4
Building, Monitoring, and Sustaining Immunization Capacity
The government role in public health provides the necessary context for private-sector activity. Government is responsible for striving to achieve a balance between the two great concerns embodied in the American public philosophy: individual liberty and free enterprise on the one hand, and just and equitable action for the good of the community on the other (IOM, 1988:46).
This context continues to shape the infrastructure of the public health system. More than a decade ago, the IOM (1988:40) defined the mission of public health as “the fulfillment of society’s interest in assuring the conditions in which people can be healthy.” This mission has been undertaken by private organizations and individuals as well as by public agencies. However, the government has the singular role of ensuring that the public health mission is sufficiently addressed and that its crucial features are implemented. To this end, the governmental public health system encompasses three key functions: assurance, assessment, and policy development. As discussed in Chapter 1, the framework for the present study represents these functions somewhat differently, identifying six specific roles of the national immunization system (see Figure 1–6):
-
vaccine purchase,
-
service delivery,
-
infectious disease prevention and control,
-
surveillance of vaccine coverage and safety,
-
efforts to improve and sustain high vaccine coverage levels, and
-
immunization finance policies and practices.
This chapter examines in turn the scope and evidentiary base of three of these roles: infectious disease prevention and control, surveillance of vaccine coverage and safety, and efforts to improve and sustain high vaccine coverage levels. The final role—immunization finance policies and practices—is reviewed in Chapter 5. The three roles discussed in this chapter complement the vaccine purchase and delivery arrangements discussed in Chapter 3, and are commonly regarded as infrastructure efforts.
Infrastructure is defined as “an underlying base or foundation” and refers to “the basic facilities, equipment, and installations needed for the functioning of a system” (Webster’s Dictionary, 1996:569). In the context of this study, infrastructure encompasses the formal set of arrangements that guide the immunization system in the United States. Although we focus principally on the public infrastructure for immunization services at the federal and state levels, we recognize that these efforts interact with local health agencies, private health care providers, and private insurers in a complex manner. Most important, the presence or absence of private health care services, including insurance coverage and standard benefits that provide immunization services for children, adolescents, and adults at reasonable cost, influences the infrastructure burden that is located within the public health sector. The scope and quality of the assessment, educational, and technical assistance efforts required within the public sector to ensure access to recommended vaccines and monitor the performance of health care providers thus depends on the extent to which the private sector can be relied upon to serve the needs of vulnerable populations.
The importance of infrastructure is not always apparent. It is often difficult to grasp, for example, why high levels of immunization coverage cannot be achieved simply by the purchase and delivery of vaccines. But sustaining high levels of immunization coverage for an increasing number of vaccines for the 11,000 children born each day, as well as a growing immigrant population, requires various forms of data collection, identification and analysis of high-risk and under served populations, and technical assistance to health care providers. Certain roles and responsibilities within the public health sector acquire greater or lesser importance as health conditions shift and private providers acquire new responsibilities for immunization services. As immunization services are integrated into routine primary care, for example, the need for precise measurement and appropriate accountability standards grows, while actual caseloads decline within the public sector. Targeting outreach and reminder services to those most in need requires reliable benchmark, baseline, and
performance monitoring data so public resources can be used wisely and efficiently.
INFECTIOUS DISEASE PREVENTION AND CONTROL
When disease incidence and burdens are high, the immunization infrastructure is often concerned with launching prominent national campaigns designed to attack infectious disease and deliver vaccines through special stand-alone and short-term programs. During active stages of disease transmission, infectious disease control includes three key components: (1) campaigns to change behavior to reduce the risk of disease transmission, (2) contact tracing, and (3) mass immunization of high-risk populations in outbreak areas.
In periods when disease burdens are low (as they are today), the use of sentinels that monitor the health of the general population acquires greater importance (IOM, 2000). These sentinels are essential to the prevention of disease outbreaks and transmission because they reveal long-term trends and provide early warnings of new patterns of disease reports. The persistent presence of infectious disease in reservoirs scattered around the world requires constant vigilance within each U.S. community until disease eradication is complete (IOM, 1992).
Although the threat of morbidity and mortality associated with vaccine-preventable diseases has decreased significantly, overall mortality from infectious diseases continues to rise as a result of the appearance of new infectious agents and the reemergence of diseases previously considered to be under control (Department of Health and Human Services [DHHS], 1998). As a group, infectious diseases were the third leading cause of death in the United States in 1992; overall mortality from infectious diseases rose 58 percent in the United States between 1980 and 1992. Although much of this increase reflects the growing burden of HIV-associated disease, the removal of HIV-associated diagnoses still leaves a 22 percent increase in mortality from infectious diseases (DHHS, 1998).
Responsibilities for prevention and control of disease outbreaks are shared among all levels of government. Local jurisdictions have on-site responsibility for dealing with outbreaks of disease in schools and the community. Each state has its own health codes and its own on-site epidemiologist, but federal expertise is often requested during outbreaks and as part of disease monitoring and reporting arrangements. Indeed, several states with unique infectious disease circumstances have disease reporting requirements that exceed those of CDC. When disease outbreaks occur, the three levels of government usually cooperate in their control efforts. States can request technical assistance from CDC to address both
acute and chronic disease patterns. CDC also monitors nationwide and international trends that have national implications.
In some cases, outbreaks can be sudden, with deadly effects (see Box 4–1). A single case of meningococcal meningitis, for example, can prompt emergency drills to identify the scores of people with whom the victim came in contact before falling ill so that each can be given immediate antibiotic therapy (Altman, 1999). To counter this threat, the Advisory Committee on Immunization Practices (ACIP) has urged the 520,000 college freshmen who live in dormitories to consider receiving the meningococcal vaccine, even though the vaccine is not expected to prevent more than a few dozen actual cases.
Disease prevention and control efforts are different from the treatment of disease itself. The latter generally falls within the domain of personal medical services and is covered by insurance; personal payments; managed care organizations; and public funding programs such as Medicaid, Medicare, and the State Children’s Health Insurance Program (SCHIP). Disease prevention and control, on the other hand, is an intrinsic role of public health agencies, which look beyond individual health to address the risk to whole populations. The major means of evaluating the impact of most vaccine-preventable disease programs is reports of the occurrence of these diseases (Orenstein and Bernier, 1990; Wharton
|
BOX 4–1 Alaskan Measles Outbreak in 1998 In late 1998 an outbreak of 33 confirmed measles cases (ages ranging from 2 to 28 years) occurred in Anchorage, Alaska, including 17 cases among a highly vaccinated high school population (CDC, 1999g). Analysis of the outbreak revealed that Alaskan schools did not require students entering kindergarten or first grade to have two doses of MMR until September 1996. Consequently, in the high school setting of 2,186 students, about half (1,057 students, or 49 percent) had received one dose of MMR, and the remaining half (1,112 students, or 51 percent) had received two or more doses (only 1 of the students had not received at least one dose of MMR before the outbreak). The Alaskan Department of Health and Social Services issued an emergency order requiring all Anchorage schoolchildren to have two doses of MMR by early January 1999. By mid-November 1998, 98.6 percent of the almost 50,000 Anchorage students were able to produce such documentation. Although no endemic measles virus is currently circulating in the United States (the outbreak was traced to importation by a 4-year-old child visiting from Japan), health officials have observed that outbreaks may continue to occur when imported cases are introduced into settings such as schools with incomplete second-dose MMR coverage. |
and Strebel, 1994). Disease control and surveillance involves not only responding to such reports, but also maintaining vigilance to ensure that appropriate sentinels are in place within an increasingly fragmented health care delivery system.
The primary mechanism for monitoring disease reports is the National Notifiable Disease Surveillance System (NNDSS), maintained by CDC. The list of reportable diseases is determined and revised collaboratively between the Council of State and Territorial Epidemiologists and CDC. Currently, 52 infectious diseases are designated as notifiable (information provided by CDC). CDC operates several additional surveillance systems as well, including a national registry for congenital rubella syndrome and surveillance systems for paralytic polio and diphtheria (Orenstein et al., 1999). CDC also sponsors efforts to collect data beyond the NNDSS for measles, pertussis, tetanus, Haemophilus influenzae type b, and hepatitis B, and relies on laboratory-based surveillance systems to monitor and confirm reports of bacterial meningitis (including Haemophilus influenzae type b and pneumococcal disease). Additional influenza surveillance is performed using a laboratory-based system, as well as death certificate data (Orenstein et al., 1999). Traditionally, influenza surveillance involved monitoring the population through the voluntary reporting of communicable diseases by practicing physicians, with no expectation of complete reporting.
Monitoring of disease reports continues to be one of the primary functions of public health across the nation. Although such efforts can be expensive, they represent an important preventive function that can result in significant health benefits and cost savings for both individuals and communities. For example, a typical case of Lyme disease (which can be prevented by vaccine) diagnosed in the early stages incurs about $174 in direct medical treatment costs. However, delayed diagnosis and treatment can result in complications that cost from $2,228 to $6,724 per patient in direct medical costs in the first year alone (DHHS, 1998). The cost of screening patients who report symptoms is an additional expense borne by those who do not experience the disease itself.
Investigations of disease reports often require independent laboratory confirmation to meet clinical case definitions, as well as epidemiological analysis to trace disease origins, pathways, and high-risk settings. Such investigations commonly involve close and swift data collection and exchange among local, state, and federal employees, who often collaborate to educate and alert the professional science and health communities about important disease patterns.
Public health laboratories have an intrinsic role in these investigations. These laboratories support surveillance activities, conduct outbreak inquiries, and monitor for new or emerging infectious diseases. Public
health laboratories provide a mechanism for developing new methods to combat disease. Studies conducted in the nation’s public health laboratories made possible identification of the organisms that cause diphtheria, cholera, tuberculosis, leprosy, and typhoid fever, which in turn enabled the development of vaccines and other treatments used to prevent and control these diseases.
Today, public health laboratories are faced with numerous challenges resulting from changes within both the public and private sectors that have made it increasingly difficult for the laboratories to fulfill their missions. In the private sector, managed care and independent laboratories are on the rise, hospital laboratories are consolidating, and both clinical and information technologies are changing rapidly. In the public sector, the public health safety net is being redefined, there is increasing reliance on managed care to address public health needs, and state budgets have become further constrained. The strategies used to achieve the goals of public health laboratories need to be altered to reflect these contextual changes. Presently, there is much variation among the states in the way the core activities of public health laboratories are carried out, and there is no unified, common theme among laboratory strategic plans. Centralized leadership will be necessary to help meet these challenges. Increased federal guidance could be particularly useful in assessing the regionalization of some laboratory services, in supporting information infrastructure development, and in facilitating cooperation between public and private concerns.
Finding 4–1. Disease sentinels and surveillance data must be reliable since infectious agents can spread rapidly in a global community. Federal officials collaborate with state and local agencies to monitor routine disease patterns and provide technical assistance during periods of outbreak, during emergency conditions when local systems are over-whelmed, or during the implementation of new surveillance systems. Efforts to collect infectious disease data require stability, consistency, and federal and state collaboration to enhance the monitoring of long-term trends and the analysis of data from different regions of the country.
SURVEILLANCE OF VACCINE COVERAGE AND SAFETY
For the first half of the 20th century, the prevention and control of vaccine-preventable disease were the frontier of the public health infrastructure. Until the 1950s, public health authorities did not monitor levels of vaccine coverage within the general population because few vaccines were available, and because relatively low levels of vaccine coverage were thought to be sufficient to control disease transmission within the general
population—a concept known as “herd immunity.” Even the national polio campaigns of the late 1950s achieved fairly low levels of vaccine coverage (in the range of 50–60 percent for the general population).
The measles outbreak of 1989–1991 exposed many incorrect assumptions behind the belief that low levels of coverage were sufficient to control the transmission of infectious disease. The changing demographics of society, the mixing of young children in day care settings, new patterns of health care delivery, high rates of uninsured children, and the shrinking size and morale of health departments all fostered circumstances in which disease transmission occurred within major metropolitan areas even though disease reports were low, and state health officials believed statewide immunization coverage was at acceptable levels (see Chapter 2). The measles epidemic demonstrated that new approaches were necessary to protect vulnerable populations from disease—approaches that depended more heavily on surveying the populations at greatest risk to determine their immunization coverage levels and to identify points of vulnerability that might emerge from a variety of causes, including shifts in population trends, disruptions in health care services, and new behaviors among providers or clients.
In the 1990s, small-area immunization coverage studies or assessments of coverage levels within specific populations (including low-income workers with private insurance, Medicaid families served by managed care organizations, and migrant farmworkers) acquired greater importance for the following reasons:
-
Small-area coverage studies reflect the quality and impact on immunization levels of national and state health finance programs, especially for programs such as Vaccines for Children (VFC), Medicaid, and SCHIP.
-
Small-area studies can demonstrate the effectiveness or limitations of other types of interventions, including outreach, education, reminder-recall, and community partnerships.
-
Despite the high rates of national coverage for preschool and school-aged children, selected regions of the United States characterized by extreme disadvantage have consistently reported low levels of coverage for routine immunizations (see Table 1–5 in Chapter 1). Studies of specific population groups can identify systemic features that need to be addressed within local, state, or national health care systems to promote quality health care, prevent the occurrence of disease, and improve immunization coverage rates.
-
High statewide coverage levels are generally sufficient to provide protection against infectious disease outbreaks, provided underimmunized individuals are dispersed among the general population. However, the
-
concentration of underimmunized individuals within specific regions increases the potential for the transmission of disease.
-
Children who receive care from more than one health provider may receive too many immunizations. One CDC study reports that 14 percent of young children (aged 19–35 months) were immunized beyond need for the vaccine to prevent polio (Feikema et al., 2000). Children seen only in public health departments were significantly less likely to be extra-immunized. Additional analysis, however, suggests that rates of extra-immunization may be overestimated as a result of documentation errors in medical records (Davis, 2000).
Virtually any and all of the data collection tools discussed below for determining immunization coverage at the national, state, and local levels can be employed or adapted to examine special populations or geographic pockets of need. In addition to these direct measures of immunization coverage, geographic pockets of need can be identified by surrogate measures, including demographic and socioeconomic variables such as average income level, percent of the population receiving Medicaid, and maternal education, all of which are associated with underimmunization (Santoli et al., forthcoming). Surrogate measures have the advantage of being readily available and inexpensive; although they do not provide direct information about immunization coverage, they can be used to identify neighborhoods at high risk for underimmunization.
Measurement of Immunization Coverage
Information about immunization coverage comes from five major sources: the National Immunization Survey (NIS), retrospective school entry surveys, special area and population surveys, Clinical Assessment Software Application (CASA) surveys of clinics and private practices, and reports from managed care plans on coverage for children in their care. The NIS and retrospective kindergarten surveys estimate the coverage of the population in a given geographical area. In contrast, the CASA and managed care assessments estimate coverage levels for particular entities responsible for the children’s care (health care providers in the former case and managed care plans in the latter).
Differences in the way immunization coverage is measured in various settings—differences in samples, antigens, and time periods, for example—inhibit comparisons across managed care plans, clinics, and private physicians’ offices. While some differences may be unavoidable, opportunities for greater comparability may be achieved through technical assistance and program leadership. Understanding the nature and origins of the differences is important if remedies to improve the current situation
are to be formulated. A brief description of the measurement strategy for each of the major sources follows.
National Immunization Survey. The NIS is a national telephone survey of households and providers that is used to estimate vaccination coverage levels for children aged 19 to 35 months (information provided by CDC). NIS studies include a two-phase design: (1) self-reports by respondents, obtained by dialing random telephone numbers, and (2) provider record checks that yield independent information on the dates of immunization for a smaller number of children identified through the household survey.
The survey is conducted in 78 Immunization Action Plan (IAP) areas, consisting of the 50 states, the District of Columbia, and 27 other large metropolitan areas. Data collection involves quarterly surveys in each of the 78 IAP areas, combined to provide annualized estimates at acceptable levels of precision. The survey asks about coverage for nine antigens1 and reports up-to-date status for a variety of combinations. Respondents are selected at random from telephone banks within the IAP areas using complex, multistage sampling techniques, including adjustment weights for the telephone bias, so the sample approximates the population in the IAP area as closely as possible.
The NIS is the primary source of both national and statewide estimates of coverage, including estimates by poverty and ethnic status. CDC relies on NIS data to monitor state progress in achieving childhood immunization objectives, to compare coverage rates across states, and to award incentive funds to CDC grantees on the basis of their immunization of certain percentages of preschool children.
Although the NIS provides reliable baseline data for the nation as a whole, the survey methodology is not designed to identify children in need of more timely immunization. For example (General Accounting Office [GAO], 1996):
-
Although the NIS includes an adjustment weight for households that do not have telephones, there is wide variation among states and metropolitan areas in the percentages of such households with children under age 2 (ranging from 2 to 25 percent across the 50 states and 28 urban areas sampled by the NIS).
-
NIS respondents, as a group, differ in some respects from the populations represented by census and vital statistics estimates. Mothers with more than 12 years of education are slightly overrepresented, and in some areas, NIS respondents are more likely to report household incomes exceeding $50,000 and less likely to report incomes below $10,000.
-
The sample size of the NIS is not large enough to provide subgroup statistics for each state or urban area. For example, one household
-
survey of central and southeast Seattle found an immunization coverage rate of 57 percent, in contrast to the 79 percent reported by the NIS for the King County area incorporating Seattle (GAO, 1996:20).
-
The absence of robust subgroup statistics within state or urban areas prevents states from linking changes in coverage rates reported by the NIS to specific programs. The NIS data are therefore not useful to states for diagnosing problems in their ongoing activities, targeting their efforts, or designing interventions (GAO, 1996:19).
Retrospective School Entry Surveys. Retrospective surveys (involving the review of school records to determine when immunizations were obtained relative to the child’s second birthday) are the most common form of local-level coverage survey. CDC provides guidelines for conducting these surveys, which involve collecting coverage data from school health records in 35 randomly selected schools for a sample of children. These studies are the least expensive and easiest to perform of the assessments reviewed here, but their data lag the period of performance by 3 to 4 years; thus, for example, they offer little help in monitoring the effects of recent immunization efforts (Orenstein et al., 1999).2
Coverage levels reported in the NIS are often higher than those in retrospective school entry surveys. Several factors help explain this difference:
-
The most important factor is that the NIS combines data from personal immunization records (and recall) with provider data, and coverage rates are often higher when immunizations from multiple sources are used.
-
The NIS is a telephone survey, and children in families with telephones have higher coverage than those without (although, as noted, an adjustment factor is calculated and applied to account for this difference).
-
Another major difference between the NIS and all other forms of assessment is the criteria for selection. The NIS uses children aged 19 to 35 months and calculates coverage at the time of the survey. Children who are 35 months old at the time of the survey have had 12 extra months in which to become up to date, while those assessed at 19 months have had 6 months less.
Special Area and Population Surveys. Determining local coverage levels in small geographic units (such as a metropolitan area, county, or census tract) or among specific populations (such as Medicaid families) requires special data analyses that have sufficient sensitivity to detect signs of change or disruption among vulnerable groups. These analyses are sometimes supported by state Section 317 grant awards. In addition, a few
health departments (New York City, Detroit, San Diego, and rural Colorado) have participated in a Community Health Network demonstration study to determine whether collaboration with academic health centers or managed care organizations would influence and improve vaccination coverage within the entire population in the designated region (A.Bauer, CDC, personal communication, May 21, 1999).
As noted above, immunization coverage studies within small geographic areas or among specific populations (including low-income workers, Medicaid or Medicare participants who are served by managed care organizations, public housing residents, and migrant farmworkers) can provide important data on variations in coverage patterns that require attention through state and national health finance and health care policies and practices. Local immunization surveys provide intelligence needed to manage the community health system effectively, target needy groups, and ensure accountability within the public and private health care sectors. However, significant barriers challenge such efforts to track vulnerable groups:
-
Surveys of baseline coverage for Medicaid populations, including the coverage rates for families served by managed care organizations, are not consistently available, nor can results be compared across survey designs.
-
The difficulty of gaining routine access to households of very poor families, due in part to the high rates of mobility among such families, poses significant methodological barriers to data collection efforts.
-
Special population studies are commonly funded for limited periods of time through research or state grant awards supported directly by CDC. These special area studies are inconsistent and often difficult to compare because they use different standards of coverage, different age groups, and different survey methods.
CASA Surveys. CASA is CDC-developed software and associated procedures for assessing coverage levels for a clinic or practice. The sample for 2-year-olds consists of children aged 24 to 35 months; coverage is calculated at the 24-month mark. Charts for children are included in the sample if there is a record of at least one medical or immunization visit; a chart can be excluded from the sample under certain stringent conditions.3 Providers sometimes complain that the inclusion criteria are overly broad.4 They report that parents often fail to notify their provider when they move, and when this happens, the chart does not bear a “moved or gone elsewhere” notation. Further, providers in birthing hospitals say patients may come to the hospital’s outpatient clinic for the first or second
visit after birth (especially if insurance is not in place for the child) and then move to a neighborhood health center or private provider.
Reports from Managed Care Plans. Managed care plans use immunization coverage as an indicator of quality, and the National Committee for Quality Assurance’s (NCQA) Health Plan Employer Data and Information Set (HEDIS) 3.0 measures include immunization rates as an accountability and performance measure for managed care plans (information provided by the National Committee for Quality Assurance). HEDIS measures of immunization coverage are available for preschool children, adolescents, and adults, and are based on claims or encounter data as well as random samples validated by chart review. While useful in monitoring overall coverage patterns, HEDIS measures have several limitations:
-
The sample consists of only those children who turned 2 in the reporting year who were continuously enrolled for 12 months prior to their second birthday (including members who had no more than one break in enrollment of up to 45 days during the reporting year).
-
Since many managed care plans report annual disenrollment rates of 35 to 40 percent, children in the HEDIS sample may represent an atypically stable group whose rates are higher than those of all participants enrolled in the managed care plans.
-
HEDIS measures for a given plan do not indicate whether the immunization was covered within the plan or was provided elsewhere.
-
HEDIS measures often do not report the timing of the first immunization or the time of completion of a series.
No national surveys describe the levels of immunization coverage among individuals enrolled in U.S. private health plans during the period 1990–1998. A few organizations (such as the Employee Benefits Research Institute and the Health Research and Educational Trust, formerly sponsored by KPMG Peat Marwick) study coverage patterns for private industry to determine whether coverage levels vary by type of plan, but these studies cannot demonstrate where certain groups or regions might be vulnerable to reduced immunization protection or disease outbreaks.
Estimating the immunization status of a plan’s participants within any given time period is extremely difficult in the absence of reliable performance measures. Health plans are not required to provide consistent data on a regular basis, and individual plans use a variety of different measures, discouraging comparisons across different payor sources. Moreover, immunization coverage rates may be considered private or proprietary information since they are often viewed as an important measure of quality for a given health plan.
Some business organizations have used immunization performance criteria to set penalties and incentives in purchasing negotiations. A study conducted by the Pacific Business Group on Health in 1996, for example, monitored the performance of 13 of California’s largest health maintenance organizations (HMOs) that had agreed to penalties (placing a specified percentage of their premiums at risk) in return for not meeting quality-of-care goals (Schauffler et al., 1999). The authors reported that 8 of the 13 plans missed their targets for childhood immunizations, falling short by 3–12 percent. Five of the plans exceeded their targets (by an average of 9.3 percent) within a range of 2–19 percent. Some HMOs excuse low performance with claims that plan participants receive immunization services outside the network of HMO providers, in settings such as county health departments or community clinics, and data on this utilization may not be reported back routinely to the plans or recorded in patients’ medical records (Schauffler et al., 1999). Many monitoring groups, however, including the Pacific Business Group on Health, hold participating plans responsible for tracking use of services, both within and outside the plan.
While some of the differences between the CASA and HEDIS criteria for inclusion are technical, the fundamental question is not. At the heart of the difference is the issue of when a provider or health plan takes responsibility for a child and when that provider or plan should be held accountable for the child’s health care, including immunization coverage. The HEDIS criteria often exclude a large segment of the population— possibly too large a segment. It does not make sense to hold a plan accountable for the services delivered to a child who has just joined, but since most children have had encounters with the health care system before joining a given plan, the criteria may be too generous. It may make more sense to hold a plan accountable for seeing that a child’s previous immunizations are included in his or her record and catching up with the remaining immunizations. On the other hand, the CASA criteria may be too inclusive. Providers have complained that they are being held accountable for children they do not see as “theirs,” that parents may come to a given provider only once for any number of reasons. Some health departments address these issues by reporting on all children selected using the CASA criteria, and then providing additional reports for children who have been followed longer.5 More attention is necessary to the issue of when providers should be held accountable for children who have visited them and how that accountability should be reflected in sample selection for coverage assessments.
Role of Registries in Documenting Coverage Levels
Recognizing that the assessment and documentation of immunization status are complicated by the movements of individuals, health care providers, and health finance systems, several states, local communities, and private health plans have initiated electronic registries to monitor immunization rates within a community or health plan. Immunization registries are “confidential, computerized information systems that contain information about immunizations and children” (National Vaccine Advisory Committee [NVAC], 1999b:2). They have also been described as “automated systems that manage immunization information” (Horne et al., forthcoming:3) and as “a computerized database that gathers immunization information on all children (with preschool children commonly a high priority) in a population defined by a specific geographic area, health maintenance organization (HMO), enrollment, etc.” (Wood et al., 1999:233).6
Most efforts to develop immunization registries in this country have focused on infants and preschool children. Some managed care companies and HMOs, however, have developed and maintain information systems that they use to monitor their effectiveness and efficiency in providing both childhood and adult immunizations to their enrolled populations.
Registry development began in 1974 when Delaware created VacAttack, a community-based registry built to collect childhood immunization data from all pediatric and family practice providers in the state (Ortega et al., 1997). In the 1980s, several large HMOs started to develop immunization registries (Wood et al., 1999). For example, Group Health Cooperative of Puget Sound created a computer-based data record for all immunizations administered to its 350,000 enrollees in the late 1980s. HMOs continued to build registries in the 1990s. In 1991, CDC collaborated with several large West Coast HMOs in the Vaccine Safety Datalink project, which established both immunization registries and systems for tracking adverse reaction events (Davis et al., 1997; Wood et al., 1999).
In 1991, the Robert Wood Johnson Foundation (RWJF) launched the All Kids Count national childhood immunization registry initiative in response to both the measles outbreak of the late 1980s and the low immunization rates of preschool children in the United States (Watson et al., 1997). These computerized information systems were designed to perform three functions: (1) to provide a computerized database for use by providers in monitoring the immunization status of individual children; (2) to identify children due or overdue for immunizations and notify their parents/guardians of the need to obtain vaccinations; and (3) to provide information for use in identifying underserved populations, targeting resources to these pockets of need, and evaluating outreach program efforts. Between 1992 and 1997, RWJF collaborated with five other private
foundations to partially fund 24 registry projects. In 1998, RWJF established the All Kids Count II project by allocating additional funds to 16 of the most advanced developing registries to help them become fully operational by January 1, 2000 (Watson et al., 1997; Wood et al., 1999; Horne et al., forthcoming).
To address the lack of consistency among different registry systems, the All Kids Count initiative developed a 20-item list of ideal components for registries (RWJF, 1996)7 The CDC National Immunization Program (NIP), in conjunction with its state and local grantees, subsequently developed a list of 12 attributes that define the minimum necessary elements of an operational registry (information provided by CDC).
The registry effort received additional support in 1993, when as part of the Childhood Immunization Initiative, President Clinton offered a challenge to create “a nationwide system of state- and community-based information systems” (Kilbourne, 1998; NVAC, 1999b). In response, the NIP assembled a task force to undertake an initiative on immunization registries, guided by an NVAC work group (NVAC, 1999b). Through public meetings and focus groups, the work group determined that registries in the United States should be a “nationwide mosaic of interoperable systems” as opposed to a federally based information system (Kilbourne, 1998:10).
The development or implementation of registries is a required condition for CDC’s state and local immunization grant awards. According to a 1999 CDC survey, 92 percent (59 of 64) of federal immunization grantees (states, cities, and territories) had met this requirement. Only a small number of these registries meet the fully functional standards set by NVAC or RWJF, however (information provided by CDC; NVAC, 1999b; Wood et al., 1999). Currently 34 states and the District of Columbia have operational registries that can import or export data from a central point (J.Harrison, CDC, personal communication, 1999). There are 9 states in which 75 percent or more of the children under age 6 are included in the state’s immunization registry. Another 5 have registries that include 50 to 74 percent of children (see Figure 4–1).8
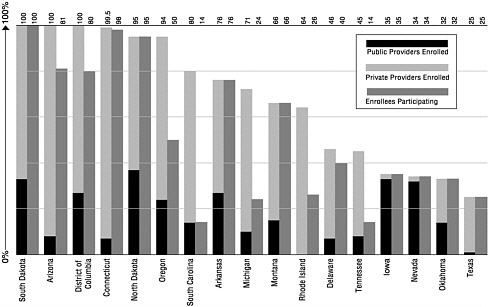
A separate measure of registry implementation is provider participation. As of January 2000, 24 states had enrolled a majority (50 percent or more) of public providers in their registries. Yet recruiting private providers has been a major barrier to registry development.9 Only 11 state registries have over 50 percent enrollment of private providers. It is important to note that provider enrollment does not ensure provider participation.10 For example, although Michigan has 71 percent of all providers enrolled in its state registry, only 34 percent of enrolled providers are actually submitting data (see Figure 4–2).
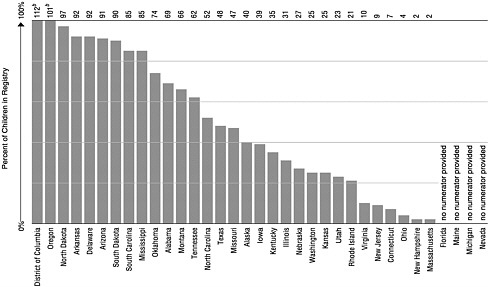
FIGURE 4–1 Enrollment of children aged 0 through 5 in immunization registries, by state (ranked by percent of enrollmenta). aEnrollment is defined as children having at least two doses of vaccine recommended by the Advisory Committee on Immunization Practices and recorded in an immunization registry’s database. bMore than 100% because of duplicate records. SOURCE: CDC, 1999h.
Role of Immunization Registries in Improving Coverage Levels. Immunization registries serve multiple purposes beyond the surveillance and assessment of current coverage rates (DeFriese et al., 1999). They can facilitate service delivery, consolidate scattered records, and simplify the assessment of need for vaccine for both private and public providers. As long as significant numbers of at-risk groups are enrolled, registries can also be used to identify pockets of need in areas where immunization coverage lags behind the current high national and/or state levels. If used appropriately, fully operational registries can be an efficient means of identifying children who require intensive services and may stimulate service responses to ensure their full immunization.
In addition to improving immunization coverage for the currently recommended immunization schedule, registries can be used to monitor and ensure the full implementation of newly recommended vaccines and vaccine schedules. Just as a series completion rate for children in the registry catchment area can be assessed, uptake of specific antigens, including those newly introduced, can be monitored and changes made in implementation strategies on the basis of populations identified as being in need. The current immunization schedule is sufficiently complex to benefit from computerized monitoring, and, as noted earlier, greater complexity is anticipated in the future.
Anecdotal reports (Fairbrother et al., 1997) indicate that immunization monitoring can also improve the identification of children who require other preventive care services (such as lead screening). It is conceivable that investments in immunization registries could benefit these programs, although the scale of this potential impact is not known. The costs and benefits of immunization registries for adult populations (particularly those over age 65) are similarly uncertain.
Barriers to Registry Development and Implementation. Barriers to registry development and implementation fall into four categories: (1) technical and operational issues, (2) privacy and confidentiality, (3) provider participation, and (4) resource requirements (NVAC, 1999b; Linkins and Feikema, 1998; Kilbourne, 1998).
Difficulties have been experienced in sharing information among community-based registries because of variations in the architecture of computer systems, including different software, hardware, data entry mechanisms, and networking resources (NVAC, 1999b). Differences also exist in the methods used to receive or send registry data and in linkages to other computer systems. CDC has encouraged its state and local grantees to endorse standard algorithms to facilitate the transfer of information between systems (NVAC, 1999b; Wood et al., 1999). Duplicate records,
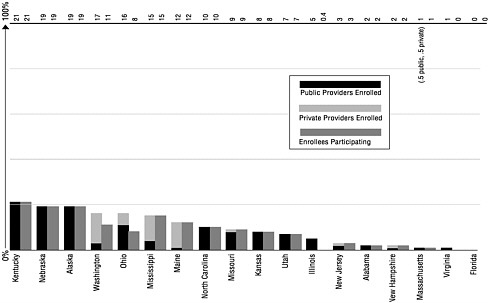
FIGURE 4–2 Provider enrollmenta and participationb in immunization registries, by state (ranked by percent of provider enrollment). aEnrollment is defined as providers who have authorized access to the registry (e.g. written agreement, passwords, rights, and responsibilities defined). bParticipation is defined as providers who have submitted data to (or accessed data from) the immunization registry in the last 6 months. SOURCE: CDC, 1999h.
transcription errors, and missing data are also a problem (NVAC, 1999b; Wilton and Pennisi, 1994).
Studies have shown that barriers to private provider participation in immunization registries include personnel time required to submit and obtain data; costs of technology and other necessary resources; liability for errors; and concerns about data accuracy, confidentiality, access, and security (Bordley et al., 1997; NVAC, 1999b; Wood et al., 1999). An Oregon study revealed that doctors are willing to spend only about “three extra seconds per patient” to participate in an immunization registry (Zablocki, 1996).
In addition to financial resources, immunization registries require a large investment in personnel. Staff are needed to design the system, develop community support, procure technological resources, connect providers to the system, train office personnel, enter and retrieve immunization data, maintain the system, and respond to any problems that might arise. A recent study of costs among a sample of All Kids Count projects showed that personnel time was more costly than computer time (Slifkin et al., 1999).
Finding 4–2. A successful immunization system must be able to monitor vaccine coverage rates in targeted areas where populations are at risk of disease outbreaks. When communities are successful in achieving high coverage rates, the task of identifying vulnerable populations becomes more difficult and expensive, especially if unprotected groups are dispersed across multiple service systems or are concentrated in hard-to-reach geographic regions (such as remote areas or households without telephones).
Finding 4–3. The shift in service delivery for low-income children from a small cadre of public health providers and community-based clinics to a larger and more diverse network of health plans and private providers has increased coverage rates, but has also created more complexity in assuring service delivery and monitoring immunization coverage. The transition in service settings has also complicated the information management and administration of populationwide services within public health agencies. Data from disparate sources must be combined; health plans must incorporate outreach and patient reminder-recall systems, measure immunization coverage, assess provider practices, and conduct record audits; and public health agencies must formulate oversight policies that can guide health care finance and delivery systems within the public sector.
Finding 4–4. Although each community may express immunization
data collection and service strategies differently, a core surveillance effort is essential in three key areas—infectious disease patterns, immunization coverage levels (especially within high-risk groups), and adverse events from vaccines within the general population—to maintain the integrity and reliability of the national immunization system. Adequate datasets in each area are necessary to allow for comparisons within and between states and to monitor trends over time, especially among vulnerable populations. Compromising the quality of data within critical areas can lead to blind spots and uncertainties that erode health care policy and practice and waste resources in both public and private settings.
Finding 4–5. Precise and consistent measures and small-area studies are necessary to monitor coverage assessments in targeted areas and to establish performance benchmarks for different health care plans. A consistent, national standard for measuring coverage levels among diverse populations and multiple health care settings does not currently exist. The result is uncertainty in comparing rates across private and public health care plans or geographic areas. Performance standards, benchmarks, and information databases that establish consistent and comparable communitywide indicators for immunization levels among children, adolescents, and adults represent important opportunities for quality-of-care initiatives, but such efforts are in the early stages of development. They should be viewed as complements to, rather than replacements for, traditional public health surveillance and assessment efforts.
Finding 4–6. Some efforts are under way to improve immunization assessments as a measure of quality of health care delivery, but no single governmental agency is responsible for collecting datasets from multiple sources (e.g., NIS, Medicaid, and SCHIP) to ensure that relevant data are made available to state health officials. In addition, the methodology used in some assessments (such as HEDIS measures) may be inadequate to determine the true depth of coverage within hard-to-reach or mobile groups.
Finding 4–7. Several states and localities have made considerable progress in implementing registries that improve the measurement of immunization levels. Those operational systems that are in place offer a number of advantages, such as the assurance of data availability in case of disaster, reductions in the cost of record verification among providers at the point of service for a given patient, and elimination of the variations that can occur in parent/patient-carried health records. Once fully operational, registries may allow communities to target their outreach and other interventions more successfully, as well as to achieve significant savings
in other surveillance programs. With the increasing importance of population-based approaches to health system planning and evaluation, immunization registries offer one of the most useful instruments for assessing the population-specific effectiveness of health and medical care programs.
Finding 4–8. The registries of most states have not achieved a level of performance that allows them to serve as effective sentinels for gaps in coverage. A number of barriers need to be addressed if registries are to be fully implemented. Broad variability, the lack of consistent technical standards, problems with access to and the operational capability of software to support registry operations, and the need to respond to the requirements of multiple jurisdictions constitute major challenges that must be resolved to improve registry performance. Making registries fully operational will entail considerable costs, as well as efforts to resolve key concerns about privacy, liability, and confidentiality.
Finding 4–9. Data gaps and unreliable measures can lead to under-estimation of coverage levels and risk of disease for vulnerable populations. These uncertainties could eventually lead to blind spots within the national immunization system that would create pressure points in policy and practice and erode the quality and integrity of the enterprise. The experience with measles outbreaks in 1989–1991 and more recent disease reports suggest that during periods of complacency, low levels of immunization coverage among vulnerable groups may remain undetected and unaddressed, and can erupt into infectious disease outbreaks (NVAC, 1991).
Monitoring Vaccine Safety
As a biological product, vaccines may cause unintended side effects, some of which can be serious. Concerns about quality, safety, and reliability can be expected to grow as use of vaccines expands, particularly as the threat of infectious disease diminishes and as knowledge of the possible long-term health consequences of vaccine use increases. The potential for adverse events argues for the need to sustain both reliable monitoring systems and sources of expertise that can investigate anecdotal and clinical reports. The ability to distinguish between causal relationships and coincidence should adverse events occur requires an evidentiary base, as well as risk assessment judgments that can guide health care and public policy decisions during periods of uncertainty. Extensive media coverage of claims about possible adverse events associated with vaccine use before research information is available to guide health professionals, policy
makers, and the public prompts skepticism and even alarm that can lead to reduced vaccine use in the absence of scientific consensus.
Recent Safety Concerns. When safety claims emerge, organizations responsible for formulating recommendations that guide the use of vaccines must determine whether the reported evidence warrants the suspension or removal of a vaccine, further study, or no action. Three recent incidents—involving concerns about the safety of rotavirus vaccine, the use of thimerosal, and the reported connections between measles, mumps, and rubella (MMR) vaccine and the onset of autism—illustrate the range of issues and responses that arise in vaccine safety discussions.
In July 1999, the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics (AAP) recommended that physicians temporarily suspend administration of rotavirus vaccine. This recommendation emerged after CDC had investigated reports of several cases of intussusception (an intestinal disorder that sometimes requires surgical intervention) during the first few days after the vaccine’s ingestion (CDC, 1999i). CDC is conducting an ongoing case-control study of the vaccine and expects to report results in 2000 (AAP, 1999a). In the interim, the rotavirus vaccine has been removed from the ACIP recommended schedule.
Also in July 1999, AAP issued a joint statement with the U.S. Public Health Service (PHS) alerting physicians and the public to concern about thimerosal, a mercury-containing preservative used in some vaccines to prevent bacterial contamination. Mercury can be toxic to children, depending on the form used, route of entry, dose, and age of exposure. AAP and PHS have recommended that government agencies work rapidly to diminish children’s exposure to mercury from all sources, including vaccines (AAP, 1999b). Although future research findings may lead to changes in the vaccine schedule or the use of selected products, the thimerosal warning has not modified current ACIP recommendations in the absence of alternative products.
More recently, a series of stories in the media have suggested the possibility of a connection between autism and vaccines, especially the MMR vaccine. Unlike the rotavirus and thimerosal cases, in which the weight of clinical evidence attracted the attention of medical organizations, safety concerns about possible links between vaccines and autism have emerged primarily from preliminary research findings that have drawn public and political attention. Since the MMR vaccine is administered at the same time that symptoms of autism may become apparent, some parents have concluded that the vaccine is responsible for the syndrome. Two congressional hearings on vaccine safety (U.S. House of Representatives, 1999b, 2000) provided an opportunity for testimony that at-
tracted media coverage and further stimulated public fears about vaccine safety, even though little is known about the biological origins of autism. Further research has been recommended on this subject, but the scientific groups responsible for vaccine recommendations have also urged the continued use of the MMR vaccine in the absence of research or clinical evidence that would establish a convincing connection between vaccine use and the emergence of this disorder.
Vaccine safety concerns related to other disorders (e.g., childhood diabetes and multiple sclerosis) have also drawn large amounts of media attention. When claims are made about possible links between specific health and behavioral disorders and vaccine use, extensive efforts are required to determine the strength of the evidentiary base that supports each claim. Future additions to the vaccine schedule may contribute to concerns about vaccine safety, increase the likelihood of adverse events, and necessitate more resources for surveillance and for such efforts to investigate claims of health risks at both the national and local levels.
Federal Role. Before vaccines reach the market, private manufacturers implement rigorous measures designed to ensure the safety of their products. Vaccine manufacturers are required to document and report in the clinical trials literature any adverse health effects that are detected prior to the approval and licensing of a new vaccine product. Once a vaccine is in widespread use, health providers that administer vaccines and vaccine manufacturers are expected to report any adverse effects to DHHS.
There are two federal programs related to vaccine safety: the Vaccine Adverse Events Reporting System (VAERS) and the National Vaccine Injury Compensation Program (VICP). Following the passage of the National Childhood Injury Act of 1986 (P.L. 99–66), DHHS established VAERS to accept all reports of suspected adverse events among all age groups after the administration of any U.S. licensed vaccine in the public or private sector. Operated jointly by the Food and Drug Administration and CDC, VAERS was created primarily to serve as a signaling system for adverse events not detected during premarket testing (Food and Drug Administration, 1998).
VAERS currently receives 800–1,000 reports each month. About 85 percent of the reports concern relatively minor adverse events, such as swelling at the injection site and ordinary fevers. The remaining 15 percent involve serious side effects, such as high fever, seizure, life-threatening illness, or death. Upon analysis of VAERS reports, the Food and Drug Administration has the authority to recall a vaccine if it represents a risk to the American public. Only three batches of vaccine have been recalled during the last 10 years (Food and Drug Administration, 1999).
The National Childhood Injury Act of 1986 also established VICP,
which became effective on October 1, 1988, as Subtitle 2 of Title XXI of the Public Health Service Act. VICP is a federal no-fault system designed to compensate individuals, or families of individuals, who have been injured by childhood vaccines, whether administered in the private or public sector. The program is a critical component of the national immunization strategy since it authorizes compensation for those with specified injuries while also allowing the manufacture of recommended childhood vaccines to continue.
DHHS, the U.S. Department of Justice, and the U.S. Court of Federal Claims administer a collaborative process for determining qualification for compensation. VICP covers adverse events caused by the following vaccines: diphtheria, pertussis, tetanus (DTP, DTaP, DT, TT, or TD); MMR or any of its components; polio (oral poliovirus vaccine [OPV] or inactivated poliovirus vaccine [IPV]), whether administered individually or in combination; hepatitis B; Haemophilus influenzae type b; varicella, and rotavirus (Health Resources and Services Administration [HRSA], 2000a).
As of February 8, 2000, 5,763 VICP petitions had been filed, and 5,079 had been adjudicated (see Table 4–1). Most (74 percent) of the petitions involved vaccines administered prior to October 1, 1988. From fiscal year
TABLE 4–1 Vaccine Injury Compensation Program Petitions Filed, Adjudications, and Awards
|
Fiscal Year |
Petitions Filed |
Adjudications Dismissed |
Compensable Adjudications |
Awards (in millions of dollars) |
|
1988 |
24 |
NA |
NA |
NA |
|
1989 |
148 |
12 |
9 |
NA |
|
1990 |
3,248 |
34 |
98 |
71.9 |
|
1991 |
962 |
446 |
142 |
79.6 |
|
1992 |
189 |
488 |
166 |
97.6 |
|
1993 |
140 |
589 |
125 |
125.4 |
|
1994 |
107 |
446 |
162 |
107.0 |
|
1995 |
180 |
575 |
160 |
108.4 |
|
1996 |
84 |
409 |
161 |
103.1 |
|
1997 |
104 |
197 |
190 |
118.6 |
|
1998 |
120 |
181 |
145 |
135.1 |
|
1999 |
411 |
143 |
97 |
101.7 |
|
2000a |
46 |
45 |
58 |
55.1 |
|
Totals |
5,763 |
3,565 |
1,514 |
$1,103.5 |
|
NOTE: NA=not applicable. aThrough February 8, 2000. SOURCE: Health Resources Services Administration, 2000b. |
||||
(FY) 1989 to February 2000, 1,514 petitions were found to be compensable, while 3,565 were dismissed.11
The funding source for VICP awards depends on the date of vaccination. For vaccine administered prior to October 1, 1988, awards are financed by federal tax dollars allocated by Congress at $110 million per year. For vaccines administered on or after October 1, 1988, awards are paid from the Vaccine Injury Compensation Trust Fund, financed by an excise tax on vaccine purchases (HRSA, 2000c). In 1997, a flat-tax rate of $0.75 per antigen was introduced for all covered vaccines.
The average FY 1999 pre-1988 injury award was $855,474 (58 cases), for a total of $51.7 million. The average FY 1999 post-1988 injury award was $1,433,319 (33 cases), for a total of $50 million. Adjudication under VICP led to payments totaling $101.7 million in FY 1999 and approximately $1.1 billion in the 1990s (see Table 4–1) (HRSA, 2000b).
Finding 4–10. Vaccine safety concerns can be expected to acquire greater importance as the incidence of infectious disease diminishes and more vaccines are approved for general use. Data monitoring and reliable reporting systems need to be in place so that public health agencies can respond appropriately to concerns and uncertainties of the public and health providers about the need for vaccines during times when disease outbreaks are not apparent. Recent experiences with substantiated and unsubstantiated reports of adverse effects associated with vaccines suggest that the media have difficulty providing reliable information on this subject, especially during periods of scientific uncertainty. The demand for informed expertise can quickly overwhelm routine assessment and data collection efforts, especially if public health officials are expected to respond to antivaccine literature and anecdotal accounts as well as clinical reports.
EFFORTS TO IMPROVE AND SUSTAIN HIGH VACCINE COVERAGE RATES
The Task Force on Community Preventive Services (TFCPS) has identified a set of strategies for improving and sustaining high levels of immunization coverage (Briss et al., 2000)12:
-
Reduce cost barriers for vaccines for disadvantaged groups.
-
Expand access to immunization services.
-
Develop provider-based interventions to address the problem of missed opportunities in health care or social service settings that serve high-risk families.
-
Increase community demand for vaccinations.
These strategies are complementary to vaccine purchase and service-delivery efforts intended to increase coverage levels within populations that require additional assistance in achieving up-to-date immunizations. Each strategy consists of several different types of interventions. TFCPS recently reviewed the available literature to determine areas of best practice (Briss et al., 2000). An abbreviated summary of the TFCPS findings is presented here to demonstrate the impact of public health infrastructure investments on immunization coverage rates.
Reducing Cost Barriers and Building Capacity
The out-of-pocket costs of immunization constitute a barrier to obtaining vaccinations for many clients (Cutts et al., 1992). Providers are more likely to refer children with less public or private insurance coverage to other sites for vaccination, and referral practices are known to have adverse effects on both the timing and rate of immunization (Bennett et al., 1994; Mainous and Hueston, 1995; Ruch-Ross and O’Connor, 1994; Taylor et al., 1997; Zimmerman et al., 1997).
Reducing out-of-pocket costs improves vaccination coverage for diverse age groups and populations in a range of settings, from individual clinics, to statewide programs, to national efforts such as the Vaccines for Children (VFC) program (Briss et al., 2000). These positive effects have been reported whether the reduced-cost intervention has been used alone or as part of a multicomponent intervention (such as client or provider reminder-recall, communitywide education, expansion of access in health care settings, and provider education). The overall median coverage difference was found to be 10 percent (range of 8 to 35 percent). On the basis of this evidence, TFCPS strongly recommended reducing out-of-pocket costs as an effective strategy to improve vaccination coverage (Briss et al., 2000).
Although poverty is commonly accepted as a significant cause of discrepancies among immunization coverage rates, low rates of immunization coverage have been reported among populations for which cost is not a barrier (Orenstein et al., 1999). In one survey of 2-year-old children of employees of a large corporation, for example, only 65 percent of the children who had medical insurance coverage for immunizations had received the 4:3:1 series (four DTP, three polio, and one MMR) (Fielding et al., 1994).13 There were also no differences in coverage found in a study that compared private practices in states with and without universal purchase policies (Taylor et al., 1997). The families surveyed in this study, however, were well educated, of moderate to high socioeconomic status, and therefore least likely to benefit from free vaccines (Orenstein et al., 1999).
Service utilization studies within public health clinics indicate that some low-income parents use public clinics because of the reduced cost, even though they might prefer to receive immunizations from their regular private providers (Lieu et al., 1994; Santoli, 1999). Studies of the implementation of VFC have indicated that referrals to health departments decrease when free vaccines are provided to private providers, suggesting that both parents and providers take advantage of the free vaccines (Zimmerman et al., 1997). Although parents may need to pay administration fees in private-care settings that are not assessed at health department clinics, this cost is usually less than the vaccine expense. Parents may also be motivated to make use of VFC in seeking vaccine services since they do not need to take additional time from work or incur further transportation or child care expenses to visit a public clinic. VFC enables them to obtain vaccines from their usual care provider (Orenstein et al., 1999).
Research has not yet demonstrated whether reduced-cost strategies will improve immunization rates or change provider referral practices for adolescents and adults below age 65. This absence of knowledge is especially important when one is considering new finance strategies for future generations of vaccines for these age groups. In particular, although the body of research on children and older adults (above age 65) strongly suggests that reduced costs for vaccines result in positive outcomes for many age groups, the adolescent and young adult population is sufficiently distinct to merit independent study.
Expanding Access to Immunization Services
Expanding Access in Health Care Settings. Many local and state health agencies have sought to improve immunization rates by enhancing access to vaccines in health care settings, whether by extending hours or adding staff; introducing express services; or adding immunization services to hospitals, pharmacies, and nursing homes. These strategies are designed to reduce the distance from the setting to the at-risk population, increase the hours during which vaccination services are offered, deliver vaccinations in clinical settings in which they were previously not provided, or reduce administrative barriers to obtaining vaccination services within clinics (e.g., by developing a drop-in clinic or an “express lane” vaccination service) (Briss et al., 2000). Some programs for expanded access are offered through special contractual agreements with local health care providers. Others require more substantial commitments, including additional personnel that may be more difficult to sustain during hiring or salary freezes.
The TFCPS report strongly recommends expanding access to immu-
nizations in health care settings as part of a comprehensive intervention, indicating that insufficient evidence exists to support this intervention when used alone (Briss et al., 2000). TFCPS notes that within a comprehensive intervention, expanded access can improve vaccination coverage among children and older adults in a range of contexts, but the contribution of individual components to the overall effectiveness of such comprehensive interventions cannot be determined. The TFCPS report further observes that there are several barriers to implementing expanded access, including difficulties of coordination among settings, a lack of appropriate records, difficulties in clients’ recall of immunization status, high numbers of clients with contraindications to vaccination, and the lack of a relationship between vaccination programs and the primary missions of other settings.
Building capacity to serve new populations, especially adolescents and young adults, is a challenge that requires major consideration of the nature of the immunization partnership and funding patterns. New vaccines may be introduced in nontraditional settings (such as school clinics, workplace sites, and pharmacies) in which strategic oversight is required to monitor coverage levels and address safety concerns, including the potential for adverse events. The scale of investment required for public health agencies to exercise a leadership role in linking programmatic efforts, guiding the implementation of future generations of vaccines, and introducing vaccines to new populations has not been addressed.
Expanding Access in Nonmedical Settings. Numerous interventions have been designed to reach important target populations in nonmedical settings where they congregate. These interventions usually involve assessment of a child’s immunization status, and the referral of underimmunized persons to appropriate providers or the provision of vaccinations on site. Four nonmedical settings have been evaluated in this regard: (1) Women, Infants, and Children (WIC) sites; (2) home visits; (3) vaccination programs in schools; and (4) child care centers.
WIC Sites. WIC is a special supplemental nutrition program for women, infants, and children, a federal grant program administered by the U.S. Department of Agriculture and implemented through state health departments and tribal organizations. The primary mission of WIC is to provide supplemental foods and nutrition education; the program can also serve as a gateway and coordinator for other health and social services, including immunizations. WIC is the single largest point of access to health-related services for low-income preschool children since it serves more than 45 percent of the U.S. birth cohort, and in some cities serves up to 80 percent of low-income infants. Participants (usually mothers with
young children) commonly visit WIC sites every 2–3 months to receive nutrition services and to pick up food vouchers; more comprehensive health status evaluations are conducted every 6–12 months.
The TFCPS report recommends vaccination programs for immunization interventions in WIC settings, but observes that certain barriers may prevent implementation of this strategy. Many WIC providers believe vaccination requirements or monthly voucher pickups constitute disincentives for WIC participation. Two evaluation studies compared dropout rates between intervention and control groups. It was concluded that small differences in dropout rates do not demonstrate a causal link between vaccination interventions and WIC dropout (Hutchins et al., 1999; Birkhead et al., 1995).
Home Visits. Home visits provide face-to-face health services to clients in their homes. These services can include education, assessment of need, referral, and provision of vaccinations. Home visiting interventions can also involve telephone or mail reminders.
The TFCPS report recommends that home visits be used to improve vaccination coverage among socioeconomically disadvantaged populations. Home visit interventions, however, when applied only to improve vaccination coverage, are highly resource-intensive relative to other available options for improving coverage since they require specialized staff training and must address concerns for staff safety.
School-Based Programs. School-based programs provide opportunities for vaccination interventions that extend beyond the simple requirement of certain vaccinations for school attendance. School interventions are intended to improve delivery of vaccinations to school attendees and to improve coverage rates among children and adolescents aged 5–18. In some cases, the programs may also target preschool siblings.
School interventions generally consist of vaccination-related education of students, parents, teachers, and other school staff; provision of vaccinations or referrals; and occasionally other components, such as incentives or written consent requirements. School-based programs often involve collaborations among schools, local health departments, private hospitals, and community clinics. They provide a unique opportunity for reaching young adolescents, since approximately 90 percent of U.S. children aged 11 and 12 attend school (Kominski and Adams, 1991). School-based vaccination programs can be used to determine each student’s immunization status, identify those who have missed doses, and ensure completion of vaccine series (especially for hepatitis B vaccine) among most students. However, TFCPS found there was insufficient evidence to
assess the effectiveness of such programs because limited studies have been conducted to evaluate their impact.
There are several potential barriers to the implementation of school-based vaccination programs. These barriers can include difficulties in coordinating among different programs, staff training requirements, disruption of school routines, and confidentiality concerns.
Child Care Centers. Children in child care centers are at increased risk for communicable diseases. In 1995, approximately one-third of preschool children (31 percent) were cared for in such settings (information provided by Children’s Health Working Group). Child care center interventions involve efforts to encourage vaccination of preschool children (younger than age 5) by assessing each child’s immunization status upon entry into child care and at some point or at periodic intervals during the child’s enrollment. Vaccination interventions can also include education or notification of parents, referral of underimmunized children to health care providers, and sometimes provision of vaccinations on site. TFCPS concluded that there is insufficient evidence available to assess the effectiveness of such interventions (Briss et al., 2000).
Addressing Missed Opportunities
Many researchers and health care providers used to attribute the lack of complete immunization coverage to poverty and the economic barriers that discouraged families from seeking vaccines or gaining access to a primary care provider. In the wake of the 1989–1991 measles outbreak, however, it was found that underimmunized children had substantially more access to the health care system than had previously been assumed. The 1988 National Health Interview Survey on Child Health, for example, revealed that 90 percent of children had a source of routine health care, although only 77 percent of 2-year-olds had achieved full immunization coverage (St. Peter et al., 1992). This finding was reinforced by a later analysis of the 1993 National Health Interview Survey, which demonstrated that 90 percent of underimmunized children reported having a usual source of health care (Tatande et al., 1996).
These findings caused many researchers and health professionals to rethink traditional strategies for improving vaccination coverage levels and to focus on addressing missed opportunities in health care and other public service settings (Santoli et al., 1998). Researchers recommended several new strategies aimed at encouraging providers to vaccinate both children and adults. These strategies included checking records of immunization status and implementing reminder-recall systems for public and
private providers; introducing immunization services in nontraditional medical settings (e.g., emergency departments or acute care clinics); linking immunization assessment efforts with other services that involved high-risk families (e.g., Head Start, WIC clinics, and the welfare system); and encouraging health professionals to offer simultaneous immunizations during acute care appointments (Orenstein et al., 1999). Four such provider-based interventions have been evaluated in the research literature (Briss et al., 2000):
-
provider reminder-recall,
-
provider assessment and feedback,
-
standing orders, and
-
provider education.
Provider Reminder-Recall. This strategy involves alerting those who administer vaccinations that individual clients are due (reminder) or overdue (recall) for particular vaccinations. Issued before, during, or after a scheduled appointment, such notices can be provided through such means as client charts, computer records, or mail.
Provider reminder-recall has been shown to improve vaccination coverage for various age groups (adults, adolescents, and children) in a range of settings and populations (Briss et al., 2000). Positive impacts have been demonstrated whether the reminder notice was used alone or as part of a comprehensive intervention. Positive results were also associated with a range of methods (e.g., computerized or simple reminders, checklists, or flowcharts).
The TFCPS task force strongly recommends the use of reminder-recall interventions to improve vaccination coverage for all age groups. However, some studies have revealed that provider offices experience difficulty with placing reminders in charts, and some health care professionals do not use reminders when provided, suggesting that the administrative burden associated with this strategy may be a major barrier to its use (Briss et al., 2000). Lack of information about vaccination status may also inhibit use of this approach. A 1992 study indicated that fewer than 20 percent of providers operated any kind of credible reminder-recall system (Szilagyi et al., 1992). A 1999 study led to a similar finding (Darden et al., 1999).
Provider Assessment and Feedback. Many providers tend to overestimate the coverage rates of their clients. In a California study, for example, physicians estimated that about 90 percent of their patients were up to date, although record audits indicated that the actual rate was well below 70 percent (Watt et al., 1998). Assessment and feedback interventions (also
called AFIX for Assessment, Feedback, Incentives, and eXchange of information) involve performing a retrospective evaluation of the performance of providers in delivering one or more vaccinations to a client population. This information is then given to providers and sometimes others (e.g., comparing performance against a goal or standard with incentives or benchmarking in a health plan). Specific interventions vary in content, intensity, and use of incentives.
Use of assessment and feedback can change provider knowledge, attitudes, and behavior, and may also stimulate additional system-level changes, such as the routine use of reminders or standing orders. TFCPS strongly recommends the use of assessment and feedback interventions, whether alone or as part of a comprehensive intervention, across a range of settings, age groups, and populations (Briss et al., 2000). At the same time, the TFCPS report notes that this intervention may constitute an administrative burden for some providers or systems, and there may also be barriers to its implementation, such as the lack of an adequate information infrastructure.
Standing Orders. Standing orders provide a protocol by which non-physician personnel prescribe or deliver vaccinations to client populations (primarily adults) without direct physician involvement at the time of the interaction. This intervention has been implemented in clinics, hospitals, and nursing homes. Standing orders are designed to improve delivery of immunizations by reducing both barriers to vaccination (such as the requirement for a physical examination) and missed opportunities (resulting from a lack of physician personnel).
TFCPS concluded that there is insufficient evidence to assess the effectiveness of standing orders in improving vaccination coverage in children (Briss et al., 2000). No studies have evaluated the effectiveness of standing orders in improving vaccination among adolescents or increasing delivery of hepatitis B or tetanus vaccinations. In contrast, TFCPS strongly recommends the use of standing orders for both influenza and pneumococcal vaccinations for adults in such settings as hospitals, clinics, and nursing homes (Briss et al., 2000). Again, however, the TFCPS report notes that the use of standing orders may be difficult. The strategy requires effective interprofessional communication and the sharing of responsibility for the associated administrative burden among busy providers and systems, especially in pediatric clinics.
Provider Education. Increasing the knowledge or changing the attitudes of providers regarding vaccination can lead to improvements in immunization coverage if providers act on this information in a positive manner. Provider education can lead to additional vaccinations; change provider-
client interactions to increase client acceptance of vaccinations; or motivate providers to implement other system-level changes, such as reminder-recall systems or standing orders.
Given the paucity of studies of this intervention, limitations in their design and conduct, and small effect sizes, TFCPS concluded that there is insufficient evidence for assessing the effectiveness of provider education interventions. The TFCPS report suggests that this approach be used only in conjunction with other interventions until better information becomes available (Briss et al., 2000).
Improving Awareness and Documentation of Immunization Status
Strategies to promote community and client awareness and documentation of individual immunization status have been proposed in the expectation that such efforts will stimulate requests for immunization services during interactions with health care providers. Three such strategies have been formulated:
-
Increasing community demand for vaccinations through various educational and reminder interventions
-
Establishing requirements or incentives for immunization
-
Enhancing awareness of immunization status through devices such as client-held medical records
Increasing Community Demand for Vaccinations. Three specific strategies are commonly used to increase community demand: client reminder-recall systems, comprehensive interventions that include education, and communitywide or clinic-based education-only interventions.
Client Reminder-Recall Systems. Reminders and recalls inform clients when vaccinations are due or overdue. They differ in content and timing and are provided in various forms, including telephone, letter, or postcard. Client reminders can be either general or specific (i.e., certain vaccinations are due on a specific date).
Client reminder-recall interventions have proven effective whether used alone or as part of a comprehensive intervention. TFCPS therefore strongly recommends the use of these interventions for both children and adults, in a range of settings and populations, and at different levels of scale (Briss et al., 2000). Barriers to implementation include lack of datasets and the administrative burden on providers or systems.
Comprehensive Interventions That Include Education. Comprehensive interventions focused on clients address health concerns and barriers
to vaccination in an integrated way. They make community members aware of vaccination services, highlight the utility and relevance of these services, and provide information that can help clients take advantage of the services. The interventions may incorporate a variety of associated strategies to improve vaccination, including client and/or provider reminders, provider education, expanded hours or access in clinical settings, lowering of out-of-pocket costs, client-held vaccination records, and WIC interventions.
Having reviewed the relevant research, TFCPS strongly recommends the use of comprehensive interventions that include education for children and adults in communitywide and clinic-based settings in a range of contexts (Briss et al., 2000). Barriers to the implementation of this intervention include the difficulties involved in coordinating strategies among various programs and administrative systems.
Communitywide or Clinic-Based Education Only. Education-only programs provide information to most or all of a target population in a geographic (communitywide) or institutional (medical or public health clinic-based) setting. The information may be provided to clients only, providers only, or both. Educational materials can take the form of brochures (including mail), videotape, posters, vaccine information statements (standardized statements often used to obtain consent for vaccination), and announcements in the media (radio, newspapers, and television). The goal of educational interventions is to increase client acceptance of and demand for vaccinations.
A limited evidentiary base exists for this intervention. TFCPS therefore concluded that there was insufficient evidence regarding the effectiveness of either communitywide or clinic-based education-only interventions (Briss et al., 2000).
Establishing Requirements and Incentives. States or local governments may develop specific requirements or incentives to ensure immunization coverage.
Vaccination Requirements for Child Care, School, and College Attendance. During the 1970s and 1980s, all 50 states adopted immunization requirements for entry to elementary school, and more than 95 percent of children are now appropriately vaccinated with recommended doses of vaccine upon entering school. The required immunization schedule varies from state to state. More recently, immunization requirements have been adopted for child care and college attendance. Enforcement levels for the latter vary greatly among the states.
Having reviewed the relevant body of evidence, TFCPS recommends
vaccination requirements for child care, school, and college attendance among all relevant populations (Briss et al., 2000). TFCPS found that the extent to which specific legal characteristics or intensity of enforcement influenced the effectiveness of state requirements could not be determined.
Client or Family Incentives. Many immunization programs offer positive incentives (e.g., baby toys, money, or discount coupons) to motivate their clients to seek vaccinations for themselves or their children. Incentives can also be negative (penalties that can lead to the client’s exclusion from a program). After reviewing the relevant literature, TFCPS concluded that insufficient evidence exists to assess the effectiveness of client incentives in improving vaccination coverage, and observed further that the potential for coercion represents a potential barrier to their implementation.
Enhancing Awareness of Immunization Status. Client-held (also called hand-held) medical records are provided to members of a target population or their families to indicate which vaccinations have been received. The records can improve a client’s awareness of vaccinations needed or due and can be used to assess immunization status in medical and other settings. Many state and local health departments and providers have encouraged use of client-held medical records to improve coverage rates by increasing clients’ knowledge about vaccinations and/or reducing missed opportunities in health care settings.
Based on the small number of studies of this strategy, limitations in study designs, and variations in the interventions and research findings, TFCPS concluded that insufficient evidence exists to assess the effectiveness of client-held medical records in improving vaccination coverage (Briss et al., 2000). Furthermore, although 80 percent of providers in one survey reported positive or very positive overall reactions to a “health diary,” 17 percent also believed that such records negatively affect client flow (Dickey and Petitti, 1992).
Finding 4–11. There is sufficient evidence in the research literature to support strongly recommending the use of certain strategies to improve immunization rates across the United States, within either the general population or targeted groups. These strategies include client and provider reminder-recall; assessment and feedback for vaccination providers; multicomponent interventions that include education; lowering of out-of-pocket costs to families for vaccinations; elimination of the gap in immunization coverage rates between minority groups and the general population; expanded access to vaccinations in health care settings; and
the use of standing orders to vaccinate adults in clinics, hospitals, and nursing homes. Other interventions—such as school or child care vaccination requirements, home visits to promote vaccinations, and vaccination programs in WIC settings—can be recommended when they are well matched to particular needs and capabilities. In other cases, additional work is necessary to determine whether a specific intervention has the potential to increase coverage levels and to be implemented successfully within different communities. Support and leadership are required from local public health agencies to test the effectiveness of and to implement promising interventions within a given community. Nationwide programs are not the best approach; instead states and local communities need to experiment with multiple strategies. Interventions designed to enhance the use of reminder-recall and assessment and feedback efforts in the private sector are likely to be highly effective. There is also a need for extensive and ongoing collaboration among local public health agencies, private health plans, and public and private health care providers to monitor and improve coverage levels.
Finding 4–12. Data on baseline and current immunization coverage levels are essential for determining the reliability or effectiveness of a selected intervention. Disruptions in data collection and assessment efforts impede the evaluation of intervention strategies and inhibit the determination of best practices.
Finding 4–13. It is unlikely that private health care plans will allocate adequate resources for populationwide services or community activities that promote the health of at-risk or hard-to-reach populations under current contractual arrangements.
SUMMING UP
The continued presence of many vaccine-preventable diseases throughout the world requires a persistent effort within each U.S. state to monitor disease reports and be prepared to respond swiftly to disease outbreaks. Similarly, surveillance and assessment of immunization coverage rates are still required so that discrepancies can be identified and resolved by reducing barriers or creating appropriate incentives within the health care system. Interventions such as assessment and feedback for public and private health care providers and client reminder-recall have been shown to improve immunization rates, and their implementation in a broad range of communities deserves full support. In areas where evidence of impact is uncertain, multiple strategies are necessary until certain approaches have achieved a significant enough impact to warrant
their implementation across multiple populations. Policy linkages are also needed to strengthen system performance and to bridge gaps between the health care finance and health care delivery systems. System-level interventions can improve immunization coverage rates when they benefit key target audiences, but such interventions can be difficult to achieve when their implementation requires changes in professional behavior or organizational practices.
Ongoing developments within the science of vaccines, changes in the organization of the U.S. health care system, and movements within the population at large all place extraordinary demands on the public health infrastructure for immunization (see Chapter 2). These demands, in turn, have generated two key forces: (1) a persistent national need for the best available expertise, technology, tools, and leadership to complement state efforts to fulfill important public health functions, and (2) a strong desire to maintain flexibility in services and policies at the state and local levels to ensure responsiveness to immediate situations and state-driven priorities. These twin forces shape the infrastructure for immunization nationwide.
Finding 4–14. The immunization infrastructure within each state needs to have the capacity to perform a set of critical surveillance, disease control, safety oversight, and immunization improvement strategies to sustain current coverage rates. Reductions in this capacity will contribute to a weakening of vaccination levels and possible disease outbreaks.
Chapter 5 considers the various forms of local, state, and federal investments in each of the three roles discussed in this chapter. Recognizing that variations in organizational style can affect the level of resources required, we examine how some states have spent federal funds to support stand-alone as well as integrated services. Determining the level of resources necessary to sustain a viable and flexible infrastructure and how such investments should be allocated across state and national budgets is essential to ensure a viable, flexible, and stable national immunization system in the future. The particular roles and contributions of the National Immunization Program and Section 317 funds are considered in this context.
ENDNOTES
|
|
example, data collected in fall 2000 on 5-year-old kindergarten entrants reflect coverage 3 years ago, when these children were age 2 (information provided by CDC). |
||||||||||||||
|
3. |
Such conditions include the following: the records were transferred to another provider; the chart indicates that the child has moved or gone elsewhere; a mailed reminder card was returned without another local forwarding address; and the chart indicates that the parent says the child is seeing another provider, or that a home or telephone visit revealed the child was seeing another provider. |
||||||||||||||
|
4. |
Examples cited are based on personal communication with providers in the New York inner-city area. |
||||||||||||||
|
5. |
The New York City Department of Health provides such separate reports. |
||||||||||||||
|
6. |
What constitutes a “fully functional registry” has been discussed and debated. Both NVAC and Wood et al. (1999:232) define a fully functional registry as one that tracks more than 95 percent of children under age 2 in the specified catchment area and provides an electronic immunization record that is accessible to providers. The Robert Wood Johnson Foundation (RWJF) considers a fully functional registry as one that includes all children in a given catchment area, with information about all doses of all vaccines delivered by all providers (NVAC, 1999b:16). |
||||||||||||||
|
7. |
The items were derived from a conceptual definition prepared by the Cecil G.Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill, which served as the national evaluation office for the All Kids Count initiative. RWJF also established a national program office for the initiative at the Task Force for Child Survival at the Carter Center in Atlanta, Georgia. |
||||||||||||||
|
8. |
Duplicate records are included in these statistics, causing some states to show more than 100 percent of their children enrolled in the state registry. |
||||||||||||||
|
9. |
The CDC survey defined public providers as “facilities operated partially or wholly with public funds (e.g., county public health clinics, community/migrant health centers, Indian Health Services, etc.) and/or the individual practitioners providing immunizations in such facilities.” Private providers are defined as “health care facilities or practices operated solely with private funds and/or the individual practitioners providing immunizations in such facilities.” |
||||||||||||||
|
10. |
For the purposes of the CDC survey, enrollment was defined as “providers who have authorized access to the registry (e.g., written agreement, passwords, rights and responsibilities defined)” (information provided by CDC). Participation entails actually having submitted data to a registry in the last 6 months. |
||||||||||||||
|
11. |
The percentage breakdown of vaccines claimed to cause adverse events is as follows:
|
||||||||||||||
|
12. |
The TFCPS report includes only three strategies (increasing community demand for vaccination, enhancing access to vaccination services, and implementing provider-based interventions). Reducing out-of-pocket costs is described in the report as one of six interventions under the strategy of enhancing access to vaccination services. Given the importance of finance approaches in the present study, out-of-pocket cost intervention is categorized as a fourth strategy in this discussion. |
||||||||||||||
|
13. |
It is possible that immunization records for these children were not available, contributing to a lower reporting rate than actually was the case. |
||||||||||||||