2
Background and Development of Electrometallurgical Technology for the Treatment of Spent Nuclear Fuel
INTRODUCTION
The electrometallurgical technique for treatment of DOE spent fuel, and in particular its application to the EBR-II Spent Nuclear Fuel Treatment Demonstration Project conducted by Argonne National Laboratory from June 1996 through June 1999, evolved in large part from ANL’s earlier work on the Advanced Liquid-Metal Reactor/Integral Fast Reactor (ALMR/IFR).1 The process developed was aimed initially at recycling IFR (and perhaps spent oxide fuels from light water reactors) into new IFR fuels, which would contain substantial quantities of uranium, plutonium, other actinides, and long-lived fission products that could then be burned in the IFR.2,3 A liquid cadmium cathode was to be used for separation of the bulk of the plutonium and the other transuranic elements (TRUs) from the bulk of the uranium, which was electrolytically deposited (separated) at a steel cathode.4 With the termination of the ALMR/IFR project, this process, with some modification, served as the basis for a proposal from Argonne in January 1995 for five major task areas: (1) treatment of metallic spent fuels; (2) recovery and treatment of canister and storage basin sludge; (3) treatment of oxide spent fuels; (4) waste treatment processes; and (5) waste form production and qualification.5 This included the use of the electrometallurgical process for the treatment of EBR-II SNF. The proposal was accepted by DOE and was to include treatment of both reactor driver fuel and uranium blanket material. The present committee as part of its task was asked to evaluate the ongoing work on electrometallurgical technology at ANL. Use of the proposed process for
oxide fuels would have required a separate front end step to convert the oxides into metal for use in the electrorefiner.6 This step consisted of reduction of the metal oxides into metal by Li, and electrochemical regeneration of metallic Li from Li2O in molten LiCl. The process has also been considered as a possible technology for disposing of excess Pu from the U.S. stockpile.7
Pyroprocessing, or molten salt electrochemical processing, has been in general use for many years for purification of materials, including plutonium.8 It involves anodization (oxidation) of a metal into a molten salt electrolyte and then reduction at a cathode to yield a more (highly) purified form. The overall process technology is diagrammed in Figure 2.1. Differences between the generic process and the pyroprocess as carried out by ANL are described below. Subsequent chapters provide additional detail.
ELECTROMETALLURGICAL TREATMENT
In the EBR-II demonstration project for the treatment of EBR-II driver and blanket assemblies, 100 driver assemblies, consisting of 410 kg of 60 to 75% highly enriched 235U, and up to 25 blanket assemblies, consisting of 1,200 kg of depleted uranium, were to be treated. (As originally proposed, the EBR-II demonstration project was to have treated 1 metric ton of driver fuel and 16 metric tons of blanket fuel, the latter in a high throughput electrorefiner.9,10 The demonstration project was limited to the size noted as the result of a revised EA.11)
As indicated in Figure 2.1, chopped driver (or blanket) fuel rod elements are placed in a steel anode basket in an electrorefiner that contains a KCl-LiCl molten salt eutectic system at upwards of 500 °C. The driver fuel is highly enriched uranium alloyed with ~10 wt % Zr; the cladding is stainless steel. The blanket fuel is depleted uranium, with stainless steel cladding. Both the driver and the blanket elements are sodium-bonded to the stainless steel. Two electrorefiners, the Mark-IV and Mark-V, were developed by ANL for use in the EBR-II demonstration project. The Mark-IV, used to electrorefine the driver elements from the EBR-II, contains a molten Cd pool—a holdover from the ALMR/IFR development—while the Mark-V, used for the blanket elements, is Cd free. The Cd pool provides a corrosion-resistant barrier to the mild steel vessel and also acts as a neutron absorber to prevent criticality problems that might result from the highly enriched uranium in the driver elements falling to the bottom of the vessel. However, the Cd pool in the Mark-IV electrorefiner is not used as a cathode, and thus no Pu separation is performed. The Mark-V differs markedly from the Mark-IV, in that it is designed to process much larger batches of material, as needed to treat the blanket elements; it is also a high-throughput electrorefiner (HTER). Nevertheless, the fundamentals of the two electrorefiners are very much the same.
An oxidant, either CdCl2, in the case of the Mark-IV, or UCl3, in the case of the Mark-V, is added to the salt prior to initiation of electrolysis. The CdCl2 oxidizes some of the U (and other active metals) from the anode baskets. Upon passage of a constant electrolysis current between the anode baskets and the steel cathode, U, Pu, transuranic elements (TRU), the alkalis and alkaline earth metals, and rare earths are oxidized into the molten salt as U3+, Pu3+, TRU, alkali and alkaline earth and rare-earth cations (Table 2.1). The stainless steel from the cladding, most of the Zr, and the noble metals remain in the anode baskets. The U3+ is reduced to the metal and
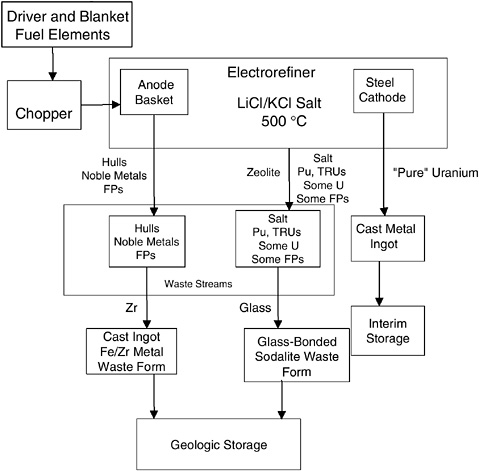
FIGURE 2.1 Overview block diagram of ANL’s electrometallurgical process for DOE spent nuclear fuel. This is the process as originally proposed by ANL for the treatment of spent nuclear fuel.
TABLE 2.1 Free Energies of Formation of SNF Chlorides (-ΔGo in units of kcal/g-eq at 500 °C)
|
Elements That Remain in Salt (very stable chlorides) |
Elements That Can Be Electrotransported Efficiently |
Elements That Remain As Metals (less stable chlorides) |
|||
|
BaCl2 |
87.9 |
CmCl3 |
64.0 |
CdCl2 |
32.3 |
|
CsCl |
87.8 |
PuCl3 |
62.4 |
FeCl2 |
29.2 |
|
RbCl |
87.0 |
AmCl3 |
62.1 |
NbCl5 |
26.7 |
|
KCl |
86.7 |
NpCl3 |
58.1 |
MoCl4 |
16.8 |
|
SrCl2 |
84.7 |
UCl3 |
55.2 |
TcCl4 |
11.0 |
|
LiCl |
82.5 |
ZrCl4 |
46.6 |
RhCl3 |
10.0 |
|
NaCl |
81.2 |
|
|
PdCl2 |
9.0 |
|
CaCl2 |
80.7 |
|
|
RuCl4 |
6.0 |
|
LaCl3 |
70.2 |
|
|
|
|
|
PrCl3 |
69.0 |
|
|
|
|
|
CeCl3 |
68.6 |
|
|
|
|
|
NdCl3 |
67.9 |
|
|
|
|
|
YCl3 |
65.1 |
|
|
|
|
|
SOURCE: Reproduced from National Research Council, An Assessment of Continued R&D into an Electrometallurgical Approach for Treating DOE Spent Nuclear Fuel, National Academy Press, Washington, D.C., 1995, p. 9. Data supplied by James Laidler, Argonne National Laboratory, January 1995. |
|||||
deposited onto the cathode in a reasonably pure state. In essence, the U is electrotransported from the chopped fuel (or blanket) elements in the anode basket to the cathode. The electrolysis is carried out under controlled current conditions such that principally U3+ is reduced at the cathode. For this to happen, a reasonably controlled amount of U3+ must be maintained in the melt. However, the build-up of sodium ion in the LiCl-KCl eutectic ultimately raises the melting point of the initial eutectic salt and requires removal of some of the salt and addition of fresh LiCl-KCl.
After a given period of electrolysis, the U cathode is removed from the Mark-IV electrorefiner, and the adherent molten salt is volatilized off in a vacuum furnace (cathode processor) and returned to the electrorefiner; the U is then cast into an ingot in a high-temperature furnace (casting furnace). (In the Mark-V the U deposits on a cathode from which it is scraped into a collection basket under the electrolysis cell). In the case of the U from the driver fuel, depleted uranium is added as well. Ultimately, the material remaining in the anode basket (the stainless steel hulls and any unoxidized material, the noble metals, and some fission products) is also subjected to treatment to volatilize off the adherent molten salts and is cast into an ingot in the casting furnace, yielding the metal waste form. The baseline metal waste form contains ~15 wt % Zr; this requires that anode basket hulls from the blanket processing have Zr added in the casting furnace. At an appropriate time, the salt containing the Pu, TRU elements, alkalis and alkaline earths, and some fission products is removed from the refiner, mixed with zeolite, heated to adsorb the salt into the zeolite, and then mixed with glass and hot isostatically pressed into a glass-bonded sodalite (GBS), the ceramic waste form.
Table 2.1 lists the free energies of formation per gram-equivalent of a number of the chlorides whose metals are important constituents of the spent nuclear fuel in the EBR-II. The elements are listed in three columns, going from those whose chlorides are most stable to those whose chlorides are least stable. On anodization of the SNF in the anode baskets, the metallic elements in the first two columns are, for the most part (the exception being Zr), converted into their chlorides and dissolve in the LiCl-KCl chloride eutectic. The metallic elements listed in the third column of Table 2.1 are sufficiently noble that they remain in the anode baskets in the metallic state. The electrolysis is carried out under a constant, controlled current. Limits are placed on the cell voltage, and thus, in effect, on both the anode and cathode potentials, such that
-
Zr is essentially not anodized into the eutectic molten salt and
-
Only the U+3 is reduced at the cathode.
If Zr were anodized into the salt, it would be reduced at the cathode. Thus, the TRUs in the first column of Table 2.1 are anodized into the molten salt but are not reduced at the cathode under the carefully controlled electrolysis conditions. As originally proposed by ANL, Pu and the bulk of the TRU elements would have been oxidized from the anode basket and deposited at the liquid Cd cathode in the Mark-IV electrorefiner (Figure 2.2); these elements form intermetallic compounds with Cd and thus could be separated from the uranium. However, the electrolysis as currently carried out (Figure 2.3) does not employ the liquid Cd as a cathode, but only as an anode to recover any U metal that is scraped off or falls from the steel mandrel and dissolves in the liquid Cd pool.
AN ELECTROMETALLURGICAL APPROACH FOR TREATMENT OF EXCESS WEAPONS PLUTONIUM
Several developments in 1995 caused the DOE to request additional evaluations by the committee. One request was to evaluate the scientific and technological issues associated with extending ANL’s electrometallurgical research and development program to handle plutonium, in the event that DOE might pursue an electrometallurgical treatment option for the disposition of excess weapons plutonium (WPu).
Initially, the electrometallurgical process under development at ANL (see Figure 2.2) was designed to separate actinide elements from fission products and direct them into separate output streams. The actinide-fission product separation was to occur during electrorefining as a consequence of the different oxidation-reduction properties of two different cathodes. Relatively pure uranium was to be deposited at a steel cathode, and the transuranic fraction was to be collected at a molten cadmium cathode.
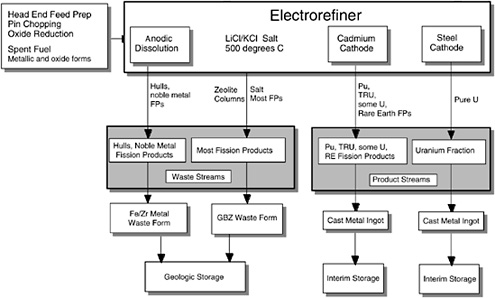
FIGURE 2.2 Original ANL electrometallurgical process scheme. In adaptation of the process for treatment of plutonium, the plutonium would be introduced at the point denoted spent fuel, metallic and oxide forms.
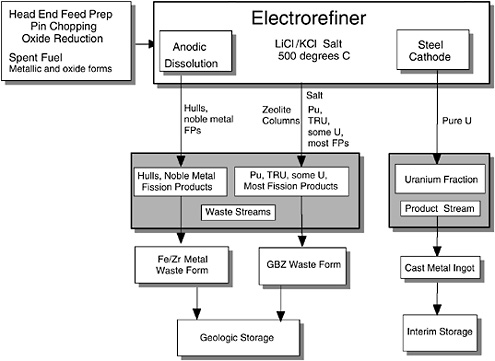
FIGURE 2.3 Current ANL electrometallurgical process scheme, in which the cadmium cathode has been removed. In adaptation of the process for treatment of plutonium, the plutonium would be introduced at the point denoted spent fuel, metallic and oxide forms.
In 1995 ANL modified its flow sheet by eliminating the cadmium cathode, with the consequence that the transuranic elements would remain in the molten salt along with the fission products, ultimately to be incorporated into the waste form derived from zeolite. The co-location of plutonium (and the other transuranics) with highly radioactive fission products suggested that the process might also be employed for disposition of excess weapons plutonium (WPu).
Earlier, in 1994 and 1995, the National Academy of Sciences (NAS) Committee on International Security and Arms Control (CISAC)12 and its associated Panel on Reactor-Related Options for the Disposition of Excess Weapons Plutonium13 had evaluated options for the disposition of plutonium. CISAC introduced the concept of the “spent fuel standard” to describe a “condition in which the WPu has become roughly as difficult to acquire, process, and use in nuclear weapons as it would be to use plutonium in commercial spent fuel for this purpose.”14 Since the amount of spent fuel was growing rapidly, the panel further concluded that “there would be very little security gain from special efforts to completely eliminate the WPu, or render it much less accessible even than the plutonium in spent fuel, unless society were prepared to take the same approach with the global stock of civilian plutonium.”
In its evaluation of EMT (generic pyroprocessing as described above) as an alternative approach for plutonium disposition, the Panel on Reactor-Related Options cited several disadvantages that it felt effectively excluded the electrometallurgical technique as a viable option in the near term. The panel concluded that the “pyroprocessing approach is not competitive with either vitrification in borosilicate glass or the use of mixed uranium-plutonium oxide fuel (MOX) in existing reactors, both of which would be likely to involve lower costs, lower technical uncertainties, and shorter delay.”15
The DOE sought advice on whether the CISAC conclusions remained valid in view of ANL’s subsequent modification of the EMT process (Figure 2.3) to capture the plutonium and other transuranic elements in a zeolite matrix along with most of the fission products. For the possible application of the electrometallurgical treatment technology to surplus fissile material disposition, ANL also proposed the addition of CsCl from capsules at DOE’s Hanford Reservation to create the radiation barrier to meet the “spent fuel standard.”
In its response to DOE, the Committee on Electrometallurgical Techniques for DOE Spent Fuel Treatment concluded that disposition of WPu would involve different feeds for use in SNF processing, raising several concerns with respect to electrometallurgical processing, zeolite loading, and waste form performance. Although ANL had at that time demonstrated an initial program in evaluating zeolite loading, considerable work would be needed to demonstrate this step in a large-scale, continuous operation with fully radioactive loadings on zeolite columns.
Introduction of WPu in the EMT process would significantly increase the demands on the technology to meet the performance requirement for waste forms relative to the use of the waste forms for ultimate disposal of fission products from SNF processing. These considerations led the committee to recommend that “greater priority should be given to the development of a strategy and a relevant test protocol to demonstrate acceptability of waste forms. This activity is of the highest importance relative to all other aspects in the development of the electrometallurgical technique for WPu disposition” (p. 8).16
The committee concurred with the earlier statements of CISAC and its Reactor Panel on excess weapons plutonium: “The existence of this surplus material constitutes a clear and present danger.”17 “The timing of disposition options is crucial to minimizing risks.”18 The urgency of moving ahead with disposing of weapons plutonium made scheduling considerations an important factor in deciding whether or not the electrometallurgical technique would be a practicable and timely solution.
The committee also noted that the potential advantage of the electrometallurgical technique for disposition of excess plutonium would depend on the availability of operational electrometallurgical process equipment. In the absence of such equipment, EMT would not be a viable approach to disposition of plutonium. The committee further concluded that until successful completion of the EBR-II fuel demonstration and treatment of additional spent fuel had been undertaken, it would be imprudent to plan for use of the electrometallurgical technique for disposition of weapons plutonium.
These conclusions led the committee to make two overall recommendations in its third report:19 First, “a decision on the use of the electrometallurgical technique for weapons plutonium disposition cannot be made until the demonstration of this technology shows whether or not this process is viable for treating DOE spent fuels. If a weapons plutonium disposition technology is to be selected for use with weapons pits before the electrometallurgical technology demonstration program is concluded, this committee recommends that the electrometallurgical technique not be included as a candidate technology” (p. 8), and second, “the potential of the electrometallurgical technique as an adjunct for long-term disposition of non-pit excess plutonium remains a possibility, but the technology is still at too early a stage of development to be evaluated relative to disposition alternatives such as glass or MOX [mixed uranium-plutonium oxide fuel]” (p. 8). Any decision to use electrometallurgical technology for WPu disposition remains dependent on establishing acceptance criteria for the waste forms.








