This paper was presented at a colloquium entitled “Vision: From Photon to Perception,” organized by John Dowling, Lubert Stryer (chair), and Torsten Wiesel, held May 20-22, 1995, at the National Academy of Sciences in Irvine, CA.
How photons start vision
DENIS BAYLOR
Department of Neurobiology, Sherman Fairchild Science Building, Stanford University School of Medicine, Stanford, CA 94305
ABSTRACT Recent studies have elucidated how the absorption of a photon in a rod or cone cell leads to the generation of the amplified neural signal that is transmitted to higher-order visual neurons. Photoexcited visual pigment activates the GTP-binding protein transducin, which in turn stimulates cGMP phosphodiesterase. This enzyme hydrolyzes cGMP, allowing cGMP-gated cationic channels in the surface membrane to close, hyperpolarize the cell, and modulate transmitter release at the synaptic terminal. The kinetics of reactions in the cGMP cascade limit the temporal resolution of the visual system as a whole, while statistical fluctuations in the reactions limit the reliability of detection of dim light. Much interest now focuses on the processes that terminate the light response and dynamically regulate amplification in the cascade, causing the single photon response to be reproducible and allowing the cell to adapt in background light. A lightinduced fall in the internal free Ca2+concentration coordinates negative feedback control of amplification. The fall in Ca2+stimulates resynthesis of cGMP, antagonizes rhodopsin's catalytic activity, and increases the affinity of the lightregulated cationic channel for cGMP. We are using physiological methods to study the molecular mechanisms that terminate the flash response and mediate adaptation. One approach is to observe transduction in truncated, dialyzed photoreceptor cells whose internal Ca2+and nucleotide concentrations are under experimental control and to which exogenous proteins can be added. Another approach is to observe transduction in transgenic mouse rods in which specific proteins within the cascade are altered or deleted.
Vision begins with the conversion of light from the outside world into electrical signals which can be processed within the retina and sent to the brain. When the conversion fails, as it does in hereditary retinal degenerations, a willing brain is left unable to see. The workings of the first step fix the absolute sensitivity, spectral sensitivity, and temporal resolution of the visual system as a whole. Our understanding of the molecular basis of visual transduction has deepened rapidly in recent years as physiology, biochemistry, and molecular biology have been brought to bear. Insights gained from the study of transduction in photoreceptor cells have helped to elucidate signaling in a wide variety of other cell types that use G-protein-coupled receptors and cyclic nucleotide cascades. My aim in this article is to review some of the accomplishments and gaps in our understanding of visual signal generation.
Electrical and Chemical Signaling in Rods and Cones
Rods and cones have the structure diagramed in Fig. 1A. The outer segment, containing the visual pigment, is connected to the inner segment, which bears a synaptic terminal contacting bipolar and horizontal cells. Light absorbed in the pigment acts to close cationic channels in the outer segment, causing the surface membrane of the entire cell to hyperpolarize. The hyperpolarization relays visual information to the synaptic terminal, where it slows ongoing transmitter release. The cationic channels in the outer segment are controlled by the diffusible cytoplasmic ligand cGMP, which binds to channels in darkness to hold them open. Light closes channels by lowering the cytoplasmic concentration of cGMP. The steps that link light absorption to channel closure in a rod are illustrated schematically in Fig. 1B.
When rhodopsin (R) absorbs a photon its 11-cis-retinal chromophore rapidly isomerizes, causing the cytoplasmic surface of the protein to become catalytically active. In this state, rhodopsin activates the GTP-binding protein transducin (T). Within a fraction of a second a single active R causes hundreds of transducins to exchange bound GDP for GTP, forming active TGTP complexes. A greatly amplified signal now passes to a third protein, cGMP PDE, which is activated by TGTP. Activated PDE hydrolyzes cGMP to 5′-GMP, which cannot open the channel. With cGMP removed, channels close, interrupting a steady inward current of Na+ and Ca2+, thus hyperpolarizing the cell. These activation steps in transduction are now well established (see ref.1 for review) and their behavior has been described quantitatively (2).
The events that terminate the response to light are not so well understood. Catalytically active rhodopsin is thought to be shut off by phosphorylation followed by binding of the soluble protein arrestin. The time course of shutoff in vivo as well as the relative importance of phosphorylation and arrestin binding in reducing rhodopsin's catalytic activity are not yet known however. Active transducin is thought to be shut off by hydrolysis of the GTP bound to it. Although this process proceeds slowly in the test tube, heat measurements on outer segment preparations indicate that it occurs on the subsecond time scale expected from the time course of the electrical response to light (3). In the intact outer segment the GTPase activity of transducin may be accelerated by a specific protein. A candidate for the accelerator is the γ subunit of PDE (4), which may act in concert with another membrane-bound protein ( 5). PDE shuts off when its inhibitory subunit, freed by deactivation of TGTP, recombines with the catalytic subunits. Finally, the cGMP concentration is restored to the dark level by cGMP synthesis, which is mediated by guanylate cyclase.
Single Photon Effect
Pioneering psychophysical experiments half a century ago indicated that a retinal rod registers the absorption of a single photon, the smallest unit of light energy (6). Electrical recordings confirm this behavior and reveal that the elementary response is highly amplified. In a mammalian rod, for example, the quantal response has a peak amplitude of about 1 pA and over the entire response the entry of about one million
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviation: PDE, phosphodiesterase.
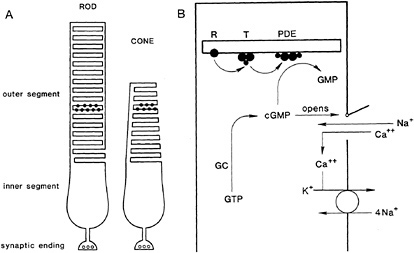
FIG. 1. Photoreceptor cells and the cGMP cascade of vision. (A) Structure of a rod and cone. Phototransduction occurs in the outer segment, which contains the visual pigment (filled circles). The synaptic terminal contacts second-order horizontal and bipolar cells (not shown). (B) cGMP cascade. The cationic channel in the surface membrane (trap door at the right) is held open in darkness by the binding of cGMP, which is synthesized from the precursor GTP by guanylate cyclase (GC). In darkness Ca2+ and Na+ enter the cell through the open channel, partially depolarizing the membrane. Photoexcitation of rhodopsin (R) causes it to catalytically activate the GTP-binding protein transducin (T), which in turn activates cGMP phosphodiesterase (PDE). Activated PDE hydrolyzes cyclic GMP to 5′-GMP (GMP), allowing the channel to close and hyperpolarize the membrane. Na+ is extruded by a pump in the inner segment (not shown), while Ca 2+ is extruded by an exchanger which is driven by entry of Na+ and efflux of K+. Continued operation of the exchanger at the onset of light produces a fall in the intracellular concentration of Ca2+.
elementary charges into the cell is blocked (7). This amplification is explained by the cascaded reactions that link rhodopsin and the cGMP-activated channels in the surface membrane. Thus, the intense cGMP sink created by activation of PDE is sufficient to close a few hundred of the 7000 or so channels that are open at any instant in darkness. Further amplification results from the sizeable rate of ion flow through the channels themselves. The single photon response of cones is typically 10–100 times smaller than that of a rod and also considerably briefer. Given these functional differences it is perhaps not surprising that many of the transduction proteins are encoded by different genes in rods and cones (see ref.1).
Because it involves enzymatic mechanisms, visual transduction proceeds relatively slowly. In a monkey rod, for instance, the single photon response resembles the impulse response of a multistage low-pass filter with an integration time of about 0.2 s (7). This interval is comparable to the integration time of rod vision measured psychophysically (8), so that transduction itself, rather than subsequent processing in the eye or brain, apparently causes the poor temporal resolution of human rod vision. Although the single photon response of cones is too small to resolve, its average form can be inferred from the shape of the response to a dim flash. In primate cones it resembles the impulse response of a bandpass filter, with a delayed s-shaped rise to a peak and a prominent undershoot on the falling phase ( 9). The amplitude spectrum of the cone flash response has a peak at a frequency of 5–10 Hz, and the form of the amplitude spectrum resembles the psychophysically determined flicker sensitivity of human cone vision measured under light-adapted conditions (10).
Dark Noise in Rods and Cones
Dark noise sets the ultimate limit on the performance of many devices that count photons, and retinal rods are no exception. The electrical noise of rods contains two dominant components that may be confused with photon responses: (a) occasional events resembling responses to single photons (the “discrete” component) and (b) a sustained fluctuation of smaller amplitude (the “continuous” component) (11). In a monkey rod the discrete noise events occur about once every 2.5 min (7). Psychophysical experiments indicate a similar magnitude for the rod “dark light,” the apparent rate of photon-like spontaneous excitations in dark-adapted rods (12,13). The temperature dependence of the rate of occurrence of discrete events gives the apparent activation energy of the process producing them as about 22 kcal mol−1 (1 kcal = 4.18 kJ) (11). This is close to the activation energy for thermal isomerization of 11-cis-retinal (14), suggesting that discrete events arise from thermal isomerization of rhodopsin's 11-cis-retinal chromophore. Additional evidence for the functional importance of thermal events is provided by behavioral experiments and recordings from retinal ganglion cells which show that the threshold for scotopic vision in toads is limited by a noise source with a very similar rate per rhodopsin molecule and temperature dependence (15). Although thermal activation occurs, it is infrequent: one calculates a 420-year average wait for a rhodopsin molecule at 37°C (7).
Cones are electrically noisier than rods, consistent with psychophysical evidence for a larger dark light in cones. In a monkey cone one component of the dark noise has a power spectrum like that of the cell's dim flash response (9). The photoisomerization rate that would produce an equivalent amount of noise is estimated as roughly 103 s−1. Bleaching a cone's pigment lowers the photon-like dark noise, suggesting that the noise may arise from thermal isomerization of pigment (9). Perhaps the red-shift in the absorption spectra of the pigments in red- and green-sensitive cones is inevitably accompanied by greater thermal instability (16).
The continuous noise of rods arises within the outer segment at a site in the transduction cascade downstream from rhodopsin (11), but the molecular mechanism has yet to be identified. The noise seems to result from shot effects of very small amplitude occurring at high frequency. The power spectrum of the continuous noise suggests that the shot effect is shaped by two of the four low-pass filter stages in an empirical quantitative model of the shape of the single photon response (11). Although the continuous component contrib-
utes more to the dark noise variance of rods than the discrete component (7), the discrete component apparently dominates the behavior measured psychophysically. It is not yet clear how this comes about, but evidence has been presented that synaptic transfer of rod signals to bipolar cells is accompanied by a temporal filtering that will help to separate the single photon response from the continuous noise (17).
A rod's electrical noise is elevated after exposure to bright light, and it has been suggested that increased noise may contribute to the elevated threshold of rod vision measured psychophysically (18). In amphibian rods the noise has a magnitude and power spectrum consistent with a superposition of shot effects like those generated by absorption of photons, and it has been proposed that the noise arises from photoexcited rhodopsin which, during the shutoff process, escapes quenching and returns to the active state (19,20). In primate rods noise after bright light results partly from anomalous photon responses, which have a rectangular waveform (see below). In psychophysical studies the briefest component of the threshold elevation following bright light decays with a time constant of 5 s (21), which matches the mean duration of the anomalous single photon responses. Perhaps anomalous events, triggered by rhodopsins which fail to be quenched properly in the first place, are responsible for the rapidly decaying threshold elevation.
Rhodopsin Quenching in Transgenic Mouse Rods
The termination of rhodopsin's catalytic activity is a key event in transduction, for as long as rhodopsin is active an amplified signal will continue to be generated by the cascade. Termination is thought to involve binding of rhodopsin kinase to the active rhodopsin, phosphorylation of the rhodopsin at one or a few sites, dissociation of the kinase, and binding of the soluble protein arrestin. Although these steps have been studied in vitro by biochemical techniques, it is not yet clear how they operate in vivo. It is not known, for instance, how rhodopsin's catalytic activity changes as each step occurs, nor whether the reactions are controlled by feedback arising from subsequent stages in the cascade. In one approach to the mechanism of control of rhodopsin 's activity, Clint Makino and I studied visual transduction in transgenic mouse rods (22). In addition to normal rhodopsin, these cells expressed a 15-amino acid truncation mutant lacking the three phosphorylation sites that biochemical experiments had previously implicated in the shutoff process (see Fig. 2). The transgenic mice were produced in Melvin Simon's laboratory by Jeannie Chen. Western blots revealed that rods of the mice utilized for the studies contained the usual amount of total rhodopsin, of which about 10% was the truncated form. A similar fraction of the single photon responses recorded from the transgenic rods failed to terminate normally, suggesting that the anomalous responses were generated by truncated rhodopsin molecules (see Fig. 3A). The anomalous responses consisted of a rounded rise to a maintained plateau which lasted on average about 20 times longer than the normal response. This behavior supports the notion that phosphorylation at one or more of the three sites within the C terminus indeed initiates rhodopsin shutoff under normal conditions. The fact that the majority of the rod's single photon responses were normal shows that the anomalous responses do not reflect a nonspecific disturbance of function in the transgenic rods. Comparison of normal and anomalous responses indicates that normal rhodopsin already begins to be quenched during the rising phase of the photon response. The functional significance of the multiple phosphorylation sites on rhodopsin remains to be determined. Can phosphorylation at a single site trigger normal shutoff? Are the three sites functionally equivalent? Rods expressing rhodopsin in which the phosphorylation sites at serines 334, 338, and 343 are removed one by one should help to answer these questions.
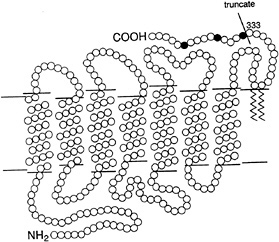
FIG. 2. Schematic cross section of rhodopsin in the lipid bilayer (horizontal lines), with amino acids drawn as circles. The mutant rhodopsin (ref.22) was truncated at the point indicated by the line. This deleted the 15 amino acids distal to residue 333, including serine residues 334, 338, and 343 (filled circles) implicated in shutoff of catalytic activity.
Truncated rhodopsin may also be present at low concentration in normal rods. About 0.1% of single photon responses in a monkey rod are grossly prolonged and closely resemble the anomalous responses of the transgenic mouse rods (7). Perhaps protein synthesis occasionally fails to reach the C terminus, producing a truncated molecule. Alternatively, proteolysis within the outer segment might occasionally remove the C terminus, producing a defective molecule which could be eliminated by outer segment renewal (23).
Feedback Control by Ca
Exposure of a photoreceptor cell to progressively higher ambient light levels causes absorbed photons to take progressively smaller bites out of the light-regulated conductance. This change, light adaptation, prevents moderate background light from closing all the cGMP-gated channels, which would defeat the cell's ability to register changes in light intensity. A light-induced fall in the cytoplasmic concentration of Ca2+ mediates light adaptation and speeds the recovery of the response to a flash presented in darkness. The fall in Ca2+ results from the mechanism diagramed in the lower part of Fig. 1B (reviewed in ref.1). In darkness, Ca2+ enters the cell through the cGMP-activated channel and is extruded by a Na/Ca-K exchanger. In light, the Ca2+ concentration falls because closure of the channel blocks Ca2+ influx while extrusion by the exchanger continues. Although the Ca2+ concentration in rod outer segments has proved difficult to measure, the free level in darkness is thought to be roughly 0.5 µM (24–26); the exchanger is thermodynamically capable of reducing the level in bright light by three orders of magnitude (27). Evidence for the key functional role of the light-induced fall in Ca2+ is that blocking the fall prevents adaptation and increases the size and duration of the flash response (28,29).
The fall in Ca2+ antagonizes the light-induced closure of channels by actions at several sites in the cascade (Fig. 4A). For instance, the channel's affinity for cGMP is lowered at high Ca2+ by a calmodulin-like protein (31). A fall in Ca2+ will increase the channel's affinity for cGMP and antagonize channel closure. The enzyme that synthesizes cGMP, guanylate cyclase, is also Ca2+ sensitive. A Ca2+-binding protein,
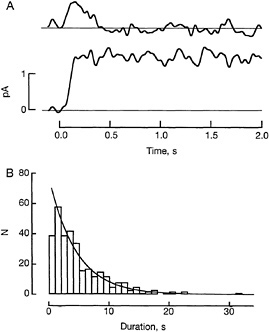
FIG. 3. Shutoff of rhodopsin activity in a transgenic mouse rod in which some rhodopsin was normal and some the truncated rhodopsin form shown in Fig. 2. (A) Illustrative dim flash responses recorded from a transgenic rod by a suction electrode. Membrane current plotted as a function of time. The flashes were delivered at time 0 on the abscissa. The upper trace is the response elicited by activation of a normal rhodopsin molecule; the lower trace is an anomalous response elicited by activation of a truncated rhodopsin molecule. (B) Distribution of durations of 325 anomalous responses from a transgenic rod (stepped histogram). The ordinate N gives the number of samples observed to have the durations shown on the abscissa. The continuous curve is an exponential with time constant 4.5 s. Reprinted with permission from ref.22 (copyright 1995, American Association for the Advancement of Science).
GCAP (32, 33) and/or GCAP2 (34), stimulates cyclase activity at low Ca2+ but not at high Ca2+. A drop in Ca2+ will thus increase the rate of cGMP synthesis, tending to reopen channels. Ca2+ also appears to control light-triggered PDE activity. Shutoff of rhodopsin by phosphorylation is inhibited at high Ca2+ by the Ca2+-binding protein recoverin, and removal of this effect at low Ca2+ may antagonize the activation of PDE by light (35). In truncated rods, Leon Lagnado and I found yet another effect of Ca2+ on light-triggered PDE activity (30). Under conditions in which cyclase activity was negligible and shutoff of the flash response was limited by rhodopsin phosphorylation, lowering Ca2+ reduced the gain of transduction without affecting the time course of response termination. Several pieces of evidence indicated that the effect was exerted at rhodopsin itself, one being that sensitivity to low Ca2+ was only present around the time of the flash (Fig. 4B). Lowering Ca2+ slightly after the flash, at a time when intense transducin and PDE activation were still present, had no effect. Recent evidence suggests that Ca2+'s effect on light-evoked PDE activation is most important in producing adaptation, while the Ca2+ effect on the cyclase mainly fixes the dark-adapted gain of the transduction mechanism (36).
Currently we are testing the role of the Ca2+-binding protein recoverin by two kinds of experiments. In one, the recombinant protein, kindly provided by Lubert Stryer, is being dialyzed into salamander rod outer segments. Leon Lagnado, Martha Erickson, and I have found that myristoylated (14-0) bovine recoverin slows the recovery of the flash response at high Ca2+ but has no effect at low Ca2+. A slowing of flash responses has previously been reported to result from addition of purified recoverin to Gekko rods through a patch pipette (37). The slowing effect in our experiments can be shown to depend on inhibition of rhodopsin shutoff, the mechanism indicated by biochemical studies (35). The Ca2+ dependence of the effect on the flash response is puzzling, however, as it occurs at unphysiologically high concentrations. In a second approach, Robert Dodd and I are studying transduction in transgenic mouse rods that do not express recoverin. These mice were made by Jeannie Chen and Melvin Simon. “Recoverin knockout” rods transduce, but their light-triggered PDE activity fails to light adapt normally. Furthermore, their Na +/Ca2+-K+ exchange current, a measure of intracellular Ca2+ kinetics, is faster than that of control rods, consistent with the absence of a buffer that binds roughly 25% of a normal rod's total intracellular Ca2+. The flash responses of knockout rods were also faster than those of control rods, perhaps because of their faster Ca2+ kinetics. Although it is not yet clear how to reconcile the results of the two kinds of experiments, one possibility is that different heterogeneously acylated forms of recoverin perform different physiological functions. Perhaps one form is active at physiological Ca2+ and mediates adaptation of light-triggered PDE activity, while another, turned on at high Ca2+, prevents rhodopsin shutoff and thus protects the cell from abnormal Ca2+ loads which might otherwise trigger cell death.
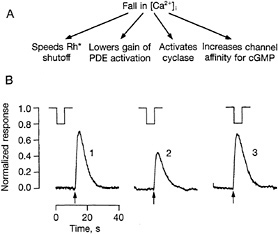
FIG. 4. Actions of Ca2+ in phototransduction. (A) Summary of proposed effects of the light-induced fall in internal [Ca2+]. Each effect antagonizes the response to light by opposing channel closure. (B) Records illustrating that a fall in [Ca2+] is only able to antagonize light-induced PDE activity near the time of light absorption, suggesting that the low [Ca2+] effect results from reduction of rhodopsin's catalytic activity. Suction electrode recordings of membrane current from an internally dialyzed, truncated salamander rod. Upper traces show the timing of a low [Ca2+] pulse delivered to the cut end of the outer segment; arrows show times at which a test flash was delivered. In record 1 the pulse just preceded the flash, in record 2 it overlapped it, and in record 3 it just followed it. Under the experimental conditions, [Ca2+] effects on the cyclase and channel were negligible; other experiments showed that the time course of shutoff of Rh* was also unaffected by the change in [Ca2+]. Reprinted with permission from ref. 30 (copyright 1994, Macmillan Magazines Limited).
Reproducibility of the Single Photon Response
An intriguing property of the visual transduction mechanism is its ability to generate a reproducible elementary response
(38), which should aid accurate photon counting. The reproducibility is illustrated in Fig. 5 A, which presents results from a recent experiment by Fred Rieke. The stepped curve in the histogram is the distribution of the amplitudes of responses evoked by repeated dim flashes of fixed applied strength. From left to right, the peaks represent 0, 1, or 2 effective photon absorptions—fluctuations expected from Poisson statistics. This distribution can be analyzed on the assumption that there is a fixed, Gaussian baseline noise variance and a similar independent variance in the elementary response itself. Reconstructing the observed distribution on this assumption (smooth curve), one finds that the peak representing single photon absorptions has a standard deviation only about onefourth the mean, indicating good reproducibility. Little would be gained if the reproducibility were better because the standard deviation of the continuous dark noise is comparable to the intrinsic fluctuation in the photon response. The shape as well as the size of the single photon response is remarkably constant from trial to trial.
How does a single rhodopsin molecule trigger a constant response? Typically active lifetimes of single molecules are highly variable because of stochastic fluctuations in the process that terminates activity. For example, the open time of single ion channels is often exponentially distributed because the open state is terminated by a memoryless, first-order transition to the closed state. Indeed, the single photon responses that arise in truncated rhodopsin molecules shut off after exponentially distributed delays with a mean of 5 s (Fig. 3B). Now if normal rhodopsin were shut off by a similar stochastic reaction that simply operated on a shorter time scale, and if it drove a chain of linear gain stages, the amplitude or time integral of the single photon response ought to fit the exponential distribution shown in Fig. 5B. Only two parts of the exponential distribution fail to fit: the right and left halves!
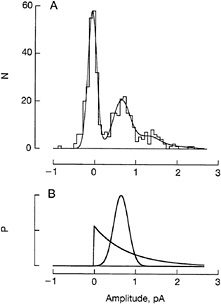
FIG. 5. Reproducibility of a toad rod's single photon response. (A) Amplitude distribution of 410 responses to a fixed dim flash that photoisomerized 0.48 rhodopsin molecule per trial on the average. The ordinate N is the number of responses that had the peak amplitude given on the abscissa. The stepped profile is the experimental distribution; the smooth curve is a theoretical fit constructed as described in the text. The peak centered at 0.65 pA results from activation of a single rhodopsin molecule. (B) Theoretical probability distributions for the single photon response amplitude. The Gaussian (smooth curve) is that assumed in constructing the theoretical curve of A. An exponential distribution (sharply peaked curve) with the same mean value and area as the Gaussian is drawn for comparison.
The absence of very small values in the experimental amplitude distribution is a strong constraint on the mechanism of reproducibility. It indicates that a single activated rhodopsin is not quenched by a first-order memoryless transition but instead shuts off along a fairly stereotypic time course. One mechanism for achieving this would be feedback control. For instance, shutoff might be disabled at the high Ca2+ level present in darkness but allowed to occur when Ca2+ falls during the flash response. By acting as a timer, this mechanism would prevent brief rhodopsin lifetimes and small responses. Somewhat against this notion is the recent finding (39) that clamping the intracellular Ca2+ at a high or low level fails to change the apparent rate of rhodopsin shutoff in bright flash responses. It might still be argued, however, that different rules apply to small responses, which were not investigated.
Alternatively, multiple steps might be required for rhodopsin's catalytic activity to be terminated. Reproducibility would then be achieved in the average activity over these steps, and reproducibility would be maximal if the events were independent and had comparable mean waiting times. A series of identical independent steps could give the exponential time course of rhodopsin shutoff derived from analysis of responses to bright flashes (40,41). What might these steps be? Perhaps kinase binding itself lowers catalytic activity somewhat, and one or two phosphorylations of rhodopsin lower it still more. Autophosphorylation of rhodopsin kinase may then occur, allowing it to dissociate from rhodopsin so that the final shutoff mediated by arrestin binding can take place.
Either feedback or multiple steps in the shutoff process might produce a distribution of photon response sizes in which small responses are absent, as in the experimental distribution of Fig. 5A. An additional mechanism is probably required to eliminate large responses from the distribution, and feedback activation of cGMP synthesis, driven by the light-induced fall in intracellular Ca2+, might accomplish this. The possibility that amplitude saturation might eliminate large responses seems unlikely because the size and duration of the single photon response increase substantially when a rod's internal Ca2+ is buffered or the response is triggered by a truncated rhodopsin. We are currently performing experiments to test whether Ca2+ or other feedback signals may contribute to reproducibility.
Transfer of Signals at the First Synapse
Generation of an amplified single photon response that exceeds electrical dark noise in the rod is an impressive feat, but it is only the first step in the chain of events leading to perception. The next step, transfer of a single photon signal across the rod's output synapse, poses novel problems because the presynaptic voltage change is very small—roughly three orders of magnitude less than the amplitude of an action potential. Such a small voltage change can produce only a small reduction in the rate of exocytosis of synaptic vesicles. This in turn requires a very high rate of resting release if the photoninduced change is to exceed statistical fluctuations. Synaptic ribbons, specialized structures found within rod and cone terminals (42), may help to support a high resting rate of a release by providing a large pool from which releasable vesicles may be drawn. The presynaptic terminals of auditory hair cells, which also generate small presynaptic voltage signals, contain dense bodies reminiscent of ribbons. If the drop in the rate of release is to be successfully detected and amplified, the elements that generate the postsynaptic response must be nicely matched to those at the presynaptic side of the junction. Remarkably, recent evidence suggests that rod bipolar cells utilize for this task a glutamate receptor coupled to a cGMP
cascade—an amplifying strategy reminiscent of that of the rods themselves (43,44). A glutamate receptor activated by the rod transmitter released in darkness appears to continually activate a G protein and in turn a cGMP PDE, which holds the level of cGMP low in darkness. A light-triggered reduction in the activity of the glutamate receptor allows cGMP levels to rise, opening cationic channels in the surface membrane and producing a depolarization which carries the message onward. It will undoubtedly be satisfying to learn more about how synaptic transmission is “ designed” to work in concert with the visual transduction mechanism. Already it appears that synaptic transmission has borrowed a successful molecular strategy from visual transduction itself.
Drs. Leon Lagnado, Clint Makino, Martha Erickson, and Robert Dodd did much of the recent work reported here, and I acknowledge their contributions with many thanks. Drs. Clint Makino, Martha Erickson, and Fred Rieke made useful comments on the manuscript. This research was supported by Grants EY01543 and EY05750 from the National Eye Institute, National Institutes of Health, as well as grants from the Alcon Research Institute, the Ruth and Milton Steinbach Fund, and the McKnight Foundation. Dr. Lubert Stryer's group is a continuing source of stimulating interactions.
1. Yau, K.-W. ( 1994) Invest. Ophthalmol. Visual Sci. 35, 9–32.
2. Pugh, E. N. & Lamb, T. D. ( 1993) Biochim. Biophys. Acta 1141, 111–149.
3. Vuong, T. M. & Chabre, M. ( 1991) Proc. Natl. Acad. Sci. USA 88, 9813–9817.
4. Arshavsky, V. Y. & Bownds, M. D. ( 1992) Nature (London) 357, 416–417.
5. Angleson, J. K. & Wensel, T. G. ( 1994) J. Biol. Chem. 269, 16290–16296.
6. Hecht, S., Shlaer, S. & Pirenne, M. H. ( 1942) J. Gen. Physiol. 25, 819–840.
7. Baylor, D. A., Nunn, B. J. & Schnapf, J. L. ( 1984) J. Physiol. (London) 357, 575–607.
8. Barlow, H. B. ( 1957) J. Physiol. (London) 136, 469–488.
9. Schnapf, J. L., Nunn, B. J., Meister, M. & Baylor, D. A. ( 1990) J. Physiol. (London) 427, 681–713.
10. Watson, A. B. ( 1986) in Handbook of Perception and Human Performance, Sensory Processes and Perception, eds. Boff, K. R., Kaufman, L. & Thomas, J. P. (Wiley, New York), Vol. 1, pp. 6 / 1–6 / 43.
11. Baylor, D. A., Matthews, G. & Yau, K.-W. ( 1980) J. Physiol. (London) 309, 591–621.
12. Aguilar, M. & Stiles, W. S. ( 1954) Optica Acta 1, 59–65.
13. Barlow, H. B. ( 1977) in Vertebrate Photoreception, eds. Barlow, H. B. & Fatt, P. (Academic, London), pp. 337–351.
14. Hubbard, R. ( 1966) J. Biol. Chem. 241, 1814–1818.
15. Aho, A.-C., Donner, K., Hyden, C., Larsen, L. O. & Reuter, T. ( 1988) Nature (London) 334, 348–350.
16. Barlow, H. B. ( 1957) Nature (London) 179, 255–256.
17. Rieke, F., Owen, W. G. & Bialek, W. ( 1991) in Advances in Neural Information Processing 3, eds. Lippman, R., Moody, J. & Touretzky, D. (Kaufmann, New York), pp. 377–383.
18. Barlow, H. B. ( 1956) J. Opt. Soc. Am. 46, 634–639.
19. Lamb, T. D. ( 1981) Vision Res. 21, 1773–1782.
20. Liebrock, C. S., Reuter, T. & Lamb, T. D. ( 1994) Vision Res. 34, 2787–2800.
21. Pugh, E. N. ( 1975) J. Physiol. (London) 248, 413–431.
22. Chen, J., Makino, C. L., Peachey, N. S., Baylor, D. A. & Simon, M. I. ( 1995) Science 267, 374–377.
23. Young, R. W. ( 1971) J. Cell Biol. 49, 303–318.
24. Lagnado, L., Cervetto, L. & McNaughton, P. A. ( 1992) J. Physiol. (London) 455, 111–142.
25. Gray-Keller, M. P. & Detwiler, P. B. ( 1994) Neuron 13, 849–861.
26. McCarthy, S. T., Younger, J. P. & Owen, W. G. ( 1994) Biophys. J. 67, 2076–2089.
27. Cervetto, L., Lagnado, L., Perry, R. J., Robinson, D. W. & McNaughton, P. A. ( 1989) Nature (London) 337, 740–743.
28. Matthews, H. R., Murphy, R. L. W., Fain, G. L. & Lamb, T. D. ( 1988) Nature (London) 334, 67–69.
29. Nakatani, K. & Yau, K.-W. ( 1988) Nature (London) 334, 69–71.
30. Lagnado, L. & Baylor, D.A. ( 1994) Nature (London) 367, 273–277.
31. Hsu, Y. T. & Molday, R. S. ( 1993) Nature (London) 361, 76–79.
32. Gorczyca, W. A., Gray-Keller, M. P., Detwiler, P. B. & Palczewski, K. ( 1994) Proc. Natl. Acad. Sci. USA 91, 4014–4018.
33. Palczewski, K., Subbaraya, I., Gorczyca, W. A., Helekar, B. S., Ruiz, C. C., Ohguro, H., Huang, J., Zhao, X., Crabb, J. W., Johnson, R. S., Walsh, K. A., Gray-Keller, M. P., Detwiler, P. B. & Baehr, W. ( 1994) Neuron 13, 395–404.
34. Dizhoor, A. M., Lowe, D. G., Olshevskaya, E. V., Laura, R. P. & Hurley, J. B. ( 1994) Neuron 12, 1345–1352.
35. Kawamura, S. ( 1993) Nature (London) 362, 855–857.
36. Koutalos, Y. & Yau, K.-W. ( 1995) J. Gen. Physiol. 106, 891–921.
37. Gray-Keller, M. P., Polans, A. S., Palczewski, K. & Detwiler, P. B. ( 1993) Neuron 10, 523–531.
38. Baylor, D. A., Lamb, T. D. & Yau, K.-W. ( 1979) J. Physiol. (London) 288, 613–634.
39. Lyubarsky, A. L., Nikonov, S. S. & Pugh, E. N. ( 1995) Invest. Ophthalmol. Visual Sci. 36, S277.
40. Pepperberg, D. R., Cornwall, M. C., Kahlert, M., Hofmann, K. P., Jin, J., Jones, G. J. & Ripps, H. ( 1992) Visual Neurosci. 8, 9–18.
41. Corson, D. W., Cornwall, M. C. & Pepperberg, D. R. ( 1994) Visual Neurosci. 11, 91–98.
42. Gray, E. G. & Pease, H. L. ( 1971) Brain Res. 35, 1–15.
43. Nawy, S. & Jahr, C. E. ( 1990) Nature (London) 346, 269–271.
44. Shiells, R. A. and Falk, G. ( 1990) Proc. R. Soc. London B 242, 91–94.






