6
Summary Findings, Conclusions, and Recommendations
Six questions posed by the U.S. Senate Committee on Appropriations and CDC established the initial framework for this study:
-
What was the extent of overall spending by all sources for immunizations in the United States during the 1990s?
-
How were new federal funds spent by the states, and to what extent did states maintain their own levels of effort over the past 5 years?
-
What are current and future funding requirements for immunization activities, and how can those requirements be met through a combination of state funding, federal Section 317 immunization grant funding, and funding available through the State Children’s Health Insurance Program (SCHIP)?
-
How should federal grant funds be distributed among the states?
-
How should funds be targeted within states to reach high-risk populations without diminishing levels of coverage among the overall population?
-
What should be the role and financing level for CDC’s current program supporting state efforts to vaccinate adults and achieve the nation’s goals for influenza and pneumococcal vaccines?1
In preparing answers to these questions, the committee examined the roles and responsibilities required for an effective national immunization system. We identified six key roles that this national system must perform:
-
Assure the purchase of recommended vaccines for the total population of U.S. children and adults, with a particular emphasis on the protection of vulnerable groups.
-
Assure access to such vaccines within the public sector when private health care services are not adequate to meet local needs.
-
Control and prevent infectious disease.
-
Conduct population wide surveillance of immunization coverage levels, including the identification of significant disparities, gaps, and vaccine safety concerns.
-
Sustain and improve immunization coverage levels within child and adult populations, especially in vulnerable communities.
-
Use primary care and public health resources efficiently in achieving national immunization goals.
The last of these roles provides overarching support for the other five, and was the focus of the committee’s charge. In conducting the study, we gave particular attention to the responsibilities of federal and state health agencies and the burden of effort required to support each of the above roles in an integrated manner. In this chapter, we apply the findings presented in Chapters 2 through 5 to answer the six questions under the committee’s charge. We then present the overall conclusions and recommendations resulting from the study, as summarized in Box 6–1.
SIX QUESTIONS AND SIX ANSWERS
Question 1. What was the extent of overall spending by all sources for immunizations in the United States during the 1990s? (Supported by Findings 3–1 through 3–6 in Chapter 3.)
The most common sources of spending for immunization in the United States during the 1990s were federal funds, state funds, private insurance reimbursements, and other private funds (e.g., foundation support for the development of registries and local outreach efforts). The federal government was and remains the primary source of assistance for both vaccine purchases and immunization programs.
Federal funding for immunization services (including vaccine purchases, infrastructure, and other grants), estimated from congressional budgets, grew from about $500 million in 1990 to more than $1 billion in 1999, an increase that reflects the expanded federal role in purchasing vaccines for disadvantaged children (see Table 1–4 in Chapter 1). Principal federal investments include the Vaccines for Children (VFC) program, Section 317 grant awards, and Medicaid reimbursements to the states for vaccine administration services. Medicare reimbursements for adult vac-
|
BOX 6–1 Conclusions and Recommendations CONCLUSIONS Conclusion 1: The repetitive ebb and flow cycles in the distribution of public resources for immunization programs have created instability and uncertainty that are eroding the continued success of immunization activities. Conclusion 2: Immunization policy needs to be national in scope. At the same time, the implementation of immunization policy must be flexible enough to respond to special circumstances that occur at the state and local levels. Conclusion 3: Federal and state governments each have important roles in supporting not only vaccine purchase, but also infrastructure efforts that can achieve and sustain national immunization goals. Conclusion 4: Private health care plans and providers have the capacity to do more in implementing immunization surveillance and preventive programs within their health practices, but such efforts require additional assistance, oversight, and incentives. At the same time, comprehensive insurance and high-quality primary care services do not replace the need for public health infrastructure. SUMMARY RECOMMENDATIONS Recommendation 1: The annual federal and state budgets for the purchase of childhood vaccines for public health providers appear to be adequate, but additions to the vaccine schedule are likely to increase the burden of effort within each state. Therefore, the committee recommends that CDC be required to notify Congress each year of the estimated cost impact of new vaccines that have been added to the immunization schedule so that these figures can be considered in reviewing the vaccine purchase and infrastructure budgets for the Section 317 program. Recommendation 2: Additional funds are needed to purchase vaccines for uninsured and underinsured adult populations within the states. The committee recommends that Congress increase the annual Section 317 vaccine budget by $50 million per year to meet residual needs for high-risk adolescents and adults under age 65 who do not qualify for other federal assistance. The committee further recommends that state governments likewise increase their spending for adult vaccines by $11 million per year. Recommendation 3: State immunization infrastructure programs require increased financial and administrative support to strengthen immunization capacity and reduce disparities in child and adult coverage rates. The committee recommends that states increase their immunization budgets by adding $100 million over current spending levels, supplemented by an annual federal budget of $200 million to support state infrastructure efforts. Recommendation 4: Congress should improve the targeting and stability of Section 317 immunization grant awards to the states by replacing the current discretionary grant award mechanism with formula grant legislation. Recommendation 5: CDC should initiate a dialogue with federal and state health agencies, state legislatures, state governors, and the U.S. Congress immediately so that legislative and budgetary reforms can be proposed promptly when Section 317 is up for reauthorization in FY 2002. Recommendation 6: Federal and state agencies should develop a set of consistent and comparable immunization measures for use in monitoring the status of children and adults enrolled in private and public health plans. |
cine purchases have been reported only recently (fiscal year [FY] 1998). The annual budget for the National Vaccine Injury Compensation Fund, administered by the Health Resources and Services Administration (HRSA), adds another $100 million annually to the federal budget, but these funds are reserved solely for injury compensation claims and are not available to support vaccine purchase, service-delivery, or immunization programs. Trend data are not available for other federal investments, such as routine vaccine purchases and administration for military personnel and their families or veterans, or vaccines dispensed through the Indian Health Service. These budgets are designed primarily for clinical services and do not supplement infrastructure efforts within the states. Some immunization services are supported through Title V grants, the Community/Migrant Health Centers grants, and the Public Health Service prevention block grants, but such budgets are not tracked separately, nor are they reported in annual executive and congressional summaries of federal expenditures for immunizations.
The proliferation of new federal funding sources for vaccine purchase and child health care services (including VFC and SCHIP) raises the question of whether these new programs have the capability to assume many functions previously supported by Section 317 funds. These newer programs have absorbed many of the costs of vaccine purchases and office visits previously covered by Section 317 or Medicaid. Even with the expansion of public and private health plans, however, pockets of need remain in which individuals are susceptible to vaccine-preventable diseases. In addition, the increasing number of new vaccines, the fragmentation of uncovered groups, and the shift to private health care providers have increased the complexity of the national immunization system, requiring additional infrastructure and oversight within the states. As Medicaid, SCHIP, and other new federal programs are fully implemented, they may be able to absorb greater responsibilities in areas such as provider audits, assessments, outreach, and education for underserved populations and their health care providers. At present, however, these newer federal programs are not designed to perform or finance these roles.
The total amount of state funds allocated for immunization activities in the 1990s is not available. In 1999, CDC required state immunization agencies to estimate other federal (non-Section 317) funds, state funds, and private sources scheduled to support their immunization efforts in calendar year (CY) 2000. Based on these self-reported data, CDC has estimated that state budgets allocate a total of about $340 million for immunization programs and services, which include vaccine purchase and infrastructure support (information provided by CDC). This figure includes a variety of revenue sources, including state-only spending, reallocated federal budgets, and intergovernmental transfers, including school health.
However, this estimate does not include state Medicaid matches, even though a portion of these funds is allocated for immunization services. Other sources of revenue within the states, such as funds available from some private-sector plans for provider reminder-recall systems, local governmental support for registry projects, and vaccine industry support for professional education, are more limited, and no national data exist that can be used to measure such investments over time.
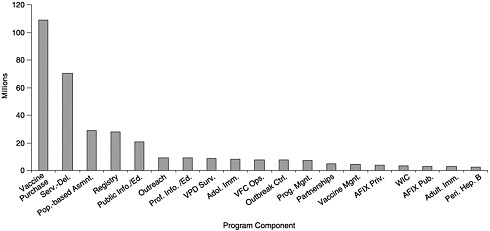
State budgets for vaccine purchase and immunization programs vary widely depending on the size of the state, the state’s poverty level, per capita investments in public health, and the state’s ability to carry out coordinated efforts that can use internal funds and cost savings efficiently. Of the total $340 million expenditures reported by the states, including state, local, other federal, and private funding (see Figures 6–1 and 6–2), states allocated approximately $109 million for vaccine purchase (including $102 million in state revenues). The remainder ($231 million) was
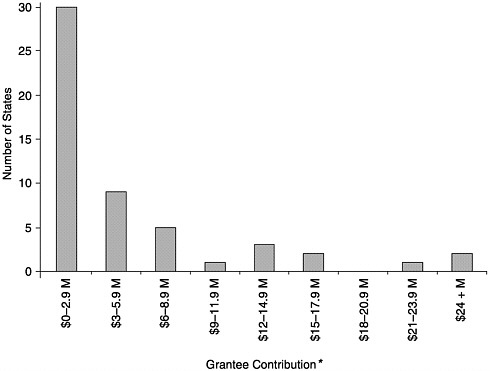
FIGURE 6–1 Level of grantee contribution by number of states, calendar year 2000. *May not include all appropriated and in-kind contributions. SOURCE: Information provided by CDC.
allocated for infrastructure efforts and program operations (information provided by CDC).
States depend heavily on federal purchases of vaccines for all age groups, and use the Section 317 funds to serve children who are not eligible for the VFC program, to support a universal purchase policy, or to purchase selected vaccines for special programmatic needs. Ten states contribute substantially—more than 30 percent—to the total amount of publicly purchased vaccines within the state. All but two of these (Georgia and Missouri) are universal purchase states, and Georgia has expanded state purchase of vaccines for underinsured children. Twenty-four states and the District of Columbia contribute less than 10 percent to all publicly purchased vaccines, and the remaining 16 states contribute between 10 and 30 percent (information provided by CDC).
Federal grants to the states under Section 317 provide the core funding for state immunization programs, most of which is passed on to local health departments to support outreach, data collection, and program oversight efforts. The states collectively spend about twice the amount provided in federal Section 317 grants to support immunization infrastructure ($231 million in state-level budgets compared with $123 million in federal assistance grants in FY 2000), but state budgets are highly variable. Only 4 states have direct state funding for a substantial portion (i.e., more than 40 percent) of their immunization program infrastructure (Freed et al., 1999). Almost half of the states (21) provide no direct state funding for immunization program infrastructure. Eleven states have, or have had, direct state funding for registry development, although the size and length of that funding varies. Ten states fund immunization program staff, while a handful of others have small amounts of general funding. Several states devote Women, Infants, and Children (WIC), Medicaid, or Maternal and Child Health (MCH) (Title V) block grant funds to immunization-related activities, but these funds largely support specific initiatives rather than broad programmatic efforts.
In most states, local health departments provide substantial in-kind contributions, ranging from facilities and overhead to locally funded staff who perform multiple duties, including the delivery of immunizations. Major in-kind contributions come from school nurses and secretaries, who conduct school-based assessments of children’s immunization status. Several states provide in-kind technical support for registry development (Freed et al., 1999).
Question 2. How were new federal funds spent by the states, and to what extent did states maintain their own levels of effort over the past 5 years? (Supported by Findings 5–8, 5–9, 5–13, 5–14, and 5–17 in Chapter 5.)
States traditionally divide their Section 317 federal immunization budgets between vaccine purchase and infrastructure or programmatic investments. Over the last 5 years, the private health care sector has assumed greater responsibility for providing immunizations to vulnerable populations (especially participants enrolled in Medicaid and SCHIP). This shift has had a profound impact on the nature of the services states are required to provide, stimulating a need for greater oversight and assessment efforts while also reducing the need, in some areas, for direct services for disadvantaged populations within public health agencies.
Vaccine Purchase. Prior to the VFC program (before FY 1995), the states routinely used Section 317 funds for vaccine purchase. In FY 1993, for example, Congress appropriated $193 million (more than 80 percent of the total Section 317 budget) for vaccine purchases by the states and $45 million for infrastructure grants (less than 20 percent of the total Section 317 budget). States were expected to gear up quickly in response to disease outbreaks, and federal funds provided them an opportunity to extend clinic hours, hire additional staff, and purchase enough vaccine to address special circumstances or short-term needs.
The implementation of VFC in the wake of the 1989–1991 measles epidemic shifted the routine purchase cost of vaccines for most Medicaid-enrolled children from state to federal funds and resulted in substantial savings to the states. The total size of these savings has not been estimated. The ways in which states used their Medicaid savings varied considerably and often depended on the state’s needs and health finance organizational structure. Eight states (California, Connecticut, Idaho, Kentucky, Massachusetts, Minnesota, Nebraska, and North Carolina) used their vaccine purchase savings to increase their reimbursement fees to Medicaid providers for administration of immunizations. Some states expanded the eligibility criteria for Medicaid programs, or used the VFC savings to purchase additional vaccines for school health programs or other groups not covered by VFC, such as the underinsured (insured children whose health plans do not cover immunizations).
While total federal immunization budgets grew significantly with the creation of VFC, federal support for immunization programs within the states decreased during the past 5 years. Although states achieved cost savings with the creation of VFC, they incurred other administrative costs within the program for which they drew on other revenue streams, including Section 317 funds. In addition, states did not apply their Medicaid savings to support other immunization roles in such areas as data collection, surveillance of coverage rates, and development of preventive interventions for the general population or high-risk groups. The administrative separation of state Medicaid offices and public health agencies
and the requirement that Medicaid funds be used only for Medicaid clients impeded the ability of most states to control and use revenue streams efficiently to support their multiple immunization roles. In general, the few states that housed their Medicaid program and department of health within the same agency had greater success in capturing VFC savings and applying this revenue directly to their immunization programs.
The creation of the VFC program did not eliminate the need for state-purchased vaccines. States continued to rely on Section 317 grants to meet the vaccination needs of children who did not qualify for federal assistance, which in some cases accounted for almost half of the state’s total vaccine purchases. For example, a recent CDC study of 12 states2 indicated that the proportion of total state vaccine purchases allocated to VFC ranged from 42 percent (Washington) to 87 percent (California) (CDC, 1998d). Similarly, the proportion of vaccine purchases allocated to Section 317 ranged from 5 percent (Florida) to 48 percent (Utah).
States continued to rely on Section 317 vaccine purchase funds for two reasons: (1) VFC eligibility requirements excluded many thousands of children (e.g., those enrolled in health plans that did not include immunization coverage) whom many states felt obligated to serve through a universal purchase program or an enhanced vaccine purchase policy, and (2) new vaccines (including rotavirus and varicella) were approved for children and adolescents during this period that were not automatically included within the VFC contract. As a result, Medicaid providers (which were bound by the Advisory Committee on Immunization Practices [ACIP] recommendations) were obligated to provide these vaccines, and states sought to make them available in public health clinics through their Section 317 purchases when they experienced delays in obtaining vaccines from the VFC program. In many cases, state Medicaid programs were also faced with reimbursing private providers for the private-sector purchase and administration to Medicaid clients of these very expensive vaccines.
In addition, despite the implementation of VFC throughout the United States, some states, particularly those with universal purchase programs, continue to allocate sizeable amounts of their own funds for vaccine purchase. Connecticut’s vaccine purchases under the federal contract, for example, amount to half of all publicly purchased vaccine in that state (information provided by CDC). Other states, such as Alabama, Michigan, and Pennsylvania, rely primarily on federal funds for vaccine purchase.
Many states have reported that the administrative burden associated with VFC has increased considerably in the past several years, both for participating providers and for the states. This increased administrative burden raises the cost of state operations and oversight efforts, since states
must provide tallies of doses administered for larger numbers of vaccines and providers, as well as estimates of VFC participants served in increasingly diverse health settings. The requirements are particularly burdensome in universal purchase programs (which were designed to reduce paperwork in determining patient or provider eligibility for state-financed vaccines). The implementation of SCHIP has added a new client base that further complicates determinations of VFC eligibility. Children who were once VFC-eligible because they were not insured now do not qualify in states that have adopted stand-alone SCHIP programs, since only Medicaid expansion programs qualify for VFC. Uncertainties about the extent to which VFC eligibility should be expanded to the entire SCHIP population have prompted requests for guidance from the Health Care Financing Administration (HCFA) (Richardson, 1999; Richardson and Orenstein, 1999), as well as legislation introduced in the Congress in September 1999 (U.S. House of Representatives, 1999a; U.S. Senate, 1999).
Infrastructure Grants. Section 317 grants to the states for infrastructure were highly unstable during the 1990s (see Figures 1–2 and 1–3 in Chapter 1). Following the 1989–1991 measles outbreak, CDC launched a national initiative designed to strengthen state immunization programs and provide resources for a broad array of direct services, outreach, and expanded access programs. The CDC budget for state infrastructure grants almost tripled between FY 1993 and 1994, growing rapidly from $45 million in new funds (FY 1993) to more than $128 million (FY 1994) (information provided by CDC). States were permitted to carry forward unobligated funds and transfer vaccine purchase funds to support infrastructure programs, further escalating the pool of funds available for state infrastructure grants in FY 1995 and 1996, even though appropriations for new awards were diminishing by this time.
Table 5–1 in Chapter 5 summarizes the principal activities to which each state directed its expanded infrastructure grants. Most commonly, states allocated their Section 317 increases to expanded service delivery, outreach and education, and development of registries. More specifically, states used this funding to improve immunization rates by, for example, undertaking collaborative efforts with groups such as WIC and Head Start to serve underimmunized clients, setting up reminder-recall systems, expanding clinic hours and locations, implementing targeted immunization campaigns for children and adolescents in pockets of need, and supporting local outreach efforts (Freed et al., 1999).
The increased Section 317 funding was viewed as a tremendous opportunity for new state efforts, especially since the creation of VFC added new responsibilities for data collection from and immunization audits of private health care providers. Yet significant barriers impeded states from reaping the full benefit of these expanded awards (Freed et al., 1999):
-
Many states had difficulty predicting the level of funding for any given year, making it problematic to create accurate budgets or engage in strategic planning for the immunization program.
-
The program year according to which CDC awards Section 317 grants is the calendar year. This matches no state fiscal year; most states’ fiscal years (46) run from July through June, with state legislative action taking place earlier in the spring. State legislatures must thus make program and budget decisions knowing only the federal award for the first 6 months (July through December) of their upcoming fiscal year.
-
Statewide hiring freezes and other administrative policies prohibited immunization programs from hiring full-time permanent staff.
-
CDC grants were commonly allocated in a piecemeal way, including multiple grants within a budget cycle and the distribution of funds late in the fiscal year.
-
States with cumbersome internal procedures for budgeting, spending, or hiring were unable to spend all their funds within a calendar year.
-
Some state legislatures meet biennially, causing delays in accommodating additional unanticipated federal grants.
-
Funds increased more quickly than states’ capacity to use them, so states retained the funding as carryover until they had established the plans and personnel necessary to implement new programs reflecting their additional responsibilities.
Many states carried forward significant amounts of federal funds from one budget cycle to the next. In 1996, for example, carryover funds represented almost half of the total federal immunization funding to the states. Congress viewed the carryover funds as excessive, and over the period FY 1996–1998 reduced the base for Section 317 infrastructure funding by almost 50 percent, from $271 million in FY 1995 to $139 million in FY 1999 and 2000 (of which about $117 million is available for actual state grant awards). Most state officials have indicated that the budget reductions occurred precisely at the point at which they believed they had made significant strides in the organization of immunization delivery and other activities (Freed et al., 1999).
In response to the budget cuts, most states reduced the scale of effort of their activities, commonly reducing outreach, education efforts, and service-delivery arrangements with outside contractors. Half of the states have reported that the budget cuts affected staffing, requiring them to reduce staff, consolidate positions, or leave vacancies unfilled (Freed et al., 1999).
States have access to other direct or indirect funding sources for infrastructure, but such funds are focused primarily on specific project efforts rather than general support. Vaccine manufacturers have assisted with
educational activities and information dissemination, particularly provider education, in 31 states. Statewide and/or local immunization coalitions are significant contributors to immunization efforts, particularly outreach activities, in 19 states. Twelve states have supported statewide registry development by All Kids Count grants from the Robert Wood Johnson Foundation, and another 11 states have received such grants for local registry efforts. Seven states have received support from insurers, managed care organizations, or other organizations (e.g., Rotary Clubs, McDonald’s, or private foundations) for specific initiatives, such as registry efforts, vaccine purchase, or outreach and education.
These other funding sources are not sufficient to maintain a viable immunization program within each state, however, and the increasing data management and service coordination demands placed on state programs exceed their current capacity:
-
Many states have discontinued funding for local organizations engaged in immunization outreach activity. Almost all program managers have reported substantial cuts in contracts with local health departments, even though they believe the most effective and critical outreach activities take place at the local level.
-
The ability of states to partner with local agencies has diminished, earlier initiatives cannot be maintained, and innovative strategies cannot be implemented.
-
Staff have increased responsibilities with little support or time to carry out their functions.
-
Several states have expressed concern that they do not have the workforce capacity to investigate disease outbreaks, work with providers, or continue registry development.
-
State officials have expressed concern that continued outreach efforts may be futile if services are not available within public health clinics to provide up-to-date vaccinations for individuals who are incompletely immunized and are not covered for such services.
Question 3: What are the current and future funding requirements for immunization activities, and how can those requirements be met through a combination of state funding, federal Section 317 immunization grant funding, and funding available through SCHIP? (Supported by Findings 2–1 through 2–4 in Chapter 2; Findings 3–2 through 3–6 in Chapter 3; and Findings 5–6, 5–7, 5–10 through 5–12, 5–15, 5–16, and 5–18 in Chapter 5.)
The funding requirements for immunization activities fall into two broad categories: vaccine purchase and infrastructure support.
Vaccine Purchase. Changes in federal and state health finance programs (including the creation of VFC and SCHIP) have improved access to vaccines for vulnerable populations within the private sector. Despite these improvements, many children continue to fall through the cracks of federal assistance programs, and new health care reforms, such as managed care, do not meet the complete immunization needs of children or adults who cycle through programs with short-term coverage arrangements. As a result, a continuing need for government-purchased vaccines remains within each state. The federal government currently spends more than $650 million annually on vaccine purchases, predominantly for childhood vaccines.3 The states estimate that they will collectively spend an additional $109 million for vaccine purchases in 2000. This combined effort appears to be sufficient to meet the current vaccine needs of uninsured and Medicaid children, but may be inadequate if new vaccines are recommended by ACIP and if initiatives to improve adult coverage levels are launched.4
States are encouraged to expand their role in purchasing vaccines to meet the needs of vulnerable underinsured populations who are not eligible for programs such as VFC or SCHIP. This role can be fulfilled in one of two ways: by purchasing additional vaccines (the committee suggests an increase of $11 million) or by requiring all private insurers within the state (including Employee Retirement Income Security Act [ERISA]-preempted health plans) to provide all ACIP-recommended vaccines to their members in accordance with state immunization standards. Federal legislation would be required, however, to establish a mandate for the inclusion of childhood immunization benefits in all private insurance plans.5
Government expenditures for adult vaccines are currently very low (less than $4 million in federal funds in FY 2000; state estimates are not available). New vaccines are being considered for adult populations, and greater emphasis is being placed on the importance of vaccines for high-risk adults under age 65 with chronic diseases (e.g., diabetes or renal failure). Additional funds will be required to support these purchases to meet residual needs among adults who lack private or public insurance coverage. As discussed in Chapter 3, the purchase of vaccines annually for the high-risk uninsured adult population aged 18–64 requires about $19 million annually for influenza vaccine and about $24.2 million for the one-time purchase of pneumococcal vaccine. If the federal government were to absorb these combined costs, an annual increase of about $50 million in the Section 317 vaccine purchase budget would be required. Future changes to the adult immunization schedule are likely to increase these costs.
As changes occur in the vaccine schedule, the pool of federal and state funds allocated for vaccine purchase will need to expand to allow the
states to continue to meet residual needs of vulnerable groups that do not have access to vaccines within private or public insurance plans. These children and adults will continue to fall through the cracks within the national immunization system unless funds are made available from programs such as Section 317 to give the states flexibility in meeting the vaccination needs of populations that cannot afford insurance, but do not qualify for federal assistance. Therefore, CDC and state health officials need to work closely and expeditiously with HCFA, state Medicaid directors, and state SCHIP program officers, as well as professional associations of health care providers, to address three objectives:
-
To ensure that the states have a pool of Section 317 funds that is sufficient to meet routine vaccine needs, as well as unexpected outbreaks.
-
To be certain that disparities do not emerge in public and private health plans in access to recommended vaccines.
-
To develop guidelines and performance measures that will encourage providers to draw on new health finance systems (e.g., VFC and SCHIP) for vaccine coverage so that Section 317 vaccines can be reserved for residual needs.
Federal agencies (particularly CDC) also require additional resources so they can provide national and international leadership; assist in the coordination of programs among states as well as other nations; and create opportunities for the exchange of technical assistance, expertise, and experience in undertaking appropriate and adequate infrastructure efforts at the state and local levels.
Infrastructure Support. Section 317 infrastructure awards currently reflect historical patterns of expenditure and are allocated largely in response to statements of need prepared by state health agencies. Recognizing that the states spent more than 90 percent of the infrastructure grant awards distributed in 1998 and 1999, the committee believes that the demands on the states exceed their current capacity, and that their ability to respond to changes in such areas as the science of vaccines and information technology could become severely compromised. In addition, managed care contracting for Medicaid has eroded the public health infrastructure and funding base in many states so that in some areas, there is no longer a sustainable volume of personal health care demand to support the provision of public health services such as immunization. As state funds with-draw into private-sector contracts, county and local health departments have fewer resources to spend on public health services. The national immunization system is weakening, and we should not have to wait for
outbreaks of vaccine-preventable disease to strengthen areas in need of support.
Current federal immunization funding for the states is significantly lower than the states’ historical expenditure levels. Based on an examination of total state expenditure histories, the committee estimates that the states require a total of about $500 million in annual infrastructure funds to sustain a national immunization system that can achieve current national health goals; respond to future developments in the areas of vaccine science, information technology, and health care delivery systems; improve the sensitivity of surveillance measures so they can identify important gaps in immunization coverage levels; and extend immunization programs to the adult population. This amount was derived from two sources: it combines (1) an estimate of what is required to support the six essential roles of a viable state infrastructure program for immunization services, as outlined above, with (2) an estimate of the base level of federal funding required to direct additional resources to areas of need without reducing current levels of support for each state (known as a “hold harmless” provision).
The committee believes the annual costs for immunization infrastructure should be shared between federal and state governments. The state share is necessary because the state is the lead public health care delivery agency in the national system, because states establish immunization requirements for their residents, and because immunization efforts require state ownership and oversight. The proposed federal government share (a total of $200 million per year, representing a $74 million annual increase over current budgets) should be administered by CDC through the Section 317 state grants program. In providing their share (a total of $331 million per year, representing a $100 million annual increase over current estimates), states should be encouraged to use their own funds as well as other federal grants (e.g., Medicaid, Title V, and SCHIP). The combined increases in federal and state support are sufficient to provide resources that can stabilize existing state-level programs, respond to the needs presented by advances in the science of adolescent and adult vaccines and information technology, and address emerging concerns about vaccine safety.
Recognizing the need for a long-term strategy to build appropriate infrastructure within each state, the committee believes federal and state agencies should make a national shared commitment to the immunization partnership by investing a total of $1.5 billion in immunization infrastructure support over the first 5-year period. This figure consists of $1 billion in federal funds (approximately $200 million each year), matched by a comprehensive effort within the states to raise an annual increase of $100 million over current spending levels.
Question 4: How should federal grant funds be distributed among the states? (Supported by Findings 4–2 and 4–14 in Chapter 4 and Findings 5–12 and 5–16 in Chapter 5.)
Each state deserves some level of federal assistance, but some states require more help than others because of the characteristics of their populations. In addition, states that have more resources because of their tax base should be expected to bear a larger burden of the costs of immunization infrastructure. Finally, states that demonstrate success in increasing their immunization coverage levels should be rewarded when such increases reflect real improvements in the status of their most vulnerable populations. Since allocation by need and by achievement may run counter to each other, specific criteria will need to be developed to balance multiple goals.
For these reasons, the committee believes federal funds should be allocated to the states on a formula basis, with a state match requirement that reflects each state’s capacity to bear part of the burden of infrastructure costs. A small proportion (perhaps 5 to 10 percent) of the total federal grant awards for infrastructure should also be set aside so that CDC will have discretionary funds available to respond to unexpected outbreaks, gaps in immunization coverage, or other exceptional circumstances within the states.
Four Basic Principles for a Federal Grant Formula
Allocating federal immunization grants to the states requires consideration of several factors: need, capacity, performance, and the determination of a base-level grant award. The committee offers four basic principles to illustrate the nature of each of these factors and the types of measures that should be considered in estimating their value within the proposed formula.
Principle 1: Each state requires a base grant award, regardless of population size, level of need, or match contribution. There is a strong federal interest in ensuring a stable immunization infrastructure within each state so that vaccine coverage levels can be sustained among vulnerable groups, and appropriate sentinels will be available to detect emerging trends or problems having national significance. The size of the base award should be sufficient to allow each state to perform the six roles of the immunization system discussed in this report. CDC may want to consider cost-indexing these awards, possibly through multiyear grants, to adjust for salary increases and variations in salary structures for public health employees in different regions of the United States.
Principle 2: States that have larger disadvantaged populations should receive greater amounts of federal assistance. The history of the Section 317 program demonstrates that the program is fundamentally designed so the federal government can share with the states the costs of enhancing access to immunizations for vulnerable and medically underserved populations, especially children, the elderly, and those who reside in areas of concentrated disadvantage. State needs differ according to population size, urban/ rural distribution, and rate of underinsured populations. States in which 20 percent of the population is without insurance, for example, require greater investments in safety net support than those that have less than 10 percent uninsured. Determining the level of need within each state requires attention to several basic components, such as the following:
-
the size of the general population (adult, adolescent, and child),
-
the size of the annual birth cohort,
-
the size of the uninsured population (adult, adolescent, and child),
-
the size of the Medicaid (and SCHIP) population,
-
the size of the immigrant population, and
-
the scope and level of benefits in insurance coverage.
Principle 3: States that have a greater capacity for addressing the needs of their citizens should do more to share the costs of vaccine purchase and infrastructure development. Estimates of need, while important, are only one of the multiple concerns that require consideration in federal funding decisions. Estimates of state capacity are commonly used when determining cost-sharing formulas in other federally funded, state-administered health programs, including Medicaid, MCH grants (Title V), and SCHIP. These capacity measures reflect per capita wealth within the state and the size of the revenue base that can be raised by the state to support health programs. Such measures facilitate comparisons among states because they can help determine what each state should be expected to spend if it is to make adequate investments of its own funds in meeting critical public health needs.
A second capacity measure that is directly relevant to immunization is more difficult to determine. The capacity of the state’s public health insurance system (including Medicaid and SCHIP) to cover immunizations and encourage the delivery of immunizations in each child’s medical home will influence the size of residual needs, reducing or expanding the population that will seek immunization services directly from safety net health centers. States that offer generous Medicaid payments to large numbers of providers and extend client eligibility for longer periods are investing their own funds to support quality health care for disadvantaged groups and thereby encouraging the participation of private health
providers in the state’s Medicaid, SCHIP, and VFC programs. If the plan participants have access to adequate immunization services from their health care providers, and there are fewer administrative barriers that restrict the plan providers from offering services to vulnerable clients, the state has a stronger primary care capacity and less need for federal safety net programs to supplement its health care system.
These two measures—per capita wealth and state health care capacity—merit explicit consideration in the allocation of federal funds, including Section 317 grants. High marks for either indicator suggest that the state has the revenue to support the cost of vaccine purchase or is already investing substantially in immunization services for disadvantaged populations, reducing the demands on the public immunization infrastructure.
Principle 4: States that achieve higher levels of performance in immunization should be rewarded for those outcomes. The increasing use of performance measures in public health programs places greater importance on the selection of appropriate indicators and measures. CDC has recognized the importance of immunization performance over the past decade (with congressional guidance) by allocating a portion of the Section 317 grant awards to incentive awards based on improvements in immunization rates.
While important, statewide immunization rates can be deceiving because they often do not reveal small areas of concentrated need within the state (see Table 1–5 in Chapter 1). The size of within-state disparities is a second important measure of performance that can demonstrate how well or how poorly the state health system is doing in providing access to immunization services among hard-to-reach populations.
A third relevant performance measure is the level of immunization coverage within Medicaid and SCHIP populations. Baseline measures are especially important in determining the extent to which health finance programs can influence access to vaccines and the size of the safety net population within each state. In addition, the size of the denominator involves careful judgments about which clients should be included in performance assessment measures: all enrolled clients, all clients who make routine visits to the provider, or clients who have visited only once?
Finally, performance measures should encompass the efficiency with which the states administer federal funds. The committee did not identify any such measures in the state survey conducted for this study. Prototype research may be necessary to design and test appropriate efficiency measures for the public health environment. Relevant studies may be available in other fields (such as education) that offer insights and experience
for comparative estimates of administrative efficiency in state spending patterns.
CDC should develop a set of performance criteria with these issues in mind that can be used to examine state immunization performance over a 5-year period. At the end of that time, a panel should be convened to consider whether changes in Medicaid, SCHIP, or other public or private health care plans have reduced the need for Section 317 vaccines, whether private health care plans are able to provide community-level measures of immunization coverage, and whether any other trends may influence the need for Section 317 funds within the states.
Proposal for a State Match Requirement
The committee believes the states bear responsibility for sharing the infrastructure costs of the national immunization system. The proposed formula for distributing federal funds should include a state match requirement—such as 75 federal/25 state—similar to that in place for Medicaid grants, Title V MCH grants, and block grants for the prevention and treatment of substance abuse. The committee believes a state match is desirable for several reasons.
First, the desirability of a state match requirement is based on the premise that immunization is a shared national partnership between federal and state governments. Allocating state funds for immunization infrastructure needs on a regular basis will create a line item in public health budgets that will add greater stability to the public health system and allow finance trends to be monitored over time. A state match requirement will also serve as an incentive for states that do not currently invest in infrastructure resources to commit funds to this area on a routine basis.
Second, the match requirement will stimulate greater attention to the dynamics of public- and private-sector involvement in providing immunizations to vulnerable groups and establish greater buy-in for public health efforts at the state and local levels. A match requirement will give state legislatures, state health finance agencies, and state governors’ offices incentives to monitor the performance of private health care plans that operate within their borders and to determine whether plans that use state funds have the capacity to keep their clients up to date on the immunization schedule. Greater oversight of the immunization budgets of public health agencies will reveal the extent to which such budgets support safety net services to meet residual needs, as well as critical surveillance and assessment functions that benefit the general population.
Third, the state match requirement does not necessarily require new sources of funds, since many states already contribute to the support of immunization efforts. The match requirement will reveal the range of
these contributions over time, and provide an opportunity for CDC to review how federal and state funds are distributed across the six roles of the immunization system discussed in this report. It is conceivable that a match requirement might prompt individual states to add their own resources or in-kind contributions to their immunization programs, or to seek such resources from outside government, since health officers will need to demonstrate a base level of grantee contribution to qualify for federal grants.
CDC has always stressed that federal funding is to be used to supplement, not supplant, each grantee’s immunization effort, but a state-level contribution is not currently required for Section 317 grants. Such grants originated in a public health environment in which it was assumed that a state match requirement would delay the use of federal funds in swiftly reducing exposure to vaccine-preventable diseases for vulnerable populations. Over time, however, immunization has become a routine part of primary care services that are financed in large measure through cooperative state and federal arrangements. Additional assessment and populationwide services have also increased the costs of sustaining and improving up-to-date coverage within the U.S. population as a whole, and the federal government should not be expected to support these costs alone.
The committee considered several arguments against a state match, but did not find them compelling. Following are these arguments and the committee’s response to each.
First, a matching requirement would necessitate changing the existing Section 317 legislation, and exposure of the legislation and approvals of annual budgetary contributions within the states could generate skepticism about the infrastructure grants program and create vulnerabilities in the political process. The committee believes broader exposure of the immunization grants program will strengthen federal and state collaboration. Although the legislative reform process may introduce undesirable or radical changes in the program’s scope, purpose, or funding approach that could create uncertainty and confusion and disrupt programmatic efforts, at least in the short term, a state match requirement is not likely to have this effect provided the rationale for the match is sound and justified.
Second, a state match requirement might create incentives for government officials or legislators to reduce grantee contributions in areas in which the match is already exceeded. In other areas, state or local public health officials or legislators might not be able or inclined to meet a matching requirement, and federal grants could be reduced or eliminated as a result. If state contributions to public health are invested wisely, evidence should be available to demonstrate why such contributions are in the state’s interest.
Third, administering a state match requirement will require additional docu-
mentation and create an administrative burden for CDC staff and state officials. It is not sound policy to introduce additional bureaucratic procedures in the public health system unless such procedures serve important goals or create new resources. State grantees will need to document their contributions to immunization infrastructure on an annual basis. This documentation will require selection criteria and finance guidelines during a start-up period, but such efforts will gradually become routine as greater familiarity is gained with the match in the budgetary cycle. Many states already have procedures for approval of match contributions, and they are accustomed to including a match in Title V and other public health grant proposals. It is doubtful that the match requirement will disrupt programmatic efforts, especially if the formula for allocation includes a base amount of federal funds for each state regardless of the size of the match contribution.
States may choose to exercise their match requirement in one of two ways: through direct or indirect support. Direct support means the states allocate their own revenues for the direct support of vaccine purchase or immunization infrastructure programs. Indirect support means the states rely on other approaches to support immunization efforts. They might use in-kind assistance in the form of contributed time from other agencies, such as the use of school nurses to monitor immunization records in the community. They might invest their own funds to provide higher fees for Medicaid and SCHIP health services and rely more heavily on these programs to cover immunizations. Indirect support might also include the oversight of private insurance plans to ensure that they provide sufficient coverage for immunization services. The direct approach involves direct payments to public health programs for immunization services, whereas the indirect approach involves greater reliance on performance assessment, incentives, and regulatory initiatives, since other agencies, including public and private health plans, are responsible for immunization services. States that cover greater numbers of children through SCHIP and serve a significant portion of their uninsured and Medicaid children with VFC through private providers may require fewer federal and state funds for Section 317 vaccine (because they receive larger amounts of federal and state funds for Medicaid and SCHIP payments). The number of children who rely on the safety net programs in these states will be smaller compared with states that use their public health infrastructure as a principal tool in serving vulnerable populations.
Determining the Appropriate Formula and Match Requirements
Although states should have flexibility in designing finance and service systems to meet their immunization needs, tracking systems should be developed that allow the states to report and compare both the scale of
their investments (in direct and indirect efforts) and the outcomes achieved by using different strategies to address common goals. Interagency collaboration at both the state and federal levels may be required to support public health efforts in the field of immunization. For example, guidelines at the national level could encourage Medicaid agencies to bear the costs of administrative services (including immunization audits of provider records and registry development) as part of case management expenditures. Whatever the source, states need to be able to predict the size of their immunization budgets on a multiyear basis, rely on steady sources of income to support both vaccine purchase and infrastructure efforts, and assess the performance of those efforts according to a consistent set of measures.
A set of proxy measures focused on need, capacity, and performance should be developed that can be monitored over time. These measures can be used to guide federal grant allocation decisions and the determination of state contribution levels, as well as programmatic and reporting requirements. A small set of comparable measures will allow federal and state agencies to monitor state need, capacity, and performance without imposing unnecessary effort on the states that restricts their ability to respond to local circumstances.
The committee believes the assignment of weights to the factors of need, capacity, and performance requires careful calculations that lie beyond the scope of this report. These calculations should be informed by a democratic process that takes individual state needs into account. The calculations required include the appropriate size of the federal base grant; appropriate “hold harmless” conditions; the nature of adequate state-level contributions; an appropriate set of proxy measures that reflect need, capacity, and performance in the field of immunization; and the appropriate multiyear finance mechanism for allocating federal funds. The calculation process requires an extensive dialogue with federal and state health agencies, state legislatures, state governors, and the U.S. Congress. The committee recommends that CDC initiate this dialogue immediately so that legislative reforms can be proposed by 2002, when Section 317 is scheduled for reauthorization.
Several different types of formula factors would alter the distribution of state grant awards relative to current allocation patterns. The factors considered in making other state health grant awards are the size of the population and federal poverty level measures, which can be set at different thresholds. The inclusion of any one formula factor would cause major shifts in the current distribution of federal funds. A small number of states (commonly states with large numbers of at-risk children, such as California, Texas, New York, and Pennsylvania) frequently rank much higher in formula distributions than smaller or more rural states. It is
important, therefore, to maintain stability by ensuring a base level of federal funds within each state while responding to specific state needs.
This need for stability in the national immunization system under-scores the importance of increasing the size of the pool of federal funds for state infrastructure awards, as discussed under Question 3 above. CDC has estimated that the use of certain formula factors in redistributing federal awards within an annual budget of $140 or $160 million for infrastructure grants would reduce the current size of a significant number of state awards, further burdening state efforts. A annual pool of $195 million, however, would minimize the need to reduce current state awards in order to increase federal assistance to states with larger poor populations (information provided by CDC).
Question 5: How should funds be targeted within states to reach high-risk populations without diminishing levels of coverage among the overall population? (Supported by Finding 3–6 in Chapter 3; Finding 4–11 in Chapter 4; and Findings 5–1 through 5–9, 5–12, 5–16, 5–18, and 5–19 in Chapter 5.)
The federal government’s role in supporting immunization activities within each state should strike a balance between helping the states achieve important national objectives and sustaining incentives for states to use their own funds to meet the needs of their residents. Given the primacy of the role of the states in public health, the federal government’s role might reasonably be restricted to certain key areas that require specific technical expertise, national data collection and analysis, and the development of benchmarks and indicators that benefit the nation as a whole. In states that do not have a sufficient revenue base to support adequate public health investments, however, the federal government has an important role to play in supplementing state funds to ensure that an adequate program is in place that can achieve national health objectives. Special considerations might include the examples that follow.
First is the size and location of the disadvantaged population within each state. Poverty remains a daunting obstacle to efforts to improve immunization coverage within any specific population. The size of a state’s population that resides in poverty and the extent to which this population is distributed or clustered within the state are important factors to consider in evaluating the size of the public health infrastructure and immunization program needed within the state.
As discussed in the response to Question 4, federal immunization investments should provide greater resources for those states that have larger pockets of need. At the same time, care needs to be taken so that federal funds are not used to support basic health services that are right-
fully the obligation of the state. Formula factors that might be built into the allocation of Section 317 grants might include, for example, the distribution of the state’s population above and below the federal poverty level, the percentage of uninsured families, the size of the child and adolescent Medicaid populations, and the size of the high-risk adult population within the state. The application of such factors would generate new winners and losers in the distribution of federal funds, possibly creating unfair discrepancies (e.g., fewer than 10 states receiving more than 90 percent of available funds). Balance needs to be achieved in leveling the playing field among the states and ensuring that each state receives a minimal grant award that is sufficient to maintain an effective partnership with the federal government.
A second special consideration might be the marginal costs of improving immunization coverage within highly disadvantaged groups. As discussed in Chapter 4, the cost of improving coverage within the final 10 percent of a total population in any given area is thought to be significantly higher than the cost of acquiring coverage for the majority of the community. However, the scale of this difference remains uncertain. Assigning costs requires consideration of such components as outreach, case management, record maintenance, disease exposure, frequency of contacts with primary care providers, and health beliefs and knowledge that influence efforts to obtain immunization.
What is known for certain is that highly disadvantaged populations seek services more frequently from multiple providers in multiple health care settings. Such populations frequently cycle among different health plans, including public and private health care finance arrangements, and are often uninsured for lengthy periods. Their case management and record maintenance costs are greater than comparable costs for individuals who remain with the same health care provider or the same practice over a period of years, especially those who remain within one health plan during the important immunization period of the early childhood years.
Despite these barriers, research has demonstrated that certain types of programs can improve immunization coverage within highly disadvantaged groups if focused on populations that have the most to gain from those programs (see Chapter 4). It is wasteful, for example, to distribute information packages and brochures about the importance of immunization within a community where parents may be illiterate or can read only in non-English languages. Similarly, it is wasteful to improve outreach and parental education programs in communities where most parents already believe in the importance of vaccines, but mistakenly believe that their children are already up to date in their immunization status.
CDC frequently relies on technical assistance to help states direct
their resources toward productive programmatic investments. However, this approach may not be sufficient in areas where states choose to distribute their own resources over a broad geographic area rather than concentrating them in areas of need where delivery systems may be weak and data collection difficult. State health agencies face difficult political obstacles when shifting public resources away from communities that have achieved high levels of coverage (sometimes with minimal state effort) so the resources can be targeted to areas where performance is poor. In such cases, the role of the federal government is to create incentives (e.g., ranking states on the basis of within-state disparities in coverage) or to provide targeted resources that enable states to do all they can to address the immunization needs of their most vulnerable citizens.
Question 6: What should be the role and financing level for CDC’s current program supporting state efforts to vaccinate adults and achieve the nation’s goals for influenza and pneumococcal vaccines? (Supported by Findings 3–3 and 3–5 in Chapter 3.)
Immunization coverage rates for adults are well below those achieved for childhood immunizations, although some progress in immunization was made in immunizing the adult population over age 65 during the 1990s. The Healthy People 2000 objective for influenza coverage levels was met for the noninstitutionalized elderly (individuals aged 65 and older) according to 1997 National Health Interview Survey (NHIS) data (see Table 3–6 in Chapter 3). The national average was 63 percent, up from 58 percent in 1995. According to 1997 data from the Behavioral Risk Factor Surveillance System (BRFSS), 45 states exceeded the goal of increasing influenza immunization levels to 60 percent among the elderly (CDC, 1998d). From 1995 to 1997, 48 states showed improvement in influenza vaccination rates for the elderly. The mean coverage level of states in 1997, 65.5 percent, was almost double the 1989 coverage level of 33 percent (CDC, 1998d). Nonetheless, in 1997, the percentage of adults aged 55–64 who received influenza vaccine ranged from 28.5 percent (Georgia) to 54.7 percent (Colorado), with a median of 38.2 percent. For persons aged 65–74, percentages ranged from 48.7 percent (Nevada) to 72.4 percent (Colorado), with a median of 63.6 percent. Among persons over age 75, percentages ranged from 51.7 percent (District of Columbia) to 82 percent (Arizona), with a median of 71.4 percent (Janes et al., 1999).
Pneumococcal immunization levels for the elderly are significantly lower than influenza immunization levels, even though Medicare covers the cost of this vaccine and its administration (Janes et al., 1999). The NHIS data show that only 42 percent of the noninstitutionalized elderly had ever received a pneumococcal vaccination by 1997 (see Table 3–6).
Although this was a large increase over the 34 percent coverage rate reported in 1995, the 1997 national average fell far below the Healthy People 2000 goal of 60 percent coverage, and this goal has still not been met. According to BRFSS, only 17 states had achieved immunization rates of 50 percent or greater among the elderly by 1997. Coverage in 1997 ranged from 9.5 percent (New York) to 30.7 percent (Alaska) among persons aged 55–64, with a median of 17.1 percent. For persons aged 65–74, percentages ranged from 30.1 percent (New Jersey) to 56.9 percent (Arizona), with a median of 42.6 percent. Finally, for persons over age 75, the percentages ranged from 31.4 percent (Louisiana) to 79 percent (Nevada), with a median of 53.3 percent (Janes et al., 1999).
Although differences in coverage rates among children of different ethnic groups have been significantly reduced, troublesome disparities remain in adult immunization coverage levels (see Table 3–6). According to 1997 NHIS data, elderly blacks had the lowest likelihood of receiving either influenza (45 percent) or pneumococcal (22 percent) immunizations. Elderly Hispanics had influenza and pneumococcal immunization coverage levels of 53 percent and 23 percent, respectively, as compared with coverage levels of 66 percent and 46 percent, respectively, for whites in 1997.
Noninstitutionalized high-risk adults aged 18–64 have extremely low immunization rates and may present the largest challenge to efforts to appropriately immunize adults (see Table 3–6). The 1997 NHIS data demonstrate that only 26 percent of this group had received an influenza vaccination, while only 13 percent had received a pneumococcal vaccination. Differences in coverage levels among races were not as great in the high-risk population aged 18–64 as in the population over age 65. Compared with the elderly, the high-risk group aged 18–64 had very low rates of immunization coverage. In 1997, among those with private health insurance, 29 percent of high-risk adults aged 18–64 and 63 percent of the noninstitutionalized elderly received an influenza vaccination. Among those with Medicaid, 26 percent of the high-risk population aged 18–64 received an influenza vaccination (see Table 3–6) (National Center for Health Statistics, 1997).
The 1997 NHIS also provides information on selected high-risk sub-groups. Coverage rates for the noninstitutionalized elderly were higher than those for the high-risk population aged 18–64 in every subgroup. In 1997, 67 percent and 44 percent, respectively, of noninstitutionalized elderly with diabetes received influenza and pneumococcal vaccinations. In contrast, only 36 percent and 19 percent, respectively, of adults aged 18– 64 with diabetes received influenza and pneumococcal vaccinations. Data on national coverage rates for adult immunizations other than influenza and pneumococcal are severely limited. According to Healthy People
2000, 67 percent of occupationally exposed workers received a hepatitis B vaccination in 1994, and 9 percent of men who had sex with men received this vaccination in 1997. NHIS data show that in 1995, 65 percent of persons aged 18–19, 54 percent of those aged 50–64, and 40 percent of those aged 65 and older had received a tetanus booster in the last 10 years (Singleton et al., forthcoming).
As with the monitoring of adult coverage levels, existing immunization finance programs tend to neglect the population aged 18–64. Adult immunizations are currently funded by a patchwork of public and private insurance that results in scattered immunization rate data, inconsistent insurance coverage among Americans, and a lack of collaborative roles and missions within the private and public health sectors.
Studies have shown that both influenza and pneumococcal vaccines are cost-effective. Yet federal funds that support adult immunization are a small fraction of the financial resources dedicated to childhood immunization. The main funding sources for adult immunization are Medicare, Section 317, and private insurance. States could spend Section 317 grants on vaccines and services for adults under age 65, but grantees have historically spent only a miniscule amount (estimated at about 2 percent) of their Section 317 funds on adult immunization. In addition, CDC did not authorize its grantees to use Section 317 funds in support of adult immunization until 1997 (information provided by CDC). Medicare has played a much larger role in adult immunization than that played by Section 317; it has covered pneumococcal vaccine since 1981 and influenza vaccine since 1993. In the future, with the dramatic rise of managed care and health maintenance organizations (HMOs) that emphasize preventive services, the committee believes adult immunizations are increasingly likely to be covered by private insurance. This trend provides an opportunity to raise awareness about the importance of immunization among health professionals who care for adults and to hold private plans and providers accountable for adult immunization performance measures.
As shown by the low coverage rates and low levels of funding, adult immunization is not a priority in the United States. Approximately 50,000 adult Americans still die each year from diseases for which both safe and effective vaccines exist, and yet as noted, only 2 percent of Section 317 funds have been dedicated to adult immunization (Poland and Miller, 2000). What is missing is a coordinated and comprehensive federal, state, and local strategy to improve adult immunization coverage levels. Health care providers are often less successful in providing age-appropriate immunizations as their clients grow from infancy through childhood to adolescence and adulthood. Immunization has not been the focus for practitioners who routinely care for adults that it has become for pediatric providers. Only rudimentary programs in state and local health depart-
ments reach out to adult populations and their health care providers regarding immunization practices. Federal and state leadership has been successful in achieving substantial coordination among the various programs devoted to specific childhood vaccine-preventable diseases. Yet the units devoted to adult vaccine-preventable diseases (e.g., influenza, pneumococcal, tetanus/diphtheria, and hepatitis B infections) typically focus on narrow goals and rarely address a comprehensive adult immunization strategy. Increased funding and coordinated programs can begin to move adult immunization beyond its current marginal status.
In the recommendations at the end of this chapter, the committee proposes a specific financing level for purchasing vaccines as part of an adult immunization program. In addition, the committee recommends that CDC develop a coordinated and comprehensive immunization effort for adults to encourage greater participation by the private and public health care sectors in achieving national goals.
CONCLUSIONS
Conclusion 1: The repetitive ebb and flow cycles in the distribution of public resources for immunization programs have created instability and uncertainty that impeded project planning at the state and local levels in the late 1990s, and delayed the public benefit of advances in the development of new vaccines for both children and adults. This instability now erodes the continued success of immunization activities.
The national immunization system that emerged in the United States in the latter half of the 20th century was created by a series of infectious disease outbreaks and governmental responses, with governmental assistance often being increased after outbreaks occurred, not to prevent them (Johnson et al., forthcoming). Substantial progress has been made in preventing and controlling disease, ensuring access to vaccines, and providing service delivery in medical homes. But three other areas require attention in renewing the national immunization partnership: improving the quality of immunization surveillance efforts and vaccine safety programs, strengthening efforts to sustain and improve immunization coverage rates, and using primary care and public health resources efficiently. The instability of funding for state immunization programs discourages the development of strategic responses designed to foster disease prevention, improve immunization coverage levels for specific populations, and ensure vaccine safety. Diminishing resources often divert attention toward protecting individual programs or interventions rather than focusing on the health and vitality of the population as a whole. The current situation is characterized by a spirit of complacency and disjointedness that creates
an unstable and unpredictable environment for immunization in the midst of rapid changes in the science of vaccines and the health care system.
Conclusion 2: Immunization policy needs to be national in scope. At the same time, the implementation of immunization policy must be flexible enough to respond to special circumstances that occur at the state and local levels.
A comprehensive strategy that clarifies the roles and responsibilities of federal and state agencies as well as private-sector providers and health plans within the national immunization system is needed to sustain an important intergovernmental partnership in the midst of change and complexity. The federal presence should be adequate and stable so that state agencies can develop strategic approaches to address local needs. The state role is to ensure that appropriate systems are in place for detecting and responding to changes in immunization coverage levels and disparities in access to immunization resources. The implementation of all six immunization roles therefore requires public attention and resources at both the state and federal levels, as well as sustained commitments within the private sector.
The eligibility requirements for VFC, for example, currently discriminate against states that choose to set up stand-alone plans rather than relying on Medicaid agencies to administer their SCHIP funds. SCHIP children who are enrolled in the stand-alone plans are considered “insured” and are therefore not eligible for benefits under the VFC program, while SCHIP children in another state may continue receiving their benefits because they are still technically enrolled in Medicaid. These types of administrative distinctions disrupt the national immunization partnership and cause states to turn unnecessarily to other federal programs (e.g., Section 317) to meet their needs.
National initiatives that provide immunization coverage for larger numbers of disadvantaged families under private and public health insurance plans require state public health responsibilities to shift from direct service delivery to oversight roles concerned with assessment, assurance, and policy development. Yet certain residual immunization needs will remain that will necessitate reliable access to vaccines within the public health sector. States need flexibility and resources to adapt to these shifts, which occur unevenly across and within state borders.
Conclusion 3: Federal and state governments each have important roles in supporting not only vaccine purchase, but also infrastructure efforts that can achieve and sustain national immunization goals.
The federal government needs to work with the states to ensure that appropriate infrastructure efforts are present within each state, to distribute national resources fairly, and to build on the strengths of the private sector in meeting community health care needs where feasible. The federal government should be the senior finance partner for the national immunization system because of the central importance of vaccines in contributing to the nation’s health, and because disease outbreaks in one region can threaten the health of another without respect for political borders. However, the federal role is to supplement and support state efforts, not replace them.
State legislatures and governments should be expected to sustain an immunization infrastructure that reflects each state’s need, capacity, and performance. Since states are the ultimate stewards of public health policy, they are responsible for delivering services to those whose immunization needs are not met by the private sector. In maintaining coverage standards for at-risk groups, state public health agencies require a surveillance capacity that allows them to measure population-based coverage rates, assess the quality of care within public and private immunization plans, offer safety net services to meet residual needs, and improve access to immunization services within many different public and private entities. Performance monitoring, including the development of immunization registries, is important to ensure that vulnerable groups have access to adequate primary health care and that residual needs are met with public resources where necessary. Finally, state agencies are also responsible for ensuring that the public and private health care sectors work collaboratively within their jurisdiction so that public resources are used efficiently. To carry out these roles, state health agencies require a national immunization policy that provides them with adequate resources, stability, and flexibility.
Conclusion 4: Private health care plans and providers have the capacity to do more in implementing immunization surveillance and preventive programs within their health practices, but such efforts require additional assistance, oversight, and incentives. At the same time, comprehensive insurance and high-quality primary care services do not replace the need for public health infrastructure.
The committee believes health plans should not have the option of providing selective coverage for vaccines once they have been recommended for widespread use, as is currently the practice in most states. A federal mandate may be necessary to achieve this goal, particularly for ERISA-exempt plans. For example, all health plans (public and private) that offer primary care benefits for children and adults should bear the
costs of integrating all vaccines recommended for widespread use into their basic health care package. Private health plans should also be expected to bear at least some of the costs of “catch-up” conditions following the licensing of new vaccines (e.g., coverage of hepatitis B vaccine for older children who were too old to have been affected by the universal recommendations). Public health agencies should not be expected to supplement public or private health insurance plans except under short-term conditions, such as responding to emergency outbreaks or reducing disparities that result from “catch-up” conditions. Coordinated efforts, such as billing practices that allow public health clinics to be reimbursed for immunizations given to individuals who are covered by private health care plans, can help reduce the burden on public health agencies. Ideally, however, immunizations should be offered routinely in each plan participant’s medical home as an integrated part of primary care benefits.
Health plan providers should also be prepared to assess immunization coverage rates among their enrollees by using measures that can contribute to accurate community health profiles at the state and local levels. These efforts require independent oversight, however, to ensure that all groups are included in such assessments and that the measures used accurately reflect the immunization profile of those not currently enrolled in public and private health plans. Public health agencies can provide important measurement and audit services, such as assessment and feedback for private providers, as an investment in the quality of community health. These and other surveillance efforts should be supported by the national immunization partnership as a national health priority, with appropriate recognition of the issues of privacy and confidentiality.
SUMMARY RECOMMENDATIONS
Recommendation 1: The annual federal and state budgets for the purchase of childhood vaccines for public health providers appear to be adequate, but additions to the vaccine schedule are likely to increase the burden of effort within each state. Therefore, the committee recommends that CDC be required to notify Congress each year of the estimated cost impact of new vaccines that have been added to the immunization schedule so that these figures can be considered in reviewing the vaccine purchase and infrastructure budgets for the Section 317 program.
The committee believes the annual allocation of federal funds for the purchase of vaccines through the VFC program ($505 million for FY 2000) and the Section 317 state grant program ($162 million per year for
FY 2000) is sufficient to meet state requests for child vaccines within the immunization schedule recommended by ACIP as of January 2000.6 But additions to the ACIP schedule will expand the burden of preventive health care costs to state and federal health agencies as well as private health plans. Such additional costs should be expected as part of the changing immunization system. Congress should anticipate such cost increases by requiring that CDC notify Congress each year of two trends: (1) the estimated cost impact of new vaccines (including administration fees) that are scheduled for consideration as additions to the recommended immunization schedule, and (2) the length of time that may be involved from the point at which such vaccines are recommended by ACIP to the establishment of a VFC contract. Federal and state vaccine purchase budgets should then be adjusted as necessary.
Recommendation 2: Additional funds are needed to purchase vaccines for uninsured and underinsured adult populations within the states. The committee recommends that Congress increase the annual Section 317 vaccine budget by $50 million per year to meet residual needs for high-risk adolescents and adults under age 65 who do not qualify for other federal assistance. The committee further recommends that state governments likewise increase their spending for adult vaccines by $11 million per year.
These estimates are based on calculations of the residual vaccine needs for uninsured and underinsured at-risk populations, including adults who are younger than age 65 and suffer from chronic disease (see Box 3–3 in Chapter 3); for hepatitis B coverage among adolescents; for adults who are at risk because of sexual behavior or occupational settings; and for tetanus coverage. Both federal and state vaccine purchase budgets will require annual adjustments as vaccine costs change or new vaccines or age groups are added to the adult immunization schedule. Therefore, CDC notification of the impact of such changes should be required annually, as indicated in Recommendation 1.
The improvement of adult immunization rates will require more than increased vaccine purchases. A comprehensive and coordinated adult immunization program needs to be initiated within each state, with leadership at the national, state, and local levels, to encourage the participation of private and public health care providers in offering immunizations to adults under the guidelines established in the ACIP schedule.
Recommendation 3: State immunization infrastructure programs require increased financial and administrative support
to strengthen immunization capacity and reduce disparities in child and adult coverage rates. The committee recommends that states increase their immunization budgets by adding $100 million over current spending levels, supplemented by an annual federal budget of $200 million to support state infrastructure efforts.
The committee believes state immunization programs could achieve stability and carry out their roles adequately through the adoption of a national finance strategy that involves investing a total of $1.5 billion in the first 5 years—an annual increase of $175 million over current spending levels—to support infrastructure efforts within the states. The federal budget figure is derived from three calculations: (1) annual state expenditure levels during the mid-1990s, (2) the level of spending necessary to provide additional resources to states with high levels of need without reducing current award levels for each state (known as a “hold harmless” provision), and (3) additional infrastructure requirements associated with adjusting to anticipated changes and increased complexity in the immunization schedule. The additional state contribution is necessary to reduce current disparities in state spending practices and to address future infrastructure needs in such areas as records management, development of appropriate performance measures and immunization registries, and outreach and education for adult vaccines. This increased budget could strengthen the state roles in immunization, with a special emphasis on infectious disease prevention and control, surveillance of vaccine coverage rates and safety, and programs to sustain and improve immunization coverage rates.
Different types of administrative support can be offered to the states to strengthen their immunization efforts. For example, federal reporting requirements for immunization grants should be reduced to six key areas that reflect the six fundamental roles of the national immunization system discussed in this report. In addition, grant budgetary cycles should be extended to 2 years to give states greater discretion and flexibility to plan and implement multiyear efforts in each area.
Recommendation 4: Congress should improve the targeting and stability of Section 317 immunization grant awards to the states by replacing the current discretionary grant award mechanism with formula grant legislation.
The formula should reflect a base level as well as factors related to each state’s need, capacity, and performance. A state match requirement should be introduced so that federal and state agencies share the total
costs of supporting the infrastructure required to operate a national immunization program and respond to the needs of disadvantaged populations.
As discussed in an earlier section of this chapter, the committee believes the states bear responsibility for sharing the infrastructure costs of the national immunization system. About half of the states currently invest in immunization programs in addition to their vaccine purchases; the remaining half do not support infrastructure costs on a routine basis.
To reduce this disparity, the proposed formula for distributing federal funds should include a state match requirement—such as 75 federal/ 25 state—similar to that in place for other federal programs, such as Medicaid grants, Title V MCH grants, and block grants for the prevention and treatment of substance abuse. The details of the match requirement and the conditions under which it would operate should be developed through a series of dialogues with state and federal officials and public health leaders to ensure that the match is fair and equitable and that it does not disrupt ongoing immunization efforts.
Recommendation 5: CDC should initiate a dialogue with federal and state health agencies, state legislatures, state governors, and Congress immediately so that legislative and budgetary reforms can be proposed promptly when Section 317 is up for reauthorization in FY 2002.
The committee believes the grant formula should include weights that reflect factors of need, capacity, and performance. The calculation of these weights and the analytical process of constructing a formula must reflect special considerations that account for individual state needs and lie beyond the scope of this report. The calculations required include estimating the appropriate size of the federal base grant; determining appropriate “hold harmless” conditions; determining the nature of adequate state-level contributions; developing an appropriate set of proxy measures that reflect need, capacity, and performance in the field of immunization; and choosing the appropriate multiyear finance mechanism for the allocation of federal funds.
Recommendation 6: Federal and state agencies should develop a set of consistent and comparable immunization measures for use in monitoring the status of children and adults enrolled in private and public health plans.
Immunization coverage measures are important for identifying both community health needs and performance outcomes for selected service interventions. Measurement research can guide future federal and state
budgetary decisions, as well as state contribution, programmatic, and reporting requirements. A small set of comparable measures that can harmonize the Health Plan Employer Data and Information Set (HEDIS) and the National Immunization Survey, for example, will allow federal and state agencies to monitor state need, capacity, and performance without imposing unnecessarily burdensome reporting efforts on the states that would restrict their ability to use federal funds productively in responding to local circumstances (Fairbrother and Freed, forthcoming). Such measures can also facilitate efforts by state and federal health officials to assess the quality of primary care health services within private-sector health plans, so that public health agencies can direct appropriate resources to areas in which private-sector plans do not have sufficient capacity to meet health care needs. Assessments of these rates should allow state and federal governments to monitor immunization levels and identify disparities in need, capacity, and performance over time and among regions, including small geographic areas and selected health plans (e.g., Medicaid, SCHIP, and private insurance).
The use of consistent immunization measures within the public and private sectors offers a valuable opportunity to conduct research on the factors that can contribute to disparities in coverage rates within different types of health plans. Finally, immunization measures offer benefit not only for immunization efforts, but also for other national programs that require national investments in primary health care.