2
Change and Complexity in the National Immunization System
As noted in Chapter 1, significant changes have occurred within the national immunization system in the past decade, including continual changes in the immunization schedule, the nature of infectious disease, and the basic demographics of the U.S. population. Immunization was once associated with emergency public health programs designed to stop the spread of infectious disease through mass campaigns conducted within a fairly short time period. Today, the process of immunization involves a series of inoculations spread out over an individual’s lifetime. This transformation has expanded the roles and responsibilities of public health agencies beyond direct service delivery to encompass records management and performance monitoring. Transformations have also occurred in the organization of U.S. health care that have redefined the responsibilities of the public and private health care sectors. These changes have added new layers of complexity to the national immunization system that must be examined with regard to their impact on coverage rates and service-delivery patterns.
Despite the stresses imposed on the system by these changes, important successes have been achieved, such as reductions in the incidence of vaccine-preventable diseases (VPD) and national increases in immunization coverage levels. Nevertheless, as discussed in Chapter 1, significant problems remain, including disparities in coverage levels for children, low adult coverage rates and ethnic disparities in adult immunization rates, and concerns about the quality of measurement tools and programmatic efforts. This chapter first describes the markers of change and com-
plexity that affect the national immunization system, and then reviews examples of success and problem areas.
KEY CHANGES
Key areas of change affecting the national immunization effort include (1) the immunization schedule, (2) the nature of infectious disease, and (3) population demographics.
Immunization Schedule
Ever since the American Academy of Pediatrics (AAP) offered the first immunization guidelines in the 1930s, scientific developments have led to regular changes in the recommended immunization schedule. The rate of change has increased dramatically in the last decade and is likely to continue accelerating in the next 20 years (see Figure 2–1). Between 1938 and 1985, five vaccines (three childhood and two adult) comprising nine different antigens were available. In the next 15 years, the number of recommended vaccines more than doubled.
To complete the current harmonized childhood immunization schedule,1 children must receive 15 to 19 doses of vaccine before 18 months of age and a total of 19 to 22 doses to be fully immunized by the age of 6 (see Figure 1–1 and Table 1–3 in Chapter 1). During some office or clinic visits, the administration of 3 or 4 separate injections is indicated. Adolescents are to receive a tetanus shot between ages 11 and 15, as well as measles, mumps, and rubella (MMR), varicella, and hepatitis B vaccinations if these were not administered at a younger age.
Immunization recommendations for adults have recently changed. The Advisory Committee on Immunization Practices (ACIP) currently recommends that all adults over age 50 receive an annual influenza vaccine (CDC, 2000a). One-time pneumococcal immunizations are recommended for all adults age 65 and over (although ACIP is considering a proposal to lower this recommendation to age 50). Both influenza and pneumococcal immunizations are recommended for anyone below age 65 with certain high-risk conditions, such as heart and lung disease, diabetes, and a compromised immune system. In certain situations, depending on age and health status, adults are also advised to receive hepatitis A, hepatitis B, tetanus, diphtheria, measles, mumps, rubella, and varicella vaccinations (see Table 1–3). Meningococcal vaccine is now recommended for college students, especially those living in dormitories.
Repeated changes to the immunization schedule in the last 5 years foreshadow the exponential changes anticipated in the future. These recent changes include the following:
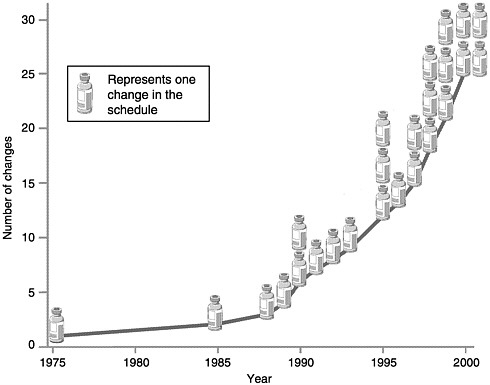
FIGURE 2–1 Changes in the childhood vaccination schedule, 1975–2000. SOURCE: Information provided by CDC.
-
Varicella vaccine was licensed in March 1995 and recommended for limited use the same year (CDC, 1995).
-
Hepatitis A vaccine gained recommended status in 1996 (CDC, 1996).
-
ACIP added rotavirus vaccine to the childhood schedule in August 1998 and then removed it in July 1999 because of indications of increased risk of bowel obstruction during the first few weeks after its administration (CDC, 1999b).
-
DTaP, with an acellular form of pertussis, has replaced DTP as the recommended vaccine against diptheria, tetanus, and pertussis (CDC, 2000b).
-
In 1999, inactivated poliovirus vaccine (IPV) was recommended for the first two doses of poliovirus vaccine instead of oral poliovirus vaccine (OPV) (CDC, 1999c). As of January 2000, IPV was recommended for all four doses (CDC, 2000b).
In addition to novel vaccines, new age groups for which vaccines are recommended have been identified. A new pediatric pneumococcal conjugate vaccine received approval for children under age 2 and high-risk children under age 5 in February 2000 (CDC, 2000d). In 1996, ACIP, AAP, the American Academy of Family Physicians, and the American Medical Association (AMA) jointly recommended immunizing all adolescents aged 11 to 12 with hepatitis B (CDC, 1998b). As mentioned above, ACIP lowered the recommended age for adult influenza vaccination from 65 to 50 years (CDC, 2000a). Many of the vaccines now in the research pipeline will be targeted at adults, and new initiatives will be required to adapt the vaccine delivery system to serve new age groups.
In the next 20 years, the number of vaccines available could triple relative to those recommended today, almost a ninefold increase since the 1950s (when only polio, diphtheria, tetanus, and pertussis vaccines were recommended) (IOM, 1999b) (see Table 2–1). While all of the vaccines that become available may not be recommended for universal use, the schedule’s complexity is certain to increase, although the creation of combination vaccines may minimize the required number of vaccine administrations and office visits.2 Moreover, in addition to the creation of new vaccine types, new forms of administration are being tested, such as the use of live, attenuated influenza virus administered by intranasal spray (Nichol et al., 1999; Poland and Couch, 1999).
With the introduction of new vaccines and changes to the immunization schedule, the cost of vaccination has fluctuated, generally increasing. A majority of new vaccines are considerably more expensive than those used previously. The catalog price for DTP increased from $11.22 in 1987 to $17 for DTaP in 1997, primarily as a result of inflation (Orenstein et al., 1999). In contrast, varicella vaccine has a per-dose catalog price of $45.56, and adolescent hepatitis B costs $20 to $24 per dose (information provided by CDC). The pediatric pneumococcal conjugate vaccine, which will prevent pneumonia, meningitis, and a limited number (about 8 percent) of ear infections, is expected to be relatively expensive, costing about $232 for a four-dose series (Lieu et al., 2000; Stolberg, 2000). In 1987, the combined catalog price for all childhood vaccines was $116; by 1997, the total price had increased to $332–$370 (Orenstein et al., 1999).
Nature of Infectious Disease
The national immunization system is also affected by the changing nature of infectious disease. Pathogens, like human populations, undergo genetic evolution. Such evolution has allowed some viruses to jump from species to species. For example, many scientists believe that smallpox made a trans-species jump into humans between 3,000 and 12,000 years
TABLE 2–1 Vaccines in Widespread Use, 1985–2020
|
1985 |
2000 |
2020a |
|
Adult influenza |
Adult influenza |
Adult influenzac |
|
Adult pneumococcal polysaccharide |
Adult pneumococcal polysaccharide |
Adult pneumococcal polysaccharide |
|
Diphtheria, pertussis, tetanus, and components |
Diphtheria, tetanus, acellular pertussis, and components b |
DTaPc |
|
Measles, mumps, and rubella |
MMRb |
Measles, mumps, rubella, and varicellac |
|
Oral poliovirus |
Inactivated poliovirusb H. influenzae type bb Hepatitis Ab Hepatitis Bb Varicellab Pediatric conjugate of pneumococcal polysaccharide Borrelia burgdorferi Meningococcal polysaccharide A,C,Y,W-135 |
Eradication of polio expected Hibc Hepatitis Ac Hepatitis Bc Varicella with MMR Pediatric conjugate of pneumococcal polysaccharidec Borrelia burgdorferi Conjugated meningococcal polysaccharide A,B,C,Y,W-135c Adult tetanus, diphtheria, acellular pertussis, and componentsc Chlamydia Coccidioides immites Cytomegalovirus Enterotoxigenic E.coli Epstein-Barr Helicobacter pyloric Hepatitis Cc Herpes simplex Histoplasma capsulatum Human papillomavirusc Child influenzac Insulin-dependent diabetes mellitus (therapeutic) Melanoma (therapeutic) Multiple sclerosis (therapeutic) Mycobacterium tuberculosis Neisseria gonorrhea Neisseria meningitidis B Parainfluenzac Respiratory syncytial virusc Rheumatoid arthritis (therapeutic) Rotavirusc Shigella Streptococcus, Group Ac Streptococcus, Group B |
|
aPriority candidate vaccines, drawn from IOM, 1999b. bVaccines covered by Vaccines for Children (VFC) as of February 2000. cVaccines likely to be recommended for universal use (including VFC coverage for childhood vaccines). |
||
ago in one of the Mesopotamian river valleys (Preston, 1999). In addition, population migrations and new areas of human habitat during the past century have led to the emergence and reemergence of pathogens unaffected by current medical treatments (IOM, 1992). The phenomenon of antibiotic resistance is alarming because antibiotic-resistant pathogens are cumulative and accelerating (IOM, 1998a; Feikin et al., 2000). The loss of treatment alternatives makes the prevention of communicable disease through immunization ever more critical.
In addition to pathogen evolution, increased global travel has changed disease patterns throughout the world. In 1998, more than 53 million individuals flew on U.S. carriers to domestic and international locations. Travelers, businesspeople, immigrants, and migrants make national and state boundaries ineffectual barriers to disease in the United States. The IOM report Emerging Infections: Microbial Threats to Health in the United States summarizes the threat posed by the increased global movements of people:
As the human immunodeficiency virus (HIV) disease pandemic surely should have taught us, in the context of infectious disease, there is no-where in the world from which we are remote and no one from whom we are disconnected. Consequently, some infectious diseases that now affect people in other parts of the world represent potential threats to the United States because of global interdependence, modern transportation, trade, and changing social and cultural patterns (IOM, 1992:v).
The September 1999 outbreak of a West Nile-like virus in New York serves as a reminder of how easily disease can spread across the global community. This was the first time the West Nile-like virus, contracted from mosquitoes that have bitten infected birds, had ever been reported in the Western Hemisphere (CDC, 1999d).
Population Demographics
The worldwide movement of people, especially through immigration and migration, continually changes U.S. population demographics, affecting susceptibility to infectious diseases and placing increased demands on the national immunization effort. For example, immigrants accounted for 35 percent (7,930) of total U.S. tuberculosis cases in 1995 (IOM, 1998b). One of every five children under age 18 in the United States (14 million) is an immigrant or has immigrant parents (IOM, 1998b). Since foreign birth has been identified as a barrier to immunization, immigrant children and adults are likely to fall further behind in vaccination coverage unless special efforts are made to integrate them into the U.S. health care system (Findley et al., 1999).
Immunizing the U.S. migrant population presents special challenges as well. Approximately 750,000 migrants live in the United States (Mountain, 1999). Since many migrants cross state borders, they are literally a moving target for health financing, service delivery, and state-based surveillance systems. In addition, a considerable number of migrants may be undocumented persons, creating ethical and political dilemmas regarding the financing of their immunizations (Mountain, 1999).
In addition to immigration and migration, the aging of the U.S. population merits consideration in the development of strategies for immunization policy and practice. As larger numbers of individuals enjoy increased life spans, the importance of vaccines such as influenza and pneumococcal will increase.
The Challenge of Change
The above changes in vaccine development, the nature of disease, and population demographics create challenges for the U.S. immunization system. First, delays and gaps in the uptake of new vaccines occur. For example, the negotiation of a federal contract price with manufacturers of varicella vaccine required 1 year, causing significant delays in the availability of publicly purchased varicella vaccine following its appearance on the market (N.Smith, CDC, personal communication, February 10, 2000). Even with this major financial barrier removed, the national pediatric coverage rate for varicella was only 43.2 percent in 1998 (CDC, 1998a). Second, the addition of new vaccines to the schedule has broadened discrepancies among state standards and coverage practices. State immunization requirements for school children vary considerably (see Appendix G). In addition, some states mandate insurance coverage of pediatric immunizations, but the policies affected and specific vaccines covered differ greatly (Freed et al., 1999). Third, a more complex immunization schedule has made it more difficult to confirm the immunization status of special groups. Identifying pockets of need has become problematic because records are scattered among public and private providers even as the number of vaccines that require surveillance has increased. Finally, the dramatic, almost exponential increase in vaccines on the horizon creates concerns about adverse reactions. As the general public becomes less familiar with the nature and threat of infectious disease, reports of adverse events associated with the use of vaccines are likely to acquire greater significance.
Finding 2–1. The rate of change in the immunization schedule, the nature of infectious disease, and population demographics increased dramatically in the 1990s and is likely to accelerate in the future. At the same
time, determining the immunization status of individuals and groups has become more difficult, especially among vulnerable populations. The complexity of the immunization schedule is likely to contribute to more missed opportunities that could decrease coverage levels and reduce the benefits of vaccines.
INCREASING COMPLEXITY
In addition to the challenges resulting from scientific advances and changes in the environment, the national immunization effort must adapt to the increasing complexity of the financing, service delivery, and public health information systems. Many vulnerable populations now receive vaccines from private health care providers, as the well-insured have long done. Yet the public sector still has primary responsibility for financing vaccine purchases and surveillance efforts for at-risk groups. A patchwork of public and private programs and funding streams that is inadequately described and poorly understood has complicated the national effort to supply, deliver, and monitor immunizations.
Vaccine Supply
The public sector currently relies on a combination of Vaccines for Children (VFC), Section 317, and state funds to purchase childhood vaccines. These programs for vaccine purchase are described in Chapter 3. VFC now provides vaccine for approximately 35 percent of the national birth cohort (National Vaccine Advisory Committee [NVAC], 1999a). In 1998, VFC purchased approximately 37 million doses of vaccine, while Section 317 funds were used to purchase about 13 million doses (information provided by CDC). States also use their own funds to buy vaccine. The states purchased a total of approximately 7 million doses through federal purchase contracts in 1998, and purchased an undetermined number of additional doses directly. Altogether, public-sector funds were used to purchase more than 57 million doses of childhood vaccine in 1998. More than half of all vaccine doses purchased in the United States in 1998 (52.4 percent) were publicly purchased through federal contracts (information provided by CDC).
Vaccine Delivery
Although the public sector purchases the majority of vaccine doses, it is not the primary source of vaccine delivery. Historically, virtually all immunizations received by public program beneficiaries were administered in the public sector. With the inception of VFC in 1994 and the rapid growth
of managed care enrollment in Medicaid over the past decade, a majority of immunizations are currently administered in the private sector.
In 1998, according to the National Immunization Survey (NIS), 54.6 percent of U.S. children received immunizations from private providers, 16.9 percent from public providers, 7.9 percent from mixed providers (public and private), and 20.5 percent from other providers3 (CDC, 1999e). In 1998, an estimated 55 percent of all U.S. children were enrolled in employer-sponsored health plans that covered pediatric immunizations (KPMG Peat Marwick, 1998; Bureau of the Census, 1999). Information on the provision of adult immunization benefits by private plans is lacking.
The rise of managed care has caused public health services, such as immunizations, to be increasingly privatized and funded through capitated arrangements, which makes it difficult to document immunization services. For example, the number of Americans that receive health care services from health maintenance organizations (HMOs) increased from 6 million in 1976 to 67.5 million in 1996 (NVAC, 1999a). The proportion of Medicaid beneficiaries enrolled in a managed care arrangement increased from less than 15 percent in 1993 to 54 percent in 1998 (Health Care Financing Administration [HCFA], 1999b). In addition, 2 million low-income children previously ineligible for Medicaid have been enrolled in the State Children’s Health Insurance Program (SCHIP) over the past 2 years, and the majority of these children receive their vaccines from private providers (HCFA, 2000a). Consequently, children who were likely to be immunized in the public sector 10 years ago may no longer be eligible for its services.
Over the past decade, both general and immunization-specific pediatric best practices have included immunizations within the child’s medical home (AAP, 1992). Administration of immunizations in a timely manner by the child’s primary care practitioner is viewed as a quality measure in its own right and as an indicator of access to a broader array of preventive and routine primary care services. There will always be conditions and population groups that require some kind of special effort, such as children attending school for the first time, adolescents who need immunizations recently added to the schedule (e.g., hepatitis B), or migrant children served by mobile clinics. However, the preferred immunization setting is the child’s regular source of primary care.
Yet the enrollment of Medicaid and SCHIP beneficiaries in private managed care organizations has led to uncertainties and tensions regarding the appropriate site for delivering publicly financed vaccines. Some clients still rely on public clinics for immunizations, either because the public clinic is more convenient (in terms of hours of service or geographic location) or because of the relatively low cost of vaccines thus
acquired. Similarly, some private health care providers find it convenient and less costly to refer their clients to public health clinics for immunizations, even though federal officials have sought to discourage this type of referral practice through advisory notices and consultations (Richardson, 1999; Richardson and Orenstein, 1999). Public health departments are facing decreased revenues from third-party payers, such as Medicaid, for immunizations because of the rapid growth in capitated arrangements. Consequently, public health officials in some areas now negotiate payment plans for immunizations with HMOs or actively discourage private provider referrals through the use of screening questions. The result is a series of mixed signals and increased paperwork for clients and public providers that can result in missed opportunities for immunization within populations with startlingly low coverage levels.
VFC has also contributed to the vaccine delivery system’s shift toward the private sector. A total of 43,000 health care provider sites have enrolled in VFC, and the majority of these (approximately 70 percent, or 30,000) are private provider sites (NVAC, 1999a). By providing free vaccine to primary care physicians, VFC attempts to keep children in their medical homes (their regular source of primary care) and decreases private provider referral of patients to public clinics for immunizations (NVAC, 1999a).
Surveillance
The complexity of vaccine supply and immunization delivery arrangements creates a dilemma for surveillance efforts. Private health plans have assumed responsibility for providing personal health services to public program beneficiaries, but are not readily held accountable for ensuring that all of their enrolled clients are kept up to date in their immunization status. Public agencies continue to deliver vaccines to disadvantaged adults and children, and also retain the responsibility for assessing records and auditing data for public program beneficiaries in both private and public health care settings. Yet the enrollment growth among Medicaid beneficiaries in capitated plans that do not bill for individual services has reduced public agencies’ ability to monitor service delivery for vulnerable populations. Record scattering and patient movement both on and off Medicaid and between health plans (known as “cycling”) have also made immunization records management more difficult. A clearer definition of responsibility for ensuring immunization services and conducting surveillance efforts is needed between the private and public sectors, as well as among public health agencies such as Medicaid, Medicare, and state immunization programs.
Roles and Responsibilities
The multiple public programs and agencies involved with vaccine purchase and administration reflect the dispersion of responsibilities within the national immunization system. This situation raises important questions about the respective leadership roles of federal and state agencies, as well as the appropriate distribution of effort between the private and public health care sectors. For example:
-
ACIP recommends vaccines for the U.S. population. ACIP’s pediatric recommendations are binding for VFC, Medicaid, and SCHIP. Most commonly, it is only after ACIP has recommended a new vaccine that CDC begins to negotiate a federal contract under which VFC and Section 317 can purchase the vaccine. Even before such contracts are in effect, however, Medicaid programs are required to reimburse providers for the newly recommended vaccine as a shared federal-state cost.
-
One strategy states have adopted to encourage private provider participation in VFC and Medicaid has been to increase Medicaid vaccine administration fees after VFC has made vaccines available to private providers at no cost. However, the impact of this strategy has been diminished by the growth of Medicaid managed care plan enrollments because these plans generally do not pay providers separately for vaccine administration.
-
Immunization records are maintained by multiple private and public parties. No single record-keeping system exists that can track clients across health care settings. For clients who use multiple providers, records are scattered, making monitoring of coverage levels as well as individual documentation difficult.
-
The array of public and private agencies involved with immunization in some manner is extensive. The public organizations include state and local public health agencies, insurance regulators, school systems, ACIP, state Medicaid agencies, HCFA (which administers Medicaid and Medicare), CDC, and Women, Infants, and Children (WIC) programs. Private agencies include individual providers, managed care organizations, health plans, and insurers. Each of these organizations has some responsibility for the immunization process, but no single entity has a universal role that allows it to establish data standards, criteria for record keeping, or performance guidelines, or to make cost allocation decisions. The result is a multifaceted enterprise that encourages diverse arrangements; tolerates discrepancies in policies and practices; and frustrates the analysis of trends and patterns, especially for vulnerable populations that depend on both public and private settings.
The scale and impact of changes in the immunization schedule, the nature of disease, and the health care delivery system have distinct implications for the efforts of various levels of government to monitor and respond to trends and shifts in immunization coverage. The states, largely through local governmental authorities, have the primary responsibility for ensuring public health and the delivery of health care services for their citizens. Infectious disease control and prevention, however, requires a nationwide effort and if only for this reason constitutes a national interest. In addition, many states require extra assistance to meet the needs of their populations, either because those needs are extraordinary or because state resources are especially limited. Finally, it is essential to acknowledge the legitimacy of state and local variations in the organization and financing of immunization services, as these services must be responsive to local needs, populations, and professional and fiscal resources. National immunization policy is better focused on goals, outcomes, and identification of successful interventions that increase immunization coverage than on “how to” prescriptions.
The changing realities of health care organization, financing, and politics have resulted in a concomitant shift in public health policy and strategy. To meet its protective responsibilities, the public health sector must work with a rapidly changing health care system, first to understand its approach, and then to apply a variety of government tools to fulfill public health objectives. These tools include (1) a regulatory environment that protects the public from dangerous or ineffective vaccines, (2) a new level of surveillance information that not only captures the peaks of epidemics but also identifies individuals who require immunization services, and (3) a quality assurance role that applies health services research and technical assistance in ensuring immunization coverage.
State and local public health agencies are taking on health system management roles and developing tools that can help them work with and through managed care to improve practices, reduce missed opportunities, and ensure timely immunization coverage. At the same time, however, public health agencies at all levels of government must remain prepared to combat disease epidemics and provide vaccines when necessary to underserved groups.
Finding 2–2. The magnitude and complexity of the modern immunization system have significant implications in terms of both cost and records management. Protecting the public’s health requires attention to multiple components of a complex system composed of numerous public and private agencies. Institutional relationships within this system are loose and disjointed, resulting in ambiguous roles and responsi-
bilities that require coordination and integration through investments in infrastructure.
Finding 2–3. The increasing complexity of the immunization schedule necessitates intensive surveillance and records management for young children, especially for vulnerable populations who may not have a regular source of health care and are therefore at greatest risk of low immunization coverage. This complexity is likely to increase during periods of reform and realignment within the health care delivery system; thus, greater oversight and monitoring are required to ensure that disparities in immunization coverage rates do not grow.
SUCCESSES AND PERSISTENT PROBLEMS
Despite the increasing change and complexity affecting the national immunization effort, the incidence of VPDs has decreased, and important objectives for national coverage were partially met in the 1990s. Yet as noted earlier, persistent problems, such as disparities in childhood coverage levels, low adult immunization rates, ethnic disparities in adult immunization rates, and concerns about the quality of surveillance tools, continue to plague the system.
Successes
The successes of the national immunization effort are most evident in the dramatic decrease in VPD incidence in the 1990s (CDC, 1999a). Only one case of diphtheria was reported in both 1998 and 1999 (CDC, 1999f; information provided by CDC). Polio has been eradicated in the Western Hemisphere, and global polio eradication is expected in this decade (information provided by CDC).
Certain childhood and adult coverage goals have been achieved as well. By 1996, more than 90 percent of American children aged 19 to 35 months had received the first and most critical doses in the primary series for DTP, Haemophilus influenzae type b (Hib), polio, and measles vaccines (NVAC, 1999a). The complete immunization of 79.2 percent of 2-year-old children for the 4:3:1:3 series (four or more doses of DTP, three or more doses of poliovirus, one or more dose of any measles-containing vaccine, and three or more doses of Hib) in 1998 is another major accomplishment, made possible by a remarkable federal-state partnership effort (CDC, 1998a), although approximately 1 million 2-year-olds still need one or more vaccine doses to be fully immunized (NVAC, 1999a). Strides have also been made in adult immunizations. In 1997, the national influenza
immunization rate for adults aged 65 and older was 63 percent, up from 58 percent in 1995 (National Center for Health Statistics, 1997).
Disparities in Childhood Coverage Levels
Despite increased national coverage levels for children and substantial reductions in disparities among racial and ethnic groups, disparities still exist among and within states, as well as within major metropolitan areas. Between 1970 and 1985, surveys of immunization coverage of pre-school-age children revealed racial and ethnic disparities in coverage levels that ranged as high as 26 percentage points (CDC, 1997). The most current available NIS data (July 1998–June 1999) show disparities of 7 to 8.6 percentage points between the immunization coverage levels for non-Hispanic white children and Hispanic and non-Hispanic black children for the 4:3:1:3 series, and lesser disparities for individual vaccine coverage rates among these groups (CDC, 2000e). Much of the difference in coverage levels among racial and ethnic groups is attributable to differences in poverty rates among these groups. Again for the latest annual period, the NIS documented a disparity of 9 percentage points between children living below and those at or above the poverty level for the 4:3:1:3 immunization series (CDC, 2000f). Concentrated poverty, along with the somewhat lower immunization levels for minority children, contributes to the lower coverage levels found in large metropolitan areas.
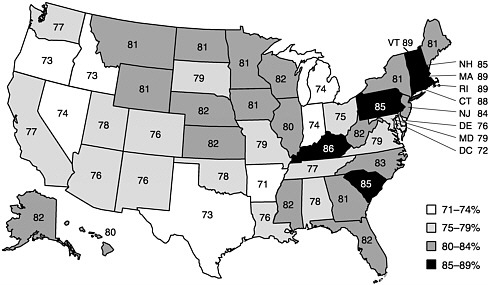
In 1999, state coverage levels for the 4:3:1:3 series ranged from 71 percent in Arkansas to 89 percent in Vermont, Rhode Island, and Massachusetts (see Figure 2–2). Also in 1999, the coverage levels for the same series were 57 percent in the City of Houston and 73 percent in Dallas County, compared with 76 percent for the rest of Texas. Newark’s coverage level was 67 percent, 18 points lower than the rate for the rest of New Jersey. Chicago’s rate was 70 percent in 1999, compared with 84 percent for the rest of Illinois (CDC, 2000c) (see Table 1–5 in Chapter 1).
Serious disparities in coverage levels also exist within certain large metropolitan areas. Several studies have demonstrated that the NIS, which collects state and county data, is often not sensitive to small area variations, which reveal significant underimmunization among the most disadvantaged populations. For example:
-
In Marion County, Indiana (Indianapolis), a special survey of poor children found their coverage rate to be 53 percent as compared with an NIS estimate of 78 percent for the county as a whole (Bates and Wolinsky, 1998).
-
A special survey of children in East Los Angeles found coverage to be 49 percent as compared with NIS data showing a coverage rate of 71 percent for the Los Angeles region (Shaheen et al., 2000).
-
A Chicago study found coverage rates of 36 percent for African American children in general and 29 percent for African American children in public housing, as compared with the NIS estimate of 59 percent coverage for children countywide (Kenyon et al., 1998).
Low Adult Immunization Coverage Rates
Although the objective that 60 percent of elderly Americans receive an influenza immunization has been met, problems in adult coverage rates persist, especially for chronically ill working-age adults who are at high risk for complications from influenza and pneumococcal disease. Many have argued that a 60 percent influenza coverage rate is too low, and the national goal has been raised to 90 percent for 2010 (Department of Health and Human Services, 2000). Pneumococcal immunization levels for the elderly are particularly low. Nationally, only 42 percent of noninstitutionalized adults over age 65 had ever received a pneumococcal vaccination by 1997 (National Center for Health Statistics, 1997). Just 17 states had achieved pneumococcal immunization rates of 50 percent or greater among elderly persons by 1997 (information provided by CDC). In addition, noninstitutionalized high-risk adults aged 18 to 64 have extremely low immunization rates. Data from the 1997 National Health Interview Survey show that only 26 percent of this group had received an influenza vaccination, and just 13 percent had received a pneumococcal vaccination (National Center for Health Statistics, 1997). Data on national coverage rates for adult immunizations other than influenza and pneumococcal are severely limited.
Disparities in Adult Coverage Levels
While the generally low adult immunization coverage levels are disconcerting, disparities in the immunization rates among ethnic populations represent an even more serious situation. In 1997, 66 percent of white adults aged 65 and over received an influenza vaccination, compared with 45 percent of black and 53 percent of Hispanic adults in the same age group (National Center for Health Statistics, 1997). The trend is similar for pneumococcal immunizations. As of 1997, 46 percent of elderly whites had received a one-time pneumococcal vaccination, but only 22 percent of elderly blacks and 23 percent of elderly Hispanics. These disparities result in communities at heightened risk for outbreaks of influenza and pneumococcal disease, in addition to other VPDs. Among persons aged 65 and over, influenza and pneumococcal disease were the fifth leading cause of death for African Americans and Hispanics as well as non-Hispanic whites. Reductions in these deaths are hindered by rela-
tively low vaccine utilization. Because of the association of appropriate health care with an individual’s economic status, race, and gender, access to immunization coverage proves difficult for many racial and ethnic minorities. For example, although rubella has been virtually eliminated within the U.S.-born population, rubella outbreaks have occurred sporadically among Hispanic populations in the last 5 years as a result of immigration from countries where rubella vaccine is not part of the childhood immunization schedule (information provided by CDC).
Finding 2–4. Important strides have been made in decreasing the incidence of vaccine-preventable diseases and increasing the immunization coverage levels for children and adults. However, sustained efforts are needed to address the troublesome disparities that remain in childhood and adult coverage levels.