3
Other Related Health-Hazard Assessment Processes
IN THIS CHAPTER, the subcommittee reviews other related health-hazard assessment (HHA) processes currently used by the consumer product and pharmaceutical industries and by the U.S. Army Center for Health Promotion and Preventive Medicine (CHPPM).
CONSUMER PRODUCT AND PHARMACEUTICAL INDUSTRIES
To provide content for critically evaluating the Navy's HHA process, the subcommittee reviewed processes, procedures, and best practices used by the consumer product and pharmaceutical industries for determining the safety of new products and materials. The information contained in this section was derived from material provided by major consumer product and pharmaceutical companies (Lilly Research Laboratories, Wolf (1987), Boylstein et al (unpublished); Eastman Kodak Company (O'Donoghue 1989); and Sanofi Research (Dean, unpublished)). Most companies subscribe to the philosophy that their product shall be perceived to be safe under conditions of intended use and reasonably foreseeable misuse. To achieve that objective, compa-
nies develop and implement human and environmental safety-assessment programs to ensure that the new products are safe. The responsibility for safety testing is given to a responsible toxicologist in each company. The detailed information needed by the toxicologist to evaluate the safety of the product or its components and to prepare the safety assessment includes the following:
-
What is the chemical composition of the product?
-
How is the product manufactured?
-
Are there known impurities or additives present in the finished product?
-
What are the chemical and physical characteristics of the product?
-
What will be the conditions of use and exposure?
-
What is the duration and route of exposure?
-
How much active material will be used in the product?
-
How much of each of the component or components can be leached or extracted from the product under consumer-relevant conditions?
-
How will the component materials be incorporated into the product?
-
Are there analytical data that could be potentially useful for assessing the expected extent of exposure?
-
What is the extent of available toxicity literature on the product or class?
Once this information is obtained and the necessary animal tests are performed, the toxicologist then conducts the human-safety assessment. This assessment involves consideration of the toxicological properties of a material and the degree of expected exposure to humans. Exposure assessment requires a realistic balance of information characterizing concentrations, route, uptake, frequency, and duration. Although the details might vary, the basic assessment process is the same for either products consumed by or applied to humans. A typical decision tree is shown (Figure 3-1). It provides the responsible toxicologist or industrial hygienist conducting the risk assessment with a system for evaluating available toxicity information and identifying data gaps.
Once the health risk assessment report is completed, it goes to a re-
port coordinator who identifies internal or external primary reviewers and coordinates the review and sign off. Usually 2 to 4 weeks are allocated for review. Any request for additional reviewers or consultations with scientific experts (secondary reviewers) is the responsibility of the primary reviewer. In most organizations, the report is shared electronically during the review process. A comment memorandum prepared by the reviewer goes to the report writer, study toxicologist, and report coordinator. Once the comments of reviewers have been incorporated into the revised report, it goes to a Chemical Safety Evaluation and Exposure Limits Committee composed of upper-level management with expertise in toxicology, industrial hygiene, environmental affairs, occupational medicine (often, a physician), pharmacology (pharmaceutical industry only), project management, and law (not at all companies). Once the exposure assessment and exposure limits are accepted by the safety committee, the information is directed to management at all sites where the material is utilized or manufactured. It should also be noted that, as additional information becomes available, the risk assessment is periodically reviewed and revised as needed. The universal challenges to risk-assessment groups in industry are to
-
provide data for safe workplace utilization or consumer consumption;
-
do it quickly and in a cost-effective manner;
-
extrapolate from oral doses in animals or humans to airborne concentrations safe for workers in the absence of inhalation data; and
-
manage requests for risk assessments with reasonable timeliness.
Most industrial groups maintain an electronic database of information on all their products, intermediates, and additives. That information is updated on a regular basis and available electronically by readaccess at all sites globally. The risk-assessment specialist takes advantage of available electronic databases to obtain the best information possible for conducting health risk assessments. Because most of the products are novel, extensive animal tests are performed by the companies either in-house or in a contract laboratory. Even if the studies are performed in contract laboratories, sufficient expertise is required by the company to develop protocols, monitor studies, and evaluate the data obtained.
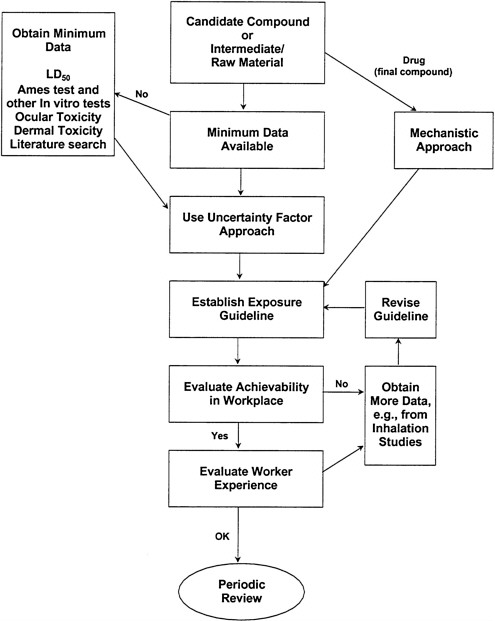
FIGURE 3-1 Typical decision tree for performing toxicological risk assessment (figure provided by R.K. Wolff, Lilly Research Laboratories).
Many companies also utilize closed-loop hazard-assessment processes such as that illustrated in Figure 3-2. In such processes, industrial hygiene data and medical surveillance information are fed back
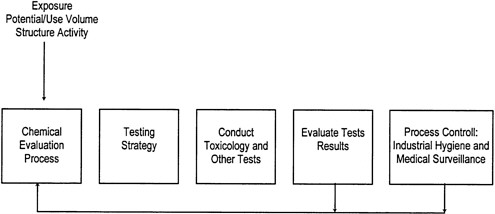
FIGURE 3-2 An example of a closed-loop hazard evaluation process. The chemical evaluation process includes collection of available information, development of a testing strategy, implementation of that strategy, and feedback to the evaluators based on industrial hygiene and medical surveillance information (modified from O'Donoghue 1989).
into the health hazard assessment process to verify the original decisions made on protective equipment and to aid in designing safety standards for new processes (O'Donoghue 1989).
U.S. ARMY CENTER FOR HEALTH PROMOTION AND PREVENTIVE MEDICINE
The subcommittee observed that there are a number of parallels between the Navy Environmental Health Center's (NEHC's) HHA process and that for the U.S. Army Center for Health Promotion and Preventive Medicine (CHPPM) (formerly the U.S. Army Environmental Hygiene Agency), which was the subject of a previous National Research Council review (NRC 1991).
In response to recommendations provided in the review (NRC 1991), CHPPM established a Peer Review Board on Toxicology in January 1992 and created the Quality Assurance Office (QAO) at the command level. CHPPM officials believe that the Peer Review Board has greatly improved the quality of the work being conducted in the organization.
Creating the QAO as a part of the command was a deliberate decision made to ensure independence from personnel engaged in the direction and conduct of toxicological studies. At the same time, the position of Quality Assurance Coordinator was also established.
Standard operating procedures (SOPs) were also developed and communicated to all pertinent staff, a systematic review of the SOP's was implemented, and third-party audits of the QAO and toxicology laboratory for good laboratory practice (GLP) compliance were initiated and maintained. Peer review of test protocols is now routine, and independent review of procedures is periodically sought. The Peer Review Board meets twice yearly to examine progress on outstanding issues, review current and planned work, provide guidance on breaking issues in the field, suggest problem resolutions, and identify useful resources and procedures for data collection and evaluation. Membership is comprised primarily of doctorate-level toxicologists with expertise in industrial toxicology, forensic toxicology, clinical toxicology, pathology, quantitative risk assessment, emergency-response planning, risk communication, analytical chemistry, preventive medicine, toxicological information storage and retrieval systems, and industrial chemical registration and preparation of health-hazard assessments under OSHA and international workplace standards.
The quality assurance coordinator has broad responsibilities to ensure that toxicological studies are performed in compliance with the GLPs, Animal Welfare Act requirements, accreditation criteria of the American Association for the Accreditation of Laboratory Animal Care, and other documented standards. The QAO's primary responsibilities are to
-
monitor toxicological studies to assure the management that the facilities,
equipment, personnel, methods, practices, records, and controls comply with the GLP monitored studies include those performed at satellite and analytical contract facilities off-site;
-
assist in developing a Facility Master Schedule Database, and maintaining a copy of that for all studies;
-
review and maintain SOPs for all toxicological studies and equipment as well as SOPs for inspection functions and responsibilities;
-
assist in, and conduct, audits to maintain International Standard-
-
ization Organization (ISO) compliance and assure follow-up on recommended corrective actions;
-
assist in the development of award contracts for services and ensure their compliance with GLP and ISO;
-
act as liaison for external Food and Drug Administration or U.S. Environmental Protection Agency inspections;
-
maintain copies of all protocols pertaining to toxicological studies for which the QAO is responsible;
-
inspect toxicological studies at intervals to ensure study integrity;
-
maintain records of inspections and report their results to management;
-
review final study reports to ensure that reported results are accurately reflected; and
-
maintain SOPs describing the functions and responsibilities of the QAO.
The QAO coordinator periodically reports to the Peer Review Board on the status of GLP compliance in the CHPPM Directorate of Toxicology. The Peer Review Board advises the QAO on possible problem resolution.
The Directorate of Toxicology also maintains its own GLP coordinator, who has responsibility for maintaining Directorate SOPs, communicating SOP requirements to investigators (via both library hard-copy and read-only electronic versions), and implementing corrective actions to resolve QAO audit findings when such actions involve SOP changes or other documentation. As SOP custodian, this individual also reviews new SOPs, maintains current SOPs, and ensures that Directorate personnel who are proficient in performing the SOP procedure review the pertinent SOPs annually for accuracy. This coordinator also ensures that all changes to SOPs or quality documentation are understood by appropriate personnel and develops and implements archiving systems. The QAO coordinator meets often with the Directorate GLP coordinator to reach joint resolution on various issues.
Currently, the Directorate of Toxicology performs health-effects research and toxicity evaluation. Health-effects research staff evaluate methods for predicting and assessing the effects of military environmental contaminants on human health and the environment, and act as consultants to installations and Department of Defense components on
environmental and ecological risk evaluations and assessments. In this capacity, CHPPM and NEHC staff have collaborated in the development and presentation of risk-communication workshops at a number of military facilities. Toxicity-evaluation staff within the Directorate identify chemical hazards and perform toxicity evaluations to develop preventive procedures for avoiding or minimizing hazardous exposures. Computerized databases, literature surveys, laboratory studies, and consultations with other health care advisors are all employed to accomplish individual assessments.
The successful results of the QAO and the Directorate GLP coordinator have led to the recent development of an overall quality plan, for the purpose of ensuring that quality assurance “responsibilities, policies and procedures . . . are identified, documented and consistently followed by each organizational element to include [the] continental United States, Subordinate Commands and CHPPM-Europe and Japan” (CHPPM 1999). This regulation also includes an appendix outlining the minimal essential elements of a quality plan. Although the CHPPM quality plan is “applicable to all USACHPPM operations, activities, and contractual services and personnel,” it should be considered only as a benchmark example and not a template for what is needed within the Navy.
The Committee on Toxicology
The National Research Council Committee on Toxicology has published several reports on methods to derive exposure guideline levels for short-term and continuous exposures (NRC 1986a, 1992, 1993, 2000a). The subcommittee recommends that NEHC routinely review those reports to help it prepare HHAs for exposure of military personnel and civilian workers to hazardous chemicals.
RECOMMENDATION
The subcommittee recommends that NEHC review HHA processes utilized by industrial and pharmaceutical companies, CHPPM, and COT in preparing its HHAs.








