Page 34
5
In Search of New Materials
Biomaterials and biologically inspired materials at the intersections of biology, medicine, nanoscience, and biomimetics have the potential to revolutionize the design and fabric of future Army systems. “Materials” form the basis for practically all biotechnologies discussed in this report, but this chapter discusses materials to meet the Army’s needs for protection and repair, which can be divided into two categories depending on whether the application is in vivo (e.g., wound healing) or external (e.g., clothing, camouflage, armor).
Materials for in vivo use, often referred to as biomaterials, must satisfy biocompatibility requirements, such as those required by the Food and Drug Administration (FDA) in the course of its approval for safety and effectiveness for intended use. Materials for external use need not be biologically compatible, but biology is still the inspiration for their design and fabrication. Synthetic materials, soft or hard, molecular scale or macroscale, that are inspired by biology are called bioinspired materials (Aksay and Weiner, 1998).
Hybrid materials, which are neither bioinspired nor biologically produced, are engineered materials that have one component that is a macromolecule (e.g., DNA, a lipid, a polysaccharide, a polymer, or a protein). Hybrid materials include functional and structural materials, such as may be used in biosensors, and engineered hard tissues, such as bone and enamel, many of which are the result of interdisciplinary developments in biomimetics and nanoscience.
This chapter describes research and development in the private sector that will be important to future Army applications. All in all, molecular-level research in materials of all kinds will be essential to the realization of most of the opportunities highlighted in this chapter (and in this report as a whole).
BIOMATERIALS FOR IN VIVO USE
A primary goal for in vivo applications is to produce self-replicating biomaterials for wound healing that can be used to heal wounds and repair bones. Materials of choice include bioactive/resorbable/degradable polymer scaffolds, bone grafts, and other materials that might assist in the regeneration of human tissues. If regeneration is not an option, tissue engineering may provide cell-integrating artificial materials (or devices) to replace or enhance the functions of human organs.
Wound Healing
Numerous, often redundant, physiologic mechanisms have evolved for the repair or regeneration of injured tissues (see Box 5-1). During repair, injured tissues are replaced with dense, organized, connective tissues (scar tissue) that may or may not return total function. During regeneration, injured tissues are replaced with structurally and functionally normal tissues. The regeneration of injured tissues is common during fetal or newborn development; scar tissue healing is more common in adults. When the reason for the change is understood, the next stage of major advancements in the treatment of defective, injured, or missing tissues and organs is likely to follow.
A variety of biomaterials are used to treat wounds, including synthetic materials, nonsynthetic materials,
Page 35
BOX 5-1Overview of Wound Healing
Regardless of the injury mechanism (e.g., infection, trauma, surgery), several critical factors are common to the wound-healing process. These factors include an adequate blood supply to the healing tissue, resolution of associated infections, infiltration of the wound site by inflammatory cells followed by mesenchymal cells, and finally the deposition of neoconnective tissues and epithelial tissues. An adequate blood supply to injured tissue has long been recognized as vital to healing. Cupping, the practice of applying a cup heated by a flame over the site of injury, was used for centuries as a means of ensuring blood flow to topical wounds. The flame consumed oxygen, creating a vacuum and thus drawing blood in the underlying tissue toward the surface. Today, angiogenic factors are delivered to the sites of injury to stimulate the formation of new blood vessels at appropriate times and locations. Hyperbaric oxygen chambers have been devised to increase oxygen concentration for cells at the site of injury and thereby increase their viability and rate of proliferation. Acupuncture, massage therapy, and a variety of poultices have been used to create the optimal wound-healing environment, especially for recalcitrant, nonhealing wounds. Wounds have been divided according to their severity, depth, and chronicity. Each category has its own standards of care. However, the principles of cleanliness, wound covering, tissue apposition, and protection from physical trauma while healing tissues return to their normal physiologic state are applicable to all wounds. A variety of coverings are used for acute and chronic wounds. Dressings range from totally occlusive dressings, which do not allow fluid (and allow little gas) to pass from the underlying wound to the outer environment, to partially occlusive or nonocclusive dressings, which remain permeable to both fluids and gases. Dressings may or may not be carriers of antiseptic or antibiotic compounds. In general, wound coverings for acute traumatic wounds are adequate for treating infections and protecting wounds from further injury. However, there is a pressing need for wound coverings that simultaneously provide, protect, and deliver a stimulus for wound healing. Stimulation for healing is especially important for large defects when “space” must be “filled.” In natural healing, large pockets at sites of injury are filled with fluid (usually plasma and/or blood) by the host, which subsequently creates a barrier to rapid healing. Therefore, dressings that not only cover the wound, but also stimulate the formation of new blood vessels and the deposition of connective tissue would greatly improve wound care. There is an inevitable gap between in vitro phenomena, which occur under carefully controlled conditions, such as ideal concentrations of growth factors that have predictable effects on selected cell lines, and practical situations, which involve the complex of mammalian systems and a plethora of different growth factors (both stimulatory and inhibitory) in environments complicated by infection, tissue necrosis, and external extremes. Although several angiogenetic growth factors have been identified, controlling their activity in vivo remains elusive, probably because of a lack of understanding of the extracellular milieu of the growth factors in vivo. Although the sources of growth factors have been identified (e.g., endothelial cells, macrophages, fibroblasts), the mechanisms that stimulate their controlled release and the three-dimensional ultrastructure in which they naturally reside are not well understood. Therefore, it should not be surprising that growth factors attached to synthetic polymers such as polylactic acid and Marlex mesh are not particularly effective. Similarly, bioartificial membranes comprised of selected molecules, such as hyaluronic acid or purified Type I collagen laced with a variety of growth factors, usually fail to produce the desired effect when applied in clinical situations. Source: Badylak, 2000. |
resorbable materials, nonresorbable materials, and materials used as carriers for biologic agents, such as growth factors, antibiotics, and procoagulants. Each biomaterial is well suited for certain uses. However, none provides an optimal environment for wound healing. Most available biomaterials are used as temporary wound coverings that are later removed to allow the body to heal itself. Future generations of biomaterials for wound care may not only protect acute wounds, but may also set the stage for accelerated healing. Biomaterials that provide a microenvironment suitable for and conducive to physiologic phenomena, such as angiogenesis and cellular proliferation and differentiation, should be targeted by the Army for development.
Biologically produced materials will offer inherent advantages over synthetic materials for wound healing, because they may be self-healing and self-replicating. The development and use of a range of cellular growth factors, as well as stem cells, to facilitate wound healing will be important areas of focus. Engineering of human tissue, both soft and hard, will be a cell-based process that is based on an understanding of how cellular systems organize and communicate at interfaces.
The next major advances in wound healing are likely to be in biomaterials that provide an appropriate environment for immediate cell attachment, proliferation, and differentiation. These advanced biomaterials will provide an
Page 36
environment for the growth of new blood vessels, mesenchymal cell infiltration and subsequent deposition of a neomatrix, and the attachment or proliferation of cells that provide protective coverings (e.g., keratinocytes in the skin, mucosal epithelial cells at other body sites).
Identifying biomaterials or biologic agents that promote organized cell proliferation and differentiation will be an active area of research. However, these agents will have to work in concert with an optimal environment (i.e., a biomaterial) to make more than an incremental improvement because they invariably work in the complex environment of the extracellular matrix ultrastructure (Badylak, 2000).
Finally, the next generation of biomaterials for the treatment of wounds will almost undoubtedly have to be resorbable. Synthetic materials, no matter how biocompatible, function as foreign bodies in the mammalian system, which reacts to them in a variety of ways, including encapsulation, infiltration with connective tissues, and/or nonphysiologic cellular response.
Fifty-five percent of battlefield mortalities are the result of excessive bleeding; therefore, soldiers must receive immediate care to survive on highly lethal battlefields (Jette, 2000). Biological materials are now known that have excellent adhesive properties and can help stop bleeding. These include adhesives from barnacles and blood fractions, such as fibrin. Biosealants with excellent adhesive properties might be developed (e.g., by modifying protein biopolymers), and individual soldiers might carry them in their back-packs. The biosealant would act as a “super glue” to stop bleeding and hemorrhaging until the injured soldier could be evacuated to a more permanent treatment setting. However, Army-sponsored research and development will probably be necessary because the private sector is not likely to pursue the specific technologies that would meet soldier needs.
Tissue Engineering
Tissue engineering, a relatively new field, involves combining synthetic materials or structures with living cells. Several research areas in tissue engineering are described below: cartilage repair and replacement; self-replicating systems; stem cells; synthetic biomaterials; bridges between electronics and the nervous system; and portable, artificial, assisting devices.
Cartilage Repair and Replacement
One example of tissue engineering would be the repair of cartilage damage. Although this might not be a lifesaving procedure, it could substantially reduce recovery time from wounds. The technology for repairing cartilage has advanced dramatically. In vitro formations of cartilage cells (chondrocytes) have been reported (Binette et al., 1998, and Guo et al., 1989). Commercial development of in vivo injections of cartilage cells for regeneration and repair is also quite advanced, and a device produced by Genzyme Tissue Repair, a company in Cambridge, Massachusetts, has received FDA approval for injection into humans. At this point, the Army has an excellent opportunity to establish partnerships with commercial developers to ensure that battlefield requirements are met.
Self-Replicating Systems
Self-replicating systems in vitro or in vivo could benefit soldiers in the field or during recovery from wounds. Advances in in vitro biomaterials have reached the commercialization stage. The cultivation of human fibroblast cells in culture has been demonstrated, and artificially produced human skins have successfully undergone clinical trials by several companies, including Advanced Tissue Sciences, San Diego, California, and Organogenesis, Canton, Massachusetts (Gentzkow et al., 1996).
The in vitro cultivation of human skin could save the lives of soldiers with severe burns or other injuries that require skin replacement. However, with present technology artificially produced skin requires stringent low-temperature storage, which would be extremely difficult to provide under battlefield conditions. Therefore, alternative methods for long-term storage will have to be developed, probably through research supported by the Army. It may be possible for a mobile unit to cultivate skin in vitro at mass casualty sites. However, the development of supporting technologies will require significant collaboration between the Army and the private sector.
Another example of a self-replicating system would be the regeneration of bones. Scientists have shown that bone morphogenesis is controlled at the genetic level, and a bone morphogenesis factor has been isolated that can regenerate bone both in vivo and in vitro. This technology is already at an advanced stage and will someday be used to regenerate bone damaged on the battlefield. A new drug that stimulates the healing of bone fractures, called OP-1, is awaiting FDA approval. However, the relatively long time required for the remediation of medium- to large-sized bone defects, especially with collateral repair of skin damage, would limit its applicability in battlefield situations.
Revascularization might be accelerated by the growth of new blood vessels (angiogenesis) in parts of the body where blood flow has been interrupted by battlefield trauma. This approach, which would require preliminary and concurrent homeostasis to avoid irreversible bleeding, could be implemented by the local administration of angiogenesis stimulants, such as vascular endothelial growth factors, or by the implantation of cells that secrete angiogenetic proteins.
Some research is focused on culturing bone marrow cells, which manufactures blood, and it should be possible to create bioreactors in which cultured bone marrow cells can be used to produce blood. In the future, bone marrow cells could be extracted from a soldier when needed, multiplied in culture, and then returned to the patient. This technology
Page 37
might also be used to treat bone marrow cells damaged by radiation.
Stem Cells
Stem cells can replace cells that die or are damaged. Some stem cells are self-renewing and can form cells like themselves; others produce specific differentiated cells. Stem cells that produce cells like themselves (e.g., sperm cells) are called omnipotent cells. Stem cells that give rise to various types of cells in the nervous system or in the blood or immune system are called multipotent cells (e.g., neural or hemopoietic cells). Immortalized pluripotent stem cells have both characteristics; they can proliferate and form more cells like themselves, and they can differentiate into other types of cells when culture conditions are modified.
Pluripotent cells cannot make an embryo on their own, however. Therefore, they are not totipotent cells, which can give rise to unlike cells and can develop into or generate a new organism or part. Current research is focused principally on pluripotent cell lines derived from mouse embryonic germ cells (other species were found to be extremely difficult to work with). In 1998, researchers at the University of Wisconsin derived human pluripotent stem cells from early human embryos either from patients undergoing treatment for infertility or from aborted fetal material. Since then, intense research has been conducted to induce differentiation in pluripotential stem cells by regulating biomolecules or by other genetic methods. Ultimately, tissues, or even organs, could be supplied to surgeons for transplantation. Possibilities include cardiac muscle cells, pancreatic islet cells for treating diabetes, liver cells for treating hepatitis, and neural cells for treating Parkinson’s disease or Alzheimer’s disease. Many ethical questions have been raised about research on and the applications of stem cell technologies, because embryonic stem cells are currently derived from early human embryos (blastocysts). Stem cells derived from human umbilical cord blood is a promising alternative; stem cells from human adults would be the least controversial.
Animal research is likely to move ahead more quickly because transferring nuclei (i.e, combining embryonic cells with unfertilized eggs) to obtain an animal with a specified trait is already widely practiced. The genotype of the cultured cells can be changed through genetic targeting to remove or replace genes. This could lead to animals with disease resistance, for example, or animals capable of producing valuable protein biopharmaceuticals in their milk. Cloning of animals has been successful for sheep, cows, goats, pigs, mice, and other animals (see Figure 5-1). Extrapolating the trend to human body parts, however, is unlikely in the near future because many clones do not make it to full term and because the low success rate (less than 2 percent) translates into very high costs.
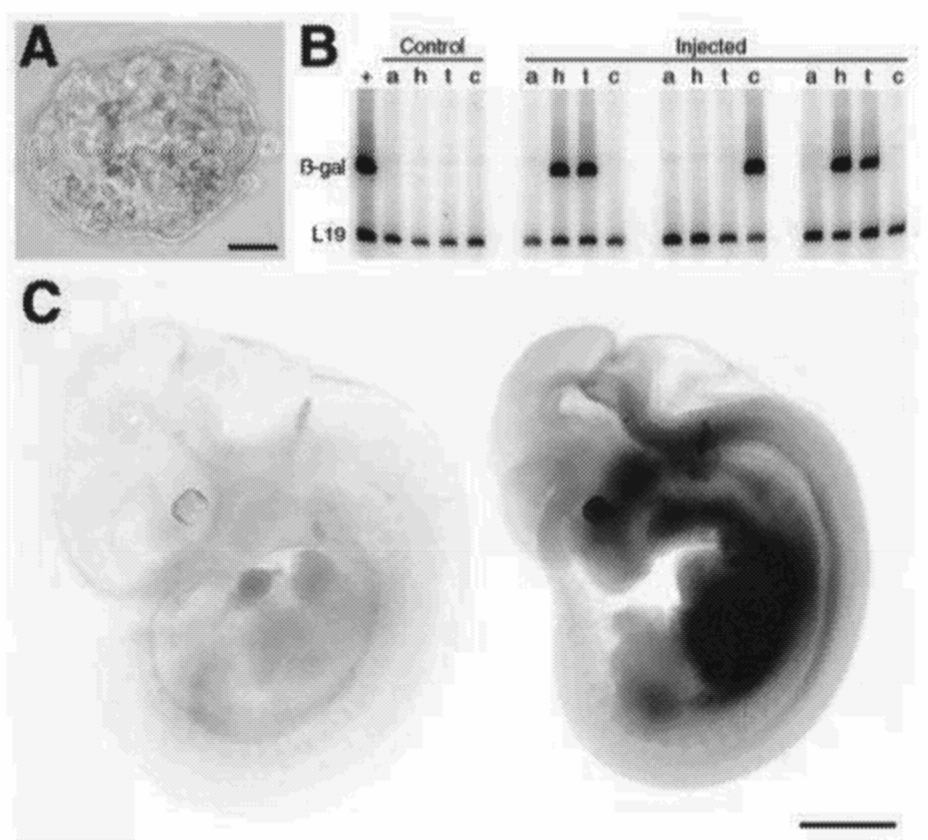
The ultimate application of stem cell research would be treating human patients directly with tissue implants, assuming that rejection could be overcome. According to a report by Clarke et al. (2000), neural stem cells from an adult mouse brain were shown to be capable of contributing to the formation of chimeric chick and mouse embryos (see Figure 5-2). This would suggest that adult animal cells are able to revert to behavior associated with stem cells.
~ enlarge ~
FIGURE 5-1 Dolly the sheep.
Source: Reprinted with permission Roslin Institute, Midlothian, United Kingdom.
Perhaps by 2025, somatic cells obtained directly from patients could be used for transfer of nuclei. Pluripotent stem cells derived from somatic cells would be antigenically identical to existing cells in the patient and, therefore, at least in theory, would not be rejected. Imagine transplants that did not require antirejection drugs or skin cells that could be rapidly regenerated to treat burn patients. However exciting the prospects, the Army should be wary of promises of immediate success but should continue to monitor technological, social, and bioethical developments. When fundamental knowledge of cell biology is improved, the possibilities can be mapped more realistically (Clarke et al., 2000; McLaren, 2000; Pennisi and Vogel, 2000).
Despite almost 30 years of active research in the field, tissue engineering to transplant islet cells destroyed by diabetes has not become a clinical reality. Low success rates in clinical trials have been compounded by the necessity of giving transplant recipients antirejection drugs that weaken the immune system and open the door to opportunistic infections or posttransplant malignant diseases. New hope has been generated by the development of a method of harvesting and injecting islet cells into the circulation in a manner that allows them to lodge stably in the liver. With this methodolology, pioneered by researchers in Edmonton, Alberta, Canada, it has been possible to render all 15 patients in a clinical study completely insulin-independent, although they are still on an antirejection regime.
Hopes have also been raised by animal experiments show-
Page 38
~ enlarge ~
FIGURE 5-2 Generation of chimeric mouse embryos. (A) X-Gal staining of a blastocyst developed in vitro from a morula aggregation experiment shows neural stem cell-derived cells in the inner cell mass. (B) RT-PCR detection of B-Gal mRNA in ROSA26-derived adult neural stem cells (+), amnion (a), head region (h), trunk (t), and caudal part (c) of a wild-type embryo (control) and embryos generated by blastocyst injection of ROSA26 neural stem cells (injected embryos other than shown in C). Primers for L19 gene were included in all reactions as an internal control. (C) X-Gal staining of an embryonic day 11 wild-type embryo (left) and a mouse embryo generated from a blastocyst into which adult neural stem cells were injected (right). Some endogenous staining is seen in the area of otic vesicle in the wild-type embryo. Bars in lower right corners denote lengths: 20 µm (A) and 1 mm (C).
Source: Reprinted with permission from Clarke et al., 2000. Copyright 2000, American Association for the Advancement of Science.
ing that implantable silicon microchips may effectively shield transplanted cells from rejection, thus opening the way for a new generation of implantable therapeutic cell bioreactors. The Army should monitor stem cell research for possible applications to trauma, the repair of wounds, and some types of cellular or organ transplants.
Synthetic Biomaterials
Another example of tissue engineering is biomaterials or compatible materials constructed of human parts, (e.g., heart valves and arteries). Development and use of these artificial devices will require a thorough understanding of biological functions, toxicity, and other factors. An understanding of surface chemistry and its implications for biological function will be essential to ensure biocompatibility. Recent advances in tailoring and characterizing surfaces at the molecular level are providing insights into how cells and tissues organize at interfaces. Continued development will lead to new methods of integrating biological and synthetic components for the generation of hybrid devices that may facilitate in vivo communications between biological events and electronic devices. Significant research in this area is already under way, and the Army should monitor developments to determine if they can be adapted to soldier applications.
One area of special interest is the transplantation of cell lines to provide an implantable biochemical factory for the production of life-sustaining or performance-enhancing molecules. Among the former are blood-clotting factors and insulin; the latter could include proteins that influence responses to fear, sleep deprivation, and fatigue. The synthetic component of these constructs is in the immunoisolating external shield, which is designed to protect them against the rejection response. Private industry is investing heavily in immunoisolating technologies (e.g., Neocrine, Inc.) thus providing the Army with opportunities for leveraging private investments.
Bridges Between Electronics and the Nervous System
For more than three decades, researchers have been exploring the coupling of electronics and the nervous system at the cellular level, both as a means of understanding neural functions and as a means of developing prostheses to mitigate, or even “cure,” a number of debilitating neural disorders (Agnew and McCreery, 1990). Considerable work is under way to develop retinal and cortical implants to restore vision to the blind, to restore movement to paralyzed limbs via functional neuromuscular stimulation, and to restore hearing to the profoundly deaf. Of these, the hearing prosthesis is the most completely developed; more than 30,000 first-generation devices have been implanted worldwide. Success rates with these implants continue to increase, enabling many patients to function normally in a hearing world. Dramatic results are expected in neural prostheses in the next decade.
As the risks and costs associated with neural implants are reduced, they may be used to increase the visual and hearing acuity of unimpaired individuals to levels well above average. Soldiers possessing these extraordinary faculties would be well suited to gathering intelligence and performing longrange reconnaissance missions. Success for some types of implants will depend on resolving biocompatibility issues. (See section Implants and Biocompatibility in Chapter 7.)
Most current neural systems use distributed arrays of individual wire electrodes (25–50µm in diameter). Future systems with higher levels of capability and more sites are likely to use micromachined electrode arrays (see Figure 5-3) (Najafi et al., 1985). After 30 years of research, three-dimensional arrays can now be formed with as many as 1,024 stimulation or recording sites spaced on 100µm–400µm centers (Bai and Wise, 2000; Rousche and Norman, 1998). With embedded circuitry, electrode sites can be positioned electronically to couple with active neurons (Ji and Wise, 1992),
Page 39
~ enlarge ~
FIGURE 5-3 Scanning electron micrograph of a multisite, micromachined, neural-recording probe. The probe size is compared to a cross section of a strand of hair at the same magnification.
Source: Courtesy Professor Ken Wise, University of Michigan.
and the resulting extracellular signals can be amplified, multiplexed, and passed to external signal processing systems. In the near future, such devices could interface to the outside world over bidirectional telemetry links that will also supply power via radio frequency coupling (Von Arx and Najafi, 1999). These arrays are potentially capable of completely instrumenting blocks of intact neural tissue while displacing less than 1 percent of the tissue volume. The hope is that these arrays will be able to record from neural networks on a very long-term basis without disturbing their normal function and will be able to insert signals into the tissue electronically. Hence, they would form a bridge between the electronic and biological worlds.
Thin-film, micromachined electrode arrays are used worldwide in acute situations (Carter and Houk, 1993), and the technology for completely implantable neuroelectronic microsystems will be available in the next decade. However, before these systems can be routinely used in prostheses, coupling between the electrodes and neuronal circuits must be made stable (Wolpaw et al., 2000). Currently, contact sites are encapsulated by cellular membranes or protein over a period of weeks or months. Although stimulating currents can pass through these layers with little effect, the films shield recording sites from the neural signals disrupting electrode-cell coupling. A variety of solutions to this problem are being explored, including improvements in site structure and site coatings based on genetically engineered polymers designed to inhibit protein adsorption and growth. Microwire electrodes have successfully recorded from neurons in vivo for periods of up to two years, permitting a simple robot arm to be controlled by signals from the motor cortex (Chapin et al., 1999; Kennedy and Bakay, 1997).
Research on neuroelectronic interfaces is significantly advancing our understanding of biological neural networks and is expected to lead to considerable progress in the treatment of neural disorders in the next decade. Although several highly sophisticated microneural probes have been developed in the past 20 years, including probes with complex electronic circuitry, significant problems with biocompatibility must still be overcome. In fact, these biocompatibility problems have limited their usefulness and applications. Long-term stability of the materials, rejection by the host cells, and loss of connections with neurons or cells still plague these promising devices. Significant improvements in biocompatibility will be necessary before the potential of these devices can be fully realized.
Page 40
Portable, Artificial, Assisting Devices
Imagine a wound received in battle damaging one or more organs vital to survival, e.g., heart, liver, lung. The development of artificial devices for treating such acute damage is in very early stages; for example, a number of artificial devices for replacing liver functions are now being tested in clinical trials. So far, questions of how these devices might be deployed on the battlefield are not being addressed. Once the Army has determined the requirements of soldiers under battlefield conditions, it should be able to capitalize on commercial developments.
Many tissue-engineered regenerative parts will certainly be available by 2025. At the same time, however, high-performance replacement parts for humans made wholly or partly of nonbiological materials may outperform tissue-engineered products. For example, a high-performance hip implant can easily last 40 years or more and may still be commonly prescribed in 2025, even if tissue-engineered products have been developed. Major trauma to the craniofacial structure, common in battlefield wounds, will also probably require non-tissue-engineered replacement products for the foreseeable future.
BIOINSPIRED AND HYBRID MATERIALS
Bioinspired materials and processes use biological principles to create synthetic analogue composites (Sarikaya and Aksay, 1995). Ideally, nanostructured organic and organic/ inorganic composites can be designed and fabricated by mimicking the processes, structures, and properties of biological materials.
Although not necessarily bioinspired, many new hybrid materials will result from biomimetics research encompassing material biosynthesis, protein selection, and other applications. Fundamental understanding of material biosynthesis includes small-particle formations (e.g., ceramics, semiconductors, and metal forming in bacteria and algae), thin films (e.g., S-layer bacteria), and shells and structures (e.g., bone, spicules, spines, dental tissues). Research on protein selection has focused on the development of genetic-engineering techniques (e.g., phage display and cell-surface display techniques) for isolation, selection, and purification of proteins for inorganic and (polymeric) surfaces. Finally, other research applications have included use of biomimetic pathways, as well as the design and synthesizing of improved (hybrid) materials.
Protein structure is closely related to protein function, which in turn is closely related to the properties of materials (see Box 5-2). New protein designs may yield materials with extremely useful properties. Materials such as bones, teeth, and shells are simultaneously hard, strong, and tough and have unique hierarchical structural motifs originating at the nanometer scale.
Nanostructured organic/inorganic materials might also fulfill the Army’s need for sensor/actuator arrays, optoelectronic devices, and medical materials. The Army is especially interested in the development of lightweight armor and other materials that can withstand the rigors of combat. Desirable materials would not only be used for weapon systems, but also for uniforms, helmets, munitions, electronics, and other critical applications.
Biocomposites
The structures of biological composites are hierarchically organized in discrete levels or scales. Virtually all biocomposite systems have at least one distinct structural feature at the molecular, nanoscopic, microscopic, and macroscopic scale. In hard materials, nature accomplishes this by growing hierarchically structured organic/inorganic composites in which soft materials (e.g., proteins, membranes, and fibers) organized on length scales of 1 nm to100 nm are used as frameworks for the growth of specifically oriented and shaped inorganics (e.g., CaCO3, SiO2, Fe3O4, hydroxyapatite) with small unit cells (~1nm) (Lowenstam and Weiner, 1989; Sarikaya and Aksay, 1992). The high-modulus inorganic phase provides stiffness while the organic phase enhances toughness. Although the principle of hierarchical design has already been applied to synthetic composites (Lakes, 1993), techniques to reduce the smallest level of hierarchy to the submicron scale are still under development. Hierarchy at the nanometer scale has led to materials properties fundamentally different from those expected based on simple rules for mixing the bulk properties of the constituents (Siegel, 1993).
Levels of structural organization are held together by specific interactions between components. For example, the structure of an abalone shell consists of layered plates of CaCO3 (∼200nm) held together by a much thinner (<10nm) “mortar” of organic template ( Figure 5-4). (Sarikaya and Aksay, 1992, 1995). Whatever the nature of the bonding between levels, adequate adhesion is required for the system’s structural integrity. Structurally organized organic surfaces induce growth of specifically oriented, dissimilar constituents catalytically or epitaxially (Sarikaya and Aksay, 1992). The structure of nacre of abalone (mother-of-pearl) is segmented laminate, a hybrid material in which the dimensions of the component phases play an important role in its impact-resistance and compressive properties.
Highly interacting levels are organized into a hierarchical composite system designed to meet a spectrum of complex functional requirements. As composite systems increase in complexity, they function at higher levels of performance (e.g., so-called intelligent materials and adaptive composite systems). A hierarchical biocomposite is more than just a material out of which larger objects can be built. It is a complete structural system in itself, and nested levels of structural hierarchy appear to yield improved dielectric and mechanical properties for particular functions (Sarikaya and Aksay, 1995; Baer et al., 1987).
Page 41
BOX 5-2Determining the Structure and Functionof Proteins
Determining the structures of new proteins is a huge project. The total output of all structural biology laboratories worldwide is currently about 1,000 new structures per year. Most new structures are determined by x-ray crystallography, which involves the basic steps shown in the figure below (Hendrickson, 1987). 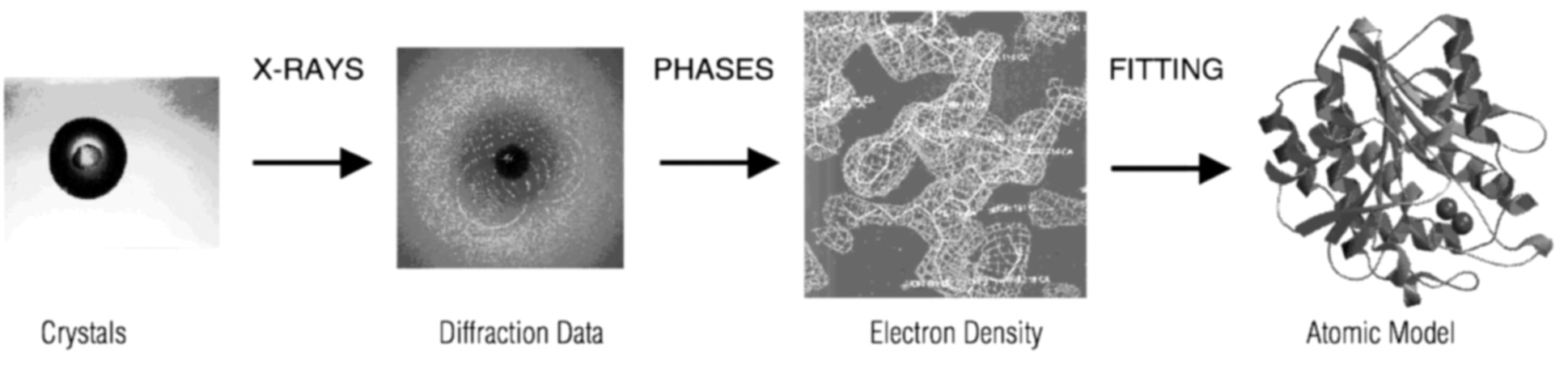
~ enlarge ~ High-throughput protein crystallography depends on several new technologies: robotics to automate protein crystallization trials; synchrotron x-ray sources to provide bright, tunable radiation; multiwavelength and purely computational approaches to overcome phase problems; and automated interpretation of electron density maps. The biggest roadblock to progress is the very beginning of the process, namely the expression, purification, and crystallization of the protein, which is both tedious and labor-intensive. Other slow steps are access to the synchrotron radiation needed for data collection at multiple wavelengths on very small crystals and the rapid interpretation of electron density maps of intermediate quality. There are only a handful of synchrotron radiation sources in the world, and these facilities are expensive to operate and in great demand (Hendrickson, 1986). In microgravity conditions, crystals can be prepared to a size and state of perfection that is otherwise difficult to achieve. Success rates for producing crystals large enough and of sufficient quality for x-ray diffraction analysis are typically less than 40 percent. Microgravity significantly reduces buoyancy-induced convection, thereby providing a more quiescent environment for crystal growth. As a result, crystals grow more slowly and, in about 25 percent of cases, they are of superior diffraction quality than their Earth-grown counterparts. The success rate and magnitude of diffraction enhancement are expected to increase when longer growth times become available via the International Space Station. Experiments on the space shuttle have demonstrated the potential benefits of microgravity. Increased diffraction resolution can often allow researchers to observe structural features that could not be seen using Earth-grown crystals. Space-grown crystals have provided information about drug-protein interactions that is vital to the design of more effective pharmaceuticals. One example of this involved the determination of the structure of human insulin complexed with a potential drug. With Earth-grown crystals diffracted to lower resolution, it was impossible to tell that two drug compounds were located in the insulin hexamer. Data from crystals grown on STS-60 allowed investigators at the Hauptman Institute to see a second drug complexed in the hexamer structure (see figure below). In addition, the overall resolution was improved considerably (more than 0.5 Å). The Hauptman Institute and a major pharmaceutical company are using this information to design new compounds in an effort to produce a longer-acting insulin formulation (Smith et al., 1996). 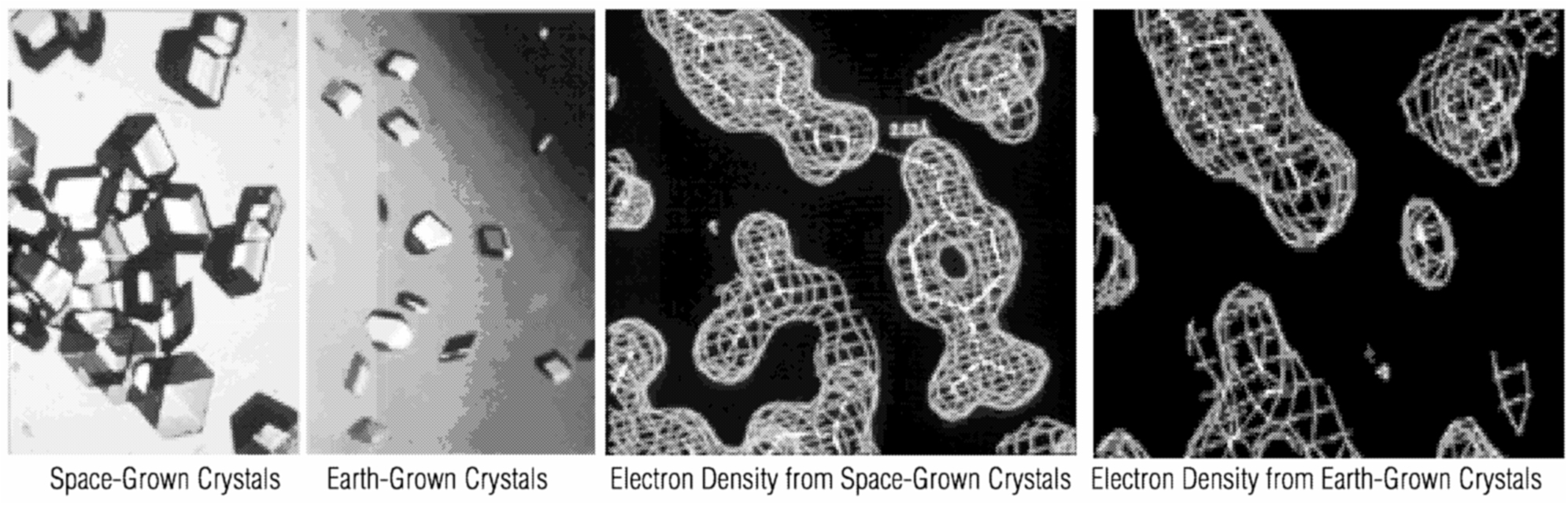
~ enlarge ~ The completion of the Human Genome Project, as well as genome projects for dozens of other species, including pathogenic bacteria and viruses, will place a significant demand on and need for crystallographic structure determinations and structure-based drug design. Given the need for methods that might improve the success rates in obtaining high-quality crystals, the microgravity environment could play a significant role in helping to address this important problem. Source: Delucas, 2000. |
Page 42
~ enlarge ~
FIGURE 5-4 Structure of an abalone shell.
Source: Reprinted with permission from Nature (Smith et al., 1999). Copyright 1999, Macmillian Magazines Ltd.
Research in hierarchical systems can provide new insight into predictions of materials performance. For example, experimental and modeling studies could be undertaken on materials and systems that have several levels of dimensional hierarchy, each dimension having separate structures and properties that are different from those at other levels. Overall, the system may have novel properties that could not be predicted using conventional approaches but could be predicted based on an understanding of cross-level interactions.
Applications of molecular biology and genetic engineering, including a large number of recombinant proteins for human therapeutics, have been focused mainly in the health care area. Many of these products, including recombinant proteins such as insulin, human growth hormone, factor VIII, erythropoietin, tissue plasminogen activator, and others, have been on the market for a number of years.
Although medical uses of biomaterials have become common, biomaterials created with recombinant DNA technology are not competitive with synthetic materials for general applications because of their high cost. This situation is likely to change in the future as nonmedical uses are found for these materials. The committee believes that biomaterials will become important for meeting the needs of the Army in 2025. Although some of these biomaterials have already been demonstrated to be technically feasible, many challenges and barriers will have to be overcome for them to be realized. A few examples, as well as some of the challenges, are described below.
Silk, a natural biopolymer, is one of the strongest fibers known on a strength-per-unit weight basis. The production of recombinant silk has been demonstrated in bacteria. Private companies (e.g., Protein Polymer Technologies, Inc., San Diego, California; DuPont, Wilmington, Delaware) and the U.S. Army Natick Laboratories have conducted research in this area. Commercial development and production of silk, however, is impractical because of costs. Silk produced by silk worms is much less expensive than recombinant protein production using a bacterial host. Nevertheless, the integration of recombinant technology with materials science and engineering appears to be a natural partnership, and the functionalities of recombinant biopolymer silk may soon surpass those of natural silk.
Professor David Tirrell of the California Institute of Technology has proposed replacing the natural amino acids in native protein polymers using both chemistry and biotechnology to generate materials with unique and added functionalities. With biological production methods, new types of polymers can be produced with well defined, selectable sequences and uniform composition. For example, modified silk may have a significantly higher strength-to-weight ratio than natural silk, increasing its utility for Army applications.
With advances in molecular biology, the sequences of protein-based polymers can be tailored to meet specific needs, and numerous copies could be produced, as desired. The ability to produce highly defined primary structures is well beyond the capability of procedures currently available in polymer chemistry. It is possible, however, that protein-based polymers with highly specific and tailored properties will have molecular weights with singular values rather than a distribution of values, as is the case with synthetic polymers. A serious challenge will be to produce large quantities of these materials so that it becomes economical to use them for specific applications, such as coatings.
Much of the organization inherent to a biological system occurs when one linear strand or chain of an entity folds into a form that features three-dimensional structure and complexity. The preferred folds are coded into the sequence of the chain by side chains with various degrees of association for one another. Although linear structures of almost any composition can now be synthesized, preparing these species
Page 43
and having them fold into structures with the desired three-dimensional structure and function can still not be done. Better computational modeling will be necessary to predict the basis of self-assembling, higher order structures.
In addition to advances in fully biologically derived materials, advances in mimicking elements of biological self-assembly will enable more combinations with synthetic materials. Research in molecular biology has produced cottage industries that can supply reagents and tags that bear a variety of biological subunits and the means to attach them to both biological and synthetic systems. These reagents provide a means to introduce characteristics of specific recognition, stimulus responsiveness, and elements of self-assembly into synthetic materials. Based on the use of biological motifs, controls over the thermal, optical, and biochemical responses of materials will soon be available. Future developments will continue to include the newest commercially available precursors, and the committee recommends that the Army inform industry of desired properties for these materials so that new designs reflect Army needs.
A major potential Army application for biomaterials is battlefield armor. For instance, on a strength-to-weight basis, the abalone shell has armor protection capabilities equal to or greater than those of existing materials. When laminated hierarchical structures of biological systems (e.g., the nacre of abalone shell) are mimicked in microlaminated ceramic-metal (Halverson et al., 1989), ceramic-organic composites (Mueller et al., 1997), or organic-organic composites (Baer et al., 1987), significant improvements in the composite mechanical properties have been observed.
Applying a simplified version of this layering to B4C/Al and other composites results in significant enhancements to their mechanical properties. The B4C/Al composites are strengthened as a result of residual stresses with nanoscale modulations in the interpenetrating network of the ceramic and metal phases (Kim et al., 1989).
Greenleaf Corporation, in Saegertown, Pennsylvania, has manufactured B4C/Al tiles for use in armor panels aboard C-130 and C-141 aircraft. The processing of these ceramic/ metal and ceramic/organic microlaminates is based on the infiltration of laminated scaffolding (e.g., ceramic) with a liquid (e.g., metal or organic polymer). The laminated composites produced by this method are similar to nacre. Although these accomplishments attest to the value of transferring lessons from biology and mimicking biological structures to create synthetic analogs, the smallest length scale in a complete system is still in the micron range because of the intrinsic limitations of the tape-casting process (Halverson et al., 1989). To capture the most important aspect of structural organization observed in biological systems, methods must be developed to process hierarchical systems with deliberately introduced designs ranging from nanometer to macroscopic dimensions.
In general, research on the biosynthesis of inorganic “biomaterials” has not been as high a priority as research in human health care. Biomaterials could have great benefits for the Army, but it may be up to the Army to demonstrate the technical and economical feasibility of biosynthesis.
Biomineralization: Organic/Inorganic Nanocomposites
The formation of bone is an example of a more general set of processes in which cells act as dynamic, local building blocks that direct the assembly of their extracellular matrix; the matrix, in turn, serves as a template for the growth and function of cells in the formation of tissues (Colognato et al., 1999; Ingber, 1997b; Schwarzbauer, 1999). The formation of bone occurs through the deposition of an inorganic phase, dahllite (carbonated hydroxyapatite), by osteoblasts on an organic template, collagen. This calcified matrix serves as the template for bone formation through the combined action of osteoblasts and osteoclasts (Erlebacher et al., 1995; Mundlos and Olsen, 1997). Bone has a number of important characteristics that could inspire materials design:
-
Complex internal structure that maximizes strength-to-weight. Bone is a sophisticated composite with several layers of hierarchical structure. In the structural phase, an inorganic material with a polymer (protein) is the matrix. The internal structure is modulated to optimize load bearing. A unique ability that may be related to this internal structure is bone’s ability to dissipate energy.
-
Adaptability. The structure of bone, like many other biological structures, is adaptive. The structure constantly remodels itself to compensate for changes in loads and stresses. On the millimeter scale, bone matrix is deposited in distinct three-dimensional patterns organized to bear mechanical loads locally. All of these mechanosensitive effects are mediated by the cells that inhabit the tissue (Ingber, 1997a).
-
Multifunctional use of internal open space. Much of the internal structure of the shaft of bone is open. In vivo, this space is largely used for microsynthesis, protection, storage of the cells of the haematopoietic system, and fuel (fat) storage. The entire bone is also permeated by a microfluidic system of blood vessels and capillaries that provide for high throughput (input and output) of chemical factors.
-
Integrated sensing. Bone contains a large number of sensors with complex control architecture to report excessive stress or damage and to control remodeling. Bone tissue, and even bone cells, are especially mechanoresponsive. Altering of stresses induces electric field (piezoelectric effect), drives internal fluid flow, modifies chemical processing rates, and changes cell growth and form.
-
Self-healing. When a bone is broken, specialized cells (osteoclasts and osteoblasts) dissolve the damaged regions and redeposit new bone. Capillary endothelial
Page 44
and smooth muscle cells that mediate the process of angiogenesis and tissue vascularization are also central to self-healing.
The processes involved in bone formation may hold the key to the development of materials with a wide range of exceptional characteristics. Materials could be engineered for Army applications as diverse as lightweight armor vehicles and “smart” clothing for soldiers.
BIOMATERIALS FOR CLOTHING AND CONCEALMENT
Biological systems can also be mimicked for the next generation of soldier camouflage uniforms. One idea is to mimic the mechanical chromatic effects that birds and fruits use. The exquisite color patterns on the feathers of birds are the result of the intricate structural pattern of each feather that enables it to diffract light. This phenomenon, mechanical chromatophores, is also exhibited by some fruits. Another natural phenomenon that might be valuable for camouflage is the biochromatic behavior of some reptiles. The chameleon, for example, can change color and patterns in accordance with the environment. Camouflage with this property would automatically change to blend with the environment, such as snow-covered terrain, desert sand, dense and light vegetation, daylight and darkness. Research in this area would require a commitment by the Army because the private sector is not likely to be very interested in these materials.
Biological means might also be useful for avoiding radar detection. Some biomolecules have long been known to be strong microwave absorbers. For example, bacteriorhodopsin has strong microwave absorptivity (3 GHz to 40 GHz). Scientists are investigating the use of chemically, and possibly genetically, modified bacteriorhodopsin protein as the active medium in microwave-absorbing paint for both tanks and planes. The absorption mechanism appears to be associated with the motion of monovalent and divalent metal cations within channels, e.g., Mg(II), Ca(II). If this theory is correct, proteins could be engineered to have precise microwave absorption bands and then fine tuned for anticipated threats in a given theater of operation. Microtubules, which are also excellent microwave absorbers, may be even better microwave absorbers and more easily fine tuned. Because much of the research in this area is classified, the committee was not able to make recommendations in this area.
The Army is always looking for ways to minimize the detection profiles of its soldiers. Because humans, tanks, and other military structures have a significantly different reflectivity than plants and trees, the enemy can easily identify military targets with inexpensive infrared lasers with wavelength-scanning capability. Even a small fraction of the target is observable because of its distinctive spectral properties. It may be possible to develop paints with terahertz and infrared reflectivity identical to trees or grass, possibly using genetically engineered plant protein as the active medium. Directed evolution could play a major role in the optimization of thermal and photochemical stability that will be needed.
Production of Biomaterials
A major barrier to the production of biomaterials is the lack of economical manufacturing processes. To overcome this barrier, agricultural biotechnology might be used for large-scale production of some new materials (see Renewable Resources in Chapter 6). For example, the protein from soybeans can be refined and sold for only pennies per pound of protein, substantially less than the cost of manufacturing equivalent synthetic polymers. The Army should include the means of production in its evaluation of the potential benefits and costs of biomaterials.
Genetically engineered crops (transgenic crops) could potentially deliver recombinant proteins directly with the food or feed products in which they are found. A recent report by Kusnadi et al. (1998) discussed the production, purification, and characterization of recombinant proteins from E. coli and chicken egg white. In this case, β-glucuronidase (GUS) and Avidin were expressed in transgenic corn seed and recovered from the seed. The Avidin made up 5.7 percent of the extractable protein, GUS was 0.7 percent of the extractable protein. Biochemical properties of these proteins were reported to be similar to those of the respective data proteins (derived from E. coli or from chicken egg white). The approach has significant potential to generate proteins cost effectively in food crops and might be used on a large scale. Given the potential for this technology to produce enhanced foods as well as specialty (therapeutic) proteins on a large scale, the Army would be well advised to monitor developments in this field.
Manufacturing Trends and Barriers
The production of large-volume, low-cost materials is increasingly based on synthetic chemistry. Although biotechnology may be cheaper and more efficient in some cases, in other cases chemical synthesis of biological materials is sometimes a better option because it can be done using the existing manufacturing infrastructure. Therefore, decisions about manufacturing processes, especially for processes that require synthetic chemistry, should include an evaluation of cost and efficiency. For example, in the production of silk or other protein polymers, biotechnology might be more cost effective than chemical synthesis.
A significant barrier to the development and use of biomaterials is that many will be considered to be, and evaluated as, medical devices by the Food and Drug Administration. Meeting FDA requirements could significantly add to development time.
Page 45
KEY RECOMMENDATIONS
The Army can best use its resources by funding basic research that addresses major impediments to the use of biomaterials, rather than for specific developments. For example, advances in understanding the effects of hierarchical structure and molecular properties on integration with biological interfaces will contribute to accelerated developments of implantable sensors, to the robustness of prosthetic devices, and to the manufacture of ultrastrong, resilient, lightweight materials for uniforms, armor, and protective gear. This research in biological interfaces will also benefit biomedical applications for advanced biomaterials for use in the treatment of wounds and trauma.
Exploiting wound-healing applications of biotechnology is of paramount importance for improving the survivability of soldiers in battle. The Army should support research and developments in wound healing in collaboration with the commercial sector to ensure that areas pertinent to battlefield conditions and postbattlefield recovery processes are addressed. Near-term projects should focus on technological barriers to the manufacture and storage of self-replicating systems, such as those used to construct human skin; the results can then be adapted for battlefield conditions. The Army should also take the lead in developing treatments for reducing the incidence of shock resulting from wound trauma; this should be a high priority for research.
Assuming that societal objections can be surmounted, research on the use of stem cells to advance developments in tissue engineering should be supported. The Army should monitor global developments in stem cell research, particularly on human adult stem cells.
The Army’s use of biochips and implantable sensors will require that these technologies be stabilized on a material substructure. For this reason, development of biocompatible materials with the properties necessary for the substructure is imperative. This area of research is especially important because the surface is the only part of the material that will interact with a biological milieu. Future engineering developments will depend heavily on reliable, robust, compatible surfaces.
Biological systems, which are models of hierarchical design with multiple functions, could be considered paradigms for the development of synthetic biomaterials. Methods of capturing the structural organization of biological systems have not been developed. The Army should support research on methods of processing hierarchical systems with deliberately introduced designs, ranging from nanometer to macroscopic dimensions, to accelerate advances in tissues in treating wounds, protective clothing, and other battlefield materials.
Such developments could also address a persistent concern expressed in briefings to the committee by the Army about reducing the 92.6-lb weight that soldiers must bear as their combat equipment load (Jette, 2000; Kern, 2000). Clothing contributes to this weight burden, and biotechnology can be used to reduce its weight and increase its utility for protection and camouflage in combat.