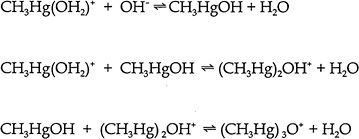2
CHEMISTRY, EXPOSURE, TOXICOKINETICS, AND TOXICODYNAMICS
THIS chapter presents background information that serves as a foundation for understanding the toxicology of MeHg. The chemical, toxicokinetic, and toxicodynamic properties of MeHg are presented. There is extensive literature on MeHg, and this review is not meant to be exhaustive. Although the primary emphasis of this report is on MeHg, this chapter includes discussions of other Hg species to provide a general review of the sources of exposure and toxicological properties of different Hg species. The emphasis is on human Hg data. Animal data are also discussed.
PHYSICAL AND CHEMICAL PROPERTIES
Chemical species of Hg that are of toxicological importance include the inorganic forms, elemental or metallic Hg (Hg0), mercurous Hg (Hg1+), and mercuric Hg (Hg2+), and the organic forms, MeHg and ethylmercury. Although there are many organic Hg compounds, the emphasis in this chapter is on MeHg. The structure, chemical formula, and physical and chemical properties of some Hg-containing compounds are shown in Table 2-1. A more complete table of physical and chemical properties of some Hg compounds can be found in the Agency of Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Mercury (Update) (ATSDR 1999). Table 2-2 summarizes the informa-
TABLE 2-1 Physical and Chemical Properties of Some Toxicologically Relevant Mercury Compounds
|
Chemical Name |
Elemental Mercurya |
Mercuric Chloride |
Mercurous Chlorideb |
Methylmercuric Chloridec |
Dimethylmercury |
|
Molecular formula |
Hg0 |
HgCl2 |
Hg2Cl2 |
CH3HgCl |
C2H6Hg |
|
Molecular structure |
Cl-Hg-Cl |
Cl-Hg-Hg-Cl |
CH3-Hg-Cl |
CH3-Hg-CH3 |
|
|
Molecular weight |
200.59 |
271.52 |
472.09 |
251.1 |
230.66 |
|
Solubility |
5.6 × 10-5 g/L at 25°C |
69 g/L at 20°C |
2.0 × 10-3 g/L at 25°C |
0.100 g/L at 21°C |
1 g/L at 21°C |
|
Density |
13.534 g/cm3 at 25°C |
5.4 g/cm3 at 25°C |
7.15 g/cm3 at 19°C |
4.06 g/cm3 at 20°C |
3.1874 g/cm3 at 20°C |
|
Oxidation state |
+1, +2 |
+2 |
+1 |
+2 |
+2 |
|
aAlso known as metallic mercury. bAlso known as calomel. cMethylmercuric chloride is used experimentally to investigate the effects of methylmercury. |
|||||
tion on some toxicologically relevant Hg compounds discussed later in this chapter.
At 25° C, elemental Hg has a water solubility of 5.6×10-5 g/L. Mercuric chloride is considerably more soluble, having a solubility of 69 g/L at 20° C. In comparison, an organic Hg compound, such as methylmercury chloride, is much less water soluble, having a solubility of 0.100 g/L at 21° C. Dimethylmercury, a very toxic by-product of the chemical synthesis of MeHg (Nierenberg et al. 1998), also has a relatively low water solubility (1.0 g/L at 21° C). Due to its low water solubility, MeHg chloride is considered to be relatively lipid soluble. As discussed later in this chapter, the solubility of the different forms of Hg might play a role in their differential toxicity.
TABLE 2-2 Summary Table Comparing Toxicologically Relevant Mercury Species
|
Methylmercury (CH3Hg+) |
Elemental Mercury (Hg0) |
Mercuric Mercury (Hg2+) |
|
Sources of Exposure |
||
|
Fish, marine mammals, crustaceans, animals and poultry fed fish meal |
Dental amalgams, occupational exposure, Caribbean religious ceremonies, fossil fuels, incinerators |
Oxidation of elemental mercury or demethylation of MeHg; deliberate or accidental poisoning with HgCl2 |
|
Biological Monitoring |
||
|
Hair, blood, cord blood |
Urine, blood |
Urine, blood |
|
Toxicokinetics |
||
|
Absorption |
||
|
Inhalation: Vapors of MeHg absorbed |
Inhalation: Approximately 80% of inhaled dose of Hg0 readily absorbed |
Inhalation: Aerosols of HgCl2 absorbed |
|
Oral: Approximately 95% of MeHg in fish readily absorbed from GI tract |
Oral: GI absorption of metallic Hg is poor; any released vapor in GI tract converted to mercuric sulfide and excreted |
Oral; 7-15% of ingested dose of HgCl2 absorbed from the GI tract; absorption proportional to water solubility of mercuric salt; uptake by neonates greater than adults |
|
Dermal: In guinea pigs, 3-5% of applied dose absorbed in 5 hr |
Dermal: Average rate of absorption of Hg0 through human skin, 0.024 ng/cm2 for every 1 mg/m3 in air |
Dermal: In guinea pigs, 2-3% of applied dose of HgCl2 absorbed |
|
Distribution |
||
|
Distributed throughout body since lipophilic; approximately 1-10% of absorbed oral dose of MeHg distributed to blood; 90% of blood MeHg in RBCs |
Rapidly distributed throughout the body since it is lipophilic |
Highest accumulation in kidney; fraction of dose retained in kidney dose dependent |
|
MeHg-cysteine complexa involved in transport of MeHg into cells |
||
|
Half-life in blood, 50 d; 50% of dose found in liver; 10% in head. |
Half-life in blood, 45 d (slow phase); half-life appears to increase with increasing dose |
Half-life in blood, 19.7-65.6 d; 1st phase, 24 d, 2nd phase, 15-30 d |
|
Readily crosses blood-brain and placental barriers |
Readily crosses blood-brain and placental barriers |
Does not readily penetrate blood-brain or placental barriers |
|
In neonate, mercuric Hg not concentrated in kidneys; therefore, more widely distributed to other tissues |
||
|
In fetus and neonate, blood-brain barrier incompletely formed, so mercuric Hg brain concentrations higher than those in adults |
||
|
Biotransformation |
||
|
MeHg slowly demethylated to mercuric Hg (Hg2+) |
Hg0 in tissue and blood oxidized to Hg2+ by catalase and hydrogen peroxide (H2O2); H2O2 production the rate-limiting step |
Hg0 vapor exhaled by rodents following oral administration of mercuric Hg |
|
Tissue macrophages, intestinal flora, and fetal liver are sites of tissue demethylation |
Mercuric Hg not methylated in body tissues but GI microorganisms can form MeHg |
|
Mechanisms of demethylation unknown; free radicals demethylate MeHg in vitro; bacterial demethylation enzymes studied extensively, none has been characterized or identified in mammalian cells |
||
|
Does not bind or induce metallothionein |
Binds and induces metallothionein |
|
|
Excretion |
||
|
Daily excretion, 1% of body burden; major excretory route is bile and feces; 90% excreted in feces as Hg2+; 10% excreted in urine as Hg2+ |
Excreted as Hg0 in exhaled air, sweat, and saliva, and as mercuric Hg in feces and urine |
Excreted in urine and feces; also excreted in saliva, bile, sweat, exhaled air, and breast milk |
|
Lactation increases clearance from blood; 16% of Hg in breast milk is MeHg |
||
|
Half-Life limination |
||
|
(Whole body) 70-80 d; dependent on species, dose, sex, and animal strain |
58 d |
1-2 mo |
|
Toxicodynamics |
||
|
Critical target organ |
||
|
Brain, adult and fetal |
Brain and kidney |
Kidney |
|
Causes of Toxicity |
||
|
Demethylation of MeHg to Hg2+ and the intrinsic toxicity of MeHg |
Oxidation of Hg0 to Hg2+ |
Hg2+ binding to thiols in critical enzyme (e.g., cysteine) and structural proteins |
METHODS OF CHEMICAL ANALYSIS
The methods used for analyzing Hg in biological samples include atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS) (Vermeir et al. 1991a, b), X-ray fluorescence (XRF) (Marsh et al. 1987), gas chromatography (GC)-electron capture (Cappon and Smith 1978), and neutron activation analysis (NAA) (Fung et al. 1995). Anodic stripping voltammetry (ASV) has also been used (Liu et al. 1990). Of those procedures, GC-electron capture is able to distinguish MeHg from other species, but only cold vapor (CV)-AAS will detect Hg at parts per billion. CV-AAS, AFS, XRF, and NAA have all been used to analyze Hg content in hair (Zhuang et al. 1989).
To measure total Hg in biological samples, the Hg must first be reduced to the elemental form. CV-AAS is most frequently used to measure Hg in urine (Magos and Cernik 1969) and blood (Magos and Clarkson 1972). For example, CV-AAS, the most commonly used method for analyzing Hg in biological samples, involves reduction of the Hg in the sample with stannous chloride to elemental Hg. To measure inorganic Hg, the analysis is carried out without chemical reduction of the sample. The difference between the total Hg concentration and the inorganic Hg concentration represents the concentration of organic Hg that was present in the sample.
Biological samples containing MeHg can also be analyzed using Pseudomonas putida strain FB1. That bacteria converts MeHg to methane gas and elemental Hg (Baldi and Filippelli 1991). This method is one of the most reliable and specific methods for MeHg quantification, because chemical interference is negligible. It can detect 15 ng of MeHg in 1 g of biological tissue with a coefficient of variation of 1.9%.
New methods for analyzing Hg in biological samples have been developed such as inductively coupled plasma-mass spectrometry (ICPMS) (Kalamegham and Ash 1992). Most of the new methods are expensive and beyond the reach of most laboratories. The cost is approximately $150,000-250,000 for the instrument and more than $35,000 a year for gases and maintenance costs.
Regardless of the analytical method used, care must be taken to eliminate or prevent contamination of the sample by Hg during preparation and analysis. All glassware and plasticware used for collection and analysis of the specimen must be acid washed. In addition, care must
be taken to avoid losses due to volatilization of elemental Hg and MeHg, especially when preserving or concentrating the samples.
Many procedures require the digestion of the sample before reduction. When attempting to quantify Hg content, especially in biological samples, data are needed to validate the procedures and their use in a given laboratory. All the methods of analysis are prone to large variations.
Biological monitoring of inorganic Hg, including elemental Hg, requires measurement of Hg concentrations in blood, urine, or both (Clarkson et al. 1988). Biological monitoring for MeHg usually involves measuring Hg content in scalp hair, blood, or both. The MeHg incorporated into hair is stable and can be used for longitudinal timing (historical record) of exposure to MeHg by analyzing segments of hair (Phelps et al. 1980; IPCS 1990; Grandjean et al. 1992; Suzuki et al. 1992). One source of error in hair Hg analysis is the presence of Hg on the hair surface due to external deposition. Adequate washing of the hair sample before analysis minimizes that error (Francis et al. 1982).
An excellent summary of the analytical methods for determining various species of Hg in biological specimens, including blood, urine, hair, breath, and tissues, as well as in environmental samples can be found in Table 6-1 in Toxicological Profile for Mercury (Update) (ATSDR 1999) and in the World Health Organization (WHO) report Methylmercury (IPCS 1990).
EXPOSURES TO MeHg IN THE U.S. POPULATION
The major source of MeHg exposure in humans is consumption of fish, marine mammals, and crustaceans. Because exposure to MeHg occurs almost entirely through fish consumption and varies according to the types of fish consumed, variations in exposure to MeHg in the U.S. population are based on individual characteristics of fish consumption. Exposure also varies according to the characteristic amounts and types of fish consumed in different regions of the United States. Hg concentrations in commercial fish and seafood in the United States span about two orders of magnitude. For example, herring contains Hg at approximately 0.01 ppm and shark contains Hg at greater than 1 ppm (EPA 1997a). Limited data suggest that coastal regions generally have
higher rates of fish consumption (Rupp et al. 1980). In addition, specific ethnic and cultural subgroups, as well as recreational fishermen, can have increased exposures (EPA 1997a). Population-based estimates of MeHg exposure in the United States have been made on the basis of dietary assessment studies, which provide information on fish consumption by species and by portion size. The combination of intake frequency by species and portion size by species for each individual consumer provides an estimate of the average mass of fish consumed (in grams per day). Summaries of such studies giving national data are provided in EPA's report to Congress (EPA 1997a). Another such dietary assessment study was conducted in New Jersey (Stern et al. 1996). To estimate population-based MeHg exposure from such studies, the gram-per-day amount of each species consumed by each individual is multiplied by the characteristic MeHg concentration of each species (microgram per gram) and then is summed across species to give the average intake of MeHg by each individual (microgram/day). The distribution of individual intakes for the study sample can then provide an estimate of MeHg intake in the underlying population. Uncertainties in such assessments include those in recall and recording of intake frequency and portion size, misidentification of the species consumed, extrapolation of short-term dietary studies to long-term average exposure, and the outdated and incomplete national database on average MeHg concentrations of different fish species. Estimates also typically vary depending on the length of time over which the fish-intake data was obtained (e.g., 1-day recall versus 1-week recall). These uncertainties are discussed by EPA (1997a) and Stern et al. (1996). Table 2-3 presents the EPA (1997c) analysis of MeHg intake for the general population and for the population of women of childbearing age based on fish-consumption data for month-long consumption. Estimates based on intake from such data are generally lower than those based on 1-day dietary data. Table 2-3 also presents data from New Jersey based on a 7-day recall survey. These data, along with the study by Rupp et al. 1980, suggest that the population in that region of the United States has higher intakes than the U.S. population in general. Estimates of population exposure and risk based on the average exposure of the U.S. population might, therefore, underestimate exposure to large subpopulations. Upon completion, data from Continuing Survey of Food Intakes by Individuals (CFSII) and National Health and Nutrition Examination
TABLE 2-3 Estimated Average MeHg Intake for the U.S. Population and for New Jersey Fish Consumers
|
Average Daly Intake of MeHg (µg/day)a |
||||
|
Percentiles of the Population |
General Population |
Women of Childbearing Age |
||
|
New Jerseyd |
||||
|
50th |
1.4 |
3.1 |
0.6 |
3.2 |
|
75th |
3.5 |
5.8 |
1.8 |
5.4 |
|
90th |
9.1 |
13.1 |
4.8 |
10.8 |
|
95th |
15.6 |
21.1 |
7.8 |
15.7 |
|
99th |
49.9 |
22.2 |
26.5 |
|
aAssuming body weight of 70 kg for the general population and 60 kg for women of childbearing age.
bData from EPA 1997a.
cUnweighted average across ethnic groups.
dData from Stern et al. 1996.
eWomen 15-45 years old.
fWomen 18-40 years old.
Survey (NHANES IV) might provide information on regional fish consumption. NHANES IV is also designed to provide information on MeHg exposure in U.S. populations.
Consumption of animals or poultry fed fish meal might increase the exposure to MeHg, but data are not available. The use of organic Hg compounds as preservatives in vaccines and medical preparations is also a source of exposure and is of particular importance in young children who might be more sensitive to those mercurials than adults. As many as 219 such products are in use (FDA 1999). Thimerosal (TM) (sodium ethylmercurithiosalicylate) and phenylmercuric acetate (PMA) are the most frequently used compounds, at concentrations of 0.01% and 0.0002%, respectively. The FDA estimates that 75-80 kg of Hg compounds are used annually by the manufacturers of those vaccines and medical preparations. The risks associated with thimerosal use in vaccines have been discussed in an interim report to clinicians (American Academy of Pediatrics 1999).
Small amounts of MeHg can be formed in the gut by intestinal bacte-
ria. A.O. Summers (University of Georgia, personal commun., Dec. 1999) estimated that 9 µg of MeHg can be formed per day in the gut of humans. That estimate is based on the bacterial species reported to occur in the human gut and assumes that there are 454 g of feces in the lower bowel of an adult human. However, not all the MeHg that is synthesized would be absorbed. Some of the methylation would occur in the colon, where absorption is less. In addition, intestinal flora can demethylate MeHg to inorganic Hg, which is poorly absorbed by the GI tract (Nakamura et al. 1977; Rowland et al. 1980).
The major source of exposure to elemental Hg in the general U.S. population is due to Hg vapor released from dental amalgams (Goering et al. 1992; Halbach 1994; Lorscheider et al. 1995). Approximately 300 metric tons of Hg are used annually by dentists for amalgams (Arenholt-Bindslev and Larsen 1996). Most amalgams used in the United States contain approximately 50% Hg (IPCS 1991; Aposhian et al. 1992a; Lorscheider et al. 1995). In a study of college students who have dental amalgams, two-thirds of the Hg excreted in the urine appeared to be derived from the Hg vapor released from their amalgams (Aposhian et al. 1992a). Evidence shows that Hg vapor from dental amalgams enters tissues, including the brain, where it is oxidized to inorganic Hg. Pregnant sheep given amalgam fillings labeled with radioactive Hg accumulated radioactivity in maternal and fetal tissues within a few days (Vimy et al. 1990). Significant positive correlations between the number of amalgams in the mouth and the mercury content of human tissues, including the brain, are also seen (Drasch et al. 1994). The mean concentration of total Hg in whole blood (in the absence of consumption of fish with high concentrations of MeHg) is probably of the order of 5-10 µg/L (IPCS 1991; Mahaffey and Mergler 1998). This concentration is most likely due to exposure to Hg vapors from amalgams, because retention of inorganic Hg is very low compared with retention of organic and elemental Hg. Furthermore, exposure to MeHg from non-fish sources is also very low (IPCS 1991).
Occupational exposure to elemental Hg has occurred because of accidents in chloralkali plants (Bluhm et al. 1992). However, there are other potential occupational exposures to elemental Hg. In addition, some Caribbean religions use elemental Hg in religious ceremonies (Wendroff 1995). Children have been known to play with elemental Hg because of its fascinating physical properties (i.e., liquid silver), possibly
severely contaminating living and play areas (ATSDR 1999). In wastewaters, the main sources of elemental Hg are dental offices, hospitals, and laboratories (Arenholt-Bindsley and Larsen 1996). Exposure of humans to mercuric Hg has occurred because of intentional or accidental (e.g., occupational exposures) poisonings with mercuric chloride (Clarkson et al. 1988).
TOXICOKINETICS
Absorption and Distribution
Methylmercury
Most fish contain MeHg. Many freshwater fish in the United States contain more than 2-3 ppm of Hg (Northeast States for Coordination of Air Use Management (NESCAUM 1998). Populations worldwide that eat fish regularly can have concentrations of more than 10 ppm in their hair (Cernichiari et al. 1995). About 95% of the MeHg in fish ingested by humans (Aberg et al. 1969; Miettinen 1973) or about 95% of methylmercuric nitrate given orally to volunteers (Aberg et al. 1969) was found to be absorbed from the gastrointestinal (GI) tract. Although MeHg toxicity following ingestion is the primary focus of this report, it should be noted that MeHg also is readily absorbed through the skin and lungs. The extent of absorption following inhalation exposure is believed to be high.
Once absorbed into the bloodstream, MeHg enters the red blood cells. More than 90% of the MeHg that is found in blood is bound to hemoglobin in red blood cells (Kershaw et al. 1980). Aberg et al. (1969) studied the distribution of Hg compounds in three healthy male volunteers administered [203Hg]-methylmercuric nitrate orally. 203Hg was found in the blood 15 min after administration and peaked within 3-6 hr. The concentration in red blood cells was 10 times greater than that in plasma. MeHg binds to cysteine residue number 104, of the α chain and numbers 93 and 112 of the β chain of hemoglobin. Numbers 104 and 112 are cysteine residues in the contact junction of the hemoglobin molecule. Number 93 is out of the junction and binds to MeHg easily because it is on the external surface of the hemoglobin molecule. The number and
position of the junctional and external cysteine residues on hemoglobin differ in animal species. An extensive table, including the data for the hemoglobin of eight animal species, can be found in Doi (1991). Some MeHg is also bound to plasma proteins. In humans exposed orally to large amounts of MeHg daily, the percentage of the total Hg found as inorganic Hg in whole blood, plasma, breast milk, liver, and urine was 7%, 22%, 39%, 16-40%, and 73% respectively (IPCS 1990). Matsuo et al. (1989) reported autopsy data on Japanese subjects. Kidney and liver contained total Hg concentrations on the order of hundreds of ng/g. Cerebrum, cerebellum, heart, and spleen contained total Hg concentrations on the order of tens of ng/g. Approximately 80% of the Hg in those organs was in the form of MeHg. In the liver, kidney medulla and kidney cortex 33%, 15% and 11% of the mercury was methylmercury, respectively. Consumption of high concentrations of MeHg in fish results in only about 5% inorganic Hg in whole blood and about 20% inorganic Hg in scalp hair (Phelps et al. 1980). It should be emphasized that the exact form(s) in which MeHg exists in the body is still unknown. MeHg ion is hydrated in aqueous solutions. There are pH-dependent reactions giving rise to Hg-substituted oxonium ions (Figure 2-1). Cotton and Wilkinson (1988), state that “the types of complexes formed by the two ions differ markedly; Hg 2+ compounds of amino acids containing SH groups are polymeric and polar, whereas the CH3HgR species are nonpolar and monomeric. For example the cysteinate is with a linear C—Hg—S.” The chemistry and formation of Hg-substituted oxonium ion complexes may affect MeHg transport, but investigators who study such transport largely ignore them.
About 10% of the body burden of MeHg is found in the brain where it is slowly demethylated to inorganic mercuric Hg (see Figure 2-2). MeHg is also readily transferred to the fetus and the fetal brain. Evidence from rat experiments suggests that MeHg transport across the blood-brain barrier occurs via a MeHg-L-cysteine complex, which is transported by the L-system (leucine preferring) amino acid carrier (Kerper et al. 1992). MeHg-cysteine is released in vitro from a MeHg-glutathione complex by the action of γ-glutamyltransferase and dipeptidases (Naganuma et al. 1988). That action suggests that glutathione might play an indirect role in the transport of MeHg into endothelial cells. The MeHg-cysteine or MeHg-glutathione complex would be expected to be water soluble. That would not support the hypothesis
that the rapid uptake of MeHg by the brain is due to lipid solubility in body tissues and fluids. Recently, Fujiyama et al. (1994) proposed that the MeHg-glutathione complex is the mechanism by which MeHg can efflux rat astroglia. Aschner et al. (1991), however, proposed that the MeHg-cysteine complex is the mechanism by which MeHg is exported from astroglia.
A case study of family members that developed classic signs of MeHg poisoning due to the consumption of contaminated pork indicates that the cerebrum and the cerebellum are particularly sensitive to MeHg (Davis et al. 1994). Analyses of various regions of the brain of one female member upon autopsy, several years later, revealed that the extent of brain damage correlated with regional-brain Hg concentrations. Inorganic Hg comprised 82-100% of the total Hg, suggesting that most of the MeHg had been converted to inorganic Hg during the period. The highest levels of Hg were found in the cerebrum and cerebellum. Magnetic Resonance Imaging (MRI) studies showed brain damage in the calcarine cortices, parietal cortices, and cerebellum of other family members. The damage in those areas is believed to underlie many of their persistent clinical signs, because those areas of the brain are responsible for coordination, balance, and sensations (see Chapter 5).
Dimethylmercury
Dimethylmercury is a supertoxic form of Hg (Gosselin et al. 1984) that has been fatal after accidental exposure. At Dartmouth College, a chemistry professor died 298 days after several drops of dimethylmercury fell on her latex gloves. The gloves did not appear to act as a barrier, and the compound was rapidly absorbed through her skin. Six to 7 months after her exposure, her blood Hg concentration was 1,000 µg/L (Nierenberg et al. 1998). Typical blood concentrations of Hg are in the range of 1 to 8 µg/L. Mouse studies suggest that the extremely toxic dimethlymercury must be metabolized to MeHg before it can enter the brain (Ostlund 1969).
Elemental Mercury
Absorption of elemental Hg vapor via the lungs is rapid. In humans,
75-85% of an inhaled dose is absorbed (Kudsk 1965; Okawa et al. 1982; Hursh 1985; Hursh et al. 1985). Elemental Hg in liquid or vapor form is not well absorbed from the GI tract (less than 0.01%) (Bornmann et al. 1970). In humans exposed to elemental Hg vapor, 97% of the absorption occurred via the lungs, and less than 3% of the total amount absorbed was via the skin (Hursh et al. 1989).
Because elemental Hg is very lipid soluble, its diffusion across the lungs and dissolution in blood lipids is rapid (Berlin 1986). The fact that it is uncharged with intermediate molecular weight and size might be another reason why it passes readily from air to blood. It is distributed throughout the body, and readily crosses the placenta and the blood-brain barrier (Vimy et al. 1997; Fredricksson et al. 1992, 1996; Drasch et al. 1994) (see Figure 2-3). Elemental Hg is oxidized to mercuric Hg. Eventually, the Hg ratio of red blood cells to plasma is 1:1.
Inorganic Mercury
Approximately 7-15% of an ingested dose of mercuric chloride is absorbed from the GI tract (WHO 1976; Miettinen 1973). Absorption is proportional to the water solubility of the mercuric salt. Mercuric Hg has a high affinity for sulfhydryl groups in the red blood cells and plasma. The half-life in the blood is reported to be 19.7-65.6 days (Hall et al. 1995).
The highest accumulation of mercuric Hg is in the kidneys. The major fraction of inorganic Hg in rat kidney is bound to metallothionein (Jakubowski et al. 1970; Wisniewska et al. 1970; Komsta-Szumska et al. 1976). In contrast MeHg has a low affinity for metallothionein (Chen et al. 1973). Because of its ionic charge, mercuric Hg does not readily penetrate the blood-brain barrier or the placenta.
Biotransformation
MeHg is converted in tissues to mercuric Hg (Magos and Butler 1972; Dunn and Clarkson 1980). The rate of demethylation in rats and most other species is very slow. The mechanisms involved in conversion of MeHg to mercuric Hg are controversial. The enzymes in mammalian
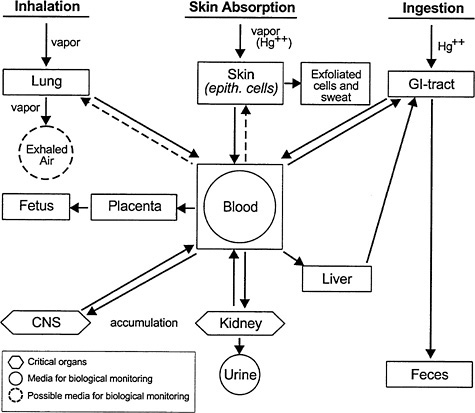
FIGURE 2-3 Inorganic mercury kinetics. This diagram is complicated by the fact that inhaled vapor is oxidized to Hg++ so that both species are present. The inhaled vapor is highly mobile, readily crosses cell membranes, the blood-brain barrier, and the placenta. The Hg++ species is much less mobile, crossing the blood-brain barrier and placenta much more slowly than dissolved vapor. Source: Elinder et al. 1988. Reprinted with permission from Biological Monitoring of Toxic Metals; copyright 1988, Plenum Publishing Corporation.
tissues believed to be responsible for the biotransformation have never been identified. Greater emphasis has been placed on investigating the possible role of a free radical mechanism (Suda and Hirayama 1992). In addition, γ-globulin and serum albumin have been shown to have
similar degradation activity that can be stimulated further by glutathione (Gage 1975). Intestinal flora, tissue macrophages, and fetal liver are all sites of tissue demethylation.
Experiments in bacteria demonstrate many different mechanisms to detoxify heavy metals. For example, some metals are actively transported out of the cell (e.g., arsenite) (Perry and Silver 1982; Mobley and Rosen 1982; Silver and Keach 1982), and other metals are sequestered by protein binding in the cell (Kägi and Nordberg 1979). Organic Hg compounds are detoxified by a microbial organomercurial resistance system (see Figure 2-4). An organomercurial lyase catalyzes the protonolysis of the carbon-Hg bond to give a hydrocarbon and a mercuric ion (Summers 1985; Robinson and Tuovinen 1984; Summers and Silver 1978). Mercuric reductase then catalyzes the reduction of mercuric Hg to elemental Hg. NADPH is the coenzyme in that reaction (Fox and Walsh 1982, 1983; Brown et al. 1983). Because elemental Hg is volatile, it evaporates from the bacterial culture.
GI absorption of MeHg is decreased by intestinal flora that convert MeHg to inorganic Hg (mercuric ion) (Nakamura et al. 1977; Rowland et al. 1980), which is poorly absorbed. Organomercurial lyase has been purified from Escherichia coli (Begley et al. 1986). The enzyme is encoded on the plasmid R831. No cofactors are required for enzyme activity, and the enzyme structure does not contain any metals. The enzyme can catalyze protonolysis of the C-Hg bond in primary, secondary, and tertiary alkyl, vinyl, allyl, and aryl organomercurial salts to the hydrocarbon and mercuric ion. A thiol must be present for activity, cysteine being the most active thiol compound, for demethylation of organic mercurials.
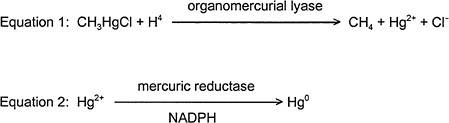
FIGURE 2-4 Organomercurical detoxification pathway in bacteria.
Enzymes similar to those found in bacteria have not been found in mammals. Demethylation of MeHg is thought to occur via a free-radical mechanism in the mammalian brain, possibly eliminating the need for those enzymes. It is also possible that the enzymes have not yet been identified.
Lefevre and Daniel (1973) examined rat, mouse, and guinea-pig liver homogenates for activity that would degrade organic Hg compounds. Although a minimum level of activity was found, phenylmercuric acetate and methoxyethylmercury chloride were degraded, but not MeHg. Fang and Fallin (1974) were able to show cleavage of phenylmercuric acetate (PMA) and ethylmercury chloride in the kidney and liver of rats, but no activity was seen against MeHg.
Elemental Hg vapor is oxidized to mercuric mercury by catalase and hydrogen peroxide (H2O2) in blood and tissues (Berlin 1986). H2O2 production is the rate-limiting step.
When mercuric Hg is administered orally to rodents, elemental Hg vapor has been detected in the expired air, indicating that some metabolism to elemental Hg must have occurred. Mammals do not methylate mercuric Hg; however, intestinal flora can methylate Hg2+ to a small extent (Rowland et al. 1977).
Excretion
Approximately 1% of the human body burden of MeHg is excreted daily (Clarkson et al. 1988). In humans, the major routes of excretion are via the bile and feces. About 90% of a given dose of MeHg is eventually excreted in the feces as mercuric Hg in humans and other species. Approximately 10% is excreted as mercuric Hg via the urine. Much of the biliary MeHg is reabsorbed; MeHg complexed with glutathione is eliminated via the bile.
Following oral administration of [203Hg] methylmercuric nitrate, only about 33% of the administered dose was excreted in 49 days, fecal excretion being the main route of excretion (Miettinen 1973). There was a 0.18% to 0.27% excretion of the dose in the urine in 10 days and 3.3% excretion in 49 days. The extent of urinary excretion continued to increase up to 71 days after ingestion. A maximum of 0.12% of the administered dose of Hg was found per gram of hair. That amount was found 40-50 days after ingestion. Using whole-body measurements, the
half-life of MeHg was 70-74 days. No methylmercuric chloride was found in the sperm, but about 50% of the body content was found in the liver and about 10% was found in the head.
In humans, the whole-body half-life of MeHg was estimated to be 70-80 days (Aberg et al. 1969; Miettinen 1973; Bernard and Purdue 1984; EPA 1997b).
The half-life in blood for MeHg as measured in blood and hair of humans ranged from 48 to 53 days (Miettinen et al. 1971; Kershaw et al. 1980; Sherlock et al. 1984; Cox et al. 1989). Elimination rates for MeHg are dependent upon species, dose, sex, and animal strain (Nielsen 1992).
It is pertinent to note that neonatal rats and monkeys are limited in their ability to excrete MeHg into the bile (Ballatori and Clarkson 1982). Therefore, it takes them longer than mature animals to excrete MeHg (Thomas et al. 1982). In addition, their intestinal flora might also be less able to demethylate MeHg during this suckling period (Rowland et al. 1977; Sundberg et al. 1998; Grandjean et al 1994). If those two phenomena are true for humans, then neonates might be particularly sensitive to exposure to MeHg. GSH may be the major cellular defense against MeHg toxicity. GSH complexation with MeHg is a major mechanism for MeHg excretion from the cell, thus protecting against MeHg toxicity (Kromidas et al. 1990).
MeHg has been measured in the breast milk of rats, humans, and guinea pigs (Sundberg and Oskarsson 1992; Yoshida et al. 1992). Therefore, breast milk is considered a route of excretion, but it is also an important route of exposure to suckling neonates. In human breast milk, 16% of the Hg was found to be MeHg (Skerfving 1988). That percent is much lower than the percent of Hg found as MeHg in whole blood. In animals, the total Hg content of breast milk was found to be proportional to the total Hg content of the plasma (Skerfving 1988; Sundberg and Oskarsson 1992).
A small amount of elemental Hg vapor is excreted unchanged in exhaled air, sweat, saliva, feces, and urine (Cherian et al. 1978). Only small amounts of elemental Hg can be detected in the urine (Stopford et al. 1978) and exhaled air (Hursh et al. 1976). Excretion via sweat and saliva is usually minimal. The hair-life for whole-body Hg excretion was 58 days in humans (Hursh et al. 1976). Elemental Hg is also oxidized in the body to mercuric Hg, which is then excreted in the feces and urine. That is demonstrated by the observation that, after exposure to Hg
vapor, the mercuric Hg content in the feces increases and is four times greater than that in the urine.
Following oral administration of mercuric Hg to humans, about 85% was excreted in the feces within a few days (Miettinen 1973). Fecal excretion of mercuric Hg occurs as the result of secretion through the small intestine epithelium and colon, and bile secretion (Berlin 1986). Mercuric Hg is also excreted in the urine, sweat, lung (Clarkson et al. 1988), and breast milk (Yoshida et al. 1992). Urinary excretion is useful for biological monitoring of inorganic Hg. Absorbed inorganic Hg has been estimated to have a half-life of 40 days (Rahola et al. 1973) or 67 days (Hall et al. 1995). WHO (IPCS 1990) reported a half-life of 35 days. Humans occupationally exposed to inorganic Hg excrete in their urine three forms of this element: a metallic form, a Hg-cysteine complex, and a large unidentified complex (Henderson et al. 1974).
MOBILIZATION OF BODY Hg
Synthetic chelating or complexing agents that compete with endogenous ligands for mercuric or organic Hg increase the urinary excretion of inorganic Hg and organic Hg and reduce the body burden (Aposhian 1983; Aposhian and Aposhian 1990). Compounds that have been used therapeutically are 2,3-dimercapto-1-propane sulfonate (DMPS, Dimaval, and Unithiol) and meso 2,3-dimercaptosuccinic acid (DMSA, succimer). DMPS and DMSA are water-soluble, less-toxic chemical analogs of 2,3-dimercapto-1-propanol (British Anti-Lewisite BAL dimercaprol). BAL is lipid soluble and must be given by deep intramuscular injection. DMPS and DMSA can be taken orally. There is an injectable preparation of DMPS. About 55% of patients administered BAL have one or more adverse reactions to it (Klaassen 1996), although most of the reactions are not serious. However, because BAL redistributes Hg, increasing brain Hg concentrations when given to Hg-intoxicated animals, its continued therapeutic use is questionable (Berlin 1986).
DMPS was introduced into the official Soviet drug armamentarium in 1958 (Klimova 1958) and to the western world in 1978. A number of reviews of DMPS and other chelating agents have appeared during the last 18 years ( Aposhian 1983; Aposhian et al. 1992b, 1995; Aaseth et al. 1995). It is approved for use by the German and Chinese equivalents of
the U.S. Food and Drug Administration (FDA). Clarkson et al. (1981) used DMPS to treat the MeHg-poisoned humans in Iraq and showed that it is more potent than D-penicillamine N-acetyl-DL-penicillamine or a thiolated resin for decreasing inorganic Hg in the blood. Elsewhere, including the United States, DMPS has been used by alternative-medicine physicians concerned with dental amalgam toxicity. It was used recently to increase the urinary excretion of Hg in eight humans exposed to mercurous Hg (Gonzalez-Ramirez et al. 1998). In contrast to BAL, DMPS does not increase brain Hg concentration in rats (Aposhian et al. 1996).
Although it has not been approved for this use, DMSA has been used to treat Hg intoxication (Aposhian 1983; Aposhian and Aposhian 1990). It was approved by the FDA in 1990 for the treatment of children with blood Pb concentrations greater than or equal to 45 µg per deciliter.
CHEMICAL FORMS OF Hg IN TOXICITY
There is ample evidence from studies of humans (Takizawa 1979; Matsuo et al. 1987; Takeuchi et al. 1989) and experimental studies using animal models (Vahter et al. 1994) that MeHg is slowly biotransformed to inorganic Hg in the brain. Although the rate of demethylation of MeHg in the brain appears to be dose related, many questions remain concerning the mechanisms by which the brain biotransforms MeHg to inorganic Hg and the slow rate at which it occurs. Davis et al. (1994) reported that a New Mexico patient who died approximately 21 years after eating MeHg-tainted pork had greatly increased total Hg concentrations in various regions of the brain (71 to 300 times greater than controls). A minimum of 82% of the Hg in the patients brain was in the inorganic form. In most regions of her brain, 100% of the Hg was in the inorganic form. Similar results were found in a Minamata patient who died 18 years after the exposure (Takeuchi and Komyo 1977).
Experimental studies have also reported a slow increase in the concentration of inorganic Hg in the brain in a number of species after administering MeHg and analyzing the brain for total and inorganic Hg from days to years after exposure (Friberg and Mottet 1989). When monkeys were exposed daily to high doses of MeHg for long periods of time, there were significant concentrations of inorganic Hg found in the brain (Lind et al. 1988). By 69-166 weeks, 10-33% of the brain Hg was
inorganic. Female monkeys (Macaca fascicularis) received daily doses of MeHg for up to 18 months (Vahter et al. 1994, 1995). When the brains were examined for Hg species, inorganic Hg made up about 9% of the total Hg after 6-12 months of exposure and 18% after 18 months of exposure. Six months after a 12-month exposure ended, it was 74%. The authors stated that they believed that inorganic Hg “was formed by demethylation of MeHg in the brain. ”
The extent to which demethylation of MeHg produces toxicity in the brain is not known. Studies by Norseth and Clarkson (1970) and Syversen (1974) indicated that MeHg itself mediates the toxicity following MeHg exposure. In addition, Magos et al. (1985) provided direct evidence that the extent of brain damage correlates better with the brain concentration of intact organic Hg than inorganic Hg when MeHg or ethyl Hg is administered to rats. A possible hypothesis is that the long half-life of inorganic Hg in the brain once demethylation occurs might be responsible for the latent or long-term MeHg effects that have been reported. No direct evidence to support that hypothesis is available at this time.
In addition to the questions regarding whether inorganic or organic Hg mediates MeHg toxicity at the cellular level, questions have also been raised regarding the species responsible for the Iraqi poisonings. In the Iraqi poisoning episode, some of the grain seeds appeared to contain phenyl Hg instead of MeHg. There is no doubt, however, that gas chromatography identified MeHg in the blood of most of the exposed population, and phenyl Hg would have been quickly converted to inorganic Hg in the blood (T.W. Clarkson, University of Rochester, personal commun., Nov. 1999). In addition, the phenyl Hg was in the barley seeds and no barley seeds were used to make bread (T.W. Clarkson, University of Rochester, personal commun., Nov. 1999).
TOXIC EFFECTS AND TARGET ORGANS
Currently, there is a general consensus that the critical organ for MeHg toxicity is the brain. Both the adult and fetal brains are susceptible to MeHg toxicity (see Figure 2-2), although the developing nervous system appears to be more sensitive. Studies of the Minamata disaster in Japan indicate that prenatal exposure causes damage throughout the fetal brain and, at high doses, results in effects in the offspring that are
largely indistinguishable from cerebral palsy caused by other factors (Harada 1995). Exposure of adults to MeHg resulted in focal lesions (Clarkson 1997). The neurotoxicity of chronic MeHg exposure at lower levels is not immediately evident. A latent period of 1 month or more usually occurs (Bakir et al. 1973; IPCS 1990). Other adverse effects (e.g., cardiovascular and immunological effects) have been reported to occur at MeHg doses lower than those producing adverse effects in the nervous system. Those effects, however, are not as well studied as the neurotoxic effects. The health effects of MeHg are discussed in more detail in Chapter 5.
The target organs of elemental Hg are the brain and kidney. The toxicity of elemental Hg is believed to be due to mercuric Hg. Inhaled elemental Hg vapor readily crosses the blood-brain barrier and is then oxidized to mercuric Hg. The latter becomes firmly bound to macro-molecules in the brain. There does not seem to be any endogenous mechanism for the rapid removal of mercuric Hg from such sites. In humans occupationally exposed to elemental Hg vapor, signs of severe exposure include tremor, psychiatric disturbances, gingivitis, and altered behavior.
The target organ of mercuric Hg toxicity is the kidney due to Hg accumulation there. The earliest signs of renal injury due to Hg compounds are increased urinary excretion of N-acetyl-β-glucoseaminidase, β2-microglobulin and retinol-binding protein. Although the exact mechanism of renal toxicity is not known, it is known that mercuric Hg has a strong affinity for sulfhydryl moieties. The formation constants of Hg sulfhydryl complexes are very high (approximately Kf = 1030) (Divine et al. 1999). The formation constant for mercuric Hg and the anionic form of a sulfhydryl group, RS-, is greater than or equal to 1010-fold higher than that for the carboxyl or amino groups (Ballatori 1991; Divine et al. 1999). Since there is a wide distribution of sulfhydryl groups in the body, especially in proteins, mercuric Hg is believed to cause toxicity by combining with the active centers of critical enzymes and structural proteins.
BIOCHEMICAL MECHANISMS OF TOXICITY
Experimental studies of the possible biochemical mechanisms of MeHg neurotoxicity have been reviewed in detail (Atchison and Hare
1994; Chang and Verity 1995; ATSDR 1999). Mitochondrial changes, induction of lipid peroxidation, microtuble disruption, and disrupted protein synthesis have all been proposed as possible mechanisms. In developmental toxicity, disruption of cell-surface recognition has also been proposed as a possible mechanism (Baron et al. 1998; Dey et al. 1999). To date, no definitive data are available that point to any one mechanism as the proximate cause for the neurotoxic symptoms associated with MeHg exposure in adults.
Exposure of rats to MeHg has long been known to cause biochemical and ultrastructural changes in the mitochondria, but the evidence is not convincing that those changes are the primary mechanism for MeHg toxicity (Denny and Atchison 1994; Yoshino et al. 1966). Sarafian and Verity (1991) showed that MeHg causes membrane peroxidation in nerve cells. Because antioxidants, such as vitamin E and selenium, offer some protection in vivo against MeHg neurotoxicity (Chang et al. 1978; Magos and Webb 1980), free-radical-induced lipid peroxidation might be involved in the cellular damage caused by MeHg. However, lipid peroxidation does not appear to be the critical mechanism that causes cell lethality for many reasons, as summarized by Atchison and Hare (1994).
MeHg disrupts protein synthesis, and disruption has been proposed as the primary mechanism of MeHg neurotoxicity. In the rat, inorganic Hg, however, was 10 times more potent an inhibitor of cell-free protein synthesis than MeHg (Sugano et al. 1975). Stimulation of protein synthesis by MeHg was also reported (Burbaker et al. 1973). Mitotic arrest is one of the most sensitive indicators of MeHg exposure in mice. A single 4-mg/kg dose MeHg on postnatal day 2 resulted in a brain Hg concentration of only 1.8 µg/g of tissue. The ratio of late mitotic figures to total mitotic figures was significantly reduced in the cerebellum of exposed mice, indicating mitotic arrest (Sager et al. 1984).
Oxidative stress might also be involved in MeHg toxicity. Glutathione is the major antioxidant of the cell. After exposure to MeHg, glutathione concentrations decline and then increase. Cells that are made resistant to MeHg toxicity had an increase in the rate of efflux of MeHg and had 4-fold higher glutathione concentrations than normal cells (Miura and Clarkson 1993).
Another proposed mechanism underlying MeHg toxicity is disruption of microtubules in the neuronal cytoskeleton (Miura and Imura 1987). Hg binds to thiols in the tubulin, the protein monomers that form micro-
tubules, and blocks the depolymerization and repolymerization of microtubules. Because the breakdown and assembly of microtubules are required for many cell functions, including cell division and migration, disruption of microtubule assembly could disrupt cellular processes. In vitro, MeHg has been shown to disrupt cell-cycle progression in primary rat brain cells (Ponce et al. 1994). The developing nervous system would be particularly sensitive to those effects due to the extensive cell division and migration that occurs during its development.
The ability to exchange between thiols forms the basis of therapeutic techniques following both MeHg exposure and exposure to Hg vapor. The neurotoxic effects of combined exposure to MeHg and Hg vapor have been reported to be similar in nature but more severe than those observed following exposure to each alone (Fredriksson et al 1996). There are many similarities in the biochemistry of the MeHg+ and the inorganic Hg cation (Hg2+), which is responsible for the toxicity following Hg vapor exposure (Clarkson 1997). Both cations exhibit a high affinity for SH groups, and association and dissociation reactions are rapid (Carty and Malone 1979). Both are found in tissues bound to large and small molecular-weight thiol-containing molecules (proteins, cysteine, and glutathione). The formation of Hg thiol bonds is believed to underlie the mobility and toxicity of Hg in the body (Clarkson 1997).
Although the exposure patterns and toxicokinetics and toxicodynamics of the different Hg species are usually studied separately, organic Hg and elemental Hg are eventually converted in vivo to inorganic Hg. The estimated average daily intake and retention of various forms of Hg are shown in Table 2-4. Estimates of the retention in the body of Hg from dental amalgams range from 3.1 to 17 µg per day. Estimates of MeHg retention range from 1 to 6 µg per day. The ratio of MeHg to total Hg will be different among those with high fish consumption. The data in Table 2-4 suggest that average exposure to Hg from dental amalgams might be considerably higher than exposure to Hg from MeHg. However, the available data are not adequate to permit a definitive comparison.
MeHg is very slowly but ultimately metabolized in situ in the brain to inorganic Hg. Elemental mercury is also oxidized to inorganic Hg in the brain. It is unclear whether MeHg toxicity at the cellular level is caused by the parent compound itself, due to the inorganic Hg that is its metabolite, or is caused indirectly by the free radicals generated by the
TABLE 2-4 Estimated Daily Intake and Retentiona (micrograms per day) of Total Hg and Hg Compounds in the General Population Not Occupationally Exposed to Hg
|
Exposure |
Elemental Hg Vapor |
Inorganic Hg Compoundsb |
MeHg |
|
Air |
|||
|
Food Sources |
|||
|
Fish |
|||
|
Non-Fish |
|||
|
Drinking water |
|||
|
Dental amalgams |
|||
|
Total |
3.9-21 (3.1-17) |
4.3 (0.3) |
1-6 (1-6) |
|
aRetention is assumed to be 95% of intake for MeHg, 80% for elemental Hg vapor, and 7% for inorganic Hg compounds. bData from IPCS 1991. cMean value. dData are for United States nationwide (per capita), calculated assuming a body weight of 70 kg (EPA 1997). eMedian value. fEquivalent data for women of childbearing age: median = 1, assuming a body weight of 60 kg. gData are for the general population of reproductive age in New Jersey fish consumers, calculated assuming a body weight of 70 kg (Stern et al. 1996). hEquivalent data for women of childbearing age: mean = 5, median = 3, assuming a body weight of 60 kg. Note: Values given are the estimated average daily intake; the figures in parentheses represent the estimated amount retained in the body of an adult. Values are quoted to two significant figures. |
|||
metabolism of MeHg to inorganic Hg. If the ultimate toxic form of MeHg is indeed its inorganic Hg metabolite, that suggests that the dose of inorganic Hg to the brain from elemental Hg exposure (particularly from dental amalgams) and MeHg might be cumulative. That is the case even if oxidation of elemental Hg in the blood before absorption to the brain is considered. Risk-assessment models for MeHg, therefore, should consider additional chronic sources of exposure to Hg such as dental amalgams.
Such considerations are complicated by uncertainty about the mechanisms by which MeHg specifically exerts its neurodevelopmental toxicity. Such mechanisms might not be the same as those responsible for adult neurotoxicity. Nonetheless, the potential implications of additive toxicity from fish consumption and dental amalgams make elucidation of the mechanisms of MeHg toxicity in the brain a critical research priority.
SUMMARY AND CONCLUSIONS
-
The major source of MeHg exposure in humans is consumption of fish, marine mammals, and crustaceans.
-
The water solubility of mercuric chloride is greater than elemental Hg. That of elemental Hg is greater than MeHg. The solubility of the different forms of Hg might play a role in their differential toxicity.
-
Elemental Hg and a portion of MeHg are converted to mercuric Hg in the body. The conversion of MeHg occurs at a very slow rate.
-
Analytical methods for analyzing Hg in biological samples include AAS, AFS, NAA, ASV, ICP-MS, and XRF. Care must be taken to prevent contamination by Hg during sample preparation and analysis.
-
MeHg is readily absorbed from the GI tract. After ingestion, 90% of the MeHg in blood can be found in red blood cells. It is bound primarily to red-blood-cell hemoglobin, but some is bound to plasma proteins.
-
Hg in blood reflects recent exposure to MeHg and inorganic Hg. The half-life in blood for humans averages 50 days but can vary substantially. Because neonates have an immature transport system, they do not excrete MeHg as rapidly as adults.
-
Hg in hair is approximately 90% MeHg. Hair measurements have the advantage of providing a historical record of MeHg exposure but do not accurately reflect exposure to inorganic Hg.
-
The daily excretion of MeHg is about 1% of the human body burden. It is excreted mainly via the bile and feces as MeHg and mercuric Hg. Complexing with GSH is involved. Urine MeHg concentrations do not accurately reflect MeHg exposure.
-
For elemental and inorganic Hg, the half-life in blood is 1-2 months. The whole-body half-life is slightly longer, but that does not take into account Hg in the brain, which is cleared very slowly. Excretion occurs primarily via urine and feces and, to a small extent, saliva, bile, sweat, and lungs.
-
DMPS and DMSA can be used to increase Hg excretion. Dimercaprol (BAL), used in the past for chelation, is contraindicated because it redistributes Hg to the brain.
-
MeHg readily crosses the blood-brain barrier. The rapid uptake of MeHg in the brain has been proposed to be due to lipid solubility, but evidence in rats suggests that the transport is due to the formation of MeHg-cysteine complexes.
-
MeHg accumulates in the brain where it is slowly converted to inorganic Hg. Whether CNS damage is due to MeHg per se, to its biotransformation to inorganic Hg, or to both is still controversial. The mechanisms and cellular site for the biotransformation in humans are not well understood. Both free-radical and enzymatic biotransformation has been proposed.
-
The critical organ for MeHg toxicity is the brain. Both adult and fetal brains are vulnerable. For elemental Hg, the critical organs are the brain and kidney. Both MeHg and elemental Hg are converted to mercuric Hg in the brain, where it is trapped. The biological mechanisms for removing mercuric Hg from the brain are limited. The critical organ for mercuric Hg toxicity is the kidney, where it accumulates.
-
There is emerging evidence that the cardiovascular and immune systems might be major sites of MeHg toxicity (see Chapter 5).
-
The high affinity of MeHg and mercuric Hg for sulfhydryl groups is believed to be a major mechanism that underlies their toxicity. If those sulfhydryl groups are in the active center of critical enzymes, severe inhibition of essential biochemical pathways occurs.
-
The toxicology of the three species of Hg — elemental Hg, mercuric Hg and MeHg — are intertwined, because MeHg and elemental Hg are transformed to inorganic Hg in the brain. Risk-assessment models for MeHg in humans are complicated because of inadequate data regarding the cumulative neurotoxic effects of MeHg per se and its biotransformation product mercuric Hg, which has a very long half-live in the brain.
RECOMMENDATIONS
-
As data become available, exposure to elemental Hg from dental amalgams should be considered in risk assessment of MeHg. Exposure to other chemical forms of Hg should also be considered.
-
Retention of inorganic Hg in the brain for years following early MeHg intake is possibly related to the latent or long-term neurotoxic effects reported. The long half-life of inorganic Hg in the brain following MeHg intake should be considered in risk assessment of MeHg.
-
The mechanisms, including any enzymes, involved in the biotransformation of MeHg to mercuric Hg in human tissues need to be investigated, especially at the subcellular level. The effects of Hg on signaling pathways and the conformation of enzymes and structural proteins should be further elucidated, because the development and function of the brain would be particularly sensitive to such effects.
-
Exposure assessment of the U.S. population — including those with high fish consumption — is needed to provide a full picture of the distribution of MeHg and total Hg exposure nationally and regionally.
REFERENCES
Aaseth, J., D. Jacobsen, O. Andersen, and E. Wickstrom. 1995. Treatment of mercury and lead poisoning with dimercaptosuccinic acid and sodium dimercaptopropane-sulfonate: A review. Analyst120(3):853-854.
Aberg, B., L. Ekman, R. Falk, U. Greitz, G. Persson, and J.O. Snihs. 1969. Metabolism of methyl mercury (203Hg) compounds in man. Arch. Environ. Health 19(4):478-484.
American Academy of Pediatrics. 1999. Thimerosal in vaccines — An interim report to clinicians. Committee on Infectious Diseases and Committee on Environmental Health . Pediatrics 104(3):570-574.
Aposhian, H.V. 1983. DMSA and DMPS: Water-soluble antidotes for heavy metal poisoning. Annu. Rev. Pharmacol. Toxicol. 23:193-215.
Aposhian, H.V., and M.M. Aposhian. 1990. Meso-2,3-dimercaptosuccinic acid: Chemical, pharmacological and toxicological properties of an orally effective metal chelating agent. Annu. Rev. Toxicol. 30:279-306.
Aposhian, H.V., D.C. Bruce, W. Alter, R.C. Dart, K.M. Hurlbut, and M.M.
Aposhian. 1992a. Urinary mercury after administration of 2,3-dimercaptopropane-1-sulfonic acid: Correlation with dental amalgam score. FASEB J. 6(7):2472-2476.
Aposhian, H.V., R.M. Maiorino, M. Rivera, D.C. Bruce, R.C. Dart, K.M. Hurlbut, D.J. Levine, W. Zheng, Q. Fernando, D. Carter, and M.M. Aposhian. 1992b. Human studies with the chelating agents DMPS and DMSA. Clin. Toxicol. 30(4):505-528.
Aposhian, H.V., R.M. Maiorino, D. Gonzalez-Ramirez, M. Zuniga-Charles, Z. Xu, K.M. Hurlbut, P. Junco-Munoz, R.C. Dart, and M.M. Aposhian. 1995. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology 97(1-3):23-28.
Aposhian, M.M., R.M. Maiorino, Z. Xu, and H.V. Aposhian. 1996. Sodium 2,3-dimercapto-1-propanesulfaonte (DMPS) treatment does not redistribute lead or mercury to the brain of rats. Toxicology 109(1):49-55.
Arenholt-Bindslev, D., and A.H. Larsen. 1996. Mercury levels and discharge in waste water from dental clinics. Water Air Soil Pollut. 86(1-4):93-99.
Aschner, M., N.B. Eberle, and H.K. Kimelberg. 1991. Interactions of methylmercury with rat primary astrocyte cultures: Methylmercury efflux. Brain Res. 554(1-2):10-14.
Atchison, W.D., and M.F. Hare. 1994. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 8(9):622-629.
ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Mercury. (Update). U.S. Department of Health & Human Services, Agency for Toxic Substances and Disease Registry , Atlanta, GA.
Bakir, F., S.F. Damluji, L. Amin-Zaki, M. Murthadha, A. Khalidi, N.Y. Al-Rawi, S. Tikriti, H.I. Dhahir, T.W. Clarkson, J.C. Smith, and R.A. Doherty. 1973. Methylmercury poisoning in Iraq. Science 181:230-241.
Baldi, F., and M. Filippelli. 1991. New method for detecting methylmercury by its enzymic conversion to methane. Environ. Sci. Technol. 25(2):302-305.
Ballatori, N. 1991. Mechanisms of metal transport across liver cell plasma membranes. Drug Metab. Rev. 23(1-2):83-132.
Ballatori, N., and T.W. Clarkson. 1982. Developmental changes in the biliary excretion of methylmercury and glutathione. Science 216(4541):61-63.
Baron Jr, S., N. Haykal-Coates, and H.A. Tilson. 1998. Gestational exposure to methylmercury alters the developmental pattern of trk-like immuno-reactivity in the rat brain and results in cortical dysmorphology. Dev. Brain Res. 109(1):13-31.
Begley, T.P., A.E. Walts, and C.T. Walsh. 1986. Bacterial organomercurial lyase: Overproduction, isolation, and characterization . Biochemistry 25(22): 7186-7192.
Berlin, M. 1986. Mercury. Pp. 387-445 in Handbook on the Toxicology of
Metals, 2nd Ed., L. Friberg, G.F. Nordberg, and V.B. Vouk, eds. New York: Elsevier.
Bernard, S., and P. Purdue. 1984. Metabolic models for methyl and inorganic mercury. Health Phys. 46(3):695-699.
Bluhm, R.E., R.G. Bobbitt, L.W. Welch, A.J.J. Wood, J.F. Bonfiglio, C. Sarzen, A.J. Heath, and R.A. Branch. 1992. Elemental mercury vapour toxicity, treatment, and prognosis after acute, intensive exposure in chloralkali plant workers: Part I: History, neuropsychological findings and chelator effects. Hum. Exp. Toxicol. 11(3):201-210.
Bornmann, G., G. Henke, H. Alfes, and H. Mollmann. 1970. Intestinal absorption of metallic mercury. [in German]. Arch. Toxicol. 26(3):203-209.
Brown, N.L., S.J. Ford, R.D. Pridmore, and D.C. Fritzinger. 1983. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry 22(17):4089-4095.
Burbaker, P.E., R. Klein, S.P. Herman, G.W. Lucier, L.T. Alexander, and M.D. Long. 1973. DNA, RNA and protein synthesis in brain, liver and kidneys of symptomatic methylmercury treated rats. Exp. Mol. Pathol. 18(3):263-280.
Cappon, C.J., and J.C. Smith. 1978. A simple and rapid procedure for the gas-chromatographic determination of methylmercury in biological samples. Bull Environ. Contam. Toxicol. 19(5):600-607.
Carty, A.J., and S.F. Malone. 1979. The chemistry of mercury in biological systems. Pp. 433-470 in The Biogeochemistry of Mercury in the Environment, J.O. Nriagu, ed. Amsterdam: Elsevier.
Cernichiari, E., R. Brewer, G.J. Myers, D.O. Marsh, L.W. Lapham, C. Cox, C.F. Shamlaye, M. Berlin, P.W. Davidson, and T.W. Clarkson. 1995. Monitoring methylmercury during pregnancy: Maternal hair predicts fetal brain exposure. Neurotoxicology 16(4):705-710.
Chang, L.W., and M.A. Verity. 1995. Mercury neurotoxicity: Effects and mechanisms. Pp. 31-59 in Handbook of Neurotoxicology, L.W. Chang, and R.S. Dyer, eds. New York: Marcel Dekker.
Chang, L.W., M. Gilbert, and J. Sprecher. 1978. Modification of methylmercury neurotoxicity by vitamin E. Environ. Res. 17(3):356-366.
Chen, R.W., H.E. Ganther, and K.G. Hoekstra. 1973. Studies on the binding of methylmercury by thionein. Biochem. Biophys. Res. Commun. 51(2):383-390.
Cherian, M.G., J.B. Hursh, T.W. Clarkson, and J. Allen. 1978. Radioactive mercury distribution in biological fluids, and excretion in human subjects after inhalation of mercury vapor. Arch. Environ. Health 33(3):109-114.
Clarkson, T.W. 1997. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 34(4):369-403.
Clarkson, T.W., L. Friberg, G. Nordberg, and P.R. Sager, eds. 1988. Biological Monitoring of Toxic Metals. New York: Plenum Press.
Clarkson, T.W., L. Magos, C. Cox, M.R. Greenwood, L. Amin-Zaki, M.A. Majeed, and S.F. al-Damluji. 1981. Tests of efficacy of antidotes for removal of methylmercury in human poisoning during the Iraq outbreak. J. Pharmacol. Exp. Ther. 218(1):74-83.
Cotton, F.A., and G. Wilkinson. 1988. Advanced Inorganic Chemistry, 5th Ed. New York: John Wiley & Sons.
Cox, C., T.W. Clarkson, D.O. Marsh, L. Amin-Zaki, S. Tikriti, and G.G. Myers. 1989. Dose-response analysis of infants prenatally exposed to methyl mercury: An application of a single compartment model to single-strand hair analysis. Environ. Res. 49(2):318-332.
Davis, L.E., M. Kornfeld, H.S. Mooney, K.J. Fiedler, K.Y. Haaland, W.W. Orrison, E. Cernichiari, and T.W. Clarkson. 1994. Methylmercury poisoning: Long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann. Neurol. 35(6):680-688.
Denny, M.F., and W.D. Atchison. 1994. Elevations in the free intrasynaptosomal concentration of endogenous zinc by methylmercury. [Abstract]. Toxicologist 14:290.
Dey, P.M., M. Gochfield, and K.R. Reuhl. 1999. Developmental methylmercury administration alters cerebellar PSA--NCAM expression and Golgi sialytransferase activity. Brain Res. 845(2):139-151.
Divine, K.K., F. Ayala-Fierro, D.S. Barber, and D.E. Carter. 1999. Glutathione, albumin, cysteine, and cys-gly effects on toxicity and accumulation of mercuric chloride in LLC-PK1 cells. J. Toxicol. Environ. Health 57(7):489-505.
Doi, R. 1991. Individual difference of methylmercury metabolism in animals and its significance in methylmercury toxicity. Pp. 77-98 in Advances in Mercury Toxicology, T. Suzuki, N. Imura, and T.W. Clarkson, eds. New York: Plenum Press.
Drasch, G., I. Schupp, H. Hofl, R. Reinke, and G. Roider. 1994. Mercury burden of human fetal and infant tissues. Eur. J. Pediatr. 153(8):607-610.
Dunn, J.D., and T.W. Clarkson. 1980. Does mercury exhalation signal demethylation of methylmercury? Health Phys. 38(3):411-414.
Elinder, C.G., L. Gerhardsson, and G. Oberdörster. 1988. Biological monitoring of toxic metals — Overview. Pp. 1-72 in Biological Monitoring of Toxic Metals, T.W. Clarkson, L. Friberg, G.F. Nordberg, and P. R. Sager, eds. New York: Plenum Press.
EPA (U.S. Environmental Protection Agency). 1997a. Mercury Study Report to Congress. Vol. IV: An Assessment of Exposure to Mercury in the United States . EPA-452/R-97-006. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1997b. Mercury Study Report for Congress. Volume V: Health Effects of Mercury and Mercury Com
pounds. EPA-452/R-97-007. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
EPA (U.S. Environmental Protection Agency). 1997c. Mercury Study Report to Congress. Volume VII: Characterization of Human Health and Wildlife Risks from Mercury Exposure in the United States. EPA-452/R-97-009. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards and Office of Research and Development.
Fang, S.C., and E. Fallin. 1974. Uptake and subcellular cleavage of organomercury compounds by rat liver and kidney. Chem. Biol. Interact. 9(1):57-64.
FDA (Center for Drug Evaluation and Research). 1999. List of Drug and Food that Contain Intentionally Introduced Mercury Compounds. Updated November 17, 1999. Online. Available:http://www.fda.gov/cder/fdama/mercury300.htm
Fox, B., and C.T. Walsh. 1982. Mercuric reductase. Purification and characterization of a transposon-encoded flavoprotein containing an oxidation-reduction-active disulfide. J. Biol. Chem. 257(5):2498-2503.
Fox, B.S., and C.T. Walsh. 1983. Mercuric reductase: Homology to glutathione reductase and lipoamide dehydrogenase. Iodoacetamide alkylation and sequence of the active site peptide. Biochemistry 22(17):4082-4088.
Francis, P.C., W.J. Birge, B.L. Roberts, and J.A. Black. 1982. Mercury content of human hair: A survey of dental personnel. J. Toxicol. Environ. Health 10(4-5):667-672.
Fredriksson, A., L. Dahlgren, B. Danielsson, P. Eriksson, L. Dencker, and T. Archer. 1992. Behavioural effects of neonatal metallic mercury exposure in rats . Toxicology 74(2-3):151-160.
Fredriksson, A., L. Dencker, T. Archer, and Danielsson. 1996. Prenatal coexposure to metallic mercury vapour and methylmercury produce interactive behavioural changes in adult rats. Neurotoxicol. Teratol. 18(2):129-134.
Friberg, L., and N.K. Mottet. 1989. Accumulation of methylmercury and inorganic mercury in the brain. Biol. Trace Elem. Res. 21:201-206.
Fujiyama, J., K. Hirayama, and A. Yasutake. 1994. Mechanism of methylmercury efflux from cultured astrocytes. Biochem. Pharmacol. 47(9):1525-1530.
Fung, Y.K., A.G. Meade, E.P. Rack, A.J. Blotcky, J.P. Claassen, M.W. Beatty, and T. Durham. 1995. Determination of blood mercury concentrations in Alzheimer's patients. Clin. Toxicol. 33(3):243-247.
Gage, J.C. 1975. Mechanisms for the biodegradation of organic mercury compounds: The actions of ascorbate and of soluble proteins. Toxicol. Appl. Pharmacol. 32(2):225-238.
Goering, P.L., W.D. Galloway, T.W. Clarkson, F.L. Lorscheider, M. Berlin, and
A.S. Rowland. 1992. Toxicity assessment of mercury vapor from dental amalgams. Fundam. Appl. Toxicol. 19(3):319-329.
Gonzalez-Ramirez, D.M., M. Zuniga-Charles, A. Narro-Juarez, Y. Molina-Recio, K.M. Hurlbut, R.C. Dart, and H.V. Aposhian. 1998. DMPS (2,3-dimercapto-propane-1-sulfonate, dimaval) decreases the body burden of mercury in humans exposed to mercurous chloride. J. Pharmacol. Exp. Ther. 287(1):8-12.
Gosselin, R.E., R.P. Smith, H.C Hodge. 1984. Clinical Toxicology of Commercial Products, 5th Ed. Baltimore: Williams & Wilkins.
Grandjean, P., P.J. Jørgensen, and P. Weihe. 1994. Human milk as a source of methylmercury exposure in infants. Environ. Health Perspect. 102(1):74-77.
Grandjean, P., P. Weihe, P.J. Jørgensen, T.W. Clarksen, E. Cernichiari, and T. Viderø. 1992. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch. Environ. Health 47(3):185-195.
Halbach, S. 1994. Amalgam tooth fillings and man's mercury burden. Hum. Exp. Toxicol. 13:496-501.
Hall, L.L., P.V. Allen, H.L. Fisher, and B. Most. 1995. The kinetics of intravenously-administered inorganic mercury in humans . Pp. 265-280 in Kinetic Models of Trace Elements and Mineral Metabolism During Development , K.N.S. Subramanian, and M.E. Wastney, eds. Boca Raton, FL: CRC Press.
Harada, M. 1995. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 25(1):1-24.
Henderson, R., H.P. Shotwell, and L.A. Krause. 1974. Analyses for total, ionic and elemental mercury in urine as a basis for biological standard. Am. Ind. Hyg. Assoc. J. 35:576-580.
Hursh, J.B. 1985. Partition coefficients of mercury (203Hg) vapor between air and biological fluids. J. Appl. Toxicol. 5(5):327-332.
Hursh, J.B., M.G. Cherian, T.W. Clarkson, J.J. Vostal, and P.V. Mallie. 1976. Clearance of mercury (Hg-197, Hg-203) vapor inhaled by human subjects . Arch. Environ. Health 31(6):302-309.
Hursh, J.B., T.W. Clarkson, T.V. Nowak, R.C. Pabico, B.A. McKenna, E. Miles, and F.R. Gibb. 1985. Prediction of kidney mercury content by isotope techniques. Kidney Int. 27(6):898-907.
Hursh, J.B., T.W. Clarkson, E.F. Miles, and L.A. Goldsmith. 1989. Precutaneous absorption of mercury vapor by man. Arch. Environ. Health 44(2):120-127.
IPCS (International Programme on Chemical Safety). 1990. Environmental Health Criteria Document 101: Methylmercury. Geneva: World Health Organization.
IPCS (International Programme on Chemical Safety). 1991. Environmental Health Criteria Document 118: Inorganic Mercury. Geneva: World Health Organization.
Jakubowski, M., J. Piotrowski, and B. Trojanowska. 1970. Binding of mercury in the rat: Studies using 203HgCl2 and gel filtration . Toxicol. Appl. Pharmacol. 16(3):743-753.
Kägi, J.H., and M. Nordberg. 1979. Metallothionein. International Meeting on Metallothionein and Other Low Molecular Weight Metal Binding Proteins. Basel, Switzerland: Birkhäuser.
Kalamegham, R., and K.O. Ash. 1992. A simple ICP-Ms procedure for the determination of total mercury in whole blood and urine. J. Clin. Lab. Anal. 6(4):190-193.
Kerper, L.E., N. Ballatori, and T.W. Clarkson. 1992. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am. J. Physiol. 262(5):R761-R765.
Kershaw, T.G., T.W. Clarkson, and P.H. Dhahir. 1980. The relationship between blood-brain levels and dose of methylmercury in man. Arch. Environ. Health 35(1):28-36.
Klaassen, C.D. 1996. Heavy metals and heavy-metal antagonists. Pp. 1649-1671 in The Pharmacological Basis of Therapeutics, J.G. Hardman, L.E. Limbird, P.B. Molinoff, R.W. Ruddon, and A.G. Gilman, eds. New York: McGraw-Hill.
Klimova, L.K. 1958. Pharmacology of a new Unithiol antidote [in Russian]. Farmakol. Toksikol. ( Moscow) 21:53-59.
Komsta-Szumska, E., J. Chmielnicka, and J.K. Piotrowski. 1976. Binding of inorganic mercury by subcellllar fractions and proteins of rat kidneys. Arch. Toxicol. 37(1):57-66.
Kromidas, L., L.D. Trombetta, and I.S. Jamall. 1990. The protective effects of glutathione against methylmercury cytotoxicity . Toxicol. Lett. 51(1):67-80.
Kudsk, F.N. 1965. The influence of ethyl alcohol on the absorption of methyl mercury vapor from the lungs of man. Acta Pharmacol. Toxicol. 23:263-274.
Lefevre, P.A., and J.W. Daniel. 1973. Some properties of the organomercury-degrading system in mammalian liver. FEBS Lett. 35(1):121-123.
Lind, B., L. Friberg, and M. Nylander. 1988. Preliminary studies on methylmercury biotransformation and clearance in the brain of primates: II. Demethylation of mercury in brain. J. Trace Elem. Exp. Med. 1(1):49-56.
Liu, K.Z., Q.G. Wu, and H.I. Liu. 1990. Application of a Nafion-Schiff-base modified electrode in anodic-stripping voltammetry for the determination of trace amounts of mercury. Analyst 115(6):835-837.
Lorscheider, F.L., M.J. Vimy, and A.O. Summers. 1995. Mercury exposure from “silver” tooth fillings: Emerging evidence questions a traditional dental paradigm. FASEB J. 9(7):504-508.
Magos, L., and W.H. Butler. 1972. Cumulative effects of methylmercury dicyandiamide given orally to rats. Food Cosmet. Toxicol. 10(4):513-517.
Magos, L., and A.A. Cernik. 1969. A rapid method for estimating mercury in undigested biological samples . Br. J. Ind. Med. 26(2):144-149.
Magos, L., and T.W. Clarkson. 1972. Atomic absorption determination of total, inorganic, and organic mercury in blood. J. Assoc. Off. Anal. Chem. 55(5):966-971.
Magos, L., and M. Webb. 1980. The interaction of selenium with cadmium and mercury. Crit. Rev. Toxicol. 8(1):1-42.
Magos, L., A.W. Brown, S. Sparrow, E. Bailey, R.T. Snowden, and W.R. Skipp. 1985. The comparative toxicology of ethyl- and methylmercury. Arch. Toxicol. 57(4):260-267.
Mahaffey, K.R., and D. Mergler. 1998. Blood levels of total and organic mercury in residents of the upper St. Lawrence River basin, Quebec: Association with age, gender, and fish consumption. Environ. Res. 77(2):104-114.
Marsh, D.O., T.W. Clarkson, C. Cox, G.J. Myers, L. Amin-Zaki, and S. Al-Tikriti. 1987. Fetal methyl mercury poisoning: Relationship between concentration in single strands of maternal hair and child effects. Arch. Neurol. 44(10):1017-1022.
Matsuo, N, T. Suzuki, and H. Akagi. 1989. Mercury concentration in organs of contemporary Japanese. Arch. Environ. Health 44(5):298-303.
Matsuo, N., M. Takasugi, A. Kuroiwa, and H. Ueda. 1987. Thymic and splenic alterations in mercuric chloride-induced glomerulopathy . Pp. 333-334 in Toxicology of Metals: Clinical and Experimental Research, S.S. Brown, and Y. Kodama, eds. Chichester, UK: Ellis Horwood Limited.
Miettinen, J.K. 1973. Absorption and elimination of dietary (Hg++) and methylmercury in man. Pp. 233-246 in Mercury, Mercurial, and Mercaptans, M.W. Miller, and T.W. Clarkson, eds. Springfield, IL: C.C. Thomas.
Miettinen, J.K., T. Rahola, T. Hattula, K. Rissanen, and M. Tillander. 1971. Elimination of 203Hg-methylmercury in man. Ann. Clin. Res. 3(2):116-122.
Miura, K., and T.W. Clarkson. 1993. Reduced methylmercury accumulation in a methylmercury resistant rat pheochromocytoma PC12 cell line. Toxicol. Appl. Pharmacol. 118(1):39-45.
Miura, K., and N. Imura. 1987. Mechanism of methylmercury cytotoxicity. Crit. Rev. Toxicol. 18(3):161-188.
Mobley, H.L., and B.P. Rosen. 1982. Energetics of plasmid-mediated arsenate resistance in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 79(20):6119-6122.
Naganuma, A., N. Oda-Urano, T. Tanaka, and N. Imura. 1988. Possible role of hepatic glutathione in transport of methylmercury into mouse kidney. Biochem. Pharmacol. 37(2):291-296.
Nakamura, I., K. Hosokawa, H. Tamura, and T. Miura. 1977. Reduced mercury excretion with feces in germfree mice after oral administration of methylmercury chloride. Bull. Environ. Contam. Toxicol. 17(5):528-533.
NESCAUM (Northeast States for Coordinated Air Use Management), NEWMOA (Northeast Waste Management Officials' Association), NEIWPCC (New England Interstate Water Pollution Control Commission) , and EMAN ( Canadian Ecological Monitoring and Assessment Network). 1998. Mercury in Northeastern freshwater fish: current level and ecological impacts. Pp. IV.1-IV.21 in Northeast States/Eastern Canadian Provinces Mercury Study — A Frame Work for Action. February, 1998.
Nielsen, J.B. 1992. Toxicokinetics of mercuric-chloride and methylmercuric chloride in mice. J. Toxicol. Environ. Health 37(1):85-122.
Nierenberg, D.W., R.E. Nordgren, M.B. Chang, R.W. Siegler, M.B. Blayney, F. Hochberg, T.Y. Toribara, E. Cernichiari, and T. Clarkson. 1998. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N. Engl. J. Med. 338(23):1672-1676.
Norseth, T., and T.W. Clarkson. 1970. Studies on the biotransformation of 203Hg-labeled methylmercury chloride in rats. Arch. Environ. Health 21(6):717-727.
Okawa, K., H. Saito, I. Kifune, T. Ohshina, M. Fujii, and Y. Takizawa. 1982. Respiratory tract retention of inhaled air pollutants. 1. Mercury absorption by inhaling though the nose and expiring through the mouth at various concentrations. Chemosphere 11(9):943-951.
Ostlund, K. 1969. Studies on the metabolism of methyl mercury in mice. Acta Pharmacol. Toxicol. 27(Suppl.1):1-132.
Perry, R.D., and S. Silver. 1982. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J. Bacteriol. 150(2):973-976.
Phelps, R.W., T.W. Clarkson, T.G. Kershaw, and B. Wheatley. 1980. Interrelationships of blood and hair mercury concentrations in a North American population exposed to methylmercury. Arch. Environ. Health 35(3):161-168.
Ponce, R.A., T.J. Kavanagh, N.K. Mottet, S.G. Whittaker, and E.M. Faustman. 1994. Effects of methyl mercury on the cell cycle of primary rat CNS cells in vitro. Toxicol. Appl. Pharmacol. 127(1):83-90.
Rahola, T., T. Hattula, A. Korolainen, and J.K. Miettinen. 1973. Elimination of free and protein-bound ionic mercury (20Hg2+) in man . Ann. Clin. Res. 5(4):214-219.
Robinson, J.B., and O.H. Tuovinen. 1984. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: Physiological, biochemical, and genetic analyses. Microbiol. Rev. 48(2):95-124.
Rowland, I., M. Davies, and J.G. Evans. 1980. Tissue content of mercury in rats given methylmercuric chloride orally: Influence of intestinal flora. Arch. Environ. Health 35(3):155-160.
Rowland, I., M. Davies, and P. Grasso. 1977. Biosynthesis of methylmercury compounds by the intestinal flora of the rat. Arch. Environ. Health 32(1):24-28.
Rupp, E.M., F.I. Miller, and C.F. Baes III. 1980. Some results of recent surveys of fish and shellfish consumption by age and region of U.S. residents. Health Phys. 39(2):165-175.
Sager, P.R., M. Aschner, and P.M. Rodier. 1984. Persistent, differential alterations in developing cerebellar cortex of male and female mice after methylmercury exposure. Brain Res. 314(1):1-11.
Sarafian, T., and M.A. Verity. 1991. Oxidative mechanisms underlying methylmercury neurotoxicity. Int. J. Dev. NeuroSci. 9(2):147-153.
Sherlock, J., J. Hislop, D. Newton, G. Topping, and K. Whittle. 1984. Elevation of mercury in human blood from controlled chronic ingestion of methylmercury in fish. Hum. Toxicol. 3(2):117-131.
Silver, S., and D. Keach. 1982. Energy-dependent arsenate efflux: The mechanism of plasmid-mediated resistance. Proc. Natl. Acad. Sci. U.S.A. 79(20):6114-6118.
Skerfving, S. 1988. Mercury in women exposed to methylmercury through fish consumption, and in their newborn babies and breast milk. Bull. Environ. Contam. Toxicol. 41(4):475-482.
Stern, A.H., L.R. Korn, and B.E. Ruppel. 1996. Estimation of fish consumption and methylmercury intake in the New Jersey population. J. Expo. Anal. Environ. Epidemiol. 6(4):503-525.
Stopford, W., S.D. Bundy, L.J. Goldwater, and J.A. Bittikofer. 1978. Micro-environmental exposure to mercury vapor. Am. Ind. Hyg. Assoc. J. 39(5):378-384.
Suda, I., and K. Hirayama. 1992. Degradation of methy- and ethylmercury into inorganic mercury by hydroxyl radical produced from rat liver microsomes. Arch. Toxicol. 66(6):398-402.
Sugano, H., S. Omata, and H. Tsubaki. 1975. Methylmercury inhibition of protein synthesis in brain tissue. I. Effects of methylmercury and heavy metals on cell-free protein synthesis in rat brain and liver. Pp. 129-136 in Studies on the Health Effects of Alkylmercury in Japan, Environmental Agency, Japan.
Summers, A.O. 1985. Bacterial resistance to toxic elements. Trends Biotechnol. 3(5):122-125.
Summers, A.O., and S. Silver. 1978. Microbial transformations of metals. Annu. Rev. Microbiol. 32:637-672.
Sundberg, J., and A. Oskarsson. 1992. Placental and lactational transfer of mercury from rats exposed to methylmercury in their diet: Speciation of mercury in the offspring . J. Trace Elem. Exp. Med. 5(1):47-56.
Sundberg, J., S. Jonsson, M.O. Karlsson, I.P. Hallen, and A. Oskarsson. 1998. Kinetics of methylmercury and inorganic mercury in lactating and nonlactating mice. Toxicol. Appl. Pharmacol. 151(2):319-329.
Suzuki, T., T. Hongo, N. Matsuo, H. Imai, M. Nakazawa, T. Abbe, Y. Yama
mura, M. Yoshida, and H. Aoyama. 1992. An acute mercuric mercury poisoning: Chemical speciation of hair mercury shows a peak of inorganic mercury value. Hum. Exp. Toxicol. 11(1):53-57.
Syversen, T.L. 1974. Distribution of mercury in enzymatically characterized subcellular fractions from the developing rat brain after injections of methylmercuric chloride and diethylmercury. Biochem. Pharmacol. 23(21):2999-3007.
Takeuchi, T., and E. Komyo. 1977. Pathology and pathogenesis of Minamata disease. Pp. 103-142 in Minamata Disease, T. Tsubake and K. Irukayama, eds. New York: Elsevier.
Takeuchi, T., K. Eto, and H. Tokunaga. 1989. Mercury level and histochemical distribution in a human brain with Minamata Disease following a long-term clinical course of 26 years . Neurotoxicology 10(4):651-657.
Takizawa, Y. 1979. Epidemiology of mercury poisoning. Pp. 325-366 in The Biogeochemistry of Mercury in the Environment, J.O. Nriagu, ed. Amsterdam: Elsevier/North-Holland Biomedical Press.
Thomas, D.J., H.L. Fisher, L.L. Hall, and P. Mushak. 1982. Effects of age and sex on retention of mercury by methylmercury-treated rats. Toxicol. Appl. Pharmacol. 62(3):445-454.
Vahter, M., N.K. Mottet, L. Friberg, B. Lind, D.D. Shen, and T. Burbacher. 1994. Speciation of mercury in the primate blood and brain following long-term exposure to methyl mercury. Toxicol. Appl. Pharmacol. 124(2):221-229.
Vahter, M.E., N.K. Mottet, L.T. Friberg, S.B. Lind, J.S. Charleston, and T.M. Burbacher. 1995. Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol. Appl. Pharmacol. 134(2):273-284.
Vermeir, G., C. Vandecasteele, and R. Dams. 1991a. Atomic fluorescence spectrometry combined with reduction aeration for the determination of mercury in biological samples. Anal. Chim. Acta 242(2):203-208.
Vermeir, G., C. Vandecasteele, and R. Dams. 1991b. Atomic fluorescence spectrometry for the determination of mercury in biological samples. Pp. 29-36 in Trace Elements in Health and Disease, A. Aitio, A. Aro, J. Jarvisalo, and H. Vainio, eds. Cambridge, UK: The Royal Society of Chemistry.
Vimy, M.J., D.E. Hooper, W.W. King, and F.L. Lorscheider. 1997. Mercury from maternal “silver” tooth fillings in sheep and human breast milk. A source of neonatal exposure. Biol. Trace Elem. Res. 56(2):143-152.
Vimy, M.J., Y. Takahashi, and F.L. Lorscheider. 1990. Maternal-fetal distribution of mercury (203Hg) released from dental amalgam fillings. Am. J. Physiol. 258(4):R939-R945.
Wendroff, A.P. 1995. Magico-religious mercury use and cultural sensitivity. Am. J. Public Health 85(3):409-410.
WHO (World Health Organization). 1976. Mercury. Environmental Health Criteria 1. Geneva: World Health Organization.
Wisniewska, J.M., B. Trojanowska, J. Piotrowski, and M. Jakubowski. 1970. Binding of mercury in the rat kidney by metallothionein. Toxicol. Appl. Pharmacol. 16(3):754-763.
Yoshida, M., H. Satoh, T. Kishimoto, and Y. Yamamura. 1992. Exposure to mercury via breast milk in suckling offspring of maternal guinea pigs exposed to mercury vapor after parturition. J. Toxicol. Environ. Health 35(2):135-139.
Yoshino, Y., T. Mozai, and K. Nakao. 1966. Distribution of mercury in the brain and its subcellular units in experimental organic mercury poisoning. J. Neurochem. 13:397-406.
Zhuang, G., Y. Wang, M. Zhi, W. Zhou, J. Yin, M. Tan, and Y. Cheng. 1989. Determination of arsenic, cadmium, mercury, copper and zinc in biological samples by radiochemical neutron-activation analysis. J. Radioanal. Nucl. Chem. 129(2):459-464.