demonstrated an inverse linear correlation between age and the apparent oral clearance of the drug (Keams et al. 1999).
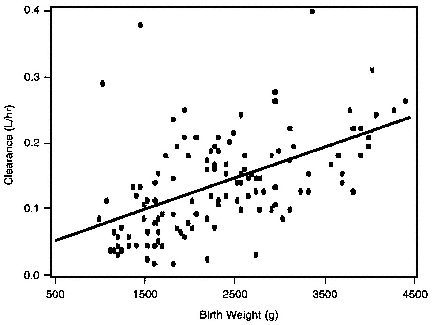
FIGURE 3-1 Midazolam clearance in newborns by birth weight. Source: Reprinted, with permission, from Burtin et al. (1994, p. 620). © 1994 by Mosby-Year Book, Inc.
In addition to the P450 cytochromes, apparent age dependence exists for several phase II enzymes that are of quantitative importance for drug biotransformation (Leeder and Kearns, 1997). Studies of N-acetyltransferase 2 (NAT2) activity using caffeine as a pharmacologic probe demonstrated attainment of adult activity by approximately 4 to 6 months of postnatal age (Pariente-Khayat et al., 1991). In contrast, the activity of thiopruine methyltransferase (TPMT) in newborn infants is approximately 50 percent higher than that observed in adults (Pariente-Khayat et al., 1991), with no discernible correlation with age demonstrated for a group of 309 Korean children between 7 and 9 years of age (McLeod et al., 1995). Studies of the pharmacokinetics of drugs that are substrates for various sulfotransferase isoforms (e.g., acetaminophen) suggest that activity of this enzyme during infancy and early childhood exceeds levels in adults (Leeder and Kearns, 1997). Finally, as recently reviewed by de Wildt et al. (1999b), ontogeny appears to have a profound effect on the activities of several isoforms of uridine-5'-diphosphate (UDP)-glucuronosyltransferases (UGTs).
Studies that have examined the effect of age on the disposition of several UGT substrates (e.g., morphine, acetaminophen, ethinylestradiol, zidovudine, propofol, lorazepam, naloxone, diclofenac, bilimbin, and chloramphenicol) suggest that isoform-specific, age-related differences in UGT activity occur. For
example, investigations of the pharmacokinetics of selected substrates for UGT2B7 (e.g., lorazepam, morphine and naloxone, chloramphenicol) support a marked reduction in the level of activity for this isoform (i.e., approximately 10 to 20 percent of the levels in adults) around birth, with attainment of competence equivalent to that in adults between 2 months and 3 years of age (de Wildt et al., 1999b). For drugs such as morphine, where UGT2B7 catalyzes the biotransformation of the drug to an active metabolite (e.g., morphine-6-glucuronide), delayed acquisition of morphine glucuronidation may have pharmacodynamic ramifications in the newborn as well. Available data concerning acetaminophen, a substrate for UGT1A6 and, to a lesser extent, UGT1A9, in children and adults suggest that the activities of these isoforms do not reach those in adults until 10 years of age (de Wildt et al., 1999b). Despite these examples, current data for the UGTs do not permit the construction of a developmental profile for these enzymes like those available for certain cytochromes P450 (e.g., CYP1A2, and CYP3A4). An information gap currently exists regarding the developmental and genetic aspects (i.e., the possible role of polymorphisms) of UGT regulation and its potential effect on pediatric drug therapy.
Despite a relative wealth of pharmacokinetic data and emerging information on isoform-specific differences in the activities of several important drug-metabolizing enzymes across the pediatric age range, there is little to no evidence that clearly describes the regulatory events at a cellular or molecular level that are responsible for producing developmental differences in drug-metabolizing enzyme activity. Although it was commonly believed that age-dependent differences in hepatic size (relative to total body size) in children was in part responsible for the apparent increased activities of many drug-metabolizing enzymes during childhood, Murry et al. (1995) demonstrated that liver volume in 16 children (3.3 to 18.8 years of age) was not associated with changes in the normalized (i.e., normalized to weight or body surface area) clearance of lorazepam, antipyrine, or indocyanine green, from plasma. In a recent study, Relling et al. (1999) examined the catalytic activities of selected pharmacologic substrates (e.g., ethoxyresorufin for CYP1A2, ethoxycoumarin for CYP2E1, midazolam or teniposide for CYP3A4/5, tolbutamide for CYP2C9 and 17α-hydroxylation of paclitaxel for CYP2C8) using hepatic microsomes obtained from children (n = 13; 0.5 to 9.0 years of age) and adults (n = 18; 13 to 52 years of age). With the exception of the slightly lower CYP2C9 activity found for children than for adults, no significant age-related differences were noted for the remainder of the P450 cytochromes when catalytic activity was examined as a maximal rate per milligram of microsomal protein. Thus, apparent increases in the activities of selected P450 cytochrome reflected by pharmacokinetic studies of certain "substrates" do not appear to be supported by these in vitro findings.
Finally, it is possible that neuroendocrine determinants of growth and maturation may, in part, be responsible for the observed developmental differences in the activities of certain drug-metabolizing enzymes. As recently postulated by
Leeder and Keams (1997), the biological effects of human growth hormone expressed during development may account for observed differences in the activity of specific drug-metabolizing enzymes. Support for this assertion was drawn from evidence that human growth hormone can modulate the effect of many general transcription factors, the demonstrated regulatory role for growth hormone in the expression of CYP2A2 and CYP3A2 in rats, the documented effects of human growth hormone treatment on the alteration of the pharmacokinetics for pharmacologic substrates of selected P450 cytochromes, and also from evidence of altered CYP1A2 activity that appears to correlate with the pubertal height spurt (Lambert et al., 1986).
In conclusion, pharmacologic and pharmacokinetic evidence supports the presence of isoform-specific developmental differences in the activities of a host of phase I and phase II drug-metabolizing enzymes. In vitro characterization of pathways for human drug metabolism combined with in vivo confirmation of quantitatively important age-related differences in drug clearance can be used together to create an effective "pattern" to examine potential developmental "breakpoints" for drug metabolism. When this approach is effectively combined with a pharmacogenetic or pharmacogenomic assessment for enzymes that are polymorphically expressed, prediction of the impact of development on drug metabolism and disposition is possible. Such data can be used to guide pharmacokinetic simulations of clinical trials and are being effectively used to design Phase land 2 clinical trials of new drugs for infants, children, and adolescents. These experimental approaches will ultimately improve pediatric drug development by focusing the pharmacologically (and biologically) relevant questions for study, streamlining the design of clinical investigations (e.g., the study of targeted pediatric populations versus the entire pediatric population), and providing increasing opportunities to control drug exposure, a determinant of both ding efficacy and safety, through enhanced design of age (i.e., developmentally)-appropriate ding dosing regimens.
The translation of data concerning developmental differences in drug metabolism to the therapeutic arena poses interesting and, in some cases, formidable challenges. Specifically, it is important to recognize that many therapeutic drugs are polyfunctional substrates for drug-metabolizing enzymes. Hence, pharmacogenetic differences between patients of the same age can have profound effects on drug metabolism (and clearance) by producing quantitatively important differences in the rates and routes of drug biotransformation. Furthermore, the apparent drug biotransformation phenotype may be influenced by disease (e.g., infection), environmental factors (e.g., diet and environmental xenobiotics compounds), and concurrent medications. Also, it must be recognized that drug response is a function of the complex interplay among genes involved in drug transport, drug biotransformation, and receptor and signal transduction processes, among others.
Finally, it is imperative that future research in the area of developmental pharmacology be focused on defining at a whole-animal, molecular, and cellular level the regulatory events that produce the age-associated differences in the activities of drug-metabolizing enzymes. Clinical investigations designed to examine pharmacokinetics must include both genotypic and phenotypic assessments so that valid biologic correlates are available to address variability in both drug disposition and, in some cases, drug response. Translational and basic research must focus on the regulatory elements of those genes that control the expression of drug-metabolizing enzymes. Such a multifaceted approach will be necessary to characterize the dynamic changes in the activities of drug-metabolizing enzymes that characterize the period of human development.
ONTOGENY OF P-GLYCOPROTEIN, AN ATP-DEPENDENT TRANSMEMBRANE EFFLUX PUMP
Presented by Jon Watchko, M.D.
Associate Professor of Pediatrics, Obstetrics, Gynecology, and
Reproductive Science, Department of Pediatrics,
University of Pittsburgh School of Medicine
A common problem in newborns is neonatal unconjugated hyperbilirubinemia. Although generally a benign developmental phenomenon, hyperbilirubinemia can become severe, particularly in the context of underlying hemolytic disorders and prematurity. In survivors it can result in kernicterus (also called hyperbilirubinemic encephalopathy) and neurologic injury, which can produce profound, long-term neurodevelopmental sequelae. Central to the development of kernicterus is the passage of bilirubin across the blood-brain barrier into the central nervous system. It is believed that bilirubin can enter the brain when it is not bound to albumin (i.e., when it is free) by passive diffusion or when the blood-brain barrier is disrupted.
Despite its high affinity for membrane lipids, bilirubin demonstrates a low level of accumulation in the brain. In this respect, bilirubin is similar to other lipophilic compounds that share its characteristic of unexpectedly low levels of accumulation in the central nervous system.
Evidence suggests that bilirubin is an endogenously generated substrate for P-glycoprotein. The expression of P-glycoprotein in brain capillary endothelial cells may play a protective role in limiting the uptake of bilirubin into the central nervous system and thus plays a role in the pathogenesis of protecting against the genesis of kernicterus (hyperbilirubinemic encephalopathy).
P-glycoprotein is expressed constitutively in many tissues, including abundant expression in the luminal aspect of brain capillary endothelial cells in the blood-brain barrier and in the brush-border epithelial cells of the small intestine. With respect to the central nervous system, P-glycoprotein limits the influx and
central nervous system retention of a wide variety of unrelated lipophilic compounds. Thus, P-glycoprotein contributes to the obstructive function of the blood-brain barrier by excluding compounds that are potentially toxic to the brain or extruding them before they have a chance to exert their cytotoxic effects. This includes the antiparasitic compound ivermectin, a well-defined P-glycoprotein substrate, various chemotherapeutic agents, and other potential neurotoxicants.
P-glycoprotein is a member of the adenosine triphosphate ATP-binding cassette or ABC superfamily of transport proteins that use ATP to translocate substrates across biologic membranes. It is encoded by a family of genes referred to as the multiple-drug resistance (MDR) genes because of their important role in contributing to resistance to multiple chemotherapeutic agents.
The MDR genes in humans are clustered on chromosome 7. The mdr1A gene encodes the P-glycoprotein isoform expressed in brain microvessels and confers a an MDR phenotype on tumors. This has two homologous halves, each of which has six hydrophobic transmembrane domains and an ATP-binding site. P-glycoprotein acts as an efflux pump, moving substrates across membranes against the concentration gradient. This has been demonstrated in tumor cells: approximately 60 percent of all tumors—solid or hematogenous—express P-glycoprotein. This has also been demonstrated in cell lines transfected with P-glycoprotein complementary deoxyribonucleic acids (cDNAs) and proteolysosomes reconstituted with P-glycoprotein.
The mechanism of action of P-glycoprotein primarily appears to be that of extracting substrates directly out of the plasma membranes before they get into the cell. Secondarily, P-glycoprotein can reduce intracellular substrate retention by pumping it out of the cell. The dependence of P-glycoprotein on ATP is absolute. P-glycoprotein has intrinsic ATP activity that can be distinguished from other ion motive ATPs and membrane-associated phosphatases. It is, in fact, stimulated by the addition of P-glycoprotein substrates. The ability of compounds to stimulate P-glycoprotein ATPase activity is directly correlated with their ability to be transported by the P-glycoprotein efflux pump. This pumping activity can be abolished by sodium azide, glucose deprivation, and mutations of the P-glycoprotein ATP-binding site.
One of the distinctive features of P-glycoprotein is its broad substrate specificity. Most of the substrates are lipophilic and amphipathic in nature, and virtually all are natural products of plants or microorganisms or are semisynthetic derivatives of such compounds. Known substrates for P-glycoprotein include the vinca alkaloids, anthracyclines, and other chemotherapeutic agents including taxol and cyclosporine; cardiovascular agents such as verapamil, digoxin, and quinidine; antibiotics; and various hormones.
Evidence suggests that bilirubin is a substrate for P-glycoprotein. This is based on observations that bilirubin will inhibit the photoaffinity labeling of P-glycoprotein by P-glycoprotein-specific photoaffinity probes. There is limited
uptake of the tritiated bilirubin by human variant multiple-drug-resistant cells compared with that by parent cells that do not express the mdr1A gene. (See discussion below.) Watchko and colleagues are testing the hypothesis that the brain capillary endothelial cell P-glycoprotein may provide an important protective effect against bilirubin toxicity by reducing brain bilirubin influx.
Additional evidence that bilirubin is a P-glycoprotein substrate includes work on brain bilirubin content in wild-type versus transgenic mdr1A null mutant P-glycoprotein deficient mice (Watchko et al., 1998). The mdr1A null mutant mice do not express P-glycoprotein in brain capillary endothelium, whereas their fvb wild-type counterparts express P-glycoprotein in abundance, as determined by both immunohistochemical staining and the Western immunoblotting technique.
With the exception of the P-glycoprotein deficiency, the integrity of the blood-brain barrier is actually maintained in the mdr1A null mutant mouse line. There is no difference in blood-brain barrier permeability to non-P-glycoprotein substrates, including fluorocine and fluorocine dextran 4000 and other blood-brain barrier integrity markers. Rhodamine 123, which is a well-defined P-glycoprotein substrate, evinces a fourfold higher concentration of P-glycoprotein in the brain, mdr1A null mutant P-glycoprotein-deficient mice than in the brains of their fvb counterparts, without differences in blood levels.
Imaging studies demonstrate that only radio-labeled or technetium-labeled P-glycoprotein substrates show enhanced accumulation in the central nervous system in vivo in the null mutant. Thus, the P-glycoprotein that crosses the blood-brain barrier may provide a protective effect against bilirubin neurotoxicity by reducing bilirubin influxinto the brain.
To understand the potential effects within the neonatal period, when the risks for hyperbilirubinemic encephalopathy seem to be the greatest, the ontogeny of brain microvessel P-glycoprotein expression must be explored. Brain microvessel P-glycoprotein expression may in fact be an early marker of blood-brain barrier development. Studies with mice have demonstrated that P-glycoprotein expression in mouse brain microvessels is limited during late embryogenesis to the newborn period, and that brain microvessel expression increases markedly with postnatal maturation. Thus, low levels of P-glycoprotein expression in the immature brain may lead to enhanced bilirubin uptake by the brain and an increased risk for hyperbilirubinemic encephalopathy in newborns. P-glycoprotein expression in the intestine is also characterized by a marked increase with postnatal maturation.
The mdr1A gene modulates the developmental expression of P-glycoprotein. It is known that the mdr1A gene is differentially expressed in normal tissues in adults and is subject to modulation by factors such as the presence of heat shock. Glucocorticoids are known to increase mdr1A gene expression in the liver and lungs in the adult. Little is known about the effects of thyroid status on mdr1A gene expression.
Preliminary studies are addressing the perinatal factors that may modulate P-glycoprotein expression, specifically, the effects of uteroplacental insufficiency on P-glycoprotein expression. Models have shown that both the gene message and protein levels are decreased by about 50 percent in the case of uteroplacental insufficiency. Thus, an intrauterine milieu induced by uteroplacental insufficiency and characterized by hypoxia acidosis and altered metabolic fuel availability is associated with a significant reduction in brain microvessel P-glycoprotein expression. These findings are of interest in relation to understanding hyperbilirubinemic encephalopathy because alleged risk factors for kernicterus include hypoxia and acidosis, and the understanding that P-glycoprotein may limit the next influx of various lipophilic compounds, including bilirubin into the central nervous system.
Factors that will alter P-glycoprotein once it is expressed include chemosensitizers, multiple-drug-resistant antagonists, or MDR reversers. These compounds will compete for P-glycoprotein binding sites and inhibit P-glycoprotein activity, and thus will limit the effectiveness of P-glycoprotein as an efflux pump. Oncologists are interested in these compounds because by attenuating the MDR phenotype they can enhance the effectiveness of the various chemotherapeutic regimens.
In sum, these findings raise more questions than answers. The following questions warrant further investigation: (1) What are the functional consequences of these developmental variations in P-glycoprotein expression in normal tissues? (2) What role does intestinal P-glycoprotein play in determining drug oral bioavailability? (3) Is there individual variability in P-glycoprotein expression as a function of gender, ethnicity, or aging? (4) Are other related transporters, such as MDR-associated protein, MDR-associated protein type II, or the canalicular multiple organic transporter in the liver also subject to developmental modulation and do they affect drug absorption, metabolism, and disposition in the neonate?
DEVELOPMENTAL ASPECTS OF GLUCOSE TRANSPORTERS
Sherin U. Devaskar, M.D.
Vice Chair of Research, Department of Pediatrics, Division of Neonatology, Mattel Children's Hospital, University of California at Los Angeles
Glucose transport is a stereospecific, saturable, carrier-mediated process of diffusion. Studies to date have primarily measured glucose transport through various glucose analogues: 2-deoxyglucose uptake and 3-O-methylglucose transport. Recently studies with humans have used 18F-deoxyglucose uptake and positron emission tomography scanning to quantify glucose uptake in both the brain and skeletal muscle.
The carrier responsible for glucose transport is classified as either the sodium glucose cotransporter or the facilitative glucose transporter. The gene of a facilitative glucose transporter has about 10 to 11 exons, depending on the isoform. The protein, a 450-amino-acid peptide, is highly conserved between species and isoforms (five proteins that have been cloned and described to date). The glucose transporter Glut-1 is responsible for basal glucose transport. It has a KM (Michaelis constant) ranging from 1 to 5 milli Molar, is present in many tissues, represents a proliferative state, and is found in fetal tissues.
Glut-2, the isoform found in the liver, the beta cells of the pancreas, and the small gut has a higher KM of about 20 to 60mM. Glut-3, which is the third isoform, is found in the brain and the placenta and is thought to be the most efficient glucose transporter, with the lowest KM for glucose. Most of the studies that have been done thus far with adults have been with Glut-4, which is the insulin-responsive type of transporter, and is found only in insulin-responsive tissues, with a KM of about 5mM. Glut-5 is a fructose transporter. Glut-6, which was cloned and which was thought to be very closely related to Glut-3, turned out to be a pseudogene. The isoforms are substrate specific, and they are not alternatively spliced products. The genes for these proteins are separate and are located on different chromosomes. Their glucose kinetics match the requirements of the tissues in which they are expressed; thus, there is tissue-specific expression. In addition, they all demonstrate a developmental pattern of expression.
Studies with animals and human autopsy tissue have found that through development, Glut-1 is found in excess, particularly in the fetus, the newborn, and most tissues that have been examined, but levels decrease with advancing age. In contrast, with advancing age there is an increase in the levels of the liver (Glut-2), brain (Glut-3), and insulin-responsive (Glut-4) forms compared with the level of Glut-1.
Glut-4 serves as a useful marker of differences in insulin responsiveness that can occur over the lifetime of an individual. For example, very little Glut-4 is present in the fetal myocardium, compared with the amount present in the adult. In the fetal heart, Glut-1 is responsible for basal glucose transport. There is a dramatic decline in Glut-4 levels in the fetus of the severely diabetic mother. Similar findings were also obtained for the skeletal muscle of the fetus, which behaved exactly like the heart with respect to Glut-1 and Glut-4. Sheep models demonstrate that in late gestation, hyperglycemia causes a time-dependent skeletal muscle Glut-1 drop, as is the case for Glut-4. Thus, there is a decrease in the skeletal muscle Glut-4 level with emerging insulin resistance.
The question that must then be answered is whether insulin has any effect on the fetus. In the rat model, insulin was administered to a 20-day-old fetus, a 2-day-old newborn, and a 60-day-old rat. A fourfold increase in the plasma insulin level was found; however, there was a concomitant 50 percent decrease in plasma glucose levels. Insulin therapy in the fetus appeared to increase the Glut-1 levels on a short-term basis, bringing it back to the baseline level. Glut-4 lev-
els in the fetus and the newborn showed no difference across the board except for a slight decline at 60 minutes in the fetal muscle.
In humans, adults have shown an increase in insulin sensitivity with increased skeletal muscle Glut-4 levels, with enhanced translocation of Glut-4. In the fetus and the newborn it appears that there is no change from that in adults in the skeletal muscle Glut-4 level, and there is no effect on translocation of Glut-4. So, in comparison with published reports in which it has been described that adults on glucocorticoid therapy have developed insulin resistance (with a decline in skeletal muscle Glut-4 levels and a diminished translocation of Glut-4), the opposite occurs in the newborn. Thus, the effect of glucocorticoids on newborn Glut-4 translocation remains to be studied.
FORMULATION
Presented by Emmett Clemente, Ph.D.
Chairman and Founder, Ascent Pediatrics, Inc., Wilimington, Massachussetts
There is a $3.7 billion market for pediatric medications, compared with an estimated total $94 billion prescription market for both pediatric and adult therapeutic categories. As a comparison, neurologic products for the adult market are approximately $7 billion, twice the entire market for pediatric medications. The largest therapeutic category in pediatric medicine is antibiotics. Anti-Infectives (antibiotics) make up 30 percent of the entire market for pediatric medications (Table 3-1). These data illustrate some of the reasons why large pharmaceutical companies have difficulty in developing pharmaceutical products for the pediatric patient: the therapeutic categories are many and market sizes are small compared to those for the adult market. To achieve an economic return, large companies would have to develop products across various therapeutic categories and enter the market with initial market potential which taken together is not feasible.
Factors that can increase the level of compliance and therefore improve the therapeutic outcome of medications in the pediatric population include dosing flexibility and dose administration. For example, most intranasal products being used for children are not specifically designed for the pediatric patient. Similarly, the volume of intraoccular medications are dosed on the basis of that for the adult eye, which can cause discomfort in the child. Palatability and appropriate dosage forms of oral medications are also important. For instance, it has been reported that up to 50 percent of children on oral steroids refuse to take their medicine because of the bitterness associated with these compounds. Because some of the alkaloids are very insoluble, their bitter taste is difficult to mask. Formulations that reduce dosing frequency are also needed.
TABLE 3-1 Important Pediatric Therapeutic Categories
|
Category |
Class of Compound |
Value of Category ($MM) |
|
Anti-Infectives |
Antibiotic |
$1,398 |
|
Cough/cold/allergy |
Antihistamine/decongestant |
385 |
|
Neurological |
CNS stimulants/anticonvuls-ants |
348 |
|
Respiratory |
Bronchodilators |
261 |
|
Dermatological |
Topical |
260 |
|
Anti-inflammatories |
Steroids |
249 |
|
Gastrointestinal |
Antispasmodis |
139 |
|
Analgesics |
Antipyretic/analgesic |
120 |
|
Hormones |
Various |
86 |
|
Ophthalmics |
Topical |
64 |
|
Antifungal |
Fungicides |
60 |
|
Subtotal |
|
3,370 |
|
All other |
|
334 |
|
Total |
|
$3,704 |
|
SOURCE: Scott-Levin, Pharmacy Acquisition Dollars, 1997. Ascent Pediatrics, Inc., Wilimington, Mass. |
||
Trytoxin is a compound with a very narrow therapeutic index. The present oral formulations include about 12 different dosage forms for the adult and the child. A neonatal patient with a nonfunctioning thyroid is administered thyroxin as a crushed tablet given in milk or water. There is no reliable way of knowing immediately that the infant has received an adequate dose. As the infant gets older, and because this compound is administered on a per kilogram basis, the accurate dosing of the child would be extremely difficult to accomplish with the present formulations. Instead, companies would need to formulate five to seven different dosage forms to accurately provide the medication to those children from birth to 6 years of age. In order to provide these formulations to the patient, formal regulatory approval is required (NDA) which includes acceptable chemistry manufacturing and control (CMC) data, as well as comparative clinical trials to demonstrate efficacy. The estimated market for this activity is between $3 million and $8 million a year, with product development time estimated to be between 5 to 7 years. These returns pose risks and returns that large companies are unwilling to undertake.
ANTI-INFECTIVES
Presented by Charles H. Ballow, Pharm. D.
Director, Anti-Infective Research, Kaleida Health Millard Fillmore Hospital
The last 10 years has been an interesting time for anti-infectives development, with the decrease in the rate at which new, unique anti-infectives were being developed occurring at the same time as an increase in the bacterial resistance rate, especially among common pathogens, including those in the pediatric population. These factors have resulted in a resurgence of interest in the development of novel compounds for the treatment of infectious diseases.
During this time ongoing research has focused on the pharmacokinetics and pharmacodynamics of anti-infectives, and has been aimed at measuring the outcome from administration of an anti-infective. Two commonly studied measures of outcome are microbiologic cure and clinical cure. An additional goal of this research has been to link dosing and concentration in serum profiles with these outcome measures. In addition, recently published data demonstrate reduced resistance development when adequate serum concentrations are achieved (Thomas et al., 1998).
The primary reason for developing new techniques for studying the pharmacokinetics and pharmacodynamics of anti-infective agents revolves around an attempt to maximize patient outcomes while minimizing toxicity. When fixed-dose strategies are used for the administration of anti-infectives, the doses will often be too high or too low for some percentage of the population, perhaps even a third of the patients, because of either age, physiologic characteristics, or bacteriologic factors. Moreover, when fixed-dose profiles and clinical cure are used as endpoints, it necessitates the use of large patient databases to demonstrate efficacy and safety. Differences between and within drug classes complicate the study of the pharmacokinetics and pharmacodynamics of anti-infective agents. The site and type of infection and the population at risk must also be considered. The primary measure of bacterial susceptibility is the minimum inhibitory concentration (MIC). Three pharmacokinetic variables are commonly evaluated relative to the MIC. These are the ratio of the maximal concentration in serum (CMAX) to the MIC (CMAX/MIC), the time that the concentrations in serum exceed the MIC (T>MIC), and the ratio of the area under the curve (AUC) to the MIC (AUC/MIC or AUIC). These parameters are useful in understanding differences in microbiologic and clinical cure between drugs, not only in terms of the extent of cure but also in terms of the cure rate. These variables can be used to determine desirable concentrations for therapy. Thus, if concentrations are too low, the percent probability of microbiologic cure will be low. If the concentrations are too high, then the patient is exposed to higher concentrations of an anti-infective agent than necessary.
In a study of patients in an intensive care unit with nosocomial pneumonia, investigators demonstrated that the rate and extent of microbiologic cure were greater when the AUICs were higher than 125 (Forrest et al., 1993). There was a further increase when the AUICs exceeded 250. The likelihood of clinical cure was also greater with higher AUICs. Thus, there are advantages in linking pharmacokinetics with pharmacodynamics in terms of the rate and extent of bacterial killing because they translate into advantages in terms of clinical cure.
Chronic bronchitis serves as another example of a lower respiratory tract model useful for performing pharmacokinetic and pharmacodynamic analyses. In a recently conducted study, patients whose cultures tested positive for Haemophilus species were administered one of two regimens for 10 days: grepafloxacin at 400 milligrams once daily or clarithromycin at 500 milligrams twice daily. Samples for pharmacokinetic studies were collected after administration of the first dose and at steady state and serial sputum cultures were taken four times on the first day of dosing and then daily for nearly 14 days (Tran et al., in press). The rate and extent of bacterial eradication differed between the two anti-infectives with grepafloxacin having a greater extent and more rapid rate of eradication. Analysis of the pharmacokinetic and pharmacodynamic variables demonstrated a much higher AUIC for grepafloxacin compared with that for clarithromycin.
Relationships between the pharmacokinetics and pharmacodynamics of anti-infective agents can be used to predict what doses an individual patient needs to maximize the likelihood of cure while minimizing the risk of toxicity. A limited number of studies in this area have been conducted with pediatric populations. The best work with pediatric populations has addressed otitis media. A retrospective pharmacokinetic-pharmacodynamic analysis conducted by Craig and Andes (1996) assessed microbiologic cure versus the time above the MIC for a number of anti-infective agents. A relationship was identified for both Streptococcus pneumoniae and H influenzae between eradication and T>MIC. When the concentrations in serum exceeded the MIC for greater than 40 percent of the dosing interval, maximal bacterial eradication was achieved. Moreover, as concentrations in middle ear fluid increased relative to the MIC, there was a nearly 100 percent likelihood of eradication.
Most of the studies of the pharmacokinetics of drugs in pediatric populations have used conventional dosing strategies, which presents a challenge because of the need to obtain multiple blood samples. Optimal sampling strategies (OSS) use applied statistical theory to identify a minimal number of key time points for drawing serum concentrations to provide the same information available by a conventional sampling design. Use of OSS may facilitate pharmacokinetic-pharmacodynamic research by making the conduct of such studies in pediatrics more feasible.
CHILDHOOD ASTHMA
Presented by Stanley J. Szefler, M.D.
Helen Wohlberg and Henry Lambert Chair in Pharmacokinetics and Director of Clinical Pharmacology, National Jewish Medical and Research Center, and Professor of Pediatrics and Pharmacology, University of Colorado Health Sciences Center
Asthma can occur early in life. Current therapy, even for children, is based on the concept that chronic inflammation is a key feature of asthma, but there is very little information on the time of onset of inflammation or the mechanism for its initiation, progression, and persistence. There is a general feeling among asthma care specialists that early childhood asthma is underdiagnosed and undertreated.
Current knowledge allows us to identify patients at high risk for asthma morbidity and mortality. Information is now developing regarding patients at risk for asthma, such as parental asthma, maternal smoking, atopic features and the presence of relevant allergens in the environment, and small lungs (National Heart, Lung, and Blood Institute, 1995, 1997). One of the consequences of undertreatment may be a loss over time of pulmonary function [FEV1] that is greater than that observed in patients without asthma and that is similar to that observed in patients with chronic obstructive pulmonary disease and cystic fibrosis (Lange et el., 1998; Peat et el., 1998; Weiss et al., 1995).
It is apparent that inhaled glucocorticoids are effective in controlling the symptoms of asthma and reducing the intensity of the inflammatory response in studies conducted with adults with asthma. This effect lasts as long as the treatment continues. No known treatment can consistently induce a lasting remission of the disease, and inhaled glucocorticoids have a relatively slow offset of effect compared with those of other long-term controller medications (Haahtela et al., 1994). Understanding the onset and progression of the inflammation, as well as its persistence, could provide insight into defining appropriate strategies for treatment depending on the stage of the disease (Peat et al., 1998).
Theories that early intervention with inhaled glucocorticoid therapy can be effective in preventing the progression of the disease and the risk for irreversible changes in the airways that could result in the persistence of symptoms have been developed (Agertoft and Pedersen et al., 1994; Haahtela et al., 1994; Overbeek et al., 1996; Selroos et al., 1995). Thus, there appears to be an effective opportunity for intervention.
Patients with ''difficult to control asthma" have evidence of persistent inflammation (Lee et el., 1996; Leung et al., 1995; Wenzel et al., 1997). Their disease often has its onset in early childhood. Does this information suggest that children who manifest persistent inflammation despite anti-inflammatory therapy are at increased risk for disease progression? If so, it will be important to
recognize these patients and provide more effective interventions at critical stages of their disease progression.
Gaps in Knowledge
Several key questions must be answered in developing treatments for asthma:
-
If asthma is inflammation-based, when does the onset of inflammation take place?
-
What cellular mechanisms are critical in the onset of inflammation? Are they the same mechanisms that allow progression of the disease? Is there a time when the disease is apparently out of control and not dependent on allergen stimulation? If so, what therapeutic interventions are appropriate for each stage of the disease? What is the role of the antigen-presenting cell in the pathophysiology of asthma? Is this cell affected by glucocorticoid therapy?
-
Is there a "window of opportunity" for intervention? If so, what is the appropriate medication or combination of medications? What is the appropriate time for intervention? What criteria should be used for the initiation, titration, and discontinuation of treatment?
-
Are inhaled glucocorticoids the drug of choice in managing the progression of early-onset childhood asthma? If so, do they affect long-term outcome? What are the risks-benefits involved in this treatment selection? What is the appropriate dose and method of administration?
-
What are appropriate outcome measures that indicate progression of the disease? Are there reliable measures of pulmonary function and markers of inflammation that could be incorporated into clinical studies on early interventions in childhood asthma? Is FEV1 an adequate measure for monitoring disease progression? How does one account for the treatment effect on measures of pulmonary function?
A recent meeting of the FDA's Pulmonary and Allergy Drug and Endocrinology Advisory Panels concluded that the limits of safe and effective doses of inhaled glucocorticoids for children have not been defined (FDA, 1998a). Moreover, insufficient information is available on the long-term effects of asthma medications, especially inhaled glucocorticoids and leukotriene modifiers, administered to children at an early age and for prolonged periods of time.
Existing Recommendations for Stepwise Therapy in Adults and Children over Age 5
In recent guidelines asthma is classified as mild intermittent, mild persistent, moderate persistent, and severe persistent on the basis of symptoms and
pulmonary function. A synopsis of recent guidelines suggests the following approach to asthma management in older children and adults (National Heart, Lung, and Blood Institute, 1995, 1997):
Intermittent: characterized as episodic bronchospasm. Therapy includes as needed β-adrenergic agonists for the relief of symptoms. One can also prevent symptoms by administering a β-adrenergic agonist before exercise or cromolynnedocromil before anticipated exposure to allergen.
Mild persistent: characterized by frequent episodes of bronchospasm, for example, more than twice per week but less than once per day, with marginal compromise in pulmonary function. First-line therapy may begin with an inhaled glucocorticoid (low dose),* cromolyn, nedocromil, or alternatively, sustained-release theophylline or a leukotriene synthesis inhibitor (zileuton [Zyflo-Abbott]) or antagonist (zafirlukast [Accolate; Zeneca]; montelukast [Singulair; Merck]). Medications can be combined to obtain beneficial effect. The doses of inhaled glucocorticoids may be increased if necessary. Inhaled β-adrenergic agonists are used as needed for breakthrough symptoms.
Moderate persistent: characterized by daily symptoms, exacerbations that affect activity and sleep, and compromised pulmonary function. Inhaled glucocorticoids (medium dose)* are the cornerstone of treatment. A long-acting bronchodilator can be used for nighttime symptoms including a long-acting inhaled β2-agonist (salmeterol), sustained-release theophylline, or long-acting oral β2-agonist.
Severe asthma: characterized by frequent symptoms, exercise-induced asthma, nocturnal exacerbations, deterioration in pulmonary function, and compromised lifestyle. Inhaled glucocorticoids at higher doses are primary therapy. Other medications are added on the basis of need, for example, a long-acting β-adrenergic agonist (salmeterol) or theophylline therapy to control night time symptoms and to prevent intermittent breakthrough. Short-acting β-adrenergic agonists (albuterol, terbutaline, pirbuterol) are used to relieve breakthrough symptoms. Nedocromil may be included in an attempt to minimize the inhaled and oral glucocorticoid dose. Oral glucocorticoids are used for severe exacerbations and are occasionally needed as maintenance therapy. Once control is established, medications are reduced in a reverse order beginning with oral glucocorticoids, then as-needed β-adrenergic agonists, followed by theophylline.
Stepwise Approach for Managing Infants and Children Under Age 5 with Chronic Asthma Symptoms
In younger children, the same classification system described above is used, but it is primarily based on symptoms, since pulmonary function is difficult to measure in young children. The following medications scheme is proposed in the National Asthma Education and Prevention Program guidelines (National Heart, Lung, and Blood Institute, 1997):
Intermittent: as-needed short-acting β-adrenergic agonists to relieve symptoms. Short-acting β-agonists are administered by a nebulizer or face mask and a spacer-holding chamber or oral liquid.
Mild persistent: first-line therapy may begin with cromolyn or nedocromil or low-dose inhaled glucocorticoid with a spacer-holding chamber and a face mask.
Moderate persistent: medium dose of inhaled glucocorticoids or, once control is established, medium dose of inhaled glucocorticoids and nedocromil or a long-acting bronchodilator (theophylline).
Severe asthma: high dose of inhaled glucocorticoids. If needed, add a systemic glucocorticoid at 2 mg/kg/day and reduce the dose to the lowest dose daily or alternate day that stabilizes symptoms.
Present Status of Inhaled Glucocorticoids as Cornerstone of Asthma Therapy
Numerous studies have shown that inhaled glucocorticoids improve asthma management and reduce inflammation in the airways. The response to inhaled glucocorticoids varies among patients. Recent observations suggest that the response to inhaled glucocorticoids is highly dependent on the time of intervention and that the earlier they are used the better (Agertoft and Pedersen et al., 1994; Haahtela et al., 1994; Overbeek et al., 1996; Selroos et al., 1995). The response to inhaled glucocorticoids is parameter specific, for example, a low dose may be effective in improving pulmonary function whereas a higher dose may be necessary to improve airway hyperresponsiveness (Pedersen and Hansen, 1995).
A high-dose, high-potency inhaled glucocorticoid (fluticasone propionate) or the use of delivery devices that improve drug delivery to the lung (budesonide with Turbuhaler) may be effective in improving pulmonary function and reducing the oral glucocorticoid requirement for patients with severe persistent asthma (Nelson et al., 1998; Noonan et al. 1995). Several recent studies have suggested that high-dose or long-term use of inhaled glucocorticoids may be
associated with a higher risk for ocular disorders, such as glaucoma or cataracts (Cumming et al., 1997; Garbe et al., 1997, 1998).
There is no practical measure of airway inflammation for clinical application; therefore, clinicians must rely on symptoms and pulmonary function to guide therapy. The best pulmonary function measure for long-term follow-up appears to be FEV1. Additional pulmonary function measures could include FEV1 and forced vital capacity ratio, morning peak flow, and peak flow variation. The effect of ongoing therapy needs to be considered in the interpretation.
In general, long-term nonsteroid controller medications (for example, theophylline, leukotriene modifiers, long-acting β2-agonists, cromolyn, and nedocromil) relieve and even prevent symptoms and also improve pulmonary function; however, their effect on long-term control of airway inflammation and disease progression is not clear. It is therefore difficult to adjust the inhaled glucocorticoid dose when indirect measures of inflammation, such as pulmonary function, can be ablated by nonsteroid long-term controllers.
Leukotriene Modifiers: An Attractive Alternative to Inhaled Glucocorticoids
Leukotrienes have been recognized as potent mediators of inflammation released by a number of cells involved in the inflammatory response to an allergic stimulus, namely, mast cells, basophils, eosinophils, neutrophils, and macrophages, all of which are present in the airways of patients with asthma. Leukotrienes are produced by the 5-lipoxygenase pathway of arachidonic acid metabolism and mediate bronchoconstriction and inflammatory changes important in the pathophysiology of asthma, such as the permeability of the microvasculature, mucus secretion, and neutrophil recruitment, and may contribute to airway edema.
In 1996, two medications in the leukotriene modifier class were approved for use in the treatment of asthma in the United States: zileuton, a 5-lipoxygenase enzyme inhibitor, and zafirlukast, a specific LTD4 receptor antagonist. In addition, another LTD4 receptor, antagonist, montelukast, was approved in 1998 by the FDA. The advantage of montelukast over the previous two medications in this class is that studies conducted with children as young as 6 years of age demonstrated efficacy (Kemp et al., 1998; Knorr et al., 1998) and were completed in children with asthma as young as 2 years of age. This has implications for application of this drug to the treatment of asthma in young children. In general, the medications in this class have the following properties:
-
immediate bronchodilator effect, as demonstrated by improvement in FEV1 by 10 to 15 percent over the baseline FEV1;
-
reduction of as-needed bronchodilator use by approximately 33 percent/day;
-
with chronic administration improvement in FEVI by approximately 10 percent over time;
-
reduction in nocturnal symptoms;
-
reduction in acute exacerbations requiting rescue medication;
-
ability to reduce inhaled glucocorticoid dose;
-
additive effect with inhaled glucocorticoid therapy;
-
additive effect with inhaled β-adrenergic agonist effect;
-
reduced cellular inflammatory response to an inhaled allergen challenge in a sensitized patient;
-
reduction in blood eosinophil count demonstrated for zileuton and montelukast, suggesting a reduced chronic inflammatory response;
-
improved exercise tolerance demonstrated with single-dose (zafirlukast) and chronic (montelukast) therapy. (The latter observation suggests a reduction in airway hyperresponsiveness with chronic therapy);
-
efficacy in blocking pulmonary response to aspirin in aspirin-sensitive patients; and
-
no indication of tolerance or tachyphylaxis to the medication with chronic administration.
Leukotriene Modifiers: Classes Within a Class of New Medications
As noted earlier, there are two subclasses of medications within the class of leukotriene modifiers: the leukotriene synthesis inhibitors and the specific leukotriene receptor antagonists. These medications differ not only in their pharmacologic activities but also in their dosage schedules, susceptibilities to drug and food interactions, and indications for use in children of various ages.
Potential Applications of Leukotriene Modifiers
The leukotriene modifiers are an interesting new class of medications that have the benefits of oral administration. This is especially useful for children in whom administration of inhaled medications may present a difficulty. These medications have various potential applications: (1) they may be used as an alternative to inhaled glucocorticoid therapy in patients with mild persistent asthma who are unable to take inhaled medication; (2) they may be used as a supplement to inhaled controller medications to reduce the need for-high dose inhaled glucocorticoid therapy; (3) a medication with a different mechanism of action could have an additive effect with other medications in improving the overall response to treatment; (4) they could be seen as a potential benefit in the management of asthma patients who are sensitive to aspirin; and (5) they may offer the opportunity to individualize the approach to therapy as the differences in asthma pathophysiology among patients begin to be understood.
Current Areas of Interest in Childhood Asthma and Opportunities for Drug Development
To date, studies have not shown a reduction in the inflammatory response in the airways as a result of chronic therapy with leukotriene modifiers, especially in patients with moderate persistent or severe persistent asthma. In addition, studies have not shown reductions in collagen and tenascin deposition with chronic leukotriene moderator therapy, as previously reported with inhaled glucocorticoid therapy (Laitinen et al., 1997; Olivierie et al., 1997). One unique property of inhaled glucocorticoids is the relatively slow offset of the effect in pulmonary function control and airway hyperesponsiveness, especially after long-term therapy. This observation suggests that inhaled glucocorticoids have provided some long-term effects on the airways. To date, most of the studies with leukotriene modifiers have been short-term, for example, 3 months, and they have not carefully evaluated the offset of the effect.
One of the more carefully studied leukotriene modifiers in relation to the resolution of airway inflammation have been pranlukast, a leukotriene antagonist. Of interest is the effect of this medication on maintaining asthma control and a number of markers of inflammation while reducing the inhaled steroid dose (Tamaoki et al., 1997), as well as the alteration of airway hyperresponsiveness (Hamilton et al., 1998) and a reduction of inflammatory cell numbers with chronic therapy (Nakamura et al., 1998a). Similar studies should be conducted with the other available leukotriene modifiers since the medications within this class may have different effects. Studies are also needed to demonstrate whether leukotriene modifiers have an effect on other allergic disorders, such as rhinitis, atopic dermatitis, conjunctivitis, and potentially, sinusitis and otitis. This would add another favorable dimension to this class of medications.
It has also been suggested that the response to these agents has been highly individualized, with some patients showing an excellent response and others showing no change in response. In general, it should be clear whether a patient is a responder or nonresponder within 2 weeks of treatment. It will be important to determine whether any difference in clinical response to these medications is related to genetic differences in the 5-lipoxygenase gene.
An understanding of the natural history of asthma would be helpful in establishing criteria for early diagnosis. Evaluation of the progressive aspects of the disease would be useful in defining appropriate measures of progression. The following areas deserve study: (1) establishment of the safety of various medications used as long-term controllers, specifically in relation to inhaled steroids and leukotriene modifiers; and (2) determination of the efficacies of certain medications in young children, including cromolyn, nedocromil, leukotriene modifiers, and inhaled steroids. For the available inhaled steroids and the respective delivery devices, research is needed to define the maximally safe
doses and the minimally effective doses for various age groups and various levels of severity.
Drug research and development is needed in the following areas:
-
dosage refinement for various drugs used in childhood asthma, especially for early intervention;
-
identification of surrogate markers for medication evaluation;
-
improved assessment of delivery systems;
-
pharmacogenetics; and
-
refined evaluation of drug metabolism and considerations of the efficacy and safety of inhaled glucocorticoids and the leukotriene modifiers.
Conclusions
Until proven otherwise, it appears that inhaled glucocorticoids are indeed the cornerstone of management for patients with moderate and severe persistent asthma. This is related to their proven effect on asthma control and their effect on resolving various measures of airway inflammation.
Leukotriene modifiers improve many measures of asthma control; however, except for pranlukast, there is limited information on their effect on resolving persistent airway inflammation. Studies are needed to determine whether the use of leukotriene modifiers alters the course of persistent asthma in a way that is comparable to that demonstrated with inhaled glucocorticoids. The role of leukotriene modifiers in mediating the course of asthma in children must be defined. Available data suggest that intervention with inhaled glucocorticoids, specifically, budesonide, has the potential to prevent the loss of pulmonary function. More studies with inhaled glucocorticoids are needed to verify this observation and to obtain comparable information for all long-term controllers.
Studies are needed to evaluate the safety of inhaled glucocorticoids with long-term treatment, considering the fact that they are now being suggested for use in young children. It must be recognized that the present concepts of treatment incorporate an earlier time of intervention and long-term treatment. Inhaled glucocorticoids with higher levels of potency (fluticasone propionate), better glucocorticoid delivery systems (Turbuhaler and hydro-fluoroalkane (HFA) propellant), and glucocorticoids that can be administered to very young children (nebulized budesonide) are now or will soon be available. As suggested by the FDA Pulmonary and Allergy Drug and Endocrinology Advisory Panels, it is important to define minimally effective and acceptably safe doses for the available inhaled glucocorticoids for various age groups including young children.
The minimal criteria for intervention with long-term therapy must be evaluated. Specifically, is the presentation of symptoms more than twice per week really an indicator for intervention with long-term controller therapy? A careful evaluation of surrogate markers and, possibly, biomarkers should be conducted
to define drug efficacy in young children, as should short-term evaluation strategies that would reliably predict long-term effects.
PEDIATRIC ONCOLOGY
Presented by David G. Poplack, M.D.
Elise C. Young Professor of Pediatric Oncology and Head, Hematology Oncology Section, Department of Pediatrics, Baylor College of Medicine and Director, Texas Children's Cancer Center
Some 8,700 new cases of childhood cancer are diagnosed annually in the United States. Pediatric cancer is the leading cause of non-accidental deaths in children less than 15 years of age. The incidence of childhood cancer increased 6 percent from the mid-1970s to the mid-1990s.
Childhood cancer differs from adult cancer in its histology, reflecting significant biological differences. In addition, carcinomas that are common in adults are rarely seen in childhood. Lifestyle-related cancers also are not normally observed in the pediatric population. Breast, lung, prostate, and colorectal cancers are the main forms of malignancies seen in adults. In contrast, acute leukemias and central nervous system tumors predominate in children. A variety of other solid tumors, including neuroblastoma, Wilms' tumor, and retinoblasroma are generally specific to children.
Over the years there has been dramatic improvement in the survival and prognosis of children with these diseases. In 1960 few children with cancer were actually cured. The current 5-year survival rate is approximately 75 percent. The mortality rate has decreased nearly 50 percent from the early 1970s to the mid-to late 1990s, and has continued to decrease in the 1990s. There has been improvement over the years even in those diseases that traditionally have been more refractory to therapy, such as neuroblastoma.
The successes with pediatric cancer treatments are the result of a highly organized national clinical trials effort that was initiated and supported by the National Cancer Institute (NCI) in the 1950s. At present, more than two-thirds of patients newly diagnosed with childhood cancer are enrolled in one or more NCI-sponsored clinical trials. Approximately 5,000 children enter treatment trials each year. The NCI has developed a consortium of approximately 40 institutions focused on conducting Phase 1 studies of new anti-cancer agents. More recently, a pediatric brain tumor consortium was formed to develop clinical studies focused on these particularly difficult cancers.
In many ways, pediatric cancer has served as a paradigm. A number of treatment concepts that are routinely applied in the treatment of adult malignancies actually evolved from developments in the treatment of pediatric malignancies. In addition, numerous drugs currently used to treat adult cancer were initially developed for the treatment of pediatric cancer. For example, many agents
originally developed for the treatment of acute lymphoblastic leukemia are now being used routinely in the treatment of a variety of adult malignancies. Nonetheless, it is important to conduct separate clinical trials for children and adults because the pharmacokinetics and pharmacodynamics of several anti-cancer agents differ between children and adults. In addition, the degree of prior therapy is frequently different, with children being more heavily treated before going on new agent trials than adults. Hence, prior therapy affects drug tolerance in children more so than in adults. For these reasons, the MTD (maximally tolerated dose) for a particular drug in children may differ significantly from that in adults. Pediatric oncologists are best qualified to prioritize, design, and implement clinical trials for children with cancer.
One of the challenges in the field is the relatively small number of patients available for pediatric new agent trials. As treatments become more successful, there will be fewer patients who relapse. Thus, there are fewer patients available for new agent studies at a time when there are more novel compounds to be studied. In part for this reason, pediatric cancer drug development is not profitable and has become, in many cases, an industry stepchild. In addition, because of the small numbers of patients available, multi-institutional trials are required. Also, because of the small patient numbers, the selection of the appropriate agents to be studied becomes critically important. In this regard there need to be better approaches to preclinical drug assessment so that only the most promising agents are advanced for clinical evaluation. Furthermore, better pre-clinical screening approaches that are more predictive for pediatric malignancies and that are also appropriate for the evaluation of the many new molecular and biologic therapies should exist. Such screening should also incorporate drug resistance models. Traditionally, in vitro screening approaches have relied on a panel of human malignancies made up almost exclusively of adult malignancies. It is important that screening be conducted using pediatric tumor cell lines.
Another important issue is how to approach the myriad drug analogues currently available. Because of limited patient numbers, pediatric clinical studies should evaluate agents with novel mechanisms of action. Assessment of analognes should be pursued using pre-clinical and animal models. Wherever possible, their utility initially should be validated in adult trials.
As the face of cancer biology changes, cancer therapy is changing. With the identification of novel molecular targets, specific targeting of pediatric malignancies has become an exciting and realistic prospect. With increasing frequency in the future, clinicians are likely to be using agents developed to target molecular targets unique for a particular pediatric cancer but not necessarily relevant to adult cancers.
Phase 3 trials for pediatric cancer have also been influenced by the improvement in overall prognosis. Most Phase 3 pediatric cancer trials are randomized studies. Over the years, as therapy has dramatically improved, the number of patients required to demonstrate a statistically significant difference
between a newer and a ''best available" therapy has increased. For example, to statistically demonstrate the increased effectiveness of adding a new agent to an acute lymphocytic leukemia induction regimen, when the current remission induction rate is well over 90 percent, would take many hundreds of patients . . For some pediatric tumors, Phase 3 trials now may take as long as four years to complete. Compounding these challenges is the fact that many new agents are available, including a variety of biologics, cytokines, differentiating agents, monoclonal antibodies, molecular therapies, and gene therapy approaches.
A major concern is the paucity of adequately trained pediatric cancer pharmacologists. Currently, Phase 1 trials are done at approximately 50 centers throughout the United States. Only a handful of these centers have active pediatric cancer clinical pharmacology laboratories; even a smaller number are actually training individuals in cancer clinical pharmacology. In many cases the type of clinical pharmacology training given is not formalized and is not adequate for the variety of new, molecularly targeted agents that will require study in the future. The pediatric cancer pharmacologist in the 21st century must not only be mined in pediatric oncology and classical clinical pharmacology but should also have an appropriate grounding in molecular biology. Training of such individuals may be the greatest single challenge for the field.



























