Necessity of Genetic and Microbiologic Quality Network from the Pharmaceutical Industry's Perspective
Naoko Kagiyama
Head, Laboratory Animal Services
Novartis Pharma K.K. Tsukuba Research Institute, Japan
BACKGROUND
Novartis Pharma, a global pharmaceutical company, has selected the Wistar Hannover rat as the standard stock for toxicology studies. The Laboratory Animal Services Group of the company expects global vendors to ensure the uniformity of animal quality between breeding sites. The guaranteed interchangeability of data obtained at each Novartis site also depends on all sites using rats supplied by the same vendor (Table 1).
ISSUES RELATED TO THE QUALITY OF OUTBRED MICE AND RATS
Genetics: Genetic Drifting and Bottleneck in Outbred Stocks
The genetic profile of a Wistar Hannover rat stock examined in 1993 is shown in Table 2. Because the HanIbm:WIST rat revealed a typical outbred gene frequency, we decided to use the rat for toxicology studies. We would now like to know how we can guarantee that the genetic profile has not changed and will not change in the future.
In Figure 1, an embarrassing experience of genetic drifting is described (Katoh and others 1991). The investigators compared the gene frequency of 23 alleles in the three Wistar stocks, A, B, and C, supplied by three different breeders. This figure summarizes the results on eight esterase-related alleles. Surprisingly, the gene frequency was quite different among the three Wistar rats, and no one would have recognized such genetic drifting if no monitoring had been done.
TABLE 1 Background
|
|
|
|
An example of bottleneck in maintaining an outbred mouse stock is shown in Figure 2 (Saitoh and Esaki 1985). The breeder producing ICR mice was faced with an infectious disease outbreak. To rederive the colony, cesarean section was performed. It is speculated that either the number of dams involved or the selection of breeding lines may have been incorrect, and the gene frequency of the hemoglobin beta chain reversed in the renewed colony. Customers complained to the breeder of significant changes in the sensitivity to chemicals and the baseline data on malformation. It is likely that more serious changes related to genetic characteristics are hidden.
Aside from inappropriate breeding and genetic contamination, evolution caused by mutation is unavoidable in any animal population. Therefore, it should
TABLE 2 Genetic Profile of HanIbm:WIST (Excerpt)
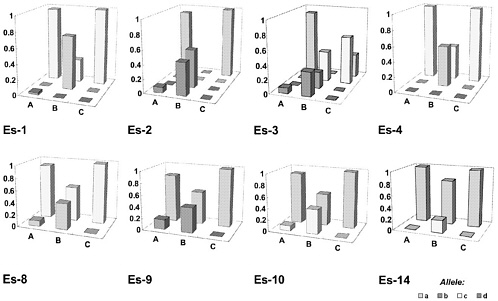
FIGURE 1 Gene frequencies in three Wistar rats, stocks A, B, and C. Reprinted with permission from Katoh H., S. Wakana, S. Utsu, and J. Yamada. 1991. Studies on the genetic monitoring of outbred mice and rats: A survey granted by the Ministry of Education, Science and Culture [in Japanese]. Tokyo, Japan.
be emphasized that monitoring of gene frequency by generation as well as by breeding site is necessary to assess the consistency of outbred stock. However, criteria have not been established for the gene frequency of each outbred stock. We also do not know whether and to what extent the difference in gene frequency is within an acceptable range of diversity. Nevertheless, it is necessary to evaluate the genetic quality of outbred stock not by the stock or vendor name but by actual monitoring results.
Microbiology: Health Profile and Checking Methods
From the viewpoint of global harmonization, three issues have been identified: (1) lack of a common, established health profile; (2) differences in sensitivity and specificity resulting from the variety of checking methods; and (3) lack of reliable monitoring results produced by inappropriate sampling. At this time, I would like to address the profile and checking methods.
For the health profile, we would like breeders to share basic monitoring items, that is, a minimum health profile for periodical monitoring. More items may be requested depending on regional situations such as biosecurity, prevalence of infectious diseases, and regulatory requirements.
For the checking method, we recommend no restriction because the method should be continually improved by experts. Instead, we would encourage refer
ence organizations to supply reference substances such as validated antigens and antisera. I believe this is the most practical approach to harmonization without jeopardizing scientific freedom.
Concerning the minimum requirement for monitoring, our group studied the selected profiles adopted by the three regional reference organizations in 1996: the FELASA, Microbiological Associates in the United States, and the ICLAS Monitoring Center in Asia. In Table 3, the results on serology for rats are presented. We were not able to prioritize among the three organizations' profiles because each had its own rationale and selected profiles reflecting the needs of the region. However, we scored the profiles as shown in the right column and finally considered 3A as the “minimum requirement” and 2A or 1A plus 2B as “recommended profiles.”
We also scored the items for bacteriology, as shown in Table 4. Unfortunately, Microbiological Associates had no checking services for bacteriology and parasitology. We considered 2A as “minimum” and 1A as “recommended.”
In Table 5, the result for rats are summarized. The 13 items on the left were taken as “minimum requirements,” including Sendai virus, sialodacryoadenitis (SDA) virus, Mycoplasma pulmonis, Bordetella bronchiseptica, Corynebacterium kutscheri, Pasteurella pneumotropica, Streptococcus pneumoniae, Salmo
TABLE 3 Microbiologic Monitoring Items for Rats (Serology)
|
Item |
FELASA (Europe) |
Microbiological Associates (US) |
ICLAS-Asia (Japan) |
Score |
|
Sendai virus |
A |
A |
A |
3A |
|
Sialodacryoadenitis virus |
A |
A |
A |
3A |
|
Pneumonia virus of mice |
A |
A |
B |
2A |
|
Mouse encephalomyelitis virus |
A |
B |
B |
1A+2B |
|
Mouse adenovirus |
X |
B |
A |
1A |
|
Minute virus of mice |
X |
X |
B |
0A |
|
Kilham rat virus |
A |
A |
B |
2A |
|
H-1 virus |
A |
A |
B |
2A |
|
LCM virus |
X |
B |
X |
0A |
|
Reo 3 virus |
A |
B |
B |
1A+2B |
|
Hantavirus |
A |
B |
A |
2A |
|
Rat cytomegalovirus |
X |
B |
X |
0A |
|
Mycoplasma pulmonis |
A |
A |
A |
3A |
|
Mycoplasma arthritidis |
X |
B |
X |
0A |
|
Clostridium piliforme (Tyzzer) |
A |
B |
A |
2A |
|
CAR bacillus |
B |
A |
B |
1A+2B |
|
Toxoplasma gondii |
A |
X |
X |
1A |
|
Encephalitozoon cuniculi |
X |
B |
X |
0A |
|
NOTE: A: basic, B: optional, X: not listed. |
||||
TABLE 4 Microbiologic Monitoring Items for Rats (Bacteriology)
TABLE 5 Microbiologic Monitoring Items for Rats
|
Minimum Requirement |
Recommended |
|
Sendai virus |
Pneumonia virus of mice |
|
Sialodacryoadenitis virus |
Mouse encephalomyelitis virus |
|
Mycoplasma pulmonis |
Kilham rat virus |
|
Bordetella bronchiseptica |
H-1 virus |
|
Corynebacterium kutscheri |
Reo 3 virus |
|
Pasteurella pneumotropica |
Hantavirus |
|
Streptococcus pneumoniae |
Clostridium piliforme |
|
Salmonella spp. |
beta-hemorrhytic streptococci |
|
Arthropods |
CAR bacillus |
|
Helminths |
Leptospira spp. |
|
Eimeria spp. |
Pseudomonas aeruginosa |
|
Giardia spp. |
Klossiella spp. |
|
Spironucleus spp. |
Encephalitozoon cuniculi |
|
Toxoplasma gondii |
|
|
Tricosomoides crassicauda |
|
|
Other flagellates |
nella, arthropods, helminths, Eimeria, Giardia, and Spironucleus. We regard the 16 items on the right as “recommended.” Similarly, we discussed monitoring items for mice and designated the 11 items on the left as “minimum” and the 17 items on the right as “recommended” (Table 6). These lists were prepared provisionally for future discussion by experts in microbiology.
CONCLUSION
Our specific proposals for the quality network include requests to breeders and reference organizations, as shown in Table 7. An adequate breeding scheme and embryo preservation for outbred stock are considered pivotal for breeders to avoid genetic drifting and backup from bottleneck. We would also like breeders to share a minimum health profile, reference substances for in-house monitoring, and ultimately establish a quality network between breeding sites or group breeders. We would like US and Japanese organizations to support our proposals by establishing an evaluation standard for genetic drifting in outbred stock, a harmonized health profile, and a list of available reference substances for validated microbiologic monitoring. I am sure that such a quality network will benefit not only humans but also the animals themselves by refining their genotype, phenotype, and dramatype.
TABLE 6 Microbiologic Monitoring Items for Mice
|
Minimum Requirement |
Recommended |
|
Sendai virus |
Pneumonia virus of mice |
|
Mouse hepatitis virus |
Mouse encephalomyelitis virus |
|
Mycoplasma pulmonis |
Minute virus of mice |
|
Corynebacterium kutscheri |
LCM virus |
|
Pasteurella pneumotropica |
Reo 3 virus |
|
Salmonella spp. |
Clostridium piliforme |
|
Arthropods |
Bordetella bronchiseptica |
|
Helminths |
Citrobactor freundii 4280 |
|
Eimeria spp. |
beta-hemorrhytic streptococci |
|
Giardia spp. |
Streptococcus pneumoniae |
|
Spironucleus spp. |
Streptococcus moniliformis |
|
CAR bacillus |
|
|
Pseudomonas aeruginosa |
|
|
Klossiella spp. |
|
|
Encephalitozoon cuniculi |
|
|
Toxoplasma gondii |
|
|
Other flagellates |
TABLE 7 Proposals for Establishing a Quality Network
|
REFERENCES
Katoh, H., S. Wakana, S. Utsu, and J. Yamada. 1991. Studies on the genetic monitoring of outbred mice and rats: A survey granted by the Ministry of Education, Science and Culture [text in Japanese]. Tokyo, Japan.
Saitoh, M., and K. Esaki. 1985. Multi-cross hybrid animals [in Japanese]. Jikken-igaku 3:564-566.









