2
Oak Ridge National Laboratory Intermetallics Program
Intermetallic compounds are a unique class of materials, consisting of ordered alloy phases formed between two or more metallic elements where the different atomic species occupy specific sites in the crystal lattice (NRC, 1984; Anton et al., 1989). Intermetallics differ from conventional alloys in that they generally possess long-range-ordered crystal structures at ambient and intermediate temperatures. Intermetallic compounds form in composition ranges close to stoichiometric ratios. Thus, although the laws of chemical valency are usually not followed, the compounds often have relatively simple chemical names like TiAl, Ti3Al, NiAl, Ni3Al, CuZn, Cu3Au, and Nb5Si3.
Intermetallics have characteristics of both metals and ceramics, and their mechanical properties are intermediate between metals (which are generally softer and more ductile) and ceramics (which are generally harder and more brittle). The predominant bonding in ceramics is covalent and ionic, as opposed to metallic bonding. Intermetallics contain both metallic and covalent bonds, depending on the constituent metals. Because of their intermediate position, the properties of intermetallics can be strongly influenced by small changes in the system (i.e., variations in the microstructure can result in changes in strength and ductility over a considerable range).
A great deal of work has been done in the last 10 to 15 years to develop and characterize intermetallics and to develop processing technologies. In response to the need for low density, high performance alloys for use in the components of airframes and turbine engines (NRC, 1993, 1996), for example, concerted efforts have been made in recent years to improve the properties of intermetallic alloys, especially alloys based on aluminides (e.g., TiAl, Ti3Al, NiAl, Ni3Al, FeAl,
Fe3Al). Their inherent oxidation resistance and retention of strength at high, homologous temperatures make them prime candidates for use at intermediate temperature ranges, where creep resistant alloys are required. The major problem with many intermetallics is that they can have extremely low ductility at ambient temperatures. Therefor, before they can be used as structural materials, intermetallics must be modified to improve their ductility and strength and to make them more resistant to oxidation and corrosion. In addition, processes must be developed for preparing and processing these materials into usable shapes.
The development program described in this report was undertaken by ORNL to increase the understanding of, and to improve the properties of, Ni3Al and other intermetallic compounds so that they could be processed and utilized as structural materials in demanding, high temperature environments in a number or applications by a number of industries.
In this chapter, the ORNL program for developing intermetallic alloy materials and processes is described; program management and interactions among the sponsoring groups within DOE are outlined; significant technical accomplishments and collaborations with industrial partners and licensees are reviewed; and conclusions and suggestions concerning the technical program are presented. The emphasis of this report is on lessons that can be derived from the development of Ni3Al alloys and processes, which have been the focus of the OIT intermetallics research program at ORNL. These lessons may be of benefit to OIT in implementing the IOF strategy throughout the OIT program.
HISTORY OF PROGRAM MANAGEMENT
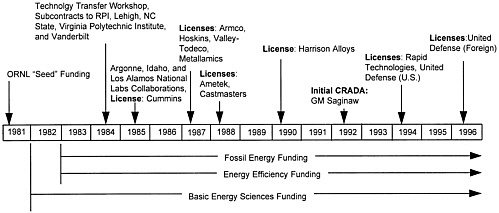
The ORNL intermetallics program is a long-term, collaborative, research and development program involving various DOE program offices. The program was initiated with ORNL “seed” funds and was subsequently funded by Basic Energy Sciences (BES), which sponsors fundamental research, and by two program offices, Fossil Energy (FE) and Energy Efficiency (EE). The intermetallics program, one of the largest, continuously-funded development programs undertaken at ORNL, has received more than $21 million since 1982. The funding profile for the DOE portion of the program is shown in Figure 2-1. In addition to the involvement of DOE organizations, the program has been supplemented by collaborations and partnerships with industry as well as by collaborative efforts with the Office of Naval Research (approximately $100,000 in both 1983 and 1984), universities, and other national laboratories. Figure 2-2 presents a time line of these interactions. Industrial interactions are described later in this chapter.
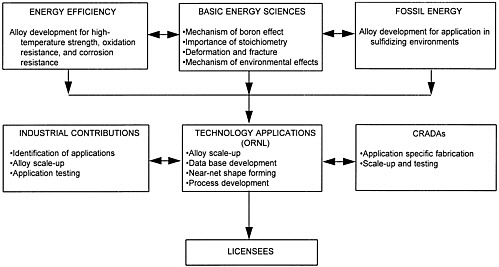
ORNL has been the lead laboratory for intermetallics research and development funded by the three DOE program offices, in collaboration with universities, industry, and other national laboratories. Figure 2-3 shows the integration of research projects and the development of mechanisms of technology transfer.
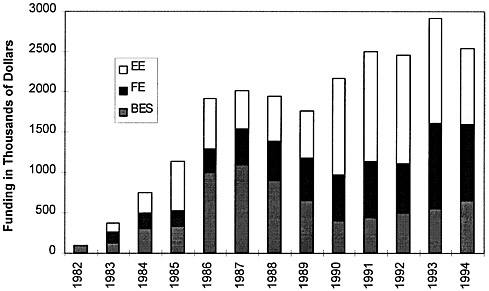
FIGURE 2-1 Profile of funding (in then-year dollars) by Basic Energy Sciences (BES), Energy Efficiency (EE), and Fossil Energy (FE) offices of DOE. Source: ORNL.
Oak Ridge National Laboratory Exploratory Studies Program
The original "seed" funding for intermetallic alloy research was provided in June 1981 through an ORNL-sponsored exploratory study. The objective was to develop a new class of structural materials for advanced energy conversion systems by developing ductile intermetallics using microalloying processes. Thus, from the beginning, the purpose of this program was not just to investigate interesting questions of materials science, but also to identify and facilitate industrial applications for the new materials.
Basic Energy Sciences
Based on the promising scientific results of the ORNL exploratory program, a nickel aluminide development program was initiated by the Division of Material Science of BES. This new program was initiated to advance the principles for alloy design and to use this knowledge to develop improved materials for high temperature structural applications (e.g., turbine and internal-combustion engine components, process tooling). Aluminides were chosen because of their potential for excellent oxidation and corrosion resistance at high temperatures. In addition, using ductile aluminides as structural materials could reduce the nation's dependence on strategic materials like chromium. Since 1982, the focus of the BES-funded program has been expanded. For example, in 1985, the research was divided into four categories, (1) boron segregation and intergranular fractures, (2) microstructures and grain boundary properties, (3) physical properties and
defect structures, and (4) coordination of an interlaboratory program. Participants in the interlaboratory program, coordinated by ORNL, include the Argonne National Laboratory, the Idaho National Engineering Laboratory, the Los Alamos National Laboratory, and the National Institute for Standards and Technology of the U.S. Department of Commerce.
Program Offices
Starting in 1983, two DOE "mission-oriented" program offices began to fund intermetallic materials and processing technology development programs at ORNL. The sponsoring programs included:
-
the Energy Conversion and Utilization Technologies (ECUT) program, renamed Advanced Industrial Materials (AIM), of EE. The AIM program is now part of the OIT research program
-
the FE Advanced Research and Technology Development Materials Program
From 1984 to 1989, much of the research and development was funded jointly by EE and FE. The program was expanded, within the funding levels shown in Figure 2-1, to include subcontract work at several universities, including Rensselaer Polytechnic Institute (mechanical properties of hydrogen-charged materials), Vanderbilt University (wear), Lehigh University (diffusion studies), North Carolina State University (mechanical alloying), and Virginia Polytechnic Institute (grain boundary modeling). In 1985, EE and FE made substantial efforts to inform industry about the program and encourage their participation. These included a workshop at ORNL and a market study funded by Martin Marietta Energy Systems. The study, performed by SRI International, looked at the near term (one to five years) and intermediate term (five to ten years) applications of Ni3Al alloys. Piston engine exhaust valves and aircraft gas turbine components were identified as having the best market potential.
Also in 1985, a licensing agreement was executed with Cummins Engine Company for the use of Ni3Al alloys in diesel engine turbochargers. Based on the Cummins license and the results of the workshop, efforts were made beginning in 1986 to support industrial applications focused on material processing technologies, including isothermal forging and rolling, near-net-shape casting and rolling, injection molding of powder into shapes, and extrusion, rolling, and forging. For the first three years, the alloy development efforts were primarily devoted to improving the durability and fabricability of wrought alloys. However, based on the results of industrial collaborations, the focus of the program was changed in 1988 to the development of castable alloys. (Industrial interactions and collaborations are described in more detail later in this chapter.)
In 1989, FE terminated work on iron-modified nickel aluminides and focused all of its funding on Fe3Al-type alloys because these had better sulfidation resistance. FE is interested in developing new structural materials for fossil energy conversion systems like coal gasifiers and fluidized-bed combustors. The FE iron aluminides program included corrosion tests, welding tests, and other development and scale-up projects to support potential industrial applications. The EE (ECUT/AIM) program has focused on the castability, weldability, and scale-up of nickel aluminides since 1989.
TECHNICAL PROGRESS AND ACCOMPLISHMENTS
From the beginning, the research and development on intermetallics has been multidisciplinary and has included basic research to increase the understanding of alloy properties, improve alloy design and properties, develop first principles theory, investigate advanced analytical techniques for characterization, and research processing and fabrication in areas such as casting and welding. These efforts have been widely recognized in the scientific and technical community (three R&D) 100 Awards; the E.O. Lawrence Award; the Humboldt Award [Germany]; and several best paper of the year citations).
Development of Nickel Aluminide (Ni3Al)
In the early 1980s, ORNL initiated a program to increase the understanding of the generally brittle behavior of intermetallic compounds and to modify that behavior by alloying and processing changes so that intermetallic alloys would be useful as structural (load bearing) materials. The focus of the initial program was on Ni3Al, one of the few materials known to exhibit a significant increase in yield strength with increasing temperature (from ambient conditions to about 800°C). The remarkable properties of nickel-based superalloys used in aircraft turbine engines result from the presence of Ni3Al. These attractive attributes provided the impetus for the development of Ni3Al alloys as structural materials for commercial applications.
Alloy Development
The initial results of the ORNL studies confirmed (as was known from the literature) that at room temperature single crystals of Ni3Al were quite ductile. The results also showed, however, that polycrystalline samples were brittle, with the failure being almost completely intergranular. In addition, polycrystalline samples exhibited a severe loss of ductility at intermediate temperatures when exposed to oxidizing atmospheres (Liu and Sikka, 1986). Reduced ductility was apparent at about 300°C, with a ductility minimum at about 750°C.
The next step in the research process (in the mid 1980s) was to develop and validate ways to improve the room- and intermediate-temperature ductility of Ni3Al (Liu, 1993). It had been previously reported that the presence of a small amount of boron (500 to 1000 ppm, by weight) in the alloy made the polycrystalline material fully ductile at room temperature (Aoki and Izumi, 1979). The ORNL team investigated this phenomenon extensively and was able to show that the ductilization effect was most pronounced in a slightly substoichiometric composition (23 to 24 atomic percent aluminum) and did not occur in a stoichiometric or a superstoichiometric composition (Liu et al., 1985). They also found that the boron was strongly partitioned to the grain boundaries, from which they inferred that the strong segregation of the boron lowered the interfacial free energy of the boundaries. In addition, they showed that the addition of chromium (about 8 percent) to Ni 3Al strongly ameliorated intermediate-temperature oxygen embrittlement, presumably by encouraging the formation of an impenetrable surface oxide layer (Liu and Sikka, 1986).
Following these discoveries, the Ni3Al intermetallic alloys were strengthened using conventional alloying techniques to produce alloys with sufficient strength to be considered as replacements for currently used materials (e.g., Fe-Ni-Cr steel alloys). The Ni3Al alloy was solid-solution strengthened by the addition of molybdenum, zirconium, and/or hafnium. Zirconium and hafnium were also found to improve castability and weldability. Finally, either carbon or additional boron was added to provide strengthening through the presence of carbide or boride dispersions.
ORNL scientists developed a number of Ni3Al-based alloys that exhibited a good balance of properties in the wrought condition. As is described later in this chapter, some of these alloys were modified to make them more amenable to casting (Sikka, 1996). Precision-, sand-, and ingot-casting processes are considered the best methods for fabricating components from nickel aluminides.
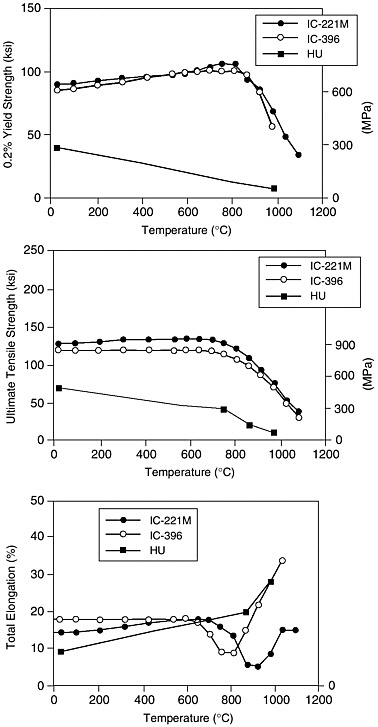
Two Ni3Al alloys now receiving focused attention in the ORNL program are IC-221M and IC-396M. The alloy compositions for these alloys are shown in Table 2-1. Figure 2-4 shows mechanical properties, including yield strength, ultimate strength, and elongation, and their variation with temperature compared with an Fe-Ni-Cr steel alloy (alloy HU) that is currently used in high temperature industrial applications. The Ni3Al alloys have superior strength and high temperature property retention compared with the current material. Both Ni3Al alloys have similar yield strength values from room temperature (590 to 620 Mpa [85 to 90 ksi]) to 926°C (565 to 590 Mpa [82 to 85 ksi]). Both have room temperature ultimate strengths in the range 830 to 900 Mpa (120 to 130 ksi). However, IC-221M exhibits an increase in strength to 930 Mpa (135 ksi) at 704°C; the strength of IC-396M decreases slowly with temperature to 810 Mpa (118 ksi) at 704°C. Above 704°C, the strength of IC-221M decreases more rapidly with temperature than IC-396M (e.g., at 926°C, the ultimate strengths of IC-221M and IC-396M are 655 Mpa (95 ksi) and 600 Mpa (87 ksi), respectively). Both alloys
TABLE 2-1 Ni3Al Alloy Compositions
|
|
Composition (weight percent) |
||||||
|
Alloy Number |
Ni |
Al |
Cr |
Mo |
Zr |
B |
Remarks |
|
IC-50 |
88.08 |
11.3 |
– |
– |
0.6 |
0.02 |
wrought |
|
IC-218 |
83.1 |
8.7 |
8.1 |
– |
0.2 |
0.02 |
wrought |
|
IC-221M |
81.1 |
8.0 |
7.7 |
1.4 |
1.7 |
0.008 |
cast |
|
IC-221W |
79.80 |
8.00 |
7.70 |
1.50 |
3.00 |
0.003 |
weld filler wire |
|
IC-396M |
80.42 |
7.98 |
7.72 |
3.02 |
0.85 |
0.005 |
cast |
|
Fe-Ni-Cr (HU) |
39.0 |
– |
18.0 |
– |
– |
– |
wrought, contains 0.55% C, balance Fe |
|
Source: Sikka, 1993. |
|||||||
show adequate ductility at room temperature (14 to 18 percent), although IC-221M exhibits a rather serious minimum in ductility (5 to 6 percent) at 871°C to 926°C. IC-396M exhibits a less serious minimum in ductility (9 percent) at 760°C to 815°C. Impact resistance values as measured by a Charpy impact test for both alloys are above 40J (30 ft-lb) from room temperature to about 593°C. The testing that has been completed was performed to provide a basic understanding of materials response in potential industrial applications, such as hot metalworking tooling, forging dies, and furnace fixtures (Sikka, 1993). Additional testing is needed to provide engineering properties, like creep, fatigue behavior, and fracture toughness, that would enable expanded use of Ni3Al alloys in more critical structural applications.
Recent alloy development at ORNL (in the late 1980s and early 1990s) has been focused on a more complete understanding of the causes of low room-temperature and intermediate-temperature ductility. Previously, it had been widely assumed that the brittle behavior of intermetallics was a result of their covalent bonding. However, some stoichiometric compounds, such as Fe3Al and single-crystal Ni3Al, are ductile at room temperature. In a series of recent experiments, ORNL researchers have shown that low room-temperature ductility was caused by the presence of ambient moisture and that specimens tested in dry gases or high vacuum were fully ductile (Liu, 1992). They later showed that moisture in the air reacted with aluminum in the alloy to produce atomic hydrogen, which diffused rapidly through the grain boundaries and caused the materials to become brittle at room temperature (Takeyama and Liu, 1992). In addition, intermediate-temperature embrittlement was shown to be a dynamic, oxygen-induced ductility loss caused by the presence of oxygen in the environment. These experiments, which were designed and performed by ORNL scientists, have resulted in a more complete understanding of the embrittlement phenomena in Ni 3Al. Additional fundamental research is needed to determine the mechanisms of ductility enhancement provided by alloying elements so that alloy composition can be further optimized.
Process Development
In addition to the alloy development described in the previous section, significant progress has been made toward the development of manufacturing process technologies for intermetallics. The ORNL process development program includes melting, casting and casting modeling, investment casting (including mold development and alloy modifications for better casting), mechanical working, rolling, and welding. Although the ORNL investigations began as a development program for wrought alloys, the investigators shifted the emphasis of the program toward the development of casting alloys and processes in response to the desire by Cummins Engine Company, an early licensee, to produce cast components. The study of the interactions between Ni3Al alloys and various casting mold media was required for the development of a compatible mold-metal system. Alloy compositions were subsequently modified to make them more castable. As a result, alloys that can be sand and investment cast are now available (Sikka, 1996). The resulting castings show considerable promise as forging dies and as hardware for heat treatment furnaces.
Two process developments are particularly noteworthy—an exothermic melting (Exo-melt®) process and welding processes. Melting Ni3Al-based alloys is difficult because of their high aluminum content and because of the great difference between the melting temperatures of the two main alloy constituents (Ni and Al). The result is that it is difficult to avoid the loss of aluminum at the temperature required to melt the nickel, zirconium, and molybdenum. ORNL scientists developed the Exo-melt process to address this problem (Sikka, 1996). The Exo-melt process uses the heat generated in the exothermic intermetallic formation reaction to melt the remaining alloy constituents and achieve good mixing of the components in a very short time. This process requires less heat than conventional melting processes, resulting in a savings of about 50 percent in both time and energy.
The second significant process development program concerned welding and weld repair processes. Many castings have surface defects that have to be repaired before the castings are ready for service. Also, it is often necessary to fabricate large, more complicated structures from smaller components. Welding is generally used for the structural and cosmetic repair of castings and is often used for the cost-effective fabrication of complex structures from smaller components. ORNL has conducted a comprehensive, 10-year program to develop knowledge and expertise in welding technologies including a basic understanding of melting and solidification processes for Ni3Al alloys along with the development of production-scale welding process methods (David and Santella, 1996).
Other Intermetallics
Based on the progress of ORNL investigators with Ni3Al alloys, the program was expanded to include the study of other intermetallic materials, including NiAl,
Fe3Al, FeAl, TiAl, Ni3Si, and Cr-Cr2Nb. Not surprisingly, each of these intermetallics was found to have unique characteristics. Applying lessons learned from work on Ni3Al was not generally useful for other compounds because they did not have comparable characteristics. For example, the addition of a small amount of boron strengthened FeAl but did not improve ductility as it had for Ni3Al.
Iron aluminide alloys are remarkably oxidation and sulfidation resistant and are therefore of great interest to the chemical and fossil fuel industries. The investigation of Fe3Al materials and process technology has been part of the ORNL intermetallics program since 1988. In that time, a family of Fe3Al alloys has been developed that includes alloy formulations optimized for room temperature ductility (5 percent chromium), sulfidation resistance (2 percent chromium), and fabricability (low aluminum content) (Liu, 1993; Sikka et al., 1993a). Although the mechanical properties of Fe3Al alloys are significantly inferior to Ni3Al, they are competitive with the ferritic and austenitic steels they are intended to replace.
In addition to alloy development, ORNL investigations of degradation mechanisms have shown that Fe3Al alloys exhibit the same kind of moisture-induced embrittlement at room temperature as the Ni3Al materials (Liu et al., 1990; McKamey et al., 1991; McKamey, 1996; Sikka et al., 1993b). Although progress has been made in the development and characterization of Fe3Al alloys, optimizing alloy composition and developing manufacturing process technology must still be done before these materials can be considered for production applications.
The ORNL investigators have also studied TiAl materials, which are of great interest within the research community because of their relatively low density and their potential for high strength. Previous work by other laboratories had shown that substoichiometric aluminum compositions were much stronger than near-stoichiometric compositions (Huang and Hall, 1991), that they were strengthened by the addition of a small amount of tungsten (Martin et al, 1983), and that adding silicon was likely to strengthen them and enhance oxidation resistance (Tsuyama et al., 1992; Kasahara et al., 1990). The most significant development by the ORNL program in the investigation of TiAl alloys resulted from a processing scheme that yields a unique microstructure with a refined colony size and ultrafine lamellar spacing. The resultant material is a TiAl-based alloy with a 1000 Mpa (145 ksi) yield strength and good creep/stress rupture properties. However, the long-term stability of this microstructure under applied load has not yet been established.
INTERACTIONS WITH INDUSTRY
ORNL has facilitated interactions with industry in the following ways:
-
collaborating with industrial researchers
-
encouraging personnel assignments and exchanges
-
making ORNL's unique research and development facilities available
-
conducting workshops
-
establishing cooperative research and development agreements (CRADAs)
-
licensing
-
subcontracting research and development
Since the program's inception in 1982, ORNL has established more than 200 research and development collaborations with academia, industry (more than 100), and other national laboratories; organized almost 40 symposia and meetings; and sponsored ORNL research involving 15 postdoctoral fellows and 25 graduate students. CRADAs and licenses have motivated efforts to further the commercialization of Ni3Al alloys. Companies that enter into CRADAs feel that they benefit from the expertise of ORNL researchers, leverage their investments (which makes them more likely to participate), and to some extent protect intellectual property and proprietary information. Carrying out application-specific development under the terms of a CRADA before manufacturing and/or licensing is attractive to industry. An example is the GM Delphi Saginaw project described later in this chapter, which began with a CRADA.
The licensing strategy now being followed by ORNL has evolved since 1985. The first license agreement, signed in 1985 with Cummins Engine Company, was for the use of Ni3Al alloys in diesel engine components. The focus was on lower temperature (760°C to 982°C) diesel engines rather than on turbine engines. The protection was exclusively for that specific use and allowed for sublicensing to material suppliers. Other licenses issued by ORNL related to Ni3Al alloys included:
-
Hoskins Manufacturing Company for heating elements (1987)
-
Metallamics, Inc., for molds and dies (1988)
-
Armco, Inc., for mill products (1988)
-
Valley-Todeco for aircraft fasteners (1988)
-
Harrison Alloys for heating element wire (1991)
The emphasis of ORNL's current licensing strategy is different in that the current strategy maintains the protection of intellectual property, allows for the termination of inactive licenses, is nonexclusive as much as possible (exclusive only when necessary as an incentive to commercialization), tries to cultivate new licensees and nurture markets for advanced materials, and helps market the technology by using CRADAs and supporting research and development to assist licensees and potential licensees in developing Ni3Al products.
Cummins Engine Company
An early, exclusive license for a specific application with Cummins Engine Company was finalized in 1985 for the use of nickel aluminide alloys for turbochargers in diesel engines. However, the license has not led to the commercialization of nickel aluminides for this application, apparently because the license was formalized before the necessary processing technology had been developed. The data indicating that nickel aluminide was a good candidate for this application was developed on wrought alloys. However, the components under investigation were best produced by investment casting, and the nickel aluminide components had to be joined to a dissimilar material by welding. Because the technologies for casting and welding were not sufficiently developed, ORNL refocused on the development of cast alloys and processing rather than on wrought materials.
Casting development at PCC Airfoils, Incorporated, (along with ORNL) under the Cummin's license resolved many of the casting issues and contributed to the development of the IC-221M alloy in 1991. Further development has led to the addition of hafnium as an alloying element to improve castability, but no production applications had been realized by the time the Cummins license expired in 1992. Nickel aluminide turbochargers had been produced for testing, but problems with weight and performance have shifted the focus of the program from nickel aluminides to titanium aluminides.
Sandusky International
Another application of nickel aluminides being evaluated is for centrifugal cast furnace rolls. In a partnership with Metallamics, ORNL, and Bethlehem Steel, Sandusky International has produced nickel aluminide furnace rolls for Bethlehem Steel with a diameter of 36.2 cm (14.25 in.) and length of 685 cm (270 in.) (Figure 2-5). This is the largest nickel aluminide centrifugal casting that has ever been made. The improved oxidation resistance of the nickel aluminide alloys can reduce the incidence of surface blemishes introduced onto furnace rolls during service and thus improve the surface quality of the steel slabs being transported by the rolls. Another potential benefit could be to reduce the furnace down time required to maintain the rolls. Nickel aluminide centrifugal casting offers Sandusky an opportunity not only to diversify its product mix and take advantage of a potential market, but also to use existing equipment more efficiently.
Two furnace rolls were cast in the summer of 1993 from IC-396M alloy. ORNL funded the production of these components for evaluation at Bethlehem Steel. The rolls were connected to sand-cast nickel aluminide trunnions and introduced into a reheat furnace in 1994. Both have been used since that time in an environment of about 900°C to 925°C, the hottest zone of the furnace.
Sandusky identified several factors that affect the success of this application. One is the surface smoothness of the furnace roll. The outer diameter (OD)
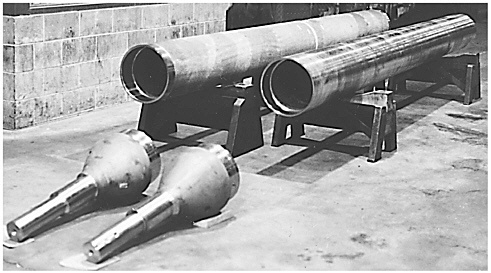
FIGURE 2-5 Cast and welded Ni3Al transfer roll (for installation at Bethlehem Steel, Chesterton, Indiana). The roll was cast by Sandusky International (Sandusky, Ohio), and the trunnions were cast by Alloy Engineering and Casting Company (Champaign, Illinois). Source: ORNL
surface of the nickel aluminide casting is not as smooth as the OD of a machined surface. However, using the roll in the as-cast condition is preferable to investing the time and expense of machining. One of the rolls now in service has an as-cast OD surface, while the other has a machined OD surface, so both conditions can be evaluated. So far, both rolls have performed satisfactorily.
Sandusky has also focused attention on the weldability of nickel aluminide alloys and the long-term performance of the welds. In this application, attaching the trunnions to each end of the furnace-roll casting was problematic. Cost considerations led to a preference for welding rather than using mechanical means of attachment. Both welding and mechanical attachments were investigated with the two rolls in service; one roll was welded to the trunnions, while the other roll was mechanically attached. Both joining approaches presented some difficulties. The welded roll had to be removed from service when cracks were found in the welds. The roll and the trunnions were secured mechanically with pins, and the roll was put back into service. The second roll had to be removed from the furnace when the pins used to attach the roll to the trunnions failed. The mechanical attachment was redesigned, and the repaired roll was subsequently returned to service.
ORNL has made significant progress in welding nickel aluminide components. For this application, Sandusky supplied parts of a centrifugally cast roll of alloy IC-221M to ORNL for the development of girth welding procedures. Industrial welding capability for nickel aluminide alloys will enhance the commercial viability of furnace rolls and be important for many other applications.
Nickel aluminide furnace rolls cost about twice as much as rolls made from steel. However, experience so far indicates that they will greatly extend the service life of the rolls. Based on the successful trials, Bethlehem Steel has ordered 32 transfer roll assemblies for delivery during 1997 to their Burns Harbor Plant.
GM Delphi Saginaw Steering Systems
Delphi Saginaw Steering Systems is investigating the use of nickel aluminides for trays and assemblies in carburizing heat-treating furnaces. The furnace assemblies (Figure 2-6) include base trays, upper fixtures, lower fixtures, and support posts. Each assembly carries about 340 kg (750 lbs) through the furnace. In comparison with the steel alloys now being used, nickel aluminides offer potential advantages of superior carburization and oxidation resistance, higher elevated temperature strength, and higher creep strength, which would reduce scheduled and unscheduled down time in heat-treating furnaces. If these advantages can be realized, an additional payoff could be savings in energy because assemblies produced with less mass require less energy to heat.
This project began in 1992 with a CRADA between Delphi Saginaw Steering Systems and ORNL. The program is being carried out in three phases. The first phase, beginning in 1992, consisted of the development of casting and molding techniques at Alloy Engineering and Casting Company (AECC), mold filling and solidification modeling at ORNL, coupon tests in a pusher-type carburizing furnace at Delphi, and the development of welding at ORNL.
The longer, second phase was an effort to evaluate trays under production conditions. A cast batch-furnace tray was successfully produced at AECC using the IC-221M alloy. This tray was used for 18 months in 1993 and 1994 in a carburizing batch-furnace, and its performance was evaluated against the performance of a conventional tray of the normally-used HU steel alloy. Both trays were exposed to approximately 1,300 furnace cycles, after which the HU tray had
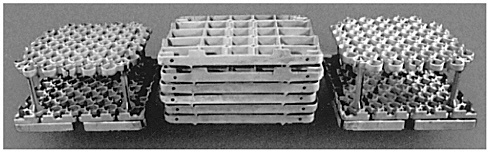
FIGURE 2-6 Ni3Al carburizing grids (cast at Alloy Engineering and Casting Company, Champaign, Illinois, for Delphi Saginaw Steering Systems, Saginaw, Michigan). Source: ORNL.
degraded to the point of failure and was removed from service. The nickel aluminide tray was in much better shape. Analyses of the tray and test coupons revealed that the nickel aluminide components were much less susceptible to carburization than the HU alloy. Also, the hardness of the nickel aluminide material was not affected, while the hardness of the HU samples increased significantly. The nickel aluminide tray will be returned to use in the furnace for additional evaluation.
In the third phase of the program, two cast nickel aluminide assemblies (IC-221M pusher trays) have been produced at AECC to be used in a carburizing furnace and compared to the current assemblies, which are made with HU material. One assembly is as-cast; the other is preoxidized. Both assemblies have been in service since January 1996 in a carburizing furnace. Six more batch-furnace trays, three as-cast and three preoxidized, have been produced by AECC for further evaluation in a batch-furnace line.
Delphi believes there is a risk in the large-scale use of nickel aluminides in production carburizing furnaces because of an incomplete understanding of the mode(s) of failure. Data from trial applications will help, but this is an area that requires further study. Based on the earlier success, Delphi has ordered 63 pusher-furnace assemblies for delivery in 1997. Although the use of Ni3Al in heat-treating furnaces looks promising, currently-used alloys are adequate, and procedures have been developed for processors to deal with weaknesses. Consequently, the rate of introduction of nickel aluminides into production applications may be slow.
United Defense LP
United Defense LP/Steel Products Division (UDLP) is committed to the commercialization of nickel aluminides (and intermetallics in general). At this time, UDLP is the only foundry licensed to melt and pour nickel aluminide-based alloys. UDLP has entered into three Ni3Al license agreements with ORNL including (1) a domestic general producer license, (2) an exclusive domestic field of use license for tooling and dies, and (3) a foreign combined general and field of use license. Both UDLP and ORNL believe that exclusive licensing to protect the licensee's initial investment from competitors is an essential incentive for commercialization. UDLP has rationalized its investment by having some exclusivity and an opportunity for a head start. In anticipation of future earnings, they have invested significantly in process development, hired scientists to focus on intermetallics, and as of May 1996, melted more than 22,690 kg (50,000 lbs) of nickel aluminide alloys. UDLP sees the role of ORNL, the licenser, as developing technology and promoting and supporting commercialization; they see their role, the licensee, as investing in the technology and producing, marketing, and profiting from products industry wants.
UDLP has received more than 250 inquiries, both domestic and foreign, and is working to develop products for many industries, including metal casting, steel, chemical, petrochemical, glass, and others. Their efforts so far have been focused on Ni3Al alloys, but there is also a good deal of interest in FeAl, Fe3Al, NiAl, and composite materials. They have identified a need for material data (for process modeling, product characterization, etc.), developing welding capability, and developing new alloys.
UDLP appears to be an effective licensee because it is a potential producer, fabricator, and end user of nickel aluminides. The company not only has melting and casting capabilities, but also mechanical presses for forging. UDLP has potential in-house applications for nickel aluminides in its heat treating and forging operations, and its parent company, FMC, may be able to use nickel aluminides in a broad range of manufacturing processes. As a producer, fabricator, and end user, UDLP has the infrastructure for integrated product development and testing.
Other Industrial Interactions
ORNL identified a number of other industrial interactions related to Ni3Al alloys that are under way. These include:
-
processing technology for sand and centrifugal castings (AECC)
-
evaluation of Ni3Al alloys for walking-beam furnace components, such as rails, support bars, and pins (Rapid Technologies, Incorporated)
-
development of walking-beam furnace parts and other components (BIMAC, an investment casting foundry, and Castmasters, a sand and investment casting foundry)
-
exploratory research in the powder metallurgy of Ni3Al alloys and possible uses, such as the production of power-cored weld wire (Ametek Specialty Metal Products Division)
-
evaluation of Ni3Al alloys for use in heat-treating trays and fixtures and for forging tooling (Wyman-Gordon Company)
Other companies working with ORNL include:
-
A. Finkl and Sons (furnace bucks and hot ingot cutting tools)
-
The Timken Company (carburizing furnace fixtures)
-
Lukens Steel (transfer rolls in steel heat-treating furnaces)
-
Chevron Corporation (tube hangers in heat recovery boilers)
-
PCC Airfoils, Incorporated (trays for ceramic mold sintering furnaces)
-
Canmet Canada (permanent molds for casting brass parts)
-
SPS Technologies, Incorporated, and Valley-Todeco, Incorporated (fastener fabrication)
-
Hoskins Manufacturing Company and Harrison Alloys (heating element wire)
-
Armco Research and Technology (supplier of cast and wrought Ni3Al alloys)
User Facility
OIT has recently established a DOE user facility at ORNL for metals processing. The facility is called the Metals-Processing Laboratory User Center. The purpose of this center is to provide industry and universities with access to technical expertise and equipment related to metals processing, joining, characterization, and process modeling. The laboratory user center provides ORNL an opportunity to work with researchers and industrial representatives to identify issues and concerns related to the industrial uses of advanced alloys.
CONCLUSIONS
This remainder of this chapter presents the panel's evaluation of the progress and accomplishments of the ORNL program to develop intermetallic alloys. The evaluation includes conclusions pertaining to program management, the conduct of the technical program, and industrial interactions. Recommendations are contained in Chapter 4.
Overall, the ORNL intermetallic alloy development program has been successful in terms of the technical goals and objectives established by the program, i.e., to develop high strength, ductile intermetallic alloys that can be processed and utilized for high-temperature structural applications. The program has been well managed, with effective integration of program elements—from basic research through production-scale demonstrations—and coordination of program goals and responsibilities among participating funding and research organizations. The program has contributed significantly to the fundamental understanding of intermetallics, the development of alloy compositions with useful ranges of properties and performance characteristics, and the development of manufacturing processes for commercial applications. The alloys have performed well in production-scale trials, but full commercialization of Ni3Al alloys has been slow. However, commercialization is the ultimate goal of the new OIT program.
Program Management
In the panel's judgment, the ORNL intermetallics program has been a successful science and technology development program for a number of reasons. These include:
-
Consistent commitment of resources. Consistent and continuous funding (since 1982) has allowed ORNL to assemble a team of outstanding researchers and to establish an international reputation in alloy and process development and characterization.
-
Effective program integration. The effective organization and management of this multidisciplinary program and the effective coordination among DOE program offices and ORNL have made the technical success of the program possible. As a lead laboratory, ORNL has coordinated the efforts of universities, other laboratories, and industry and has facilitated interactive and iterative activities between basic and applied research and development.
-
Flexibility. Throughout the history of the program, research efforts have been reoriented and refocused in response to promising results or identified needs, without compromising scientific excellence.
-
Industry involvement. Partnerships and collaborations with industry have helped the program identify industry needs and establish practical goals for technology development.
Technical Program
The ORNL intermetallic alloy development program has made significant technical advances since its inception—from basic exploratory research and characterization through process development and scaling. The early decision to focus on Ni3Al and to concentrate on optimizing alloy composition, characterizing material behavior, and developing production-scale processing methods has been critical to the success of the program.
Technical accomplishments in material characterization and in the development of Ni3Al alloy compositions include:
-
the identification of brittle grain-boundary fracture mechanisms at ambient temperatures and the substantial loss of ductility at intermediate temperatures as major material deficits
-
the determination of causes for brittle ambient-temperature fracture (moisture-induced embrittlement) and intermediate-temperature ductility loss (dynamic oxygen-induced degradation)
-
the improvement of ductility by microalloying with boron and chromium
-
the improvement of elevated temperature strength and processibility using standard alloying techniques, including solid-solution strengthening (Mo), dispersion strengthening with carbides and borides (C, B), and improving strength, weldability, and castability (Zr, Hf)
-
ORNL researchers have attempted to apply lessons-learned to other intermetallic compounds but with limited success.
In the panel's judgment, some of the most significant accomplishments of the intermetallic alloy research program have been in the development of manufacturing processes. Developments in this area include:
-
development of production-volume melting process that maintains aluminum concentration while melting higher-temperature-melting constituents (Exo-melt process)
-
development and testing of methods and alloy modifications for low-cost casting processes
-
development of materials (e.g. weld wire) and processes for structural welds and weld repairs
Interaction with industrial participants throughout the program have been critical to identifying the needs and priorities for process development.
Although considerable progress has been made, significant work must still be done to enable Ni3Al alloys to be used for commercial production, including:
-
fundamental research to determine the mechanisms of ductility enhancement provided by alloying elements so that alloy composition can be further optimized
-
characterization testing to provide engineering properties, for example creep, fatigue behavior, and fracture toughness
-
development of data to model solidification (casting and welding) processes and to establish production processing standards and the development of methods for machining and welding nickel and iron aluminides
REFERENCES
Anton, D.L., D.M. Shah, D.N. Duhl, and A.F. Giamei. 1989. Selecting high-temperature structural intermetallic compounds: The engineering approach. Journal of Materials 41(9):12-17.
Aoki, K., and O. Izumi. 1979. Improvement of room temperature ductility of the LI2 intermetallic compound Ni3Al by boron addition. Nippon Kinzoku Gakkaishi 43(12):1190-1196.
David, S.A., and M.L. Santella. 1996. Joining. Pp. 655-676 in Physical Metallurgy and Processing of Intermetallic Compounds, N.S. Stoloff and V.K. Sikka, eds. New York: Chapman and Hall.
Huang, S.-C., and E.L. Hall. 1991. Plastic deformation and fracture of binary TiAl-base alloys. Metallurgical Transactions A 22A:427-439.
Kasahara, K., K. Hashimoto, H. Doi, and T. Tsujimoto. 1990. High temperature oxidation behavior of TiAl-base alloys with additions of third elements. Journal of the Japan Institute of Metals 54(8): 948-954.
Liu, C.T. 1993. Ni3Al aluminide alloys. Pp. 365-377 in Structural Intermetallics, Proceedings of the First International Symposium on Structural Intermetallics, R. Darolia, J.J. Lewandowski, C.T. Liu, P.L. Martin, D.B. Miracle, and M.V. Nathal, eds. Warrendale, Pennsylvania: The Minerals, Metals and Materials Society.
Liu, C.T. 1992. Environmental embrittlement and grain boundary fracture in Ni3Al. Scripta Metallurgica et Materialia 27(1):25-28.
Liu, C.T., and V.K. Sikka. 1986. Nickel aluminides for structural use. Journal of Metals 38(5):19-21.
Liu, C.T., C.G. McKamey, and E.H. Lee. 1990. Environmental effects on room-temperature ductility and fracture in Fe3Al. Scripta Metallurgica et Materialia 24(2):385-389.
Liu, C.T., C.L. White, and J.A. Horton. 1985. Effect of Boron on Grain-Boundaries in Ni3Al. Acta Metallurgica 33(2):213-229.
Martin, P.L., M.G. Mendiratta, and H.A. Lipsitt. 1983. Creep deformation of TiAl and TiAl+W alloys. Metallurgical Transactions A 14A (10):2170-2174.
McKamey, C.G. 1996. Iron aluminides Pp. 351-391 in Physical Metallurgy and Processing of Intermetallic Compounds, N.S. Stoloff and V.K. Sikka, eds. New York: Chapman and Hall.
McKamey, C.G., J.H. DeVan, P.F. Tortorelli, and V.K. Sikka. 1991. A review of recent developments in Fe3Al-based alloys. Journal of Materials Research 6(8):1779-1805.
NRC (National Research Council). 1996. Coatings for High-Temperature Structural Materials: Trends and Opportunities. National Materials Advisory Board. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1993. Materials Research Agenda for the Automotive and Aircraft Industries. National Materials Advisory Board. Washington, D.C: National Academy Press.
NRC (National Research Council). 1984. Structural Uses for Ductile Ordered Alloys. National Materials Advisory Board. Washington, D.C: National Academy Press.
Sikka, V.K. 1996. Processing of aluminides. Pp. 561-604 in Physical Metallurgy and Processing of Intermetallic Compounds, N.S. Stoloff and V.K. Sikka, eds. New York: Chapman and Hall.
Sikka, V.K. 1993. Data Package on Ni3Al and NiAl-based Alloys Developed at ORNL. Oak Ridge, Tennessee: Martin Marietta Energy Systems, Incorporated.
Sikka, V.K., S. Viswanathan, and C.G. McKamey. 1993a. Development and commercialization status of Fe3Al-based intermetallic alloys. Pp. 483-491 in Structural Intermetallics, Proceedings of the First International Symposium on Structural Intermetallics, R. Darolia, J.J. Lewandowski, C.T. Liu, P.L. Martin, D.B. Miracle, and M.V. Nathal, eds. Warrendale, Pennsylvania: The Minerals, Metals and Materials Society.
Sikka, V.K., S. Viswanathan, and S. Vyas. 1993b. Acceptable Aluminum Additions for Minimal Environmental Effect in Iron-Aluminum Alloy. Pp. 971-976 in High Temperature Ordered Intermetallic Alloys V, Proceedings of the Materials Research Society Symposium, Vol. 288, I. Baker, R. Darolia, J.D. Whittenberger, and M.H. Yoo, eds. Pittsburgh, Pennsylvania: The Materials Research Society.
Takeyama, M., and C.T. Liu. 1992. Elevated-temperature environmental embrittlement and alloy design of L12 ordered intermetallics. Material Science and Engineering A A153(1-2):538-547.
Tsuyama., S., S. Mitao, and K. Minakawa. 1992. Alloy modification of gamma-base titanium aluminide for improved oxidation resistance, creep strength, and fracture toughness . Materials Science and Engineering A A153 (1-2):451-456.