31 Gasoline Toxicity
|
Environmental ALERT…
|
This monograph is one in a series of self-instructional publications designed to increase the primary care provider’s knowledge of hazardous substances in the environment and to aid in the evaluation of potentially exposed patients. See page 21 for more information about continuing medical education credits and continuing education units.
|
Guest Contributor: |
David Logan, MD, MPH |
|
Guest Editor: |
Richard Dart, MD, PhD |
|
Peer Reviewers: |
John Ambre, MD, PhD; Charles Becker, MD; Jonathan Borak, MD; Alan Hall, MD; Richard J.Jackson, MD, MPH; Jonathan Rodnick, MD |
|
Air Force Peer Reviewer: |
Bruce Poitrast, MD, MPH |

U.S. DEPARTMENT OF HEALTH & HUMAN SERVICES
Public Health Service
Agency for Toxic Substances and Disease Registry
Case Study
A 29-year-old auto mechanic with headaches, irritability, and forgetfulness
A 29-year-old man is brought to your office by his wife. He is reluctant to provide a history but does admit that he has been having frequent headaches for the past month. His wife indicates that at times he is somewhat confused and forgetful. She feels these symptoms have developed since they moved to their new home 6 months ago. She tells you that she has also been irritable and that several of their neighbors have been complaining of a variety of nonspecific symptoms, including headaches and forgetfulness. The wife also says that a nearby gas station was recently fined for having a leaking underground storage tank, and she feels that her family and her neighbors are being poisoned by contaminated drinking water.
The patient’s medical history is unremarkable. After completing high school, he entered the Air Force, where he was trained as a mechanic. When he left military service, he and his family moved back to this rural community to be close to his wife’s family. He has taken a job at the hardware store as a temporary position and plans to open an automotive repair shop within the next year. He has had an interest in old automobiles since high school. His new position at the hardware store has allowed him to spend an increasing amount of time working on old cars in his garage in back of the house.
The patient’s physical examination is unremarkable except for mild dermatitis on the hands, which he attributes to grease and the dirty auto parts he handles. Results of laboratory screening tests, including CBC and liver function tests, are within normal limits.
![]()
(a) What further questions would you ask regarding the wife’s and neighbors’ symptoms?
_________________________________________________________________
_________________________________________________________________
(b) What further questions would you ask regarding the patient’s work at the hardware store and his automobile-related hobby?
_________________________________________________________________
_________________________________________________________________
(c) What additional data would be informative regarding the leaking storage tank mentioned by the patient’s wife?
_________________________________________________________________
_________________________________________________________________
Answers to the Pretest questions are on page 19.
Exposure Pathways
❑ The principal exposure to gasoline for the general population is through vapor inhalation during automobile refueling.
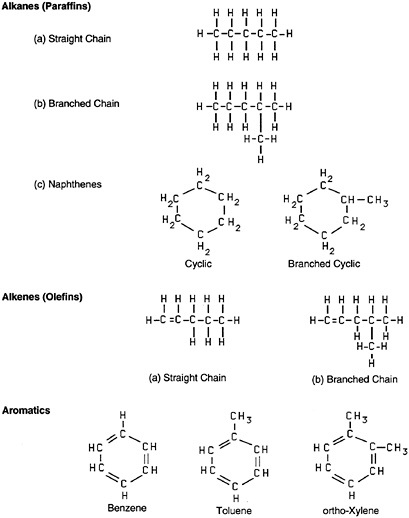
Gasoline is a refined petroleum product that is used as a motor fuel. It is highly flammable and potentially explosive. It contains more than 250 hydrocarbons and small quantities of additives and blending agents. The composition of gasoline varies depending on geographic region, season, performance requirements, and blending stocks. The typical hydrocarbon content of liquid gasoline (% volume) is as follows: approximately 60% to 70% alkanes (straight chain, branched chain, and cyclic), 5% to 10% alkenes (straight chain and branched chain), and 25% to 30% aromatics (Figure 1).
Benzene, a known hematotoxic agent, is present at an average concentration of approximately 1% in U.S. gasoline but can be as high as 5% concentration in European formulations. In addition to hydrocarbons, gasoline contains additives to improve its performance as a motor fuel and to enhance its stability. These additives include antioxidants, metal deactivators, antirust agents, anti-icing agents, detergents, dyes, and antiknock agents (which prevent detonation during combustion in internal-combustion engines and ensure smooth, even burning within the combustion cylinder).
❑ Gasoline misuse or abuse is a serious exposure concern.
Gasoline that contains more than 0.05 grams of lead per gram of gasoline is considered leaded gasoline. Organic lead is added to enhance a fuel’s octane rating. (The octane rating compares the antiknock properties of a liquid motor fuel to an industry standard; the higher the rating, the more likely the fuel will burn evenly in the combustion chamber.) In 1989, only 10% of the gasoline purchased in the United States was leaded gasoline. Due to the mandated phaseout of leaded gasoline in the United States, this percentage is rapidly decreasing. By 1997, the use of leaded gasoline in this country will have virtually ceased. However, organic lead compounds are still added to gasoline in other parts of the world. The presence of organic lead compounds in gasoline requires the addition of lead-scavenging agents such as ethylene dibromide (EDB). The replacements for organic lead antiknock agents include ethanol, methanol, methyl tertiary butyl ether (MTBE), and tertiary butyl ether (TBE), which are typically added in concentrations of 5% to 15%.
The composition of gasoline vapor differs considerably from the composition of liquid gasoline. In general, vapor pressures of the individual ingredients of liquid gasoline determine the composition of the gasoline vapor. Analysis of the major components in the liquid and vapor phases of a typical blend of the gasoline shows that while the liquid contains about 60% total alkanes, the vapor consists of nearly 90% alkanes (mainly the lighter C4-C5 components). Aromatic compounds represent 30% of the liquid phase, compared with only about 2% of the vapor phase. Benzene that is 2.1 % of the liquid phase constitutes about 0.9% of the vapor phase. n-Hexane,
a neurotoxic agent, is about 2.7% of the liquid phase and 0.9% of the vapor phase. Gasoline additives can also be present in the vapor phase but usually in small amounts.
Exposure to Gasoline Vapor
Gasoline exposure to the general population occurs primarily through inhalation of the vapor during automobile refueling. Of the more than 300 million gallons of gasoline used in the United States annually, the Environmental Protection Agency (EPA) estimates that about 4 million gallons were emitted into the atmosphere as vapor in 1982 alone. About 40% of the vaporization occurred at automotive service stations. Self-service gasoline customers typically experience short-term exposures during refueling of approximately 200 parts per million (ppm) gasoline hydrocarbons and less than 1 ppm benzene for periods of about 2 minutes. The Occupational Safety and Health Administration (OSHA) short-term exposure limit (STEL) averaged over 15 minutes is 500 ppm for gasoline hydrocarbons and 5 ppm for benzene. Thus, the exposure during self-service refueling of automobiles is not likely to be a significant hazard to the public.
Inhalation is the main exposure route for employees of the petroleum and automotive industries. Gasoline vapor is released into the air during refining of crude petroleum, bulk transfer of gasoline, and leaks from storage containers and loading equipment, as well as during refueling of vehicles. Eight-hour time-weighted average measurements for service-station attendants, tank-truck drivers, and refinery workers have shown that potential exposures up to 6 ppm exist for total hydrocarbons and less than 0.1 ppm for benzene. (The occupational permissible exposure limit [PEL] averaged over an 8-hour day is 300 ppm for total gasoline hydrocarbons and 1 ppm for benzene.) Depending on activities, transient exposures for these workers can be much higher.
Exposure to Liquid Gasoline
Human exposure to liquid gasoline occurs by unintentional or intentional ingestion, accidental skin contact, or by misuse of the solvent. Misuse of gasoline, especially to clean and degrease floors, tools, and machine parts, represents the single most important health risk from gasoline for the general public. Gasoline kept in the home for degreasing and to power lawn tools, boats, motorcycles, and recreational vehicles is both a fire and toxic hazard. It should be stored in a properly labeled, tightly sealed, metal container out of the reach of children. Gasoline improperly stored in containers such as soft drink or milk bottles can lead to unintentional ingestion, especially by children. Adults have also unintentionally ingested gasoline while attempting to siphon the fuel.
Contaminated water is a potential source of exposure for the general public, not only through ingestion, but also through inhalation and dermal absorption during bathing and laundering. Gasoline can migrate to groundwater from the soil surrounding a spill or a leaking underground storage tank or pipeline. Leaks from below-ground storage tanks are an increasing environmental problem. Of the estimated 1.4 million underground gasoline storage tanks in the United States, approximately 85% have no protection against corrosion. By one estimate, as many as 100,000 tanks leak millions of gallons of gasoline to groundwater each year; approximately 2,000 leaks are reported each year in New Jersey alone. The state of Maine estimates that leaking underground storage tanks are responsible for the release to groundwater of about 11 million gallons of gasoline each year in that state alone.
Even though chronic ingestion of gasoline through contaminated drinking water is a potential health concern, the federal government has no safety standards for gasoline in water. (EPA has set a maximum contaminant level [MCL] for many of the components of gasoline, but it has no MCL for the mixture.) At three sites near the unintentional release of 1900 metric tons of gasoline into Block Island Sound in Rhode Island, the total levels of C8 to C12 hydrocarbons ranged from 5 to 20 parts per billion (ppb) at a depth of 3.5 meters in the water column. Other unintentional releases to large bodies of surface water have resulted in levels in the range of ppb, but the significance of these levels to human health is not known.
Degradation of gasoline in groundwater and surface waters is expected to be rapid under conditions favorable to microbial activity (i.e., neutral to high pH, moderate temperature, and low salinity). Components such as alkanes, which are not very water soluble, are likely to be degraded by microorganisms, with removal time of days to weeks. Some high molecular-weight components may be ingested by fish and bioconcentrated.
|
(1) What are the potential sources of gasoline exposure for the patient described in the case study? _________________________________________________________________ _________________________________________________________________ (2) What steps can be taken to further evaluate the patient’s gasoline exposure? _________________________________________________________________ _________________________________________________________________ |
Who’s at Risk
❑ Workers in bulk terminals and on marine tankers, tank-truck drivers, auto mechanics, and service-station attendants have the greatest occupational risk of gasoline exposure.
The gasoline manufacturing and distribution system involves production of gasoline at oil refineries; transportation via truck, rail, barge, or pipeline to bulk terminals; and further movement from bulk terminals to retail gasoline stations. Refinery workers, persons associated with transportation, gasoline service-station attendants, and workers involved in removal and maintenance of underground storage tanks are at greatest risk of exposure to gasoline. Persons living near gasoline production and distribution systems also have potential exposure. Health risk among consumers and the general public from gasoline-vapor exposure during refueling at self-service gasoline stations is probably negligible.
Persons who misuse gasoline to clean garage floors or use gasoline-soaked rags to clean hands or machinery parts risk toxicity from both inhalation and dermal absorption. Persons who unintentionally ingest gasoline while siphoning and those who intentionally inhale gasoline vapors to obtain euphoric effects risk serious health consequences. The highly flammable and explosive nature of gasoline increases its risk, especially during misuse. Although it is illegal to smoke near a gasoline source, many persons do so, placing themselves and others in great danger. Using gasoline to ignite fires or to increase their rate of burning is also hazardous.
❑ Persons who misuse gasoline as a recreational drug are at substantial risk for adverse effects.
Persons who ingest gasoline-contaminated groundwater are at risk of exposure, but the health risks of chronic ingestion of gasoline are unknown. Although numerous cases of leaking underground storage tanks have been documented, contaminated water supplies have rarely contained the individual components of gasoline at concentrations that exceed the EPA maximum contaminant levels.
Persons susceptible to gasoline toxicity include the very young, the very old, pregnant women, and persons suffering from malnutrition.
Biologic Fate
❑ The most common routes of gasoline exposure are inhalation and dermal absorption.
The data on the toxicokinetics of gasoline mixtures in humans or animals are sparse. Studies on several of the hydrocarbon components of gasoline are readily available (see Case Studies in Environmental Medicine: Benzene Toxicity and Case Studies in Environmental Medicine: Toluene Toxicity) and on some additives (see Case Studies in Environmental Medicine: Methanol Toxicity and Case Studies in Environmental Medicine: Lead Toxicity). However, the interaction of the components in a gasoline mixture may influence the absorption, distribution, metabolism, and elimination patterns of the individual components.
Gasoline Hydrocarbons
❑ The metabolic pathway for gasoline may be different from the metabolic pathways of individual components of gasoline.
Gasoline can be absorbed by inhalation, ingestion, and dermal exposure routes. In general, the hydrocarbon components with higher blood/gas partition coefficients in the lungs (e.g., xylene, benzene, toluene) have a higher absorption rate during inhalation than components with lower coefficients (e.g., cyclohexane, ethane, ethylene). In time, ingested gasoline is probably absorbed completely because of the high lipophilic properties of the hydrocarbon compounds and the large surface area of the gastrointestinal tract. The rate of dermal absorption is low compared with absorption after ingestion, although the aromatic hydrocarbons, such as benzene, are expected to have higher skin penetration than the alkanes.
Data on the biologic fate of gasoline in humans have been obtained primarily through autopsies. In a male who died after unintentional ingestion of gasoline, the highest gasoline concentrations were in the liver, gastric wall, and lungs. Service-station attendants who were exposed to gasoline, most likely by dermal contact as well as inhalation, had elevated blood levels of hydrocarbons such as benzene, toluene, pentane, and hexane.
The metabolism of gasoline mixtures in humans is unknown. However, the interaction of the components will most likely influence the metabolizing enzymes, thereby altering the elimination rate of a component. The increased metabolism of antipyrine in humans and experimental animals exposed to gasoline vapors suggests that mixed-function oxygenase activity is accelerated by gasoline. Some gasoline hydrocarbons are oxidized by liver microsomal-enzyme systems to products that are readily excreted in the urine.
No specific data exist on excretion patterns of gasoline mixtures after exposure. Because alkanes are stable, saturated compounds, they generally are not metabolized. Most of what is systemically absorbed is excreted unchanged through the lungs. Urinary phenol, a biologic indicator of benzene exposure, was found to be elevated in gasoline-pump workers (average 40 milligrams/liter [mg/L]) compared with persons with no occupational exposure to gasoline (less than 20 mg/L).
Gasoline Additives
Tetraethyl lead and tetramethyl lead can be rapidly absorbed through inhalation and skin contact. After absorption, these organic lead compounds are rapidly dealkylated by the liver to trialkyl metabolites that are toxic. The trialkyl metabolites, which are water soluble, can accumulate in the brain; they are slowly metabolized to inorganic lead. Approximately 76% of the lead in these compounds is ultimately excreted as inorganic lead in the urine, 16% in gastrointestinal secretions, and 8% in epithelial structures and sweat.
Methanol is readily absorbed after inhalation or ingestion. A small portion is eliminated unchanged in the breath and urine, but most is metabolized in the liver to formaldehyde, formate, formic acid, carbon dioxide, and water. Formate is the intermediate metabolite believed to be responsible for the delayed effects of methanol poisoning. Recent research shows that formate accumulation in the blood of monkeys parallels the development of ocular disturbances and acidosis. The metabolism of formic acid and formate is dependent, in part, on the vitamin folate. The low level of methanol that could be absorbed during automobile refueling would be metabolized readily to nontoxic compounds through this folate-dependent pathway in most people. (About 10% of the population may be folate-deficient.) However, ingestion of large amounts of gasoline can result in absorption of methanol in a quantity sufficient to quickly overwhelm the folate-dependent metabolic pathway and produce severe toxicity. (See Case Studies in Environmental Medicine: Methanol Toxicity.)
Ethanol may be blended into gasoline at concentrations of 5% to 10% as an oxygenate replacement for MTBE. Ethanol is readily absorbed by the gastrointestinal tract and the lungs, whereas absorption through the skin is usually negligible. Approximately 90% of an absorbed ethanol dose is metabolized in the liver to acetaldehyde and acetic acid, and finally to carbon dioxide and water. Ethanol toxicity in humans is rarely attributed to gasoline inhalation exposure.
Depending on the dose and exposure route, 20% to 70% of absorbed MTBE is rapidly exhaled. The remaining MTBE is either metabolized or excreted unchanged in the urine. When metabolized, MTBE is oxidized to formaldehyde and demethylated to tertiary butyl alcohol, which may then be further oxidized to 2-methyl-1,2-propanediol and alpha-hydroxyisobutyric acid. These oxidation products are excreted in the urine.
Physiologic Effects
The major target organ of gasoline exposure is the central nervous system (CNS). Inhalation is the most common route of exposure. Ingestion can result in severe toxicity; single oral doses of approximately 10 milliliters per kilogram (mL/kg) body weight (or about 700 mL for an adult) may be fatal. Much smaller amounts, if aspirated into the lungs, may lead to lipoid pneumonitis.
Contact with liquid gasoline can cause an acute burning sensation in the skin, eyes, and mucous membranes. Prolonged contact with liquid gasoline can defat the skin and cause irritation and dermatitis. Absorption of gasoline probably increases if the skin is broken. Systemic gasoline poisoning due solely to skin exposure has not been documented conclusively.
Central Nervous System Effects
❑ Prolonged overexposure to high concentrations of gasoline vapor may cause CNS toxicity.
The major systemic effect of acute gasoline overexposure is CNS depression. Overexposure can lead to facial flushing, ataxia, vertigo, mental confusion, headaches, blurred vision, slurred speech, and difficulty swallowing. At very high concentrations, coma and death can occur within a few minutes without any accompanying respiratory depression or anoxia. In laboratory studies, human volunteers exposed to gasoline vapor developed dizziness and headaches at concentrations greater than 1800 ppm. A 1-hour exposure to 900 ppm caused slight dizziness and irritation of the eyes, nose, and throat. At 10,000 ppm, nose and throat irritation developed within 2 minutes, dizziness within 4 minutes, and signs of intoxication in 4 to 10 minutes. Humans exposed to high, nonlethal concentrations of gasoline usually recover completely, although rare cases of permanent brain damage after massive exposure have been reported.
❑ Relatively little is known about the potential neurotoxicity of gasoline vapors after prolonged exposures to low concentrations.
Chronic intentional abuse (e.g., sniffing or “huffing”) of gasoline can result in death. The cause of death has been postulated to be either CNS depression, leading to respiratory failure, or a lowering of the myocardial threshold to the dysrhythmogenic effects of circulating catecholamines, leading to fatal dysrhythmia. Chronic abuse of leaded gasoline may cause a range of neurologic effects including encephalopathy, ataxia, and tremor. Among 73 chronic sniffers of leaded gasoline (age range: 4 to 20 years), 69 users (95%) showed definite neurologic effects and had blood lead levels ranging from 30 to 344 micrograms per deciliter (µg/dL). The neurologic effects may have been due to the action of aliphatic and aromatic hydrocarbons, tetraethyl lead, or both.
The potential neurotoxicity associated with repeated low-level exposure to gasoline is undetermined. Appropriate occupational studies are not available and the results of experimental animal studies do not provide a dose-response relationship sufficient to determine a no-observed-adverse-effect level (NOAEL). Subtle neurotoxic effects have been reported in two animal studies. At 8000 ppm, a reduction in overall activity was observed in rats, whereas at the lower concentrations tested (i.e., 4000 ppm and 800 ppm), an increase in physical activity was observed.
Respiratory Effects
❑ Chemical pneumonitis from aspiration of gastric contents after gasoline ingestion is a concern.
At high concentrations, gasoline vapor is a respiratory-tract irritant. Pulmonary congestion, edema, acute exudative tracheobronchitis, and intrapulmonary hemorrhage were found when autopsies were performed on persons who died from gasoline overexposure. The lungs of rats chronically exposed to intermediate levels of gasoline vapor showed a progression of lesions characteristic of fibrosing alveolitis (interstitial fibrosis and alveolar collapse). Concurrent with
this finding was the observation that surfactant levels in the lung were markedly decreased, suggesting that surfactant deficiency may be involved in the pathogenesis of lung damage due to gasoline inhalation exposure.
Gasoline contains many low-viscosity compounds, which pose a serious pulmonary aspiration hazard. If such compounds are introduced directly into the lung or aspirated during emesis, a severe chemical pneumonitis characterized by pulmonary edema, hemorrhage, and tissue necrosis can result. Pulmonary aspiration of gasoline is a particular concern after ingestion exposure in children, which may occur when gasoline is stored in improperly labeled or inappropriate household containers. Ingestion and pulmonary aspiration may also occur from siphoning gasoline.
Hematopoietic Effects
❑ Gasoline contains a small percentage of benzene, which is a known hematotoxic agent.
Several case reports describe hematologic effects in persons with known long-term exposure to gasoline vapor. In these case reports, the blood dyscrasias described (i.e., anemia, hypochromia, thrombocytopenia, and neutropenia) were thought to be due to the benzene in gasoline mixture. Benzene does not pose an identifiable risk to consumers during normal gasoline use because of its low concentration, although it can cause serious damage to the hematopoietic system (see Case Studies in Environmental Medicine: Benzene Toxicity).
Carcinogenic Effects
❑ Lifetime exposure to gasoline vapor causes cancer in experimental animals, but extensive studies do not generally support a similar cancer hazard in humans.
More than 55 epidemiologic studies of workers exposed occupationally to hydrocarbons have been published. These studies, in general, have not substantiated the carcinogenic effects observed in experimental animals.
A recent case-control study examined kidney cancer deaths reported in refinery workers and concluded there was no association between kidney cancer and exposure to gasoline-like vapors in refineries. Studies of workers in the British and Canadian gasoline distribution systems (from the refinery to the service-station pump) showed instances of excess cases of kidney cancer and leukemia, but these findings were not statistically significant and did not appear related to exposure levels.
❑ Epidemiologic studies suggest that certain cohorts of refinery workers employed before 1940 may have had an elevated risk of leukemia due to relatively large exposures to benzene.
A recent study of U.S. gasoline workers in land-based distribution and marine operations found no evidence of increased cancer risk associated with gasoline exposure. A small excess of a subtype of leukemia was seen in the land-based group but was not related to gasoline exposure levels. This observation and the small excess of leukemia cases observed in distribution workers in Britain and
❑ IARC has classified gasoline as a possible human carcinogen based on the presence of benzene and 1,3-butadiene.
Canada suggest a possible association that needs further evaluation. A meta-analysis in 1989 of several epidemiologic studies did not reveal any clear association between gasoline exposure and leukemia. The authors of the analysis did conclude that some refinery workers, particularly those employed before 1940, may have been at increased risk for developing leukemia caused by the relatively large exposures to benzene that employees experienced in that era.
In 1989, the International Agency for Research on Cancer (IARC) reviewed the world literature on gasoline and concluded that the experimental animal data provide only limited evidence of carcinogenicity and that human epidemiologic studies were inadequate because of lack of complete exposure data, concurrent exposures to other chemicals, and other confounding factors. However, because of the limited evidence from experimental animal studies and the presence of benzene and 1,3-butadiene in gasoline, IARC concluded that gasoline is possibly carcinogenic to humans.
Reproductive and Developmental Effects
❑ Several constituents of gasoline can cause reproductive or developmental toxicity in experimental animals but probably do not pose a risk to humans under normal use conditions.
No studies are currently available to evaluate the potential reproductive toxicity of gasoline. Ethanol is the only major gasoline additive with unequivocal evidence of human reproductive or developmental toxicity. Ingestion of as little as one ounce of ethanol daily by pregnant women can cause adverse effects in offspring (e.g., fetal alcohol syndrome). However, gasohol—a blend of gasoline and ethanol—is a very unlikely vehicle for such exposure. A person would have to ingest or inhale large amounts of hydrocarbons to receive sufficient ethanol exposure from gasohol, and the hydrocarbons themselves would cause acute toxicity.
There is evidence that methanol, toluene, benzene, xylene, 1,3-butadiene, and methyl-t-butyl ether can cause reproductive or developmental effects in experimental animals under various exposure situations. Low-level exposure to those chemicals during normal use and handling of gasoline, however, should not pose human reproductive or developmental health risks.
|
(3) What components of gasoline might explain the patient’s symptoms? _________________________________________________________________ _________________________________________________________________ (4) What other factors might explain the patient’s symptoms and those of his wife and neighbors? _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ |
Clinical Evaluation
History and Physical Examination
❑ The history and physical examination should focus on the CNS.
When patients have acute symptoms from gasoline overexposure, it is usually apparent where and how the exposure occurred. Persons who are inadequately protected have been overcome by gasoline vapors while cleaning or working in gasoline storage tanks, although this occurrence is rare. Occasionally, emergency spills or leaks can also lead to acute overexposure. Persons who abuse gasoline through intentional inhalation can most often be identified from the history. More problematic are cases in which patients have vague or nonspecific symptoms, and no obvious exposure incident can be elicited.
Specific questions should be asked regarding mouth siphoning of gasoline and use of gasoline as a solvent to clean tools, hands, automobile parts, or garage floors. Open containers of gasoline in a confined space, such as a garage, cannot only pose a serious fire or explosion hazard but can result in potentially toxic vapor concentrations. Because of gasoline’s defatting properties, persons who misuse gasoline as a solvent often have dermatitis on their hands.
The physical examination should emphasize the neurologic system because it is the principal target organ for gasoline toxicity. In severe overexposures, life-threatening CNS depression and potential respiratory arrest can occur.
When ingestion of gasoline is suspected, the patient should be evaluated for possible pulmonary aspiration, which may lead to complications of chemical pneumonitis, pulmonary edema, and pulmonary hemorrhage. Ingestion can also cause gastrointestinal disturbances.
Signs and Symptoms
Acute Exposure
❑ Serious acute exposures are infrequent and usually related to misuse or emergencies involving spills, leaks, or exposures in a confined space.
Acute gasoline toxicity occurs only rarely today and is most often associated with emergencies involving the cleaning or maintenance of storage tanks, exposures related to large spills or leaks, intentional inhalation of gasoline vapors to obtain euphoric effects, deliberate ingestion in suicide attempts, or unintentional ingestion during siphoning, or misuse of gasoline as a solvent.
The signs and symptoms that develop after acute exposure depend on the route of exposure and the dose absorbed. High-concentration exposures by any route cause CNS depression, which results in confusion, tinnitus, disorientation, headache, drowsiness, weakness, seizures, and coma. Inhalation may produce respiratory-tract irritation, resulting in dyspnea, tachypnea, and rales that may progress rapidly to massive pulmonary edema; a burning sensation in the chest may be present. Ingestion may cause pain and irritation of the mucous membranes, resulting in nausea, vomiting, abdominal pain, and diarrhea. Irritation and dermatitis can occur after skin contact, and conjunctivitis can occur after eye contact.
Chronic abuse of gasoline through sniffing has been reported to cause cardiac dysrhythmias and tachycardia.
Chronic Exposure
❑ Repeated exposures to gasoline through refueling of automobiles or ingestion of contaminated water have not been reported to cause chronic health effects.
Studies have clearly demonstrated that exposures to gasoline and its constituents, including benzene, n-hexane, and 1,3-butadiene during refueling of motor vehicles, are not a serious health hazard for consumers. Organic lead compounds can produce chronic neurologic toxicity, but exposure to such compounds in gasoline is currently negligible in the United States. Potential lead toxicity remains a concern in countries that continue to formulate or use leaded gasoline.
Ethanol, methanol, and other additives in gasoline pose potential exposure risks, particularly through unintentional ingestion or suicide attempts. These materials, however, are not hazardous to consumers in the amounts volatilized when gasoline is used as a motor fuel. Hydrocarbon toxicity is likely to occur before toxicity from these additives occurs.
Chronic exposure to gasoline through contaminated drinking water could pose a health risk if concentrations are excessive, especially if the gasoline has a high methanol content; chronic exposure to methanol poses a risk because of its high toxicity after ingestion.
Laboratory Tests
Direct Biologic Indicators
No biologic indicators exist for gasoline that would be of definite value in assessing a person’s exposure to gasoline.
Indirect Biologic Indicators
❑ Appropriate laboratory testing depends on the severity of exposure and existing symptoms.
Appropriate laboratory evaluation depends on the severity and route of exposure, and on the patient’s symptoms. Patients whose symptoms suggest CNS toxicity should have a neurologic evaluation. This evaluation might include neurobehavioral testing and an EEG. After severe, acute overexposure to gasoline, degenerative changes may occur in the liver and kidneys; these effects should be evaluated through routine laboratory testing.
Persons with suspected ingestion should also have a careful pulmonary evaluation, including a baseline chest radiograph to assess possible aspiration. A follow-up chest X ray should be obtained in about 6 hours if pulmonary symptoms develop. Pulse oximetry or arterial blood-gas analyses may be needed to assess oxygenation if significant pulmonary symptoms or cyanosis are present.
Prolonged ingestion of drinking water contaminated with relatively high levels of gasoline may pose a small risk related to benzene exposure. Although no definitive evidence exists to indicate that such exposures are associated with any increased risk of hematologic disorders, in some limited circumstances, periodic hematologic monitoring has been suggested. It is unclear whether such testing can detect early development of leukemia. (See recommendations in Case Studies in Environmental Medicine: Benzene Toxicity.)
|
(5) What further medical workup is indicated for the patient described in the case study? _________________________________________________________________ _________________________________________________________________ Additional information for the case study: Several drinking-water analyses indicate levels of benzene ranging from non-detectable to 0.1 ppb, ethyl benzene from nondetectable to 25 ppb, total xylenes of up to 100 ppb, and toluene up to 10 ppb. (6) Is it likely that the patient’s complaints and those of his wife and neighbors are from gasoline contamination of the drinking water? _________________________________________________________________ _________________________________________________________________ (7) Careful history indicates that the patient frequently uses gasoline to clean his hands and to degrease automobile parts. Could this exposure to gasoline account for his complaints? _________________________________________________________________ _________________________________________________________________ |
Treatment and Management
Acute Exposure
❑ Treatment of a patient with acute gasoline exposure is supportive.
No specific antidotes exist for gasoline; medical management for exposed persons is supportive. After severe inhalation exposure, affected persons should be moved to safety, and resuscitated if necessary. Respiratory compromise may require intubation or surgical creation of an airway.
In cases of ingestion, vomiting should not be induced because of the risk of pulmonary aspiration. Patients should be watched for signs of chemical pneumonitis (coughing and dyspnea) whenever pulmonary aspiration is a possibility. Activated charcoal is of limited use; spontaneous vomiting and diarrhea are likely to occur if a massive dose of gasoline has been ingested.
If skin or hair has been in contact with liquid gasoline, remove clothing and flush skin and hair with water (preferably under a shower) for 2 to 3 minutes. Wash with mild soap; rinse thoroughly with water. If eye contact has occurred, the eye should be flushed with water for at least 5 minutes or until pain resolves.
Chronic Exposure
❑ Avoidance of further exposure is the most important intervention in cases of gasoline misuse.
The most important intervention in situations where gasoline is misused either to obtain euphoric effects or as a solvent is to ensure that further exposures do not occur. This can usually be accomplished through education and counseling. Persons who intentionally inhale gasoline may require intensive psychological therapy. In most cases, exposure cessation usually leads to complete recovery, even for patients who have evidence of CNS toxicity.
Defatting of the skin and dermatitis that can occur with repeated and prolonged skin contact is managed using skin moisturizers and barrier creams, as well as avoidance of further exposure. For persistent symptoms, a dermatologist should be consulted.
|
(8) What treatment will you recommend for the patient described in the case study? _________________________________________________________________ _________________________________________________________________ |
Standards and Regulations
Workplace
Air
❑ The current OSHA workplace standard is 300 ppm (8-hr TWA).
The Occupational Safety and Health Administration (OSHA) mandates permissible limits for occupational exposures. The permissible exposure limit (PEL) for gasoline is 300 ppm as an 8-hour time-weighted average (TWA) and 500 ppm as a short-term exposure limit (STEL). (See Table 1.)
Table 1. Standards and regulations for gasoline
|
Agency* |
Focus |
Level |
Comments |
|
|
ACGIH |
Air—workplace |
300 ppm 500 ppm |
Advisory; TWA† Advisory; STEL§ |
|
|
NIOSH |
Air—workplace |
No criteria |
Advisory; as low as possible, based on carcinogenic risk |
|
|
OSHA |
Air—workplace |
300 ppm 500 ppm |
Regulation; PEL¶ Regulation; STEL |
|
|
EPA |
Air—environment |
No criteria |
||
|
Drinking water |
||||
|
Gasoline |
No criteria |
|||
|
Gasoline components |
|
|||
|
Benzene |
0.005 mg/L |
(5 ppb) |
Regulation; MCL** |
|
|
Toluene |
1.0 mg/L |
(1000 ppb) |
Regulation; MCL |
|
|
Ethyl benzene |
0.7 mg/L |
(700 ppb) |
Regulation; MCL |
|
|
Total xylene |
10.0 mg/L |
(10,000 ppb) |
Regulation; MCL |
|
|
Ethylene dibromide |
0.05 µg/L |
(0.05 ppb) |
Regulation; MCL |
|
|
*ACGIH=American Conference of Governmental Industrial Hygienists; EPA=Environmental Protection Agency; NIOSH =National Institute for Occupational Safety and Health; OSHA=Occupational Safety and Health Administration †TWA (time-weighted average)=concentration averaged over a normal 8-hour workday and 40-hour workweek to which nearly all workers may be repeatedly exposed. §STEL (short-term exposure limit)=concentration to which workers may be exposed on a short-term basis (usually determined by a 15-minute sampling period). ¶PEL (permissible exposure limit)=level of contaminant in air, averaged over an 8-hour workday (TWA), to which a worker may be exposed. **MCL (maximum contaminant level)=highest concentration of a contaminant allowed in public drinking water supplies. |
||||
Environment
Air
The Clean Air Act Amendments of 1990 mandate that gasoline be reformulated in the nine air quality control regions in the United States with the worst pollution (specifically those that have not attained mandated ozone levels) and provide the option for other areas to adopt these requirements. By 1995, reformulated gasoline will have to meet the following characteristics: 1% maximum benzene content and 15% reduction in total emissions of benzene, 1,3-butadiene, polycyclic organic matter, formaldehyde, and acetaldehyde.
Drinking Water
EPA has not established a maximum contaminant level (MCL) for gasoline in public water systems. EPA has established MCLs, however, for some of the constituents of gasoline. (See Table 1.)
Suggested Reading List
General Reviews
Edminster SC, Bayer MJ. Recreational gasoline sniffing: acute gasoline intoxication and latent organolead poisoning. J Emerg Med 1985;3:365–70.
Harrington JM. Health experience of workers in the petroleum manufacturing and distribution industry: a review of the literature. Am J Ind Med 1987;12:475–97.
Page NP, Mehlman M. Health effects of gasoline refueling vapors and measured exposures at service stations. Toxicol Ind Health 1989;5(5):869–90.
Weaver NK. The petroleum industry. State Art Rev Occup Med 1988:3(3).
Exposure Assessment
Kearney CA, Dunham DB. Gasoline vapor exposures at a high-volume service station. Am Ind Hyg Assoc J 1986;47(8):535–9.
Smith TJ. An exposure assessment for marketing and marine distribution workers in the petroleum industry with potential exposure to gasoline. Washington, DC: Am Petroleum Institute, 1992.
Carcinogenicity
International Agency for Research on Cancer. Occupational exposures in petroleum refining; crude oil and major petroleum fuels. Lyon: IARC 1989:159–201. (IARC monographs on the evaluation of the carcinogenic risks to humans; vol 45.)
Poole C, Satterfield MH. A case-control study of kidney cancer among petroleum refinery workers. Washington, DC: Am Petroleum Institute, 1990.
Wong O, Harris F. A mortality study of marketing and marine distribution workers with potential exposure to gasoline in the petroleum industry. Washington, DC: Am Petroleum Institute, 1992.
Wong O, Raabe GK. Critical review of cancer epidemiology in petroleum industry employees, with a quantitative meta-analysis by cancer site . Am J Ind Med 1989;15:283–310.
Related Governmental Publications
Northeast States for Coordinated Air Use Management, Air Toxics Committee. Evaluation of the health effects from exposure to gasoline and gasoline vapors. Final Report. Boston: NESCAUM 1989.
Environmental Protection Agency. A toxicological assessment of the unleaded gasoline contamination of drinking water. Washington, DC: US Environmental Protection Agency, 1989.
Sources of Information
More information on the adverse effects of gasoline and treating cases of gasoline exposure can be obtained from ATSDR, your state and local health departments, and university medical centers. Case Studies in Environmental Medicine: Gasoline Toxicity is one of a series. To obtain other publications in this series, please use the order form on the inside back cover. For clinical inquiries, contact ATSDR, Division of Health Education, Office of the Director, at (404) 639–6204.
Answers to Pretest and Challenge Questions
Pretest questions are on page 1. Challenge questions begin on page 5.
Pretest
-
Because an environmental exposure is being considered, further information should be obtained about the reported symptoms of the wife and the neighbors.
Detailed questions regarding the woman’s irritability would be appropriate. A review of neurologic symptoms is indicated. In addition, the relationship of those symptoms to the family’s relocation should be explored. Because there may be other explanations for her complaints, discuss her recent relocation and adjustment to the new community. Are there new stresses in her life that could account for the symptoms? Explore recent onset of any unrelated diseases and possible pregnancy. A detailed description of the nonspecific symptoms of the neighbors should also be elicited from the couple if possible. Until there is further evidence to indicate a potential environmental concern, the neighbors should not be interviewed directly. Follow-up with the neighbors or the health department will be appropriate when a potential public health problem has been established.
-
The patient’s potential occupational exposures at the hardware store should be explored (e.g., filling propane tanks, mixing paints, cleaning recent spills). Do coworkers have any related symptoms?
In addition, working on old cars in a garage can involve exposure to many materials. Detailed information should be obtained regarding the solvents, paints, welding fumes, greases, or other agents that the patient may encounter.
The composition of the materials used or handled, the quantities involved, the duration of exposure, and any protective measures that are used should be considered in making an overall assessment.
-
Because the safety of the drinking water has been questioned, information should be obtained regarding the water supply to the house. Is the water supplied by a public water system or from a local or household well? If the water is supplied through a public water system, it is highly unlikely that a gasoline storage-tank leak near the patient’s house could lead to contaminated water. If the water is obtained through a local well (probable in the case study because they live in a rural community), a potential leak should be investigated. A call to the regional EPA office could provide specific information about the water system and the results of any water analyses.
Challenge
-
The patient has potential gasoline exposure from the following sources:
-
automobile refueling
-
consuming contaminated drinking water
-
working on old cars
-
Exposure of the general public to gasoline vapor through automobile refueling is low and does not pose an identifiable health risk to consumers. The patient’s exposure to gasoline through contaminated drinking water is possible, although it is unlikely that levels of benzene, toluene, or other constituents would be at a level that could produce toxicity. The lack of eye and respiratory-tract irritation from volatilization of gasoline in the home
-
water supply during showering, dishwashing, laundering, and other activities does not indicate significant exposure by this route. To evaluate this exposure route, levels of gasoline constituents in the household drinking water should be measured and more information about the storage-tank leak should be obtained.
The potential for gasoline or hydrocarbon-based solvent exposure through the patient’s hobby should be explored carefully. Detailed information should be obtained regarding the patient’s use of gasoline as a solvent to clean his hands or automobile parts. What is the frequency and duration of exposure? Is gasoline stored in open containers in the garage? Is the garage ventilated while he is working? If there is any indication of recreational gasoline abuse, his symptoms could be related to overexposure.
-
Several of the hydrocarbons in gasoline can produce CNS toxicity. The most likely components, based on their percentage volume, would be toluene and xylene. n-Hexane can also cause CNS, as well as peripheral nerve, toxicity; however, the low concentration of n-hexane in gasoline makes it an unlikely candidate.
-
Nonspecific symptoms can be very difficult to evaluate. If there is no objective evidence of disease and no laboratory or physical abnormalities, the clinician should consider other contributory factors. Are the readjustment stresses to the new community? Are there financial or marital difficulties or other external considerations?
-
If a careful history indicates that the patient has had recent onset of frequent headaches, as well as other neurobehavioral symptoms, a thorough neurologic examination should be performed. If deficits are demonstrated, further neurologic workup, such as scans, EEG, and neurobehavioral testing, is indicated. If gasoline toxicity is a consideration, liver and kidney function should be evaluated, although abnormalities are unlikely unless there has been severe acute overexposure.
-
No, the measured results are well within normal limits and do not indicate a toxic exposure. Given the potential variability of water analyses, it would be appropriate to confirm these insignificant levels by performing two or three analyses.
-
Occasional misuse of liquid gasoline to clean hands or machinery parts is unlikely to cause significant toxicity, although the practice may present a serious fire or explosion hazard. Repeated skin contact can lead to defatting of the skin and dermatitis. The dermatitis on the patient’s hands in this case study could indeed be from dermal contact with liquid gasoline.
Prolonged and repeated misuse of gasoline as a solvent or cleaning agent can, however, cause significant toxicity. If the patient has frequent extensive skin contact with liquid gasoline or is frequently exposed to high concentrations of gasoline vapors via open containers of gasoline in a confined space, his headaches, confusion, and forgetfulness could be from gasoline overexposure.
-
The single most important intervention in this case would be to counsel the patient on the hazards of gasoline and to eliminate further misuse. Removal of exposure would most likely lead to a complete resolution of symptoms without further sequelae. In a few cases, some residual deficits might persist.