5
Crystalline Silicotitanate Ion Exchange
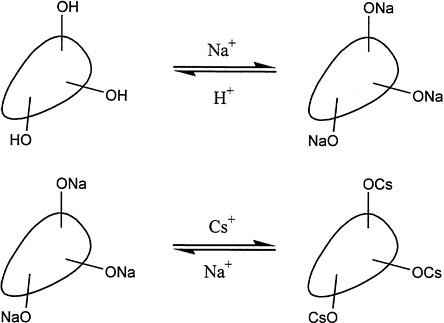
Ion exchange has been in commercial use for over 100 years to remove ionic species from aqueous solutions (e.g., deionization of water). As illustrated in Figure 5.1, a typical process for exchange of cations employs insoluble polymeric beads (inorganic materials, such as zeolites, are also widely used) that have a number of exchangeable sites (shown in the Figure 5.1 as either OH or ONa). Ion exchange processes typically involve equilibrium reactions, and the separated ions are usually eluted from the ion exchange material using a dilute acid (e.g., elution of sodium ions in the reverse reaction for Figure 5.1) or a salt solution (e.g., elution of cesium ions in the reverse reaction of Figure 5.1). The elution sequence allows the ion exchange material to be reused in multiple cycles.
Although the underlying technology is well established, ion exchange for cesium removal from high-level waste at the Savannah River Site (SRS) and other U.S. Department of Energy (DOE) sites poses many challenges. The ion exchange material must withstand both high alkalinity and high radiation fields, while at the same time exhibiting selectivity for cesium in the presence of much greater concentrations of chemically similar ions such as sodium and potassium.
A promising ion exchange material, crystalline silicotitanate (CST), was investigated by workers at Sandia National Laboratory and Texas A&M University (Sherman, 1999). CST is an outgrowth of earlier work at Sandia on amorphous hydrous titanium oxide (HTO) in the 1960s and 1970s (Walker, Taylor, and Lee, 1999). An ion exchange medium based on CST, known as TAM-5, was developed in the early 1990s at Sandia and Texas A&M under the auspices of the DOE Environment Management —Office of Science and Technology. Subsequent product development and commercial manufacture was carried out by UOP of Des Plaines, Illinois, under a cooperative research and development agreement with Sandia (Sherman, 1999). The commercial material is sold under the name IONSIV®. The product in current use is designated as IONSIV® IE-911. An earlier product, which was a powdered form of the material, was called IONSIV® IE-910.
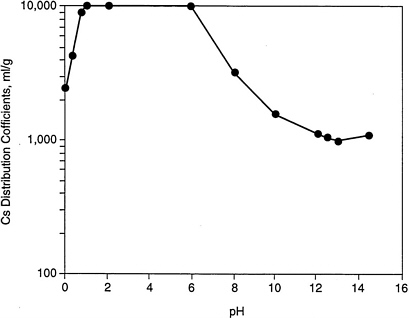
Silicotitanates have received considerable attention as ion exchange materials for nuclear waste applications, in particular because they exhibit very high selectivity for cesium ions (Cs+) and also show specificity for strontium. CST is unusual in that it exhibits high selectivity for cesium ions (Gu et al., 1997) in salt solutions (Anthony et al., 1994) and for cesium over a wide pH range from acidic to basic solution as shown in Figure 5.2 (Zheng, Gu, and Anthony, 1995; Anthony et al., 1994). It also exhibits high stability to radiation (Zheng et al., 1996). Though maximum selectivity is reported at pH ≤6, the reported distribution coefficient (Kd) for cesium under alkaline conditions is still high in comparison to other ion exchange materials. According to Anthony et al. (1994), the decrease in Cs+ selectivity at high pH is not desirable in applying TAM-5 to defense waste in its current form.
CST is also unusual in that cesium is not easily removed from the material [i.e., the reaction rate for the reverse process (Figure 5.1) is low], and the process is described as nonelutable ion exchange. Consequently, it is not practical to recycle the CST, and the loaded CST would need to be incorporated into the high-level waste stream. This, in turn, raises questions of the effects of higher concentrations of titanium on the stability of borosilicate glass.
SRS first evaluated the use of CST for removal of cesium from SRS high-level waste (HLW) using simulants in 1995 and found it to be “very effective for cesium removal” (McCabe, 1995). In 1997, small-scale tests were performed with actual SRS waste (McCabe, 1997). Also, in 1997, SRS collaborated with Oak Ridge National Laboratory (ORNL) to successfully demonstrate a glass wasteform formulation that could incorporate the higher titanium loadings required by the use of nonelutable CST (Andrews and Workman, 1997). SRS has been performing research and development (R&D) on CST as the back-up for small tank precipitation for the last two years.
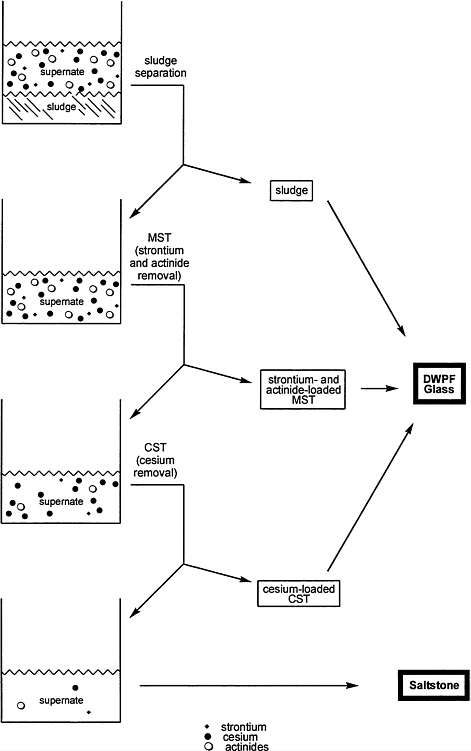
The proposed CST ion exchange process for treatment of SRS HLW is summarized in Figure 5.3. The process involves the following steps, beginning with separation of the supernate from any sludge in the tank. A slurry of monosodium titanate (MST) would then be added to the waste to sorb strontium, plutonium, and other actinides, and the resulting slurry would be filtered to remove insoluble MST and entrained sludge from the prior step. The insoluble solids would then be washed and transferred to Defense Waste Processing Facility (DWPF) for incorporation into borosilicate glass. The clarified salt solution from filtration would be passed through a series of columns packed with CST to remove cesium. The cesium-loaded CST would be transferred to DWPF for incorporation into glass, while the decontaminated salt solution would be treated as a low level waste and disposed of as saltstone (Figure 5.3).
Outside the SRS program, CST in the form of IE-911 has been incorporated into the Cesium Removal Demonstration Project at ORNL. Approximately 1.2 × 105 liters of supernate, obtained from the Melton Valley storage tanks, were processed during the demonstration. Some 265 liters of sorbant were successfully used to remove 1.1 × 103 Ci of 137Cs from the supernate (Walker, Taylor, and Lee, 1999; Lee et al., 1997). The task at SRS is considerably more challenging, with a total free supernate and saltcake volume of 1.2 × 108 liters containing 8.8 × 107 Ci. In other words, the volume of waste to be treated is larger by a factor of 1,000, and the amount of cesium is higher by nearly five orders of magnitude.
In contrast, the Hanford Site used an elutable ion exchange resin to make capsules of cesium. In the early 1980s, the Dupont Chemical Company (then the management and operating contractor at SRS for DOE) conducted a cost analysis of building new facilities for both a vitrification and an ion exchange process. The analysis showed that an ion exchange facility, based on the Hanford process, would cost about the same as the vitrification facility—almost a billion dollars each. By contrast, the tetraphenylborate (TPB) process for cesium removal appeared to offer a way to avoid the cost of a new ion exchange facility by separating the cesium from the waste in the already-existing tanks (U.S. General Accounting Office, 1999). The issue of catalytic decomposition of TPB was not an issue at that time.
PHYSICAL, CHEMICAL, AND MINERALOGICAL CHARACTERISTICS OF CST
Crystalline siliotitanate ion exchange materials have been synthesized in a nominal three-component system Na2O-TiO2-SiO2 (Balmer et al., 1997). A fourth, proprietary dopant component (Savannah River Site, 1999) is used in the commercially available material, and a five-component system is produced by cesium exchange. Partial data in the three-component system, Na2O-TiO2-SiO2, have been reported (Levin, Robbins, and McMurdie, 1979, Figure 531) but there is little information available for the four- and five-component systems. Relatively few details are available on the synthesis of CST. Its preparation by a hydrothermal process has been described (Anthony et al., 1994), but reaction conditions were not reported. A more detailed preparation was reported via a sol-gel route where the precursor materials were mixed for 15 hours and dried in air at room temperature. The amorphous, homogeneous precursor was subsequently heated in air at 800 °C for at least one hour to form crystalline CST (Balmer et al., 1997). In all of the studies reported, the starting materials are titanium isopropoxide and silicon ethoxide in alkaline aqueous medium (Anthony et al., 1994; Balmer et al., 1997).
As indicated previously, CST is a multi-component material for which details of composition and structure are not well defined. The commercial material, IE-911, includes two proprietary components, a dopant and a binder (Savannah River Site, 1999; W. Wilmarth, November 21–22, 1999, personal
communication), which has further limited the ability of this committee to assess its physical, chemical, and mineralogical characteristics. Anthony et al. (1994) reported that hydrothermal synthesis produced a material, TAM-5, that was fine grained and consisted of >99 percent phase-pure aggregates of small crystalline particles with an average size <100 nm. The variants of TAM-5 and IE-910 have been reported as the same materials and described as an “… inorganic ion exchanger with a well-defined crystal structure …” (Zheng, Gu, and Anthony, 1995). Commercial manufacture produces CST in the form of extremely fine particles (IE-910), which must be agglomerated into beads (IE-911) for the intended use.
Su, Balmer, and Bunker (1997) conducted studies of the thermal stability of cesium loaded TAM-5 to 1000 °C, and they concluded that the material exists, as binary mixtures of phases, not phase-pure materials. This observation is consistent with information presented to the committee (W. Wilmarth, November 21–22, 1999, personal communication) suggesting that there are two crystalline phases with known structures, at least one crystalline phase with unknown structure, and a non-crystalline fourth phase in commercial CST (IE-911). Su, Balmer, and Bunker (1997) reported that the two crystalline materials are “known sodium- and sodium-titanium-containing phases that also contain a proprietary component.” Six hydrous sodium titanosilicate compounds are reported in the open literature for this chemical system, including three mineral phases (Sokolova et al., 1985; Khalilov, 1965; Dadchov and Harrison, 1997) and three synthetic phases. Two of the reported synthetic materials are hydrous sodium titanosilicate phases (Poojary, Cahill, and Clearfield, 1994; Clearfield, Poojary, and Bortun, 1996) described as having ion exchange properties.
ISSUES TO BE ADDRESSED FOR CST
Several key issues have been identified (Savannah River Site, 1999) by SRS that would need to be resolved if CST ion exchange were to be implemented at SRS. These include the interface with MST processing for strontium and actinide removal, effect of variation in waste-feed composition, column design parameters, influence of glass formulation with higher titanium loadings on waste form performance, stability of the loaded material as a function of temperature, reproducibility of the manufactured CST, and reaction of CST in alkaline solution to form new solid phases. The committee's interim report highlighted the last two concerns, specifically noting that they would need to be resolved before the CST process could be deployed. In addition to these concerns, the large column sizes proposed for CST processing (discussed later) will result in high radiation fields with potential problems from radiolytic gas generation and heating. Work is ongoing in each of these areas and important findings are described below.
Potential problems with MST processing are discussed in Chapter 3. That treatment is intended to remove strontium and actinide ions, but it is not entirely clear how that step will interface with the CST process. It has been
reported (National Research Council, 1996) that silicotitanates are selective for both cesium and strontium, although the committee has not received information about its selectivity for actinides. Consequently, the interplay between the sequential steps of supernate treatment with MST and CST could allow some flexibility in the overall processing.
Using the existing models developed at Texas A&M, the IE-911 variant of CST was evaluated (Savannah River Site, 1999) at SRS. The goal of this effort was to “… determine the performance of the CST in column applications using SRS simulated waste to determine agreement with computer modeling.” The results of two column tests indicated agreement with the Texas A&M modeling, although the assumption of a 30 percent reduced cesium exchange capacity was required to model the higher flow rate (Wilmarth et al., 1999). Further evaluation during the “decision phase” of the study resulted in an unexpected increase in experimental breakthrough in the column experiment (Walker, 1998). Conflicting interpretations of the loss of exchange capacity have also been reported (McCabe, 1997; Walker et al., 1998).
Uncertainty in the nature of the ion exchange material is illustrated by a report (Walker et al., 1998) on the results for both batch and column testing for IE-911 from “batch 2” using SRS supernatant from Tank 22H. If CST continues to be an option for processing the SRS waste, SRS plans to engage ORNL and UOP to examine revised manufacturing processes to improve and ensure consistency of the CST product. Cross-laboratory comparisons of material performance are also currently underway. The Savannah River Technology Center (SRTC) also plans to “perform an evaluation of various tank wastes during the next several years, ” the purpose of which is to “catalogue the cesium removal efficiencies of the currently marketed CST versus the chemical compositions of F- and H-area wastes” (Savannah River Site, 1999).
Reasons for the concern about the stability of loaded ion exchange material are illustrated by a batch test of CST IE-911. The CST exposed “… to salt solution at elevated temperature … for long duration resulted in a loss of cesium sorption capability. When the slurry cooled to room temperature, cesium did not adsorb to the IE-911.” The reason for this irreversible behavior is not understood. Problems of stability in caustic solution will be addressed by long-term exposure testing to be performed by ORNL. SRTC believes that desorption may result from the formation of sodium aluminosilicate, and it plans to conduct experiments on the effects of silicon and aluminum. In addition, UOP has plans to modify its manufacturing procedures to avoid the presence of other materials.
In general it is expected that inorganic ion exchange materials, such as CST, would exhibit high stability to radiation. However, the current plant design places very stringent requirements on both the material and the process. The current column design is based on an assumed 2.5–5.8 MCi of cesium-137 in a 5-foot (1.5-meter) diameter by 16-foot (4.9-meter) long column. A 5.8 MCi loading corresponds to a radiation field of 0.66 Mrad/hr within the column, and a total dose of 1.4 × 109 rad, assuming that the column stays
loaded for 3 months (Jones, 2000a). The column design and size were determined on the basis of the required processing rates and decontamination factor (Jones, 2000a). Radiation stability tests in “various waste simulants” have been conducted at the Sandia National Laboratory on IE-910 and IE-911 up to 1.2 × 109 rads at 1.4 Mrad/hr at room temperature with no observed decrease in performance. Although this provides information on radiation stability, no tests on actual wastes that would assess column flow and the effects of heat loading have been performed (or are planned) at levels close to plant design.
Work has been done to investigate potential gas generation due to radiolysis in the high fields expected to be present in the columns. Irradiation tests on IE-911 slurries have shown that hydrogen, oxygen, and nitrous oxide are produced. Gas generation tests were conducted to provide information on how gases are retained and released in the column bed. Though some issues have been identified with respect to hydrogen generation and hydrogen peroxide poisoning, no major poisoning problems were identified, and further tests are planned. SRS plans to conduct additional tests to examine cesium removal performance in the presence of gas generation, but the specific tests to be conducted have not yet been decided. Possible tests include use of hydrogen peroxide for a non-radioactive test and the use of ORNL's High Flux Isotope Reactor for a radiation exposure test, although the applicability of such reactor-based tests has not been established.
The non-elutable nature of CTS used in this ion-exchange process would necessitate that the CST as well as the cesium salts be incorporated into borosilicate glass at the DWPF. This introduces questions about the effect of higher titanium content on the stability of the glass. SRS performed a systematic survey of ten experimental glass compositions (Andrews and Workman, 1997). CST was varied from 5 to 22 weight percent, sludge content was varied from 25 to 35 weight percent, and glass frit made up the difference in the bulk composition. This survey established that a glass formulation with 5 percent CST and 28 percent sludge, formed a durable glass with viscosity and liquidus properties within the acceptable processing ranges. Edwards, Harbour, and Workman (1999) identified the ineffectiveness of modeling homogeneity. They concluded that the viscosity model used in previous studies appears to be acceptable and, in fact, over-predicts the measured viscosities. SRS plans to conduct studies on crystal growth kinetics, the effects of variation of chemical constituents, liquidus temperature bounds, and phase separation of the CST.
ANALYSIS
It is clear from the literature that CST contains multiple phases, including proprietary material that is introduced in the manufacturing process. The variability in composition of CST poses a major impediment to interpretation of the large body of data in the literature, yet these data form the basis
for acceptance of CST in the proposed application. Additional uncertainty is introduced by variability in the manufacture and production of CST. In comparison to the samples studied and reported in the peer-reviewed literature, material produced commercially has been prepared by different set of chemical reactions. The form of CST for use with tank wastes is described in the literature as a sodium salt, but it is manufactured and distributed by UOP in a protonated form at pH 3; pretreatment is needed to convert it to the sodium form (Figure 5.1). The pretreatment may be contributing to the currently observed CST instability. A further problem exists in labeling these materials, which sometimes carry the same designations even when derived from different processes. The problem has been exacerbated by inconsistencies in protocols that have been used at SRS.
It is not uncommon to experience difficulties in scaling up chemical engineering processes from bench-scale to pilot-scale to full-scale operations, and this may explain some of the problems observed at SRS. But other difficulties may be related to the composition—or variability in composition—of the CST. It was reported at the committee meeting in November (W. Wilmarth, November 21–22, 1999, personal communication) that several batches of the CST were recalled because of quality acceptance and quality control difficulties. These difficulties could readily account for the loss of capacity. It also appears that the proprietary binder has not yet been adequately evaluated for its solubility at the high pH found in SRS wastes; the reaction of commercial CST in alkaline solution to form new solid phases may be responsible for observed decreases in column performance. Some of the observed difficulties may be the result of lot-to-lot variations or perhaps the change from titanium isopropoxide to chloride and then to nitrate precursors.
To resolve these questions, it will be essential to establish the relationship between the composition of CST and its performance in the ion exchange process. An assessment of the source of the inconsistency in the performance of CST must be a shared responsibility between the manufacturer and SRS. For example, it is possible that some of the observations of poor CST performance may not be caused by variations in the manufactured material but could instead result from failure on the part of SRS to prescribe and follow a uniform pretreatment protocol for the CST. It is appropriate that SRS seek assistance from Sandia National Laboratory to develop an understanding of the mechanism that governs the CST function. Since the modeling effort relies very heavily upon the studies from Texas A&M University, and in light of the observed discrepancies between the performance of CST and the modeling of the performance, a careful re-examination of the assumptions that went into the modeling is in order.
FINDINGS AND CONCLUSIONS
From the preceding discussion, it is clear that a number of issues must be addressed before CST ion exchange can become a viable technol-
ogy for removing cesium from SRS HLW. A review of the available literature on CST suggests several categories of problems:
-
The issues identified with reproducibility of CST performance (for example, variability in exchange capacity and possible dissolution/reprecipitation of the proprietary components) are not well understood. These have been tentatively traced to manufacturing variability at production scale, but they could also be a consequence of the variations in pretreatment of the material at SRS or in the testing used to characterize the materials.
-
The column design for the CST process was based on typical parameters such as throughput and decontamination factors, but do not appear to be optimized for thermal loadings and expected radiation fields. The combination of particle size of the ion exchange material and the high thermal and radiation loading causes the committee to have concerns with the large-column concept and the use of this concept to drive future development.
-
The possible problems of radiolytic gas generation have not been resolved; formation of gas inside the columns could disrupt the flow of liquid and reduce the efficiency of the ion exchange process.
-
The potential effects of incorporating loaded CST into the borosilicate glass stream are not well understood. But the cesium loading capacity of the CST columns will affect the quantity of material and titanium content of the glass feed (see Chapter 8).
RECOMMENDATIONS
-
Efforts should be made—in conjunction with the manufacturer of CST—to ensure that a consistent and reproducible material is obtained for use in the ion exchange process. The basis for consistency and reproducibility should include clear and relevant acceptance criteria for the manufactured material.
-
Uniform CST pretreatment and testing protocols should be developed.
-
Column ion exchange design (and other possible processing streams using CST, such as a slurry) should be reevaluated and optimized, as should the elutable ion exchange resin.
-
An R&D effort should be undertaken to determine the performance characteristics of the standard material. This should include study of temperature effects, radiation effects, capacity, flow, gas generation, and other effects, including time, that are important for process and column operation and design.