Concept for Establishment of Rat Outbred Global Standard Strains
Tatsuji Nomura
Central Institute for Experimental Animals
Kawasaki, Japan
INTRODUCTION
Accuracy and quality standards for reagents and measuring scales are very important for the reproducibility of experimental results, and methods to verify such standards must be established. The need for verification also applies to experiments involving the genetic quality of laboratory animals.
Outbred rats are the most common animals used in drug safety testing at this time. Reproducibility of animal experimental results obtained using outbred rats can be expected only if the genetic quality of the rats is guaranteed. However, a genetic testing system to verify the genetic quality of outbred rats has not been established.
Outbred rats are often used in bioassays such as carcinogenicity studies. According to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (DeGeorge 1997), future carcinogenicity testing applications must consist of one set of animal studies for joint new drug applications, which must be submitted simultaneously in each country. The animals for this purpose must be “carefully selected standardized outbred rats of the same strain,” which can be used on an international level in safety studies to achieve reproducibility and comparability of results. This condition requires reliable genetic and microbiologic quality standards and test methods. In particular, a genetic testing system that can monitor the quality of these outbred rats (technically difficult to achieve in the past) must be established.
HISTORY OF GENETIC QUALITY CONTROL
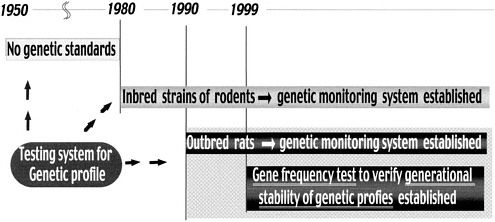
As shown in Figure 1, from the time the laboratory animal modernization movement started in 1950 until 1980, there were no quality concepts and test methods to confirm genetic profiles. Animals were used only on the basis of strain names. At the end of the 1970s, the genetic profile concept and monitoring system as a genetic testing system using biochemical and immunologic markers for inbred strains was established, and mice and rats covered by the genetic monitoring system became available. In 1979, the ICLAS Monitoring Center was founded in CIEA (Figure 1).
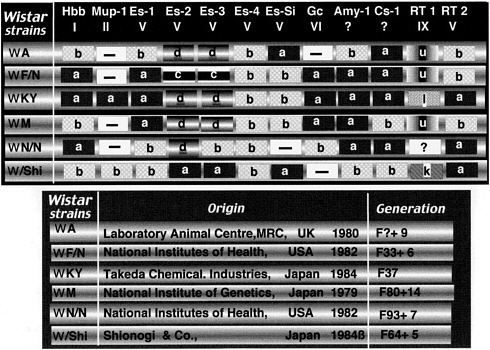
In 1985, the genetic profiles of six strains of Wistar inbred rats from various countries around the world were examined, and different results were obtained (Figure 2). Before this study, they were considered to be the same. These results revealed to most users the importance of genetic quality testing. However, outbred rats used at present are not subject to genetic quality control because such control is difficult compared with inbred or hybrid rats. The genetic background of outbred stocks is not clear, and genetic changes leading to genetic drift might occur due to an accidental infection.
QUALITY STANDARDS
The quality standards or specifications for these animals must be set by users of the animals (that is, pharmaceutical companies and the regulatory authorities) and not by breeders. In addition, the regulatory authorities and pharmaceutical companies must use the same scales to evaluate animal experiments. Our objective is to develop a testing system by which the genetic background of the outbred rats can be clearly understood so that it is possible to perform genetic control of the outbred stocks and to produce the animals in large numbers with a uniform genetic structure and permanent characteristics. Our objective is to establish the generational stability of characteristics.
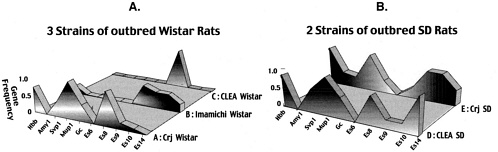
Global standards of outbred rat strains cannot be established without evaluation of genetic profiles as a scale; however, no methods have been available to establish such profiles in outbred rats. In 1990, we studied outbred stocks of rats using the genetic monitoring system, and genetic testing methods to confirm the genetic quality of outbred stocks were established (Katoh and others 1998). The results of tests on three Wistar (A, B, and C) and 2 SD (D and E) strains showed different genetic profiles in animals with the same strain name, which was considered to be due to the bottleneck effect or artificial selection, as seen in Figure 3, A and B.
CONCEPT OF GLOBAL STANDARDS FOR OUTBRED RATS
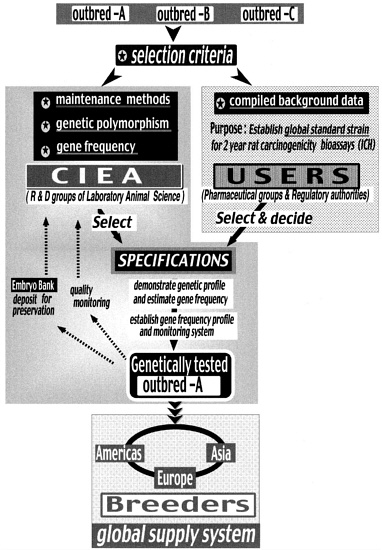
As shown in Figure 4, CIEA concluded in 1999 that it is essential to establish global standards of outbred rats based on the following concept. In using the
several theoretical methods available (Katoh 2000) for global standardization of outbred stocks (such as the multicross hybrid method), the great amount of time required to achieve results is not practical. Not only one but several global standard strains are required. It is necessary to prepare standard strains as soon as possible by selecting the most suitable strains of outbred rats from the several strains available at present. The selection criteria are established by the users and laboratory animal research and development (R&D) group as follows:
-
Specifications (standards) Selected by Users Based on the Objective
-
Compiled background data are used as selection criteria showing that the animals are appropriate for the objective (2-year carcinogenicity study on rats, in this case).
-
-
Selection Criteria for the Laboratory Animal R&D Group
-
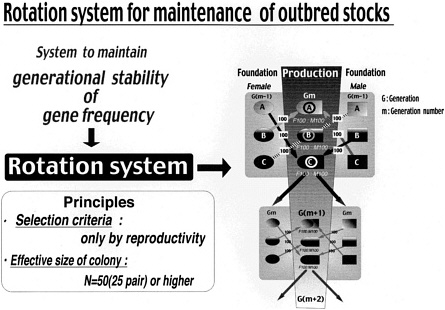
Reliable genetic control must be possible by some method (rotation system) for maintenance of the outbred stock (Figure 5).
-
Establish a production and maintenance system of colonies to maintain the quality standards (genetic profile).
-
Establish reliable genetic test methods for evaluating whether the production and maintenance system of the colonies is appropriate.
-
-
Genetic polymorphisms
-
Generational stability of gene frequency
-
Stability of the gene frequency profile must be assured over time by the established test methods.
-
-
SELECTION OF A GLOBAL STANDARD OUTBRED RAT STRAIN
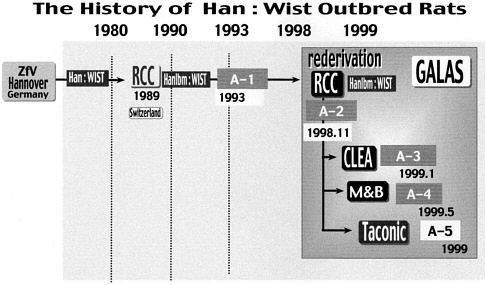
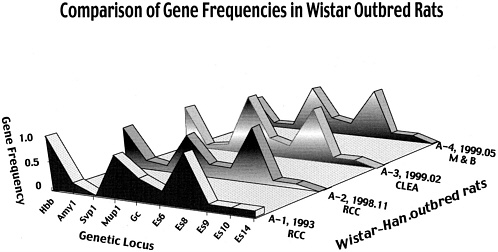
One standard strain was selected by the following method based on the concept described above. As shown in Figure 6, Wistar Hannover stock maintained in RCC by the rotation system was selected on the basis of genetic characteristics and the opinion of users. Four colonies from RCC, CLEA, M&B, and Taconic underwent the gene frequency test in 1993, 1998, and 1999. As can be seen in Figure 7, the gene frequencies shown by various markers were stable in the stocks.
SUMMARY
Genetic quality of outbred rats can now be standardized, which was impossible in the past. CIEA has established the world's first method of assessing the genetic quality of outbred rats using the gene frequency test (including the embryo bank system) to verify generational stability of genetic profiles of outbred rats. With the development of this genetic testing and monitoring method, it has become possible to establish a genetic control system (rotation system shown in Figure 6) for outbred rats and to confirm reliably that Han lbm:WIST rats are
|
Compiled Background Data of Wistar Hannover Rats (Hanlbm:WIST) ( RCC. 1981-1998 )
2 year study data on control rats (N=120, 1996-9)
|
FIGURE 8 Compiled background data of Wistar Hannover rats (Hanlbm:WIST)
appropriate as a standard strain. More than 1,000 pages of data on spontaneous abnormal findings, malformation rates, and 2-year carcinogenicity studies (including control data) as required for ordinary toxicity tests have been collected on Han ibm:WIST outbred rats since 1981. These background data have served as selection criteria for users, as shown in Figure 8. The selection criteria for genetic quality standards (Figure 4) have also been met. It should be possible to confirm from discussions with the users that the Han ibm:WIST outbred rat can be used as one of the standard strains of rats for evaluation of carcinogenicity based on a general evaluation of the data.
We plan to establish global standard rats for the 2-year carcinogenicity bio-assay on rats specified by ICH as well as an integrated system for global distribution of these rats as follows:
-
Establish genetic control of outbred rats.
-
Maintain and produce the animals, applying the rotation system on the basis of genetic quality control.
-
-
Confirm genetic profiles and generational stability by a genetic monitoring system.
-
Preserve the foundation stocks by cryopreservation for emergencies.
-
Establish a global distribution system of standard outbred rats.
A global distribution system of quality controlled standardized outbred rats covering Europe, the Americas, and Asia must be established. Breeders that can assure reliable quality control must be selected in each region, and an alliance should be formed to supply outbred rats of uniform quality.
REFERENCES
DeGeorge, J. 1997. Proceedings of the Fourth International Conference on Harmonization, Brussels, 1997. Carcinogenicity Testing: A New Approach. Belfast: The Queen's University of Belfast. p. 261-263.
Katoh, H. 2000. International harmonization of laboratory animals. In: Microbial Status and Genetic Evaluation of Mice and Rats: Proceedings of the 1999 US/Japan Conference. p. 85-96.
Katoh, H., S. Wakana, M. Ebukuro, and T. Nomura. 1998. Existence of outbred substock demonstrated using genetic monitoring system. Rat Genome 4:120-125.