This paper was presented at a colloquium entitled “Vision: From Photon to Perception,” organized by John Dowling, Lubert Stryer (chair), and Torsten Wiesel, held May 20–22, 1995, at the National Academy of Sciences in Irvine, CA.
Emergence of order in visual system development
CARLA J. SHATZ
Howard Hughes Medical Institute and Division of Neurobiology, Department of Molecular and Cell Biology, LSA 221, University of California, Berkeley, CA 94720
ABSTRACT Neural connections in the adult central nervous system are highly precise. In the visual system, retinal ganglion cells send their axons to target neurons in the lateral geniculate nucleus (LGN) in such a way that axons originating from the two eyes terminate in adjacent but nonoverlapping eye-specific layers. During development, however, inputs from the two eyes are intermixed, and the adult pattern emerges gradually as axons from the two eyes sort out to form the layers. Experiments indicate that the sorting-out process, even though it occurs in utero in higher mammals and always before vision, requires retinal ganglion cell signaling; blocking retinal ganglion cell action potentials with tetrodotoxin prevents the formation of the layers. These action potentials are endogenously generated by the ganglion cells, which fire spontaneously and synchronously with each other, generating “waves” of activity that travel across the retina. Calcium imaging of the retina shows that the ganglion cells undergo correlated calcium bursting to generate the waves and that amacrine cells also participate in the correlated activity patterns. Physiological recordings from LGN neurons in vitro indicate that the quasiperiodic activity generated by the retinal ganglion cells is transmitted across the synapse between ganglion cells to drive target LGN neurons. These observations suggest that (i) a neural circuit within the immature retina is responsible for generating specific spatiotemporal patterns of neural activity; ( ii) spontaneous activity generated in the retina is propagated across central synapses; and (iii) even before the photoreceptors are present, nerve cell function is essential for correct wiring of the visual system during early development. Since spontaneously generated activity is known to be present elsewhere in the developing CNS, this process of activity-dependent wiring could be used throughout the nervous system to help refine early sets of neural connections into their highly precise adult patterns.
The formation of precise connectivity is generally thought to involve two distinct sets of mechanisms: those that require neuronal activity and those that are activity independent (reviewed in ref.1). Many studies in both vertebrates and invertebrates suggest that the early events of axon outgrowth, pathfinding, and target selection are relatively accurate and do not require action potentials and synaptic transmission. These studies have shown that axon growth cones can select correct pathways and targets by responding to a variety of specific molecular cues laid out on cell surfaces, in the extracellular matrix, or even diffusing from distant sources. Once axons invade their targets, however, the initial connections they make with individual target neurons frequently are not accurate. Rather, the process of forming the adult precision of connectivity involves the correction of many initial errors; this process of error correction almost always requires neural activity.
Here, I wish to consider how neural activity contributes to the emergence of the adult pattern of precise connectivity in the mammalian visual system. In the adult visual system, information about the world is sent from the eye to more central visual structures via the output neurons of the retina, the ganglion cells (2). Axons of the retinal ganglion cells project to several visual relay structures within the brain, such as the lateral geniculate nucleus (LGN). The connections between ganglion cells and LGN neurons are precise and stereotyped. One important example of this precision is that the connections are topographically organized; neighboring retinal ganglion cells send their axons to contact the same or nearby target neurons within the LGN, setting up a retinotopic map. In addition, within the LGN, retinal ganglion cell axons from one eye are strictly segregated from those arising from the other eye to form a series of alternating eye-specific layers. There are several pairs of LGN layers because each pair receives inputs from a subset of functionally distinct retinal ganglion cells (reviewed in ref.3).
LGN Layers Are Not Present Initially in Development
How is the segregated pattern of eye input from ganglion cells to the LGN wired up during development? Many lines of experiments now indicate that when connections between retinal ganglion cells and LGN neurons first form they are not as precise as in the adult. Intraocular injections of anterograde tracers (4–6) or filling of individual ganglion cell axons with horseradish peroxidase (HRP) (7) reveal that the eye-specific layers are not present initially; ganglion cell axons from the two eyes are intermixed with each other throughout a large portion of the LGN (Fig. 1). With ensuing development, the layers emerge as axons gradually remodel their branches by retracting sidebranches from inappropriate LGN regions and growing extensive terminal arbors within the region appropriate to their eye of origin.
The presence of extensive intermixing of retinogeniculate axons, followed by segregation into layers, suggests that the process by which segregation is achieved may involve competitive interactions between ganglion cell axons from the two eyes. Such interactions might permit right- and left-eye axons to compete for LGN neurons that themselves are not intrinsically different from each other with respect to eye of origin. Some of the first evidence in favor of the idea that competitive interactions between retinal ganglion cell axons give rise to the eye-specific layers has come from studies in many species in which one eye is removed during development and the pattern of the retinogeniculate projection from the remaining eye is examined at later developmental times or in adulthood. The
The publication costs of this article were defrayed in part by page charge payment. This article must therfore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: LGN, lateral geniculate nucleus; TTX, tetrodotoxin; En, embryonic day n; Pn, postnatal day n; HRP, horseradish peroxidase; EPSC, excitatory postsynaptic current.
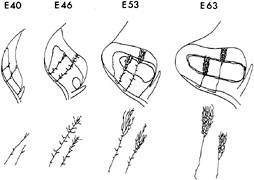
FIG. 1. The relationship between the changes in the global pattern of the retinogeniculate projection and the development of individual retinogeniculate axons is summarized. Shaded areas indicate regions within the LGN simultaneously occupied by ganglion cell axons from the two eyes. Stick figures show the appearance of representative axons from the ipsilateral (shorter axon) and contralateral eyes at each age, based on their appearance by using the in vitro HRP-filling technique. By embryonic day 63 (E63), just before birth, the eye-specific layers are almost completely formed: axons from the contralateral eye have terminal arbors largely restricted to LGN layer A (top layer), while those from the ipsilateral eye are largely restricted to layer A1 (middle layer). (Modified from ref. 7.)
results are generally consistent in showing that axons from the remaining eye are capable of occupying the entire LGN, including territory that normally would have been innervated by the enucleated eye (refs. 8 and 9; see ref.10 for review and ref.11 for a possible exception). These observations indicate that inputs from both eyes are necessary for segregation to occur, and they suggest that LGN neurons themselves are not rigidly specified with respect to the ocular identity of their retinal innervation.
Studies, both of the mammalian visual system and elsewhere in the central and peripheral nervous systems, suggest that the transformation from a mixed to a segregated state occurs during a period in which the axonal inputs destined to segregate from each other are first capable of forming functioning synaptic connections with common postsynaptic target cells (for reviews see refs.12–16). This evidence has generated the current hypothesis, considered below, that segregation is achieved via an activity-mediated competitive process requiring the formation and elimination of synaptic connections.
In the developing retinogeniculate pathway, the cellular machinery necessary to sustain activity-driven synaptic competition is present. Ultrastructural examination of identified retinogeniculate axons has demonstrated directly that ganglion cell axons from each eye form many synapses, both in territory ultimately destined to become innervated by that eye and also in the territory that will come to belong to the other eye (17). Not only are synapses present during the fetal period, but they are also capable of functional transmission (ref.18; see also below). By electrically stimulating the optic nerves and recording from LGN neurons with extracellular microelectrodes in vitro, we found that even before the onset of segregation there is functional synaptic transmission. Moreover, during the period of extensive anatomical intermixing (E40–E59 in cat), about 90% of the LGN neurons studied physiologically received convergent excitatory inputs from stimulation of both optic nerves. In contrast, in the adult, the vast majority of LGN neurons receive excitatory input from only one nerve. The most reasonable interpretation of these observations, particularly in the context of the anatomical experiments considered above, is that prior to the completion of segregation many LGN neurons indeed receive monosynaptic excitation from both nerves. The emergence of the eye-specific layers is then accompanied by a functional change in the synaptic physiology of the retinogeniculate pathway: from binocular to monocular excitation.
Action-Potential Activity Is Required for the Formation of the Eye-Specific Layers
It is important to note that in every mammalian species studied so far the eye-specific layers form during a period in which vision is not possible: the photoreceptor outer segments are not yet present or functional (19,20). Therefore, unlike other developing systems, such as the neuromuscular junction or the primary visual cortex, in which action potentials are evoked via use-dependent activity, here, it is necessary to postulate that activity is present as spontaneously generated action potentials. Elegant experiments by Galli and Maffei (21) indicate that this is indeed the case. They made extracellular microelectrode recordings from fetal rat retinal ganglion cells in vivo and demonstrated that ganglion cells indeed can fire spontaneously. The nature of this spontaneous activity and whether it is relayed to the LGN neurons will be considered more fully below.
There are now several excellent examples in which activity-dependent competition is known to be required for the final patterning of axonal connections in the vertebrate visual system. These include the postnatal development of the system of ocular dominance columns in layer 4 of the visual cortex (14,22–24) and the experimentally induced formation of eye-specific stripes in the optic tecta of frogs (25,26) and goldfish (27,28). In each instance, blocking retinal ganglion cell activity [by means of injections of tetrodotoxin (TTX), a blocker of voltage dependent sodium channels] or blocking synaptic transmission (by the use of glutamate receptor blockers such as 2-amino-5-phosphonovaleric acid) prevents segregation of eye input (reviewed in refs.15 and 16). By analogy with these examples, it should be possible to prevent or at least delay retinogeniculate segregation by blocking retinogeniculate transmission. Blockade was achieved by implanting osmotic minipumps in utero in cat fetuses and infusing TTX intracranially for the 2-week period during which the eye-specific layers largely form (between E42 and E56; Fig. 1). Infusions of TTX (but not vehicle) prevented the segregation of ganglion cell axons into layers (29). Moreover, as shown in Fig. 2, the axons were not simply stunted or arrested in their growth, but, rather, they grew extensively and, in fact, were about 35% larger in total linear extent than were untreated axons (30).
These observations indicate that TTX can affect the development of two basic features of ganglion cell arbor morphology: the shape of the axon (normally restricted to a cylindrical terminal arbor) and its location (normally within a single eye-specific layer). One possible explanation for how TTX has exerted its effect is that it has acted in a nonspecific manner to deregulate the growth state of retinal ganglion cells, a possibility suggested by studies showing that action potentials can have an inhibitory effect on axonal growth in vitro (31,32). However, the effect cannot be entirely nonspecific since ganglion cell axons come to an abrupt halt at the LGN border, indicating that they can still respond to many cues even in the presence of TTX. In the context of all the evidence presented above, the most reasonable explanation for the alteration in axon arbor morphology following TTX treatment is that it has acted to block spontaneously generated action potentials and synaptic transmission, which in turn are required for the formation of the normal specific patterns of axonal arborization. It is as if, in the absence of activity, the normal elimination of side branches fails to occur, and, instead, each sidebranch continues to elongate to form a significant portion of the terminal arbor. If so, then the conservative remodeling of axon arbors seen during normal retinogeniculate development is
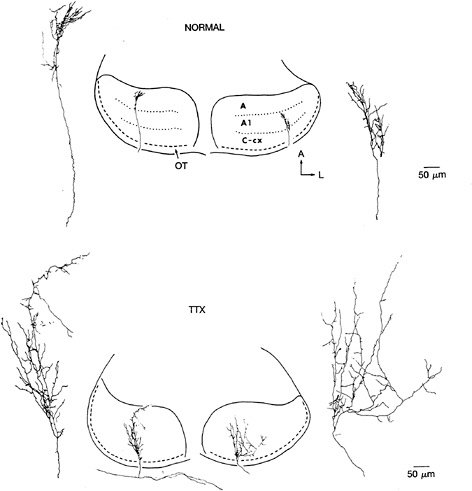
FIG. 2. Examples of the terminal arborizations of retinogeniculate axons at E58 in normal animals (Upper) and in animals that received minipump infusion of TTX between E42 and E58 (Lower). In both cases, axons were labeled by using the in vitro HRP-filling technique. In the normal case, axons from the contralateral (Left) and ipsilateral (Right) eyes are shown, and their restricted arborization within the appropriate LGN layer (either layer A or layer A1) is indicated (Center). With TTX treatment, axons branch extensively and indiscriminately within the LGN without regard for (implied) laminar borders. [Reproduced with permission from ref.30 (copyright 1988 Macmillan Magazines Limited).]
unlikely to reflect a highly prespecified intrinsic program of axonal growth. Rather, such economical growth is more likely due to the presence of intense competitive interactions that are activity dependent.
The Spontaneous Firing of Neighboring Retinal Ganglion Cells Is Correlated
How can the firing of retinal ganglion cells give rise to a segregated pattern of eye input within the LGN? Action potentials by themselves are not sufficient. It is the precise temporal and spatial patterning of neural activity, in conjunction with the presence of special kinds of synapses, that are necessary (14,33,34). For example, Stryker and Strickland (35) have shown that if all of the axons in the optic nerves from both eyes are electrically stimulated synchronously during the postnatal period in which the ocular dominance columns normally form in layer 4 of primary visual cortex, segregation of the LGN axons representing the two eyes is prevented, just as occurred with TTX. In contrast, if the two nerves are stimulated asynchronously, such that ganglion cell axons in one nerve never fire at the same time as those in the other nerve, then segregation to form the columns in layer 4 proceeds. This important experiment illustrates the fact that “cells that fire together wire together,” in the sense that the timing of action potential activity is critical in determining which synaptic connections between LGN neurons and layer 4 cortical neurons are retained and strengthened and which are weakened and eliminated. (Note that at the postnatal age that these experiments were performed, retinal ganglion cell axons have already segregated to form the eye-specific layers in the LGN and the stimulation does not affect the LGN layers.) Similarly, Eisele and Schmidt (36) demonstrated that strobe rearing (in which the activity of all ganglion cells becomes correlated due to the visual stimulus), like TTX, blocks the sharpening of the regenerating retinotectal projection in goldfish. Although in these particular experiments all of the ganglion cells in one eye are experimentally made to fire simultaneously, under normal circumstances, vision itself acts naturally to correlate the activity of neighboring ganglion cells since they receive inputs from the same or immediately adjacent parts of the visual world. Thus, these required correlations are normally generated as a consequence of visual experience.
What about earlier in development, when ganglion cell axons segregate into the layers within the LGN but the photoreceptors are not present? Even without vision, somehow neighboring ganglion cells within each eye ought to fire in near synchrony with each other, while the firing of cells in the two eyes should be asynchronous. To search for spatiotemporal patterns of spontaneous firing within the retina, it is necessary to monitor simultaneously the action potential activity of many ganglion cells in the developing retina during the early times when the eye-specific layers are forming. Two recent technical advances have made it possible to record simultaneously from many ganglion cells in the developing retina: multielectrode recording and optical recording. In these experiments, we studied ferret retinas because, as in other mammalian retinas, ferret ganglion cell axons sort to form the eye-specific layers prior to vision (5,37,38). The ferret, however, has the advantage of being born in a very immature state, almost a month before the cat, thereby obviating the need for fetal surgery.
By placing neonatal ferret retinas onto a special 61-electrode array (39) in vitro, we were able to record extracellularly the spontaneously generated action potentials from up to 100 cells simultaneously (40,41). In confirmation of the in vivo recordings of Galli and Maffei (21), each cell on the multielectrode array fired in a very stereotyped and rhythmic pattern: high-frequency bursts of action potentials lasting several seconds, followed by long silent pauses lasting 0.5 –2 min in duration. The biggest surprise came when the spatiotemporal firing patterns on the array were analyzed. First, we discovered that almost all of the cells on the array fired action potentials within about 5 s of each other and then paused together for up to 2 min before firing again. This observation showed that the activity of ganglion cells is indeed correlated. Further analysis demonstrated that the activity of neighboring cells on the array is more highly correlated than that of distant cells. Even more remarkable, as shown in Fig. 3, the spatial pattern of firing resembled a wave of activity that sweeps across the retina at about 200 µm/s. Each “wave” is followed by a silent period lasting up to 2 min, after which another wave is generated but in a completely different and apparently random direction. Finally, we found that these spontaneously generated retinal waves are present throughout the period when eye-specific layers form in the ferret between P1 and P21, but they then disappear by P30, just before eye-opening and the onset of visual function.
From an engineering standpoint, these waves seem beautifully designed to provide the postulated local correlations in the firing of nearby ganglion cells while also ensuring a sufficient time delay so that the firing of more distant cells, also correlated with each other, is not synchronized across the entire retina. Such a pattern of firing could help to refine the topographic map conveyed by ganglion cell axons to each eye-specific layer in the LGN. Moreover, the fact that wave direction appears to be entirely random during each successive burst implies that ganglion cells in corresponding locations in the two retinas are highly unlikely ever to fire synchronously—a requirement for eye-specific layers to form. Thus, even before the onset of vision, the retina spontaneously generates stereotyped patterns of correlated firing that are entirely appropriate to subserve the process of activity-dependent sorting of connections. Experiments aimed at disrupting the waves are now needed to demonstrate that they are required for the formation of the layers in the LGN.
These observations naturally raise the question of what neural substrate is responsible for generating the observed correlations in the firing of retinal ganglion cells? During the period when the waves are present, the outer retina, including photoreceptors and bipolar cells, is very immature (37). The principal synaptic inputs to retinal ganglion cells at these ages appear to be from the amacrine cells (42,43), suggesting that they too might participate in a circuit that generates correlations in firing. Another possibility is that nonsynaptic mechanisms, such as the release of a diffusible, excitatory substance—e.g., potassium, an excitatory amino acid, etc.—might contribute. And finally, the coupling of neuronal firing via gap junctions, known to be present between subsets of ganglion cells and amacrine cells in the adult mammalian retina (44), could also occur.
We have begun to investigate some of these possibilities by pursuing two separate lines of experiments. First, using optical recording techniques that permit dynamic changes in intracellular calcium to be monitored, we have found that not only ganglion cells but also retinal interneurons—most likely amacrine cells based on their size and location—undergo spontaneous calcium bursting that is correlated among many near neighbors (Fig. 4; ref.45). Recent whole-cell recordings from ganglion cells in the neonate combined with calcium imaging also indicate that the ganglion cells receive a barrage of excitatory postsynaptic currents (EPSCs) during each wave (46). Second, intracellular injections of neurobiotin, an agent known to cross gap junctions, reveals tracer coupling between retinal ganglion cells and amacrine cells from P1 onwards in ferret retinas (47). Neurobiotin coupling at the earliest ages is rare, but it becomes quite extensive by the third postnatal week. Taken together, these results imply that an early tangential network of amacrine cells and ganglion cells may act together to generate the synchronized patterns of spontaneous activity in the developing retina.
Retinogeniculate Synapses Can Undergo Activity-Dependent Strengthening
A major issue raised from the experiments described above is whether the correlations in the firing of neighboring ganglion
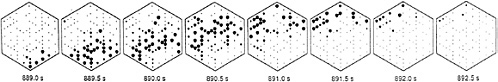
FIG. 3. The pattern of spike activity over the multielectrode recording array is plotted for eight successive intervals during one burst of ganglion cell firings covering the time interval from 889 s to 893 s during the recording session. Each frame shows the averaged firing rate during an 0.5-s interval. Each of 82 neurons is represented with a small dot at its approximate spatial location on the electrode array. The dot area for each cell is proportional to its average firing rate during the relevant 0.5-s interval: the larger the diameter, the higher the average firing rate. During this recording, ganglion cells located in the lower right hand corner of the array commenced firing together at the beginning of a burst (889.0 s), and then activity progressed in a wave-like fashion across the array so that at the end of the burst period (892.5 s), ganglion cells at the upper left hand edge of the array were active. Recordings are from a postnatal day 5 (P5) ferret retina. [Reproduced with permission from ref.40 (copyright 1991 American Association for the Advancement of Science).]
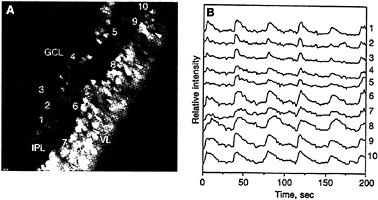
FIG. 4. Both ganglion cells and amacrine cells show correlated calcium oscillations. (A) Cross-sectional view of a P10 retina showing spontaneous activity both in the ganglion cell layer (GCL, cells 1–5) and in the forming amacrine cell layer (cells 6–10). Spontaneously active cells are shown in red, whereas inactive ones are coded in blue. IPL, inner plexiform layer; VL, ventricular layer. (B) Recordings of the spontaneous fluctuations in intracellular calcium of the 10 cells marked in A. (Bar = 10 µm.) [Reproduced with permission from ref.45 (copyright 1995 Macmillan Magazines Limited).]
cells can be detected and used within the LGN to cause strengthening of appropriate synapses and weakening of inappropriate ones. Two lines of preliminary experiments suggest that this is likely to be the case. To address the question of whether the bursts of action potentials generated by retinal ganglion cells are relayed to LGN neurons, an obvious experiment would be to record from the LGN neurons in vivo at the appropriate ages and see if they are driven to spike by the retinal inputs; however, the spontaneously generated retinal activity is sensitive to anesthetics (R. O. L. Wong, D. A. Baylor, M. Meister, and C.J.S., unpublished results). To circumvent this problem, my colleagues R. Mooney, A. A. Penn, and R. Gallego and I developed a preparation in which the entire visual pathway from retina to LGN is dissected intact and placed in vitro. Extracellular microelectrode recordings from the optic nerves or LGN (Fig. 5) indicate that the retinal ganglion cells not only are active and generate bursts of action potentials with a period similar to that observed in vivo, but also that LGN neurons are driven to fire spikes (48). Thus, it is highly likely that the retinal waves are relayed across the synapse to LGN neurons during the period in which the eye-specific layers form.
As mentioned above, for an activity-dependent mechanism to operate in the formation of the eye-specific layers, there must be special synaptic mechanisms at the retinogeniculate synapse to strengthen connections when action potentials from different presynaptic inputs arrive within near synchrony of each other and also to weaken them if cells fire asynchronously. Many years ago, Hebb (49) proposed the existence of synapses that could undergo strengthening whenever activity in a presynaptic cell occurred simultaneously with that in the postsynaptic cell. Such “Hebb synapses” have the property that if many inputs coincide in activating a cell, then they all undergo strengthening. Clear evidence showing that synapses with this special property actually exist in the central nervous system comes from studies of the hippocampus and the phenomenon of long-term potentiation, in which the pairing of pre- and postsynaptic activity can cause increases in the strength of synaptic transmission specifically between the paired cells that can last from hours to days (reviewed in refs.50 and 51). Hebb synapses are almost certainly also present in the visual cortex during the postnatal period in which ocular dominance columns form, although their properties are less-well understood than those in the hippocampus (52–55).
Can the synapses established between retinal ganglion cells and LGN neurons undergo activity-dependent changes at the relevant developmental ages? To examine this question, we prepared slices of the LGN from ferrets between P1 and P21
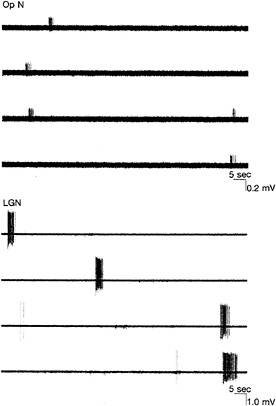
FIG. 5. Examples of extracellular microelectrode recordings from a single axon in the optic nerve (Op N) (Upper) or from a single postsynaptic neuron in the LGN (Lower) of a neonatal mouse brain preparation consisting of the retinas, Op Ns, and LGNs dissected and maintained in vitro. Spontaneously generated bursts of action potentials in both Op N and LGN neuron occur about once per minute. Note different voltage scales in the two examples. (Data are from A. A. Penn, R. Gallego, R. Mooney, and C.J.S.)
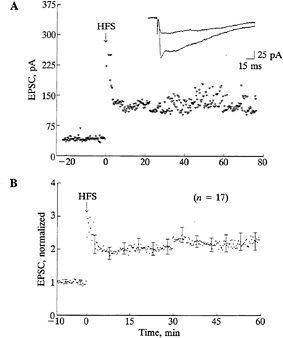
FIG. 6. Retinogeniculate synaptic transmission can undergo longlasting enhancement. (A) Example from an LGN slice from a P21 ferret. At the beginning of the recording, single shocks delivered to the optic nerve evoked monosynaptic EPSCs in the LGN neuron. Following several bursts of high-frequency stimulation (HFS) of the optic tract, a long-term increase in the amplitude of the evoked EPSC that lasted for up to 80 min resulted. Recordings were made in the presence of picrotoxin. EPSCs were recorded with perforated-patch, whole-cell techniques. Superimposed EPSCs shown at the top of the figure were evoked before and 60 min after HFS. (B) Ensemble average of 17 positive cases of synaptic enhancement observed in LGN slices from P6–P21 ferrets. For each case the amplitude of the EPSC at each point was normalized to the average of 40 traces immediately preceding the test stimulus. [Reproduced with permission from ref. 56 (copyright 1993 Cell Press).]
and made whole-cell voltage clamp recordings in vitro to monitor the efficacy of synaptic transmission (56). Ganglion cell axons were stimulated by inserting electrodes into the optic tract, just adjacent to the LGN in the slice. Three to six bursts of high frequency stimulation of the optic tract caused a significant enhancement of retinogeniculate synaptic transmission that lasted for several tens of minutes to an hour in about 40% of all the cells recorded. Examples of such enhancement of transmission, recorded from ferret LGN slices, are shown in Fig. 6. In some instances, we could also prevent this increase by using 2-amino-5-phosphonovaleric acid to block N-methylD-aspartate (NMDA) receptors, known to be present in LGN neurons at this age (56–58). Blockade of NMDA receptors in vivo between P14 and P21 is also known to prevent the final refinement of retinal ganglion cell axons into sublayers receiving input from on-center or off-center ganglion cells (38). Thus, these observations suggest that the activity-dependent synaptic enhancement observed in vitro could indeed represent a cellular mechanism underlying the process of segregation of ganglion cell axons within the LGN.
In some ways these experiments have raised more questions than they have answered. Is the stereotyped bursting pattern of firing of retinal ganglion cells the most effective pattern for evoking synaptic enhancement? What are the long-term consequences of enhancement of synaptic transmission —e.g., does it result in morphological change? To what extent is the cellular mechanism underlying synaptic strengthening during development similar to that known to occur in the hippocampus during long-term potentiation (59)? And finally, is there a process of activity-dependent weakening of synaptic transmission since, as discussed above, some of the synaptic contacts between retinal ganglion cells and LGN neurons that are present early on are ultimately eliminated.
Concluding Remarks
The development of retinogeniculate connections in mammals is one of many examples in which the adult pattern of connections is not established initially but rather is sculpted from an immature pattern. Here, I have put forward the argument that the formation of the adult pattern of the eye-specific layers in the mammalian LGN requires activitydriven competitive interactions between ganglion cell axons from the two eyes for common postsynaptic neurons. Of course, this cannot be the whole story since the layers always form in the same pattern and since there is also a segregation of subtypes of functionally distinct retinal ganglion cell axons within different layers and from each other even within the same layer. Just how many of these other important details of LGN organization may be accounted for by timing differences in the generation of different classes of retinal ganglion cells (60), by competitive interactions between ganglion cells within the same eye (61), or by specific molecular differences between cells remain to be determined. Nevertheless, the implication that activity-driven competition plays an essential role in wiring of the visual system even before vision raises the possibility that spontaneous neural activity may shape connections elsewhere in the nervous system during fetal development.
The requirement for neuronal activity in producing the adult precision of connections is a genetically conservative means of achieving a high degree of precision in wiring. To specify precisely each neural connection between retina and LGN by using specific molecular markers would require an extraordinary number of genes, given the thousands of connections that are formed. The alternative, to specify precise pathways and targets with molecular cues and then using the rules of activity-dependent sorting to achieve ultimate precision in connectivity, is far more economical. Indeed, once axons recognize and grow into their appropriate targets, the same general rules of activity-dependent competition can apply throughout the nervous system. A major challenge for the future will be to elucidate the cellular and molecular bases for these rules.
I wish to thank the many colleagues and collaborators whose essential contributions made this review article possible, including D. Baylor, G. Campbell, M. Feller, R. Gallego, P. A. Kirkwood, D. V. Madison, M. Meister, R. Mooney, A. Penn, M. Siegel, D. W. Sretavan, M. P. Stryker, R. O. L. Wong, and R. M. Yamawaki. The original research work presented in this article was supported in part by grants from the National Institutes of Health (EY02858 and MH48108), the National Science Foundation (IBN92-12640), and the March of Dimes. C.J.S. is an investigator of the Howard Hughes Medical Institute.
1. Goodman, C. S. & Shatz, C. J. ( 1993) Cell 72/ Neuron 10, 1–20.
2. Rodieck, R. W. ( 1979) Annu. Rev. Neurosci. 2, 193–255.
3. Sherman, S. M. ( 1985) Prog. Psychobiol. Physiol. Psychol. 11, 233–313.
4. Rakic, P. ( 1977) Philos. Trans. R. Soc. London B 278, 245–260.
5. Linden, D. C., Guillery, R. W. & Cucchiaro, J. ( 1981) J. Comp. Neurol. 203, 189–211.
6. Shatz, C. J. ( 1983) J. Neurosci. 3, 482–499.
7. Sretavan, D. W. & Shatz, C. J. ( 1986) J. Neurosci. 6, 234–251.
8. Rakic, P. ( 1981) Science 214, 928.
9. Chalupa, L. M. & Williams, R. W. ( 1984) Hum. Neurobiol. 3, 103–107.
10. Shatz, C. J. & Sretavan, D. W. ( 1986) Annu. Rev. Neurosci. 9, 171–207.
11. Guillery, R. W., Lamantia, A. S., Robson, J. A. & Huang, K. ( 1985) J. Neurosci. 5, 1370–1379.
12. Purves, D. & Lichtman, J. W. ( 1980) Science 210, 153–157.
13. Fawcett, J. W. & O'Leary, D. D. M. ( 1985) Trends Neurosci. 8, 201–206.
14. Miller, K. D., Keller, J. B. & Stryker, M. P. ( 1989) Science 245, 605–615.
15. Constantine-Paton, M., Cline, H. T. & Debski, E. ( 1990) Annu. Rev. Neurosci. 13, 129–154.
16. Shatz, C. J. ( 1990) Neuron 5, 1–10.
17. Campbell, G. & Shatz, C. J. ( 1992) J. Neurosci. 12, 1847–1858.
18. Shatz, C. J. & Kirkwood, P. A. ( 1984) J. Neurosci. 4, 1378–1397.
19. Donovan, A. ( 1966) Exp. Eye Res. 5, 249–254.
20. Robinson, S. R. ( 1991) in Vision and Visual Dysfunction, ed. Cronly-Dillon, J. (Macmillan, New York), Vol. 3, pp. 69–128.
21. Galli, L. & Maffei, L. ( 1988) Science 242, 90–91.
22. LeVay, S., Stryker, M. P. & Shatz, C. J. ( 1978) J. Comp. Neurol. 179, 223–244.
23. LeVay, S., Wiesel, T. N. & Hubel, D. H. ( 1980) J. Comp. Neurol. 191, 1–51.
24. Stryker, M. P. & Harris, W. ( 1986) J. Neurosci. 6, 2117–2133.
25. Reh, T. A. & Constantine-Paton, M. ( 1985) J. Neurosci. 5, 1132–1143.
26. Cline, H. T., Debski, E. A. & Constantine-Paton, M. ( 1987) Proc. Natl. Acad. Sci. USA 84, 4342–4345.
27. Meyer, R. L. ( 1982) Science 218, 589–591.
28. Boss, V. C. & Schmidt, J. T. ( 1984) J. Neurosci. 4, 2891–2905.
29. Shatz, C. J. & Stryker, M. P. ( 1988) Science 242, 87–89.
30. Sretavan, D. W., Shatz, C. J. & Stryker, M. P. ( 1988) Nature (London) 336, 468–471.
31. Cohan, C. S. & Kater, S. B. ( 1986) Science 232, 1638–1640.
32. Frank, E. ( 1987) Trends Neurosci. 10, 188–190.
33. Willshaw, D. J. & von der Malsburg, C. ( 1976) Proc. R. Soc. London B 194, 431–445.
34. Mastronarde, D. N. ( 1989) Trends Neurosci. 12, 75–80.
35. Stryker, M. P. & Strickland, S. L. ( 1984) Invest. Ophthalmol. Visual Sci. 25, Suppl., 278.
36. Eisele, L. L. & Schmidt, J. T. ( 1988) J. Neurobiol. 19, 395–411.
37. Grenier, J. V. & Weidman, T. A. ( 1981) Exp. Eye Res. 33, 315–332.
38. Hahm, J. O., Langdon, R. B. & Sur, M. ( 1991) Nature (London) 351, 568–570.
39. Regher, W. G., Pine, J., Cohan, C. S., Mischke, M. D. & Tank, D. W. ( 1989) J. Neurosci. Methods 30, 91–94.
40. Meister, M., Wong, R. O. L., Baylor, D. A. & Shatz, C. J. ( 1991) Science 252, 939–943.
41. Wong, R. O. L., Meister, M. & Shatz, C. J. ( 1993) Neuron 11, 923–938.
42. Maslim, J. & Stone, J. ( 1986) Brain Res. 373, 35–48.
43. Wong, R. O. L., Yamawaki, R. M. & Shatz, C. J. ( 1992) Eur. J. Neurosci. 4, 1387–1397.
44. Vaney, D. I. ( 1991) Neurosci. Lett. 125, 187–190.
45. Wong, R. O. L., Chernjavsky, A., Smith, S. J. & Shatz, C. J. ( 1995) Nature (London) 374, 716–718.
46. Feller, M. B., Wellis, D. P., Werblin, F. S. & Shatz, C. J. ( 1995) Soc. Neurosci. Abstr. 21, 1504.
47. Penn, A. A., Wong, R. O. L. & Shatz, C. J. ( 1994) J. Neurosci. 14, 3862–3880.
48. Penn, A. A., Gallego, R., Mooney, R. & Shatz, C. J. ( 1995) Soc. Neurosci. Abstr. 21, 1504.
49. Hebb, D. O. ( 1949) The Organization of Behavior (Wiley, New York).
50. Madison, D. V., Malenka, R. C. & Nicoll, R. A. ( 1991) Annu. Rev. Neurosci. 14, 379–397.
51. Malenka, R. C. ( 1994) Cell 78, 535–538.
52. Artola, A. & Singer, W. ( 1987) Nature (London) 330, 649–652.
53. Komatsu, Y., Fujii, K., Maeda, J., Sakaguchi, H. & Toyama, K. ( 1988) J. Neurophysiol. 59, 124–141.
54. Shultz, D. & Fregnac, Y. ( 1992) J. Neurosci. 12, 1301–1318.
55. Kirkwood, A., Dudek, S. M., Gold, J. T., Aizenman, C. D. & Bear, M. F. ( 1993) Science 260, 1518–1521.
56. Mooney, R., Madison, D. V. & Shatz, C. J. ( 1993) Neuron 10, 815–825.
57. Esguerra, M., Kwon, Y. H. & Sur, M. ( 1992) Neuroscience 8, 545–555.
58. White, C. A. & Sur, M. ( 1992) Proc. Natl. Acad. Sci. USA 89, 9850–9854.
59. Kandel, E. R. & O'Dell, T. J. ( 1992) Science 258, 243–245.
60. Walsh, C., Polley, E. H., Hickey, T. L. & Guillery, R. W. ( 1983) Nature (London) 302, 611–614.
61. Dubin, M. W., Stark, L. A. & Archer, S. M. ( 1986) J. Neurosci. 6, 1021–1036.







