5
Biological Impacts of Species Invasions: Implications for Policymakers
KAREN GOODELL
Department of Ecology and Evolution, State University of New York at Stony Brook
INGRID M. PARKER
Department of Biology, University of California, Santa Cruz
GREGORY S. GILBERT
Department of Environmental Science, Policy, and Management, University of California, Santa Cruz
The processes that control the transport, establishment, spread, and impact of invasive organisms underlie many of our concerns about sanitary and phytosanitary risk. In the United States and worldwide, our perception of risk has expanded from primarily economic and human health concerns to include risks to natural ecosystems. The effects of invasive non-indigenous species comprise one of the most apparent risks of globalization of international trade to both agricultural and natural ecosystems. Since Elton's (1958) first formal treatment of biological invasions as an ecological problem, ecologists have made great strides in understanding patterns of non-indigenous species introduction and establishment of self-sustaining populations (Williamson, 1996). Once a novel species is introduced to a region, the risk it poses depends on whether it will establish there and what impact it could have once established. There has been considerable progress in understanding which traits confer invasiveness (Rejmánek and Richardson, 1996; Reichard and Hamilton, 1997), but until very recently, much less attention has been given to developing rules for predicting, or even quantifying, impacts. Such rules could guide managers and policy makers in deciding which species can be safely introduced and which should be avoided, and help them set priorities to protect ecosystems.
In this paper, we review the impacts of invasions resulting from both planned and unplanned introductions from a biological perspective, pulling together examples and lessons learned from both agricultural science and the
ecology of natural ecosystems. Although agricultural impacts and ecological impacts are rarely discussed in concert, we feel that the two are mutually illuminating; together they form a continuum that presents an array of challenges to managers and policy makers. First, we review the diversity of impacts and how they are measured from a strictly anthropocentric view, followed by a strictly ecological view, and then discuss what occurs when the two views are incongruent. In addition, we emphasize the utility of identifying trade and transportation pathways as a way to organize the diversity of potential invaders. Using vectors of introduction to define groups of invaders with similar biology and potentially similar impacts on natural systems may prove an efficient approach to identify targets for regulation and control. Often targeting the vector is easier than targeting each individual species.
We then focus on three aspects of predicting the risk of impacts from introductions of particular species: (1) predicting the successful establishment of invaders, (2) predicting the impact of species that establish themselves, and (3) assessing the uncertainty associated with these predictions. Progress in predicting establishment of certain invaders shows promise for informing policy on planned introductions, but relies heavily on detailed biological and geographical information about the species. Forecasting which invaders will then have the biggest impacts is even more challenging. Factors such as host range and dispersal ability can help gauge how big of a problem an invader will become. The prevalence of ecological idiosyncrasies, complex indirect effects and the possibility for synergistic effects among invaders, however, hinder our ability to predict ecological impacts. We finish with a discussion of prioritizing the management and control of invasive species based on impact, and the need for more consistent measures of impact.
To illustrate the main points that we emphasize in this paper, we have provided three detailed case histories of invasions. Through these case histories, we attempt to convey the importance that a biological perspective plays in assessing the degree of impact, as well as understanding the mechanisms behind that impact. We have intentionally chosen case histories that involve species native to the United States that have become problematic invaders in other parts of the world. This admittedly biased selection represents a suite of invaders less publicized within the United States that may feature prominently in current or future trade disputes.
IMPACT FROM AN ANTHROPOCENTRIC PERSPECTIVE
Impact from an anthropocentric view is often equated with the economic losses caused by a non-indigenous species or by the cost of its control or eradication. For example, the Office of Technology Assessment report on non-indigenous species lists as ''high impact" those species that are significant pests of agriculture, rangelands, or forests and those that seriously foul waterways or power plants (U.S. Congress, 1993). Among several proposed measures of impact of non-indigenous species, Williamson (1998) quoted costs in pounds sterling for control of different weed species in the United Kingdom. Just as
some environmentalists strive to attract wider public support for environmental protection measures by calculating the economic value of the services provided to humankind by biodiversity (e.g., Constanza et al., 1997; Daily, 1997), so have scientists raised public and political consciousness of the problem of non-indigenous species by pointing to the $100 million annual cost of fighting weeds in the United States (U.S. Congress, 1993) or the $400 million impact of zebra mussels in the Great Lakes over a five-year period (O'Neill, 1996).
Perhaps even more important to most people than their wallet is their health. Extensive research documents the negative impacts of non-indigenous pests and pathogens on human health, although, unfortunately, there is little sharing of information between the medical, public health, and epidemiological literatures on the one hand, and ecological and biological invasions literature on the other. Only occasionally does a medically oriented contribution appear in volumes devoted to invasion biology (e.g., Craig, 1993). Interestingly, invaders that cause human health hazards can have a psychological impact out of proportion to their real risks. A good example is the Africanized honey bee (Apis mellifera scutellata), a non-indigenous species made notorious by a relatively small number of deaths and immortalized by its nickname "killer bee."
In our review of the biological impacts of invasions, we include impacts on human health, on agricultural ecosystems, and on natural ecosystems. Throughout, however, we focus primarily on ecological impacts, both in natural and agricultural ecosystems, because here the diversity of interactions and the complexity of the issues best inform us of the range of risks we face and the sources of uncertainty in predicting consequences of biological invasions.
CASE STUDY 1: THE GRAPE ROOT LOUSE PHYLLOXERA: THE IMPORTANCE OF RECOGNIZING AND REGULATING VECTORS
The most devastating impacts of some invasive species have been economic and social, rather than environmental. Introduced agricultural pests, for example, cost millions in agricultural losses and control programs and have lasting effects on human populations. Such is the case of the grape root louse phylloxera (Daktulospharaira vitifoliae), an insect pest in the aphid family native to the Eastern United States. This pest first became problematic in its native range as European settlers in Eastern North America began to develop a wine-making industry in the seventeenth to nineteenth centuries. Viticulturalists discovered that European grape vines that had been imported because of their superior flavor (Morton, 1985) grew poorly in North American soils. In particular, the vines often shriveled and died within a few years of planting. Native American grapes, however, did not succumb to this affliction. The cause of the grape vine affliction was not discovered until it had been introduced into France and wiped out ancient vineyards. Exactly how phylloxera arrived in France is unknown. American vines had been introduced into France as early as the sixteenth century, but it was not until the advent of steamships allowing
rapid crossings of the Atlantic that phylloxera could survive the trip (Stevenson, 1980). In the mid-1800s, colonists made several shipments of cuttings of American grapes to vine breeders in Southern France with the hopes of hybridizing the European species to the American. These vines were the probable vectors of phylloxera (Morton, 1985).
In 1863, centuries-old vineyards in the Rhone Valley began to show the effects of the phylloxera infestation. Within the next 10 years, not only had most of the vineyards in the Rhone Valley perished, but the infestation had spread throughout France, affecting more than 600,000 ha of vineyards (Pouget, 1990). By 1900, there remained only a few vineyards in all of France, and phylloxera had spread, presumably through the shipment of vine cuttings, to the rest of Europe (Oestreicher, 1996), Australia (Desdames, 1984; Buchanan, 1987), and South Africa (Oestreicher, 1996; Van Zyl, 1984). Later, phylloxera appeared in California, South America, New Zealand, and Japan.
The economic and social effects of the early European infestation of phylloxera were severe. Historical sources report the abandonment of entire communities in places like the Midi in Southern France where viticulture comprised the sole industry. The inhabitants migrated to Algeria, Argentina, or Chile, seeing little option for a livelihood in France or even Europe (Pouget, 1990). In fact, some historians have likened the social implications of the phylloxera epidemic in France to those of the potato blight in Ireland (Lukacs, 1996).
The French government responded to the crisis by appointing a special commission to study the pest and find a remedy. By 1887 they had discovered that grafting the European species to the rootstock of North American species provided a vine resistant to phylloxera but with the high-quality fruit of the European stock (Pouget, 1990). As the technology for grafting and selecting appropriate rootstock developed and farmers replanted, the wine industry slowly rebuilt.
Phylloxera, although still spreading in regions such as Australia and New Zealand (King and Buchanan, 1986), presents much less of a threat today because the cultural practice of grafting onto resistant roots has mitigated its impact. However, the more recent and disturbing discovery of a phylloxera strain in California that has overcome resistant rootstock threatens a resurgence of the phylloxera problem (Granett et al., 1985).
Phylloxera probably had very little direct ecological impact because of aspects of its biology. The host specificity of phylloxera and its probable mode of long-distance transport on grape vine cuttings, rather than autonomous flights, at least in some regions (King and Buchanan, 1986), confined the infestation to highly modified agricultural landscapes, some of which had been under cultivation for thousands of years. Had a more generalized pest been introduced or had phylloxera evolved the ability to use novel host plant species, its impacts may have extended to other agricultural systems or noncultivated ecosystems. To date, however, no research has looked for ecological impacts of the phylloxera invasion.
One unexpected negative effect of the phylloxera epidemic was the introduction of downy mildew into European vineyards, probably when
American rootstock was imported as the French sought to solve the phylloxera problem (Cowling, 1978; Lukacs, 1996). Thus the solution to one problematic invader served as the vector for introducing a second problematic invader—a story that has been repeated in other contexts as well (Simberloff and Stiling, 1996a).
The lessons to be learned from a biological perspective on the phylloxera invasion of the wine-producing regions of the world are threefold. First, to manage invasions we need to look for them and know what we are looking for. Although this idea may seem self-evident, it remains poorly implemented worldwide, partly because of a lack of well-trained taxonomists at points of inspection. The indispensability of well-trained taxonomists at all levels of the inspection service has a recent illustration in the discovery of Karnal bunt on wheat in the United States. Good taxonomic treatments coupled with modern diagnostic techniques led to a reliable program for detecting Karnal bunt and differentiating it from similar fungi. This program has saved the $5 billion wheat export market from international prohibition of cereal and grass seed from the United States (Palm, 1999). Second, we recognize that ecological and evolutionary interactions between pests and their hosts play an important role in phytosanitary risk, as they do in the health risks of emerging human diseases (Ewald, 1994). Third, our strategies for managing the impacts of an invasion often carry with them their own invasion risks, such as the downy mildew associated with American grape vines. All three of these lessons can be understood in the context of vectors, or routes of introduction, in ways that increase our ability to predict potential invasions.
VECTORS
By examining routes of introduction of invaders, planned or unplanned, we find patterns that may help us predict the likelihood of particular kinds of impacts associated with different kinds of introductions. In many cases, regulating the vectors may prove easier than regulating the organisms themselves. A vector-based perspective can help organize the complexity of invasion impacts into approachable subunits, thereby potentially providing useful generalizations to guide policy decisions. Specifically, analysis of vectors offers two advantages: (1) identifying a restricted group of taxa likely to have similar impacts that can be managed in a particular way and (2) using the vector to manage the risks of a particular species.
Identifying Patterns of Species Introductions and Impacts
One of the most well-known examples of a vector for introducing non-indigenous organisms is ballast water. Ballast water taken on by ships in one part of the world, then expelled in another region, can efficiently transport a diversity of marine and freshwater organisms including bivalves, crustaceans,
and fish (Mills et al., 1993; Carlton and Gellar, 1993; Cohen and Carlton, 1998). Locke et al.(1993) estimated that 800 million liters of ballast water are dumped into the North American Great Lakes each year. The impacts of the organisms that have established through such means can include mechanisms generally important in all ecosystems, such as competition and predation, as well as mechanisms specific to that suite of organisms, such as the voracious filter feeding of the Asian clam (Potamocorbula amurensis) (Werner and Hollibaugh, 1993) or the fouling of industrial pipes by the invasive zebra mussel (Dreissena polymorpha) in the Great Lakes (MacIsaac, 1996). Ballast water is a good example of a vector targeted for regulation, as the zebra mussel disaster resulted in the passage of federal regulations requiring the exchange of fresh water ballast for salt water ballast before ships enter the Great Lakes (U.S. Coast Guard, 1993). These regulations, in theory, should greatly reduce the risk of invasion. Nevertheless, it comes as little surprise that several prolific invertebrates have invaded the Great Lakes since the implementation of preventative measures (Ricciardi and MacIsaac, 2000). Lack of complete compliance with ballast exchange policy (Locke et al. 1993), and the possibility that nonindigenous organisms may be transported on the hull or other parts of ships, suggest that we may need to reevaluate the implementation and enforcement of the policy periodically.
The most obvious vector for a pest or pathogen is its own host species, and consequently many phytosanitary regulations have focused on this pathway. Perhaps surprisingly, hosts as vectors may take menacingly varied forms. For example, New Zealand officials intercepted the fungus Bipolaris maydis, which caused the epidemic of Southern corn leaf blight that devastated the United States corn production in 1970, in packages of popcorn imported from the United States (Scott, 1971). We generally expect new pests and pathogens to come from the area of origin of the host species (Thomas, 1973). Because the majority of important crop species originated outside of North America, the United States may seem an unlikely origin of pests in this regard. However, the U.S. Department of Agriculture (USDA) Germplasm Resource Information Network (GRIN) lists 418 species or subspecies of flowering plants native to the United States with a recognized economic importance for food, construction, fuel, or forage (see Table 5-1).
Table 5-1. Counts of Plant Species Native to the United States That Have a Known Economic Importance
|
Economic Importance |
Number of Species |
|
Food |
105 |
|
Construction |
206 |
|
Fuel |
28 |
|
Forage |
126 |
|
Weeds |
284 |
|
Alternate disease host |
14 |
|
Source: USDA Agricultural Research Service 1999. |
|
It is important to note that pests and pathogens commonly show rapid evolution (Ebert, 1998; Thomson, 1998), and host shifts can occur unexpectedly (Strong 1984) leading to disease or infestations of introduced crops by pathogens from the new range of the crop. Novel pathogens can then be transported back to the region of origin of the crop, or to other regions through unexpected vectors. The fire-blight pathogen (Erwinia amylovora) in New York illustrates such a transfer. This pathogen shifted from its previously known host, wild crabapple (Crataegus sp.), to cultivated apples, which had been introduced from Eastern Europe. Following the subsequent introduction of the pathogen to England, it spread throughout Europe, causing extensive losses in the apple industry (Van der Zwet and Beer, 1992). Similarly, the introduction of North American rainbow trout (Onchorynchus mykiss ) to Europe for trout farming was followed by an apparent host switch of the Eurasian fish parasite (Myxobolus cerebralis) that causes whirling disease (Hedrick et al., 1998). This parasite was then transported to North America via infected live or frozen trout, where it imperils both commercial rearing operations and natural populations (Hoffman, 1970). Host switching and rapid evolution of pests and pathogens, coupled with global distributions of many crops and noxious invasive species, complicates our ability to predict routes of introduction and invasion.
Cargo and luggage also provide passage for a wide diversity of "hitchhikers" that may represent serious invasion threats. Similarly, some kinds of agricultural or horticultural introductions (e.g., rootballs packed with soil) are of particular concern not because of the plants themselves, but because they may harbor a diversity of unexpected organisms. Of the potential pest organisms that the Australian Quarantine Inspection Service (AQIS) intercepted on entry to Australia from the United States between 1990 and 1998, 80 percent were found in shipping containers or sea freight, and an additional 15 percent were found in air freight (AQIS, 1999). Intercepted organisms ranged from nematodes to frogs, associated with everything from pottery to logs. Of 8,243 interceptions during that period, 42 percent were encountered on fruits (60 percent mites, 10 percent hemipteran bugs, and 10 percent thrips) and 37 percent on timber (92 percent beetles).
Using Vectors to Regulate Specific Risks
A second use of vectors as an approach to understanding and regulating invasions involves the management of particular risks associated with a particular species. For example, brown tree snakes were introduced into Guam from New Guinea or Australia as stowaways in shipments of derelict war equipment shortly after World War II (Fritts and Rodda, 1998). By the 1980s, these predators had extirpated or drastically reduced population sizes of most of the 10 species of native birds, as well as reduced the populations of native fruit bats and lizards (Fritts and Rodda, 1998; Savidge, 1987). Brown tree snakes also have had major economic impacts on poultry operations and caused power outages by climbing on transformers (Fritts and Rodda, 1998). Now, Hawaii and
other Pacific islands are eyeing the problems on Guam with alarm. Flights and cargo from Guam into Hawaii undergo mandatory inspection to prevent the introduction of the brown tree snakes. As a tribute to their success, Hawaii remains free of the snake, although six snakes were intercepted between 1981 and 1994 on incoming airplanes (Fritts, 1999).
Another cargo hitchhiker most effectively regulated through its vector is the Asian long-horn beetle (Anoplophora glabripennis). Beetle larvae burrow in untreated wood used in packing crates in shipments from China and have emerged as adult beetles in several U.S. cities. The mobile beetles are voracious pests of a number of important tree species and have caused widespread death of trees in New York and elsewhere (Haack et al., 1997).
IMPACT FROM AN ECOLOGICAL PERSPECTIVE
Measuring the Ecological Impact of Invaders
A biologist trying to determine the impact of an invader on native species and ecosystems faces a more difficult challenge than an economist evaluating that invader in financial ("pest") terms. There are many different approaches to measuring impacts, and there appears to be little agreement among ecologists over how impacts should be quantified and compared among invading taxa (Parker et al., 1999).
The simplest measure of impact is the area of land occupied by an invader (Dombeck, 1996; Schmitz et al., 1997). Using range to represent impact, however, assumes that all invaders have effects of a similar magnitude on local biological communities, which is clearly not the case (Williamson, 1996; Wonham et al., 2000). A complete assessment of impact would incorporate the range of the invader, its abundance, and its local effects (Parker et al., 1999). However, the local effects depend on the ecological interactions between an invader and its host community or ecosystem (Drake, 1983). Determining local effects of an invader, therefore, represents the greatest challenge to predicting the impacts of a particular invader in an ecosystem. Local impacts can be measured at five scales of ecological organization: traits of individuals, genetic characteristics of populations, abundance and dynamics of populations (within species), communities of multiple species, and ecosystem processes.
Individuals
Invaders can have a variety of impacts on the characteristics of individuals. For example, invaders can compete with natives causing poor growth and reduced individual size (Gentle and Duggin, 1997) or altered morphology, such as rooting depth (D'Antonio and Mahall, 1991). Invaders can also cause changes in behavior of native animals, such as invading predacious fish that alter the habitat use and diet of native fish in rivers (Brown and Moyle, 1991). Impacts on individuals of a species can have important implications for the
viability or dynamics of the whole population as well, but can be easier to measure than population parameters.
Genetics
Sometimes non-indigenous species are introduced to areas already inhabited by closely related native species. If these native-non-indigenous pairs of species can interbreed, then genetic interchange between two species can lead to a loss of the unique genetic makeup of the species (Echelle and Conner, 1989; Rhymer and Simberloff, 1996). Such hybridization and introgression can lead to a virtual extinction of native species through "genetic pollution," especially when the invader becomes much more common than the native species. In fact, of 24 federally listed species in the United States that have become extinct since the enactment of the Endangered Species Act, three have done so through hybridization with non-indigenous species (McMillan and Wilcove, 1994).
Populations and Species
Species-level measures of impact are often used in ecology and conservation. Extinction of native species is arguably the most dramatic impact of invasive species. Recall, for example, the brown tree snake that caused the extinction, or near extinction, of most of the native bird species of Guam (Savidge, 1987). Extinction of native species, however, characterizes relatively few invasions (Simberloff, 1981). Reduced population sizes or local extinctions appear more common, but changes in population sizes of native species after invasion by a non-native can vary greatly in magnitude and even direction. Researchers documented this wide variation with changes in abundance of terrestrial arthropod species before and after invasion of the fire ant (Solenopsis invicta) in Hawaii (see Figure 5-1). Although most species showed reduced abundance because of competition or predation by fire ants, other species, such as the scarab beetle (Myrmecaphodius vaticollis) increased in abundance (Porter and Savignano, 1990).
Communities
Community measures, such as changes in species diversity or richness, incorporate effects on many individual species, yet provide a simple and interpretable summary of impact. Although these measures do not reflect the magnitude of change in any one species, they do provide a sense of how much an invader interacts with community members. Community measures have the further advantage that they are potentially comparable across communities with different species assemblages.
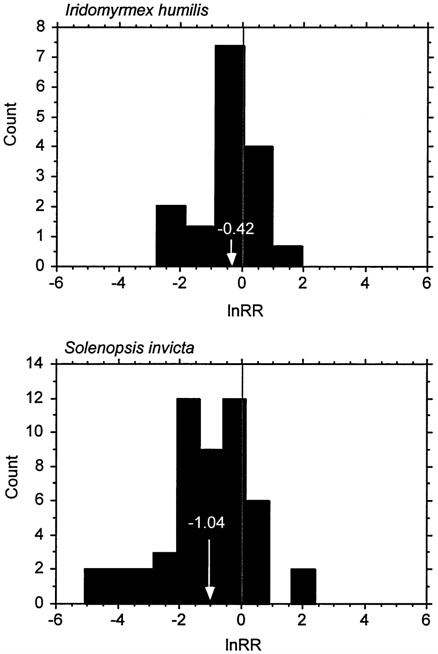
FIGURE 5-1. Frequency Histogram of the log Response Ratio (lnRR) for Ant Impacts. lnRR is a measure of effect size used in meta-analysis(Hedges, 1996), here defined as lnRR = ln(abundance with the invader/abundance without the invader). Each bar represents the number of sampled resident invertebrate species that responded to the invasion by one of two introduced ant species at each effect size category. When lnRR = 0, there was no change in density of the native species, when lnRR < 0 the native species density declined in the presence of the invader, and when lnRR > 0 the resident species increased in density in the presence of the invader. Arrows and numbers in white show means. Data on Iridomyrmex humilis were taken from Cole et al., (1992); data on Solenopsis invicta were taken from Porter and Savignano (1990).
Ecosystems
Invaders that affect ecosystem processes such as nutrient cycling or disturbance regimes are viewed by some ecologists as the most problematic (Vitousek and Walker, 1989; Mack and D'Antonio, 1998). These biologists reason that changing ecosystem processes "changes the rules of the game" in a way that influences many, if not all, of the component species. For example, Myrica faya, an introduced nitrogen-fixing shrub in Hawaii, colonizes the nutrient-poor soils of recent lava flows and increases soil nitrogen levels (Vitousek and Walker, 1989). These changes in the nutrient cycle are thought to affect patterns of succession on the lava flows (Vitousek et al., 1987). The large amount of water used by the invasive saltcedar trees (Tamarix ramossissima) (Carman and Brotherson, 1982) lowers the water table in ephemeral or permanent wetlands in North American arid zones and can eliminate habitat for native migratory birds (Neill, 1983).
CASE STUDY 2: THE MOSQUITO FISH: WHEN ANTHROPOCENTRIC AND ECOLOGICAL PERSPECTIVES CLASH
The ecological impacts of some biological invasions contrast with their economic and social impacts. This situation characterizes some invaders that also serve as biological control agents. Resolutions of such conflicts require policymakers to carefully weigh very different sorts of impacts.
From a purely ecological perspective, the mosquito fish (Gambusia affinis affinis and Gambusia affinis holbrooki, two subspecies) exemplifies a biological control agent gone awry. After the discoveries in 1898 that mosquitoes transmitmalarial parasites to humans, and in 1900 that a species of mosquito carries yellow fever, interest in mosquito control grew (Krumholz, 1948). The mosquito fish, native to the southeastern United States, quickly rose as a popular control measure. True to their name, mosquito fish consume large numbers of aquatic mosquito larvae. The American Red Cross, the International Health Board, and the Rockefeller Foundation jointly mounted a program to control mosquitoes by introducing mosquito fish to many regions of the world, including parts of the United States where the fish was not native (see Figure 5-2). This program gave little thought to potential negative ecological effects (Lloyd et al., 1986).
Some mosquito fish introductions studied not only effectively controlled mosquito populations (Gerberich, 1946; Krumholz, 1948), but reduced incidence of malaria (Howard, 1922; Gerberich, 1946), and in one case even increased the rate of human population growth (Holland, 1933). Mosquito fish proved prolific and tolerant of a wide range of environmental conditions, including pollution (Krumholz, 1948; McKay, 1984; Lloyd et al., 1986). In retrospect, it comes as little surprise that some of the same characteristics that made the mosquito fish a successful biocontrol agent also made it a successful
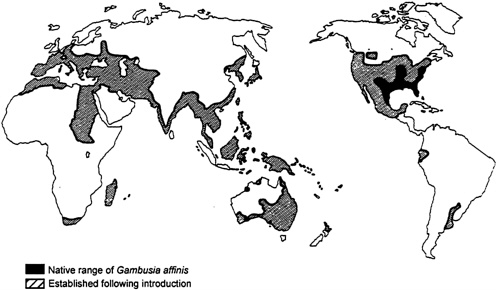
Figure 5-2.
Worldwide Distribution of Gambusia affinis. Modified from Lloyd et al. (1986) showing that G. affinis now enjoys a strikingly broader distribution than its native distribution. Shaded regions representing establishment following introduction indicate that G. affinis occupies suitable habitats in those regions.
Reproduced by permission of John Wiley & Sons, Australia.
invader. Their broad environmental tolerances allowed them to thrive in many regions of the world. Their rapid population growth and large appetite put them in competition with native surface-feeding fish. In addition, mosquito fish consume eggs and young of native fish and amphibians. Australian researchers, in particular, have conducted detailed research on mosquito fish impacts, carefully documenting declines in small surface-inhabiting native fishes, as well as juveniles of important game and food fish species (McKay, 1984; Howe et al., 1997). Mosquito fish have been implicated in the decline of 35 fish species throughout the world (Lloyd, 1989), as well as three Australian frog species (Webb and Joss, 1997). At least one of these frogs, Litoria aurea, is listed as threatened (Morgan and Buttemer, 1996). Experimental research on the impact of mosquito fish suggests that it could have ecosystem impacts by consuming aquatic invertebrates and thus altering the trophic balance of still-water systems. Ultimately these changes could lead to accumulation of nutrients, increased biotic growth, and oxygen-deficient water (i.e., eutrophication; Hurlburt et al., 1972).
To evaluate whether the net consequence of introducing mosquito fish turned out, on balance, positive or negative, one must weigh the ecological effects against the human health effects. To put the negative effects in perspective, it is instructive to compare the ecological impacts of using mosquito fish to control mosquitoes with those of alternative methods. Previous methods were either less effective and more expensive, such as applying oil films to the surface of still water (De Buen, 1929, in Gerberich, 1946), or
imposed a different suite of ecological and human health risks, such as the application of DDT (Krumholz, 1948). In comparison to the impact of other control measures, the impact of mosquito fish may seem less egregious. Fish biologists in Australia, however, contend that several native Australian species could serve in mosquito control, obviating the need to introduce non-native species (Lloyd et al., 1986). In this case, knowledge of ecological impacts combined with a good understanding of the biology of the system could motivate more appropriate and safer alternatives that were overlooked or unavailable in the past. Even with all of the available information on the negative ecological impacts of the mosquito fish, however, policymakers may still decide to introduce the fish into new areas. Although ecological information may not always drive policy, it is critical to ''have all the cards on the table" for society to make informed decisions.
The mosquito fish represents just one of many invaders for which we will have to develop regulations that weigh the relative costs and benefits to society. Of the 284 species of important international weeds that are native to the United States, GRIN (USDA, 1999) lists 39 species that have both weed potential and important economic uses. This duality characterizes the well-known case of the Eurasion honey bee, Apis mellifera, which was brought to North America by European colonists for honey and wax production. Apiculture has since burgeoned into a $10-billion industry, playing a key role in the pollination of many insect-pollinated crop plants (Robinson et al, 1989). However, ecologists have documented negative effects of honey bees on native pollinators and plants, through competition for floral resources and inadequate pollination (Roubik, 1982; Kato et al., 1999; Goodell, 1998; Paton, 1997).
PREDICTING OUTCOMES OF SPECIES INTRODUCTIONS
When we consider both economic and ecological impacts, the uncertainty involved in predicting the impacts of unplanned introductions can be overwhelming, even if we employ a vector-based approach to focus on a subset of potential invaders with similar impacts (e.g., ballast water introductions). One attempt to quantify risk at the level of a vector was an assessment of the potential cost posed by untreated Siberian timber to North American forest ecosystems and the U.S. timber industry (USDA, 1991; Ruesink et al., 1995). This assessment suggested that losses due to unintentional introduction of organisms associated with larch could have reached $58 billion (USDA, 1991).
Planned introductions, for which we at least have an identified organism, may provide a better starting point to test our ability to predict the impacts of species introductions. Intentionally introduced organisms offer us a variety of appealing traits—an attractive shrub, an herbivorous insect that controls a noxious weed, or an affectionate pet. Yet these desirable organisms may sometimes themselves become invasive pests. We discuss three components important to assessing the risk of a planned introduction: (1) predicting which species will successfully establish in a new region, (2) predicting which of those
species will have large impacts, and (3) assessing the degree of uncertainty involved in the prediction.
Quantitative and Experimental Approaches to Identifying Potential Invaders
Predicting the outcome of species introductions is an area of active research in ecology. So far, few general rules of thumb regarding which introduced species will establish self-sustaining populations apply to all kinds of nonindigenous organisms. For some taxonomic groups or ecologically similar groups of species, however, biologists have identified characteristics that fairly consistently correlate with invasiveness. The methods biologists use to reach these conclusions typically involve scoring introductions of known outcome as invasive or not invasive, then looking for correlations between invasiveness and various attributes of the introduced species, such as life history traits or biogeography, using multivariate statistics (e.g., Bergelson and Crawley, 1989; Perrins et al., 1992; reviewed in Ruesink et al., 1995).
At the taxonomically broadest end of the spectrum, Reichard and Hamilton (1997) developed a decision analysis to evaluate which characteristics were associated with invasiveness of woody plant species that had been introduced into the United States. Starting with a multivariate approach that included many different traits, they found a strong tendency for woody plant species that were invasive in other parts of the world also to be invasive in North America. Some attributes proved more useful for regional models than for the continental-scale model. For example, having an Asian origin indicated non-invasiveness in the large-scale model but invasiveness in a regional model using data from the southeastern United States. Their results suggest that models incorporating geographic attributes may apply only to the region for which they were developed and may not be generalizable to other geographic regions.
Reichard and Hamilton (1997) then used these distinguishing attributes of invaders to construct a "decision tree" for use in deciding which woody tree species should be introduced and which should not. The decision tree resembles a flow chart and presents a series of questions regarding the presence of particular traits, starting with those most strongly associated with invasiveness. The answers to these yes/no questions form dichotomous branches leading to either a question about the next most important trait or a recommendation to accept, reject, or further study the species proposed for introduction. Their decision tree relies on information about the introduced species that is relatively easily obtained from the literature or herbarium records, which makes it a practical tool for managers. In validating their decision tree using all woody plant introductions, invasive and non-invasive, they correctly rejected 88 percent of the pest species and unconditionally accepted for admission only 7 percent of the invasive species. Predictive power for non-invaders was lower with 46 percent admitted unconditionally, 18 percent rejected, and 36 percent recommended for further analysis. The decision analysis approach offers an efficient and flexible screening process for proposed, planned introductions.
Because traits associated with invasiveness often differ among taxa, the greatest predictive power may lie within narrow taxonomic groups. Rejmánek and Richardson (1996) examined pine trees (Pinus sp.) for traits predictably associated with invasiveness. They used multivariate statistical techniques to distinguish between invasive and non-invasive pine species on the basis of ecological and life history characteristics. According to their model, invasive pines share characteristics associated with long-distance dispersal and rapid individual and population growth rates (e.g., small seeds, short minimum juvenile period, and a short time interval between large seed crops). The results appeal to our ecological sensibilities because they underscore traits that influence processes of species establishment and spread, as well as formation of persistent, self-sustaining populations. Exceptions to the pattern, such as Pinus pinea, a large seeded, vertebrate-dispersed tree species, also had relatively accessible ecological explanations. Despite its more specialized mode of dispersal, this species has become moderately invasive in regions of the endemic South African fynbos ecosystem where introduced squirrels (Sciurus carolinensis) disperse its seed (Richardson et al., 1990).
In an attempt to generalize from a taxonomically narrow model, Richardson et al. (1990) used the life history criteria that distinguish invasive and non-invasive pine species to categorize Banksia spp. into functional (ecologically similar) groups. Some of these native Western Australian trees and shrubs have begun to arrive in South Africa and share similar suites of attributes with other shrubs invasive in the African fynbos. Functional groups that possess traits associated with invasive pines are identified as high risk, but the authors point out that belonging to a functional group does not necessarily guarantee success or failure of an invasion. As in the pine and squirrel case, idiosyncrasies of the invader or its interactions with the recipient community often play an important role in success or failure. Of course, we might successfully predict some of those idiosyncrasies if we knew the biology of the system well enough. For example, some Banksia identified as high risk are susceptible to a pathogenic fungus already present in South Africa, which may inhibit potential invasions (Richardson et al., 1990). Richardson et al. (1990) took advantage of the large body of knowledge about these two groups generated primarily by their economic importance. Clearly, a sound understanding of the basic biology, ecology, and natural history of any potential invader and recipient community is requisite for making accurate predictions. Sadly, we often lack this seemingly basic information.
Some researchers have tried to gather some of the missing ecological information needed for predictive models. Forcella et al. (1986) used biogeography and empirical ecophysiology combined with multivariate statistics to examine the relationship between species characteristics and invasiveness in Echium, a genus of herbaceous and shrubby plants. Species known to be invasive in Australia had broad native distributions, which may reflect their probability of introduction. They also showed rapid seed germination under a range of conditions, which may reflect their probability of establishment. Their model could serve to evaluate species of Echium of unknown invasiveness,
although the empirical studies needed for each unknown species would require substantial time and funding.
The results of the above predictive models highlight the importance of good ecological, biogeographical, and historical data. These models can best serve managers and policymakers when the information required about the species in question can be obtained relatively easily. For instance, the woody tree species example requires information about the invasiveness of the species in question in other parts of the world. Biogeography of Echium proved useful in the model by Forcella et al. (1986). This type of information is easily amenable to Internet-accessible databases. The further development of such predictive models will depend on the creation, maintenance, and accessibility of large databases of invasive species worldwide.
Experimental plantings of individual species into areas beyond their native range potentially offer an alternative to predictive models and show promise in determining if a particular species will become invasive in a region. Experimental plantings could have application in fields such as agriculture and horticulture, in which planned introductions are the rule. This approach may prove especially powerful if combined with manipulative studies to test the success of introductions over a variety of environmental conditions (Mack, 1996). The drawback of conducting experimental transplants of non-indigenous plants or animals outside of their native range is the risk of escape. The unfortunate consequences of this risk are manifested in the escape of the gypsy moth and the Africanized honey bee, both of which were brought to the Americas to investigate their cultivation for commercial purposes. In making use of experimental introductions, researchers must take extreme precautions to prevent escapes (including the escape of genes from introduced plants, Ellstrand and Hoffman, 1990), and the implementation of these experiments should be regulated by agencies designed to monitor nonindigenous species introductions. Conducted properly, these experiments will be costly, but could provide the desired predictive power for assessing risk of introductions
Predicting the Impacts of Those Invaders
Predicting invasion success is a necessary requirement for predicting impact, but it is not sufficient. Within groups of successful invaders, only a proportion will have a large impact (Williamson, 1996). Defining impact, of course, is essential to evaluating it (Parker et al., 1999). When measuring impact of an invader, the parameters chosen can affect greatly the magnitude of the impact detected. To illustrate this point we refer again to ant invasions, specifically two studies on the invasive ant species Iridomyrmex humilis (Cole et al., 1992) and Solenopsis invicta (Porter and Savignano, 1990); in each case the change in abundance associated with invasion was measured for a large collection of resident invertebrates species. For both studies, the size and even direction of the effect varied greatly among response species for both invaders (see Figure 5-1).
Lessons about predicting impact for introduced species come from the regulatory process for evaluating potential biological control agents, that is, non-indigenous species introduced to control a specific weed or pest (DeBach and Rosen, 1991). Before introduction, researchers screen potential biocontrol agents to see if they are likely to survive and produce viable populations in the new range (i.e., invasion success; Waage and Greathead, 1988). Although these tests are themselves time and resource intensive, a further step would be to evaluate whether sustained populations of the control agent would really inflict a significant demographic impact on the weed or pest. Currently, researchers rarely consider this last step before releasing biological control agents (McEvoy and Coombs, 1999). The process for evaluating or predicting impact seems complex for biological control agents, yet with other non-indigenous species the process becomes even more complex because we have to consider impacts on many more than just one target species, as well as community or ecosystem effects.
Ecologists have made little progress in predicting which invaders will have a big impact (Parker et al., 1999). One early idea proposed by Darwin (1859) and Gause (1934) suggests that ecologically similar species will interfere with each other more strongly than ecologically different species. Therefore, if an introduced species successfully establishes, it might have the biggest impact on species that are most closely related to it if related species play similar ecological roles. A recent meta-analysis (Goodell et al., 2000) combined results from seven studies showing responses of 61 resident species to a variety of insect invasions and found support for the idea that invaders have larger competitive impacts on confamilial resident species than on more distantly related species (see Figure 5-3). The "close relatives" generalization aids us less in predicting which non-indigenous species will have big impacts overall, and more in predicting which species may suffer the largest impacts by an invader. From a practical perspective, this generalization may contribute most to protecting particular suites of rare or sensitive native species from specific introductions.
Another fairly well-accepted idea in invasion biology is that introduced species have the greatest impacts when the invader performs a novel function in the recipient community (Elton, 1958; Simberloff, 1991). The idea is that these novel types of species can dramatically change the ecological context for many species at once. Because the "close relatives" generalization specifically applies to competition between ecologically similar pairs of species, it does not necessarily conflict with the "novel function" generalization. Predators on oceanic islands (Elton, 1958) and nitrogen-fixing shrubs in nutrient-poor systems (Vitousek and Walker, 1989) comprise some classic examples of species that perform novel functions. A related idea suggests that species that dramatically change disturbance regimes (e.g., frequency of fires or floods) impose very large ecosystem-level impacts (Mack and D'Antonio, 1998) and will also have large impacts on all the component species in a system.
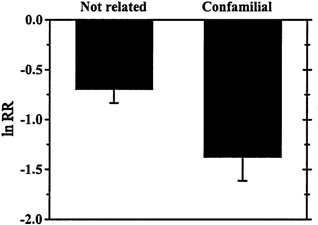
FIGURE 5-3. Mean Effect Sizes (±1 SE) of Insect Invaders on Resident Confamilial Species (n=27) Versus More Distantly Related Species (n=34). Data compiled from seven studies of impact and represented as log response ratios (see Figure 5-1 legend). These data only consider competitive interactions. Note that smaller (more negative) numbers indicate that invaders were associated with larger reductions in population abundances.
These generalizations are interesting and represent an important first step in defining what is a large impact, but they guide prediction only in a very limited context. That is, identifying the potential change in population dynamics of a target host plant, identifying the species ecologically most similar to the invader, or identifying novel functions can be done only in the context of a particular natural community. In fact, many have argued that we should only study invasions as an interaction between the invader and the host community (Drake, 1983; Simberloff, 1986). In light of the great diversity of ecological communities within the boundaries of the United States alone, matching potential introductions with native communities seems daunting indeed.
Before we give up hope on producing generalizations about which species tend to have large impacts, it is important to realize that invasion biology is an extremely young science. So far, there have been almost no attempts to synthesize information because, until recently, we did not have very extensive data on the impacts of different invaders. Scanty published information even for the most infamous non-indigenous species (Hager and McCoy, 1998) serves as a reminder that many basic questions remain unanswered. The poverty of our knowledge of the biology and natural distributions of insects and pathogens adds another dimension of difficulty to predicting which invaders will inflict the most damage.
CASE STUDY 3: THE CRAYFISH PLAGUE AND THE SIGNAL CRAYFISH: LIMITS TO PREDICTION WHEN SPECIES INTERACT SYNERGISTICALLY
Crayfish plague is a disease of crayfish native to the United States. This oomycete pathogen (Aphanomyces astaci) was first reported in Europe in 1860 where it devastated populations of the native Noble crayfish (Astacus astacus) in Northern Italy (Laurent, 1997). The exact mode of introduction is unknown, but crayfish plague most likely crossed the Atlantic in fresh ballast water. The disease subsequently spread throughout the eastern parts of Europe, Russia, and the Baltic states from the 1870s through the 1920s (Alderman, 1996). The mode of spread likely included human transport of crayfish for trade and aquaculture within Europe, transport of infected fishing equipment (Unestam, 1973), and possibly mammalian and bird predators of crayfish that move between isolated bodies of water (Taugbol and Skurdal, 1993).
Native European crayfish play an important ecological role in freshwater ecosystems. Crayfish are thought to keep aquatic plant growth in check and their absence is associated with overgrowth of lakes (Unestam, 1973). At one time, several species also were important in fisheries. All five species of native European crayfish show extreme susceptibility to crayfish plague. As the crayfish plague spread through Europe, reduced population sizes and local extinctions of native species occurred, causing a marked decline in overall crayfish densities. This decline undoubtedly had ecological effects in addition to collapsing the crayfishing industry (Unestam, 1973; Nylund and Westman, 1992).
In the mid-1960s a new phase of commercial interest in crayfish bloomed in Europe, which included the introduction of the Signal crayfish (Pacifasticus leniusculus) native to the western United States. The large size and overall hardiness of the Signal crayfish made it an attractive commercial species. In addition, its natural resistance to the crayfish plague pathogen offered it as an ideal replacement for the native species in the plague-ravaged waters of Europe.
Although importing Signal crayfish has undoubtedly bolstered the commercial crayfish industry, it has brought a double dose of harmful effects on native species. The most damaging impact of the Signal crayfish has been its role as a vector of crayfish plague. These introductions brought with them several new infestations of crayfish plague. Transport of the Signal crayfish among European countries has facilitated spread of the pathogen. DNA evidence has linked recent range extensions of crayfish plague into Spain in 1965 (Diéguez-Uribeondo et al., 1997) and Britain in the 1980s (Lilley et al., 1997) to these new introductions, although other new outbreaks are thought to stem from recent Signal crayfish introductions as well (e.g., Greece in 1982; Lowery and Holdich, 1988).
Increased spread of crayfish plague via Signal crayfish introductions appears to have increased the frequency of infections and also may have prevented the extinction of the pathogen with its dying native hosts in isolated bodies of water. This pattern of extinction has been observed in Ireland, where
alien crayfish have not been imported (Matthews and Reynolds, 1992). Beyond its role as a disease vector, Signal crayfish also directly affects aquatic organisms in the areas where it has been introduced. It is larger and more aggressive than its European counterparts and can have a larger impact on its competitors and prey. These attributes have reduced densities of native fish, as well as further reduced native crayfish densities. For example, Signal crayfish both eats and competes for shelter with native British fishes: the stone loach and the bullhead. Together these effects cause a negative relationship between crayfish density and the density of these stream fish (Guan and Wiles, 1996). In a Swedish lake, Signal crayfish outcompeted native Noble crayfish for shelter, leading to greater perch predation on the native species (Söderbäck, 1994).
The evidence suggests that the crayfish plague and the introduced Signal crayfish have synergistic impacts. This phenomenon was named "invasional meltdown" in a recent paper by Simberloff and Von Holle (1999). We do not know how generally this pattern applies to invasions, but it certainly represents a type of unexpected effect that could foil attempts to predict impact of an invasive species.
Indirect Effects of Invaders May Limit Predictability
Like the Signal crayfish and its impact on natives through shared pathogens, species can have far-reaching indirect effects mediated through a complex of ecological interactions. For example, a seldom recognized threat of introduced plant diseases comes from alternate hosts (i.e., obligate second host in a pathogen life cycle) or alternative hosts (i.e., additional host to a pathogen of a crop species). GRIN lists 14 plant exports native to the United States in this category (Table 5-1; USDA, 1999). Of particular interest are plants of potential horticultural value that serve as alternate hosts for heteroecious rust fungi that attack other economically important plants. In such cases, the rust fungi must pass alternately through two host species to complete their life cycle. Introduction of the alternate host could potentially introduce the pathogen as a hitchhiker. Even if the pathogen is not introduced on the alternate host, the presence of the alternate host creates the opportunity for disease development should the pathogen be introduced independently at some time in the future. If strong indirect effects of invaders are common, efforts to predict the impact of invasive species will be particularly difficult (Simberloff and Stiling, 1996b).
Assessing the Degree of Uncertainty
Because biological systems are complex and never fully understood, predictions of risk will always carry with them some uncertainty. As shown by the above examples, indirect effects can be extremely difficult to forecast a priori without detailed information about both the organism and the recipient ecosystem. We believe that certain types of invasions are more likely than others to have indirect effects, or highly unpredictable effects, and that explicitly
identifying this degree of uncertainty forms an important step in risk assessment. We do not suggest that more predictable invasions necessarily will have smaller impacts, only that the impacts will be easier to project and possibly quantify.
Organisms with the highest degree of predictability in terms of impact include highly host-specific pathogens or pests in agroecosystems. For host-specific, seedborne pathogens, the introduced pathogens will not spread beyond the field planted with the seeds; thus their impacts represent a predictable quality control issue with minimal economic or ecological importance. Similarly, we also expect to have reasonably high predictive power for impacts of host-specific pests and pathogens that cause epidemics in previously unaffected geographic areas. Many problematic agricultural invasions fall into this category, such as zonate leaf spot of sorghum (caused by the fungus Gloeocercospora sorghi), which arrived in Venezuela on seed sent by the USDA for experimental purposes and subsequently became widespread (Ciccarone, 1949). In cases such as these, as well as for releases of biological control agents, the final impact on a host can vary in different areas and under different ecological conditions. In addition, as we have discussed, it is difficult to eliminate the possibility of a future switch in host use. Nevertheless, their impact should follow more predictable patterns than that of pests, pathogens, or other introduced organisms with multiple hosts.
Of intermediate predictability are impacts of host-specific pests or pathogens (or parasites or predators) whose host species is a dominant species or "keystone species" (Power et al., 1996) in the invaded natural ecosystems. The Chestnut blight pathogen (Cryphonectria parasitica), introduced to the United States on European logs, led the American chestnut to the brink of extinction and changed the dominant tree over millions of hectares of forest (Anagnostakis, 1987; von Broembsen, 1989; Jarosz and Davelos, 1995). Also of intermediate predictability are impacts of species that attack not just one but rather a fairly restricted suite of hosts, such as biological control agents with undesirable effects on nontarget species (Louda et al., 1997; Louda, 1998). We may not predict their impacts perfectly, but with good basic information about the likelihood of alternate host use, we should make reasonable assessments of the risks.
Among introduced species that likely affect many resident species, our best chance of predicting impacts lies in those invaders with restricted habitat use. For example, many introduced organisms will persist only in highly disturbed agroecosystems. Similarly, we might anticipate fairly easily the impact of invasive aquatic plants such as the water hyacinth (Eichhornia crassipes) because of their restricted habitat use and consequently restricted set of interacting species (Schmitz et al., 1997).
In contrast, the species with the most difficult impacts to assess, and therefore those with the biggest uncertainty, are species that can invade a variety of natural ecosystems and that interact with many different native species. The oomycete pathogen Phytophthora cinnamomi has had a devastating impact on the eucalyptus forests in Western Australia, where the pathogen attacked more than a third of the species present in the forest (Shearer and Dillon, 1995),
causing a wholesale reshuffling of the ecological interactions within that plant community. Opportunistic predators such as feral house cats eat small mammals, songbirds, reptiles, and insects in proportion to their availability in the environment (Pearre and Maass, 1998). Where introduced or domesticated animals are abundant, they comprise the bulk of the cats' diet (Langham, 1990), but in other areas cats may be important predators of endemic animals, such as the endemic lizard, Urosaurus auriculatus, on Socorro Island, Mexico (Arnaud et al., 1993). The highly variable impact of an organism like the house cat, then, will hinge on characteristics of the particular area into which it is introduced.
SETTING PRIORITIES FOR MANAGEMENT OF INVASIVE SPECIES
At this time, predicting the impacts of invasive species seems a lofty longterm goal, toward which we have made little progress. Although we should not be paralyzed by the apparent complexity of predicting invasions, perhaps a more realistic and optimistic short-term goal of invasion ecology lies in analyzing impacts of currently widespread invaders for broad taxonomic, trophic, or geographic patterns. Such a synthesis of empirical work may tell us something about how we should manage already widespread invasive species. For instance, do certain sorts of habitats suffer more damage from invaders than others, or do invasive predators tend to have stronger effects on recipient communities than invasive herbivores? The answers to these types of questions could eventually focus our efforts on the types of invaders or types of invaded communities that need the most immediate attention.
Meta-analysis, a technique gaining popularity in ecology (Gurevitch and Hedges, 1993), eventually may prove useful for synthesizing data gathered on impacts of invaders. This statistical technique takes advantage of multiple studies that address a similar question, in this case, the measured effect of the introduction of an invasive species into a system (Wonham et al., 2000). The strength of meta-analysis lies in its ability to combine many disparate studies to gain insight into very large-scale questions that would be beyond the scope of any individual research program. A meta-analysis on the impacts of invaders can address questions at a level beyond the interaction between an insect and its specific host plant, or between a species and a congeneric competitor. Therefore, it should be helpful in providing insight at the level of national policy regarding the risk of species introductions.
One of the frustrations of constructing a meta-analysis using the currently available data is that little concordance exists among studies in how results are communicated (Gurevitch et al., 1992), let alone how impact is measured (Wonham et al., 2000). At the very minimum, authors need to report basics, such as the number of replicates used in experiments, the unit of replication, and measures of variance around summary statistics. As we have alluded to above, the diversity of measures of impact make this task more difficult because the impact of an invader can depend on how it is quantified. A concerted effort among those researchers seriously studying the impact of an invasive species to
employ a standard set of measures in their studies could benefit efforts to compare results from different studies and different systems.
For ecologists studying biological invasions, comparability among measures of impact allows the exploration of predictions about species interactions or the way that communities and ecosystems are structured. Comparability among measures has more than academic appeal, however. Those in the position to make decisions regarding regulation, control, or allocation of funding to solve problems generated by invasive species must often decide which problems to solve now and which can be passed over. It is hard to imagine how these decisions are made in the absence of information regarding the effects of the invaders in question. Ideally, we would like to rank invaders by their impact and choose the highest ranked cases as priority for management efforts. Recent work by Williamson (1998) explored correlations among measures of the impact of introduced weeds in Britain; he found strong concordance among some measures, but not others We may also obtain valuable predictions about the comparability among measures of impact from community models (Parker et al., 1999). Modeling, although a complement rather than a replacement for empirical work, has the advantage of being an efficient, low-cost technique for making unlimited numbers of comparisons and is not subject to the same logistical constraints as empirical studies.
CONCLUSIONS
In the United States, as throughout the world, our perception of sanitary and phytosanitary risk of species introductions and invasions has expanded from concern about agriculture and human health to concern about natural ecosystems and communities. As a result of this shift, regulatory bodies now must strive to incorporate appropriate levels of protection for natural systems, as well as agriculture and human health, in the development of new policy. Key to understanding the risk that invasive species pose to natural environments is understanding what types of ecological effects current invaders have on natural ecosystems. We have stressed that ecological impacts can take many forms and that sometimes they not only conflict with more anthropocentric impacts, but also with each other. Because some introductions and invasions by agricultural pests have been relatively well documented and well studied, they provide sound information about the biology of invaders, invaded systems, and the mechanisms of interaction between them from which to launch needed investigations into ecological impacts of invaders. Often these investigations of ecological impact follow earlier studies of economic or agricultural impact of the same invader, as was the case for the mosquito fish. We find it interesting that common problems plague efforts to quantify and predict the risk of invaders in both agricultural and ecological contexts: a poor understanding of the basic biology, ecology, and taxonomy of invasive, or potentially invasive species (especially for insects, pathogens, and marine invertebrates); inconsistency in data collection and reporting; and lack of complete, accessible databases of
invaders. These problems existed in the nineteenth century when phylloxera jeopardized our chances of tasting a fine Bordeaux claret, and they still exist today as new pests, such as Asian long-horn beetles, monopolize media on environmental problems. Today, these deficiencies are particularly crippling because the rate of introductions has greatly accelerated due to increased human traffic (Lövel, 1997; Cohen and Carlton, 1998).
Several approaches may help both ecologists and policymakers make some sense of the complexity of ecological impacts. Focusing on vectors can help to define suites of invaders with similar biology and impacts and to increase our efficiency in evaluating their risk. In regulation, vectors may provide an efficient point of attack, as opposed to a species-by-species approach. Our ability to predict the establishment of certain types of invaders shows some promise and may be especially useful in the context of intentional introductions. As of yet, we are unable to predict which successful invasions may have the biggest impacts. Nevertheless, in the short term, it makes sense for managers, policymakers, and ecologists to work together in prioritizing control and prevention of current invaders by the impact they have on natural systems. Meanwhile, the difficult task of predicting impacts of potentially invasive organisms should continue through attempts to synthesize broad patterns of invader impacts (e.g., with meta-analysis), community modeling, and, of course, continued empirical research on as many systems as possible.
ACKNOWLEDGEMENTS
We are grateful for helpful comments and careful editing by John Hunter, Dan Simberloff, and anonymous reviewers. Some of the ideas presented here were developed in conjunction with the Invasions Working Group (1998–1999) that was supported by the National Center for Ecological Analysis and Synthesis, a center funded by the National Science Foundation (NSF) grant DEB-94-21535, the University of California at Santa Barbara, and the state of California. The completion of this paper also benefited from NSF grant DEB-98-01274 to K. Goodell, and NSF grant DEB-98-08501 to I. M. Parker, and NSF grant DEB-98-06517 to G. S. Gilbert. In addition, K. Goodell was supported by NSF grant DEB-97-07330 to J. D. Thomson during the preparation of this manuscript.
REFERENCES
Alderman, D.J. 1996. Geographical spread of bacterial and fungal diseases of crustaceans. Revue Scientifique et Technique International Office of Epizootics 15:603–632.
Anagnostakis, S.L. 1987. Chestnut Blight: the classical problem of an introduced pathogen. Mycologia 79(1):21–37.
AQIS (Australian Quarantine Inspection Service). 1999. PDI Database. Australian Department of Agriculture, Fisheries and Forestry, Canberra, Australia.
Arnaud, G., A. Rodriguez, A. Ortega-Rubio, and S. Alvarez-Cadenas. 1993. Predation by cats on the unique endemic lizard of Socorro Island (Urosaurus auriculatus), Revillagigedo, Mexico. Ohio Journal of Science 93:101–104.
Bergelson, J., and M. Crawley. 1989. Can we expect mathematical models to guide biological control programs? A comment based on case studies of weed control. Comments on Theoretical Biology 1:197–216.
Brown, L.R., and P.B. Moyle. 1991. Changes in habitat and microhabitat partitioning with an assemblage of stream fishes in response to predation by Sacramento squawfish (Ptychocheilus grandis). Canadian Journal of Fisheries and Aquatic Sciences 48:849–856.
Buchanan, G.A. 1987. The distribution of grape phylloxera, Daktulosphaira vitifolii (Fitch) in central and north-eastern Victoria. Australian Journal of Experimental Agriculture 27:591–595.
Carlton, J.T., and J.B. Gellar. 1993. Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82.
Carman, J.G., and J.D. Brotherson. 1982. Comparisons of sites infested and not infested with saltcedar (Tamarix pentandra) and Russian olive (Eleagnus angustifolia). Weed Science 30:360–364.
Ciccarone, A. 1949. Zonate leaf spot of sorghum in Venezuela. Phytopathology 39:760–761.
Cohen, A.N., and J.T. Carlton. 1998. Accelerating invasion rate in a highly invaded estuary. Science 279:555–558.
Cole, F.R., A.C. Medeiros, L.L. Loope, and W.W. Zuehlke. 1992. Effects of the Argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology 73:1313–1322.
Constanza, R., R. d'Arge, R. de Groot, S. Farber, M. Grasso, B. Hannon, K. Limburg, S. Naeem, R. O'Neill, J. Paruelo, R.G. Raskins, P. Sutton, and M. van den Belt. 1997. The value of the world's ecosystem services and natural capital. Nature 387:253–260.
Cowling, E.B. 1978. Agricultural practices that favor epidemics. In J.G. Horsefall and E.B. Cowling, eds., Plant Disease: An Advanced Treatise, Vol. II. New York: Academic Press.
Craig, G.B.J. 1993. The diaspora of the Asian tiger mosquito. Pp. 101–120 in B. N. McKnight, ed., Biological Pollution: The Control and Impact of Invasive Exotic Species. Indianapolis: Indiana Academy of Sciences.
Daily, G.C., ed. 1997. Nature's Services. Washington, D.C.: Island Press.
D'Antonio, C.M., and B.E. Mahall. 1991. Root profiles and competition between the invasive, exotic perennial, Carpobrotus edulia and two native shrub species in California [USA] coastal scrub . American Journal of Botany 78:885–894.
Darwin C. 1859. On the Origins of Species by Means of Natural Selection, 1st ed. London: John Murray.
DeBach, P., and D. Rosen. 1991. Biological Control by Natural Enemies, 2nd ed. Cambridge, U.K.: Cambridge University Press.
Desdames, C. 1984. Victor Pulliat, viticulteur du beaujolais, vainqueur du phylloxera. Historia 454:77–78.
Diéguez-Uribeondo, J., C. Termiño, and J.L. Múzquiz. 1997. The crayfish plague fungus (Aphanomyces astaci) in Spain. Bulletin Français de la Pêche et de la Piciculture 347:753–763.
Dombeck, M. 1996. Noxious and invasive weeds: should we have a national policy for a national problem? Proceedings of the Western Society of Weed Science 49:5–9.
Drake, J.A. 1983. Invasibility in Lotka-Volterra interaction webs. In D. D'Angelis, W.M. Post, and G. Siguhara, eds., Current Trends in Food Web Theory. Oak Ridge, Tenn.: Oak Ridge National Laboratory.
Ebert, D. 1998. Evolution-experimental evolution of parasites. Science 282:1432–1435.
Echelle, A.A., and P.J. Conner. 1989. Rapid, geographically extensive genetic introgression after secondary contact between two pupfish species (Cyprinodon, Cyprinodontidae). Evolution 43:717–727.
Ellstrand, N.C., and C.A. Hoffman. 1990. Hybridization as an avenue of escape for engineered genes—strategies for risk reduction. Bioscience 40:438–442.
Elton, C.S. 1958. Ecology of Invasions by Plants and Animals., London: Metheun.
Ewald, P.W. 1994. Evolution of Infectious Diseases. Oxford, U.K.: Oxford University Press.
Forcella, F., J.T. Wood, and S.P. Dillon. 1986. Characteristics distinguishing invasive weeds within Echium (Bugloss). Weed Research 26:351–364.
Fritts, T.H. 1999. A summary of documented arrivals of Brown Tree snakes (and sightings on Saipan likely to represent Brown Tree snakes) to islands and the continental United States. U.S. Geological Survey, Patuxent Wildlife Research Center. Available online at http://www.pwrc.nbs.gov/btdisp2.htm.
Fritts, T.H., and G.H. Rodda. 1998. The role of introduced species in the degradation of island ecosystems: a case history of Guam. Annual Review of Ecology and Systematics 29:113–140.
Gause, G.F. 1934. The Struggle for Existence., Baltimore, Md: Williams & Wilkins (reprinted in 1964 by Hafner, New York).
Gentle, C.B., and J.A. Duggin. 1997. Allelopathy as a competitive strategy in persistent thickets of Lantana camara L. in three Australian forest communities. Plant Ecology 132:85–95.
Gerberich, J.B. 1946. An annotated bibliography of papers relating to the control of mosquitoes by the use of fish. The American Midland Naturalist 36:87–131.
Goodell, K. 1998. Impacts of introduced honeybees on native solitary bees. Bulletin of the Ecological Society of America Abstracts: 62.
Goodell, K., M. Wonham, B. Von Holle, and I.M. Parker. 2000. Trophic and taxonomic patterns of impact of invasive species: a meta-analysis. Bulletin of the Ecological Society of America Abstracts. In press.
Granett, J., P. Timper, and L.A. Lider. 1985. Grape phylloxera (Daktulosphaira vitifoliae) (Homoptera:Phylloxeridae) biotypes in California. Journal of Economic Entomology 78:1463–1467.
Guan, R.-Z., and P.R. Wiles. 1996. Ecological impact of introduced crayfish on benthic fishes in a British lowland river. Conservation Biology 11:641–647.
Gurevitch, J., and L.V. Hedges. 1993. Meta-analysis: combining the results of independent experiments. Pp. 378–398 in S. M. Scheiner and J. Gurevitch, eds., Design and Analysis of Ecological Experiments. New York: Chapman & Hall.
Gurevitch, J., L.L. Morrow, A. Wallace, and J.S. Walsh. 1992. A meta-analysis of competition in field experiments. American Naturalist 140:539–572.
Haack, R.A., K.R. Law, V.C. Mastro, H.S. Ossenbruggen, and B.J. Raimo. 1997. New York's battle with the Asian long-horned beetle. Journal of Forestry 95(12):11–15.
Hager, H.A., and K.D. McCoy. 1998. The implications of accepting untested hypotheses: a review of the effects of purple loostrife (Lythrum salicaria) in North America. Biodiversity and Conservation 7:1069–1079.
Hedges, L.V. 1996. Statistical considerations. Pp. 29–38 In H. Cooper and L. V. Hedges, eds., The Handbook of Research Synthesis. New York: Russell Sage Foundation.
Hedrick, R.P., M. El-Matbouli, M.A. Adkison, and E. MacConnell. 1998. Whirling Disease: reemergence among wild trout. Immunological Reviews 166:365–376.
Hoffman, G.L. 1970. Intercontinental and transcontinental dissemination and transfaunation of fish parasites with emphasis on whirling disease (Myxosoma cerebralis). Pp. 69–81 in Diseases of Fish and Shellfish, S.F. Snieszko, ed. Washington, D.C.: American Fisheries Society.
Holland, E.A. 1933. An experimental control of malaria in New Ireland by distribution of Gambusia affinis. Transactions of the Royal Society of Tropical Medicine and Hygiene 26:529–538.
Howard, H.H. 1922. An indigenous fish used in combating malaria. Nation's Health 4:65-69, 139–143.
Howe, E., C. Howe, R. Lim, and M. Burchett. 1997. Impact of the introduced poeciliid Gambusia holbrooki (Girard, 1859) on the growth and reproduction of Pseudomugil signifer (Kner, 1865) in Australia. Marine and Freshwater Research 48:425–434.
Hurlburt, S.H., J. Zelder, and D. Fairbanks. 1972. Ecosystem alteration by mosquitofish (Gambusia affinis) predation. Science 175:639–641.
Jarosz, A.M., and A.L. Davelos. 1995. Tansley Review No. 81: Effects of disease in wild plant populations and the evolution of pathogen aggressiveness. The New Phytologist 129:834–841.
Kato, M.; A. Shibata, T. Yasui, and H. Nagamasu. 1999. Impact of introduced honeybees, Apis mellifera upon native bee communities in the Bonin (Ogasawara) Islands. Res. Popul. Ecol. 41:217–228.
King, P.D., and G.A. Buchanan. 1986. The dispersal of phylloxera crawlers and spread of phylloxera infestations in New Zealand and Australian vineyards. American Journal of Ecology and Viticulture 37:26–33.
Krumholz, L.A. 1948. Reproduction in the western mosquito fish Gambusia affinis affinis (Baird and Girand) and its use in mosquito control. Ecological Monographs 18:1–43.
Langham, N.P.E. 1990. The diet of feral cats (Felis catus) on Hawke's Bay farmland, New Zealand. New Zealand Journal of Zoology 17:243–256.
Laurent, P.J. 1997. Introductions d'ecrevisses en France et dans le monde, historique et conséquences. Bulletin Français de la Pêche et de la Piciculture 344/345:345–356.
Lilley, J.H., L. Cerenius, and K. Söderhäll. 1997. RAPD evidence for the origin of crayfish plague outbreaks in Britain. Aquaculture 157:181–185.
Lloyd, L. 1989. The ecological implications of Gambusia holbrooki with Australian native fishes. Pp. 94–97 in D. A. Pollard, ed., Introduced and Translocated Fishes and their Ecological Effects. Canberra: Australian Government Publishing Service.
Lloyd, L., A.H. Arthington, and D.A. Milton. 1986. The mosquito fish--a valuable mosquito-control agent or a pest? Pp. 5–25 in R. L. Kitching, ed., The Ecology of Exotic Animals and Plants: Some Australian Case Histories. New York: John Wiley & Sons.
Locke, A., D.M. Reid, H.C. VanLeeuwen, W.G. Sprules, J.T. Carlton. 1993. Ballast water exchange as a means of controlling dispersal of fresh-water organisms by ships. Canadian Journal of Fisheries and Aquatic Sciences 50(10):2086–2093.
Louda, S.M. 1998. Population growth of Rhinocyllus cinicus (Coleoptera: Curculionidae) on two species of native thistles in prairie. Environmental Entomology 27:834–841.
Louda, S.M., D. Kendall, J. Conner, and D. Simberloff. 1997. Ecological effects of an insect introduced for the biological control of weeds. Science 277:1088–1090.
Lövel, G. 1997. Global change through invasion. Nature 388:627–628.
Lowery, R.S., and D.M. Holdich. 1988. Pacifasticus leniusculus in North America and Europe, with details of the distribution of introduced and native crayfish species in Europe. Pp. 283–308 in D.M. Holdich and R.S. Lowery, eds., Freshwater Crayfish: Biology, Management and Exploitation. London: Croom Helm Ltd.
Lukacs, P. 1996. How America saved Europe's vineyards. American Heritage 47:89.
Macisaac, H.J. 1996. Potential abiotic and biotic impacts of zebra mussels on the island waters of North America. American Zoologist 36:287–299.
Mack, M.C., and C.M. D'Antonio. 1998. Impacts of biological invasions on disturbance regimes. Trends in Ecology and Evolution 13:195–198.
Mack, R.N. 1996. Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biological Conservation 78:107–121.
Matthews, M., and J.D. Reynolds. 1992. Ecological impact of crayfish plague in Ireland. Hydrobiologia 234:1–6.
McEvoy, P.B., and E.M. Coombs. 1999. A parsimonious approach to biological control of plant invaders. Ecological Applications 9:387–401.
McKay, R.J. 1984. Introductions of exotic fishes in Australia. Pp. 177–199 in W.R. Courtenay, Jr. and J. Stauffer Jr., eds., Distribution, Biology, and Management of Exotic Fishes. Baltimore, Md: Johns Hopkins University Press.
McMillan, M., and D. Wilcove. 1994. Gone but not forgotten: why have species protected by the Endangered Species Act become extinct? Endangered Species Update 11:5–6.
Mills, E.L., J.H. Leach, J.T. Carlton, and C.L. Secor. 1993. Exotic species in the Great Lakes: a history of biotic crisis and anthropogenic introductions. Journal of Great Lakes Research 19:1–54.
Morgan, L.A., and W.A. Buttemer. 1996. Predation by the non-native fish Gambusia holbrooki on small Littoria aurea and L. dentata tadpoles. Australian Zoologist 30:143–149.
Morton, L.T. 1985. Winegrowing in Eastern America., Ithaca, N.Y.: Cornell University Press.
Neill, W. 1983. The tamarix invasion of desert riparian areas. Educational Bulletin of the Desert Protective Council, Vol. 83–84. Spring Valley, Calif: Educational Foundation of the Desert Protective Council.
Nylund, V., and K. Westman. 1992. Crayfish diseases and their control in Finland. Finnish Fisheries Research 14:107–118.
Oestreicher, A. 1996. La crisis filoxérica en España (estudio comparativo sobre las consecuencias socio-económicas de la filoxera en algunas regiones vitivinícolas Españolas). Hispania 1/2:587–622.
O'Neill, C.R. 1996. Economic Impact of Zebra Mussels: the 1995 National Zebra Mussel Information Clearinghouse Study., Ithaca, N.Y.: Cornell Cooperative Extension.
Palm, M.E. 1999. Mycology and world trade: a review from the front line. Mycologia 91:1–12.
Parker, I.M., D. Simberloff, W.M. Lonsdale, K. Goodell, M. Wonham, P.M. Kareiva, M.H. Williamson, B. Von Holle, P.B. Moyle, J.E. Byers, and L. Goldwasser. 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions 1(1):3–19.
Paton, D.C. 1997. Honeybees Apis mellifera and the disruption of plant-pollinator systems in Australia. Victorian Naturalist 114:23–29.
Pearre, S., and R. Maass. 1998. Trend in the prey size-based trophic niches of feral and house cats Felis catus L. Mammal Review 28:125–139.
Perrins, J., M. Williamson, and A. Fitter. 1992. A survey of differing views of weed classification: implications for regulation of introductions. Biological Conservation 60:47–56.
Porter, S.D., and D.A. Savignano. 1990. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 71:2095–2106.
Pouget, R. 1990. Histoire de la Lutte Contre le Phylloxera de la Vigne en France. Versailles: Institut Nacional de la Recherche Agronomique.
Power, M.E., D. Tilman, J.A. Estes, B.A. Menge, W.J. Bond, L.S. Mills, G. Daily, J.C. Castilla, J. Lubchenco, and R.T. Paine. 1996. Challenges in the quest for keystones: identifying keystone species is difficult--but essential to understanding how loss of species will affect ecosystems. BioScience 46:609–620.
Reichard, S.H., and C.W. Hamilton. 1997. Predicting invasions of woody plants introduced into North America. Conservation Biology 11:193–203.
Rejmánek, M., and D.M. Richardson. 1996. What attributes make some plant species more invasive? Ecology 77:1655–1661.
Rhymer, J.M., and D. Simberloff. 1996. Extinction by hybridization and introgression. Annual Review of Ecology and Systematics 27:83–109.
Ricciardi, A., and H.J. MacIsaac. 2000. Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends in Ecology and Evolution 15(2):62–65.
Richardson, D.M., R.M. Cowling, and D.C. Le Maitre. 1990. Assessing the risk of invasive species in Pinus and Banksia in South African mountain fynbos. Journal of Vegetation Science 1:629–642.
Robinson, W.S, R. Nowogrodzki, and R.A. Morse. 1989. The value of honeybees as pollinators of U.S. crops. American Bee Journal 129:411–423.
Roubik, D.W. 1982. Ecological impact of Africanized honeybees on native neotropical pollinators. Pp. 233–247 in: Social Insects in the Tropics, Vol. 1., P. Jaisson, ed. Paris, France: Université de Paris.
Ruesink, J.L., I.M. Parker, M.J. Groom, and P.M. Kareiva. 1995. Reducing the risks of nonindigenous species introductions: guilty until proven innocent. BioScience 45:465–477.
Savidge, J.A. 1987. Extinction of an island avifauna by an introduced snake. Ecology 68:660–668.
Schmitz, D.C., D. Simberloff, R.H. Hofstetter, W. Haller, and D. Sutton. 1997. The ecological impact of nonindigenous plants. Pp. 39–61 in D. Simberloff, D.C. Schmitz, and T.C. Brown, eds., Strangers in Paradise: Impact and Management of Nonindigenous Species in Florida., Washington, D.C.: Island Press.
Scott, D.J. 1971. The importance to New Zealand of seedborne infection of Helminthosporium maydis. Plant Disease Reporter 55:966–968.
Shearer, B.L., and M. Dillon. 1995. Susceptibility of plant species in Eucalyptus marginata forests to infection by Phytophthora cinnimomi . Australian Journal of Botany 43:113–134.
Simberloff, D. 1981. Community effects of introduced species. Pp. 53–81 in M.H. Nitecki, ed., Biotic Crises in Ecological and Evolutionary Time. New York: Academic Press.
Simberloff, D. 1986. Introduced insects: a biogeographic and systematic perspective. Pp. 3–26, in: Mooney, H.A. and J.A. Drake, eds. Ecology of Biological Invasions of North America and Hawaii. Springer-Verlag, Berlin.
Simberloff, D. 1991. Keystone species and community effects of biological introductions. In L.R. Ginzburg, ed., Assessing Ecological Risks of Biotechnology. Boston, Mass.: Butterworth-Heinemann.
Simberloff, D., and P. Stiling. 1996a. How risky is biological control? Ecology 77:1965–1974.
Simberloff, D., and P. Stiling. 1996b. Risks of species introduced for biological control. Biological Conservation 78:185–192.
Simberloff, D., and B. Von Holle. 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1(1):21–32.
Söderbäck, B. 1994. Interactions among juveniles of two freshwater crayfish species and a predatory fish. Oecologia 100:229–235.
Stevenson, I. 1980. The diffusion of disaster: the phylloxera outbreak in the département of the Hérault, 1862–1880. Journal of Historical Geography 6:47–63.
Strong, D.R., J.H. Lawton, and T.R.E. Southwood. 1984. Insects on Plants. Cambridge, Mass: Harvard University Press.
Taugbol, T., and J. Skurdal. 1993. Crayfish plague and management strategies in Norway. Biological Conservation 63:75–82.
Thomas, W. 1973. Seed-transmitted squash mosaic virus. New Zealand Journal of Agricultural Research 16:561–567.
Thompson, J.N. 1998. Rapid evolution as an ecological process. Trends in Ecology and Evolution 13:329–332.
Unestam, T. 1973. Significance of diseases on freshwater crayfish. International Symposium on Freshwater Crayfish 1:135–150.
U.S. Coast Guard. 1993. Ballast water management for vessels entering the Great Lakes. Code for Federal Regulations 33-CFR Part 151.1510. Effective May 10, 1993.
U.S. Congress. 1993. Harmful Nonindigenous Species in the United States. Washington D.C.: Office of Technology Assessment.
USDA (U.S. Department of Agriculture). 1991. Pest Risk Assessment of the Importation of Larch from Siberia and the Soviet Far East, Vol. Publ. No. 1495. Washington, D.C.: U.S. Government Printing Office.
USDA (U.S. Department of Agriculture), Agricultural Research Service. 1999. National Genetic Resources Program. Germplasm Resources Information Network (GRIN). Online Database National Germplasm. http://www.ars-grin.gov
Van der Zwet, T., and S.V. Beer. 1992. Fire Blight—Its Nature, Prevention, and Control. A Practical Guide to Integrated Disease Management. USDA Agricultural Information Bulletin No. 631. Washington, D.C.: Government Printing Office.
Van Zyl, D.J. 1984. Phylloxera vastatrix in die kaapkolonie, 1886-1900: voorkoms, verspreiding en ekonomiese. South African Historical Journal 16:26–48.
Vitousek, P.M., and L.R. Walker. 1989. Biological invasions by Myrica faya in Hawaii: Plant demography, nitrogen fixation, ecosystem effects. Ecological Monographs 59:247–265.
Vitousek, P.M., L.R. Walker, L.D. Whiteaker, D. Mueller-Dombios, and P.A. Matson. 1987. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 238:802–804.
von Broembsen, S.L. 1989. Invasions of natural ecosystems by plant pathogens. Pp. 77–83 In J.A. Drake, H.A. Mooney, F. di Castri, R.H. Groves, F.J. Kruger, M. Rejmánek, and M. Williamson, eds., Biological Invasions: A Global Perspective. Scientific Committee on Problems of the Environment (SCOPE) of the International Council of Scientific Unions. New York: John Wiley & Sons.
Waage, J.K., and D.J. Greathead. 1988. Biological control and opportunities. Philosophical Transactions of the Royal Society of London B. 318:111–128.
Webb, C., and J. Joss. 1997. Does predation by the fish Gambusia holbrooki (Atheriniformes: Poeciliidae) contribute to declining frog populations? Australian Zoologist 30:316–324.
Werner, I., and J.T. Hollibaugh. 1993. Comparison of clearance rates and assimilation efficiency for phytoplankton and bacterioplankton. Limnology and Oceanography 38:949–964.
Williamson, M. 1996. Biological Invasions. London: Chapman & Hall.
Williamson, M. 1998. Measuring the impact of plant invaders in Britain. Pp. 57–68 in U. Starfinger, K. Edwards, I. Kowarik, and M. Williamson, eds., Plant Invasions: Ecological Consequences and Human Responses. Leiden, The Netherlands: Backhuys.
Wonham, M., K. Goodell, B. Von Holle, and I.M. Parker. 2000. Ecological and geographic patterns of impact in invaded communities: a meta-analysis. Bulletin of the Ecological Society of America Abstracts. In press.































