This paper was presented at a colloquium entitled “Vision: From Photon to Perception,” organized by John Dowling, Lubert Stryer (chair), and Torsten Wiesel, held May 20–22, 1995, at the National Academy of Sciences in Irvine, CA.
Primate photopigments and primate color vision
(opsin genes/polymorphism/cones/evolution)
GERALD H. JACOBS
Neuroscience Research Institute and Department of Psychology, University of California, Santa Barbara, CA 93106
ABSTRACT The past 15 years have brought much progress in our understanding of several basic features of primate color vision. There has been particular success in cataloging the spectral properties of the cone photopigments found in retinas of a number of primate species and in elucidating the relationship between cone opsin genes and their photopigment products. Direct studies of color vision show that there are several modal patterns of color vision among groupings of primates: (i) Old World monkeys, apes, and humans all enjoy trichromatic color vision, although the former two groups do not seem prone to the polymorphic variations in color vision that are characteristic of people; (ii) most species of New World monkeys are highly polymorphic, with individual animals having any of several types of dichromatic or trichromatic color vision; (iii) less is known about color vision in prosimians, but evidence suggests that at least some diurnal species have dichromatic color vision; and (iv) some nocturnal primates may lack color vision completely. In many cases the photopigments and photopigment gene arrangements underlying these patterns have been revealed and, as a result, hints are emerging about the evolution of color vision among the primates.
The generalization that color vision is a more developed and acute capacity in primates than it is in other mammals came from a consideration of the natural history of mammals (1). There is now extensive experimental support for this proposition (2), but a surprise from the results of color-vision studies of the past two decades is that primate color vision is not monolithic. The substantial variations in color vision that have been revealed, both among the members of some species of primate and between various groupings of species, have provided the opportunity to examine in greater detail the biological mechanisms that underlie color vision, particularly the photopigments of cone photoreceptors and the genes crucial for the production of these photopigments. These findings also provide leads about the evolution of primate color vision, and they have served to reawaken interest in understanding the functional utility of color vision.
Cone Photopigment Polymorphism
The biological process that results in color vision is initiated by the neural comparison of signals from classes of cone photoreceptor that contain spectrally distinct photopigments. Among other things, the nature of the color vision that ensues depends on the number of such classes of photopigment, the spectral separation of the photopigments, and the relative representation of the different pigments among the population of photoreceptors. An elegant feature of color vision is that variations in the number of types of cone pigment found in the retina normally map directly into the dimensionality of color vision as defined by the standard behavioral test of color matching —i.e., two classes of cone pigment underlie dichromatic color vision; three classes of cone pigment yield trichromatic color vision. This compulsive linkage between behavioral test results and pigment measurements means that measurements of either kind can be used to draw inferences about the other.
New World Monkeys. Although there were plenty of earlier hints that the color vision of New World monkeys differs from that of normal human subjects ( 3,4), it was unexpected to find the biggest difference was the degree of intraspecies variation in color vision. Direct behavioral tests of color vision in squirrel monkeys (Saimiri sciureus) showed that there were striking individual variations in this species (5). For instance, in one test monkeys were required to discriminate various additive mixtures of middle- and long-wavelength lights from a light of a fixed intermediate wavelength. Tests of this sort were first devised more than 100 years ago by Lord Rayleigh (6) and are commonly called Rayleigh matches. The results of this test indicated that many squirrel monkeys were quite unable to make the discrimination. Behavior of this sort is diagnostic of dichromatic color vision. Others monkeys succeeded at this discrimination, but there was further individual variation in the nature of the mixtures that could and could not be discriminated. These and other tests made it clear that while many squirrel monkeys have dichromatic color, others are trichromatic. In fact, results from a battery of tests of sensitivity and color vision led to the conclusion that six distinct forms of color vision could be found in this species of primate.
Since the variations in color vision among these monkeys were defined by variations in color matching, the implication, as noted above, was that the differences in color vision probably reflected differences in the types of cone pigment found in individual animals. Microspectrophotometric measurements of the cone pigments in the retinas of animals of known color vision verified that prediction (7–9). The retinas of all squirrel monkeys were found to contain a photopigment with peak sensitivity in the short wavelengths (S pigment). These monkeys also had three possible cone pigments with maximum absorption in the middle (M) to long (L) wavelengths; the average peak values (λMAX) of these are at about 535, 550, and 563 nm. Individual animals had any one of these three, or they had any pair. The former have trichromatic color vision; the latter are dichromats. The variation in photopigment complement accounts completely for the substantial variations in color vision in these monkeys.
Subsequent investigations have shown that this pattern of cone pigment and color vision polymorphism is common
The publication costs of this article were defrayed in part by page charge payment. This article must therfore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: L, long-wavelength-sensitive; λMAX, wavelength of peak sensitivity; M, middle-wavelength-sensitive; S, short-wavelength-sensitive.
among New World monkeys (10–14). Although there is some limited variation in the set of M/L photopigments for different species, in each case the sorting of these pigments among individuals appears to be the same as we found for the squirrel monkey. Because there are still many species that have not been investigated, we do not yet know if this pattern is universal for New World monkeys.
A second surprise about New World monkeys was that their color vision variations have a singular sex-linked component. Although individual female monkeys can have either dichromatic or trichromatic color vision, all the males are dichromats (15,16). The genes that specify the opsins required to produce M and L cone photopigments are on the X chromosome. This fact suggested a simple model to explain the polymorphism of cone pigments and color vision in these New World monkeys (7,17). The idea is that there is a single locus on the X chromosome of these monkeys with three allelic versions of the opsin gene. Each gene specifies one of the three possible M/L pigments. Male monkeys have one of these three genes; in combination with the S-cone pigment [the opsin of which derives from a gene on chromosome 7 (18)] males thus get one of three types of dichromatic color vision. Homozygous female monkeys will also have dichromatic color vision, but heterozygous females inherit genes for two spectrally distinct M/L cone pigments. The mechanism of X-chromosome inactivation sorts these two into separate cone classes and trichromatic color vision emerges. Studies employing both classical pedigree analysis (15) and molecular genetic approaches (16,19) have provided strong support for this model.
Old World Monkeys and Apes. Color vision in Old World monkeys and apes presents a quite different picture. As far as we know, all the species from these two groups have trichromatic color vision (20). Direct measurements of the M/L photopigments in these species are rather sparse, but it appears that the λMAX values for two types of pigment are at about 530 and 560 nm, respectively (21–23). The opsins for these pigments arise from the activity of two different types of gene on the X chromosome (24–26). There is so far a remarkable absence of any evidence for polymorphism of these photopigments and consequent individual variations in color vision in any of the Old World monkeys or apes.
Classical Variations in Human Cone Pigments. Polymorphic variations in M/L cone photopigments are common among people (affecting a total of about 4% of the population). These lead to the color vision defects and anomalies that have been the subject of intensive study for many years (27). As estimated from a variety of different experimental approaches, the M/L cone pigments of normal human trichromats have spectral peaks of about 530 and 560 nm (28–31). Absence of either of these types leads to dichromatic color vision —deuteranopia and protanopia, respectively. A second major class of polymorphic variation in the M/L pigments produces the most common color vision defects, the anomalous trichromacies. In this case, the standard explanation has been that either the normal M or the normal L pigment is replaced by an “anomalous” pigment, and this anomalous pigment is peak-shifted so as to be very close in spectral position to that of the remaining normal pigment (32). The reduced spectral separation of the two pigments, perhaps in combination with other factors, accounts for the aberrant color discrimination that is characteristic of these individuals. The actual spectral positions of these anomalous pigments are not as securely established, but by many accounts the peak separation between these pigments and the remaining normal pigments may be about 6 nm (e.g., see ref.33).
A compilation of the measurements of M/L cone pigments in nonhuman and human primates suggests that all primate color vision in this part of the spectrum is subserved by a restricted set of available pigment types. There may be only six of these. Fig. 1 shows the absorption spectra for these pigments, and it is noteworthy that the same pigment positions are represented in many different species of primate. For instance, every Old World monkey, the apes, people, and (with one known exception) all New World monkeys share in common a version of the M/L pigment that has a spectral peak at about 560 nm. The mechanism controlling spectral positioning of primate pigments must be conservative.
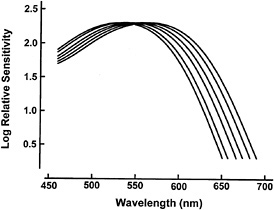
FIG. 1. Absorption spectra for primate M/L cone pigments having λMAX of 530, 535, 543, 549, 556, and 562 nm, respectively. These six have been found in a variety of different primates, and they may represent the full set of available primate photopigments in this portion of the spectrum. The actual λMAX values obtained vary somewhat depending on the measurement techniques. The values specified here come from electrophysiological measurements made by me and my colleagues.
Additional M/L Pigment Polymorphism in Humans. Evidence has accumulated over the past decade to indicate that there are measurable variations in the spectra of human M and L pigments beyond those that produce the classical color vision defects. It has long been apparent that human subjects of the same color vision phenotype often make reliably different color matches. In a thorough series of psychophysical experiments, Alpern and his colleagues documented these individual variations in color matching, and in so doing convincingly demonstrated that they must be attributed to individual variations in the spectral positioning of the M and L cone pigments (reviewed in ref.34). Although most psychophysical experiments have confirmed the presence of variation in the spectral positioning of the human M/L pigments, both the extent and nature of the variation have been subjects of spirited debate (for a recent review of this work see ref.35). On the one side are experiments involving Rayleigh matching in which the distribution of matches made by trichromatic subjects is multimodal (36–38); in other experiments, however, the match distribution is found not to be multimodal (39,40). The interpretational difference between these two sets of experiments is whether or not the match variations of normal human subjects can be considered to reflect an additional polymorphism of the human M and L photopigments. That these behavioral experiments have not yielded a common outcome could well reflect the small size of the variations that are being measured and the inevitable differences arising from variations in experimental techniques. Although the behavioral experiments are ambiguous on the possibility of additional polymorphism of human M/L pigments, recent work on human cone opsin genes and their pigment products shows in convincing fashion that such pigment polymorphism does exist.
Cone Opsin Polymorphism
In a stunning achievement of a decade ago, Nathans and co-workers isolated and sequenced the genes encoding the human cone opsins ( 18,41). The X-chromosome genes for the M and L cone opsins were found to be highly homologous and to lie close together in tandem array. A surprise was that rather than two opsin genes, as predicted from most classical theories about the inheritance of color vision, there was individual variation in the total number of genes. Recent work supports this finding, suggesting that many individuals may have multiple copies of either or both of the M and L cone opsin genes (42). The high homology of these genes, their physical proximity, and the variation in number provides a mechanism for producing variations in color vision. The idea is that unequal homologous recombinations can result in variation both in the number and, as a result of intragenic exchange of sequence, in the identity of genes on the X chromosome. Although accounts differ in detail, these resortings are argued to yield the various phenotypes of human color vision (35,41,43).
The great similarity among the X-chromosome opsin genes implies that only a small number of changes in gene sequence likely account for the differences in the absorption spectra of the M and L cone pigments. The clear variations in the pigments of the New World monkeys suggested it would be profitable to correlate spectral positioning with sequence differences among several different phenotypic versions of the M and L cone pigments in these animals. Accordingly, we made sequence comparisons for a total of eight different opsin genes, six from two species of New World monkey and two from human dichromats (44). The results indicated that as few as three amino acid substitutions were sufficient to explain the variations in the spectra of these pigments. In each case, replacement of a nonpolar with a hydroxyl-bearing amino acid appeared to result in a spectral shift of the pigment toward the long wavelengths. Each individual change was associated with a spectral shift of a different magnitude and, somewhat surprisingly, it seemed that the effects of changes at the three locations were approximately additive. A subsequent comparison of sequences for three additional genes from two other species of New World monkey lent support to these conclusions (45).
These intuitions about the control of spectral tuning in M/L pigments have been largely confirmed by more direct experiments in which mutant pigments have been expressed and examined in vitro. In these studies (31,46), as in the examination of the naturally occurring polymorphisms, three amino acid substitutions are identified as being involved in spectrally tuning these pigments. In addition, the in vitro experiments suggest the possibility that changes at a restricted number of other sites could potentially cause other small (4 nm or less) shifts in the spectral peaks of these M/L pigments.
Particular interest has been focused on one of these changes (a Ser/Ala substitution at position 180) because it is a naturally occurring polymorphism in the M/L cone pigments of human populations. In the in vitro experiments, as well as in those involving comparisons of genes from different primates, this substitution leads to a spectral shift in the pigment of perhaps 5–7 nm. Are there measurable differences in the vision of individuals who have these two different versions of these genes? Apparently so, for measurements yield a significant positive correlation between the polymorphic version of the genes present and the derived color matches (37,47). Furthermore, this same polymorphism occurs in human dichromats, and here the gene variation can be shown to cause a shift in the measured spectral sensitivity of the eye (48). There seems little doubt that in addition to the opsin gene polymorphisms associated with color vision defects there are more subtle variations that also can be shown to have an impact on human vision. It is provocative that the Ser/Ala-180 polymorphism so apparent in the human M/L opsin genes is absent in our closest relative, the chimpanzee (25).
S-Cone Genes and Photopigments
In addition to one or more M/L pigments, most primates also have an S-cone photopigment. For reasons that include the restricted number of S cones in primate retinas and the difficulties of isolating their activity in intact eyes, specification of primate S cone spectra is more tentative than for the M and L cones. For instance, the λMAX values offered for the human S cone cover a range of about 20 nm. Similar uncertainty exists for some other primate species. There do, however, appear to be two supportable conclusions about the primate S-cone pigments: first, not all primate S cones have identical spectral positioning (23) and, second, unlike the case for M/L cone pigments, there is no evidence for any S-cone polymorphism in any primate species. With respect to the first of these possibilities, it has been suggested that the spectral positioning of the S-cone pigment, like that of M and L pigments, is controlled by discrete amino acid substitutions (in this case at only two sites) that also involve the gain or loss of a hydroxyl group (49).
A recent discovery is that at least some nocturnal primates appear to lack a population of S cones. Evidence derived from electrophysiological, behavioral, and immunocytochemicallabeling studies shows that the retinas of both the owl monkey (Aotus), the only nocturnal simian, and a nocturnal prosimian (the thick-tailed bushbaby, Otolemur crassicaudatus) contain no S cones (50–52). However, individual animals from each of these species were found to have an S-cone opsin gene. Sequence analysis suggests a reason why the bushbaby fails to express S-cone pigment. Exon 4 of the bushbaby S-cone pigment gene, the region coding for one of the transmembrane segments of the pigment, contains a two-nucleotide insertion followed by a single deletion. These changes shift the reading frame and introduce a stop codon (53). Even though these primates do have a single type of M/L cone pigment, the absence of S cones sentences them to complete color blindness (although there is the possibility that they may be able to make some pure-spectral discriminations based on comparison of signals from rods and the single cone type—see ref.51).
Summary and Prospects
The variations in primate cone opsin genes, cone photopigments, and color vision thus far documented suggest the summary classification of Table 1. This account is subject to the qualification that we still lack much essential information. Given that, five different patterns emerge. The predominant arrangement among nonprimate mammals is a retina containing two classes of cone photopigment that supports dichromatic color vision (2). There appears to be little or no polymorphic variation in these animals. At least some diurnal prosimian species follow this model (54). Some nocturnal primates have a similar arrangement, except that their S-cone opsin gene has acquired a deleterious mutation that precludes the possibility of a second class of cone pigment; it renders these animals monochromatic. The polymorphic confusion of photopigments and color vision among many New World monkeys has been extensively documented. Less extensively documented, but thus far without exception, is the routine trichromacy found among Old World monkeys and apes. Although broadly similar to these primates in having routine trichromacy, our species shows significant photopigment polymorphisms, including those that lead to dramatic variations in color vision and the much smaller changes that appear to have little or no practical impact.
One possible scenario for the evolution of primate color vision starts from the view expressed above: that the norm for
Table 1. Summary of primate opsin genes, cone photopigments, and color vision
|
Group |
Chromosome 7 genes |
X-chromosome genes |
Photopigments (λMAX, nm) |
Color vision |
|
Some nocturnal primates |
Defective S-opsin gene |
Single M/L |
543 |
Monochromatic |
|
Some diurnal prosimians |
S |
Single M/L |
≈430 + 543 |
Dichromatic |
|
Many New World monkeys |
S |
Polymorphic |
420−435 + (535, 543, 550, 556, 562) |
Dichromatic/trichromatic |
|
All (?) Old World monkeys; apes |
S |
M + L (multiple copies) |
430 + 530 + 562 |
Uniformly trichromatic |
|
Humans |
S |
M + L (multiple copies/polymorphisms) |
410−430 + 530/535 + 556/562 |
Uniformly trichromatic; significant polymorphisms |
|
The λMAX values for the M and L cones represent averages obtained from electrophysiological measurements (see Fig. 1). Those for the S cones are taken from a variety of different types of measurement; a range indicates that there is uncertainty or that there may be alternative pigments in different species in the group. The alternative possibilities suggested for the human M and L cones reflect a polymorphism of the cone opsins. |
||||
mammals is two classes of cone pigment and dichromatic color vision (2). Some contemporary primates conform to this norm; others would be this way except that mutational changes have rendered their S-cone opsin gene nonfunctional. Molecular comparisons of cone opsin genes suggest that the divergence that led to two separate M and L cone pigments occurred about 30 million years ago (17,55). Presumably this was subsequent to the separation of New and Old World primate lineages but prior to the separation of cercopithecoid and hominoid primates (26). This divergence event yields the photopigment basis for routine trichromatic color vision. It can be argued that the arrangement of genes and cone pigments in the New World monkeys provides a blueprint as to how this may have happened. In a routinely dichromatic species, only a single nucleotide substitution in an opsin gene is required to yield a novel M/L pigment. When the novel gene appears in a heterozygous female she will produce two spectrally discrete M/L pigments and, if her visual nervous system is arranged like that of many New World monkeys, trichromatic color vision emerges. Additional altered genes can increase the frequency of female trichromacy; for instance, with three alleles two-thirds of all female New World monkeys can achieve that status. To make trichromacy routine requires a second gene locus. This could have come either from an unequal crossover between chromosomes having different alleles or through gene duplication and subsequent gene conversion.
Although these ideas provide the mechanics for the evolution of trichromatic color vision, they fail to reveal the selective pressures that conditioned these changes. A standard idea is that trichromatic color vision substantially enhances one's ability to detect, identify, and evaluate objects in the environment (56). In the case of primates, the objects of concern were probably colored fruits. For instance, the trichromatic color vision of primates will allow a rapid and accurate detection of yellow and orange fruit hidden among the abundant green foliage of tropical forests. In turn, the primate harvester then serves as an agent to disperse seeds to new locations. Although definitive proof is lacking, it seems likely that this contractual interaction may have provided the setting for the evolution of primate trichromacy.
Two things are missing from the current picture. First, there remains a dearth of information about cone opsin genes and color vision for many species of primate. Second, although informed discussions about the functional utility of color vision now appear with increasing frequency (e.g., refs.57–61), we still lack a detailed understanding of the many ways in which primates use spectral information in their successful dealings with the environment.
Over the years I have received indispensable help on this project from the following collaborators: B. Blakeslee, J. K. Bowmaker, M. A. Crognale, J. F. Deegan II, J. D. Mollon, M. Neitz, and, especially, J. Neitz. My work summarized here was funded by grants from the National Science Foundation and the National Eye Institute.
1. Walls, G. L. ( 1942) The Vertebrate Eye and Its Adaptive Radiation (Cranbrook Institute of Science, Bloomfield Hills, MI).
2. Jacobs, G. H. ( 1993) Biol. Rev. 68, 413–471.
3. Grether, W. F. ( 1939) Comp. Psychol. Monogr. 29, 1–38.
4. Jacobs, G. H. ( 1963) J. Comp. Physiol. Psychol. 56, 616–621.
5. Jacobs, G. H. ( 1984) Vision Res. 24, 1267–1277.
6. Strutt, J. W. ( 1881) Nature (London) 25, 64–66.
7. Mollon, J. D., Bowmaker, J. K. & Jacobs, G. H. ( 1984) Proc. R. Soc. London B 222, 373–399.
8. Bowmaker, J. K., Jacobs, G. H., Spiegelhalter, D. J. & Mollon, J. D. ( 1985) Vision Res. 25, 1937–1946.
9. Bowmaker, J. K., Jacobs, G. H. & Mollon, J. D. ( 1987) Proc. R. Soc. London B 231, 383–390.
10. Jacobs, G. H. & Neitz, J. ( 1987) Vision Res. 27, 1263–1268.
11. Jacobs, G. H., Neitz, J. & Crognale, M. ( 1987) Vision Res. 27, 2089–2100.
12. Travis, D. S., Bowmaker, J. K. & Mollon, J. D. ( 1988) Vision Res. 28, 481–490.
13. Tovee, M. J., Bowmaker, J. K. & Mollon, J. D. ( 1992) Vision Res. 32, 867–878.
14. Jacobs, G. H. & Deegan, J. F. ( 1993) Invest. Ophthalmol. Visual Sci. 34, 749 (abstr.).
15. Jacobs, G. H. & Neitz, J. ( 1987) Proc. Natl. Acad. Sci. USA 84, 2545–2549.
16. Jacobs, G. H., Neitz, J. & Neitz, M. ( 1993) Vision Res. 33, 269–274.
17. Jacobs, G. H. & Neitz, J. ( 1985) Vision Res. 25, 141–144.
18. Nathans, J., Thomas, D. & Hogness, D. H. ( 1986) Science 232, 193–202.
19. Williams, A. J., Hunt, D. M., Bowmaker, J. K. & Mollon, J. D. ( 1992) EMBO J. 11, 2039–2045.
20. Jacobs, G. H. ( 1990) in Inherited and Acquired Colour Vision Deficiencies: Fundamental Aspects and Clinical Studies, ed. Foster, D. H. (Macmillan, London), pp. 199–214.
21. Baylor, D. A., Nunn, B. J. & Schnapf, J. L. ( 1987) J. Physiol. (London) 357, 145–160.
22. Bowmaker, J. K., Astell, S., Hunt, D. M. & Mollon, J. D. ( 1991) J. Exp. Biol. 156, 1–19.
23. Bowmaker, J. K. ( 1991) in From Pigments to Perception, eds. Valberg, A. & Lee, B. B. (Plenum, New York), pp. 1–9.
24. Ibbotson, R. E., Hunt, D. M., Bowmaker, J. K. & Mollon, J. D. ( 1992) Proc. R. Soc. London B 247, 145–154.
25. Deeb, S. S., Jorgensen, A. L., Battisti, L., Iwasaki, L. & Motulsky, A. G. ( 1994) Proc. Natl. Acad. Sci. USA 91, 7262–7266.
26. Dulai, K. S., Bowmaker, J. K., Mollon, J. D. & Hunt, D. M. ( 1994) Vision Res. 34, 2483–2491.
27. Pokorny, J., Smith, V. C., Verriest, G. & Pinckers, A. J. L. G. ( 1979) Congenital and Acquired Colour Vision Defects (Grune & Stratton, New York).
28. Dartnall, H. J. A., Bowmaker, J. K. & Mollon, J. D. ( 1983) Proc. R. Soc. London B 230, 115–130.
29. Schnapf, J. L., Kraft, T.W. & Baylor, D. A. ( 1987) Nature (London) 325, 439–441.
30. Merbs, S. L. & Nathans, J. ( 1992) Nature (London) 356, 433–435.
31. Asenjo, A. B., Rim, J. & Oprian, D. D. ( 1994) Neuron 12, 1131–1138.
32. Piantanida, T. P. ( 1976) Am. J. Optom. Physiol. Opt. 53, 647–657.
33. DeMarco, P., Pokorny, J. & Smith, V. C. ( 1992) J. Opt. Soc. Am. 9, 1465–1476.
34. Alpern, M. ( 1987) in Frontiers of Visual Science: Proceedings of the 1985 Symposium, eds. National Research Council Committee on Vision (Natl. Acad. Press, Washington, DC), pp. 169–193.
35. Neitz, J. & Neitz, M. ( 1994) in Molecular Genetics of Inherited Eye Disorders, eds. Wright, A. F. & Jay, B. (Harwood, Reading, U.K.), pp. 217–257.
36. Neitz, J. & Jacobs, G. H. ( 1990) Vision Res. 30, 621–636.
37. Winderickx, J., Lindsey, D. T., Sanocki, E., Teller, D. Y., Motulsky, A. & Deeb, S. S. ( 1992) Nature (London) 356, 431–433.
38. Ji, C. H. & Shevell, S. K. ( 1994) Vision Res. 34, 367–376.
39. Lutze, M., Cox, N. J., Smith, V. C. & Pokorny, J. ( 1990) Vision Res. 30, 149–162.
40. Jordan, G. & Mollon, J. D. ( 1995) Vision Res. 35, 613–620.
41. Nathans, J., Piantanida, T. P., Eddy, R. L., Shows, T. B. & Hogness, D. S. ( 1986) Science 233, 203–210.
42. Neitz, M. & Neitz, J. ( 1995) Science 267, 1013–1016.
43. Deeb, S. S., Lindsey, D. T., Hibiya, Y., Sanocki, E., Winderickx, J., Teller, D. Y. & Motulsky, A. G. ( 1992) Am. J. Hum. Genet. 51, 687–700.
44. Neitz, M., Neitz, J. & Jacobs, G. H. ( 1991) Science 252, 971–974.
45. Hagstrom, S. A., Teunnisen, D. L., Neitz, M., Deegan, J. F., II, Jacobs, G. H. & Neitz, J. ( 1993) Invest. Ophthalmol. Visual Sci. 34, 809.
46. Merbs, S. L. & Nathans, J. ( 1992) Science 258, 464–466.
47. Neitz, J., Neitz, M. & Jacobs, G.H. ( 1993) Vision Res. 33, 117–122.
48. Neitz, M., Neitz, J. & Jacobs, G. H. ( 1995) Vision Res. 35, 2095–2103.
49. Hunt, D. M., Bowmaker, J. K., Patel, R., Appukuttan, B. & Mollon, J. D. ( 1995) Invest. Ophthalmol. Visual Sci. 36, S889 (abstr.).
50. Wikler, K. C. & Rakic, P. ( 1990) J. Neurosci. 10, 3390–3401.
51. Jacobs, G. H., Deegan, J. F., II, Neitz, J., Crognale, M. A. & Neitz, M. ( 1993) Vision Res. 33, 1773–1783.
52. Deegan, J. F., II, & Jacobs, G. H. ( 1994) Am. J. Primatol. 33, 205 (abstr.).
53. Jacobs, G. H., Neitz, M. & Neitz, J. ( 1995) Am. J. Primatol. 36, 129 (abstr.).
54. Jacobs, G. H. & Deegan, J. F., II ( 1993) Am. J. Primatol. 30, 243–256.
55. Yokoyama, S. & Yokoyama, R. ( 1989) Mol. Biol. E vol. 6, 186–197.
56. Jacobs, G. H. ( 1981) Comparative Color Vision (Academic, New York).
57. Goldsmith, T. H. ( 1990) Q. Rev. Biol. 65, 281–322.
58. Mollon, J. D. ( 1991) in Evolution of the Eye and Visual System, eds. Cronly-Dillon, J. R. & Gregory, R. L. (CRC, Boca Raton, FL), pp. 306–319.
59. Endler, J. A. ( 1992) Am. Nat. 139, S125–S153.
60. Nagle, M. G. & Osorio, D. ( 1993) Proc. R. Soc. London B 252, 209–213.
61. Jacobs, G. H. ( 1995) Evol. Anthropol. 3, 196–205.





