Page 93
6
Advanced Engine and Fuel Systems Development for Minimizing Carbon Dioxide Generation
James A. Spearot
General Motors Corporation
It's a pleasure to have the opportunity to participate in this “roundtable” and to provide General Motors' perspectives on the issues and research opportunities related to the concept of carbon management.
The purpose of this chapter is not to discuss the science of global warming or to take part in a debate over predicted ambient temperature increases. General Motors' (GM's) position on these subjects has been presented before. Specifically, “We believe there is enough cause for concern to warrant responsible actions to reduce global greenhouse gas emissions.” Since reductions in carbon dioxide emissions are an essential part of this objective, I would like to discuss some of the options available to us in developing advanced engine and fuel systems for minimizing CO2 generation.
The objectives here are the following:
-
Review potential changes in energy utilization during the next century.
-
Review research goals and promising vehicle technologies for reduced CO2 generation from light-duty vehicles.
-
Identify future transportation fuel needs, particularly if vehicles are to produce no net increase in CO2.
-
Summarize probable engine and fuel systems for the twenty-first century.
I would like to begin by providing a historical perspective. The complex issues related to selecting an optimum engine and fuel system are not new.
“Fuel today is a subject that is engrossing the minds of many men with an investigative turn. It is a subject, too, that requires quite a bit of figuring, for it is a serious problem.”
“There are a number who are working out new methods of producing gasoline, as well as inventing new liquid fuels. Each is absolutely sure his method will eventually solve the present problem, opening up to the motoring public a source of cheap fuel that will last for many, many years to come.”
The author of these quotes was Charles F. Kettering, the founder of the Dayton Engineering Laboratory (DELCO) and of General Motors Research Laboratories, as published in the DELCO magazine in 1916.
Page 94
Although these quotes were written early in the twentieth century, they provide a fairly good summary of where we are at the beginning of the twenty-first century. The technical issues related to operation of engine/fuel systems are different, the societal issues driving change are different, the sophistication of engine and fuel technology is much greater, but the search for better engine and fuel systems continues.
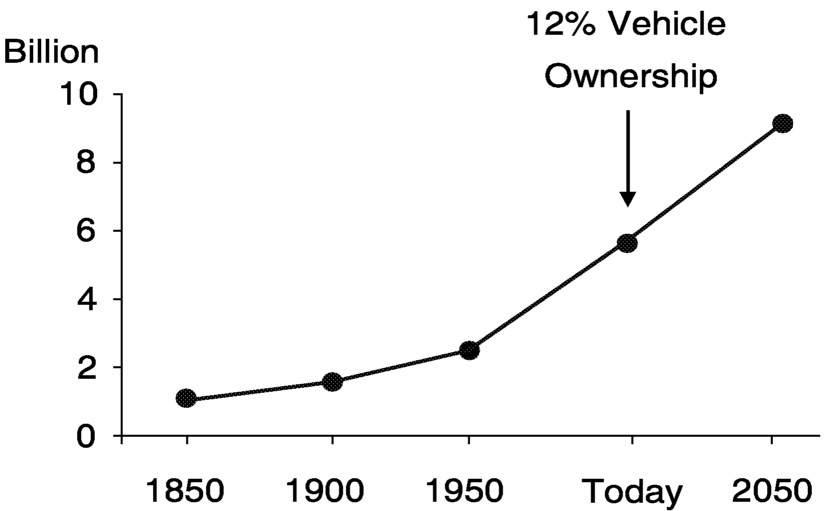
Today, Earth's population tops 6 billion people and more than 700 million cars and trucks are in use ( Figure 6.1). This means that only 12% of us are realizing the benefits provided by engine-powered vehicles. No other transportation technology gives us the freedom to go wherever we want, whenever we want, with whomever we choose, carrying whatever we need. This connectivity is what drives the nearly universal aspiration people have for “auto-mobility.”
To satisfy this demand in the future, we must realize sustainable auto-mobility. With the world's population predicted to reach 7 billion by 2015, the global car park will grow to nearly 850 million vehicles over the next 15 years if the ownership rate remains at 12%. While this represents significant growth in the demand for our products, as an industry we need to be thinking bigger than this. If the ownership rate increases just 3 points—to 15%—the car park in 2015 would exceed 1 billion vehicles.
We will not be able to take advantage of this market opportunity unless auto-mobility is truly sustainable. Energy forms that are renewable and vehicle technologies that have zero impact on the ambient environment will be required for society to continue to enjoy and expand the benefits of personal mobility.
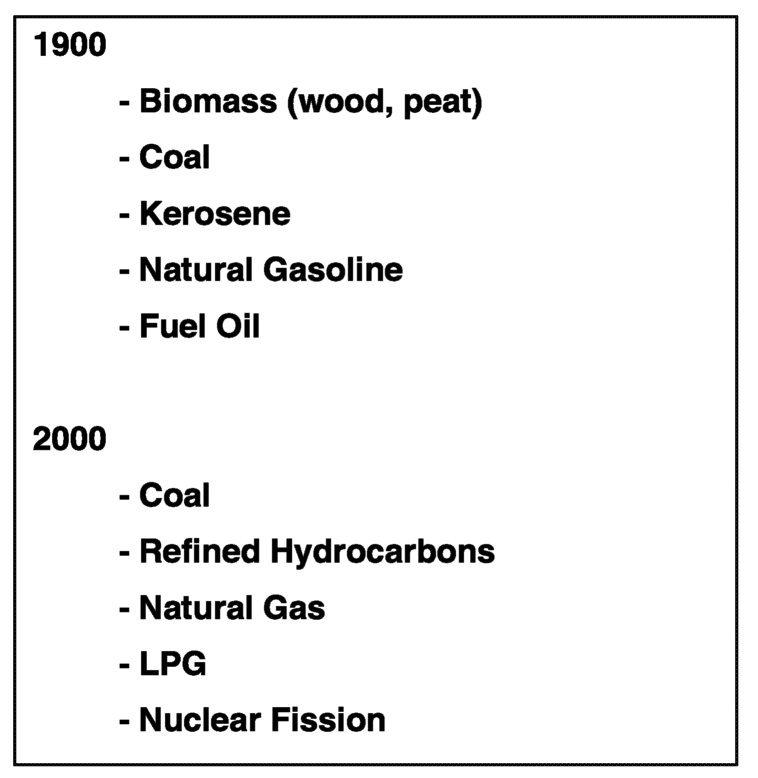
One way to understand how the nature of supplied energy for both mobile and stationary sources might change during the next 100 years is to compare the primary energy forms used at both the beginning and the end of the twentieth century ( Figure 6.2). Although the changes might not appear substantial at first glance, the transition in energy forms has been significant. Why did the shift occur? It occurred because human needs for greater amounts of practical energy and reduced environmental impact demanded the shift and technological advances enabled the changes. As a result, society is better off due to the transition that took place.
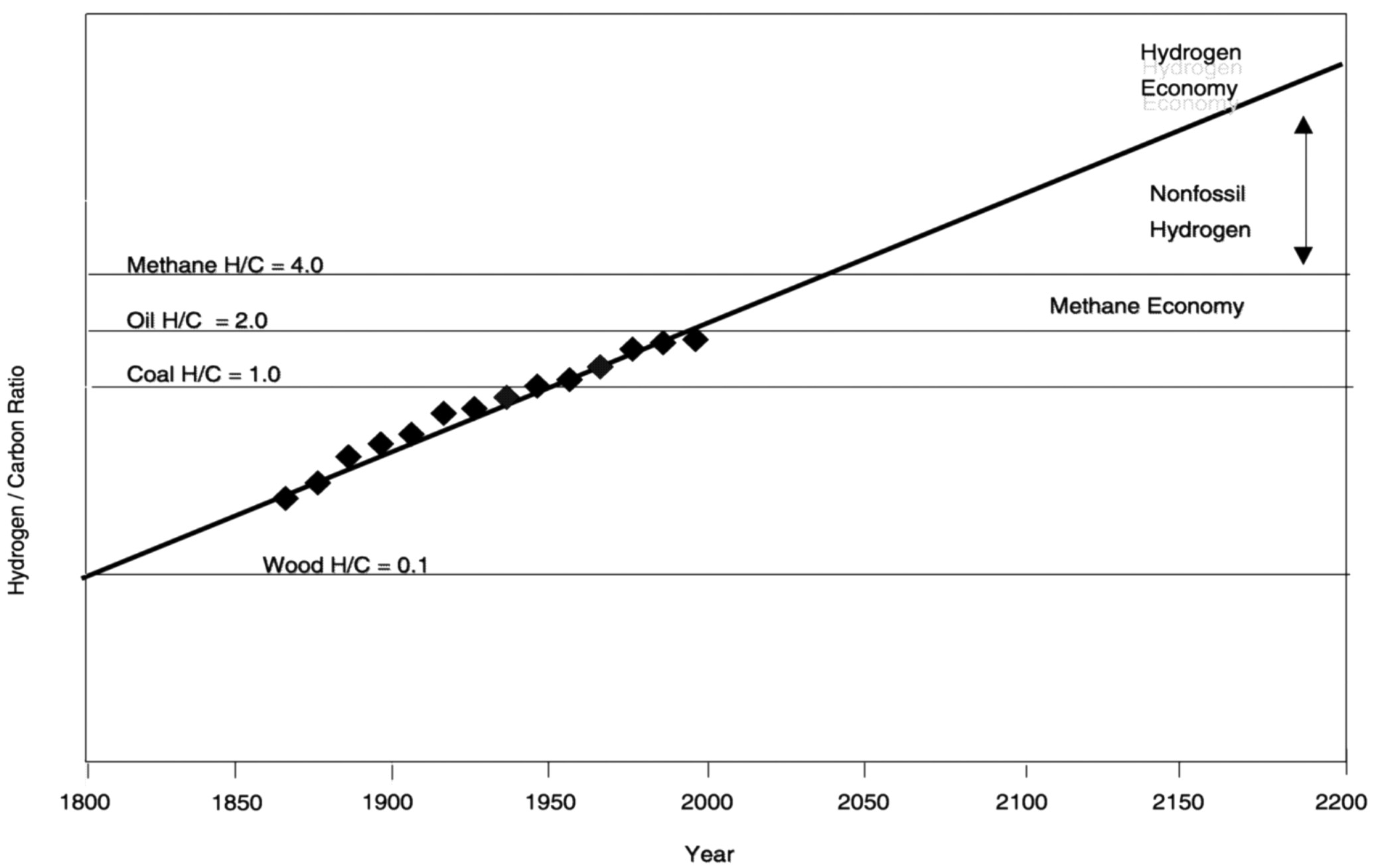
Where are we headed in the next 100 years? My version of Figure 6.3 was published in 1996 in Daedalus, the journal of the American Academy of Arts and Sciences. A figure from the paper suggests that the calculated hydrogen-to-carbon (H/C) ratio of total energy consumed in the world is increasing at an exponential rate. Although this assumption can surely be debated, I believe the important point of this figure is the trend in the data, not the mathematical relationship used to fit the trend. General Motors believes this trend will continue well into this century, culminating at the point of its implied conclusion—a hydrogen economy. Extrapolating the data in this plot 100 years into the future takes “fortune-
~ enlarge ~
FIGURE 6.1 World population and vehicle ownership.
Page 95
~ enlarge ~
FIGURE 6.2 Energy sources at the beginning and the end of the twentieth century. NOTE: LPG = liquefied petroleum gas.
telling” to new heights. Yet, it is my contention that if carbon emissions are to be mitigated or managed, the auto and the energy industries will have to work together to make this prediction a reality. The concept of a renewable hydrogen economy and, specifically of hydrogen being used for transportation energy offers the promise of sustainable auto-mobility.
One method for reducing carbon emissions is by improving the efficiency of future vehicles and propulsion systems. However, this statement begs the question, What will become the dominant engine technology in the next century? Only the marketplace can answer this question, but several clues on possibilities for future engine technologies can be obtained from the efforts of the auto industry-government program Partnership for a New Generation of Vehicles (PNGV).
There are three research goals ( Figure 6.4) for the PNGV program, which is looking at the most advanced technologies aimed at improving the energy efficiency of the transportation fleet. The first is the development of new manufacturing techniques to reduce the costs of advanced automotive technologies and improve the efficiency of vehicle manufacturing operations. The second is to develop and introduce new fuel efficiency and emissions reduction technologies on current design vehicles as quickly as possible. The third, and most ambitious-goal is the one most people focus on: to develop a new class of vehicle that provides up to three times the fuel efficiency of today's family sedan. The term “comparable vehicle” means that in addition to providing three times the fuel efficiency of today's cars, this new class of vehicle must also provide the same performance, load-carrying capacity, and range. These conditions must be in place while meeting applicable safety and emissions standards at the adjusted cost of a 1994 midsize sedan.
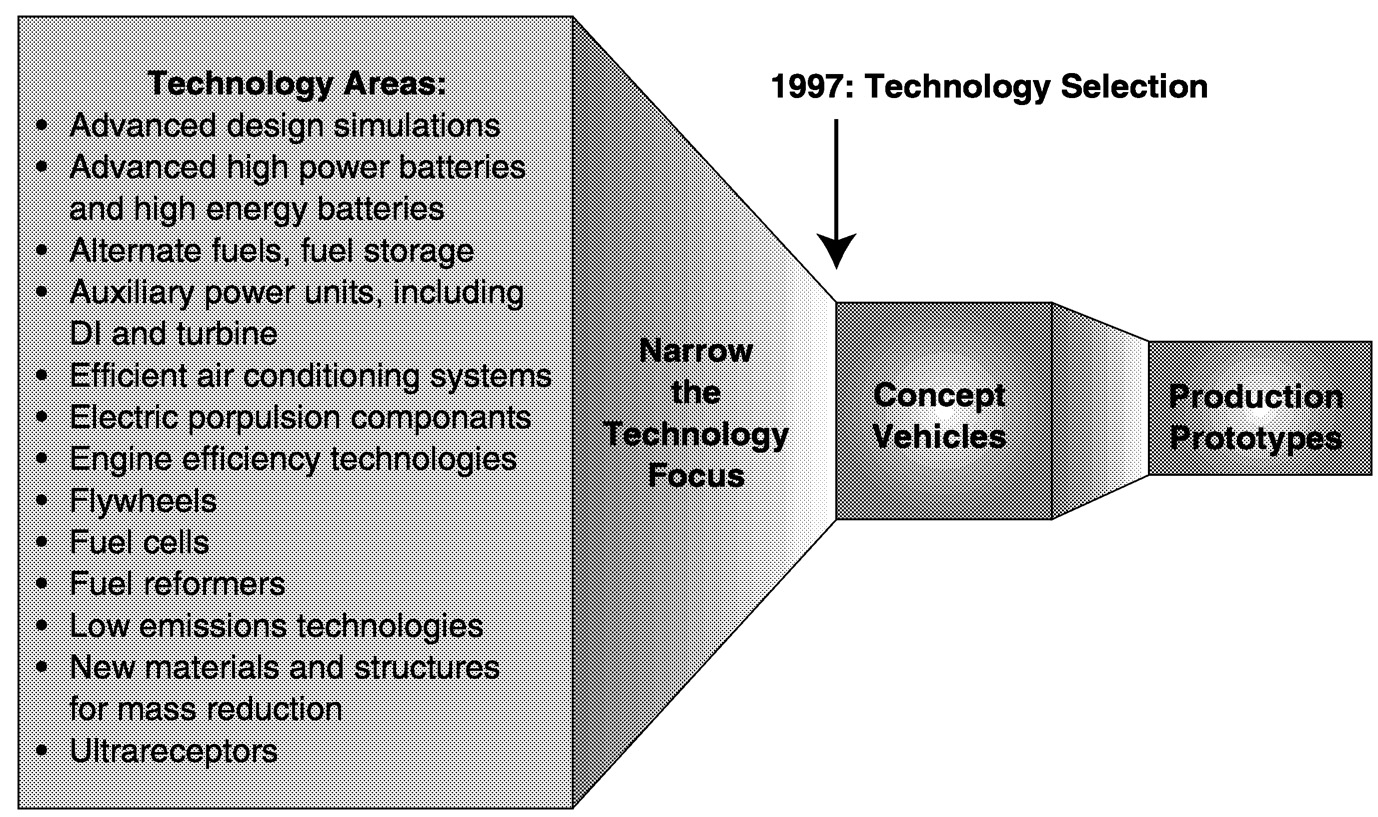
To be able to demonstrate vehicle prototypes that meet the third goal of the partnership by 2004, the PNGV program embarked on a plan entitled “Invent on Schedule.” ( Figure 6.5). The first phase of this plan called for compiling the technologies that might contribute to meeting program goals. The second phase focused on eliminating those technologies that were clearly not going to be practical during the “agreed-to” time period of the program. The output of this process has led to the identification of several key technologies that are being pursued with great effort and intensity. The next phase of the program was directed at the development of several vehicle prototypes that have been presented to the public this
Page 96
~ enlarge ~
FIGURE 6.3 The trend in hydrogen-to-carbon ratio in global energy consumption. NOTE: y-axis is logarithmic. SOURCE: Nakicenovic, N. (1996) Freeing energy from Carbon. Daedalus 125(3): 95-112.
Page 97
~ enlarge ~
FIGURE 6.4 Research goals for the PNGV program.
past year. Although these prototypes are impressive examples of automotive technology, they do not meet all of the goals of the program. They indicate that progress has been made as a result of this industry-government partnership and where additional improvements are needed to develop a production prototype in 2004.
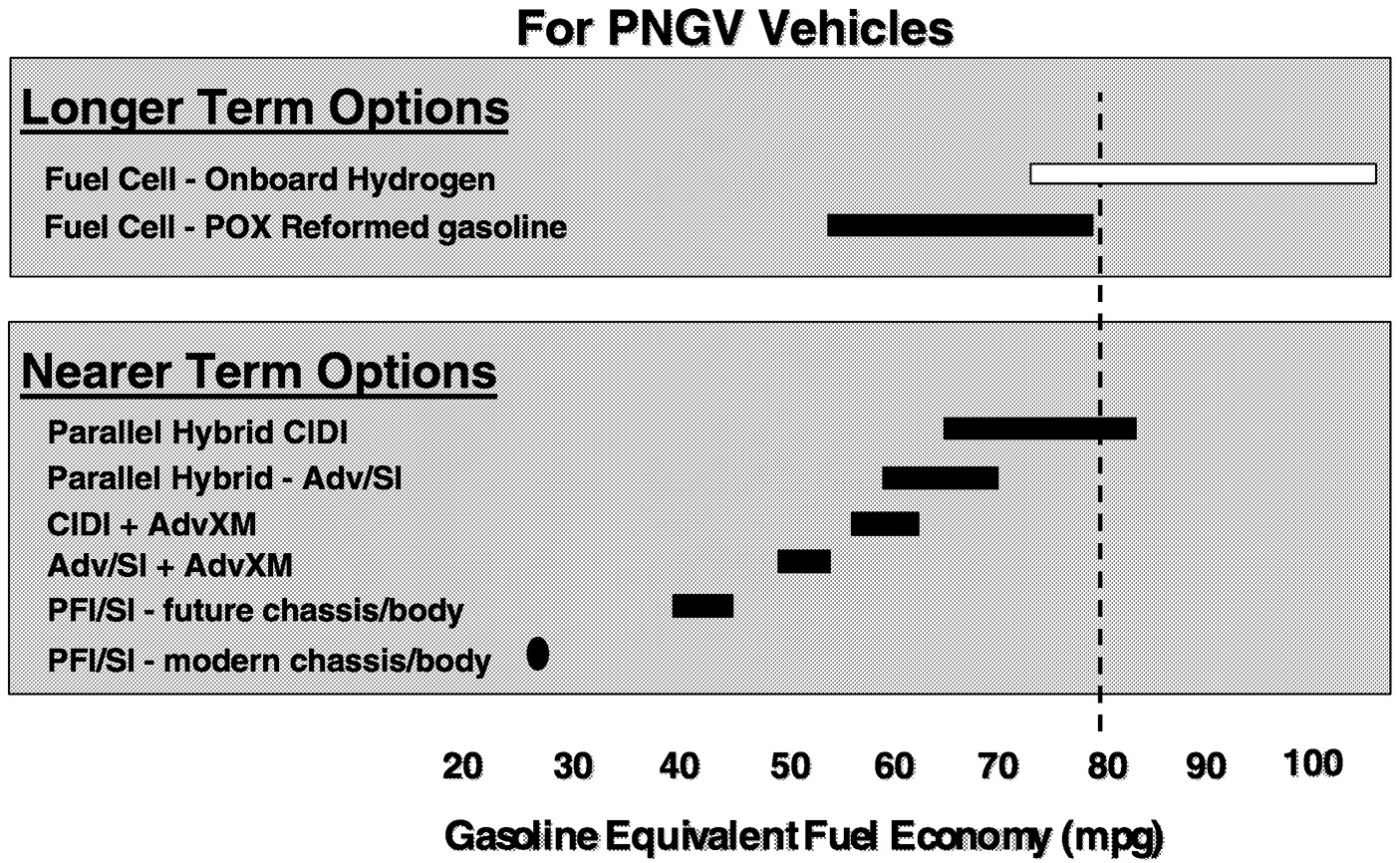
During the first two phases of the PNGV program, considerable use of analytical models helped define the expected fuel efficiency of different vehicle-powertrain-fuel combinations ( Figure 6.6). The baseline fuel economy calculations in this figure correspond to a 1994 midsize sedan equipped with a port fuel-injected, spark ignition engine operating on a reformulated gasoline. Various improvements in fuel economy can be obtained through use of lightweight bodies, advanced spark ignition engine technologies such as direct injection, and advanced transmissions. The upper and lower ranges for each
~ enlarge ~
FIGURE 6.5 The “invent on schedule” plan for the PNGV program.
Page 98
~ enlarge ~
FIGURE 6.6 Fuel economy projections of different PNGV technologies. NOTE: CIDI = compression-ignited direct injection engine, MPG = miles per gallon, PFI = port fuel injected, POX = partial oxidation, SI = spark ignited, XM = transmission.
bar in this figure are based respectively on aggressive or conservative assumptions regarding the efficiency to be gained with each technology. Compression ignition engines provide a substantial increase in fuel efficiency, particularly when installed in hybrid powertrain configurations. In a separate category from internal combustion engines are fuel cell-powered vehicles operating on either gasoline or hydrogen. The results of such modeling efforts have led the PNGV program to identify key promising technologies that must be developed if the program is to meet its stated goals.
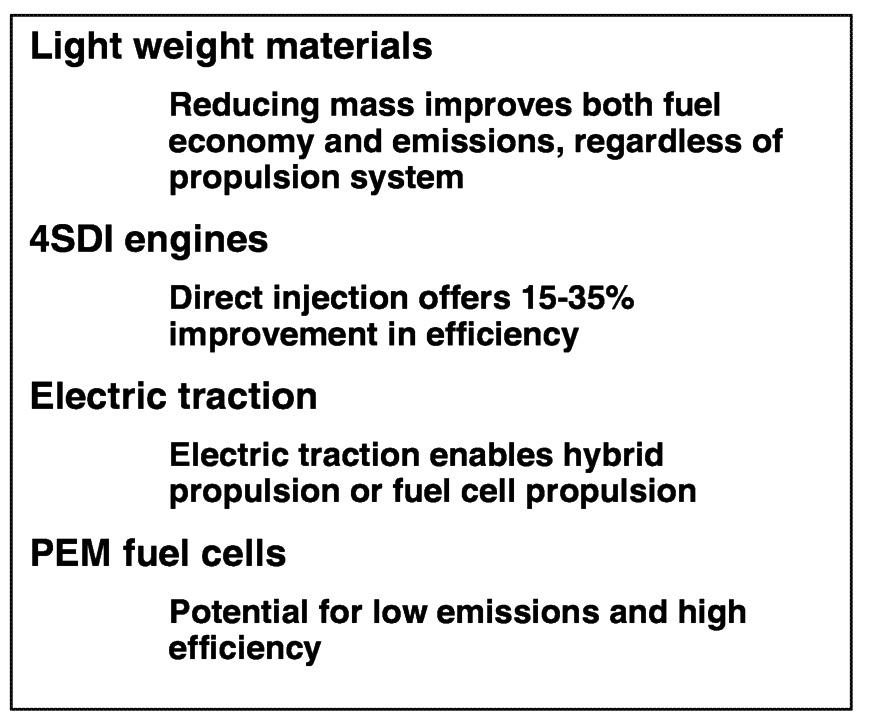
The four technologies critical to the program success are shown in Figure 6.7; these include light-weight materials, four-stroke direct injection engines (4SDI), electric traction systems, and proton
~ enlarge ~
FIGURE 6.7 Four technologies critical to the success of the PNGV program.
Page 99
exchange membrane (PEM) fuel cells. Although not explicitly stated, it should be clear that the fuel needed for both 4SDI engine and fuel cell operation is a critical aspect of the development of these two propulsion technologies.
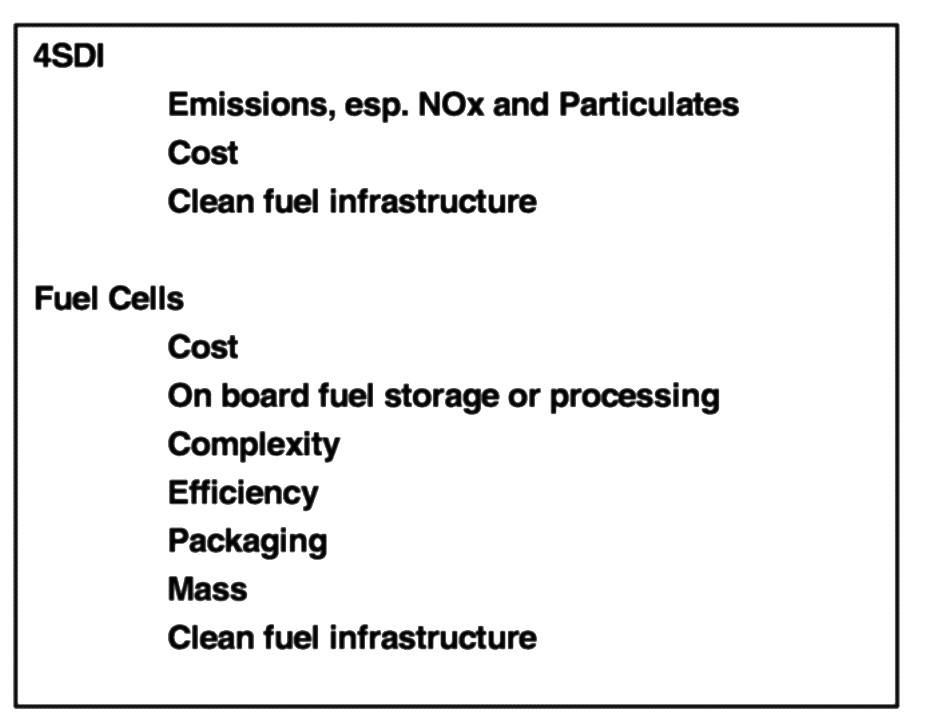
A list of the challenges associated with these propulsion technologies is shown in Figure 6.8. Because it has been a viable, fuel-efficient power source for many years, compression ignition engines have been identified as the leading 4SDI configuration. However, challenges remain regarding meeting future emissions standards, particularly for NOx and particulates, achieving cost targets, and identifying fuel compositions that provide the best fuel efficiency-emissions trade-off. Fuel cells are clearly a riskier propulsion choice. Significant questions remain regarding cost, ability to reform fuels on-board the vehicle to produce hydrogen, which fuel to use, complexity, efficiency, packaging, and mass of the final system.
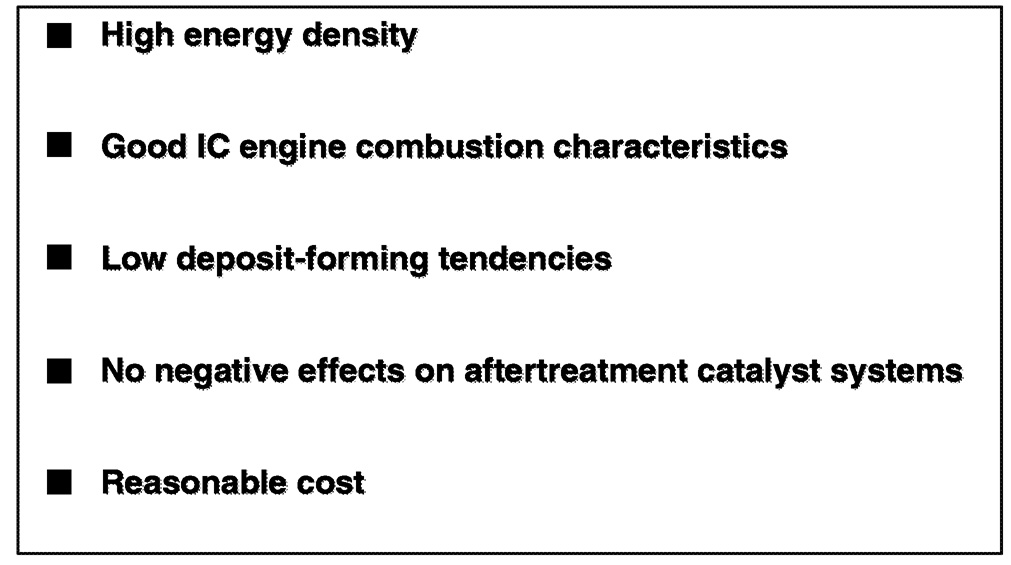
Given that fuel composition can affect the ability of engine or fuel systems to meet fuel efficiency and emissions goals, it is worth considering what fuel characteristics will be needed to support the introduction of advanced engine technologies ( Figure 6.9). Generally, there are several key characteristics that automotive engineers will always want in any fuel. First, it should have the highest energy density possible for maximum range and customer convenience. For internal combustion engines, it should provide good combustion characteristics—that is, the proper octane for spark ignition engines, and high cetane for compression ignition engines. The fuel should have a low tendency to form deposits throughout the fuel, reformer, combustion, and exhaust emissions control systems. The fuel should have no instantaneous or long-term deleterious effect on pre- or aftertreatment catalyst systems. And finally, the fuel should have a reasonable cost.
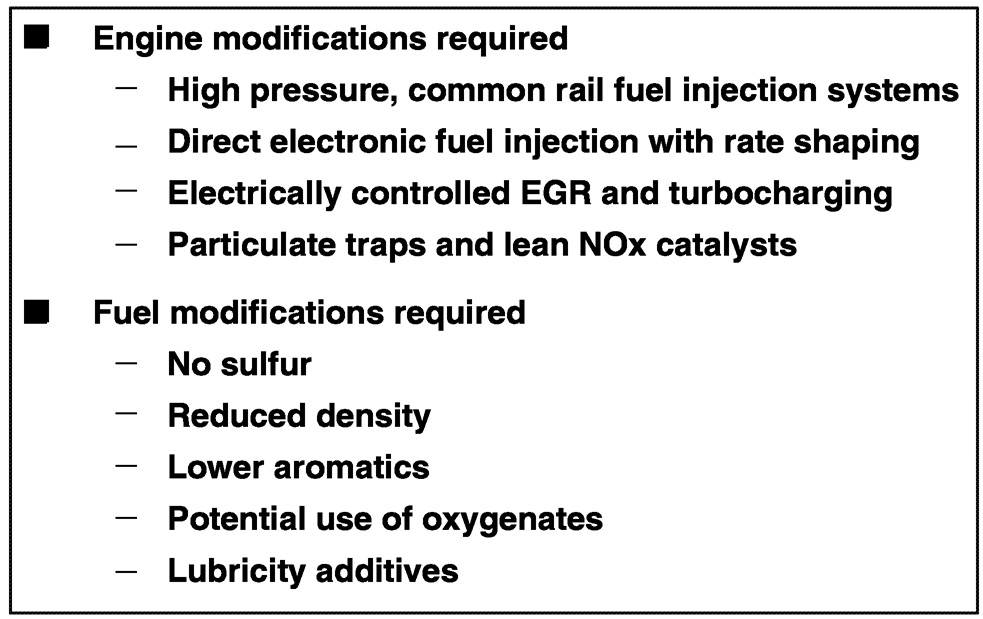
Within the context of these general characteristics, the nature of a specific fuel depends greatly on the engine technology in which it will be used. For example, in the case of compression ignition engines ( Figure 6.10), mechanical and electrical control technologies such as common rail fuel injection systems, direct electronic fuel injection with rate shaping, electronically controlled turbocharging and exhaust gas recirculation, and lean NOx catalysts and particulate traps will be required to meet emissions goals. These technologies will require fuels that contain virtually no sulfur (certainly less than 15 parts per million [ppm]), reduced density, lower aromatics, potentially an oxygenate component, and increased use of lubricity additives for good long-term durability.
~ enlarge ~
FIGURE 6.8 Challenges associated with four-stroke direct injection and fuel cell propulsion technologies.
Page 100
~ enlarge ~
FIGURE 6.9 Desired characteristics for transportation fuels. NOTE: IC = internal combustion.
In contrast as shown in Figure 6.11, for vehicle powertrains that employ a PEM fuel cell and a liquid fuel reformer to produce the required hydrogen, liquid fuels will be required to have zero sulfur (this means less than 10 ppm in the fuel, possibly coupled with an on-board sulfur trap resulting in less than 0.5 ppm delivered to the reformer or stack), as well as the highest H/C ratio possible and an acceptable energy density. There will be no octane requirement, and vapor pressure limits will be needed only to control evaporative emissions from the fuel tank. Some oxygenated components in the fuel blend might be used for a variety of performance attributes, but there cannot be any contaminants that would interfere with or poison the fuel cell stack.
Based on these fuel attributes and engine requirements, the engine-fuel combinations shown in Figure 6.12 are the most likely to achieve substantial market penetrations in different global markets at different times during the twenty-first century. Although each combination is technically possible during the next century, the extent to which any of these combinations will be successful in the marketplace depends on a combination of social, economic, and/or regulatory events.
In the case of spark ignition engines, the best options for fuels include highly reformulated gasoline with virtually no sulfur; alcohols derived from biomass; natural gas; or as the infrastructure develops, hydrogen.
~ enlarge ~
FIGURE 6.10 Advanced engine and fuel requirements for reduced emissions from compression ignition engines. NOTE: EGR = exhaust gas recirculation.
Page 101
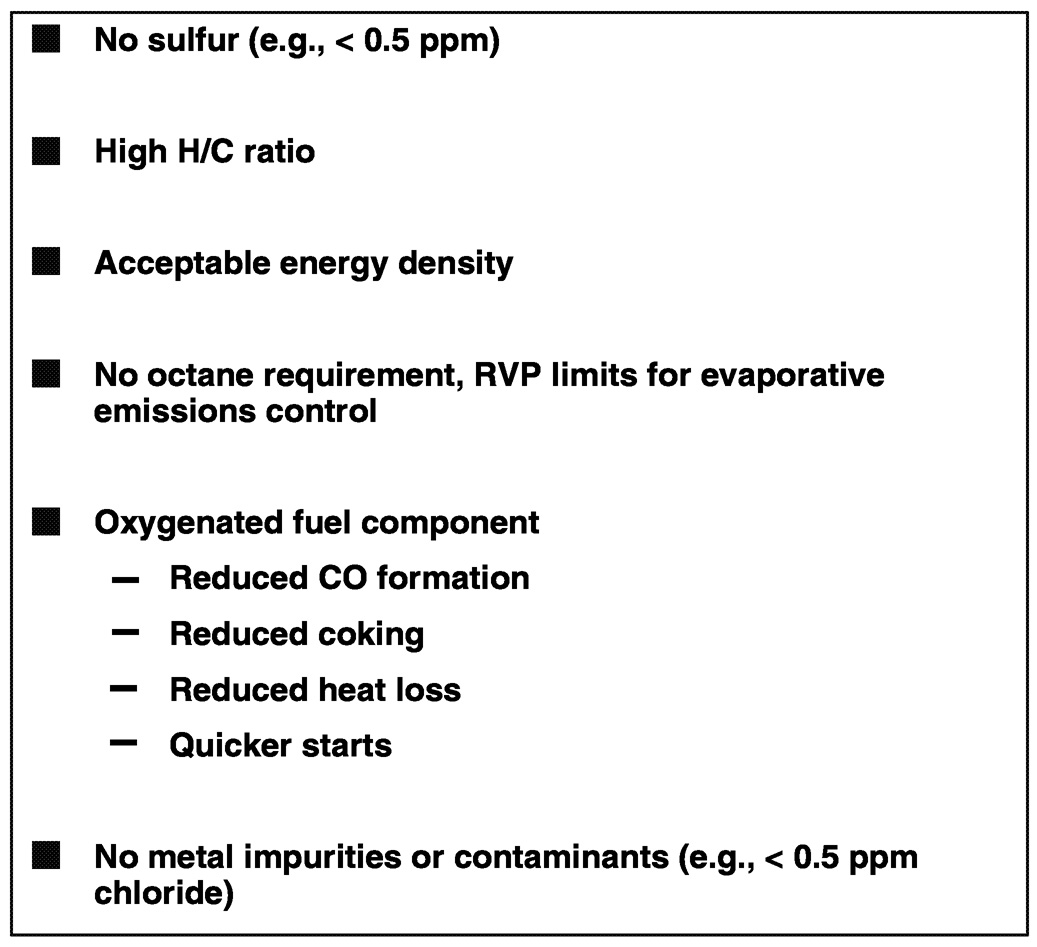
~ enlarge ~
FIGURE 6.11 Fuel attributes for good on-board fuel processor operation. NOTE: RVP = vapor pressure.
For compression ignition engines the most promising fuels include highly reformulated diesel fuel with virtually no sulfur; liquid hydrocarbons derived from natural gas; diesel fuels blended with specific oxygenate components; alcohols derived from biomass; or natural gas possibly blended with some percentage of hydrogen.
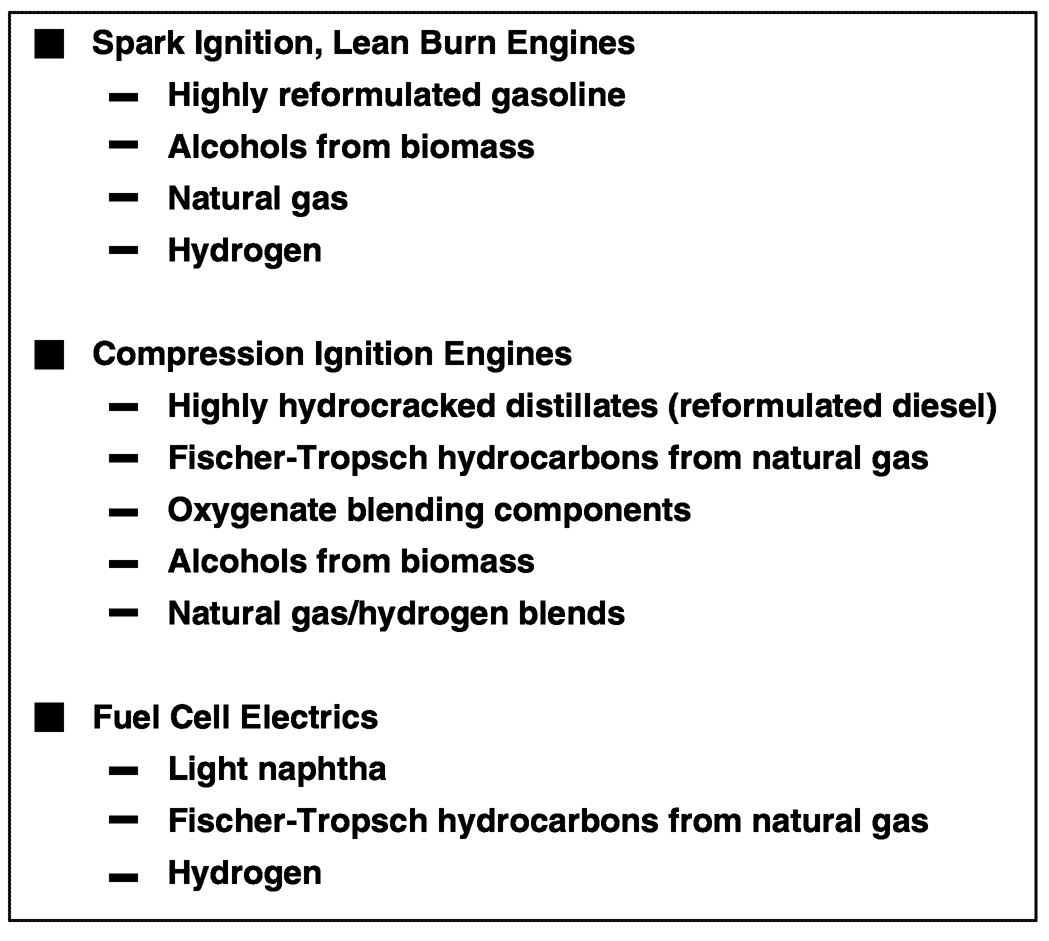
~ enlarge ~
FIGURE 6.12 Potential engine-fuel combinations for the twenty-first century.
Page 102
Finally, the best alternatives in the near term for fuel cell electric vehicles include light naphtha or alkylate refinery streams; liquid hydrocarbons derived from natural gas; or as production, storage, and distribution systems develop, hydrogen.
The desire to “manage” carbon emissions as future vehicles are added to the global car park suggests that some of these listed options are more desirable than others. If, in addition to meeting engine or propulsion system requirements and customers' expectations, we add the goal of no net CO2 emissions from the vehicle fleet, then the best long-term options in my perspective are renewable hydrogen or biomass-derived ethanol.
I have not included the processing of fossil fuels to produce hydrogen coupled with “decarbonization” or sequestration facilities at centralized plants for capturing solid carbon or CO2 emissions. Technology does exist for capture of these H2- manufacturing plant by-products, and several strategies have been suggested for their disposal. Although such technologies could be a possible near-term mechanism for lowering CO2 emissions and speeding introduction of H2, both decarbonization and sequestration strategies suffer from significant drawbacks. Collection and disposal of such by-products add substantial effort, inefficiency, and cost to the production of transportation fuels that are avoidable if renewable, non- or low-carbon fuels are produced in the first place.
It's worth reviewing the importance of each of these options. In the case of hydrogen, I've already pointed out that it could turn out to be the “ultimate” fuel. Today's fuels are increasing in hydrogen content, hydrogen can be made without generating any CO2 emissions, and I believe the energy industry would be receptive to the use of hydrogen since it would represent the last fuel change needed.
In addition, hydrogen enables a variety of advanced engine or propulsion technologies including “zero-emission” fuel cell vehicles, simplified fuel cell vehicle designs without the need for a fuel reformer, and internal combustion engines that have both improved efficiency and very low emissions ( Figure 6.13).
The concept of hydrogen-powered fuel cell vehicles is far more than a futuristic prediction. General Motors introduced a significant prototype minivan at the 2000 Geneva Motor Show that operates on cryogenic hydrogen fuel. More than an auto show concept, the Zafira minivan ( Figure 6.14) is a fully operational, five passenger vehicle that has been demonstrated in five different countries, including pacing the men's and women's marathons at the Sydney Olympics.
Although the current Zafira is designed to utilize liquid hydrogen, General Motors is undecided on an optimum form of hydrogen storage for future vehicle applications ( Figure 6.15). We are currently investigating storage methods that utilize compressed gas, cryogenic liquid, and solid adsorption tech-
~ enlarge ~
FIGURE 6.13 Why hydrogen is important to General Motors. NOTE: ZEV = zero emission vehicle.
Page 103
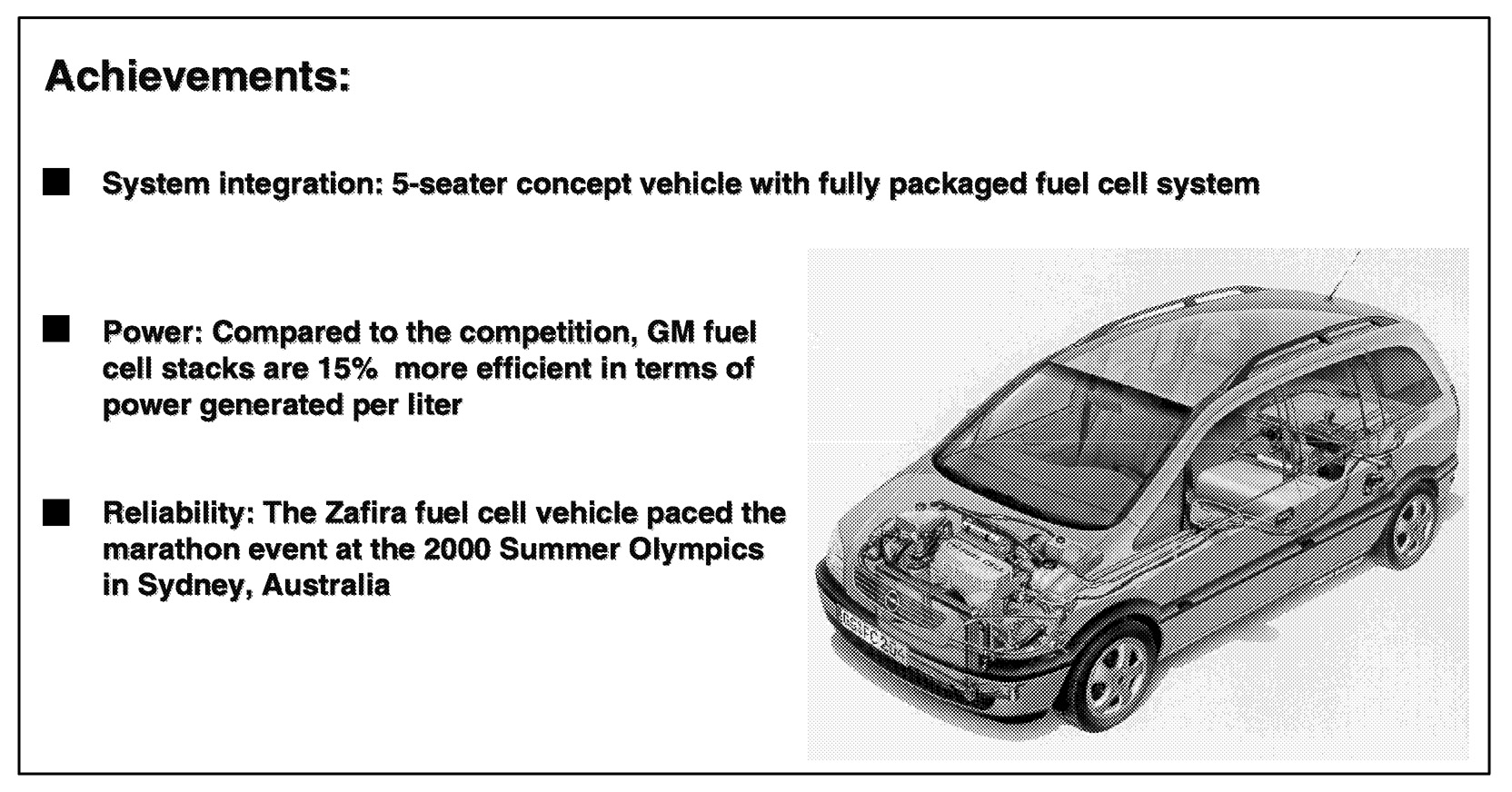
~ enlarge ~
FIGURE 6.14 The hydrogen-powered Opel Zafira at the Geneva Motor Show 2000.
nologies. We are also comparing the efficiencies and utility of such storage methods with gasoline reformer-equipped vehicle designs. It's claimed that advanced tank designs for both gaseous and liquid storage, as well as advanced solid adsorption materials, can store between 5 and 10% hydrogen calculated as a fraction of the overall weight of the total fuel storage system. Such technologies still store substantially less energy than is possible from liquid hydrocarbon fuels, even when the weight of the reformer system is added in. Advanced materials research is needed to improve both the materials of construction for gaseous and cryogenic tanks and new solid formulations for adsorbing greater amounts of hydrogen.
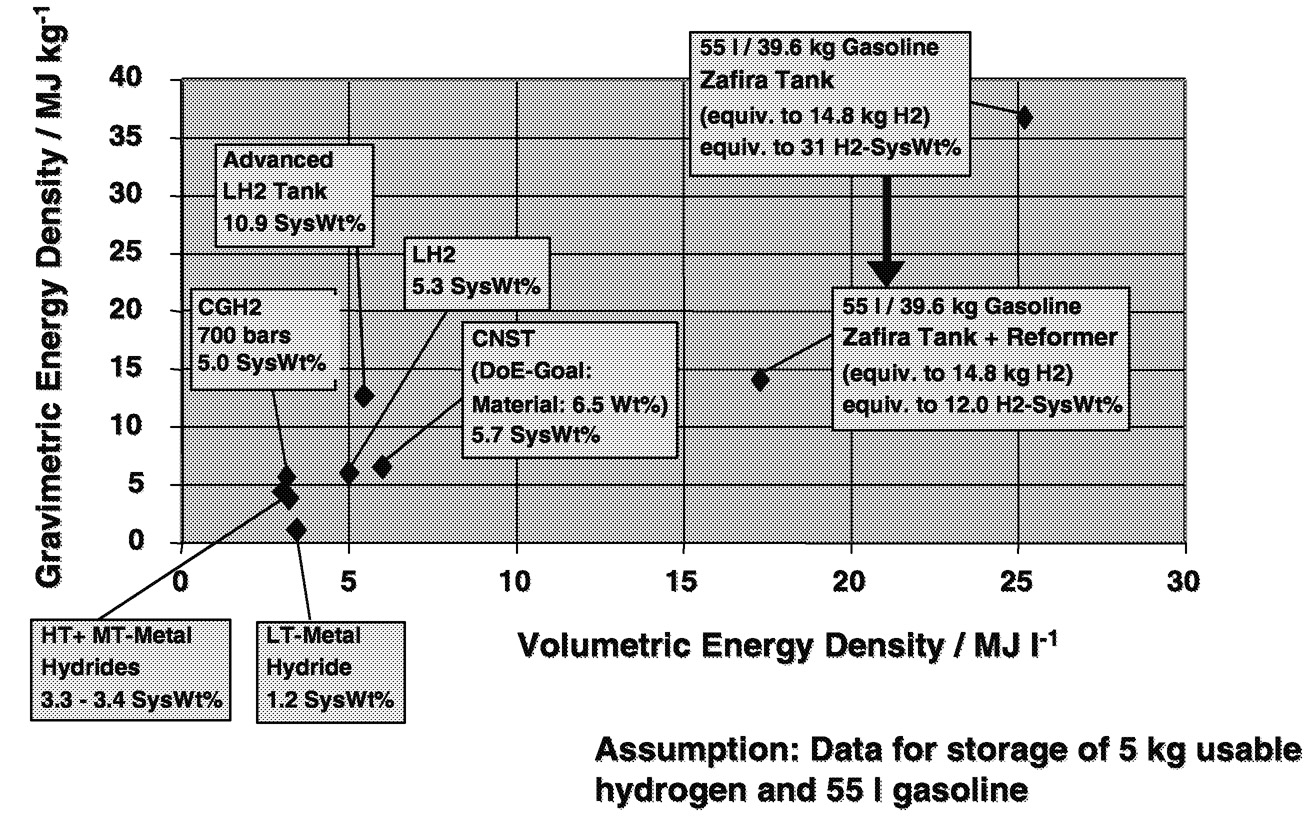
~ enlarge ~
FIGURE 6.15 Energy storage comparison: gravimetric versus volumetric energy density of hydrogen storage.
Page 104
To demonstrate that solid adsorption hydrogen storage systems are technically feasible, a version of the Precept ( Figure 6.16), GM's PNGV technology demonstration vehicle, was equipped with a fuel cell and a low-temperature hydride storage system. It is useful to note that the gasoline-equivalent fuel economy for this vehicle is calculated as 108 miles per gallon (mpg). Although successful as a developmental prototype, substantial improvements in many aspects of fuel cell vehicle design are needed prior to any future production vehicle program.
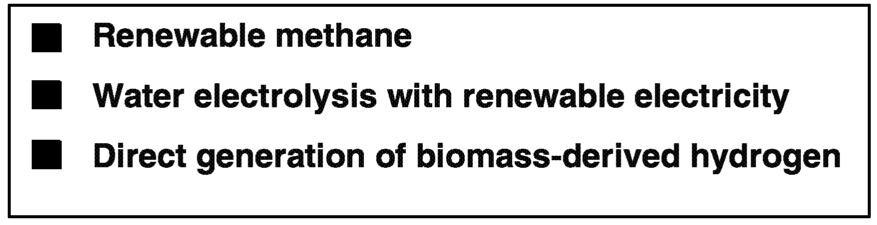
Developing a suitable hydrogen storage system is only part of what it takes to create a hydrogen transportation fuel industry. The other necessary components are the production technology used to create renewable hydrogen ( Figure 6.17) and the infrastructure to deliver it to the driving public. The generally accepted technique for producing hydrogen today is through steam reforming of natural gas. In the short-term, this process can be coupled with decarbonization techniques to produce hydrogen with little CO2 generation. In the longer term, however, this technology could be transferable to the production of hydrogen using biomass-derived or sewage- and garbage-derived methane. Carbon dioxide generation could be substantially reduced if not brought to a CO2-neutral balance point through the use of biomass-derived hydrogen.
Longer-range hydrogen generation techniques could include both electrolysis of water using renewable electricity and direct generation of hydrogen through use of bacteria and algae systems. Both of these technologies require significant development to further improve generating efficiencies and reduce production costs.
The other critical component of a hydrogen fuel transportation system is an infrastructure designed to compete with other forms of energy ( Figure 6.18). This can be achieved if we provide value to the driving customer by creating a distribution and storage system that is safer than gasoline, has widespread availability, meets customer expectations for value, and is easy to use. There is no room for compromise on any of these issues. Gasoline and diesel fuels are very good energy sources for transpor-

~ enlarge ~
FIGURE 6.16 The Precept at the Detroit Motor Show 2000.
Page 105
~ enlarge ~
FIGURE 6.17 Necessary ingredients to create renewable hydrogen.
tation systems. They meet many of the criteria that automotive engineers desire in a fuel. Customers will not accept a new fuel unless it provides the same or greater levels of safety, convenience, and value.
In the end, the use of hydrogen in transportation applications will depend as much on research and development of production and delivery technologies as any issue associated with hydrogen use onboard the vehicle.
The other fuel option identified as a possibility for management of carbon emissions is biomass-derived ethanol. The concept of biomass-derived ethanol can be focused further to ethanol derived from cellulose ( Figure 6.19). Currently the ethanol used for blending with gasoline in many states in the United States is derived primarily from glucose (starch) in the corn kernel. General Motors does not believe that a large percentage of U.S. transportation fuel needs could be produced from this source of biomass. Furthermore on a societal basis, we do not believe that transportation energy needs should compete with human food production. Thus, we conclude that ethanol used for transportation needs should be derived from cellulose.
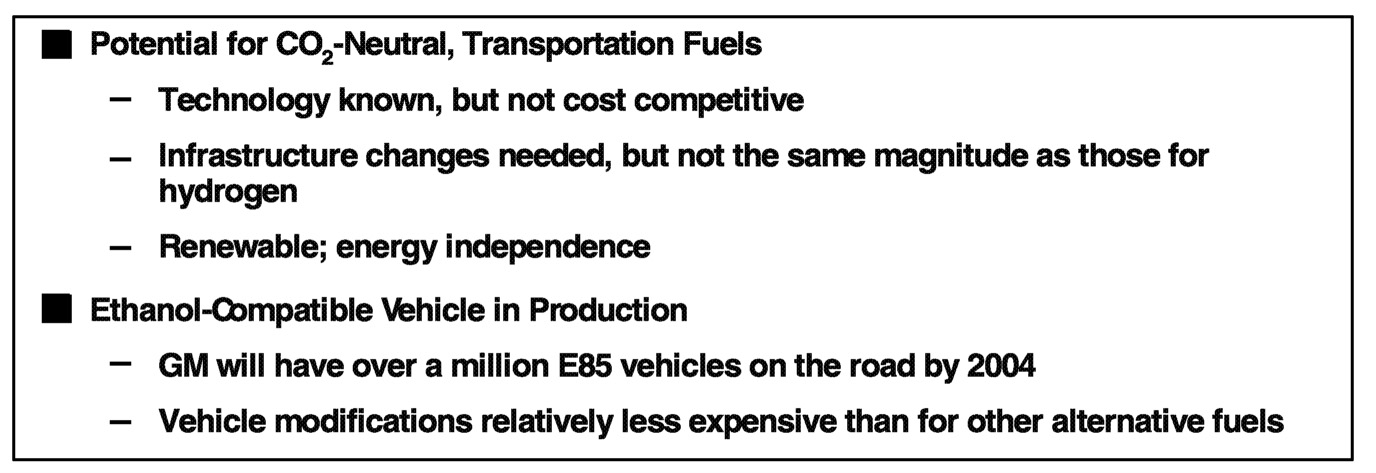
Cellulose-derived ethanol could provide a source of fuel that would consume as much CO2 as it produces during the combustion process, thus making the transportation sector CO2 neutral. Technologies for the production of ethanol for cellulose are known, but they are not cost competitive with production from glucose. Infrastructure changes are needed to minimize contamination of ethanol by water-soluble components, but the changes would be of less magnitude than for introduction of a hydrogen infrastructure. Ethanol can be produced from the cellulose of renewable crops such as switch grass and poplar trees. Calculations have shown that there is sufficient land available in the United States to replace the 100 billion gallons of gasoline used each year, thereby also providing substantial improvements in U.S. energy independence.
From a vehicle perspective, the introduction of fuels containing large amounts of ethanol is a much simpler task than producing hydrogen-fueled vehicles. As allowed by current law, several manufacturers have introduced E-85 vehicles into the marketplace in exchange for limited corporate average fuel
~ enlarge ~
FIGURE 6.18 Necessary ingredients to create a hydrogen infrastructure.
Page 106
~ enlarge ~
FIGURE 6.19 Ethanol derived from cellulose: its importance to General Motors.
economy (CAFE) credits. GM will have more than a million E-85 vehicles on the road by 2004. These vehicles are designed to operate on 100% gasoline or any blend of ethanol and gasoline up to 85% ethanol. Admittedly, most of these vehicles currently operate on straight gasoline, but that's only because the number of E-85 stations is limited. The modifications of an E-85 vehicle are relatively less expensive than those for alternative fuel candidates.
An example of GM's current E-85 vehicle offerings is the Chevrolet S-10 pickup truck. E-85 versions of the Chevrolet Suburban and Tahoe and GMC Yukon and Yukon XL sport utility vehicles equipped with the 5.3 liter V-8 have been added in model year 2001.
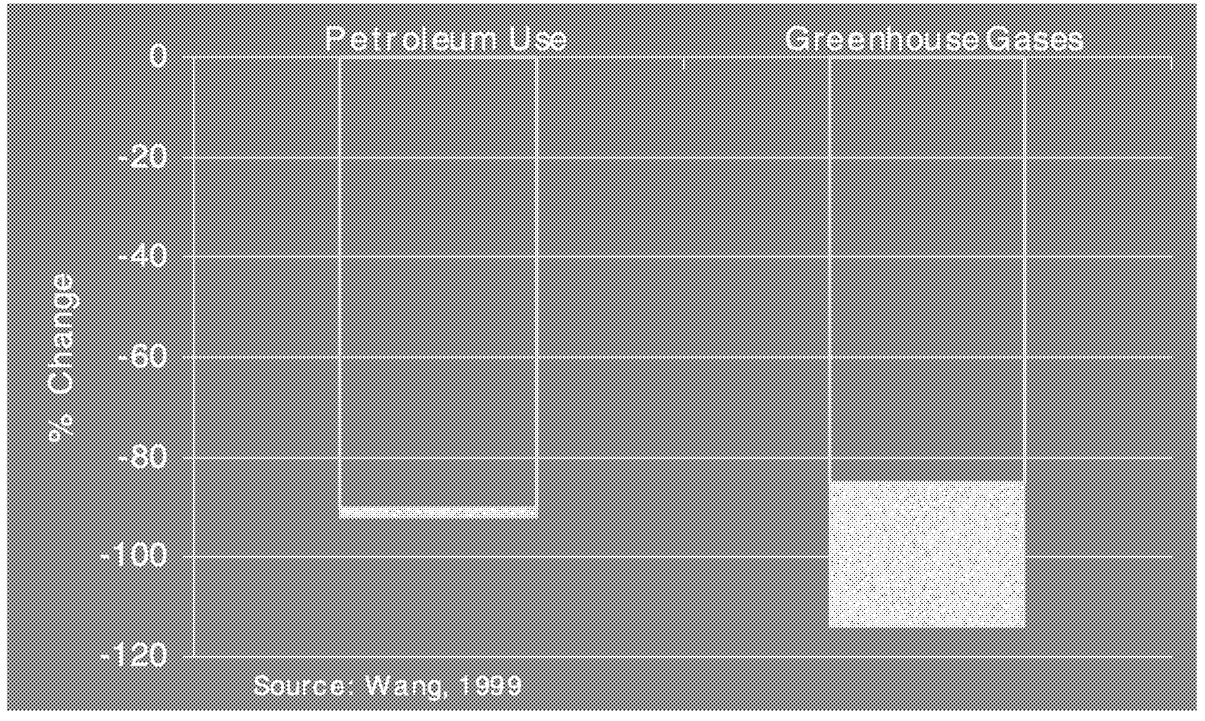
Finally, it is worth noting that calculations conducted at Argonne National Laboratories have demonstrated that it is possible to reduce petroleum consumption by approximately 90% and essentially eliminate all of the CO2 generated by mobile sources through use of ethanol generated from cellulose ( Figure 6.20). The range of estimates depends on whether herbaceous or woody biomasses are being used as a source of cellulose. Reductions in greenhouse gases can exceed 100% due to natural, long-term carbon sequestration in the soil. Given the installed capital base associated with refineries and petroleum pipelines, converting the transportation system to an alternative fuel such as ethanol derived from cellulose would require a number of years. If managing carbon is decided to be in the national
~ enlarge ~
FIGURE 6.20 Potential future impact of ethanol from cellulose. Adapted from M. Wang, C. Saricks, and D. Santini (1999). Effects of fuel ethanol use on fuel-cycle energy and greenhouse gas emissions, Argonne National Laboratory Report ANL/ESD-38, January 1999.
Page 107
interest, ethanol from cellulose needs to be given serious consideration as a technologically achievable solution.
In summary, General Motors believes that carbon management will be a major driver for development of future engine-fuel systems. It is expected that future transportation fuels will continue the trend toward lower density and higher H/C ratios. Reformulated gasoline and diesel fuels will provide the opportunity to reduce CO2 generation through improved internal combustion engine efficiency meeting applicable emissions standards. The best longer-range options for achieving a CO2-neutral state include (1) renewable hydrogen and (2) ethanol derived from cellulose. Both additional research efforts and a national long-range energy plan (longer than one administration) are needed to achieve future carbon management objectives.
DISCUSSION
Richard Wool, University of Delaware: Significant advances have been made in genetically engineered plant oils, and at the same time, some advances have been made with bio-diesel. Have you looked at this possibility?
James Spearot: Bio-diesel has some interesting aspects associated with it, but right now it is not cost competitive. If we decide as a nation and as a global society that we need to control carbon—that wasn't the point of my talk here—then we have to go far beyond bio-diesel.
Now, let me say something about genetically modified crops. I think this is a technology that deserves its day in the sun. Unfortunately, I am concerned that it will run into some of the environmental concerns that have been generated with other advanced technologies. I hope this doesn't happen. We would argue that grasses could be grown for fuel production and kept out of the food system, but unfortunately, as you know, once you put things out into nature, it is difficult to keep them segregated.
David Keith, Carnegie Mellon University: It is clear that there is great potential for biomass. Current estimates put the price of biomass at about $2.50 per gigajoule of primary energy. By comparison, the price of energy, particularly if we require liquid fuels, from other non-fossil renewables is substantially higher. Yet I also want us to think hard about the environmental impact of biomass use, because this is the metric that counts, not just CO2 or renewability.
Consider the fact that, in the United States, where we eat a lot of meat, it takes roughly a hectare per person to feed ourselves and we use 10 kw of primary power each. The largest energy flux you get out of biomass is a watt or two per square meter, which equals about a hectare per person. So, that means if you wanted to use biomass for the third of our energy consumption that is used in transportation, you need increase our current total cropland use by about by about one-third. I think we must think hard about whether we want this future.
Arguably, our land use is the biggest single environmental impact we make. You need only fly over the middle part of this country to see the level of current impact. Think about what a substantial increase in land use would mean. So, I realize that this is not really a fair question for you because it is not GM's responsibility to grapple with these problems, but I would like you to comment on the potential of biomass.
James Spearot: You helped me answer the question with your last sentence. Your question was, What are our options for transportation fuels? I believe that ethanol derived from cellulose is a viable option that should be looked at. Clearly, there is no option that is obvious or easy. Every option has pluses and
Page 108
minuses associated with it, and you have identified some of the minuses of deriving liquid fuels from agricultural crops.
I think it has been shown that there is enough land available, but it is fair to ask the question of whether we want to devote all that land to switch grass or poplar trees. This is something that society as a whole will have to make a decision on when the time comes.
Alex Bell, University of California at Berkeley: The hydrogen fuel cell has always been an attractive option for automotive driving force. The issue is one of materials of construction. When I was serving on the Partnership for a New Generation of Vehicles Review Committee, it became apparent that with the current loadings of precious metals required for the fuel cell, even excluding the reformulation apparatus, you could probably build on the order of a million cars a year. This is one-twentieth of what we produce in the United States alone.
So, what is the long-term viability of looking at hydrogen fuel cells for the world, given that the resources of these precious metals don't reside here in the United States?
James Spearot: We are now working on our sixth-or seventh-generation fuel cell stack. We have lowered the noble metal content per power output on every generation. In fact, the numbers are coming down dramatically.
I can't quote the numbers from memory, but I wish the cost, the size, and the complexity of the fuel reformer were coming down as quickly as the efficiency of the fuel cell stack is improving. I think we will get to the point where we can have a manageable amount of noble metal in the fuel cell. It may be that the first few fuel cell vehicles sold are over-designed in terms of noble metal usage, but we believe that fuel cells are a viable option. We are going to have to solve that problem if we want to produce these vehicles.
That is the approach we are taking to this particular issue, but there is a critical need for additional electrochemical research to develop fuel cell systems that have reduced amounts of noble metal. We are lowering the amount, but it is not where it needs to be yet.
Tobin Marks, Northwestern University: Let me make sure I understand. The ethanol from cellulose, what is the process for that? Is it fermentation or some other process?
James Spearot: Can any of the life science people answer this? I am a chemical engineer with a background in gasoline and refinery operations. My understanding is that it consists of a variety of processes put together. They are well-known processes. It is just that they are more expensive and energy intensive than making ethanol from corn. My limited understanding is that there is significant research to be done on enzyme processes to speed the organic chemistry going on.
Tobin Marks: Many are not optimized yet.
James Spearot: Well, they have been working on optimizing it for a long time, but the hope is that they can still make further improvements.
Klaus Lackner, Los Alamos National Laboratory: The issue of whether to produce hydrogen from electrolysis or through some fossil fuels, where you then have to go through the sequestration side, ultimately boils down to a cost issue. For comparison, if you take gasoline at $1.50, there are several hundred dollars per ton of CO2 right in the cost of the fuel. So if you now estimate $30 per ton of CO2
Page 109
for sequestration, this is on the order of a 10% correction. If you start with electricity as the basis for making hydrogen, you start with a very expensive source of energy. Since there is a finite efficiency in that process, the total amount of energy consumption is even higher. No matter which way you go, electrolysis is an expensive way of making hydrogen.
James Spearot: In some parts of the world, there is cheap hydrogen available where there are hydroelectric capabilities. I am one of the people who believes that eventually we are going to have to look at additional nuclear energy sources in the future. In fact, I am concerned that we might be falling behind countries such as France and Korea, which are very interested in nuclear energy.
So, today, you are absolutely right. I don't argue with where we are today, but again we are looking out over a period of time. I don't even mind starting from producing hydrogen from steam reforming with sequestration as a bridge technology to get the hydrogen infrastructure in place. I think that is a viable option, but I think, in the long term, we have to figure out how to make renewable hydrogen in a cost-effective manner. That is the only point I am trying to make.
Brian Flannery, Exxon Mobil: We have certainly looked at fuels from biomass, which are often promoted as being potentially net CO2 free, but in current practice, they are very far from it. In fact, they are virtually identical to using fossil fuels directly because of the enormous energy that goes into the cultivation, harvesting, refining, and processing, et cetera.
So, I think an option that needs to be explored very carefully is real systems and how they could work in practice. We need to find out whether a system has the potential to be as effective as some ultimate optimum potential might be.
However, the use of biomass does raise huge questions of land use and other environmental impacts that I think need to be on the table along with the climate change issues and the environmental impacts that may be associated with that.
James Spearot: I don't disagree with anything you have said, Brian. I will argue only that the economics and the CO2 savings that we are looking at today are based on ethanol from corn and ethanol blended primarily to 10% in some gasoline. I think the economics will change dramatically with different crops and with larger amounts of ethanol use, as will the amount of CO2 that is saved.
However, it is one of the options and I believe it ought to be on the table. I guess we can agree to disagree because I believe sequestration has some significant environmental issues associated with it also.
Alan Wolsky, Argonne National Laboratory: I recollect estimating that using today's technology and today's electricity prices, hydrogen from electrolysis of water was $11 per million British thermal units. That is expensive. I am not aware of any nuclear advocate who thinks that electricity from nukes is cheaper than electricity from gas turbines.
James Spearot: I don't have figures to refute anything you have said. I would argue only that as we go forward during the next century, there is a reasonable hope that technology will bring hydrogen production cost down.
The German government believes that hydrogen is, in fact, competitive today with gasoline in Germany at $4 a gallon gasoline price. I don't know whether this is right or not. I don't know where they are planning to get their hydrogen from, but they have a very real interest in the use of hydrogen in Germany.
Page 110
In fact, I suspect that if GM produces fuel cells at some point in time, we will see them in Europe before we see them in the United States because of the higher fuel costs there.
John Stringer, Electric Power Research Institute: The cost of electricity from nuclear plants, as I said before, depends very much on the price of capital. If the capital cost is low enough, then the electro-steel research science is less, but that is not the point. The point is that if you have a nuclear station and you are able to run at base load all the time, then the comments that you made are quite right.
However, if—and this is usually the case—the plant doesn't go on at base load all the time, the period when it isn't going at base load can be used to generate hydrogen at a low marginal cost. That is the issue, I think.
Now, how that works out for an economy requiring hydrogen in large quantities is a matter of the scenario in which you put it, but there are scenarios in which you can get very cheap hydrogen by use of marginal hours from nuclear power.
Tom Rauchfuss, University of Illinois: I have a question about what progress you have made on high-density storage of hydrogen. Are there new materials that perhaps store more hydrogen than palladium, which is maybe 1%.
James Spearot: Unlike many of the reports that are surfacing all over the world, we have not been very successful in identifying materials that absorb large amounts of hydrogen. We are still working on this particular issue. I don't want to discredit anybody's work, but we are concerned about some of the reports because we are not able to reproduce some of the work that is being done, and we are trying very hard to reproduce it.
The most positive news we have in terms of storage is some of the numbers that are currently being evaluated in prototyped, compressed-hydrogen tanks. Right now, tanks with capability of 5,000 pounds per square inch (psi) are almost validated, and tanks with a capacity of 10,000 psi are being looked at, although they are a long way from being validated. With these two technologies, you can begin to achieve 5 or 6% hydrogen storage range.
Participant: When we talk about producing energy from agricultural sources, whether it be biomass or anything else, we have to take into consideration the energy balance between growing, harvesting, and transporting these materials so that they can be converted, let's say, to ethanol. This energy balance has been negative in the past. Is there a change now?
James Spearot: I can't answer your question specifically with numbers. All I can tell you right now is that our analyses have been based on total life-cycle energy and materials costs, and based on these total life-cycle analyses, we believe that ethanol from biomass is competitive. Ethanol from cellulose is more expensive than petroleum-based energy forms today, but there is a very real reason to think that improvements can be made in cellulose processing techniques that would allow us to bring the cost of the ethanol derived from cellulose down to a competitive level.
In terms of CO2, the life-cycle analysis has said that there are benefits from utilizing ethanol derived from cellulose, and work done at Argonne supports those numbers.