1
INTRODUCTION
MERCURY (Hg) is a persistent substance that comes from natural and anthropogenic sources. Hg that enters our oceans, lakes, and rivers is converted to methylmercury (MeHg) by aquatic biota and bioaccumulates in aquatic food webs including fish and shellfish. Humans and wildlife are exposed to MeHg primarily through the consumption of contaminated fish, 1 particularly large predatory fish species such as tuna, swordfish, shark, and whale. In humans, MeHg is known to be neurotoxic. The fetus is more sensitive to those effects than the adult (EPA 1997a).
In 1997, the U.S. Environmental Protection Agency (EPA) issued two reports on Hg and its effects on public health to the U.S. Congress. The first of these reports, the Mercury Study Report to Congress (EPA 1997a,b,c), assessed the source and amount of Hg emissions in the United States, the detrimental effects of Hg on humans and wildlife, and the feasibility of control technologies. The second report, the Study of Hazardous Air Pollutant Emissions from Electric Utility Steam Generating Units. Final Report to Congress (EPA 1998), looked specifically at emissions from utility companies and cited Hg as a major contaminant,
|
1 |
In this report, the term fish includes shellfish and marine mammals, such as the pilot whale, that are consumed by certain populations. |
especially in emissions from coal-fired power plants. Because concerns have been raised about Hg exposure levels in the United States, particularly among sensitive populations, questions have arisen among federal agencies over what is an acceptable level of exposure to MeHg.
Due to disagreement over the appropriate level of concern for MeHg exposure, the potentially widespread implications for human health, and the challenges associated with further regulating Hg emissions, Congress directed EPA in the House Appropriations Report for EPA 's Fiscal 1999 funding to contract with the National Research Council (NRC) to prepare recommendations on the appropriate value for a Hg exposure reference dose (RfD). In response, the NRC convened the Committee on the Toxicological Effects of Mercury, whose membership includes experts in toxicology, pharmacology, medicine, epidemiology, developmental psychology, neurophysiology, neuropsychology, public health, nutrition, statistics, exposure assessment, and risk assessment. The committee was charged with the following specific tasks:
-
Evaluate the body of evidence that led to the EPA-derived MeHg RfD. Human epidemiological and animal toxicity data should be the basis of the evaluation. The evaluation should determine the appropriateness of the critical study, end point of toxicity, and uncertainty factors used by EPA in deriving the RfD for MeHg. Sensitive populations should be considered.
-
Evaluate any new data (e.g., mechanistic data) that were not considered in EPA's 1997 Hg report that are relevant to EPA's MeHg RfD for protecting human health.
-
Consider exposure pathways (especially from the consumption of MeHg in fish) in evaluating likely human exposures, especially exposures of sensitive subpopulations. The evaluation should focus on those elements of exposure relevant to the establishment of an appropriate RfD.
-
Identify data gaps and make recommendations for future research.
Although the committee name, the Committee on the Toxicological Effects of Mercury, does not limit the scope of this report to MeHg, the committee focused on the health effects of this organic form of Hg because the toxicity due to this form is of greatest concern. In addition,
the committee did not attempt to establish an RfD for MeHg. Instead, the committee provides guidance to EPA on the data sets, exposure-assessment approaches, modeling techniques, and statistical analysis that should be considered in deriving an appropriate Hg RfD.
SOURCES OF Hg
In the environment, Hg comes from natural and anthropogenic sources. Mercuric sulfide, or Hg in cinnabar, is the natural form of Hg. The concentration of cinnabar varies greatly with the location of deposits. Hg can be released into the air through weathering of rock containing Hg ore or through human activities, principally incineration and burning of fossil fuels. Hg is a global pollutant, that once released to the air can travel long distances and impact distant sites. Water contamination can occur from run-off water, contaminated by either natural or anthropogenic sources, or from air deposition. Potential sources of general population exposure to Hg include inhalation of Hg vapors in ambient air, ingestion of drinking water and foodstuffs contaminated with Hg, and exposure to Hg from dental amalgams and medical treatments. Dietary intake is one of the most important sources of non-occupational exposure to Hg, fish and other seafood products being the dominant source of Hg in the diet. Most of the Hg consumed in fish or other seafood is the highly absorbable MeHg form. The substantial variation in human MeHg exposure is based on the differences in frequency and amount of fish consumed and Hg concentration in the fish. MeHg exposure is a major problem in some populations, especially subsistence fish eaters who consume large amounts of fish (EPA 1997a). Intake of elemental Hg from dental analgams is another major contributing source to the total Hg body burden in humans in the general population (IPCS 1990, 1991).
The World Health Organization (WHO) has estimated that anthropogenic sources, mainly the combustion of fossil fuels, contribute 25% of the overall (natural and anthropogenic) Hg emissions to the atmosphere (ATSDR 1999). EPA has estimated that those sources account for 50% to 75% of the total yearly input of Hg into the atmosphere (EPA 1997a). In the United States, the majority of Hg emissions are from
combustion sources. Medical and municipal waste incinerators and coal-fired utility boilers account for greater than 80% of the Hg emitted from point sources (EPA 1997b; ATSDR 1999).
FATE AND TRANSPORT
Hg has three valence states (Hg0, Hg1+, Hg2+) and is found in the environment in the metallic form and in various inorganic and organic complexes. The natural global bio-geochemical cycling of Hg is characterized by degassing of the element from soils and surface waters, atmospheric transport, deposition of Hg back to land and surface water, sorption of the compound onto soil or sediment particles, and revolatilization from land and surface water (see Figure 1-1). This emission, deposition, and revolatilization creates difficulties in tracing the movement of Hg to its sources (ATSDR 1999). Once in the environment, interconversion between the different forms of Hg can occur. Particulate-bound Hg can be converted to insoluble Hg sulfide and precipitated or bioconverted into more volatile or soluble forms that re-enter the atmosphere or are bioaccumulated in aquatic and terrestrial food chains. Conversion of inorganic Hg to MeHg occurs primarily in microorganisms especially in aquatic systems. Once in its methylated form, Hg bioaccumulates up the food chain; the microorganisms are consumed by fish, and the smaller fish are consumed by larger fish. Such bioaccumulation can result in very high concentrations of MeHg in some fish, which are one of the main sources of human and piscivorus wildlife exposure to MeHg.
HEALTH EFFECTS
Human exposure to MeHg from contaminated fish and seafood can pose a variety of health risks. A spectrum of adverse health effects has been observed following MeHg exposure, with the severity depending largely on the magnitude of the dose. Fatalities and devastating neurological damage were observed in association with the extremely high exposures that occurred during the Minamata and Iraqi poisoning
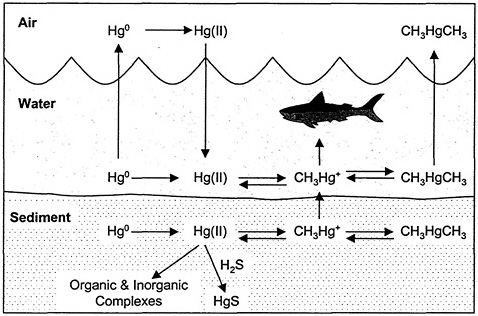
FIGURE 1-1 Cycling of Hg in aquatic system. CH3Hg+, methylmercury ion; CH3HgCH3, dimethylmercury; Hg(ll), mercuric mercury; Hg0, elemental mercury; H2S, hydrogen sulfide; HgS, cinnabar. Source: Adapted from EPA 1997b.
episodes. The fetus is considered much more sensitive than the adult. Prenatal exposures interfere with the growth and migration of neurons and have the potential to cause irreversible damage to the developing central nervous system (EPA 1997a). Infants exposed in utero to MeHg during the Minamata and Iraqi episodes were born with severe disabilities, such as mental retardation, seizure disorders, cerebral palsy, blindness, and deafness. At much lower doses that result from chronic maternal fish consumption, infants might appear normal during the first few months of life but might later display deficits in subtle neurological end points (e.g., IQ deficits, abnormal muscle tone, decrements in motor function, attention, and visuospatial performance).
Exposures that occur during childhood and adulthood can also cause damage to the central nervous system, as evidenced by human poison-
ing incidents in Japan, Iraq, and the United States, in which the first signs of toxicity often appear several months after exposure has ended (EPA 1997b, Davis et al. 1994).
There is evidence that MeHg also effects other systems. In 1995, researchers in Finland found a correlation between consumption of MeHg-contaminated fish and the risk of acute myocardial infarction (Salonen et al., 1995). This prospective study of 1,833 fishermen was intended to confirm previous studies in which fish consumption was associated with a reduced risk of heart disease. Instead, they discovered that hair Hg levels above 2 parts per million (ppm), or daily ingestion of more than 30 grams (g) of fish, increased the risk of acute myocardial infarction (AMI) or cardiovascular death 2- to 3-fold. The estimated daily dietary Hg intake ranged from 1.1 µg to 95.3 µg (mean, 7.6 µg). The investigators theorized that the cardiovascular effects of MeHg might be caused, at least in part, by the ability of Hg to enhance lipid peroxidation via a Fenton-type reaction.
Inorganic and organic forms of Hg are also well-known renal toxicants. Human case investigations and animal feeding studies have repeatedly confirmed that effect. Human exposures to organic Hg have resulted in symptoms of polyuria and albuminuria (Jalili and Abbasi 1961; Cinca et al. 1979). Autopsies of patients who died following ingestion of alkyl Hg revealed nephritis and tubular degeneration (Al Saleem 1976; Cinca et al. 1979). Animal studies have shown that MeHg damages the proximal tubules in the kidney (Mitsumori et al. 1990).
During the past decade, researchers have studied the effects of MeHg on immune function and blood-pressure regulation. After administering MeHg to mice for 12 weeks, IIbäck (1991) noted changes in the thymus and natural killer-cell activity. Sørensen et al. (1999) found an association between prenatal exposure to MeHg and childhood blood pressure. Diastolic and systolic blood pressures, measured at age 7, increased 13.9 millimeters (mm) and 14.6 mm, respectively, as cord-blood Hg concentrations rose from 1 to 10 micrograms per liter (µg/L).
EXPOSURE EVENTS AND STUDIES
Between 1950 and 1975, several MeHg poisoning incidents occurred in Japan and Iraq. Scientists who investigated those events identified
developmental neurotoxicity as the health effect of greatest concern following high-level episodic exposures. Individuals poisoned by MeHg through consumption of contaminated fish in Japan exhibited paresthesia, ataxia, sensory disturbances, tremors, impairment of hearing, and difficulty walking (Harada 1995). In Iraq, exposure was due to the consumption of home-made bread that was made with grain treated with MeHg as a fungicide. In that outbreak, the most common symptom in adults was paresthesia; the most severely affected individuals exhibited ataxia, blurred vision, slurred speech, hearing difficulties, blindness, deafness, and death (Marsh et al. 1987). In both Iraq and Japan, the effects in offspring who were exposed to MeHg in utero were more serious, and in some cases seen at lower doses, than in adults. Both exposure episodes have been studied to determine the doses and the effects resulting from exposure to MeHg. Although the doses that produced those effects in the Japanese and Iraqi populations were undoubtedly quite high, precise dose-response relationships have not been established, and the exposure scenarios are not comparable to the low-dose chronic exposure that the general population in North America might experience.
In an attempt to establish dose-response relationships, three large prospective epidemiological studies have evaluated subtle end points of neurotoxicity. One study was conducted in the Republic of the Seychelles, a nation of islands located in the Indian Ocean off the coast of East Africa (Davidson et al. 1995, 1998). Another major study was conducted in the Faroe Islands (part of Denmark), which are located in the North Sea between Scotland and Iceland (Grandjean et al. 1997, 1998, 1999). The other major study was conducted in New Zealand (Kjellström et al. 1986, 1989). The populations of the Seychelles, Faroe Islands, and New Zealand were chosen for study, because their dietary dependence on fish and marine mammals provides an ongoing source of exposure to MeHg. Prenatal MeHg exposures in those populations were within the range of at least some U.S. population exposures. All three studies evaluated large numbers of subjects.
The 66-month study of 711 children in the Seychelles islands assessed the effects of prenatal MeHg in tests of global intelligence and developmental milestones. No adverse effects were seen that could be attributed to MeHg. Maternal hair samples collected at birth contained Hg concentrations that ranged from 0.5 to 27 ppm (mean, 6.8 ppm). Meanwhile,
scientists working in the Faroe Islands found that children whose prenatal exposures were similar to those observed in the Seychelles population had subtle developmental dose-related deficits that were apparent at 7 years of age. Abnormalities were seen in tests of memory, attention, and language and, to a lesser extent, in neurophysiological end points. Measurements of blood pressure, heart rate, and heart-rate variability were also taken when the children reached 7 years of age. Researchers found that diastolic and systolic blood pressures increased, and hfeartrate variability decreased as cord-blood Hg concentrations rose from 1 to 10 µg/L.
A prospective study carried out in New Zealand (Kjellström et al. 1986, 1989) examined the effects in offspring exposed in utero to MeHg via maternal consumption of fish. Scores on the Denver Developmental Screening Test (DDST), a standardized test for childhood mental and motor development, were compared in groups of children 4 years of age categorized by maternal Hg exposure (as measured in parts per million in maternal hair) (Kjellström et al. 1986). At 6 years of age, a battery of specific cognitive tests was administered (Kjellström et al. 1989). At both ages, the researchers found significant decrements in test performance in the children exposed to moderate-to-high doses of MeHg prenatally (more than 6 ppm).
A correlation was demonstrated between hair Hg concentrations and neurophysiological effects in a study of an adult population in the Amazon, where gold-mining activities have resulted in fish highly contaminated with Hg (Lebel et al. 1996). In that study population, it is likely that the adult population was also exposed to MeHg in utero.
The studies of the Iraqi, Amazon, Seychelles, and Faroe Islands populations were reviewed by an expert panel that met in Raleigh, North Carolina, at the Workshop on the Scientific Issues Relevant to Assessment of Health Effects from Exposure to MeHg. A report of that workshop has been published (NIEHS 1998). In suggesting possible explanations for the discrepant findings of the Seychelles and Faroe studies, the panel pointed to differences in sources of exposures or exposure measures, differences in the neurobehavioral tests used or their administration or interpretation, influences of confounders and covariates, and biostatistical issues involved in the analysis of the data. The differences between those studies are discussed further in Chapter 6.
SUMMARY OF RISK ASSESSMENTS FOR MeHg
State and national governments as well as international organizations have recommended acceptable levels of Hg exposure that are thought to be protective against adverse effects (see Table 1-1). General risk assessment approaches used by the various agencies are described in NRC (1983) and NRC (1984). In this report, information on how EPA derives an RfD can be found in the section on Risk Assessment for Noncancer End Points in Chapter 7. Specific details on the derivation of EPA's MeHg RfD can be found in the section on The Current EPA Reference Dose in Chapter 8. In the United States, responsibility for regulating Hg is shared by two federal agencies: the Food and Drug Administration (FDA) and EPA. FDA is responsible for ensuring that Hg concentrations in commercially sold fish and seafood do not exceed what the agency defines as an action level for this contaminant (FDA 1979). EPA monitors Hg concentrations in the environment and regulates industrial releases to air and surface water. Although not a regulatory agency, the Agency for Toxic Substances and Disease Registry (ATSDR) evaluates the potential for humans to be exposed to MeHg and investigates reported health effects. Currently, each of these agencies uses a different guideline to assess exposure to toxicants.
The differences in guidelines among the agencies are due to the use of different risk-assessment methods, data sets, and uncertainty factors and the different mandates of each agency (EPA 1984, 2000; FDA 1979; ATSDR 2000). For example, EPA used data from the 1971 Iraqi poisoning incident to derive an RfD of 0.1 microgram per kilogram (µg/kg) of body weight per day for MeHg (EPA 1997a). The reference dose was calculated using a benchmark dose of 1.1 µg/kg per day. That benchmark dose was divided by uncertainty factors(UF) to account for the variability in the human population (UF of 3) and for the lack data on reproductive effects, sequelae, and adult paresthesia (UF of 3). Although MeHg is classified by the agency as a possible human carcinogen, no uncertainty factor was used to protect against that effect. The RfD calculated by EPA was in the range of other values obtained by EPA using similar analysis of other data sets.
In 1998, ATSDR used the Seychelles study (Davidson et al. 1998) as the starting point for estimating a minimal risk level for exposure to MeHg (ATSDR 1999). In this study, the investigators examined
TABLE 1-1 Summary of Risk Assessments for Methylmercury
|
Agency |
Key Studies |
End Points |
Biomarker and Exposure Level |
Critical Dose |
Uncertainty Factors |
Acceptable Level |
|
EPAa |
Iraqi study (Marsh et al. 1987) |
Combined instance of neurological effects following in utero exposure b |
Maternal hair, 11 ppm; equivalent to intake of 1.1 µg/kg/d |
Benchmark dose, 1.1 µg/kg/dc |
UF, 10d |
RfD, 0.1 µg/kg/d (based on fetal effects) |
|
ATSDR |
Seychelles study (Davidson et al. 1998) |
Developmental neurotoxicity measured by neurological evaluation, behavioral, psychological tests |
Maternal hair, 15.3 ppm; equivalent to intake of 1.3 µg/kg/d |
NOAEL, 1.3 µg/kg/d |
UF, 4.5e |
MRL, 0.3 µg/kg/d |
|
FDA |
Japanese data (Friberg et al. 1971) |
Overt neurological symptoms in adults |
Adult blood, 0.2 ppm; equivalent to intake of 300 µg/d |
LOAEL, 4.3 µg/kg/d |
SF, 10f |
Action level in fish, 1 ppm in edible portiong (equivalent to 0.5 µg/kg/d) |
|
JECFAh |
Japanese data (Friberg et al. 1971) |
Overt neurological symptoms in adults |
Adult blood, 0.2 ppm; equivalent to intake of 300 µg/d |
LOAEL, 4.3 µg/kg/d |
SF of 10i |
pTWI, 3.3 µg/kg/wk (equivalent to 0.5 µg/kg/d) |
|
Health Canada |
Seychelles study (Davidson et al.1998); Faroe Islands (Grandjean et al. 1997); New Zealand (Kjellstrom 1986, 1989) |
Developmental neurotoxicity |
Maternal hair, 10 ppm; equivalent to intake of 1 µg/kg/d |
Benchmark dose, 1 µg/kg/dj |
UF, 5k |
pTDI, 0.2 µg/kg/d (for women of childbearing ages, infants, and young children) l |
|
North Carolina |
Seychelles study (Davidson et al. 1998) |
Developmental neurotoxicity |
Maternal hair, 6.8 ppm; equivalent to intake of 0.5 µg/kg/d |
Arithmetic mean,m 0.5 µg/kg/d |
Modifying factor, 3 |
RfD, 0.2 µg/kg/d (for women of childbearing age and developing fetuses)n |
|
Washington State |
Faroe Islands (Grandjean et al.1997) |
Impaired neurological development and longterm or delayed sequelae in children |
Maternal hair, 4.3-10 ppm; equivalent to intake of 0.35-0.8 µg/kg/d |
Daily intake range,0 0.35-0.8 µg/kg/d |
UF, 10p |
TDI, 0.035-0.08 µg/kg/d |
|
aEPA report to Congress states that “a number of additional studies of human populations generally support the dose range of the benchmark dose level for perinatal effects. ” The agency is awaiting the results of this NRC report before updating its RfD based on more recent data. bThe data for delayed onset of walking and talking, neurological scores of less than 3, mental symptoms, and seizures were grouped together for this analysis. cEPA carried out the analysis using the polynomial model and the Weibull model. The results of the two models were within 3% of each other. EPA based its analysis on the Weibull model due to goodness of fit and history of use. The Benchmark dose is an estimate of an experimental dose associated with a specified low incidence of adverse effects. dAccording to the Integrated Risk Information System (IRIS), the following uncertainty factors were applied: 3 for the variability in human population (variability in the half-life of methylmercury and in hair-to-blood ratio) and 3 for the lack of a two-generation reproductive study and data on the effect of exposure duration on sequelae of the developmental neurotoxicity effects and on adult paresthesia. eThe following uncertainty factors were applied: 1.5 for human pharmacokinetic variability, 1.5 for human pharmacodynamic variability and 1.5 to account for domain-specific findings in the Faroe study. fArbitrary value; the Federal Register states that, in cases in which human data are available, the safety factor used is 10. gThis conversion was calculated using fish consumption data from the National Marine Fisheries Service. hThe Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) concluded in 1999 that “the information available was insufficient for evaluating the neurodevelopmental effects on offspring of mothers with low intakes of methylmercury. ” |
||||||
the correlation between subtle neurological effects and low-dose chronic exposure to MeHg. No correlation between Hg concentrations and neurological effects was seen. ATSDR determined a minimal risk level of 0.3 µ g/kg per day, based on a dose of 1.3 µg/kg per day, which reflects the average concentration of the upper quintile of the exposed population but does not necessarily correspond to a no-observed-adverse-effect level (NOAEL). The agency used two uncertainty factors of 1.5 each to account for pharmacokinetic and pharmacodynamic variability within the human population. A modifying factor of 1.5 was applied to account for the possibility that domain-specific tests used in the Faroe Islands study might have allowed detection of subtle neurological effects that were not evaluated in the Seychelles cohort. Although the conventional risk-assessment approach is to multiply uncertainty factors, the agency summed these factors to develop an overall safety factor of 4.5.
According to Tollefson and Cordle (1986), FDA used data from the Minamata Bay poisoning episode to determine the action level of 1 ppm (in the edible portion of fish), which corresponds to a daily intake of 0.5 µg/kg (Friberg et al. 1971). FDA followed the approach taken by the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA), who had determined a provisional tolerable weekly intake (pTWI) of 0.5 µ g/kg in adults and stated that the fetus and children might be more sensitive but that the data are insufficient to determine a safe intake in these populations (JECFA 1972). That pTWI was recently confirmed at the JECFA meeting in June 1999 (JECFA 1999). Canadian recommendations are based on the JECFA pTWI in adults; however, Canada also has a provisional tolerable daily intake of 0.2 µg/kg per day for children and women of child-bearing years, an intake based on a qualitative assessment of available data (M.-T. Lo, Food Directorate, Health Canada, personal commun., June 1999). The effect on public health of using one dose rather than another to set acceptable exposure levels might be substantial, leaving open the question of which value best ensures public safety. Differences in acceptable levels can affect many government programs, including state fish advisories, and regulation of such industries as commercial fishing and electric power plants (Renner 1999).
SCIENTIFIC CONTROVERSIES AND SOURCES OF UNCERTAINTY
Many controversies surround the determination of what is an acceptable level of exposure to MeHg. Some of these controversies stem from the science underlying the toxicity data base for MeHg. For example, there is disagreement over which studies and which end points of concern should be used to derive an acceptable level. There is emerging evidence of potential effects on both the immune and cardiovascular systems at low doses. The contradictory findings from the Seychelles and Faroe Islands studies have made it difficult to determine an appropriate point of departure for risk assessment. Scientists also do not agree on whether Hg in hair or blood is the more appropriate biomarker or measurement of exposure. There is debate over the assumptions on the disposition and metabolism of MeHg that are used to extrapolate from a measured biomarker value to a corresponding Hg exposure level. In addition, there is debate over the assumptions on fish intake and the concentration of Hg in the fish that are used to determine a safe amount of fish for consumption. The choice of dose-response model and uncertainty factors, if any, is also controversial.
ORGANIZATION OF THE REPORT
The remainder of this report is organized into six chapters and an appendix. In Chapter 2, information on the chemistry, toxicokinetics, toxicodynamics, and exposure of MeHg is presented. Chapter 3 presents a discussion on toxicokinetic variability and other factors that influence variation in human sensitivity to MeHg. Those factors include age, genetics, and nutrition. In Chapter 4, issues involved in assessing MeHg exposure and dose are presented. The focus is on the selection and interpretation of dose metrics and the implications of the possible dose metrics for dose-response assessment and nutritional assessment. The health effects associated with the ingestion of MeHg are discussed in Chapter 5. Emphasis is placed on the more-recent studies with respect to the choice of end points, possible confounders, and sensitive subpopulations. Evidence from experimental animal studies is also discussed. In Chapter 6, a comparison of studies that are appropriate for risk assessment for MeHg is presented. Chapter 7 provides an evalua-
tion of the various data sets and statistical approaches for deriving an acceptable Hg exposure level. Further details of one approach are provided in the appendix. In Chapter 8, the risks from ingestion of MeHg and the sources of uncertainty are characterized and the adequacy of the EPA MeHg RfD for protecting human health is evaluated. The public-health implications of exposure to MeHg, including the implications of choosing one Hg exposure level over another, and how these relate to state and federal concerns, such as fish advisories and consumption, are also addressed.
REFERENCES
Al-Saleem, T. 1976. Levels of mercury and pathologic changes in patients with organomercury poisoning. Bull. WHO 53(Suppl.):99-104.
ATSDR (Agency for Toxic Substances and Disease Registry). 1997. Toxicological Profile for Mercury. (Update). Draft. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, GA.
ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Mercury. (Update). U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, GA.
Cinca, I., I. Dumetrescu, P. Onaca, A. Serbanescu, and B. Nestorescu. 1979. Accidental ethyl mercury poisoning with nervous system, skeletal muscle, and myocardium injury. J. Neurol. Neurosurg. Psychiatry 43(2):143-149.
Davidson, P.W., G.J. Myers, C. Cox, C.F. Shamlaye, D.O. Marsh, M.A. Tanner, M. Berlin, J. Sloane-Reeves, E. Cernichiari, O. Choisy, A. Choi, and T.W. Clarkson. 1995. Longitudinal neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from maternal fish ingestion: outcomes at 19 and 29 months. Neurotoxicology 16(4):677-688.
Davidson, P.W., G.J. Myers, C. Cox, C. Axtell, C. Shamlaye, J. Sloane-Reeves, E. Cernichiari, L. Needham, A. Choi, Y. Wang, M. Berlin, and T.W. Clarkson. 1998. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: Outcomes at 66 months of age in the Seychelles Child Development Study. JAMA 280(8):701-707.
Davis, L.E., M. Kornfeld, H.S. Mooney, K.J. Fiedler, K.Y. Haaland, W.W. Orrison, E. Cernichiari, and T.W. Clarkson. 1994. Methylmercury poisoning:Long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann. Neurol. 35(6):680-688.
EPA (U.S. Environmental Protection Agency). 1997a. Mercury Study for
Congress. Volume I: Executive Summary. EPA-452/R-97-003. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1997b. Mercury Study for Congress. Volume V: Health Effects of Mercury and Mercury Compounds. EPA-452/R-97-007. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1997c. Mercury Study Report to Congress. Volume VII: Characterization of Human Health and Wildlife Risks from Mercury Exposure in the United States. EPA-452/R-97-009. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1998. Study of Hazardous Air Pollutant Emissions from Electric Utility Steam Generating Units. Final Report to Congress. EPA-453/R-98-004a,-b. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
FDA (U.S. Food and Drug Administration). 1979. Action level for mercury in fish, shellfish, crustaceans and other aquatic animals. Withdrawal of proposed rulemaking. Dept of Health, Education and Welfare. Fed. Regist. 44(14):3990-3993. Jan. 19.
Friberg, L. (Swedish Expert Group). 1971. Methylmercury in fish: A toxicological-epidemiologic evaluation of risks report from an expert group. Nord. Hyg. Tidskr. 4(Suppl.):19-364.
Grandjean, P., E. Budtz-Jørgensen, R.F. White, P. Weihe, F. Debes, and N. Keiding. 1999. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am. J. Epidemiol. 150(3):301-305.
Grandjean, P., P. Weihe, R.F. White, F. Debes, S. Araki, K. Yokoyama, K. Murata, N. Sørensen, R. Dahl, and P.J. Jørgensen. 1997. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 19(6):417-428.
Grandjean, P., P. Weihe, R.F. White, N. Keiding, E., Budtz-Jørgensen, K. Murato, and L. Needham. 1998. Prenatal exposure to methylmercury in the Faroe Islands and neurobehavioral performance at age seven years. Response to workgroup questions for presentation on 18-20 Nov. 1998 . In Scientific Issues Relevant to Assessment of Health Effects from Exposure to Methylmercury. Appendix II-B. Faroe Islands Studies. National Institute for Environmental Health Sciences. Available: "http://ntp-server.niehs.nih.gov/ Main_Pages/PUBS/MethMercWkshpRpt.html"
Harada, M. 1995. Minamata disease: Methylmercury poisoning in Japan
caused by environmental pollution. Crit. Rev. Toxicol. 25(1):1-24.
Ilbäck, N.G. 1991. Effects of methylmercury exposure on spleen and blood natural killer cell activity in the mouse. Toxicology 67(1):117-124.
IPCS (International Programme on Chemical Safety). 1990. Environmental Health Criteria Document 101: Methylmercury. Geneva: World Health Organization.
IPCS (International Programme on Chemical Safety). 1991. Environmental Health Criteria Document 118: Inorganic Mercury. Geneva: World Health Organization.
Jalili, H.A., and A.H. Abbasi. 1961. Poisoning by ethyl mercury toluene sulphonanilide. Br. J. Indust. Med. 18(Oct.):303-308.
JECFA (Joint FAO/WHO Expert Committee on Food Additives). 1972. Evaluation of Certain Food Additives and the Contaminants Mercury, Lead, and Cadmium. World Health Organization Technical Series No. 505. Geneva: World Health Organization.
JECFA (Joint FAO/WHO Expert Committee on Food Additives). 1999. Joint FAO/WHO Expert Committee on Food Additives. 53rd meeting. Rome, 1-10 June, 1999. Online. Available:"http://www.who.int/pes/jecta/jecta.htm"
Kjellström, T., P. Kennedy, S. Wallis, and C. Mantell. 1986. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish. Stage I: Preliminary tests at age 4. National Swedish Environmental Protection Board Report 3080. Solna, Sweden.
Kjellström, T., P. Kennedy, S. Wallis, A. Stewart, L., Friberg, B. Lind, T. Wutherspoon, and C. Mantell . 1989. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish. Stage II: Interviews and psychological tests at age 6. National Swedish Environmental Protection Board Report 3642. Solna, Sweden.
Lebel, J., D. Mergler, M. Lucotte, M. Amorim, J. Dolbec, D. Miranda, G. Arantes, I. Rheault, and P.Pichet. 1996. Evidence of early nervous system dysfunction in Amazonian populations exposed to low-levels of methylmercury. Neurotoxicology 17(1):157-168.
Marsh, D.O., T.W. Clarkson, C. Cox, G.J. Myers, L. Amin-Zaki, and S. Al-Tikriti. 1987. Fetal methylmercury poisoning: Relationship between concentration in single strands of maternal hair and child effects. Arch. Neurol. 44(10): 1017-1022.
Mitsumori, K., M. Hirano, H. Ueda, K. Maita, and Y. Shirasu. 1990. Chronic toxicity and carcinogenicity of methylmercury chloride in B6C3F1 mice. Fundam. Appl. Toxicol. 14(1):179-190.
NIEHS (National Institute of Environmental Health Sciences). 1998. Scientific Issues Relevant to Assessment of Health Effects from Exposure to Methyl-
mercury. Report of the Workshop on Scientific Issues Relevant to Assessment of Health Effects from Exposure to Methylmercury, Nov. 18-10, 1998, Raleigh, NC.
Renner, R. 1999. Consensus on health risks from mercury exposure eludes federal agencies . Environ. Sci. Technol. 33(13):269A-270A.
Salonen, J.T., K. Seppänen, K. Nyyssönen, H. Korpela, J. Kauhanen, M. Kantola, J. Tuomilehto, H. Esterbauer, F. Tatzber, and R. Salonen . 1995. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in Eastern Finnish men. Circulation 91(3):645-655.
Sørensen, N., K. Murata, E. Budtz-Jørgensen, P. Weihe, and P. Grandjean. 1999. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 10(4):370-375.
Tollefson, L., and F. Cordle. 1986. Methylmercury in fish: A review of residue levels, fish consumption and regulatory action in the United States. Environ. Health Perspect. 68:203-208.


















