Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
THE DEVELOPING BRAIN 183 expressed in the brain. This promises a dramatic expansion in the ability to understand the interweaving of genetic and environmental influences as they affect both brain and behavioral development (see Nelson and Bloom, 1997). Growth in brain knowledge naturally leads to questions about what it means for raising children and, specifically, for improving their develop- ment. Accordingly, efforts to translate this emerging knowledge for public consumption have proliferated in recent years. Some of this information has been portrayed well and accurately, but some has not. The challenge of deciphering what this information means for what parents, guardians, and teachers of young children should do is enormous. There are actually few neuroscience studies of very young children, and those that exist have not usually focused on the brain regions that affect cognition, emotions, and other complex developmental tasks. Much of the fundamental knowledge about brain development is based on experimental studies of animals. The translation of this information from basic neuroscience into rules for application to humans can be quite straightforward when the mechanisms involved are very similar in humans and animals, as is the case with the developing visual system. But the interpretation of other data from animals, or even some data from humans (such as estimates of the density of synapses in various brain regions at various ages), can be extraordinarily complex or inappropriate when the brain mechanisms of cognition, language, and social-emotional develop- ment are addressed. In this context, it is essential to balance excitement about all the new learning with caution about the limits of what is under- stood today. This chapter about the developing brain focuses on the role of experi- ence in early brain development. Following a brief discussion of how to study the developing brain is an overview of early brain development from conception through the early childhood years. We then turn to a discussion of how early experiences contribute to brain development. Four themes run throughout this section: 1. Developmental neuroscience research says a great deal about the conditions that pose dangers to the developing brain and from which young children need to be protected. It says virtually nothing about what to do to create enhanced or accelerated brain development. 2. The developing brain is open to influential experiences across broad periods of development. This openness to experience is part of what ac- counts for the remarkable adaptability of the developing mind. Although there are a few aspects of brain growth that require particular kinds of experience at particular times, as far as we know at present, this is more the exception than the norm for human brain growth.
184 FROM NEURONS TO NEIGHBORHOODS 3. The kinds of early experiences on which healthy brain development depends are ubiquitous in typical early human experienceâjust as nature intended. This means, however, that concern should be devoted to children who, for reasons of visual impairment, auditory processing problems, ma- jor perceptual-motor delays, and other basic deficits cannot obtain these experiences on which the developing nervous system depends. 4. Abusive or neglectful care, growing up in a dangerous or toxic envi- ronment, and related conditions are manifest risks for healthy brain devel- opment. Beyond these extremes, the nature and boundaries of the environ- mental conditions necessary for healthy brain growth are less well known, partly owing to the complexity and the cumulative achievements of cogni- tive, language, and socioemotional growth. Exploration in this area is cutting-edge research. STUDYING THE DEVELOPING BRAIN Neuroscience techniques have advanced significantly, rendering studies of young childrenâs brains more feasible and informative than in the recent past. These techniques have enabled scientists to learn more about how babiesâ brains change with development and how vulnerable or resilient they are to environmental harm. However, the repertoire of techniques that can be used with preschool-age and even younger children is still limited. Some of the more direct methods (i.e., looking into the brain) are either invasive (e.g., positron emission tomography requires the injection of a radioactive substance) or require long periods of remaining still (e.g., functional magnetic resonance imaging). Nevertheless, by tracking the brainâs activity from the outside with the electroencephalogram, event- related potentials, and magnetic encephalography, researchers can learn about brain functioning in very young children. For instance, scientists can record the electrical or magnetic activity of the brain while the child is presented with different stimuli (e.g., speech sounds) and identify which parts of the brain are active and how active they are when children are doing different things. This approach has been used to reveal that the neural substrate for recognizing faces and facial expressions is remarkably similar in infants and adults (de Haan and Nelson, 1997, 1999), and that babiesâ brains change as they learn their native language (Neville et al., 1998). In addition, children with localized brain damage can be studied using neuropsychological tools. These entail giving young children behavioral tasks that have been shown to involve specific brain functions (e.g., work- ing memory, spatial planning) and observing how performance varies with the particular part of the brain that is damaged (Luciana and Nelson, 1998). This approach, used in a longitudinal study of language develop-
THE DEVELOPING BRAIN 185 ment in children who suffered focal brain damage in the first months of life, revealed the extensive capacity for recovery of language functioning in these children (Bates and Roe, in press). Finally, among children whose medical conditions have required that their brains be studied, positron emission tomography has revealed metabolic patterns consonant with syn- aptic growth and pruning occurring in early development (Chugani and Phelps, 1986). (See Appendix B, as well as Nelson and Bloom, 1997, for a fuller discussion of technologies for studying the developing human brain.) WHAT DEVELOPS IN EARLY BRAIN DEVELOPMENT? The development of the brain has a long trajectory, beginning within a few days after conception and continuing through adolescence and beyond. The nervous system undergoes its most dramatic development during the first few years of life. Yet the processes that establish the structure and functioning of the brain, made possible by the developing networks of synapses that interconnect nerve cells and by the progressive fine-tuning of the neurons for the roles they will play within their synaptic networks, continue well into adolescence. The milestones of brain development from the prenatal period until school entry involve the development and migra- tion of brain cells to where they belong in the brain, embellishments of nerve cells through the sprouting of new axons or by expanding the den- dritic surface; the formation of connections, or synapses, between nerve cells; and the postnatal addition of other types of cells, notably glia. Fasci- nation with the earliest stages of brain development is understandable. During this period, the spinal cord is formed, nearly all of the billions of neurons of the mature brain are produced, the dual processes of neural differentiation and cell migration establish the neuronâs functional roles, and synaptogenesis proceeds apace. These processes represent an elaborate interplay between gene activity and the surrounding environments both inside and outside the child. There have been significant changes over time in the aspects of brain development that have captured public attention. Twenty years ago, people were fascinated by the ability to measure developmental changes in the degree to which neurons in different areas of the brain become wrapped in the white, fatty matterâmyelinâthat insulates nerve cells and affects the speed with which nerve impulses are transmitted from one cell to another. Myelination is, in fact, affected by the young childâs behavioral experiences and nutrition, as discussed below. Today, the public is more focused on information, not all of it new, about the rate of synapse development, particularly on studies showing that there is a tremendous burst of synapse formation early in life, followed by a decline in synapse number, apparently extending into adolescence in some areas of the brain. Combined with
186 FROM NEURONS TO NEIGHBORHOODS evidence that synapses that are used are retained and those not used are eliminated, there has been a frenzy of concern expressed as âuse it or lose itâ in the first years of life. It turns out, however, that synapse elimination is a normal part of development. In comparison to the brainâs wiring, far less attention has been paid to the neurochemistry of early brain develop- ment, which is essential to the brainâs capacity to learn from experience and is likely to play an important role in the regulation of behavior. Development of the Brainâs Wiring Diagram Brain development proceeds in overlapping phases: making the brain cells (neurulation and neurogenesis), getting the cells to where they need to be (migration), growing axons and dendrites, which are structures needed to link with other nerve cells (neuronal differentiation and pathfinding), developing synapses or points of communication with other cells (synapto- genesis), refining those synapses (maturation and pruning), and, finally, forming the supportive tissue that surrounds the nerve cells and makes for efficient communication among them (gliagenesis or myelination). The brain and spinal cord arise from a set of cells on the back (dorsal part) of the developing embryo called the neural plate. Two rows of rapidly dividing cells arise from the plate on each side along its length and fold over centrally into the neural tube. The anterior or head end of the neural tube forms a set of swollen enlargements that give rise to the various parts of the brainâthe forebrain containing the cerebral hemispheres, the midbrain containing important pathways to and from the forebrain, and the hind- brain containing the brainstem and cerebellum. The remainder of the neural tube becomes the spinal cord, peripheral nerves, and certain endo- crine, or hormone, glands in the body. Under the control of regulatory genes, the brain cells migrate to where they belong in accord with the functions they will ultimately serve. These genes provide developmental directions to particular groups of cells, which tell them what to do and where to go in the embryonic brain. Within the neural tube, the innermost cells divide repeatedly, giving rise first to the cells that primarily become nerve cells, or neurons, and later giving rise to both neurons and the supportive tissue components called glia. Once the nerve cells are formed and finish migrating, they rapidly extend axons and dendrites and begin to form connections with each other, called synapses, often over relatively long distances. These connections allow nerve cells to communicate with each other. This process starts prenatally and continues well into the childhood years. There is evidence in many parts of the nervous system that the stability and strength of these synapses are largely determined by the activity, that is, the firing, of these connections. The speed with which neurons conduct nerve impulses is
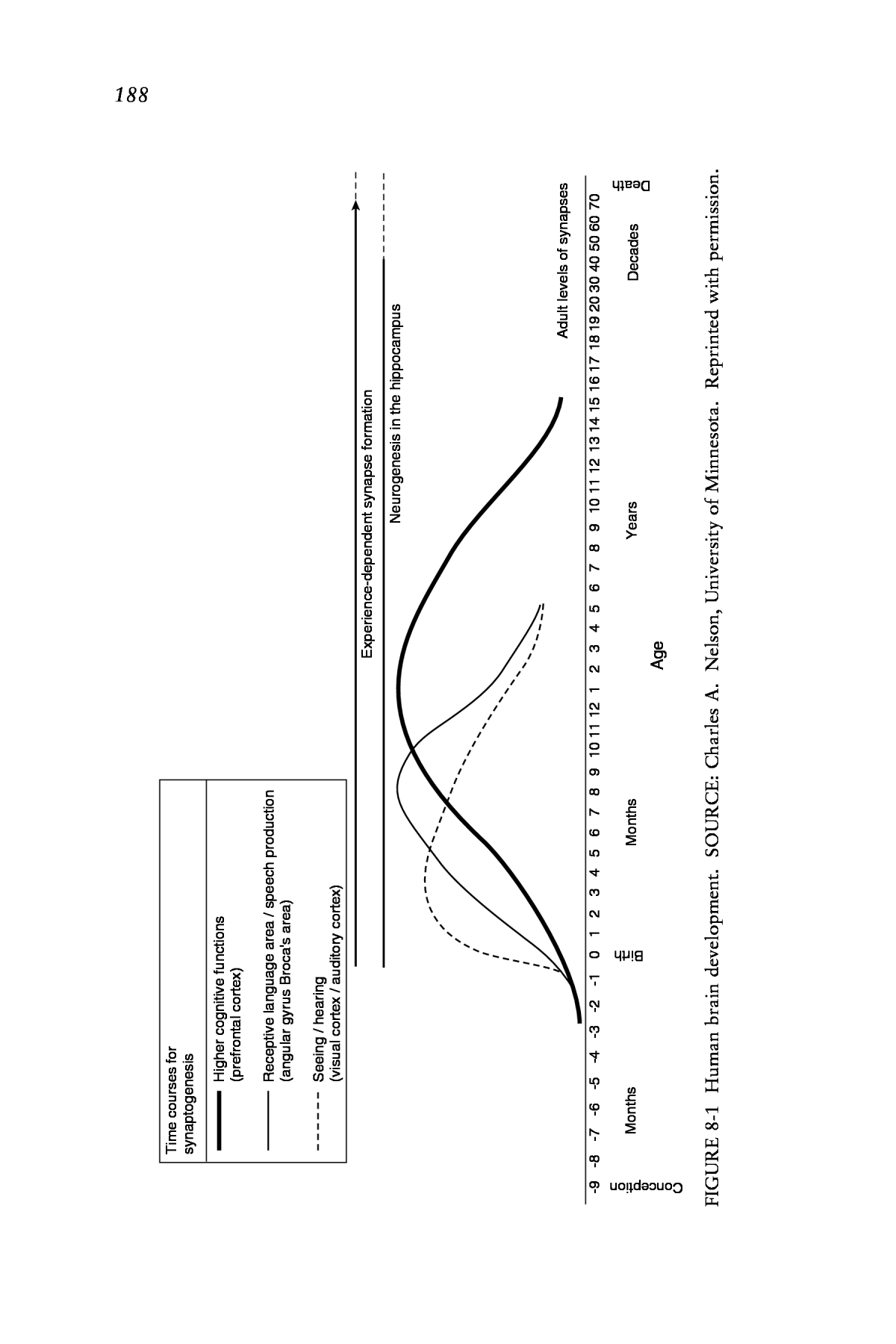
THE DEVELOPING BRAIN 187 determined by the development of myelin, a substance that wraps itself around nerve axons. By insulating the nerve cell axon, myelin increases conduction velocity. The development of myelin is a protracted process extending well into the postnatal period. The rate and extent of myelina- tion is also affected by experience. Most myelinated pathways are laid down in the early years, but for some, as in the frontal cortex, myelination continues into the third decade of life. The unique wiring diagram that brain development produces in each individual brain guides thoughts, memories, feelings, and behaviors. Synaptic Overproduction and Loss Beginning 20 years ago, Huttenlocher (e.g., Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997) first showed that there is a pattern to synaptogenesis in the human cerebral cortex characterized by the rapid proliferation and overproduction of synapses, followed by a phase of syn- apse elimination or pruning that eventually brings the overall number of synapses down to their adult levels. This process is most exuberant during the first few years of life, although it can extend well into adolescence. Within this developmental span, however, different brain regions with dif- ferent functions appear to develop on different time courses (see Figure 8-1). Huttenlocher estimated that the peak of synaptic overproduction in the visual cortex occurs about midway through the first year of life, fol- lowed by a gradual retraction until the middle to the end of the preschool period, by which time the number of synapses has reached adult levels. In areas of the brain that subserve audition and language, a similar although somewhat later time course was observed. However, in the prefrontal cortex (the area of the brain where higher-level cognition takes place), a very different picture emerges. Here the peak of overproduction occurs at around one year of age, and it is not until middle to late adolescence that adult numbers of synapses are obtained.1 Scientists have pondered the purpose of synaptic overproduction and loss for a very long time. One of the earliest observations was made by 1Many of the human findings regarding synaptic overproduction and loss were based on measurements of the density of synapses, rather than on measurements of the actual number of synapses. Density measures reflect both how many synapses are present and how many other things (e.g., nerve cell bodies, dendrites and axons, glial cells, and blood vessels) are present in addition to synapses. The human brain adds lots of cells to the cerebral cortex postnatally (almost two-thirds of the mass of the cerebral cortex is added after birth), and this makes density estimates very difficult to interpret. Thus, evidence available to date does not enable determination of how ubiquitous synapse overproduction and loss are in brain devel- opment generally or in humans specifically.
188 N eu ro ge ne sis in th e hi pp oc am pu s Ex pe rie nc e- de pe nd en t s yn ap se fo rm at io n Ad ul t l ev el s of s yn ap se s Death D ec ad es Ye ar s M on th s Birth M on th s Conception - 9 - 8 - 7 - 6 - 5 - 4 - 3 - 2 - 1 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 30 40 50 60 70 H ig he r c og ni tiv e fu nc tio ns (pr efr on tal co rte x) R ec ep tiv e la ng ua ge a re a / s pe ec h pr od uc tio n (an gu lar gy rus B roc a's ar ea ) Se ei ng / he ar in g (vi su al co rte x / au dit ory co rte x) Ag e Ti m e co ur se s fo r sy na pt og en es is FI G U R E 8 -1 H um an b ra in d ev el op m en t. SO U R C E : C ha rl es A . N el so n, U ni ve rs it y of M in ne so ta . R ep ri nt ed w it h pe rm is si on .
THE DEVELOPING BRAIN 189 Spanish neuroanatomist and Nobel laureate Santiago Ramon y Cajal (Ramon y Cajal, 1989[1917]): I noticed that every ramification, dendritic or axonic, in the course of formation, passes through a chaotic period, so to speak, a period of tri- als, during which there are sent out at random experimental conductors most of which are destined to disappear. . . . What mysterious forces precede the appearance of the processes, promote their growth and ram- ification . . . and finally establish those protoplasmic kisses, the inter- cellular articulations, which seem to constitute the final ecstasy of an epic love story? A more modern formulation of the love story began with the Cragg (1975) report that the cat visual cortex produced a greater number of synapses during development than it actually retained into adulthood. Sub- sequent work in monkeys and cats by Hubel and Wiesel and their collabo- rators (e.g., LeVay et al., 1980) demonstrated that as the physiological functioning of the visual cortex became more refined and precise, the ana- tomical synaptic connections were also refined. Those that fit the intended pattern were retained, and those that did not were eliminated. Scientists also showed that visual experience played a necessary role in this process. If experience was distorted, so that one eye got much more stimulation than the other, for example, its connections were pared back less drastically than usual, and the connections with the inexperienced eye were pruned more than usual. In short, the development of patterned organization in the visual cortex was dependent on visual experience and involved the selective loss of connections that were not appropriate to the pattern. Synapses appear to be programmed to be eliminated if they are not functionally confirmed, based on some not fully known aspects of their activity history. In general, frequently active connections, like those of the more experienced eye, are more likely to survive. While the data are not as complete for other senses, it is reasonably clear that building the organized neural systems that guide sensory and motor development involves the production of excess connections followed by some sort of pruning that leaves the system in a more precisely organized pattern. Moreover, in both humans and animals, the effects of experience on these systemsânormal or abnormalâbecome increasingly irreversible over time. In kittens, irreversible deficits in vision will result with depriva- tion lasting for only 2-3 months after birth. In humans, irreversible deficits in vision are present when corrections for such optical conditions as strabis- mus (in which, due to muscular weakness, one eye deviates from and can- not be brought into alignment with the other normally functioning eye) are not made by the time the child reaches elementary school. Deficits become more pronounced with more prolonged visual deprivation. Thus, a sensi-
190 FROM NEURONS TO NEIGHBORHOODS tive period exists for vision, but rather than being sharply demarcated, it gradually tapers off. A useful way to consider how experience becomes incorporated into the developing synaptic connections of the human brain, discussed briefly in Chapter 2, has been offered by Greenough and Black (Black and Greenough, 1986; Greenough and Black, 1992). They distinguish between experience-expectant and experience-dependent mechanisms guiding brain development. Experience-expectant synaptogenesis refers to situations in which a species-typical experience (that is, something that all members of a species experience, barring highly aberrant conditions) plays a necessary role in the developmental organization of the nervous system. Normal brain growth relies on these forms of environmental exposure. For ex- ample, the visual cortex âexpectsâ exposure to light and patterned visual information and is genetically programmed to utilize these inputs for nor- mal development. Deprivation of these ubiquitous and essential forms of environmental input can permanently compromise behavioral functioning, which is why it is essential to detect and treat early sensory deficits (e.g., cataracts, strabismus, auditory deficits) that interfere with the detection and registering of expected experiences. Experience-dependent synaptogenesis, in contrast, refers to encoding new experiences that occur throughout life, foster new brain growth and the refinement of existing brain structures, and vary for every individual. This process optimizes the individualâs adaptation to specific and possibly unique features of the environment. Whereas in experience-expectant de- velopment, all brains depend on the same basic experiences to develop normally, in experience-dependent development, individual differences in brain development depend on the idiosyncratic experiences that are en- countered across the life span. Experience-dependent development is also linked to synaptogenesis, but in this case all we know is that experience triggers more plentiful connections among neurons. We do not know if this occurs through a process of overproduction and pruning, or if a more continuous pattern of growth is involved. Whatever the specific mecha- nism, experience-dependent brain development is a source of enduring plas- ticity and of adaptability to the demands of everyday life. And it is impor- tant to note that there appears to be no abrupt transition from utilization of experience-expectant processes to utilization of experience-dependent pro- cesses of brain development. In fact, it seems likely that the greater poten- tial for recovery from deprivation or damage that characterizes young ani- mals probably reflects the availability of both mechanisms. Postnatal Neurogenesis We now need to add the possibility of postnatal neurogenesisâthe postnatal production of new nerve cellsâto the repertoire of mechanisms
THE DEVELOPING BRAIN 191 by which the human brain continues to develop after the early childhood years. Prevailing knowledge about brain development, notably that the adult human brain does not produce new neurons, has recently been chal- lenged by new insights into adult brain development. Specifically, impor- tant forebrain regions, such as the hippocampal dentate gyrus (which is involved in establishing memory for facts and relationships among events and places in oneâs experience), continue to receive new nerve cells into adulthood in humans (e.g., Eriksson et al., 1998). Recent findings in mon- keys indicate that new neurons are also being formed each day and migrat- ing to areas that include the prefrontal cortex, the seat of planning and decision making (Gould et al., 1999). Although it remains to be determined how significant neuronal additions in adulthood are to the functioning of the brain, it certainly lends further support to the argument that the brain continuously remodels itself. Neurochemistry of Early Brain Development The sending and receiving of messages in the nervous system depends on chemical messengers. A number of these chemical messengers affect gene expression in nerve cells in ways that have long-lasting effects on how nerves grow, respond to stimulation, and function. They are thus inti- mately involved in the growth and development of the nervous system and in neural plasticity. The past two decades have seen an explosion of infor- mation about these chemical messengers. In addition to the classic neurotransmitters, over 60 other peptide and steroid molecules have been identified that have direct effects on the brain. Currently, what can be confidently applied from this field directly to human development is lim- ited. However, the study of neurochemistry is already revolutionizing the way people think about the nervous system, and a brief overview of some basic ideas from this work is warranted. Chemical messengers that affect the brain operate through receptors, most of which are located in the dendrites and synapses of nerve cells. Like locks and keys, the physical structure of the messenger (the key) has to fit the physical structure of the receptor (the lock) for the chemical messenger to have any effect on the nerve cell. Receptors are specific. They typically recognize or bind with only one natural molecule. For many years, this type of specificity gave rise to the hope that science would be able to link specific neurochemicals to specific behaviors, allowing highly focused ma- nipulations of behavior through drug therapy. However, despite what filters its way into the popular press (e.g., low serotonin levels cause aggres- sion), the way the biochemistry of the brain operates is vastly more com- plex than a match of one chemical with one behavior. For example, it now appears that many of the chemicals that affect brain function are able to unlock several different receptors. This allows the same (or quite similar)
192 FROM NEURONS TO NEIGHBORHOODS chemical to have different functions and to play a role in multiple (often related) behavioral systems. The brain is also able to alter its sensitivity to a chemical messenger by changing the presence, conformation (structure), and availability of the chemicalâs receptors. Receptor changes often reflect the history of the nerve cellâs experience with its neurochemical. High levels of the chemical oper- ating on the receptor frequently result in a decrease in the nerveâs receptors for that chemical (a process called down-regulation); sometimes a dearth of a chemical important in a nerveâs functioning results in an increase in receptor number (i.e., up-regulation). Up- and down-regulation takes place over hours and days, partially explaining why some psychoactive drugs take time before they begin to influence behavior and why some drugs, with time, need to be taken in higher and higher dosages to have the same effects. Some of these shifts in chemical messenger-receptor systems appear to be relatively permanent, perhaps especially those that occur during periods of rapid development; others are more transient, reflecting the normal turn- over (production, decline, replacement) of receptors. This complexity may complicate things for those who are trying to decipher the mysteries of the brain, but it does allow the brain to be highly plastic, toning its functioning in highly nuanced ways, often quite rapidly. Neurochemical-receptor systems also lie at the heart of how the brain alters its physical structure. A variety of different nerve growth factors (i.e., chemicals that play a role in the growth of dendrites and synapses) have been identified. These growth factors are present in different quantities and locations at different points in development of the brain, regulated by genes involved in normal brain development. They also change in their concen- tration in response to nerve damage, playing a role in the brainâs attempts to adapt to and restore functioning following trauma. Receptor systems play critical roles in both experience-dependent and experience-expectant neural plasticity. The NMDA (N-methyl-D-aspartate) receptor is one re- ceptor, but not the only one, that plays a role in neural plasticity. It appears to support learning by helping to foster what is termed âlong-term poten- tiation.â Long-term potentiation, a memory âmodelâ involving increased synaptic strength, is brought about by sustained, rapid activity in the neural circuits involved in newly acquired information, analogous to repeating a new phone number in order to memorize it. It also appears that at critical points in the development of neural systems, there is sometimes an increase in NMDA receptors. This increase seems to open the window for the development of that neural system, allowing stimulation to have large ef- fects, with the window closing when the number of NMDA receptors de- creases. Changes in chemical messenger systems and their receptors tend to tone
THE DEVELOPING BRAIN 193 the nervous system, altering sensitivity to stimuli and probabilities of re- sponses, rather than necessarily causing particular behaviors. The follow- ing thought experiment provides a good example. You have been on a low- calorie diet (and have stuck to it) for several weeks. Numerous neurochemical changes in your brain have been set into motion by this semi-starvation. All of these changes do not mean that you will eat that luscious steak the waiter just set in front of you (the fact that you are dieting, are a vegetarian, and did not order the steak will hopefully rule the day). But the myriad of neurochemical changes in your brain set into motion by semi-starvation will probably make you more sensitive to how good the steak smells, make you salivate more, make you remember that steak for a long time, and so on; all changes orchestrated to help increase the probability that you will break down and eat the steak that your body might, in fact, âneed.â As this thought experiment indicates, the behavioral impact of changes in neurochemistry are dependent on the context and the individualâs history. Like oneâs temperament, the changes tend to orches- trate a bias or propensity to respond in particular ways rather than rigidly determine that a behavior will always be expressed. A number of research- ers believe that in order to understand the neural bases of temperament and emotions, they will need to understand the genetic and experiential pro- cesses that regulate these complex neurochemical systems of the brain throughout development. Characteristically, the neurochemical systems of the brain are open both to input from the environment and to events occurring in the body other than the brain. There is increasing animal evidence that the environ- ment plays a role in regulating aspects of brain neurochemistry. For ex- ample, the licking and grooming that the mother rat does of her pups (infant rats) appear to enhance the production of serotonin and thyroid hormone, both important in the neurochemistry of brain development. There is also increasing evidence that elements of early caregiving may help modulate the neurochemicals involved in pain and distress. Thus, the fats and sugars in breast milk appear to stimulate taste receptors linked to central opioid (natural painkiller) pathways, stimulating mild analgesia. Similarly, tactile stimulation of the mouth appears to operate through neu- rochemical mechanisms, not involving opioids, that affect brain pathways controlling distress. Some of these effects have been demonstrated in hu- man infants. The evidence that the regulation of neuroactive chemical systems extends into basic caregiving activities is exciting, even though much of it still has been demonstrated only in animals. This evidence promises to help explain how alterations in the environment early in life may have wide-ranging effects on brain development and may alter pat- terns of behavioral responding for children with different rearing histories.
194 FROM NEURONS TO NEIGHBORHOODS HOW THE BRAIN IS AFFECTED BY EARLY EXPERIENCE This account of early brain development emphasizes the ways in which the nervous system is designed to recruit and incorporate experience into its developing architecture and neurochemistry. Normal experience (e.g., good nutrition, patterned visual information) supports normal brain develop- ment, and abnormal experience (e.g., prenatal alcohol exposure, occluded vision) can cause abnormal neural and behavioral development (Black et al., 1998). Plasticity is a double-edged sword that leads to both adaptation and vulnerability. The process of synaptic overproduction and loss is de- pendent on environmental information, although the evidence is largely restricted to sensory systems. Similarly, the brainâs neurochemistry is ex- quisitely sensitive to behavioral and environmental stimuli. Scientists are far, however, from linking specific types or amounts of experience to the developing structure or neurochemistry of the immature human brain, and, conversely, from understanding how early brain development affects the ways in which young children process the abundance of information and experiences that their environments present to them. Answers to questions about when during development particular experiences must occur and when, in fact, timing is important and when it is not also lie, to a large extent, beyond the boundaries of current knowledge. Research on the developing brain can nevertheless provide a framework for considering the effects of early experience on development more generally. The questions that have been asked by neuroscientists have their parallels in research on behavioral development. Two issues have played pivotal roles in guiding scientific inquiry about early experience and the brain. The first concerns the nature of early experiences. Those who raise and work with young children are deeply concerned about whether they are providing them with the right experi- ences and protecting them from harmful ones. What harm is done by exposure to inappropriate experiences, and how reversible are the effects? What degree of enhancement can be achieved by exposure to enriched experiences, and how long do beneficial effects last? Much more is known about the negative consequences for brain development of harmful environ- ments than about the benefits of advantageous environments. And rela- tively more is known about the effects of pre- and perinatal environments on the developing nervous system than about environmental influences after the first few months of life. The second issue concerns the timing of experience and is often ex- pressed in terms of critical or sensitive periods. Much of the contemporary discussion of the importance of the first three years of life is framed in the terminology of sensitive periods. But does it really matter when the child is exposed to particular experiences? Do specific experiences need to occur
THE DEVELOPING BRAIN 195 during specific windows of time in order for the brain to develop normally? Can the brain recover or compensate when critical experiences are missed? In addition to the examples regarding the visual system described above, there are some very dramatic instances of timing effects, again primarily in other species. For example, an injury to the ratâs cortex on the first day after birth causes more ultimate damage to brain tissue and greater loss of normal behavioral functioning than a similar injury on day 5 (Kolb and Whishaw, 1998). The presence of testosterone in the third trimester of human fetal development organizes the physiological characteristics of brain regions such as the hypothalamus in the male direction, so that release of hormones that govern sexual and reproductive functions follows the noncyclic pattern seen in the post-adolescent male (Cooke et al., 1998). Although estrogen and testosterone can affect neural structures after this time, nothing can duplicate or reverse the effects of this in utero hormone exposure. Normal development of the zebra finchâs song (reviewed in Clayton, in press) requires exposure of the young male to an adult tutor during a sensitive period in juvenile life (Immelmann, 1969). The shortest period demonstrated to be sufficient for development of a relatively normal song extends from approximately day 20 to day 35 (Böhner, 1990). Zebra finches continue to be sensitive to the effects of further tutoring up to the age of about 65 days (Jones et al., 1996). In developmental science, the term âsensitive periodâ is generally pre- ferred to âcritical periodâ because it implies less rigidity in the nature and timing of formative early experiences (Immelmann and Suomi, 1982). Sen- sitive periods can be defined as unique episodes in development when spe- cific structures or functions become especially susceptible to particular ex- periences in ways that alter their future structure or function (Bornstein, 1989; Thompson, in press(a)). This susceptibility can operate in two ways: first, certain early experiences uniquely prepare the young children for the future by establishing certain capabilities at a time when development is most plastic and responsive to stimulation. Second, the young child is highly vulnerable to the absence of these essential experiences, and the result may be permanent risk of dysfunction. In fact, it is extraordinarily difficult to study issues of timing in human development given that it is profoundly unethical to deprive children of needed experiences in order to introduce them at different developmental stages. We are thus dependent on animal studies, which are generating fascinating evidence of timing effects (see, for example, Bornstein, 1989; Knudsen, 1999) but have limited translations to humans, and on so-called experiments of nature, such as prenatal exposures that occur at different points in fetal development (discussed below) and research on children with sensory deficits, such as the case of deaf children who do not experience normal spoken language inputs, and children who have sustained brain
196 FROM NEURONS TO NEIGHBORHOODS injuries. In the latter case, as we saw in Chapter 6, unilateral brain lesions incurred prior to age 5 or 6 appear to have few lasting effects on language development, whereas when damage occurs after this age language devel- opment is often compromised. However, there can be significant deficits in certain aspects of memory and verbal functioning when these lesions are accompanied by seizure disorders and these deficits do not appear to be sensitive to the age at which the seizures occur (Vargha-Khadem et al., 1997, 1992). This exemplifies the complexity of what is presently known about sensitive periods in childhood. Within these limitations, it is well known that a variety of environmen- tal factors play a significant role in modulating early brain development. Some of the greatest insights have come from research on the detrimental consequences of early biological insults, deprivations, and stress. We have also learned a great deal from research on the neurobiological consequences of prematurity. We turn to this research following a brief overview of the studies that generated excitement about the brainâs receptivity to environ- mental influence. The Contribution of Environmental Variation Documented differences in the brains and behaviors of animals that have experienced markedly discrepant early environments have emerged from the laboratory of Greenough and his colleagues (Black and Greenough, 1998; Black et al., 1998; Greenough and Black, 1992). Rats, not babies, were the subjects of study. They were either housed from the time of weaning or placed as adults in cages that varied in the degree of stimulation offered. The âcomplexâ cages contained play objects and other animals. Animals reared since weaning or placed in these cages as adults outper- formed rats raised alone or placed in typically barren laboratory cages on a variety of learning and problem-solving tasks. The brains of the rats reared in the complex environments also showed more mature synaptic structure, more dendritic spines, larger neuronal dendritic fields, more synapses per neuron, more supportive glial tissue, and increased capillary branching that increases blood volume and oxygen supply to the brain (see Box 8-1). It is important to note that these effects did not appear to be character- ized by a critical period. The indicators of both superior performance and more developed brains characterized the rats exposed to the complex envi- ronments as adults, as well as those housed in these environments since weaning. Both early and later exposure to greater environmental stimula- tion had beneficial consequences, although the effects occurred more rap- idly and to a greater degree in the younger animals. Moreover, while long- term neuron and synapse studies have not been conducted, the effects of exposure to a complex environment on learning ability diminished over
THE DEVELOPING BRAIN 197 time if the rats were removed from the environment. The intervention provided by the complex cages thus functioned more like the tetanus vac- cine, which requires regular boosters, than the smallpox vaccine, which inoculates against disease with a single injection. As this book shows, most early interventions for humans act more like the tetanus than the smallpox vaccine. Studies of complex environments in rats have also revealed the role that such environments can play in processes of recovery. For example, the detrimental behavioral effects of prenatal exposure to low to moderate levels of alcohol in rats (e.g., motor dysfunction and impairments in learn- ing spatial tasks) can be greatly attenuated by raising the animals in a complex environment (Hannigan et al., 1993). A program of forced motor skill training in alcohol-exposed rats nearly eliminated motor dysfunction, and it also increased synapse number in their cerebellar cortex (Klintsova et al., 1997, 1998). Finally, increasing the complexity of the environment before or after brain damage in developing and adult rats enhanced recov- ery from the impairments produced by damage to various brain areas, probably through mechanisms that involve the development of alternative strategies rather than the direct recovery of lost functions (see Kolb and Whishaw, 1998). This research on complex environments certainly suggests that better BOX 8-1 Experience, Learning, âExercise,â and the Brain Some neuroscientists are trying to understand learning at the level of the nerve cells and the synaptic connections through which they communi- cate. As noted in the text, early work found that rats reared in a complex environment exhibited substantial increases in the numbers of synapses in various parts of the brain. These studies have shown, however, that formation of new synapses is probably one of the mechanisms underlying memory. Follow-up studies examining motor skill learning in adult rats found that animals that performed a lot of effortful exercise without signif- icant learning formed new blood vessels in their brains but no new syn- apses. If they learned motor skills, with minimal exercise, they formed synapses but not new blood vessels (Black et al., 1990). This shows both that (1) there are components to the brainâs adaptation to the environ- ment beyond neurons and synapses and that (2) making new synapses is associated with learning. In addition, this research has shown that the ability to add synapses in response to housing in a complex environment or learning something new is a lifelong property of the brainânot some- thing lost at an early ageâwhich is precisely what we would expect for memory.
198 FROM NEURONS TO NEIGHBORHOODS environments with greater diversity can have beneficial effects. However, it would be misleading to locate the complexity of the animal environments near the enriched part of the continuum from deprivation to enrichment. In fact, the environments in these experiments were probably less complex than the ratsâ natural wild habitats. Nevertheless, these studies do point to the existence of a multidimensional continuum of environments and indi- cate that development (and recovery) is improved as one moves toward the anchor point of enrichment. Moving from these animal studies to research on the neurological aspects of human cognitive, linguistic, and social-emo- tional development is a big leap, but one that warrants a major investment of time and resources. The need for research that can illuminate how environments that exceed some minimal threshold of adequacy affect hu- man brain development is especially needed, in light of the fact that most of the research on how experience affects the developing brain explores the detrimental consequences of harmful experiences. Early Biological Insults and the Developing Brain Research on early biological insults provides fundamental insights into the vulnerability and resilience of the developing central nervous system. This area of research also offers a compelling illustration that plasticity cuts both ways, leaving the developing fetus and young child simultaneously vulnerable to harm and receptive to positive influences. It also suggests that the current emphasis on the years from birth to age 3 may have unwittingly bypassed an important stage of development: the prenatal period is when damaging environmental conditions may have some of the most devastat- ing effects on development and, consequently, is when preventive efforts may have the greatest benefits. Environmental factors that play a significant role in modulating prena- tal and early postnatal brain development include substances and circum- stances that are necessary for normal brain development, as well as expo- sures to chemicals, diseases, and stressors that are toxic or disruptive. As with psychosocial risks, such as poverty and family violence, their effects on development are probabilistic; they increase, but do not seal, the odds of impaired development. Table 8-1 lists some of the environmental factors that are beneficial and some that are detrimental. The factors listed, by no means exhaustive, are examples selected on the basis of clinical importance, availability of basic research on brain effects, and existence of relevant clinical studies of human infants. We consider below a few of these detri- mental conditions and substances in more detail: an infectious disease (ru- bella); a developmental neurotoxin (alcohol), and a nutrient deficiency (lack of iron).
THE DEVELOPING BRAIN 199 TABLE 8-1 Conditions and Substances that Affect the Developing Brain Needed for Normal Brain Development Detrimental or Toxic Oxygen Alcohol Adequate protein and energy Lead Micronutrients, such as iron and zinc Tobacco Adequate gestation Prenatal infections Iodine Polychlorinated biphenyls (PCBs) Thyroid hormone Ionizing radiation Folic acid Cocaine Essential fatty acids Metabolic abnormalities (excess phenylalanine, ammonia) Sensory stimulation Aluminum Activity Methylmercury Social interaction Chronic stress Note: The listed factors are not intended to be exhaustive. Infectious Disease Rubella (German measles) is a classic example of an infectious disease that causes harm in utero. Exposure to rubella early in prenatal develop- ment affects the organs (e.g., eyes, ears) that are developing at the time that the virus crosses the placental barrier. Because the development of most organs is largely complete by the end of the first trimester, fetal develop- ment during the second and third trimesters of pregnancy is relatively more protected from the negative effects of the rubella virus. The rubella story demonstrates how long it has often taken to recognize that a particular condition or exposure can put the fetus or child at risk. It was widely believed that few diseases were as benign as rubella until 1942, when the first report of the devastating effects of maternal infection during pregnancy was published (Gregg, 1942). One of the puzzles is why the medical community did not figure out the link between maternal rubella and congenital malformations earlier. Some qualities of rubella, which exist in other conditions as well, made it difficult to make the connection (Beswick et al., 1949). For example, it is not always clear that a fetus has been exposed to a particular infectious illness or toxic agent during preg- nancy. In the case of rubella, there are many causes of fever, rash, and other symptoms that are seen. To complicate matters further, effects on the developing fetus or child may also be quite variable. For instance, rubella may affect the fetusâs eyes, ears, brain, or heart, among other organs. Furthermore, the very idea that the fetus could be vulnerable to harm was novel before the rubella syndrome was accepted. This is now known to be true for many conditions, such as some of those in Table 8-1.
200 FROM NEURONS TO NEIGHBORHOODS The rubella story also illustrates a triumph in prevention. As better methods of diagnosing rubella became available in the 1960s (Forbes, 1969), there was more certainty about which rashes and nonspecific symptoms in early pregnancy were due to rubella and which were not. Today, public health policy requiring universal immunization against rubella has virtually eliminated the problem of the congenital rubella syndrome in the United States. Developmental Neurotoxins Substances such as drugs and chemicals that are damaging to the devel- oping nervous system are known as developmental neurotoxins. Table 8-1 indicates a number of these agents. Their effects on brain and behavior have been summarized in several comprehensive volumes (Kimmel et al., 1990; Slikker and Chang, 1998), as well as in thousands of original re- search reports. We use prenatal alcohol exposure as an example of this class of early biological insult. The effects of prenatal alcohol have been studied extensively, and the current state of knowledge was recently consid- ered in depth in an Institute of Medicine report (Institute of Medicine, 1996). Major points related to questions of early brain and behavior development are highlighted here. The adverse effects of prenatal alcohol exposure are now so widely known and accepted that it is hard to believe that the first report was issued only 30 years ago. Fetal alcohol syndrome was first described in the En- glish-language medical literature in 1973 (Jones and Smith, 1973). Mater- nal alcohol consumption during pregnancy can lead to facial deformities, loss of neurons, severe neurobehavioral impairment, and impaired cogni- tive functioning, among other problems. Its consequences appear to persist throughout life (Connor and Streissguth, 1996; Institute of Medicine, 1996; Jacobson et al., 1993; Sampson et al., 1994; Streissguth et al., 1996a). They are not, however, inevitable. One of the perplexing aspects of fetal alcohol exposure is that, even with high doses of alcohol, not all fetuses develop symptoms of fetal alcohol syndrome or alcohol-related neuro- developmental disorder (see below). Its importance lies in its prevalence and preventability, not its inevitability. Nonetheless, this is a very common cause of harm to the fetus that can be prevented. Survey data collected by the Centers for Disease Control and Preven- tion show that the incidence of drinking at levels sufficient to put the fetus at risk for neurobehavioral impairment was 3.5 percent in 1995 (the most recent year for which data are available), with binge drinking the predomi- nant pattern (87 percent of the cases) (Ebrahim et al., 1998). The propor- tion of women who consume alcohol during pregnancy has decreased since the mid-1980s (Serdula et al., 1991), although much of the decline is due to
THE DEVELOPING BRAIN 201 the changed habits of light drinkers. Women who drink heavily, who pose the greatest risk to their fetus, appear to be more resistant to prevention efforts. Heavy drinking and the consequent incidence of fetal alcohol syndrome are much higher among black Americans than among white Americans (Abel, 1995; Faden et al., 1997) and are also high among Ameri- can Indians (Duimstra et al., 1993). Fetal alcohol syndrome is the most severe form of prenatal alcohol effects. Defined by a specific pattern of facial and other physical deformi- ties accompanied by growth retardation, fetal alcohol syndrome identifies a relatively small proportion of children prenatally affected by alcohol. The Institute of Medicine (1996) recently suggested that the term âalcohol- related neurodevelopmental disorderâ be used to focus specifically on brain dysfunctions in the presence of significant prenatal alcohol exposure but without physical deformities. Fetal alcohol syndrome is estimated to occur at a rate of 1-3 per 1,000 live births; alcohol-related neurodevelopmental disorder is estimated to be at least 10 times more prevalent. Brain dysfunc- tions in alcohol-exposed children without fetal alcohol syndrome are often as severe as those in children with the full impairment. A variety of neurobehavioral changes have been observed in children exposed to alcohol prenatally. These effects range from problems with attention and memory to poor motor coordination to difficulty with prob- lem solving and abstract thinking. Infants and toddlers may be delayed in reaching important milestones, may have difficulty tuning out excess sen- sory stimuli, and often are hyperactive. About half of all individuals with fetal alcohol syndrome are mentally retarded (IQ < 70). Both severely and more mildly affected children demonstrate slower information processing and longer reaction times and appear to have specific problems with arith- metic (Jacobson et al., 1994). These effects have been documented through the early adolescent years and into adulthood. Such results demonstrate the importance of assessing functions other than IQ. In fact, these measures often detect effects of early biological insults in the absence of IQ differ- ences, and behavioral disturbances may create more functional impairment than a lower IQ. In addition, more specific and sensitive measures may indicate differing effects of various developmental neurotoxins (Jacobson, 1998). The importance of considering timing (when a condition occurs during development), severity (degree or dose), and chronicity (how long it lasts) in attempting to understand the effects of early biological insults is well illus- trated by prenatal alcohol exposure. In general, the prenatal period ap- pears to be distinguished by its sensitivity to a large array of harmful conditions. But even within the prenatal period, timing matters. For instance, alcohol exposure early in gestation has different effects on the developing brain from similar exposure later on. Case reports from autop-
202 FROM NEURONS TO NEIGHBORHOODS sies and, more recently, neuroimaging studies (Riley et al., 1995; Sowell et al., 1996; Swayze et al., 1997) give an indication of central nervous system effects in humans. However, animal modelsâwith experimental manipula- tion of alcohol exposure and direct examination of brain tissueâcontinue to provide crucial information. In the mouse, for example, exposure to alcohol on days 7 and 8 of gestation results in not only the typical facial deformities of fetal alcohol syndrome but also brain anomalies, such as small overall size and deficiencies in cerebral hemispheres, striatum, olfac- tory bulbs, limbic structures, the corpus callosum, and lateral ventricles. Exposure later in gestation generally does not produce such gross structural malformations but nonetheless kills nerve cells and interferes with synapto- genesis, formation of myelin, and other biochemical processes, including reduction of NMDA receptor binding in the hippocampus. Research with humans also shows that the timing of prenatal alcohol exposure has differential effects (Connor and Streissguth, 1996; Institute of Medicine, 1996; Jacobson et al., 1993, 1998; Sampson et al., 1994; Streissguth et al., 1996a, 1996b). The unusual facial features of fetal alcohol syndrome in the human infant (e.g., low-set ears, short philtrum, cleft palate, cleft lip) appear to be due to heavy exposure early on, in the first trimester, when the structures that come together to form the face are developing. Fetal exposure to alcohol during the second and especially the third trimester of pregnancy appears to be a time of particular vulnerability for the impaired neurobehavioral development, although some data suggest that these effects extend throughout pregnancy. Dividing cells appear to be particularly sensitive to the toxic effects of alcohol, and hence a period during which extensive neurogenesis occurs would be a time of acute sensi- tivity to the effects of alcohol. The cognitive effects associated with expo- sure to alcohol later in pregnancy, for example, may be associated with the high level of neuronal cell division in pertinent parts of the brain that occurs during the third trimester. The severity of exposure is another important factor in understanding ill effects, perhaps as important as the timing. For prenatal alcohol use, greater exposures are associated with worse effects. In addition, episodic binge drinking appears to be more harmful to the developing brain than equivalent levels of alcohol consumed steadily. Experimental animal stud- ies indicate that ingestion of a given dose of alcohol over a short period of time generates a greater peak blood alcohol concentration than the same dose ingested over several days (Bonthius and West, 1990). Thus, the developing fetus is actually exposed to a higher level of alcohol in binge drinking and has been found in animal research to experience greater neu- ronal (Bonthius and West, 1990) and behavioral (Goodlett et al., 1987) impairment. In humans, binge drinking is more of a problem than is usually recognized, because moderate drinkers, who consume 1-2 drinks
THE DEVELOPING BRAIN 203 per day on average, in fact, tend to concentrate their drinking on 1-2 days per week, thus drinking 4 or more drinks per occasion (Jacobson and Jacobson, 1999). When juxtaposed with evidence on the timing of alcohol exposure, the detrimental effects of binge drinking suggest that any bouts of drinking during pregnancy run the risk of damaging some aspect of the developing brain. Chronicity is another important factor in understanding the effects of early biological insults. In the case of prenatal alcohol exposure, it appears that the effects on the fetus worsen with successive pregnancies. Specifi- cally, older mothers who are moderate-to-heavy drinkers are at higher risk for having an affected offspring (Institute of Medicine, 1996). This may be due to reduced ability to metabolize alcohol by women who have been drinking heavily for several years (Jacobson et al., 1993, 1994). In the case of alcohol exposure, chronicity should thus be thought of as a dimension of risk both within and between pregnancies. Research on early biological insults has also yielded information on modifiability or brain plasticity. Environmental interventions to reduce the effects of alcohol exposure (other than specific treatment of a toxin or deficiency) have been studied for only a few conditions. Prenatal alcohol exposure is perhaps the best researched in recent years. In animal models, a variety of interventions has been shown to ameliorate some of the central nervous system effects of alcohol (Greenough and Black, 1992; Hannigan et al., 1993; Klintsova et al., 1997; Weinberg et al., 1995). Effective inter- ventions include motor training, procedures that enhance maternal care- giving behaviors, and a postweaning environment that is physically and socially stimulating. However, one should not conclude that the process is trivial. For instance, getting a rat to do motor training may require quite heroic efforts on the part of the investigator, and interventions typically do not bring the brain and behavior of exposed animals fully back to the levels of animals who never experienced the biological insult. As common sense would suggest, protecting the developing brain from early biological insults is a more desirable and effective strategy than trying to correct the deficits once they have occurred. Fetal alcohol research provides a particularly compelling case for preventive interventions, as well as for early detection and treatment of associated difficulties. Nutrient Deficiency Both before and after birth, nutritional adequacy is important for opti- mal brain development and function (see Georgieff and Rao, 1999, and Morgan and Gibson, 1991, for recent reviews). The effects of generalized undernutrition (lack of sufficient protein, energy, and other nutrients) on the developing brain have been studied extensively over several decades
204 FROM NEURONS TO NEIGHBORHOODS (Dobbing and Smart, 1974; Morgane et al., 1993; Strupp and Levitsky, 1995; Winick and Rosso, 1969). This research has demonstrated that the timing of nutrient supplementation or deficiency is important. For ex- ample, nutritional deprivation in the second trimester of pregnancy has been shown to result in deficient numbers of neurons, whereas deprivation in the third trimester affects numbers of glial cells and the maturation of neurons (e.g., Dickerson, 1981). Postnatal nutrition also appears to show timing effects, with the first 2 to 3 years of life being an especially vulner- able time for effects on brain growth. The earlier the malnutrition occurs, the greater the reduction in brain size, and the longer the malnutrition continues, the greater the effect on the brain (Morgan and Winick, 1985; Winick, 1976). Nevertheless, as the literature on orphanage-reared infants illustrates (reviewed in the next chapter), young children can show remark- able recovery in growth and behavior even after gross early (postnatal) generalized malnutrition when they are fed adequately. Although sufficient nutrient intake is important throughout life, certain nutrients have a more profound effect on the developing brain than others. The following discussion summarizes research on iron deficiency, an area in which there has been a recent burst of relevant research. Iron deficiency is probably the worldâs most common single nutrient disorder. Approxi- mately 20 to 25 percent of babies worldwide have iron-deficiency anemia, and a much higher proportion have iron deficiency without anemia (deMaeyer and Adiels-Tegman, 1985; Joint Committee on Health Policy of the World Health Organization and UNICEF, 1994). The latter is common even in countries where public health interventions have reduced anemia. In the United States, for instance, the prevalence of iron-deficiency anemia has decreased dramatically (Looker et al., 1997), due to fortification of infant formula and cereal and increased breast-feeding, among other fac- tors. However, poor and minority children are still at considerable risk for iron deficiency with or without anemia (Ogden, 1998). In a recent U.S. national survey, nonpoor white toddlers had the lowest prevalence of iron deficiency (about 3 percent), while Mexican-American toddlers were at highest risk regardless of economic status, affecting approximately 18 per- cent of poor and 12 percent of nonpoor Mexican-American children (Ogden, 1998). Altered behavior and development are among the most worrisome con- cerns about iron deficiency in infancy. Iron-deficient anemic infants gener- ally test lower in mental and motor development (see review by Nokes et al., 1998). Other behavioral differences, such as increased fearfulness, fatigue, and wariness, have also been noted (Honig and Oski, 1984; Lozoff et al., 1985, 1986, 1996, 1998; Walter et al., 1983, 1989). Although one study reported that test scores improved with a full course of iron treatment (Idjradinata and Pollitt, 1993), the other available studies found that a
THE DEVELOPING BRAIN 205 majority of infants with iron-deficiency anemia continued to have lower developmental test scores (Aukett et al., 1986; Lozoff et al., 1987, 1996; Walter et al., 1989), despite iron therapy for 2-6 months and correction of anemia; other behavioral differences were also still observed (Lozoff et al., 1998). Differences thus appear to persist. Follow-up studies have sought to determine if differences persist be- yond infancy. Several studies have shown that, at early school age, children who were anemic as infants continue to have lower test scores than their peers who did not experience anemia (Dommergues et al., 1989; Lozoff et al., 1991; Palti et al., 1983, 1985; Walter et al., 1990). A comprehensive follow-up at the transition to adolescence (Lozoff et al., 2000) found that children who had been treated for severe, chronic iron deficiency in infancy still scored lower on measures of mental and motor functioning, specifically in arithmetic achievement and written expression, motor functioning, and some specific cognitive processes such as spatial memory and selective re- call. They were also more likely to have repeated a grade. Parents and teachers rated the formerly iron-deficient children as showing more anxiety or depression, social problems, and attention problems. In a different, population-based study (Hurtado et al., 1999), children who were anemic in infancy (presumably due to iron deficiency) were at increased risk for mild to moderate mental retardation at age 10. Thus, severe, chronic iron deficiency in infancy identifies children who continue to be at developmen- tal and behavioral risk more than 10 years later. Basic research and animal studies indicate some possible mechanisms for such behavioral and developmental differences. Iron is required for many processes, including neurotransmitter synthesis (dopamine being the most studied), myelination, and oxidative metabolism (reviewed in Georgieff and Rao, 1999). Maximal transport of iron into the brain corre- sponds with the brain growth spurt, and iron deficiency during this period results in a deficit of brain iron in animal models. These observations suggest that the developing brain may be particularly vulnerable to the effects of this nutrient deficiency. Conversely, free or excess iron is toxic to cell membranes and may contribute to neuronal damage following a brain injury. New studies that utilize neurophysiological and electrophysiological methods are now providing data on iron-deficient human infants and dem- onstrating close links to results in animal models. In one such study (Roncagliolo et al., 1998), 6-month-old infants with iron-deficiency anemia had slower nerve conduction in the auditory pathway. Differences in nerve conduction velocity between anemic and nonanemic infants increased over the following year despite iron therapy. A disruption or defect in myelina- tion was considered to be a promising explanation, given that brain iron is required for myelination, young iron-deficient animals have been noted to
206 FROM NEURONS TO NEIGHBORHOODS be hypomyelinated, and the auditory system is rapidly myelinating in the first two years after birth in the human infant (reviewed in Roncagliolo et al., 1998). The hippocampus, which is a key structure in the circuit that subserves recognition memory, also appears to be vulnerable to early iron deficiency (de Ungria et al., 2000; Erikson et al., 1997). In animal models, iron deficiency results in markedly reduced neuronal metabolism (as indicated by cytochrome coxidase activity) in all subareas of the hippocampus and other regions involved in higher cognitive functions (de Ungria et al., 2000). Preliminary evidence from a study of infants of diabetic mothers (who are at risk for lower levels of iron in the liver, heart, and brain in addition to hyper- and hypoglycemia and hypoxia), using electrophysiological tech- niques, has revealed impaired recognition memory despite normal iron status at 6 to 8 months of age (de Regnier et al., in press). These findings are consistent with a hippocampally based memory deficit, and iron defi- ciency may be a contributing factor. Disruptions in recognition memory, in turn, may be a subtle early effect that could contribute to learning disabili- ties later on. It is important to emphasize that early biological risks and insults, such as iron deficiency, often do not occur in isolation (see Figure 8-2). In fact, they typically are increased among infants who also grow up in disadvan- taged environments. Iron deficiency, for example, is more prevalent among poor infants in the United States (McLoyd and Lozoff, 2000). Thus, in human studies, it can be exceedingly difficult to disentangle poor develop- ment and behavioral outcomes that are due to the biological exposure, from those due to the problematic environment. Prematurity and Early Brain Development One of the true marvels of human brain development is that an infant can be born prematurely in the early part of the third trimester and not only survive, but also achieve something resembling his or her potential in men- tal and motor behavior. Highly sophisticated intensive care techniques have improved survival rates of premature infants. The KidsCount data of the Annie E. Casey Foundation shows that the percentage of low-birth- weight babies increased in each of the 50 states between 1990 and 1997 (Annie E. Casey Foundation, 2000). Low-birthweight babies were 7.5 percent of all births in 1997, compared to 7.0 percent in 1990. This represents a 7 percent increase over just this 7-year period (National Center for Health Statistics of the Centers for Disease Control, 1993, 1999). The borders of viability (approximately 24 weeks of gestation), how- ever, have not changed since 1980 (Richardson et al., 1998). Greater than 95 percent of infants born after 28 weeks of gestation and greater than 50
THE DEVELOPING BRAIN 207 percent of infants born at 24-28 weeks survive (Hack et al., 1991). At the very borders of viability (22-24 weeks of gestation), mortality remains high. Of the infants who survive, a high percentage have sustained damage to developing neurological structures and have significant neurological mor- bidity (Allen et al., 1993). Moreover, recent research with toddlers suggests that even low-risk preterm infants (those born between 27 and 34 weeks gestational age) cannot be assumed to have caught up with their full-term counterparts in all aspects of cognitive development (de Haan et al., 2000). Nevertheless, it is safe to say that, over the past decade, neonatology has begun to concern itself less with survival (mortality) and more with out- come (morbidity) (Richardson et al., 1998). The corresponding challenges that these infants at the border of viability present to society are only beginning to be addressed. It is useful to consider preterm infants as fetuses who develop in extrau- terine settings at the time when their brains are growing more rapidly than at any other time in their life (Als, 1997; McClellan, 1972). Prematurity Iron deficiency anemia Decreased brain iron Hypo- myelination Stress vulnerability Behavior pattern Delayed neuromaturation Impaired dopaminergic function Poor feeding practices Functional isolation Poorer outcome Learning experiences Disadvantaged environment Limited support for child dev. Parenting FIGURE 8-2 Model of interaction of environment, nutrition, parenting, and child characteristics on outcomes. SOURCE: Lozoff et al., 1998. Reprinted with per- mission.
208 FROM NEURONS TO NEIGHBORHOODS has two main negative effects on brain development. First, premature birth predisposes the infant to pathological events that directly injure the brain. These events can be thought of as damage committed by factors that the human at this gestational age would not normally encounter. These can be as seemingly benign as the wrong mixture of nutrients to more obvious neuropathologies such as intracranial hemorrhage. Second, premature birth interrupts the normal process of intrauterine brain development by denying it expected intrauterine stimuli and factors important for growth (e.g., nutrients such as docosohexaenoic acid). One can consider this to be disruption due to omission of factors that are critical for normal develop- ment. Ultimately, the morbidity seen at any gestational age is the result of the combination of the number and severity of exposure to both types of influence. The first principle of assessing the effect of prematurity on neurological outcome is to note that the childâs general developmental status and intelli- gence scores decrease with reductions in gestational age (Saigal et al., 1991). Thus, an infant born at 24 weeks is at greater risk than an infant born at 26 weeks, who in turn is at higher risk than an infant born at 28 weeks. Infants born at 24 weeks not only have a less complete brain than those born at 26 weeks, but they also are far more prone to intracranial hemor- rhage, hypoglycemia, and postnatal malnutrition, all of which adversely affect the more primitive parts of the brain. Once one moves out of the high-risk groups, however, outcomes become highly variable. Insults Due to Prematurity The literature on neonatal outcomes is replete with studies assessing the effects of intracranial hemorrhage (Papile et al., 1983), periventricular leucomalacia (Feldman et al., 1990; Lowe and Papile, 1990), hypoglyecmia (Duvanel et al., 1999), and malnutrition (Georgieff et al., 1985, 1989; Hack and Breslau, 1986) on head growth and developmental outcome. Besides gestational age and socioeconomic status, the next most important factor in assessing risk of adverse neurological outcomes is the degree of illness of the infant during the newborn period. Infants whose overall physiology is more compromised are more developmentally delayed at 2 years and appear to be at greater risk of prefrontal deficits at age 8 (Brazy et al., 1991; Luciana et al., 1999). Intracranial hemorrhage (also known as intraventricular hemorrhage) is the most extensively studied noxious event that affects the premature infantâs brain. This is probably due to the fact that it is easily visualized by cranial ultrasonography and quantifiable into Grades I (least severe) to IV (most severe). Approximately 20 percent of infants between 28 and 34 weeks gestation have intraventricular hemorrhage, with the vast majority
THE DEVELOPING BRAIN 209 (> 60 percent) rated as Grade I or II. In contrast, 60 percent of infants born between 24 to 28 weeks have intraventricular hemorrhage, and their hem- orrhages tend to be the more severe Grade III and IV varieties. Accord- ingly, the risk of major handicaps, both motor and cognitive, is increased. Infants with lower-grade hemorrhages do not appear to be at any greater risk of major handicap (cerebral palsy, mental retardation) than infants who did not bleed (Papile et al., 1983), although they are at higher risk of minor handicaps (e.g., behavior problems, attention problems, memory deficits) (Lowe and Papile, 1990; Ross et al., 1996). Omission of Factors Important for Normal Brain Development A premature infant with a benign neonatal course nevertheless remains at increased risk of neurological morbidity. Although one can never be assured that all noxious events (both prenatal and postnatal) have been accounted for in any given study, there is mounting evidence that transfer- ring brain growth and development from an intrauterine to an extrauterine environment prematurely is less than optimal even in the absence of other definable neurological risk factors (Chapieski and Evankovich, 1997; Cherkes-Julkowski, 1998; Huppi et al., 1996). Recent research, for ex- ample, has demonstrated poorer performance on elicited imitation tasks (a medial temporal lobe function) at age 18 months in 27- to 34-week gesta- tional age preterm infants with completely benign neonatal courses com- pared with term infants tested at the same post-conceptional age (de Haan et al., 2000). These emerging data strongly suggest that the human brain continues to develop in a unique way in utero until the end of gestation and that early termination of pregnancy disrupts that development with subse- quent behavioral consequences. A more pernicious effect of extrauterine life on brain development in small preterm infants is the general problem of malnutrition. Neonatal illness not only predisposes preterm infants to definable adverse events (e.g., intraventricular hemorrhage, hypoxia) but also blocks provision of adequate nutritional substrates to promote normal brain growth and devel- opment. Studies have estimated that greater than 50 percent of very low- birthweight infants fall below the 5th percentile for head growth sometime during their hospitalization (rendering them, by definition, microcephalic) (Georgieff et al., 1985). Fortunately, one of the most amazing aspects of early human life is the ability of the head (and brain) to demonstrate catch- up growth. After a period of no growth, the head exhibits a remarkable increase in growth velocity to double or triple normal rates, given adequate protein-energy intakes (Georgieff et al., 1985; Sher and Brown, 1975). There is, however, a point of diminishing return. If the infant has had no growth for more than a month, the subsequent catch-up rate is markedly
210 FROM NEURONS TO NEIGHBORHOODS reduced, almost as if the potential for catch-up has been lost (Georgieff et al., 1985; Hack and Breslau, 1986; Sher and Brown, 1975). Premature infants with more striking postnatally acquired microcephaly due to malnu- trition indeed have smaller head circumferences and poorer scores on the Bayley Scales of Infant Development at age 12 months (Georgieff et al., 1985). Reduced head circumference at 8 months postnatally bodes poorly for developmental outcomes measured at age 3 and 8 years (Hack and Breslau, 1986). These studies suggest that although catch-up head growth is a marvelous compensatory response, it is better to have never experi- enced the growth deficit in the first place. Extrapolating further, it argues for important windows of opportunity for brain growth late in the third trimester that, if interrupted by premature birth and lack of head growth, may result in the brain being âconstructedâ in an alternative manner (de Haan et al., 2000). In sum, prematurity confers a significant risk to the developing brain. The risk emanates from both insults that arise during the course of illness in the premature infant and from interruptions of the provision of the ex- pected substrates and environment apparently necessary for normal brain development. We have used examples for which there is a substantial literature (e.g., intraventricular hemorrhage), but hasten to add that other potentially neuropathological factors that are more difficult to isolate and quantify (e.g., hypoxia-ischemia, hypoglycemia, neurotoxic medications such as steroids) are likely to play important roles as well. The ultimate risk to any single premature infant is likely to be a composite of all the known and unknown risk and protective factors that characterize that infant, and on the infantâs general extent of biological and environmental vulnerability. Thus the premature infant born to a lower-income mother with few re- sources who received poor prenatal care is likely to have a much more difficult neonatal course, and therefore be at higher neurodevelop-mental risk, than an infant of the same gestational age born to a mother who received better prenatal care and has more resources. Perhaps this helps explain the overall down-shifting of developmental scores in premature infants from families of lower socioeconomic status (Saigal et al., 1991). Growing awareness of environmentally based differences in the out- comes of premature infants has fueled multiple intervention efforts ranging from dramatic changes in the care these infants receive in neonatal intensive care units (see reviews by Als, 1997; Hernandez-Reif and Field, 2000) to comprehensive initiatives that provide a range of services to the infants and their families from the time they leave the hospital to several months or years after discharge. The best known of the comprehensive approaches is the Infant Health and Development Program (see Box 8-2) (Gross et al., 1997), which included a randomized trial and extensive follow-ups of the participating families.
THE DEVELOPING BRAIN 211 BOX 8-2 Infant Health and Development Program Premature babies with low birthweight are more likely than babies with normal birthweight to have a range of health and developmental prob- lems, including lower IQ, cerebral palsy, less emotional maturity, less social competence, and attentional difficulties. Many low-birthweight, pre- mature infants are also considered doubly vulnerable because they are also more likely to experience environmental risks such as living in pover- ty, having a single parent, or being the child of a teenage mother. The Infant Health and Development Program was a large, randomized clinical trial to determine the efficacy of an intervention designed to pro- mote the physical health, and cognitive and socioemotional development of low-birthweight, premature children. The program provided services for 985 children from birth through age 3 at 8 different sites throughout the United States. All of the children received pediatric surveillance and community referral services. The families of one-third of the children also received family support through home visits throughout the program. Beginning at age 1, the children from these families participated in full- day educational child care in eight child development centers, and their parents participated in regular group meetings. Data on the health, be- havior, and cognitive development of all of the children were collected during the 3 years of the program, as well as at ages 5 and 8. Infants participating in the intervention demonstrated improved behav- ioral functioning (e.g., higher IQ scores, vocabulary gains, and fewer be- havioral problems) at the conclusion of the intervention, when they were 3 years old (Infant Health and Development Program, 1990). At age 5, only the heavier low-birthweight infants (i.e., 2,000-2,500 grams) contin- ued to show gains that distinguished them from the children that did not receive the intervention (Brooks-Gunn et al., 1994). By age 8, even the gains of the heavier infants had been substantially diminished (McCarton et al., 1997). The authors have speculated about the outcomes that might have emerged if they had continued the program up to school entry. SOURCES: Brooks-Gunn et al. (1994); Gross et al.(1997); McCarton et al. (1997). The evaluation literature on these interventions offers good news about the capacity of early childhood programs, which emphasize individualized developmental care, as well as initiatives focused on parental coping and training in optimal parenting skills, to improve health outcomes and de- crease developmental delays in premature infants. It thus appears that the developmental problems associated with prematurity and low birthweight can be mitigated by intervention. However, this is such a complex biologi- cal phenomenon that relatively nonspecific interventions may not be the
212 FROM NEURONS TO NEIGHBORHOODS most productive approach. Moreover, virtually all experts in this area agree that efforts focused on preventing low birthweight need to be the top priority. Stress and the Developing Brain Research on premature infants has provided substantial evidence of the importance of the caregiving environment for the babyâs later progress. This theme emerges, as well, from research on animals regarding how stress affects the developing brain. This research provides preliminary insights into how alterations of the early caregiving environment affect neurochemi- cal aspects of early brain development. Extending this evidence to the human species is not yet warranted, however. There is, for example, only one scientifically reviewed study that has imaged the brains of maltreated children (De Bellis et al., 1999a) (discussed in Chapter 9). The animal evidence, however, is suggestive of the physiological processes that may underlie associations found between highly dysfunctional caregiving and problematic child outcomes, particularly those that lie in the realm of self- regulatory behaviors. This, in turn, points to promising directions for fu- ture collaborative research among behavioral and brain scientists. The term âstressâ is used by psychologists, physiologists, and the lay public and means different things to each (Engle, 1985). In this report, stress refers to the set of changes in the body and the brain that are set into motion when there are overwhelming threats to physical or psychological well-being (Selye, 1973, 1975). Stress can have dramatic effects on health and development (Johnson et al., 1992). This happens because the physiol- ogy of stress produces a shift in the bodyâs priorities. When threats begin to overwhelm oneâs immediate resources to manage them, a cascade of neuro- chemical changes that begin in the brain temporarily puts on hold the processes in the body that can be thought of as future-oriented: finding, digesting, and storing food; fighting off colds and viruses; learning things that donât matter right now but may be important sometime in the future; reproducing and rearing offspring. Many of these neurochemical changes take place in the very same brain structures (e.g., hypothalamus and brain- stem) that function to regulate heart rate, respiration, food intake and digestion, reproduction, growth, and the building up versus breaking down of energy stores (Stratakis and Chrousos, 1995). These brain regions also play a role in regulating the production of stress responses in the rest of the body. Specifically, the adrenal glands, located on the top of the kidneys, produce adrenaline and cortisol (Axelrod and Reisine, 1984). Adrenaline is part of the sympathetic nervous system. Increases in sympathetic nervous system activity support vigilance, focus attention, increase heart rate, shunt blood to muscles and away from the
THE DEVELOPING BRAIN 213 digestive system, break down fat stores making energy available to cells, and dampen activity of the immune system. Cortisol is a steroid hormone that plays a myriad of roles in stress physiology. It helps to break down protein stores, liberating energy for use by the body. It suppresses the immune system, suppresses physical growth, inhibits reproductive hor- mones, and affects many aspects of brain functioning, including emotions and memory. Current understanding of how psychological stimuli, such as experi- ences of fear and anxiety, set in motion stress physiology is centered on an area of the brain called the amygdala (Miller and Davis, 1997; Rolls, 1992; Schulkin et al., 1994), which has close back-and-forth communication with areas of the brain involved in attention, memory, planning, and behavior control. In animals, experimentally causing a hyperstimulation of the amygdala (a process termed âkindlingâ) seems to create a hypersensitiza- tion of the fear-stress circuits of the brain and changes in behavior that look like an animal version of posttraumatic stress disorder (Rosen et al., 1996). It is as if the fear circuits get locked in the âonâ mode and have trouble shutting off. These circuits course through the amygdala and an area called the bed nucleus of the stria terminalis. They appear to be pathways through which circumstances outside the body set in motion the cascade of events inside the body and the brain that undergird fear-stress responses. These events involve the elevation of cortisol and stimulation of the sympathetic arm of the stress response. In animals, flooding the brain with cortisol for prolonged periods of time produces changes in this process that may lower the threshold for activating the fear-stress system (Makino et al., 1994). The result is an animal that more readily experiences fear, anxiety, and stress and may have a harder time dampening or regulating these responses. The amygdala is a fairly mature brain area at birth in humans and seems to be fully mature at least as early as a childâs first birthday. All anatomical evidence suggests that by the end of the first year, young chil- dren should be capable of experiencing psychologically driven fear, anxiety, and stress. Indeed, fear reactions to strangers (Bronson, 1971; Schaffer, 1966; Waters et al., 1975) and anxiety reactions to separation from famil- iar caregivers (Ainsworth and Bell, 1970; Bowlby, 1973; Sroufe, 1979) are hallmarks of emotional development in late infancy. Brief periods of stress are not expected to be problematic. Indeed, survival requires the capacity to mount a stress response. However, because the stress system functions to put growth-oriented processes on hold, frequent or prolonged periods of stress may negatively affect development. Evidence from research on rodents and primates suggests that experi- ences of neglect early in life constitute the kinds of stressful experiences to which young offspring are especially sensitive and may result in a more reactive stress system. In studies of rats, for example, when experimenters
214 FROM NEURONS TO NEIGHBORHOODS do things to the nest that affect maternal behavior (such as handle the pups), they can affect the development of the ratâs stress system (Denenberg, 1999; Levine and Thoman, 1970). Doing things to the nest that result in better organized maternal behavior results in infant rats that develop into less fearful, less stress-reactive adults, whereas doing things that disrupt maternal behavior results in more fearful and stress-reactive adult rats. Researchers have also shown that strains of rodents that are known to be more stress-reactive are characterized by maternal care that involves less licking and grooming (Liu et al., 1997; Meaney et al., 1996; Plotsky and Meaney, 1993). Cross-fostering genetically high stress-reactive infants to mothers from low stress-reactive strains results in the development of a more stress-resilient animal. These effects of early experience in the rat appear to operate through the development of the receptor system in the brain that influences the reactivity of the fear-anxiety circuits. Plenty of input early in life that keeps the stress system dampened down results in the development of a stress-modulating receptor system that can quickly turn off stress reactions. Without this input, the fear-stress system appears to get âshapedâ so that the rat pup becomes a more highly reactive adult who has difficulty modulating these responses. In short, the development of a less stress-reactive rat seems to revolve around enhancing and supporting quali- ties of the caregiving environment. There are monkey analogues of these rat studies, although details of the biobehavioral mechanisms have not been worked out as thoroughly. Infant monkeys deprived of normal social stimulation grow into socially incompe- tent, fearful adults (Harlow et al., 1971; Young et al., 1973). More recent studies have documented that monkeys reared on cloth surrogates, but exposed every day to several hours of play with other infant monkeys, are not as socially incompetent as monkeys raised in isolation, but they show numerous physiological signs of being very anxious and fearful (Suomi, 1991). They produce higher levels of stress hormones when threatened and they have high levels of anxiety-related brain neurochemicals in the cere- brospinal fluid, which bathes and nourishes the brain and spinal cord. Monkeys reared only with other infant monkeys (i.e., no cloth surrogates to call their own), show similar patterns of high reactivity to stress (Champoux et al., 1989, 1992). A high stress-reactive adult monkey can also be produced by proce- dures that cause stress to its mother (Coplan et al., 1995, 1996; Rosenblum and Andrews, 1994; Rosenblum et al., 1994; Schneider, 1992a, 1992b; Schneider et al., 1992, 1998). One technique for stressing the mother is to make her food resources unpredictable. This has the effect of deeply dis- turbing the motherâs social relationships with other adult monkeys in her group. The infant monkeys in these unpredictable food studies (who are
THE DEVELOPING BRAIN 215 roughly equivalent in developmental age to 1- to 2-year-old human chil- dren) experience high levels of stress hormones (like their mothers) and grow up into highly fearful, socially less competent adult animals (Rosenblum and Andrews, 1994; Rosenblum et al., 1994). These effects were obtained even though food was never uncertain for the young mon- keys themselves, and thus seem to be influenced by what this uncertainty and disturbance in the social environment does to their mothers. There is a great deal to learn about how the social environment con- nects with the biology of growth and the regulation of stress physiology in human infants and children. Intriguing research is emerging, however, to suggest that the development of stress regulation in young children may be a very promising place to look for brain-experience dynamics. For ex- ample, both failure to thrive and psychosocial dwarfism (Gohlke et al., 1998; Skuse, 1985), in which childrenâs pituitary glands fail to secrete sufficient growth hormone (Skuse et al., 1996), are associated with failures in the social environment (Alanese et al., 1994). Removing the child from the problematic social system reverses the disorder and growth increases rapidly. This research, as well as that on orphanage-reared infants dis- cussed in Chapter 9, raises extremely important questions about the plastic- ity and self-righting tendencies inherent in the human (as well as the ani- mal) brain. In general, there is much to learn about the extent to which the neurological pathways between caregiving environments and dysfunctional behavior that are emerging in the animal literature apply to human off- spring and about the effects of remedial experiences that attempt to en- hance the development of children from early abusive and neglectful envi- ronments. In sum, neuroscience evidence from animal research is increasingly pointing to experiences of neglect, stress, and trauma within the caregiving environment as a source of compromised brain development. Research on rodents and primates indicates that the ways in which the brain learns to respond to stressful and fear-inducing circumstances are profoundly af- fected by the capacity of the infantâs caregivers to regulate the developing stress system. Disruptions to the caregiving environment that produce stress in the mother appear to alter the offspringâs developing reactivity to stress, as seen behaviorally in high levels of fearfulness and neurologically in how the brain releases and modulates stress hormones. Alternatively, supportive and nurturant caregiving can protect offspring from these conse- quences. Although this evidence is compelling with regard to the signifi- cance of early rearing environments as they affect the developing brain, we are barely at the beginning of exploring these issues in human babies (Kimmel et al., 1990; McLoyd and Lozoff, 2000; Morgane et al., 1993).
216 FROM NEURONS TO NEIGHBORHOODS SUMMARY AND CONCLUSIONS Basic research on the development of the brain is a rapidly moving frontier. Abundant evidence indicates that brain development begins well before birth, extends into the adult years, and is specifically designed to recruit and incorporate experience into its emerging architecture and func- tioning. For some systems, environmental inputs need to occur prenatally or relatively early in life, after which time the brain becomes decreasingly capable of developing normally. But available evidence indicates that such critical periods are more exceptional than typical in human development. For the vast majority of brain development, including areas of the brain involved in cognitive, emotional, and social development, either questions regarding critical or sensitive periods have not been explored or it appears that the brain remains open to experiences across broad swaths of develop- ment. This makes sense. Adaptation depends on the rapid consolidation of capabilities essential to survival and the life-long flexibility to adjust to changing circumstances and learn new skills. As a result, assertions that the die has been cast by the time the child enters school are not supported by neuroscience evidence and can create unwarranted pessimism about the potential efficacy of interventions that are initiated after the preschool years. Nevertheless, what happens early matters. Concerns about protecting the developing brain need to begin well before birth. During the prenatal months, the developing brain is highly vulnerable to intrinsic hazards (such as errors of neural migration) and external insults resulting from drug or alcohol exposure, viral infection, malnutrition, and other environmental harms. This directs attention to efforts to protect brain development dur- ing pregnancy and the earliest months of life, including the importance of prenatal and postnatal medical care, as well as expanded public health efforts to improve nutritional quality and reduce drug and viral exposure. It also argues for continued efforts to reduce the incidence of premature births and to ameliorate the adverse consequences of prematurity. Neuro- science evidence also directs attention to the early detection, identification, and treatment of problems such as visual impairments, auditory deficits, and major perceptual-motor delays that have profound effects on childrenâs capacity to access and incorporate the stimulation needed to organize the developing nervous system. For these aspects of development, there is solid evidence that the timing of corrective efforts matters a great deal. Beyond this evidence regarding detrimental influences on brain devel- opment, neuroscience offers few insights into how early environments can function to enhance development beyond what might otherwise be ex- pected. The experiments with complex environments conducted on rats reveal the benefits of more enriched environments, indicate that younger
THE DEVELOPING BRAIN 217 brains react more rapidly and to a greater degree to environmental varia- tion, and suggest that removal from complex environments results in de- creasing benefits over time. Nevertheless, we do not yet have the evidence on infant brains to translate these findings from animal research into tan- gible recommendations for early interventions aimed at childrenâs cognitive or social-emotional development. For these insights, additional behavioral evidence from human development is needed. A final implication of research on early brain development concerns the detrimental effects of early and sustained stressful experiences, particularly those that derive from aberrant or disrupted caregiving environments. Evi- dence from research on animals suggests that such experiences overactivate neural pathways that regulate fear-stress responses in the immature brain, perhaps placing them on a âhigh alertâ setting that may alter patterns of behavioral responding in adult animals with different rearing histories. Translations of these findings to human development are largely specula- tive. However, emerging evidence regarding the physiology of children subjected to serious deprivation and trauma early in life are consistent with the animal studies, as is the richer body of behavioral data on young chil- dren exposed to such early adverse experiences. This is an especially prom- ising area for research that integrates animal and human studies, using both neuroscience and behavioral approaches, and explores not only the nega- tive consequences of early stress and trauma but also the capacity of the brain to reorganize itself following highly depriving circumstances early in life. In sum, the neuroscientific research on early brain development says that the young children warranting the greatest concern are those growing up in environments, starting before birth, that fail to provide them with adequate nutrition and other growth-fostering inputs, expose them to bio- logical insults, and subject them to abusive and neglectful care. Children with undetected sensorimotor difficulties (whose developing brains may not receive the stimulation they need) also warrant concern. The brain research also reassures that brain development is probably on course for the vast majority of young children who are protected from these conditions and, in many instances, can be affected positively by timely corrective interventions focused on early insults and deficits.
E219 The Context for Early Development III arly interventions are premised on a belief in the power of environmental influences on early develop- ment. Our review of the research on early development in areas as disparate as behavior genetics, neurobiology, and social and cognitive development has supported this belief. Genetic susceptibilities are activated and dis- played in the context of environmental influences. Brain development is exquisitely attuned to environmental inputs that, in turn, shape its emerg- ing architecture. The environment provided by the childâs first caregivers has profound effects on virtually every facet of early development, ranging from the health and integrity of the baby at birth to the childâs readiness to start school at age 5. Documenting and understanding environmental influ- ences, however, are not the same tasks as changing environments. Indeed, as we discuss in this report, it is decidedly not a simple task to shift the developmental pathways of young children through interventions that af- fect their environments, particularly when the interventions are modest in scope, poorly implemented, and inadequately staffedâwhich is all too of- ten the case. In Part III we expand our lens on young children to encompass the contexts that influence early development. We start with the nurturing relationships that are forged between the growing child and his or her caregivers at home. Early development is inextricably tied to this most proximal, interpersonal context. In fact, active debates now characterize discussions of the extent to which parenting and the family environment affect child development (Harris, 1995, 1998; Rowe, 1994). Our reading of