CHAPTER 8
DEEP ECOLOGY MEETS THE DEVELOPING WORLD
JAMES D.NATIONS
Director of Research, Center for Human Ecology, Austin, Texas
There is a movement afoot in the United States that environmentalists call deep ecology (Tobias, 1985). In a nutshell, its basic tenet is that all living things have a right to exist—that human beings have no right to bring other creatures to extinction or to play God by deciding which species serve us and should therefore be allowed to live. Deep ecology rejects the anthropocentric view that humankind lies at the center of all that is worthwhile and that other creatures are valuable only as long as they serve us. Deep ecology says, instead, that all living things have an inherent value—animals, plants, bacteria, viruses—and that animals are no more important than plants and that mammals are no more valuable than insects (Blea, 1986). Deep ecology is similar to many Eastern religions in holding that all living things are sacred. As a conservationist, I am attracted to the core philosophy of deep ecology. Like the Buddhists, and Taoists, and supporters of the Earth First! movement, I also believe that all living things are sacred. When human activities drive one of our fellow species to extinction, I consider that a betrayal of our obligation to protect all life on the only planet we have.
Where I run into trouble with the philosophy of deep ecology is in places like rural Central America or on the agricultural frontier in Ecuadorian Amazonia—places where human beings themselves are living on the edge of life. I have never tried to tell a Latin American farmer that he has no right to burn forest for farmland because the trees and wildlife are as inherently valuable as he and his children are. As an anthropologist and as a father, I am not prepared to take on that job. You could call this the dilemma of deep ecology meeting the developing world.
The dilemma is softened somewhat by the realization that the farmer in the developing world probably appreciates the value of forest and wildlife better than we do in our society of microwave ovens and airplanes and plastic money. The Third-World farmer appreciates his dependence on biological diversity because that
dependence is so highly visible to him. He knows that his life is based on the living organisms that surround him. From the biological diversity that forms his natural environment he gathers edible fruit, wild animals for protein, fiber for clothing and ropes, incense for religious ceremonies, natural insecticides, fish poisons, wood for houses, furniture, and canoes, and medicinal plants that may cure a toothache or a snakebite.
There are indigenous peoples in some parts of the world who have an appreciation for biological diversity that puts our own conservation theorists to shame. I stayed once in southeastern Mexico with a Maya farmer who expressed his view this way:
“The outsiders come into our forest,” he said, “and they cut the mahogany and kill the birds and burn everything. Then they bring in cattle, and the cattle eat the jungle. I think they hate the forest. But I plant my crops and weed them, and I watch the animals, and I watch the forest to know when to plant my corn. As for me, I guard the forest.”
Today, that Maya farmer lives in a small remnant of rain forest surrounded by the fields and cattle pastures of 100,000 immigrant colonists. He is subjected to the development plans of a nation hungry for farmland and foreign exchange. The colonists have been forced by population pressure and the need for land reform to colonize a tropical forest they know nothing about. The social and economic realities of a modern global economy are leading them and their national leaders to destroy the very biological resources their lives are based upon.
The colonists are fine people who are quick to invite you to share their meager meal. But if you want to talk with them about protecting the biological diversity that still surrounds them, be prepared to talk about how it will affect them directly. If you look a frontier farmer in the eye and tell him that he must not clear forest or hunt in a wildlife reserve and that the reason he must not do these things is because you are trying to preserve the planet’s biological diversity, he will very politely perform the cultural equivalent of rolling his eyes and saying, “Sure.”
But he will not believe you. Instead, you should be prepared to demonstrate how he can produce more food and earn more money by protecting the biological resources on his land. The developing world colonist may understand his dependence on biological diversity, but his interest in protecting that diversity lies in how it can improve his life and the lives of his children. Colonists on the agricultural frontier do not have the luxury of debating the finer points of deep ecology.
The same thing can be said for the government planner in the nation where the pioneer farmer lives and the development banker in Washington, D.C. The planner and the banker may appreciate the moral and aesthetic values of biological diversity. They may lament the eradication of wilderness and wildlife. But if you want them to protect a critical area of forest or place their hydroelectric dam outside a protected area, be prepared to talk about the economic value of watersheds, income from tourism, and cost-benefit analysis.
In the developing world, as well as in our overdeveloped world, we are obligated to present economic, utilitarian arguments to preserve the biological diversity that ultimately benefits us all. Deep ecology makes interesting conversation over the seminar table, but it won’t fly on the agricultural frontier of the Third World or in the board rooms of the Inter-American Development Bank.
The day may come when ethical considerations about biological diversity become our most important reason for species conservation. But in the meantime, if we want to hold on to our planet’s biological diversity, we have to speak the vernacular. And the vernacular is utility, economics, and the well-being of individual human beings.
In the 1980s, the question seems to be, “What has biological diversity done for me lately?” The good news is that the answer to that question is, “Plenty, and more than you realize.” Our lives are full of examples of the logic of preserving the plants and animals that we depend upon as a species.
Our food is a good example. Human beings eat a wealth of plants and animals in the home-cooked meals and restaurant dinners that we live on day-to-day. Yet one of the most immediate threats posed by the loss of biodiversity is the shrinkage of plant gene pools available to farmers and agricultural scientists. During the past several decades, we have increased our ability to produce large quantities of food, but we have simultaneously increased our dependence on just a few crops and our dependence on fewer types of those crops. As much as 80% of the world food supply may be based on fewer than two dozen species of plants and animals (CEQ, 1981). We are eroding the genetic diversity of the crops we increasingly depend upon, and we are eradicating the wild ancestors of those crops as we destroy wilderness habitats around the world.
We are dependent on biological diversity in ways less visible than the plants and animals we eat and wear. We also depend on them for raw materials and medicines. We depend on the diversity of plants and animals for industrial fibers, gums, spices, dyes, resins, oils, lumber, cellulose, and wood biomass. We chemically screen wild plants in search of new drugs that may be beneficial to humankind. We import millions of dollars worth of medicinal plants into the United States and use them to produce billions of dollars worth of medicines (OTA, 1984).
We use animals in medical research as well, though sometimes with brutal results. We import tens of thousands of primates for drug safety tests and drug production (OTA, 1984). We use Texas armadillos in research on leprosy. When human activities threaten the survival of these animals and their wild habitats, they threaten human welfare as well.
At the same time, we have to acknowledge that we will never be able to demonstrate an immediate, utilitarian reason for preserving every species on Earth. Some of them may have no use for humankind beyond being part of the great mystery. But who will tell us which species are unimportant? Who can tell us which level of extinction will seriously disrupt the web of life that we depend upon as human beings?
Environmental writer Erik Eckholm says that one of the key tasks facing both scientists and governments is to identify and protect the species whose ecological functions are especially important to human societies. And “in the meantime,” Eckholm continues, “prudence dictates giving existing organisms as much benefit of the doubt as possible” (Eckholm, 1978).
One of the important factors in providing those species with the benefit of the doubt they deserve is educating ourselves and our governments’ policy makers about our dependence, as human beings, on biological diversity. That education tends
to emphasize the utilitarian value of species protection. One of the results is that there is a growing, pragmatic ethic among scientists and conservationists. It is an ethic that centers on the realization that our ability to preserve biological diversity depends on our ability to demonstrate the benefits that diversity brings to human beings (Fisher and Myers, 1986).
On one level, these benefits take the form of immediate economic income through activities like wildlife harvesting, tourism, and maintaining agricultural production. On another level, they focus on unfulfilled potential—new crops, new medicines, new industrial products. Taken together, the benefits of biological diversity provide short-term income to individual people and improve the long-term well-being of our species as a whole.
These two levels of benefits work together in the sense that if we hope to see the long-term benefits of biological diversity, we have to focus first—or least simultaneously—on the immediate, short-term benefits to individual people. Few of the wild gene pools—the raw materials for future medicines, food, and fuels—are likely to survive intact in places where people have to struggle simply to provide their basic, daily needs (Wolf, 1985).
One of our long-term goals as a species is to enjoy the uncounted benefits that our planet’s biological diversity can eventually bring us. But in the short term, at a minimum for the next few decades, our basic strategy must concentrate on ensuring that people here and on the frontiers of the developing world receive material incentives that will allow them to prosper by protecting biological diversity rather than by destroying it (Cartwright, 1985). That done, we can return to the ethical and aesthetic arguments of deep ecology with the knowledge that when we look up from our discussion, there will still be biological diversity left to experience and enjoy.
The authors of the three chapters that follow are counted among the most successful and most dedicated of the scientists now working to point out the short-term and long-term benefits of biological diversity—three scientists who are working as quickly as possible to discover the unread books of our planet’s genetic diversity and to translate those discoveries into practical advantages for their fellow human beings.
REFERENCES
Blea, C. 1986. Individualism and ecology. Earth First! Journal 6(6):21, 23.
Cartwright, J. 1985. The politics of preserving natural areas in third world states. Environmentalist 5(3):179–186.
CEQ (Council on Environmental Quality). 1981. The Global 2000 Report to the President, Vol. II. Council on Environmental Quality and the U.S. Department of State, Washington, D.C.
Eckholm, E. 1978. Disappearing Species: The Social Challenge. Worldwatch Paper 22. Worldwatch Institute, Washington, D.C. 38 pp.
Fisher, J., and N.Myers. 1986. What we must do to save wildlife. Int. Wild. 16(3):12–15.
OTA (Office of Technology Assessment). 1984. Technologies to Sustain Tropical Forest Resources. OTA-F-214. Office of Technology Assessment, U.S. Congress, Washington, D.C. 344 pp.
Tobias, M., ed. 1985. Deep Ecology. Avant Books, San Diego, Calif. 285 pp.
Wolf, E.C. 1985. Challenges and priorities in conserving biological diversity. Interciencia 10(5):236–242.
CHAPTER 9
SCREENING PLANTS FOR NEW MEDICINES
NORMAN R.FARNSWORTH
Research Professor of Pharmacognosy, Program for Collaborative Research in the Pharmaceutical Sciences, University of Illinois at Chicago, Chicago, Illinois
The U.S. pharmaceutical industry spent a record $4.1 billion on research and development in 1985, an increase of 11.6% from 1984 (Anonymous, 1986). In the same year, the American consumer purchased in excess of $8 billion in community pharmacies for prescriptions whose active constituents are still extracted from higher plants (Farnsworth and Soejarto, 1985). For the past 25 years, 25% of all prescriptions dispensed from community pharmacies in the United States contained active principles that are still extracted from higher plants, and this percentage has not varied more than 1.0% during that period (Farnsworth and Morris, 1976). Despite these data, not a single pharmaceutical firm in the United States currently has an active research program designed to discover new drugs from higher plants.
THE GLOBAL IMPORTANCE OF PLANT-DERIVED DRUGS
Approximately 119 pure chemical substances extracted from higher plants are used in medicine throughout the world (Farnsworth et al., 1985) (see Table 9–1). At least 46 of these drugs have never been used in the United States. For the most part, the discovery of the drugs stems from knowledge that their extracts are used to treat one or more diseases in humans. The more interesting of the extracts are then subjected to pharmacological and chemical tests to determine the nature of the active components. Therefore, it should be of interest to ascertain just how important plant drugs are throughout the world when used in the form of crude extracts. The World Health Organization estimates that 80% of the people in
TABLE 9–1 Secondary Plant Constituents Used as Drugs Throughout the World, Their Sources and Uses
|
Compound Name |
Therapeutic Category in Medical Science |
Plant Sources |
Plant Uses in Traditional Medicine |
Correlation Between Two Usesa |
|
Acetyldigitoxin |
Cardiotonic |
Digitalis lanata Ehrh. (Grecian foxglove) |
Not used |
Indirect |
|
Adoniside |
Cardiotonic |
Adonis vernalis L. (Pheasant’s eye) |
Heart conditions |
Yes |
|
Aescin |
Antiinflammatory |
Aesculus hippocastanum L. (Horse chestnut) |
Inflammations |
Yes |
|
Aesculetin |
Antidysentery |
Fraxinus rhynchophylla Hance (variety of Fraxinus chinensis Roxb.) |
Dysentery |
Yes |
|
Agrimophol |
Anthelmintic |
Agrimonia eupatoria L. (Common agrimony) |
Anthelmintic |
Yes |
|
Ajmalicine |
Circulatory stimulant |
Rauvolfia serpentina (L.) Benth. ex Kurz (Indian snakeroot) |
Tranquilizer |
Indirect |
|
Allantoinb |
Vulnerary |
Several plants |
Not used |
No |
|
Allyl isothiocyanateb |
Rubefacient |
Brassica nigra (L.) Koch (Black mustard) |
Rubefacient |
Yes |
|
Anabasine |
Skeletal muscle relaxant |
Anabasis aphylla L. (Tumbleweed) |
Not used |
No |
|
Andrographolide |
Antibacterial |
Andrographis paniculata Nees. (Karyat) |
Dysentery |
Yes |
|
Anisodamine |
Anticholinergic |
Anisodus tanguticus (Maxim.) Pascher (Zàng qiè) |
Meningitis symptoms |
Yes |
|
Anisodine |
Anticholinergic |
Anisodus tanguticus (Maxim.) Pascher (Zàng qiè) |
Meningitis symptoms |
Yes |
|
Arecoline |
Anthelmintic |
Areca catechu L. (Betel-nut palm) |
Anthelmintic |
Yes |
|
Asiaticoside |
Vulnerary |
Centella asiatica (L.) Urban (Indian pennywort) |
Vulnerary |
Yes |
|
Atropine |
Anticholinergic |
Atropa belladonna L. (Belladonna) |
Dilate pupil of eye |
Yes |
|
Benzyl benzoateb |
Scabicide |
Several plants |
Not used |
No |
|
Berberine |
Antibacterial |
Berberis vulgaris L. (Barberry) |
Gastric ailments |
Yes |
|
Bergenin |
Antitussive |
Ardisia japonica Thunb. (Japanese ardisia) |
Chronic bronchitis |
Yes |
|
Borneolb |
Antipyretic; analgesic; antiinflammatory |
Several plants |
Not used |
No |
|
Bromelain |
Antiinflammatory; proteolytic |
Ananas comosus (L.) Merrill (Pineapple) |
Not used |
Indirect |
|
Caffeine |
Central nervous system stimulant |
Camellia sinensis (L.) Kuntze (Tea) |
Stimulant |
Yes |
|
Camphor |
Rubefacient |
Cinnamomum camphora (L.) Nees & Eberm. (Camphor tree) |
Not used |
No |
|
(+)-Catechin |
Hemostatic |
Potentilla fragarioides L. (Cinquefoil) |
Hemostatic |
Yes |
|
Chymopapain |
Proteolytic; mucolytic |
Carica papaya L. (Papaya) |
Digestant |
Yes |
|
Cocaine |
Local anesthetic |
Erythroxylum coca Lam. (Coca) |
Appetite suppressant; stimulant |
Yes |
|
Codeine |
Analgesic; antitussive |
Papaver somniferum L. (Opium poppy) |
Analgesic; sedative |
Yes |
|
Colchiceine amide |
Antitumor agent |
Colchicum autumnale L. (Autumn crocus) |
Gout |
No |
|
Colchicine |
Antitumor agent; anti-gout |
Colchicum autumnale L. (Autumn crocus) |
Gout |
Yes |
|
Convallatoxin |
Cardiotonic |
Convallaria majalis L. (Lily-of-the-valley) |
Cardiotonic |
Yes |
|
Curcumin |
Choleretic |
Curcuma longa L. (Turmeric) |
Choleretic |
Yes |
|
Cynarin |
Choleretic |
Cynara scolymus L. (Artichoke) |
Choleretic |
Yes |
|
Danthron (1,8-dihydroxyanthraquinone)b |
Laxative |
Cassia species (Senna) |
Laxative |
Yes |
|
Demecolcine |
Antitumor agent |
Colchicum autumnale L. (Autumn crocus) |
Gout |
No |
|
Deserpidine |
Antihypertensive; tranquilizer |
Rauvolfia tetraphylla L. (Snakeroot) |
Not used |
Indirect |
|
Deslanoside |
Cardiotonic |
Digitalis lanata Ehrh. (Grecian foxglove) |
Not used |
Indirect |
|
Digitalin |
Cardiotonic |
Digitalis purpurea L. (Common foxglove) |
Cardiotonic |
Yes |
|
Digitoxin |
Cardiotonic |
Digitalis purpurea L. (Common foxglove) |
Cardiotonic |
Yes |
|
Digoxin |
Cardiotonic |
Digitalis lanata Ehrh. (Grecian foxglove) |
Not used |
Indirect |
|
Compound Name |
Therapeutic Category in Medical Science |
Plant Sources |
Plant Uses in Traditional Medicine |
Correlation Between Two Usesa |
|
L-Dopab |
Antiparkinsonism |
Mucuna deeringiana (Bort) Merr. (Velvet bean) |
Not used |
No |
|
Emetine |
Amebicide; emetic |
Cephaelis ipecacuanha (Botero) A. Richard (Ipecac) |
Amebicide; emetic |
Yes |
|
Ephedrineb |
Sympathomimetic |
Ephedra sinica Stapf (Ma-Huang) |
Chronic bronchitis |
Yes |
|
Etoposideb |
Antitumor agent |
Podophyllum peltatum L. (May apple) |
Cancer |
Yes |
|
Galanthyamine |
Cholinesterase inhibitor |
Lycoris squamigera Maxim. (Ressurection lily; magic lily) |
Not used |
No |
|
Gitalin |
Cardiotonic |
Digitalis purpurea L. (Common foxglove) |
Cardiotonic |
Yes |
|
Glaucarubin |
Amebicide |
Simaruba glauca DC. (Paradise tree) |
Amebicide |
Yes |
|
Glaucine |
Antitussive |
Glaucium flavum Crantz (Horned poppy, sea poppy) |
Not used |
No |
|
Glaziovine |
Antidepressant |
Ocotea glaziovii Mez (Yellow cinnamon) |
Not used |
No |
|
Glycyrrhizin (Glycyrrhetic acid) |
Sweetener |
Glycyrrhiza glabra L. (Licorice) |
Sweetener |
Yes |
|
Gossypol |
Male contraceptive |
Gossypium species (Cotton) |
Decreased fertility observed |
Yes |
|
Hemsleyadin |
Antibacterial; antipyretic |
Hemsleya amabilis Diels (Luó guō di) |
Dysentery |
Yes |
|
Hesperidin |
Capillary antihemorrhagic |
Citrus species (Citrus, e.g., orange, lemon) |
Not used |
No |
|
Hydrastine |
Hemostatic; astringent |
Hydrastis canadensis L. (Golden seal) |
Astringent |
Yes |
|
Hyoscyamine |
Anticholinergic |
Hyoscyamus niger L. (Henbane) |
Sedative |
Yes |
|
Kainic acid |
Ascaricide |
Digenea simplex (Wulf.) Agardh (Red alga) |
Anthelmintic |
Yes |
|
Kawainb |
Tranquilizer |
Piper methysticum Forst. f. (Kava) |
Euphoriant |
Yes |
|
Khellin |
Bronchodilator |
Ammi visnaga (L.) Lamk. (toothpick plant) |
Asthma |
Yes |
|
Lanatosides A, B, C |
Cardiotonic |
Digitalis lanata Ehrh. (Grecian foxglove) |
Not used |
Indirect |
|
Lobeline |
Respiratory stimulant |
Lobelia inflata L. (Indian tobacco) |
Expectorant |
Yes |
|
Mentholb |
Rubefacient |
Mentha species (Mint, e.g., peppermint, spearmint) |
Carminative |
No |
|
Methyl salicylateb |
Rubefacient |
Gaultheria procumbens L. (Wintergreen) |
Carminative |
No |
|
Monocrotaline |
Antitumor agent (topical) |
Crotalaria spectabilis Roth (Rattlebox) |
Skin cancer |
Yes |
|
Morphine |
Analgesic |
Papaver somniferum L. (Opium poppy) |
Analgesic; sedative |
Yes |
|
Neoandrographolide |
Antibacterial |
Andrographis paniculata Nees (Karyat) |
Dysentery |
Yes |
|
Nicotine |
Insecticide |
Nicotiana tabacum L. (Tobacco) |
Narcotic |
No |
|
Nordihydroguaiaretic acid |
Antioxidant (lard) |
Larrea divaricata Cav. (Creosote bush) |
Antitussive |
No |
|
Noscapine (narcotine) |
Antitussive |
Papaver somniferum L. (Opium poppy) |
Analgesic; sedative |
Yes |
|
Ouabain |
Cardiotonic |
Strophanthus gratus (Hook.) Baill. (Twisted flower) |
Arrow poison |
Indirect |
|
Pachycarpine [(+)-sparteine] |
Oxytocic |
Sophora pachycarpa Schrenk ex C. A. Meyer (Pagoda tree) |
Not used |
No |
|
Palmatine (fibraurine) |
Antipyretic; detoxicant |
Coptis japonica Makino (Goldthread) |
Not used |
No |
|
Papain |
Proteolytic; mucolytic |
Carica papaya L. (Papaya) |
Digestant |
Yes |
|
Papaverineb |
Smooth muscle relaxant |
Papaver somniferum L. (Opium poppy) |
Sedative; analgesic |
No |
|
Phyllodulcin |
Sweetener |
Hydrangea macrophylla (Thunb.) Seringe (Hydrangea) |
Sweetener |
Yes |
|
Physostigmine (eserine) |
Anticholinesterase |
Physostigma venenosum Balf. (Ordeal bean) |
Ordeal poison |
Indirect |
|
Compound Name |
Therapeutic Category in Medical Science |
Plant Sources |
Plant Uses in Traditional Medicine |
Correlation Between Two Usesa |
|
Picrotoxin |
Analeptic |
Anamirta cocculus (L.) Wright & Arn. (Fish berry) |
Fish poison |
Indirect |
|
Pilocarpine |
Parasympathomimetic |
Pilocarpus jaborandi Holmes (Jaborandi) |
Poison |
Indirect |
|
Pinitolb |
Expectorant |
Several plants |
Not used |
No |
|
Podophyllotoxin |
Escharotic |
Podophyllum peltatum L. (May apple) |
Cancer |
Yes |
|
Protoveratrines A & B |
Antihypertensive |
Veratrum album L. (False hellebore) |
Hypertension |
Yes |
|
Pseudoephedrineb |
Bronchodilator |
Ephedra sinica Stapf (Ma-Huang) |
Chronic bronchitis |
Yes |
|
Pseudoephedrine, nor-b |
Bronchodilator |
Ephedra sinica Stapf (Ma-Huang) |
Chronic bronchitis |
Yes |
|
Quinidine |
Antiarrhythmic |
Cinchona ledgeriana Moens ex Trimen (Yellow cinchona) |
Malaria |
No |
|
Quinine |
Antimalarial; antipyretic |
Cinchona ledgeriana Moens ex Trimen (Yellow cinchona) |
Malaria |
Yes |
|
Quisqualic acid |
Anthelmintic |
Quisqualis indica L. (Rangoon creeper) |
Anthelmintic |
Yes |
|
Rescinnamine |
Antihypertensive; tranquilizer |
Rauvolfia serpentina (L.) Benth. ex Kurz (Indian snakeroot) |
Tranquilizer |
Yes |
|
Reserpine |
Antihypertensive; tranquilizer |
Rauvolfia serpentina (L.) Benth. ex Kurz (Indian snakeroot) |
Tranquilizer |
Yes |
|
Rhomitoxin |
Antihypertensive; tranquilizer |
Rhododendron molle G. Don (Yellow azalea) |
Contraindicated in low blood pressure |
Yes |
|
Rorifone |
Antitussive |
Rorippa indica (L.) Hiern (Nasturtium) |
Chronic bronchitis |
Yes |
|
Rotenone |
Piscicide |
Lonchocarpus nicou (Aubl.) DC. (Cubé root) |
Fish poison |
Yes |
|
Rotundine [(+)-tetrahydropalmatine] |
Analgesic; sedative; tranquilizer |
Stephania sinica Diels (Chinese stephania) |
Sedative |
Yes |
|
Rutin |
Capillary antihemorrhagic |
Citrus species (Citrus, e.g., orange, lemon) |
Not used |
No |
|
Salicin |
Analgesic |
Salix alba L. (White willow) |
Analgesic |
Yes |
|
Sanguinarine |
Dental plaque inhibitor |
Sanguinaria canadensis L. (Bloodroot) |
Not used |
No |
|
Santonin |
Ascaricide |
Artemisia maritima L. (Levant wormseed) |
Anthelmintic |
Yes |
|
Scillaren A |
Cardiotonic |
Urginea maritima (L.) Baker (Squill) |
Cardiotonic |
Yes |
|
Scopolamine |
Sedative |
Datura metel L. (Recurved thornapple) |
Sedative |
Yes |
|
Sennosides A & B |
Laxative |
Senna alexandrina Miller (Alexandria senna) |
Laxative |
Yes |
|
Silymarin |
Antihepatotoxic |
Silybum marianum (L.) Gaertn. (St. Mary’s blessed, milk, or holy thistle) |
Liver disorders |
Yes |
|
Sparteine |
Oxytocic |
Cytisus scoparius (L.) Link (Scotch broom) |
Not used |
No |
|
Stevioside |
Sweetener |
Stevia rebaudiana Hemsley (Sweet herb; Caa-hê-hê) |
Sweetener |
Yes |
|
Strychnine |
Central nervous system stimulant |
Strychnos nux-vomica L. (Nux vomica) |
Toxic stimulant |
Yes |
|
Teniposidec |
Antitumor agent |
Podophyllum peltatum L. (May apple) |
Cancer |
Yes |
|
Δ9-Tetrahydrocannabinol |
Antiemetic; decrease ocular tension |
Cannabis sativa L. (Marijuana, hemp) |
Euphoriant |
No |
|
(±)-Tetrahydropalmatine |
Analgesic; sedative; tranquilizer |
Corydalis ambigua Cham. & Schltdl. (Birthwort) |
Sedative |
Yes |
|
Tetrandrine |
Antihypertensive |
Stephania tetrandra S. Moore (Fāng jĩ, turtle twig) |
Not used |
No |
|
Theobromine |
Diuretic; vasodilator |
Theobroma cacao L. (Cocoa, cacao) |
Diuretic |
Yes |
|
Theophylline |
Diuretic; bronchodilator |
Camellia sinensis (L.) Kuntze (Tea) |
Diuretic; stimulant |
Yes |
|
Thymol |
Antifungal (topical) |
Thymus vulgaris L. (Common thyme) |
Not used |
No |
|
Compound Name |
Therapeutic Category in Medical Science |
Plant Sources |
Plant Uses in Traditional Medicine |
Correlation Between Two Usesa |
|
Trichosanthin |
Abortifacient |
Trichosanthes, kirilowii Maxim. (Chinese snake gourd) |
Abortifacient |
Yes |
|
Tubocurarine |
Skeletal muscle relaxant |
Chondodendron tomentosum R. & P. (Curare) |
Arrow poison |
Yes |
|
Valepotriates |
Sedative |
Valeriana officinalis L. (Valerian) |
Sedative |
Yes |
|
Vasicine (peganine) |
Oxytocic |
Adhatoda vasica Nees (Malabar nut) |
Expectorant |
No |
|
Vincamine |
Cerebral stimulant |
Vinca minor L. (Common periwinkle, running myrtle) |
Cardiovascular disorders |
Yes |
|
Vinblastine (vincaleukoblastine) |
Antitumor agent |
Catharanthus roseus (L.) G. Don (Madagascar periwinkle) |
Not used |
No |
|
Vincristine (leurocristine) |
Antitumor agent |
Catharanthus roseus (L.) G. Don (Madagascar periwinkle) |
Not used |
No |
|
Xathotoxin (ammoidin; 8-methoxypsoralen) |
Pigmenting agent |
Ammi majus L. (Bishop’s weed) |
Leukoderma; vitiligo |
Yes |
|
Yohimbine |
Adrenergic blocker; aphrodisiac |
Pausinystalia johimbe (K. Schum.) (Pierre ex Beille) |
Aphrodisiac |
Yes |
|
Yuanhuacine |
Abortifacient |
Daphne genkwa Sieb & Zucc. (Pinyin; Yuán huā) |
Abortifacient |
Yes |
|
Yuanhuadin |
Abortifacient |
Daphne genkwa Sieb. & Zucc. (Pinyin; Yuán huā) |
Abortifacient |
Yes |
|
aYes indicated a positive correlation between the traditional medical use of the plant and the current therapeutic use of the chemical extracted from the plant. No indicated that there is no correlation as indicated previously. bNow also synthesized commercially. cA minor synthetic modification over a natural product. |
||||
developing countries of the world rely on traditional medicine1 for their primary health care needs, and about 85% of traditional medicine involves the use of plant extracts. This means that about 3.5 to 4 billion people in the world rely on plants as sources of drugs (Farnsworth et al., 1985). Specific data in support of these estimates are difficult to find, but the few examples that are available are quite revealing.
THE IMPORTANCE OF HERBAL DRUGS
In Hong Kong
In the small British colony Hong Kong (1981 population, 5,664,000), there were at least 346 independent herbalists and 1,477 herbal shops in 1981 (Kong, 1982); that same year, there were 3,362 registered physicians and 375 registered pharmacies. Chinese herbalist unions in Hong Kong claim to have a membership of about 5,000 (Kong, 1982). It is claimed that Hong Kong is the largest herbal market in the world, importing in excess of $190 million (US) per year (Kong, 1982). About 70% of these herbal products are used locally, and 30% are reexported. They fall into three roughly equal categories: ginseng products, crude plant drugs other than ginseng, and over-the-counter drugs and medicated wines (Kong, 1982). By comparison, about $80 million worth of Western-style medicines were imported into Hong Kong during the same period. Kong (1982) calculated that the average Hong Kong resident spends about $25 (US) per year for Chinese medicines.
In Japan
The system of traditional medicine in Japan, known as Kampo, is an adaptation of Chinese traditional medicine. Kampo formulations are essentially multicomponent mixtures of natural products, primarily plant extracts. In 1976 more than 69 kinds of Kampo formulae were introduced into the National Insurance Scheme in Japan, and this number has doubled since that time. The total expenditure for all types of pharmaceutical products in Japan was approximately $8.3 billion (US) in 1976, whereas only about $12.5 million (US) was spent on Kampo medicines. Thus in that year, Kampo medicines in the Japanese health care system amounted to only about 0.15% of total pharmaceutical expenditures. In 1983, total pharmaceutical expenditures in Japan were valued at about $14.6 billion (US) and those for Kampo medicines increased to about $150 million (US). Hence, in 7 years, expenditures for Kampo medicines in the Japanese health care system increased to about 1% of total pharmaceutical expenditures (Terasawa, 1986).
In a survey of 4,000 Japanese clinicians conducted in 1983, 42.7% of the respondents reported that they used Kampo medicines in their daily practices. As with most systems of traditional medicine, the applications of Kampo are most
successful in the treatment of chronic diseases, most of which are difficult to treat successfully with Western type medicine. Conditions for which traditional medicine is most frequently used include chronic hepatitis, climacteric disorders, common cold, bronchial asthma, high blood pressure, constipation, autonomic insufficiencies, allergic rhinitis, diabetes mellitus, gastritis, headache, and bowel dysfunction (Terasawa, 1986).
In the People’s Republic of China
The People’s Republic of China includes one-fourth of the world’s population. In 1974 I was privileged to visit that country as a member of the Herbal Pharmacology Delegation—the third of nine scientific exchange delegations set up by former President Nixon when he first visited that country. Since then, I have returned to the PRC in 1980 and again in 1985. It is obvious that the system of Chinese traditional medicine, in which the use of plant extracts to treat disease is extremely important, remains today as an important element in providing adequate primary health care for this populous country. Some of the value of Chinese medicine is most likely its use as a placebo, but I for one am convinced that the vast majority of plants used in this system have constituents that produce real therapeutic effects.
THE SEARCH FOR NEW PLANT DRUGS
There is a great deal of interest in and support for the search for new and useful drugs from higher plants in countries such as the People’s Republic of China, Japan, India, and the Federal Republic of Germany. Virtually every country of the world is active in this search to a limited degree. However, in light of its size and resources, the United States must be regarded as an underdeveloped country with regard to productivity and programs designed to study higher plants as sources of new drugs, both in terms of industrial and university-sponsored research.
Estimates of the number of higher plants that have been described on the face of the Earth vary greatly—from about 250,000 to 750,000. How many of these have been studied as a source of new drugs? This is an impossible question to answer for the following reason. The National Cancer Institute in the United States has tested 35,000 species of higher plants for anticancer activity. Many of these have shown reproducible anticancer effects, and the active principles have been extracted from most of these and their structures determined. However, none of these new drugs have yet been found to be safe and effective enough to be used routinely in humans. The question then arises, could any of these 35,000 species of plants contain drugs effective for other disease states, such as arthritis, high blood pressure, acquired immune deficiency syndrome (AIDS), or heart trouble? Of course they could, but they must be subjected to other appropriate tests to determine these effects. In reality, there are only a handful of plants that have been exhaustively studied for their potential value as a source of drugs, i.e., tested for several effects instead of just only one. Thus, it is safe to presume that the entire flora of the world has not been systemically studied to determine if its
constituent species contain potentially useful drugs. This is a sad commentary when one considers that interest in plants as a source of drugs started at the beginning of the nineteenth century and that technology and science have grown dramatically since that time.
As shown in Table 9–1, the 119 plant-derived drugs in use throughout the world today are obtained from less than 90 species of plants (Farnsworth et al., 1985). How many more can be reasonably predicted to occur in the more than 250,000 species of plants on Earth?
Use of the NAPRALERT Data Base
It is possible to present certain types of data showing the relative interest in studying natural products as a source of drugs by means of the NAPRALERT data base that we maintain at the University of Illinois at Chicago (Farnsworth et al., 1981, 1983; Loub et al., 1985). This specialized computer data base of information on natural products was derived from a systematic search of the world literature. Data that can be retrieved from the system include folkloric medicinal claims for plants, the chemical constituents contained in plants (and other living organisms), the pharmacological effects of naturally occurring substances, or the pharmacological effects of crude extracts prepared from plants. More than 80,000 articles have been entered into the data base since 1975, and about 6,000 new articles are added each year. The system contains folkloric, chemical, or pharmacological information on about 25,000 species of higher plants alone.
Pharmacological Interest in Natural Products
To give some idea as to the interest (or lack thereof) in studying the pharmacological effects of natural products, we can cite the following data from NAPRALERT. In 1985, approximately 3,500 new chemical structures from natural sources were reported. Of these, 2,618 were obtained from higher plants, 512 from lower plants (lichens, filamentous fungi, and bacteria), and 372 from other sources (marine organisms, protozoa, arthropods, and chordates) (Table 9–2). A significant 56.6% of the new chemicals obtained from lower plants (primarily antibiotics produced in industrial laboratories) were reported to have been tested for biological effects. About 23.9% of those obtained from marine sources, protozoa, arthropods, and chordates were studied for biological effects, but only 9.5% of the new structures obtained from higher plants were tested for pharmacological effects. The probable reasons for the low, 9.5% figure are that a majority of these discoveries were reported from university laboratories where the interest is mainly on chemistry, where there is less interdisciplinary research (i.e., botanists, chemists, and biologists working in collaboration), and where routine testing services for pharmacological activity are not readily available.
Why is there so little interest and activity in plant-derived drug development in the United States? An attempt will be made to answer this question, but first it is important to describe briefly some of the more fruitful approaches to drug discovery from higher plants.
TABLE 9–2 New Chemical Structures of Natural Origin Reported in 1985a
Approaches to Drug Discovery from Plants
There are many approaches to the search for new biologically active principles in higher plants (Farnsworth and Loub, 1983). One can simply look for new chemical constituents and hope to find a biologist who is willing to test each substance with whatever pharmacological test is available. This is not considered to be a very valid approach. A second approach is simply to collect every readily available plant, prepare extracts, and test each extract for one or more types of pharmacological activity. This random collection, broad screening method is a reasonable approach that eventually should produce useful drugs, but it is contingent on the availability of adequate funding and appropriate predictable bioassay systems. The last major useful drugs to have reached the marketplace based on this approach are the so-called vinca alkaloids, vincristine sulfate (leurocristine) and vinblastine sulfate (vincaleukoblastine). Vincristine is the drug of choice for the treatment of childhood leukemia; vinblastine is a secondary drug for the treatment of Hodgkin’s disease and other neoplasms.
Vincristine was discovered by Gordon H.Svoboda at the Lilly Research Laboratories. In January 1958, Svoboda submitted an extract of the Madagascan periwinkle plant [Catharanthus roseus (L.) G. Don] to a pharmacological screening program at Lilly (Farnsworth, 1982). This was the fortieth plant that he selected for inclusion in the program. Vincristine was marketed in the United States in 1963, less than 5 years after a crude extract of C. roseus was observed to have antitumor activity. In 1985, total domestic and international sales of vincristine
(as Oncovin®) and vinblastine (as Velban®) were approximately $100 million, 88% of which was profit for the company (G.H.Svoboda, personal communication, 1986).
This discovery of new drugs from higher plants is one of the few that has evolved from a random-selection broad pharmacological screening program. For example, in the very expensive research and development effort undertaken by the National Cancer Institute described above, not one useful drug has emerged.
Recently we analyzed information on the 119 known useful plant-derived drugs to determine how many were discovered because of medicinal folkloric information on the plants from which they were isolated. In other words, what correlation, if any, exists between the current medical use of the 119 drugs and the alleged medical uses of the plants from which they were derived? As shown in Table 9–1, 74% of the 119 chemical compounds used as drugs have the same or related use as the plants from which they were derived. This does not mean that 74% of all medical claims for plants are valid, but it surely points out that there is a significance to medicinal folklore that was not previously documented.
Thus, in my opinion, future programs of drug development from higher plants should include a careful evaluation of historical as well as current claims of the effectiveness of plants as drugs from alien cultures. Such information is rapidly disappearing as our own culture and ideas permeate the less developed countries of the world where there remains a heavy dependence on plants as sources of drugs.
LACK OF INTEREST IN NEW DRUG DISCOVERY PROGRAMS FROM PLANTS
Why is there such a reluctance to initiate new programs involving plants as sources of drugs in the United States, where we have the most sophisticated pharmaceutical industry in the world and where expenditures for drug development are staggering? In my conversations with staff from U.S. pharmaceutical companies, the following reasons seem to be consistent:
-
To recover the costs of developing such drugs, solid patent protection must be secured. It is generally believed that natural products cannot be patented with the same degree of assurance as can synthetic compounds. This of course cannot be a valid deterrent, since patent protection for vincristine and vinblastine was sufficiently secure that the Eli Lilly Company had exclusive marketing rights to these substances for the full term of patent protection.
-
Most promising plants seem to be indigenous to developing countries, many of which do not have stable governments and thus cannot provide assurance that there will be a continued supply of the raw material needed to produce the useful drugs. This of course may be true in a strict sense; however, as history shows, it is rare when a useful plant grows only in one isolated developing country. In the course of developing a full program involving plants as a source of raw material, it would be normal logic to immediately seek sources of the useful plant from a large number of geographic areas. Cultivation programs should also be initiated. In the early stages of development of vincristine and vinblastine, the plant source
-
C. roseus was collected from many different countries of the world and was also cultivated in eastern European countries and in the United States.
-
There is reputed to be biological variation from lot to lot of plant drugs, but scientific documentation for this statement is difficult to find. This does not appear to be a problem affecting any of the plant sources required for production of the 119 drugs listed in Table 9–1.
What really seems to be the problem is that most pharmaceutical firms, as well as decision-making offices in government agencies, lack personnel who have a full understanding and appreciation of the potential payoff in this area of research. For example, new programs in drug development are usually initiated by the presentation of a proposal by a research staff member before a group of peers and research administrators. Following is one possible scenario: Dr. E.Z.Greenleaf prepares his arguments for a new drug development program at the ABC Pharmaceutical Corporation in which he proposes to study plants as a source of new drugs. His approach to the program is to examine written medicinal folklore to obtain information on plants allegedly used by primitive peoples for certain specified diseases. He might even be brave enough to suggest that the ABC Pharmaceutical Corporation hire one or two physicians to travel to Africa, Borneo, New Caledonia, or other exotic areas to live with the people for a year or so. During this period, Drs. U. Canduit and I.M.Reliant would observe the witch doctors treating patients and then would make their own diagnoses of each patient and conduct follow-up observations on outcome. When improvement is noted, they would record which plants had been used to treat the patients. These plants would then be collected and sent to the Research Laboratory of the ABC Pharmaceutical Corporation located in Heartbreak, Colorado, for scientific studies. Total cost of such a 5-year program would be less than the cost of a new jet fighter.
The second scientist from the ABC Pharmaceutical Corporation to make a new program presentation is Dr. Adam N.Molecule. He uses a long sequence of chemical equations to illustrate his theory that he can synthesize a series of chemical analogs based on computer analysis of structure-activity relationships in which his theoretical compounds will react favorably with specific receptor sites. He illustrates his plan with a full color videotape presentation of the computerized sequence of events that he hopes will take place at the molecular level. There is nothing left to the imagination. Molecule’s computer produces a flowchart projecting the full costs of each stage of the synthesis at 2-month intervals. Everything is predictable, based on a percentage of projected sales should the end product prove to be a useful drug, and ensuring at least a 75% profit margin.
At the end of the two presentations, management must decide on whether to follow the folkloric line of Dr. E.Z.Greenleaf or the molecular biology-computer graphic-theoretical approach of Dr. Adam N.Molecule. Since Dr. Greenleaf is probably the only person in the room with a background and appreciation for his approach and most of the scientists in attendance are well trained and highly skilled synthetic chemists, biochemists, and molecular biologists, it is not difficult to predict which program will be approved and implemented.
SUMMARY
Higher plants have been described as chemical factories that are capable of synthesizing unlimited numbers of highly complex and unusual chemical substances whose structures could escape the imagination of synthetic chemists forever. Considering that many of these unique gene sources may be lost forever through extinction and that plants have a great potential for producing new drugs of great benefit to mankind, some action should be taken to reverse the current apathy in the United States with respect to this potential.
REFERENCES
Anonymous. 1986. Pharmaceutical R&D Spending by US Industry Hits $4.1 Billion, Setting Record, as do Sales. P. 5 in Chem. Mark. Rep. February 3, 1986.
Farnsworth, N.R. 1982. Rational approaches applicable to the search for and discovery of new drugs from plants. Pp. 27–59 in Memorias del 1er Symposium Latinoamericano y del Caribe de Farmacos Naturales, La Habana, Cuba, 21 al 28 de Junion, 1980. Academia de Ciencias de Cuba y Comisin Nacional de Cuba ante la UNESCO, UNESCO Regional Office, Montevideo, Uruguay.
Farnsworth, N.R., and W.D.Loub. 1983. Information gathering and data bases that are pertinent to the development of plant-derived drugs. Pp. 178–195 in Plants: The Potentials for Extracting Protein, Medicines, and Other Useful Chemicals. Workshop Proceedings. OTA-BP-F-23. U.S. Congress, Office of Technology Assessment, Washington, D.C.
Farnsworth, N.R., and R.W.Morris. 1976. Higher plants—the sleeping giant of drug development. Am. J. Pharm. Educ. 148(Mar.–Apr.):46–52.
Farnsworth, N.R., and J.M.Pezzuto. 1983. Rational approaches to the development of plant-derived drugs. Pp. 35–63 in Proceedings of the Second National Symposium on the Pharmacology and Chemistry of Natural Products, Joao Pessoa, Brazil, November 3–5, 1983. Paraiba University, Joao Pessoa, Brazil.
Farnsworth, N.R., and D.D.Soejarto. 1985. Potential consequences of plant extinction in the United States on the current and future availability of prescription drugs. Econ. Bot. 39(3):231–240.
Farnsworth, N.R., W.D.Loub, D.D.Soejarto, G.A.Cordell, M.L.Quinn, and K.Mulholland. 1981. Computer services for research on plants for fertility regulation. Korean J. Pharmacogn. 12:98–110.
Farnsworth, N.R., O.Akerele, A.S.Bingel, D.D.Soejarto, and Z.-G.Guo. 1985. Medicinal plants in therapy. Bull. WHO 63:965–981.
Kong, Y.-C. 1982. The control of Chinese medicines—a scientific overview. Yearb. Pharm. Soc. Hong Kong 1982:47–51.
Loub, W.D., N.R.Farnsworth, D.D.Soejarto, and M.L.Quinn. 1985. NAPRALERT: Computer handling of natural product research data. J. Chem. Inf. Comput. Sci. 25:99–103.
Terasawa, K. 1986. The present situation of education and research work on Traditional Chinese Medicine in Japan. Presentation at the International Symposium on Integration of Traditional and Modern Medicine, Taichung, Republic of China, May 22, 1986.
CHAPTER 10
SERENDIPITY IN THE EXPLORATION OF BIODIVERSITY
What Good Are Weedy Tomatoes?
HUGH H.ILTIS
Director, University of Wisconsin Herbarium, Madison, Wisconsin
For someone studying natural history, life can never be long enough
(Miriam Rothschild, British entomologist, television interview on Nova, 1986).
Biodiversity is out there in nature, everywhere you look, an enormous cornucopia of wild and cultivated species, diverse in form and function, with beauty and usefulness beyond the wildest imagination. But first we have to find these plants and animals and describe them before we can hope to understand what each of them means in the great biological—and human—scheme of things.
The classification of biodiversity is the job of taxonomists who, born as packrats and inspired by a compulsion to explore and collect the world’s biological riches, will risk life and limb to solve the great puzzles of biogeography, ethnobotany, and evolution.
But for taxonomists these are paradoxical times. Were Alexander von Humboldt or Charles Darwin (two of our godfathers) alive today, they would marvel at our knowledge and technology, and the relative ease with which we can now explore the most inaccessible places, enabling us to bring back biological treasures even from the darkest jungles of Africa and the greenest hells of Amazonia. That’s the good news.
The bad news is that the same roads that allow us to drive jeeps into the rain forests or up the highest tundras of the Andes, and the very technologies that land helicopters on the mist-shrouded mesas of Mt. Roraima in Venezuela’s Lost World, also bring in a flood of land-hungry squatters, ambitious cattle ranchers, and greedy
corporations, often under the auspices of international promoters of development such as the world’s multilateral development banks. All are recklessly destructive of nature and in an orgy of environmental brutality, clearcut the forests, burn the trees, and plow up the land to grow more food or graze more cattle, even before any scientist has had a chance to find out what lives there. In the name of growth, progress, and development, and with a colossal self-confidence, we humans are now messing up even the last wild lands and damming the last wild rivers, oblivious of the irreplaceable biological treasures that are being destroyed.
In short, our twentieth-century civilization still pretty much reflects the shortsighted seventeenth-century pragmatism of Cotton Mather (1663–1728), the witch hunter of Salem, Massachusetts, who proclaimed: “What is not useful, is vicious.” But who is to say what is useful and what is not, especially about species not yet discovered that, unknown and unstudied, fall prey to plow or cow? And who can predict the value of a monkey, a butterfly, or a flower? Or of intact ecosystems, to which we are inseparably linked, whether we acknowledge this or not?
Mankind depends on plants for food, fiber, drugs—and a livable world. But more than that, our children will want nature to experience while growing up—to explore, love, and enjoy its beauty and diversity. Corn and cows, concrete and cars are not enough to sustain and empower a human psyche that until only a few generations ago lived in daily contact with a variety of plants and animals, a psyche that, winnowed and sifted by natural selection, is genetically programmed to respond positively to nature and its patterns (Iltis et al., 1970; Wilson, 1984). By destroying so much of the natural environment, we humans are now destroying crucial parts of our own psychological as well as physical habitat. For those in the know, it is a gloomy picture indeed.
Like most taxonomists, I am by nature a born collector, first of stamps, then of plants—a botanical adventurer excited by the prospects of finding species no one has ever seen before. Unlike some botanists, I have never had a compelling interest in increasing the world’s food supply. After all, is it not now obvious that the world hunger problem cannot be solved by growing more food, but only by growing fewer people, and that more food will always result in still more people, who in turn will devastate ever more nature, inevitably exterminate ever more plant and animal species, and in the long run, make life for themselves and their children ever more difficult? It is then quite ironical that by hunting for the evolutionary origin of potatoes and maize, I was involved in the discovery of two new species of agricultural significance, both splendid examples of wild biodiversity directly useful to humans.
THE DISCOVERY OF A NEW TOMATO
In December 1962, Don Ugent (now a botany professor at Southern Illinois University in Carbondale) and I were collecting wild and weedy potatoes and associated plants in the Peruvian Andes for the University of Wisconsin Herbarium at Madison (Iltis, 1982).
For a month we had studied potato populations in the mountains east of Lima to determine how the modern cultigen might have evolved. In fact, its exact origin
is still an ethnobotanical mystery (Ugent, 1970). Was it in Peru, Bolivia, or Chile that people first collected the bitter wild tubers and selected edible potatoes?
We traveled to the Peruvian city of Cuzco by way of a gravelly back road, which crossed the Andes east of Pisco and then traversed above Puquio the vast and unending altiplano, an arid tundra-like grassland called the puna. A cold 3,500 to 4,500 meters above sea level, and therefore often higher than Pike’s Peak, the puna is covered with a fantastic collection of cushion plants, including white fuzzy cacti that look like sleeping sheep, all adapted to withstand grazing by domesticated llamas and alpacas and the rare, wild vicuñas.
On the eastern slope of these gigantic mountains, within sight of snow-covered peaks, this so-called road (at times not much more than a footpath) dipped dizzily down from 4,260 meters to 1,800 meters at Abancay in only 25 kilometers, then crossed the Apurimac River below Curahuasi (where once stood The Bridge of San Luis Rey of Thornton Wilder’s novel), and eventually wound its way up again to the altiplano and on to Cuzco, the capital both of Inca kings and of wild and cultivated potato diversity.
A rest in Abancay was welcome after freezing nights tenting above timberline and being miserable with siroche (mountain or altitude sickness). On December 21, the early morning was spent packing some 1,500 dried herbarium specimens of the 296 different species collected the week before and getting ready for the push to reach Cuzco in time for Christmas and a hot bath. Then off we drove to the Hacienca Casinchihua in the Rio Pachachaca valley to look for a rare, wild potato species cited by Correll (1962) in a monograph published a short time before.
It was the beginning of the rainy season, and this deep valley was now bursting into bloom. Most memorable were pendent, 4-inch-long, orange trumpet flowers of a Mutisia, a gorgeous daisy named by Linnaeus’s son for the eighteenth-century Spanish botanist Don José Celestino Mutis.
Above the hacienda, our jeep was soon stopped by a landslide. There was nothing to do but hike along that old Inca road until high above the river we stopped to eat our lunch of avocados, oranges, cheese, and small, boiled Peruvian potatoes, yellow and rich in protein.
All around us was a floristic wonderland, full of rare and beautiful plants. In fact, these arid inter-Andean valleys are veritable biogeographic islands, each with many endemic (i.e., unique) species and isolated from other such valleys by wet tropical forests below and cold Andean tundras above, a situation favoring speciation and, hence, biodiversity. In a nearby gully, iridescent green and blue hummingbirds hovered and flitted about, piercing with their bills the cardinal-red flower tubes of a bushy sage, Salvia oppositifolia, one of several hundred (!) Andean species of this prolific genus.
So here we spent the rest of the day, always collecting five specimens of each plant—one each for the University of Wisconsin, the University of San Marcos in Lima, and the U.S. National Herbarium in Washington, D.C., and one or two for botanists specializing in that particular plant family, who would tell us exactly what we had collected. This must be done, for there are no accurate, usable books on the 30,000 species of Peruvian plants, a flora so rich it staggers the imagination.
(The northeastern United States is much larger than Peru but has only about 5,000 species of plants; yet here we have many up-to-date botanical compendia called floras by which plant species may be identified.)
Presently we noticed a tangled, yellow-flowered, sticky-leaved, ratty-looking wild tomato, not much different from the weedy tomatillo (Lycopersicon peruvianum) so widespread in Peru. Nevertheless, we took immediate notice of it, for tomatoes belong to the potato family and this was a relative of a cultivated species. And wild or weedy tomatoes must always be taken seriously!
Not only did we collect herbarium specimens of this weedy tomato, describing it in our notebook under the serial number 832 (i.e., the 832nd collection of this expedition), but we also gathered two dozen of its green-and-white striped berries, which are smaller than cherries. We smashed the berries between newspapers to dry their seeds, and weeks later, we mailed them together with other tomato seed samples to Charles Rick, tomato geneticist at the University of California, Davis, who, we had heard, would want to grow them in his experimental plots.
This is an old story, of course, and illustrates the network nature of the study of natural history. Taxonomists do this sort of thing for each other all the time, unasked and as a matter of course, whether they know each other personally or not. “I will collect seeds for you of your special plant group, if you will collect seeds for me of mine.”
Back at the University of Wisconsin, a thank-you note from Prof. Rick informed us that our No. 832 was most unusual and perhaps useful in plant breeding. Not until 1976, however, after 14 years of research, did Rick (1976) publish this as a new species, naming it Lycopersicon chmielewskii in honor of the late Tadeusz Chmielewski, a Polish tomato geneticist and Rick’s associate. Another of our tomato collections, obtained below Curahuasi, he described as yet another new and local species, Lycopersicon parviflorum. That certainly made us feel good: to have been involved in the discovery of two new species in this small though important genus. Previously, taxonomists recognized only seven species of wild tomato, and now there were nine! Our story could have ended here, of course, and still be a good one, what with us showing off the type of specimens housed in the University of Wisconsin Herbarium to interested students and telling tall expedition tales of haciendas and vicuñas, potatoes and tomatoes. But there was more to come.
HOW MUCH IS A WILD TOMATO WORTH?
In July 1980, a letter from Dr. Rick told the following story. When 17 years before, he had received our seeds numbered 832, he crossed their progeny with a commercial tomato variety to improve the latter’s characteristics. After nearly 10 (!) generations of back-crossing the first-generation (F1) hybrids, and with subsequent selection, Rick was able to produce several new tomato strains with larger fruit and a marked increase in fruit pigmentation. But most importantly, they had greatly increased the content of soluble solids, mainly fructose, glucose, and other sugars, all attributes of prime importance to the tomato industry. While the usual type of tomato contains between 4.5 and 6.2% soluble solids, the genes from our
No. 832 increased the content in the new hybrids to 6.6 to 8.6%. In a paper published in 1974, Rick summarized this work as follows:
An attempt was made to combine the high soluble-solids content of ripe fruits of the small, green-fruited Lycopersicon [chmielewskii] with the horticulturally desirable characteristics of a standard L. esculentum cultivar. By backcrossing from the former to the latter, and by subsequent pedigree selection, pure-breeding lines in which soluble-solids content was elevated to 7–7.5 percent—at least 2 percentage points above that of the recurrent parent—were synthesized (Rick, 1974, Abstract).
Parts of Rick’s letter to us are worth reproducing:
In our assays of [Iltis and Ugent No. 832 from Hacienda Casinchihua], we discovered that its fruits have a very high sugar content [to 11.5%] as assayed by refractometer readings. Since this species is readily hybridized with the cultivated tomato and the crosses yield relatively fertile hybrids, we initiated a program to introgress the genes responsible for high soluble solids from No. 832 to horticultural lines of [tomatoes. Thus] it was possible to transfer at least some of the genes for this character to produce large, red-fruited lines with significantly elevated sugar content. These derived lines have been widely distributed to tomato workers, some of whom have been exploiting them with the aim of improving sugar content of new tomato cultivars.
The concentration of soluble solids in raw tomatoes is a matter of great economic importance to the processing industry. A number of years ago an expert estimated that each 0.5% increase in soluble solids would be worth about a million dollars. Greatly improved flavor is another benefit. I thought you might be interested in this use of your valuable collection and want to thank you again for your trouble and foresight in sharing it with us.
To make a long story short, and adjusting for inflation to 1987 U.S. dollars, the value to the tomato industry of the genes found in collection No. 832 could, if widely incorporated, be worth about $8 million dollars a year or, to bask in the glory of larger numbers, about $80 million over a decade!
The yearlong expedition (including the jeep) and 3 years of follow-up research cost the National Science Foundation only $21,000, a small amount in the great scheme of things, and yielded more than 1,000 different numbered herbarium collections, a total of 8,000 specimens now scattered in many major herbaria of the world. In other words, each of our collection numbers cost the U.S. government on the average only $21 (1962 value), including Iltis and Ugent 832 and any of the other species previously unknown to science.
In fact, perhaps the most significant values stemming from our expedition are yet to come, possibly from the high-protein potatoes we collected or from the hundreds of bits and pieces of botanical information we passed along to colleagues, graduate students, and others. But as in the case of our tomato, collected in 1962, commercially utilized a decade later, and not described as a new species until 1976, the practical value of an organism can often not be recognized except after years of work, even for plant groups with known economic use that have been well studied by teams of specialists (which does not apply to most taxonomic groups because of lack of funds to support such large efforts).
For this reason, among others, I have no patience with the phony requests of developers, economists, and humanitarians who want us biologists to “prove” with
hard evidence, right here and now, the “value” of biodiversity and the “harm” of tropical deforestation. Rather, it should be for them, the sponsors of reckless destruction, to prove to the world that a plant or animal species, or an exotic ecosystem, is not useful and not ecologically significant before being permitted by society to destroy it. And such proof, of course, neither they nor anybody else can offer!
The benefits of even the most unimportant research are often quite unexpected. Who could have predicted that these tiny, slimy seeds of a useless, ugly weed, stuck to an old newspaper and costing no more than a few dollars and 30 minutes of our time, might enrich the U.S. economy by tens of millions of dollars—in other words (using 1986 dollars in the calculation), a potential $8 million-a-year gain on a one-time $42 investment? Not bad for a government agency (the National Science Foundation) sometimes maligned for supporting such old-fashioned research. Pretty good for a band of field biologists not even wearing white lab coats.
Finally, this discovery is not exceptional. As Rick pointed out, “the literature is replete with examples of the transfer from the [wild species] to acceptable [tomato] cultivars of desirable new traits—mostly resistance to diseases and other pests—often of enormous economic value” (Rick, 1974, p.493). Sweet indeed are the uses of biodiversity!
A NEW SPECIES OF WILD MAIZE
The sensational story of Zea diploperennis, a wild species of maize (teosinte) recently discovered in the Mexican state of Jalisco, has often been told (Iltis et al., 1979; Vietmeyer, 1979). We cannot here even begin to outline the unlikely events that led a Mexican undergraduate to find this, the fourth species of the genus Zea, which includes maize (Zea mays)—the world’s third most important crop with the enormous 1986 global value of more than $50 billion. But although our new tomato was collected through pure serendipity, the diploperennial teosinte owes its discovery to many people, all of whom shared a consuming interest in the Mexican flora and in the mysterious origin of maize (Iltis, 1983).
This story has a very happy ending. Because of the spectacular beauty of the 10,000-foot (2,886-meter)-high Sierra de Manantlán and the potential agricultural value of this rare, perennial, virus-resistant teosinte, which grows here on only 6 hectares and nowhere else on Earth, Mexican botanists and others have worked tirelessly, and successfully, to establish the Las Joyas biological field station of the Universidad de Guadalajara and a 135,000-hectare (350,000-acre) Reserva Biosfera de la Sierra de Manantlán under State of Jalisco and UNESCO Man in the Biosphere (MAB) auspices. The dedication of this enormous reserve on March 5, 1987, by Mexico’s President Miguel de la Madrid H. was very gratifying, because the new reserve will now protect the whole, vast, intact biodiversity of that mountain chain, not only the world’s only wild populations of this teosinte but also the parrots and jaguars, the orchids and ocelots, the crested guans and giant magnolias, and 10,000 lesser species. Moreover, it will allow all these organisms, including the flagship species Zea diploperennis, to survive in the very environment to which they are
evolutionarily adapted. In the long run, such in situ (in place) preservation of whole ecosystems in very large nature reserves is really the only effective way these, or any other species, can be assured survival.
THE CONTINUING IMPORTANCE OF BOTANICAL EXPLORATION
The new species of tomato and wild maize are just two examples of valuable plants saved in the very nick of time before their minuscule populations faced extinction. And there are tens of thousands of unknown species yet to be discovered! Biologists, therefore, must insist that the days of exploration are far from over and that the study of biological diversity, and its preservation in situ, is one of their primary scientific responsibilities.
In the final analysis, the necessary investments in nature preserves and in such noncommercial activities as biological expeditions, herbaria, zoological museums, and training of field biologists (especially in the tropics), inexpensive as these are, are far wiser uses of tax dollars than the billions that are so readily spent on space flights or Star Wars. The Moon and the planets will be out there forever, but the Earth’s biological diversity is being exterminated now. It is therefore imperative that we study and carefully preserve nature on this planet now, for this will be our last chance to ensure that biodiversity will survive for future generations. Protection of biodiversity needs to receive top priority in national and international planning. But if nature preservation is to be effective and long-lasting, it must become codified into law and incorporated into ethics and organized religion. Not only biologists and agriculturists, but every thinking citizen, every responsible politician and religious leader, has here an indispensable role.
REFERENCES
Correll, D.S. 1962. The Potato and Its Wild Relative. Texas Research Foundation, Renner, Texas. 606 pp.
Iltis, H.H. 1982. Discovery of No. 832: An essay in defense of the National Science Foundation. Desert Plants 3:175–192.
Iltis, H.H. 1983. From teosinte to maize: The catastrophic sexual transmutation. Science 222:886–894.
Iltis, H.H., O.L.Loucks, and P.Andrews. 1970. Criteria for an optimum human environment. Bull. At. Sci. 26:2–6.
Iltis, H.H., J.F.Doebley, R.Guzmán M., and B.Pazy. 1979. Zea diploperennis (Gramineae): A new teosinte from Mexico. Science 203:186–188.
Rick, C.M. 1974. High soluble-solids content in large-fruited tomato lines derived from a wild green-fruited species. Hilgardia 42(15):492–510.
Rick, C.M. 1976. Genetic and biosystematic studies on two new sibling species of Lycopersicon from interandean Peru. Theor. Appl. Genet. 47:55–68.
Ugent, D. 1970. The potato: What is the botanical origin of this important crop plant, and how did it first become domesticated? Science 170:1161–1166.
Vietmeyer, N.D. 1979. A wild relative may give corn perennial genes. Smithsonian 10:68–79.
Wilson, E.O. 1984. Biophilia, the Human Bond with Other Species. Harvard University Press, Cambridge, Mass. 157 pp.
CHAPTER 11
THE OUTLOOK FOR NEW AGRICULTURAL AND INDUSTRIAL PRODUCTS FROM THE TROPICS
MARK J.PLOTKIN
Director, Plant Conservation, World Wildlife Fund-U.S., Washington, D.C., and Research Associate, Harvard Botanical Museum, Cambridge, Massachusetts
Many of the initial international wildlife conservation efforts focused on attractive species of endangered mammals—the so-called charismatic megafauna. Although a number of these programs have proven to be extremely successful, the modus operandi was clearly not entirely applicable to the conservation of all organisms: “Save the Sedges!” is just not as stirring a battle cry as “Save the Tiger!” We cannot save the pandas, however, unless we save the bamboos on which they feed. Furthermore, human existence is much more dependent on the plant kingdom than on animals. Plants are indeed the roots of life.
Because of the sheer diversity of plant life—especially in the tropics—many conservationists in the recent past have had some difficulty trying to decide where to begin. Faced with an area like the Amazon, home to tens of thousands of species of plants, many of which have yet to be discovered by modern scientists, it is clearly impractical to evaluate the conservation status and potential utility of each species on an individual basis. Consequently, there has been a perceptible shift in emphasis toward plants that are either useful or potentially useful to people. The concept of protecting a plant because it shows promise for aiding human well-being seems to have a much wider appeal than preserving a species for purely aesthetic or academic purposes.
Conservationists generally divide useful plants into three categories: medicinal, agricultural, and industrial. Of these three groupings, medicinal plants tend to attract the most attention from the media. There is no denying the appeal of the modern ethnobotanist’s ventures into the jungle to work with witch doctors to find healing herbs. Due to a variety of factors—factors that are expected to change in
the near future—there has been relatively little development of new wonder drugs from tropical plants during the last decade (Tyler, 1986; see also Farnsworth, Chapter 9 of this volume). The recent history and predicted future for new agriculture and industrial plant products from the tropics have been very positive (Balick, 1985; Schultes, 1979).
AGRICULTURE
The greatest service which can be rendered any country is to add a useful plant to its culture. From Thomas Jefferson, 1821.
A hungry people listen not to reason, nor care for justice, nor are bent by prayers. From Seneca, ca. 60 A.D.
A hungry mob is an angry mob. From Bob Marley, 1979.
Tropical forest plants can be of use to modern agriculture in three different ways: as sources of new crops that can be brought into cultivation; as source material for breeding improved plant varieties; and as sources of new biodegradable pesticides.
NEW CROPS
Only a very small proportion of the world’s plants have ever been used as a food source on a large scale. Of the several thousand species known to be edible, only about 150 have ever become important enough to enter into world commerce (R.E.Schultes, Harvard Botanical Museum, personal communication, 1986). In the movement toward a global economy, there has been a trend to concentrate on fewer and fewer species. Today, less than 20 plant species produce most of the world’s food (Vietmeyer, 1986b). Furthermore, the four major carbohydrate crop species—wheat, corn, rice, and potatoes—feed more people than the next 26 most important crops combined (Witt, 1985).
The obvious place to turn for new crops to reduce our heavy reliance on such a relatively small number of species is the tropics. North America north of Mexico has contributed relatively little to the storehouse of economically important crop plants. If we had to live on plants that originated in the United States, our diet would consist of pecans, sunflower seeds, cranberries, blueberries, grapes, wild rice, pumpkins, squashes, and Jerusalem artichokes. Caufield (1982) estimated that 98% of U.S. crop production is based on species that originated outside our borders. Of our common foodstuffs, corn, rice, potatoes, sweet potatoes, sugar, citrus fruit, bananas, tomatoes, coconuts, peanuts, red pepper, black pepper, nutmeg, mace, pineapples, chocolate, coffee, and vanilla all originated in tropical countries. A typical American breakfast of cornflakes, bananas, sugar, coffee, orange juice, hot chocolate, and hash brown potatoes is based entirely on tropical plant products.
Few people realize how much of our diet today has been determined by exploitation patterns developed when tropical countries were colonies of Europe. In many cases, the advantage that some current crop staples have over other, less-exploited tropical species is the disproportionate amount of research to which they have been subjected. Under the colonial system, only a few key species were chosen for export, and the establishment of a market for these species determined future cultivation
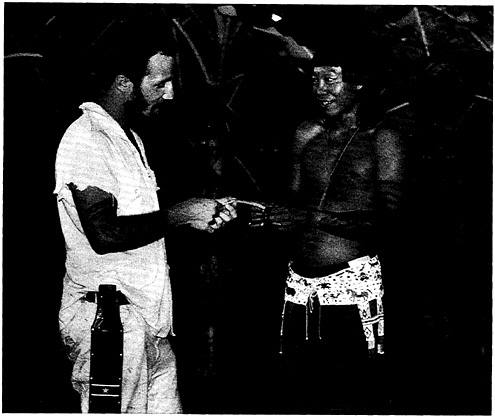
The author collecting medicinal plants with local Indian guide, in southern Suriname.
and research priorities, which excluded lesser-known species. This overreliance on a few species was maintained even after independence, since developing countries had to depend on preexisting markets and technicians trained in temperate countries (NRC, 1975).
Many currently underexploited tropical species will become common sights in the produce sections of our supermarkets during the next decade. Because those species are often best known to aboriginal or peasant peoples, they have often been stigmatized as slave foods in their country of origin. This has impeded the development of these crops, which often tend to be robust, productive, self-reliant, free of indigestible compounds with relatively high nutritive value, and suitable for growing in some sort of agricultural system.
The demand for tropical cuisine continues to grow in this country. The Los Angeles area is said to have more than 200 Thai restaurants, and Mexican fast-food outlets have become a $1.6 billion industry (Vietmeyer, 1986a). Even a short walk down M Street in Washington, D.C., will take you past Chinese, Vietnamese, Thai, Filipino, Mexican, Central American, and South American restaurants. Kiwi fruit from China was not introduced into this country until 1962, yet last year they were purchased by more than 10 million Americans. Furthermore, do-
mestic demographic trends will add to the demand for tropical produce: the U.S. population has increased 17% since 1970, whereas the Hispanic population has risen 87% and the Asian population, 127% (Vietmeyer, 1986a).
The more promising species include the following:
-
The uvilla (Pourouma cecropiaefolia; family Moraceae). The uvilla is a medium-size tree native to the western Amazon. Both harvested from the wild and cultivated by local Indians as a doorstep crop, it yields fruit in only 3 years time. The tasty fruits can be eaten raw or made into a wine (Balick, 1985; Prance, 1982).
-
The lulo (Solanum quitoense; family Solanaceae). The lulo, or naranjilla, is one of the most highly prized fruits in Colombia and Ecuador. It is a shrubby perennial bearing pubescent, yellow-orange fruits. The greenish flesh is made into an exceptionally delicious drink. The lulo has already been introduced in Panama, Costa Rica, and Guatemala, where it is being marketed as a frozen concentrate (Heiser, 1985).
-
The pupunha (Bactris gasipaes; family Palmae). Native to the northwest Amazon, the pupunha, or peach palm, is a 20-meter-tall palm widely cultivated in both South and Central America. Each year this palm can yield up to 13 bunches of fruit, which contains carbohydrates, protein, oil, minerals, and vitamins in nearly perfect proportions for the human diet. Under cultivation, the tree will produce more carbohydrate and protein per hectare than does corn (Balick, 1985; NRC, 1975; Vietmeyer, 1986b).
-
The amaranths (Amaranthus spp.; family Amaranthaceae). The three major species of amaranths (Amaranthus caudatus, A. cruentus, and A. hypochondriachus) are rapidly growing cereal-like plants that have been cultivated in Central and South America since Pre-Columbian times. The ancient Aztecs considered amaranth a sacred plant and consumed cakes made of ground amaranth seeds and human blood. Because of this religious practice, the Spanish severely suppressed the cultivation of this plant. Amaranth seeds have extremely high levels of total protein and of the nutritionally essential amino acid lysine, which is usually lacking in plant protein (NRC, 1984; Vietmeyer, 1986b). Amaranth is currently being marketed in this country as breakfast cereal and is now being sold in many health food stores.
-
The guanabana (Annona muricata; family Annonaceae). The guanabana, or soursop, is a medium-size tree native to tropical America. Throughout the year the tree produces fruit whose delicious white flesh has a unique smell and a texture that can be best described as a sort of fibrous pineapple custard. Already popular in China, Australia, Africa, and the Philippines, guanabana can be eaten raw or made into a delicious drink or yogurt (NRC, 1975).
-
The buriti palm (Mauritia flexuosa; family Palmae). A veritable tree of life to many Amazonian Indians, the buriti palm produces a fruit said to be as rich as citrus in vitamin C content. Its pulp oil is believed to contain as much vitamin A as carrots and spinach. A starch extracted from the pith is used to make bread. An edible palm heart can be extracted from the shoots. The Indians also make wine from its fruit, sap, and inflorescences (NRC, 1975). A strong fiber is obtained from the young leaves, and a useful cork-like material is extracted from the petioles.
-
The wood of the trunk is used in light construction. Furthermore, the buriti palm thrives in Amazonian swamps of little use for intensive agriculture (Schultes, 1979).
IMPROVEMENT OF CROP SPECIES THROUGH CROSS-BREEDING
Relatives of commercial species must continuously be crossbred with these species to improve crop yield, nutritional quality, durability, responsiveness to different soils and climates, and resistance to pests and diseases (IUCN, 1980). Since many of the world’s most important crop species originated in the tropics, we must look to the equatorial regions for wild or semidomesticated relatives of commercial species to maintain or improve our crops.
A barley plant from Ethiopia has already provided a gene that protects a $160-million barley crop in California from the lethal yellow dwarf virus. A wild relative discovered by Iltis in the Peruvian Andes has increased the sugar content of the domestic tomato which has resulted in an increased commercial value estimated at $5 to $8 million per year (Witt, 1985; see also Iltis, Chapter 10 of this volume). In fact, tomatoes are one of the world’s most important crops, yet they could not be grown commercially in the United States without the genes provided by wild relatives (Harlan, 1984). Rice grown in Asia is protected from the four main rice diseases by genes provided by a single wild species from India. In both Africa and India, yields of cassava—one of the most important crops throughout the tropics—have been increased up to 18 times because of the disease resistance provided by genes from wild Brazilian cassava. Disease resistance provided by wild Asian species of sugarcane have saved the sugarcane industry in the southeastern United States from total collapse (Prescott-Allen and Prescott-Allen, 1983). Perennial corn, discovered by Guzmán in Mexico in 1977, has proven to be immune or resistant to the seven major diseases of domesticated corn (Witt, 1985).
Although the use of wild and semidomesticated relatives is already extensive, it will undoubtedly increase in the near future because of the wider availability of these plants and the growing documentation of their potential utility (Frankel, 1983; Prescott-Allen and Prescott-Allen, 1983). Rapid advances in genetic engineering will also provide greater access to certain gene pools, which can now only be taken advantage of with special techniques (Frankel, 1983).
Following are some good examples of the types of plants that may prove useful for future breeding purposes:
-
Coffee (Coffea spp.; family Rubiaceae) is a mainstay of the economy of several tropical countries, yet it is rather susceptible to certain fungal diseases. Although Africa is home to most commercial species (particularly C. arabica from Ethiopia), the island of Madagascar has approximately 50 wild species of Coffea. Some of these species may prove important for commercial breeding not only for their potential resistance to fungal infections but also because they produce beans with little or no caffeine (Guillaumet, 1984; Plotkin et al., 1985).
-
Two wild species of potatoes (Solanum spp.; family Solanaceae) have leaves that produce a sticky substance that traps predatory insects, which subsequently
-
die of starvation. This type of self-defense could conceivably reduce or negate the need for using pesticides on cultivated potatoes (Gibson, 1979; Harlan, 1984).
-
Formerly one of the most important timber trees of western coastal Ecuador, Persea theobromifolia, also called Caryodaphnopsis theobromifolia (family Lauraceae) has been pushed to the very brink of extinction by overexploitation. When it was finally described in 1979, it was found to be a relative of the common avocado (Persea americana) and might one day prove useful as rot-resistant root graft stock for the cultivated species (Gentry and Wettach, 1986).
NATURAL PESTICIDES
Many tropical plants have developed chemical defenses to deter predation by herbivorous animals. Tropical people possess a sophisticated knowledge of these plants, often using them as medicines or poisons. The calabar bean (Physostigma venenosum) was traditionally used as an ordeal poison in West Africa, and studies of the active principle of this species led to the development of methyl carbamate insecticides. World trade in daisy flowers (Chrysanthemum cinerariaefolium), the source of insecticidal pyrethrum extracts, is a multimillion dollar business (Oldfield, 1984). This plant was first discovered because of its use by African tribal peoples to control insect pests.
South American Indians use Lonchocarpus, a forest vine, as a poison to stun fish. Today we import the roots of this plant as a source of rotenone, a biodegradable pesticide. Other plants used by tribal people as fish poisons have yet to be evaluated for their potential as pesticides. Plants used to make arrow poisons or curares also bear looking into, since one such species, Chondrodendron tomentosum already provides us with d-tubocurarine—an anesthetic administered during abdominal surgery. Not only do we need to investigate the individual components used in the manufacture of the many different types of curare but we must also study the interactions among different species that are sometimes used together. In the northeast Amazon, the preparation of an arrow poison may involve the mixing of seven different species, and the Indians insist that each plant changes and amplifies the toxicity of the poison.
Yet another category of potentially useful natural pesticides are allelochemicals. These are chemicals produced by plants that inhibit the growth of other plants and of soil microorganisms. Allelochemicals include a number of different types of chemicals and may one day be used directly or serve as models for seminatural or wholly synthetic compounds (Balandrin et al., 1985).
Species that might prove useful as sources of biodegradable pesticides in the future include the following:
-
Piquiá (Caryocar spp.; family Caryocaraceae). One Amazonian species of Caryocar produces a compound that seems to be toxic to the dreaded leaf-cutter ant (Atta spp.). This insect is the scourge of South American agriculture, causing millions of dollars of damage each year.
-
Guaraná (Paullinia cupana; family Sapindaceae). This woody vine is native to central Brazil. It is grown on plantations near Manaus for use in Brazilian soft
-
drinks. Guaraná contains three times as much caffeine as does coffee, and recent tests at Harvard have shown that caffeine and some synthetic analogs can kill or inhibit the growth of mosquitoes and other insects (J.Nathanson, Harvard, personal communication, 1986). Should further testing prove caffeine to be an effective insecticide, guaraná could become a major crop throughout the tropics.
INDUSTRY
Development of native indigenous plants, particularly with reference to tropical and subtropical soils, will be beneficial at a variety of economies of scale. In some instances, their development will be small and amenable to utilization by individual farmers or farming groups. On the other hand, there will be instances where development will be large scale and have international implications. From McKell, 1980.
During the Arab oil embargo of 1973, the U.S. community was faced not only with the loss of a major energy source but also with the loss of its most important raw material for the manufacture of innumerable synthetic products. Few realize how many of our everyday products are made from petroleum and petroleum by-products, such as plastics, fertilizers, lubricants, and adhesives, to name only a few. It has recently been estimated that almost one-fifth of the petroleum used in this country is devoted to industrial nonfuel purposes (White, 1979). Between 1973 and 1976, the annual use of petroleum-based chemicals in the United States was more than 100 billion pounds (Princen, 1977), yet the majority of these substances can now be synthesized from plant products (Wang and Huffman, 1981). These so-called botanochemicals are destined to become increasingly important as raw materials for industry.
Until 1985, the reasons for reducing our dependence on fossil fuels were obvious. At present, the price of oil has dropped sharply, and there are those who believe that the heyday of the OPEC cartel is over. Nonetheless, experts disagree sharply about predictions of future price trends for petroleum. Since oil is a nonrenewable resource, and since the largest reserves lie in one of the most politically unstable regions of the world, we should try to reduce our dependence on petroleum whenever it is economically feasible.
FATS AND OILS
Approximately 3 million tons of vegetable fats and oils are used each year in the manufacture of coatings, lubricants, plasticizers, and many other products (Prescott-Allen and Prescott-Allen, 1982). In the past, industrial usage of these vegetable products has suffered from competition with cheap synthetic petroleum products (Wang and Huffman, 1981), but this trend is expected to change due to the uncertainty about the future of the petroleum market. Between 1973 and 1981, the price of petrochemicals increased more than 700%, whereas that of vegetable oils rose less than 100% (Prescott-Allen and Prescott-Allen, 1982). Even in the industrialized world, commercial demand for oils for use as a food and in industry continues to grow, and demand often exceeds supply (Schultes, 1979).
The supply of edible oils is seriously inadequate to meet human nutrition requirements, especially in underdeveloped tropical regions. However, several tropical forest species have been used by tribal peoples as sources of edible oils for thousands of years. These oils contain vitamins and minerals and are necessary for cooking in areas where butter or lard are either unavailable or in short supply. There has been little attempt to domesticate some of these species, although ambitious efforts are under way in Brazil, Colombia, and Malaysia (M.Balick, New York Botanical Garden, personal communication, 1986). Domestication would increase yield, lower production costs, and reduce or eliminate characteristics that might inhibit harvesting on a commercial scale while providing a steady supply of edible and/or industrial oils. Some of these plants, e.g., bacabá (Oenocarpus bacaba) and patauá (Jessenia bataua), can grow in both the forest and on semiforested plantations and thus seem to be potential crop species of great importance in the tropics. Some of the more promising tropical oil plants include the following:
-
The patauá palm (Jessenia bataua; family Palmae). The patauá palm grows to a height of 20 meters and is found in the lowlands of tropical South America. The oil of the fruit is almost identical to olive oil in its chemical and physical properties, and the biological value of its protein is almost 40% higher than that of soybean protein (Balick, 1985; Balick and Gershoff, 1981).
-
The babassú palm (Orbignya spp.; family Palmae). The South American babassú palm may reach 60 meters in height. A single tree may produce up to a half ton of a fruit that resembles the coconut, although babassú has a higher oil content. This oil can be refined into an edible oil or used to make plastics, detergents, soap, margarine, and shortening. The seedcake is 27% protein and is an excellent fertilizer and animal feed. Its ability to colonize and thrive in deforested areas makes it an ideal species for turning degraded areas into productive lands (Balick, 1985; Schultes, 1979).
-
The vine (Fevillea; family Cucurbitaceae). Seeds of the fruits of these vines have a higher oil content than that of any other dicotyledenous plant. Gentry and Wettach (1986) theorized that if naturally occurring lianas in a rain forest were cut and replaced by Fevillea, a per-acre oil yield comparable to those obtained in the most productive plantations might be obtained without felling a single tree.
FIBERS
Fiber plants are second only to food plants in terms of their usefulness to humans and their influence on the advancement of civilization. Tropical people use plant fibers for housing, clothing, hammocks, nets, baskets, fishing lines, and bowstrings. Even in our industrialized society, we use a wide variety of natural plant fibers: for ropes, brooms, brushes, and baskets. In fact the so-called synthetic fibers now providing much of our clothing are only reconstituted cellulose of plant origin. [Cellulose is produced in far greater quantities by the world’s plants than any other organic compound—up to 3 billion tons a day, according to R.E.Schultes of the Harvard Botanical Museum (personal communication, 1986)]. Several trees in
tropical South America could be exploited for the fiber that they produce; their commercial potential is, at this point, unrealized. Promising species include:
-
The tucúm palm (Astrocaryum tucuma; family Palmae). The tucum palm reaches a height of 20 meters and is native to the western Amazon. Its fiber is considered to be among the finest and most durable of the plant kingdom and is highly valued by Amazonian Indians. Furthermore, the tucúm produces an edible palm heart and a fruit that contains three times more vitamin A than do carrots (Balick, 1985; Schultes, 1977).
-
Rattans (Demoncus spp.; family Palmae). Rattans are climbing palms native to the Asian tropics. Trade in rattan end products amounts to more than $1 billion a year. Unable to afford imported rattan, Peruvian peasants have begun to use Demoncus, a local climbing palm that has proven to be a very satisfactory substitute (A.Gentry, Missouri Botanical Garden, personal communication, 1986).
THE ROLE OF THE ETHNOBOTANIST
Tropical forest peoples are the key to understanding, utilizing, and protecting tropical plant diversity. Virtually every plant mentioned in this paper—not only the lesser-known species like the tucum palm and the buriti but also the well-known ones like corn and chocolate—were first discovered and utilized by indigenous peoples. Although it may come as a surprise to many that modern botanists are learning about useful plants from primitive peoples (the science known as ethnobotany), we are in fact just getting started. A single tribe of Amazonian Indians may use more than 100 different species of plants for medicinal purposes alone, yet very few tribal populations have been subjected to a complete ethnobotanical analysis and the need to do so becomes more urgent with each passing year. As we struggle to protect the dwindling tropical rain forest and find new and useful plant species for the benefit of modern human beings, the people who best understand these forests are dying out. More than 90 different Amazonian tribes are said to have disappeared since the turn of the century (G.Prance, New York Botanical Garden, personal communication, 1986). Through extinction and tribal acculturation, true forest peoples are dying out, and their oral traditions are disappearing with them. Each time a medicine man dies, it is as if a library has burned down.
Conservationists often talk about the problem of disappearing species, but the knowledge of how to use these species is disappearing much faster than the species themselves. In order to collect this information, we need to expand ethnobotanical field research. Organizations like the World Wildlife Fund and the National Geographic Society, together with leading botanical institutes like the Harvard Botanical Museum, the New York Botanical Garden, and the Missouri Botanical Garden, are working to document ethnobotanical lore (Figure 11–1). The results of this type of research are not only lists of useful species but also data on potentially useful wild and cultivated varieties as well as ecological information on how to best utilize tropical ecosystems in a sustainable manner. The collection of this type of information, combined with expanded programs bringing some of the more
promising species into cultivation, will eventually enrich our diets, and reduce our overdependence on current crop species and nonrenewable industrial materials.
REFERENCES
Balandrin, M., J.Klocke, F.E.Wurtele, and W.Bollinger. 1985. Natural plant chemicals: Sources of industrial and medicinal materials. Science 228:1154–1160.
Balick, M. 1985. Useful plants of Amazonia: A resource of global importance. Pp. 339–368 in G. Prance and R.Lovejoy, eds. Key Environments—Amazonia. Pergamon Press, Oxford.
Balick, M., and S.Gershoff. 1981. Nutritional evaluation of Jessenia bataua: Source of high quality protein and oil from Tropical America. Econ. Bot. 35(3):261–271.
Caufield, C. 1982. Tropical Moist Forests: The Resource, the People, the Threat. International Institute for Environment and Development, London. 67 pp.
Frankel, O. 1983. Genetic principles of in-situ preservation of plant resources. Pp. 55–65 in S.Jain and K.Mehra, eds. Conservation of Tropical Plant Resources. Proceedings of the Regional Workshop on Conservation of Tropical Plant Resources in South’East Asia, New Delhi, March 8–12, 1982. Botanical Survey of India, Howrah.
Gentry, A., and R.Wettach. 1986. Fevillea—A new oil seed from Amazonian Peru. Econ. Bot. 40(2):177–185.
Gibson, R. 1979. The geographical distribution, inheritance and pest-resisting properties of stick-tipped foliar hairs on potato species. Potato Res. 22:223–236.
Guillaumet, J.L. 1984. The vegetation: An extraordinary diversity. Pp. 27–54 in A.Jolly, P. Ogberle, and R.Albignac, eds. Key Environments—Madagascar. Pergamon Press, Oxford.
Harlan, J. 1984. Evaluation of wild relatives of crop plants. Pp. 212–222 in J.Holden and J.Williams, eds. Crop Genetic Resources: Conservation and Evaluation. Allen and Anwin, London.
Heiser, C. 1985. Ethnobotany of the naranjilla (Solanum quitoense) and its relatives. Econ. Bot. 39(1):4–11.
IUCN (International Union for the Conservation of Nature). 1980. World Conservation Strategy. International Union for the Conservation of Nature, Gland, Switzerland. 55 pp.
McKell, C.M. 1980. Native plants: An innovative approach to increasing tropical food production. Pp. 349–382 in Background Papers for Innovative Biological Technologies for Lesser Developed Countries. Office of Technology Assessment, Washington, D.C.
NRC (National Research Council). 1975. Underexploited Tropical Plants with Promising Economic Value. National Academy of Sciences, Washington, D.C. 187 pp.
NRC (National Research Council). 1984. Amaranth: Modern Prospects for an Ancient Crop. National Academy Press, Washington, D.C. 76 pp.
Oldfield, M. 1984. The Value of Conserving Genetic Resources. U.S. Department of the Interior, National Park Service, Washington, D.C. 360 pp.
Plotkin, M., V.Randrianasolo, L.Sussman, and N.Marshall. 1985. Ethnobotany in Madagascar. Report submitted to the International Union for the Conservation of Nature, Merges, Switzerland. 657 pp.
Prance, G. 1982. The increased importance of ethnobotany and underexploited plants in a changing Amazon. Pp. 129–136 in J.Hemming, ed. Change in the Amazon Basin. Vol. I: Man’s Impact on Forests and Rivers. Manchester Press, Manchester.
Prescott-Allen, R., and C.Prescott-Allen. 1982. What’s Wildlife Worth? Economic Contributions of Wild Plants and Animals to Developing Countries. International Institute for Environment and Development, London. 92 pp.
Prescott-Allen, R., and C. Prescott-Allen. 1983. Genes from the Wild. Using Genetic Resources for Food and Raw Materials. International Institute for Environment and Development, London. 101 pp.
Princen, L. 1977. Potential wealth in new crops: Research and development. Pp. 134–148 in D. Siegler, ed. Crop Resources. Proceedings of the 17th Annual Meeting of the Society for Economic Botany, the University of Illinois, Urbana, June 13–17, 1976. Academic Press, New York.
Schultes, R.E., 1977. Promising structural fiber palms of the Colombian Amazon. Principes 21(2):72–82.
Schultes, R.E. 1979. The Amazonia as a source of new economic plants. Econ. Bot. 33(3):259–266.
Tyler, V. 1986. Plant drugs in the twenty-first century. Econ. Bot. 40(3):279–288.
Vietmeyer, N. 1986a. Exotic edibles are altering America’s diet and agriculture. Smithsonian 16(9):34–43.
Vietmeyer, N. 1986b. Lesser-known plants of potential use in agriculture and forestry. Science 232:1379–1384.
Wang, S., and J.B.Huffman. 1981. Botanochemicals: Supplements to petrochemicals. Econ. Bot. 35(4):369–382.
White, J. 1979. The growing dependency of wood products on adhesives and other chemicals. For. Prod. J. 29:14–20.
Witt, S. 1985. Biotechnology and Genetic Diversity. California Agricultural Lands Project, San Francisco. 145 pp.










































