1
Introduction and Background
The purpose of this report—one of a series resulting from a comprehensive effort initiated by the Institute of Medicine's Food and Nutrition Board to expand the approach to the development of dietary reference standards—is to assist nutrition and health researchers and other professional users of dietary reference standards in the transition from using the former Recommended Dietary Allowances (RDAs) and Canadian Recommended Nutrient Intakes (RNIs) to using all of the new Dietary Reference Intakes (DRIs) appropriately (a detailed discussion of the origin and framework for development of the DRIs is presented in Appendix A). This report reviews the scientific literature regarding the uses of dietary reference standards and their applications, and provides guidance on the application of DRIs to assess the nutrient intakes of groups and individuals. Application of DRIs in planning diets of groups and individuals will be presented in a subsequent report.
PURPOSE OF THE REPORT
This report focuses on application of the DRIs in dietary assessment and is meant as both a “how to” manual and a “why” manual. In this light, specific examples of both appropriate and inappropriate uses of the DRIs in assessing the nutrient adequacy of intakes for groups and for individuals are included. The statistical background that justifies the use of DRIs as described in this report is also included. The detailed statistical approaches for the methods described here have been grouped into appendixes; the text in the main body of
the report is precise, but should not require extensive background in statistics to be useful.
An important consideration in the application of the DRIs in both assessment and planning is that a nutrient requirement is defined as the lowest continuing intake level of a nutrient that will maintain a defined level of nutriture in an individual. The criterion of nutritional adequacy on which requirements are based differs among nutrients, and may also differ for a given nutrient depending on the life stage of individuals. The criterion used, the rationale for its selection, and any functional indicators are described in depth in each of the nutrient reports in this series (IOM, 1997, 1998b, 2000). The criterion or criteria chosen for a specific nutrient is for the healthy U.S. and Canadian populations and may not be the most appropriate criterion for other populations. This has important implications for those using the DRIs in assessment or planning. For example, agreement between assessment of dietary intake and assessment of nutritional status cannot be expected if the criterion used to determine the requirement and the criterion used in clinical and biochemical examination for other purposes are not the same.
For the DRIs published at the time this report went to press, the requirement for each nutrient is presented as a single reference intake (amount) for various life stage and gender groups rather than as multiple endpoints. This approach differs from that of the joint World Health Organization and Food and Agriculture Organization Expert Consultation on requirements of vitamin A, iron, folate, and vitamin B12 (FAO/WHO, 1988), which recommended both a basal requirement (the amount of nutrient needed to prevent clinically detectable impairment of function) and a normative storage requirement (the amount of nutrient needed to maintain a desirable level in tissues). The single endpoints established for DRIs currently available are more in keeping with a normative storage requirement than a basal requirement.
WHAT ARE DRIs?
Dietary Reference Intakes (DRIs) are relatively new to the field of nutrition. The DRIs are a set of at least four nutrient-based reference values that can be used for planning and assessing diets and for many other purposes. They are meant to replace the former Recommended Dietary Allowances (RDAs) in the United States and Recommended Nutrient Intakes (RNIs) in Canada. The DRIs differ from the former RDAs and RNIs in that (1) where specific data on
safety and efficacy exist, reduction in the risk of chronic degenerative disease—rather than just the absence of signs of deficiency—is included in the formulation of the recommendation; (2) where data are adequate, upper levels of intake are established to prevent risk of adverse effects; and (3) components of food that may not fit the traditional concept of an essential nutrient but are of possible benefit to health will be reviewed and if sufficient data exist, reference intakes will be established.
Where adequate information is available, each nutrient will have a set of DRIs. A nutrient will have either an Estimated Average Requirement (EAR) and RDA, or an Adequate Intake (AI). When an EAR for the nutrient cannot be determined (and therefore, neither can the RDA), then an AI is provided for the nutrient. In addition, most nutrients will have a Tolerable Upper Intake Level (UL). Like the former RDAs and RNIs, each type of DRI refers to the average daily nutrient intake of apparently healthy individuals over time, although the amount may vary substantially from day to day without ill effect in most cases.
In developing recommended intakes, emphasis is placed on the reasons underlying the particular criterion of adequacy used to establish the requirement for each nutrient. A table of the recommended daily intakes developed using the DRI process, at the time this report was printed, can be found at the end of this book.
The EAR
The EAR1 is the median usual intake value that is estimated to meet the requirement of half the healthy individuals in a life stage and gender group. At this level of intake, the other half of the individuals in the specified group would not have their needs met. The EAR is based on a specific criterion of adequacy, derived from a careful review of the literature. Reduction of disease risk is considered along with many other health parameters in the selection of that criterion. The EAR is used to calculate the RDA.
|
1 |
It is recognized that the definition of the EAR implies a median as opposed to a mean or average. The median and average would be the same if the distribution of requirements followed a symmetrical distribution such as the normal, and would diverge as a distribution became skewed. Two considerations prompted the choice of the term EAR: (1) data are rarely adequate to determine the distribution of requirements, and (2) precedent has been set by other countries that have used the term EAR for reference values similarly derived (COMA, 1991). |
The RDA
The RDA is the average daily dietary intake level that is sufficient to meet the nutrient requirement of nearly all healthy individuals in a particular life stage and gender group. If the distribution of requirements in the group is assumed to be normal, then the RDA is the value that exceeds the requirements of 97 to 98 percent of the individuals in the group (Figure 1-1). Under the assumption of normality, the RDA can be computed from the EAR and the standard deviation of requirements (SDREQ) as follows:
RDA = EAR + 2 SDREQ
If the distribution of requirements is normal, 97 to 98 percent of the individuals in the group will have a requirement that is below the RDA. The RDA is intended for use primarily as a goal for usual intake of individuals. Because the RDA is derived directly from the EAR, if data are insufficient to establish an EAR, no RDA can be set.
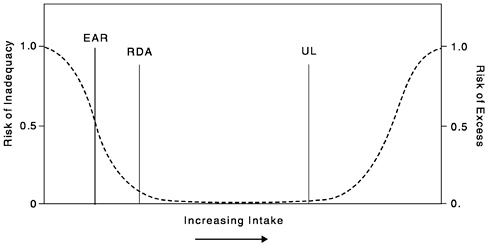
FIGURE 1-1 Dietary reference intakes. This figure shows that the Estimated Average Requirement (EAR) is the intake at which the risk of inadequacy is 0.5 (50 percent) to an individual. The Recommended Dietary Allowance (RDA) is the intake at which the risk of inadequacy is very small —only 0.02 to 0.03 (2 to 3 percent). The Adequate Intake (AI) does not bear a consistent relationship to the EAR or the RDA because it is set without being able to estimate the requirement. At intakes between the RDA and the Tolerable Upper Intake Level (UL), the risks of inadequacy and of excess are both close to 0. At intakes above the UL, the risk of adverse effects increases.
The AI
If sufficient scientific evidence is not available to establish an EAR and set an RDA, an AI is derived instead. The AI is based on experimentally derived intake levels or approximations of observed mean nutrient intakes by a group (or groups) of apparently healthy people who are maintaining a defined nutritional state or criterion of adequacy. Examples of defined nutritional states include normal growth, maintenance of normal levels of nutrients in plasma, and other aspects of nutritional well-being or general health.
The AI would not be consistently related to the EAR and its RDA even if they could be established. For example, for young infants, the AI is usually based on the daily mean nutrient intake supplied by human milk for healthy, full-term infants who are exclusively fed human milk. For adults, the AI may be based on data from a single experiment (e.g., the AI for choline [IOM, 1998b]), based on estimated dietary intakes in apparently healthy population groups (e.g., the AIs for biotin and pantothenic acid [IOM, 1998b]), or result from a review of data from different approaches (e.g., the AI for calcium, based on calcium retention, factorial estimates of requirements, and limited data on bone mineral density and bone mineral content changes in adult women [IOM, 1997]). The AI is expected to exceed the EAR and the RDA for a specified criterion of nutritional adequacy. When an RDA is not available for a nutrient (since there is no EAR), the AI can be used as the goal for an individual's intake. However, as is explained later in this report, the AI has limited uses in assessment.
The issuance of an AI indicates that more research is needed to determine, with some degree of confidence, the mean and distribution of requirements for that specific nutrient. When this research is completed, it should be possible to replace estimates of AIs with EARs and RDAs.
The UL
The UL is the highest level of continuing daily nutrient intake that is likely to pose no risk of adverse health effects in almost all individuals in the specified life stage group (Figure 1-1). As intake increases above the UL, the potential risk of adverse effects increases. The term tolerable intake was chosen to avoid implying a possible beneficial effect. Instead, the term is intended to connote a level of intake with a high probability of being tolerated biologically. The UL is not intended to be a recommended level of intake. Unless
specifically identified in the nutrient reports (e.g., for folate in the prevention of neural tube defects [IOM, 1998b]), there is no currently established benefit to healthy individuals associated with ingestion of nutrients in amounts exceeding the RDA or AI.
The UL is based on an evaluation conducted using the methodology for risk assessment of the adverse effects of nutrients (IOM, 1998a). The need to establish ULs grew out of the increasingly common practice of fortification of foods with nutrients and the increased use of dietary supplements. For some nutrients, data may not be sufficient for developing a UL. This indicates the need for caution in consuming high intakes and should not be interpreted as meaning that high intakes pose no risk of adverse effects.
General Properties of DRIs
Unless otherwise stated, all values given for EARs, RDAs, AIs, and ULs represent the total quantity of the nutrient or food component to be supplied by foods (including nutrients added to foods) and by nutrients ingested as supplements. These values are also based on usual or continuing intakes. The DRIs apply to the apparently healthy population. RDAs and AIs are not expected to replete individuals who are already malnourished, nor are they intended to be adequate for those who may have increased requirements because of certain disease states. Appropriate goals for intake should be provided to those with greatly increased nutrient requirements. Although the RDA or AI may serve as the basis for such guidance, qualified medical and nutrition personnel should make necessary adaptations for specific situations.
Comparison of the AI with the RDA
In general, both values are intended to cover the needs of nearly all members of a life stage group. For both RDAs and AIs, values for children and adolescents may be extrapolated from adult values if no other usable data are available. However, there is much less certainty about an AI value in comparison to an RDA value.
The RDA is based on specific knowledge of the requirement and assumptions about its distribution and is set to meet the requirements of almost all (97 to 98 percent) of the population. In contrast, the AI is an experimentally derived or observed mean intake that appears to maintain a specific criterion of adequacy in a group of apparently healthy people. Therefore, by definition, the RDA incorporates only the estimated variability in requirements, where-
as the AI, if based on observed mean intakes, incorporates the variability of both requirements and intake. The AI represents an informed judgment about what seems to be an adequate intake for an individual based on available information, whereas the RDA is a more data-based and statistically relevant estimate of the required level of intake for almost all individuals. For this reason, AIs must be used more carefully than RDAs.
Criteria of Adequacy
In the derivation of the EAR or AI, close attention has been paid to determining the most appropriate criteria of adequacy. A key question is, Adequate for what? In many cases a continuum of benefits may be ascribed to various levels of intake of the same nutrient. Each EAR and AI is described in terms of the selected criterion or, in some cases, criteria. For example, the EAR, and thus the RDA, for folate for women of childbearing age is based on a combination of biochemical indicators or criteria. A separate recommendation is made for women capable of becoming pregnant to reduce the risk of a neural tube defect in the offspring if pregnancy occurs. There are many possible and equally legitimate criteria of adequacy. The criteria are discussed in each nutrient report as part of the rationale for the DRIs developed (IOM, 1997, 1998b, 2000).
Uncertainty in Requirement Estimations
The task of setting both median requirements (EARs) and ULs for apparently healthy persons of all ages and both genders in various physiological states is ambitious. Ideally, data from the target population on intakes at various levels and the functional effects of these intakes would be available. In reality the information base is often limited, and its reliability varies from nutrient to nutrient. These limitations are discussed in detail in each of the nutrient reports (IOM, 1997, 1998b, 2000). Users of these reports should recognize that the DRIs are estimates based on available data, and that even when an EAR, RDA, and a UL for a nutrient are provided for a life stage and gender group, there is considerable uncertainty about these values. The DRIs will continue to evolve as better information becomes available. When interpreting the results of assessments of individuals or groups, it is appropriate to consider possible limitations in the information base that was used to generate the relevant DRIs.
ORGANIZATION OF THE REPORT
This report is organized to take the user step-by-step through methodology for using the Dietary Reference Intakes (DRIs) to assess the adequacy of nutrient intakes. An overview of the concept of using dietary reference standards along with the identification of their past uses (specifically the former Recommended Dietary Allowances [RDAs] and Recommended Nutrient Intakes [RNIs]) is presented in Chapter 2.
Chapter 3 describes how DRIs can be used for assessing the apparent nutrient adequacy of individuals, and includes a discussion of obtaining and interpreting information on individual intakes and the effect of the large within-person variation. Examples of specific applications are also provided.
Chapter 4 provides the statistical basis for the use of the Estimated Average Requirement (EAR) in assessing nutrient adequacy of groups. The chapter begins with a basic discussion of the concept of assessing the prevalence of inadequate nutrient intakes and then develops the statistical approaches for estimating this prevalence. Assumptions required for the use of the statistical models are discussed, as is the need for adjusting intake distributions.
In Chapter 5, the focus is on group-level assessment of nutrient adequacy using the Adequate Intake (AI). Chapter 6 provides guidance on the extent to which the Tolerable Upper Intake Level (UL) can be used to estimate the prevalence of potential risk for adverse effects in groups.
Specific guidance with examples on appropriate applications of the DRIs for group assessment purposes is provided in Chapter 7— the methodological approaches described in Chapter 4, Chapter 5 and Chapter 6 are applied to some of the specific uses of dietary reference standards reported in Chapter 2. Three specific applications are presented and discussed.
A brief description of limitations in the measurement of intakes and requirements, and the importance of accurate sampling techniques are highlighted in Chapter 8. Chapter 9 provides recommendations for research needed to improve and refine nutrient assessments.








