6
Caustic Side Solvent-Extraction Process
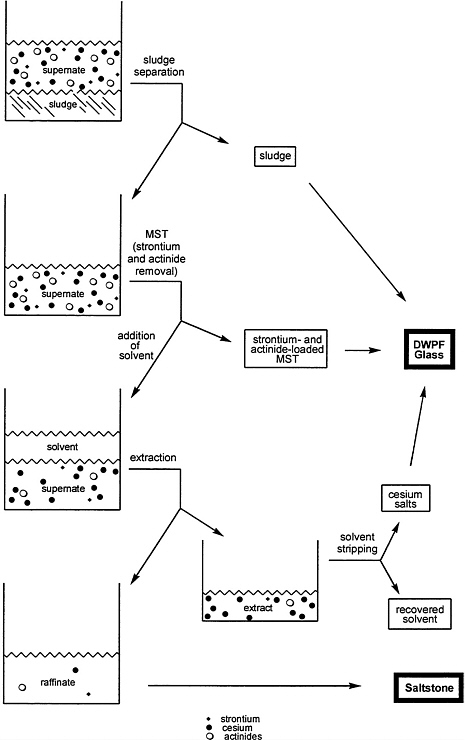
The goal of the proposed solvent-extraction process is to extract cesium ions from the aqueous supernate into a second (nonaqueous) solvent, thereby reducing the radionuclide content of the supernate to a sufficiently low level that it could be directed to the saltstone facility for disposal as a low-level waste. A schematic description of the process is shown in Figure 6.1.
A typical solvent-extraction process for hydrometallurgical operations includes several steps. First, the aqueous feed stream is contacted with an organic solvent that is immiscible with the aqueous phase. During this contact—called extraction—one or more target components undergo transfer from the aqueous stream to the organic solvent, now called the extract, while other components remain in the aqueous phase. Subsequently, the loaded solvent is sent to a scrubbing and stripping operation in which the impurities are captured and returned to the feed stream. The target component(s) are transferred to a separate aqueous stream. Scrubbing and stripping both work through extraction in the opposite direction, transferring components from the organic solvent back into an aqueous solution that may differ from the original aqueous feed in pH or concentrations of other ionic species. The stripped organic solvent can then be recycled, and the target components can be treated by whatever process is appropriate. In this fashion a process can be designed to allow continuous extraction of the target components from an input stream and produce an output stream called the raffinate that is nearly free of the target components.
For industrial and other large-scale processes (i.e., larger than laboratory scale), extraction typically is conducted in a continuous process rather than the batch process depicted schematically in Figure 6.1. The continuous extraction process—a countercurrent extraction—often employs a vertical column, with one phase moving up the column and the second (more dense) phase moving down the column. In such an arrangement, multiple stages can be employed, and very high removals of target component(s) can often
be attained. In the process proposed by the Savannah River Site (SRS), countercurrent extraction would be accomplished with the use of a version of the centrifugal contactors developed and operated at SRS since 1956, in which efficient mixing is accomplished by rotating the vessel containing the aqueous and organic phases and separating them by centrifugal force.
Solvent extraction has had a long history of successful use in the nuclear industry for operations such as spent fuel reprocessing and plutonium recovery. This experience includes exposing various organic solvents to high-radiation fields without experiencing catastrophic degradation rates. Previous experience has been mainly with aqueous feed streams that are acidic, and the key to all solvent extraction operations usually centers on the selective transfer of specific nuclides into the organic solvent.
DESCRIPTION OF THE PROCESS
According to Savannah River, the solvent-extraction process for cesium removal must remove approximately 99.998 percent of the cesium (which will require a decontamination factor, or DF, of approximately 50,000) from an aqueous, tank-waste feed stream to meet the regulatory requirements for saltstone. In the present case, the aqueous solution (produced by solvent stripping) that contains the target components—cesium ions—would be directed to the Defense Waste Processing Facility (DWPF). The cesium-free raffinate would be sent to the saltstone facility.
The use of solvent extraction for removal of cesium from aqueous tank waste at the SRS is made more difficult than from waste at other locations because of two factors. First, the SRS feed stream is highly alkaline, which renders problematic the use of the organic solvents used in many other nuclear-industry separations that employ an acidic feed. And second, the solvent must be highly selective for cesium ions as compared especially to sodium and potassium ions. All three are chemically similar, but their concentrations vary widely. The concentration of sodium ions in the supernate is typically higher than that of cesium by four orders of magnitude. The potassium ion concentration, while lower, is for many of the tanks still two orders of magnitude higher than that of cesium. Consequently, a non-selective solvent would also extract the vast fraction of the sodium and potassium ions, thereby dramatically—and unacceptably—increasing the quantity of solids to be processed in the DWPF.
Several potential challenges might be expected in developing a solvent system that is highly selective for cesium and also is thermally, chemically and radiologically stable. In addition, there is concern that insoluble material may build up at the interface between the aqueous and organic phases, a known processing issue in industrial solvent extraction that may be exacerbated by the high alkalinity of the supernate. Nevertheless, there are several potential advantages for using such a process at SRS. These include the following:
-
As a general method of separation, solvent extraction is a mature technology and has been used in the nuclear industry for over 50 years (although primarily with acidic media).
-
All processing occurs in liquid phases—an inherently simpler operation than performing a separation in a solids-containing stream—and no additional solids are formed. This contrasts with other alternatives for cesium removal: the small tank TPB process would generate additional solids that would need to be catalytically destroyed in subsequent processing, and the loaded inorganic ion-exchange material from the CST process would need to be sent to the DWPF.
-
The process would interface well with the existing high-level waste facility.
-
Solvent extraction has a wide range of operability as illustrated by its use in spent fuel reprocessing with primary feeds that have main constituents varying from aluminum to zirconium to uranium.
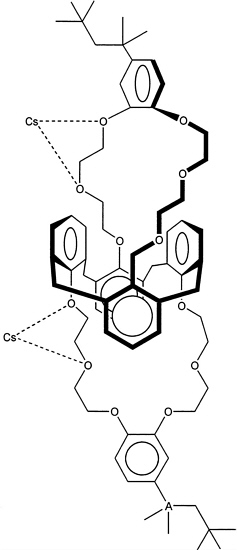
The proposed process uses a chemically complex, cesium-selective solvent system in which the cesium selective component is a calixarene crown ether, in which two crown ethers bridge the calix[4]arene component. The specific material proposed for use at SRS is called BoBCalixC6, for short. Other components in the solvent system include a proprietary modifier, a tertiary amine, and a hydrocarbon diluent. The calixarene crown ether appears to work through a combination of effects to generate a cavity that preferably incorporates cesium relative to other ions. The general structure shown in Figure 6.2 shows that there are two cavities, each of which is defined by a polyether macrocyclic ring containing six oxygen atoms capable of solvating the included ion. In addition, each cavity is further restricted in shape and size by two aromatic rings in the central belt of the molecule to which the macrocyclic ether rings are anchored.
Thorough demonstration of the process on an experimental scale that can confirm whether it should be seriously considered as a primary method for cesium removal from the SRS high-level tank waste has only been partially accomplished. The solvent extraction step has been demonstrated in a batch operation at Oak Ridge National Laboratory (Delmau et al., 1999)—but not continuously—using simulated feed streams. Originally, there was a problem with stripping of the cesium from the complex, but the source of the problem has since been identified. It can be solved by the addition of a tertiary amine to the solvent mix. A patent application (Moyer et al., 1999) has been submitted on this methodology.
Some continuous solvent-extraction studies have been performed at Argonne National Laboratory (ANL) on a simulated feed stream using laboratory centrifugal contactors (Leonard et al., 1999). These tests seem to have gone reasonably well, and the required separation performance was approached closely. Highly efficient, low-residence-time contactors such as those tested are absolutely essential in order to minimize the inventory of the very expensive solvent mixture. Scaled up versions of these contactors were installed for other purposes in the canyons at SRS and have been operating successfully for a number of years.
The latest complexing agent/diluent/modifier blend appears to be reasonably stable in the presence of radiation and the chemical environment it would encounter in the extraction process (Delmau et al., 1999). However, this recently-developed solvent system was not used in the centrifugal contactor studies at ANL described above.
ANALYSIS, FINDINGS AND CONCLUSIONS
One chief finding has been that the technical maturity of this particular solvent-extraction process lags significantly behind the technical maturities of its two competing processes. Most of the questions to be answered, however, might be called operating concerns. They can only be answered through extensive testing of the complete process. Specific questions to be addressed include:
-
Can the solvent be successfully cleaned after it becomes contaminated?
-
Will “crud” build up at the interface between the two phases (a common experience in solvent-extraction processes) and thereby limit the simple separation of these phases? If encountered during processing, this could cause an unacceptable loss of solvent as well as reduce the cesium-removal efficiency.
-
How will changes in the feed composition, pH, temperature, ratios of solvent constituents, and other operating variables affect the separation efficiency and capacity of the system? Will the extraction, stripping, and scrubbing operations be so delicate as to require especially sophisticated control systems to maintain steady-state, high DF operation?
-
Will various ions from the tank waste or other materials in the feed stream tend to build up in the solvent phase and reduce separation efficiency or reduce the carrying capacity of the calixarene crown ether for cesium?
-
Will a reliable supply (and supplier) become available for the large quantities of the calixarene crown ether that would be needed?
In spite of these concerns, the potential advantages offered by the solvent-extraction process lead the committee to conclude that it is too early to remove it from consideration and that it should remain on the list of potentially viable options.
RECOMMENDATIONS
-
“Cold” demonstration on a modest scale of the solvent extraction process should be made as soon as possible. The objectives of the demonstration should be to show that the process is adequately robust. In particular, it would need to (i) identify and solve problems such as those mentioned in the previous section and (ii) show that the required DF could be achieved for different feed compositions representative of the range expected for processing of the SRS tank waste. Other important engineering variables should also be investigated. Especially important would be the identification of “show stoppers” which could eliminate solvent extraction from consideration. During this stage, laboratory work should continue on demonstrating thermal, chemical, and radiolytic stabilities of all components of the solvent. Degradation pathways and products should be identified and these products tested for adverse effects. From this work, realistic solvent makeup volume requirements should be established.
-
Design of a hot laboratory demonstration process—using real tank waste—on a scale sufficient to define the final process should begin immediately. If solvent extraction remains in contention as a possible alternative, implementation of the hot demonstration should begin as soon as a high degree of confidence in the feasibility of the process is achieved from the cold demonstration and solvent-stability tests.
-
Work should begin immediately on defining the production capability and economics for commercial quantities of the calixarene crown ether. Purity and other specifications relevant to performance of the solvent system should be developed. Suppliers should demonstrate their ability to produce batches of solvent components that conform to specifications.