1
Introduction and History
NATURALLY OCCURRING INFECTIOUS DISEASES IN THE U.S. MILITARY
Despite the tremendous strides that have been made in public health, the control of infectious diseases, and preventive medicine during the past century, infectious agents remain a substantial threat to the operational capacity of military forces at the onset of the new millennium for three distinct reasons: (1) new recruits are trained in groups under crowded conditions, increasing the risk of spread of infectious agents; (2) warfighters, as a result of deployments, may come into contact with pathogens with which they have no prior experience and, therefore, no immunity; and (3) warfighters, along with others, may face the intentional use of weaponized infectious agents.
Until World War II, deaths due to infectious diseases outnumbered those due to direct combat injuries (Gordon, 1958). A large body of historical literature exists describing the importance of infectious diseases in deciding the results of military campaigns. Napoleon ceased his advance in the eastern Mediterranean when faced with a plague outbreak in Jaffa. Florence Nightingale achieved fame by addressing the fundamental hygiene problems that had caused the extraordinarily high rates of injury-related gas gangrene during the Crimean War. She, along with William Farr, compared mortality data for soldiers against a civilian standard. Finding that men of military age in England and Wales had an annual mortality of 9.2/1,000 compared to one of 35.0/1,000 for servicemen, Farr and Nightingale showed that most of the excess mortality among members of the military was due to contagious diseases and crowding (Curtin, 1989). Modern conflicts have been no different, as evidenced by the experiences of the Axis
forces with infectious hepatitis during the North African campaign and the wide variety of infectious diseases that affected American warfighters in Vietnam. Although the use of vaccines against plague and cholera significantly minimized the incidence of those diseases among U.S troops in Vietnam (Ellenbogen, 1982; Ognibene, 1987), diseases for which vaccines were not available—for example, leptospirosis, meliodosis, and shigellosis—were prevalent (Ognibene, 1987). Even in recent years, U.S. troops have been deployed to geographic regions where there exist endemic infectious disease agents against which the U.S. military does not have immediately available either suitable, safe, and effective vaccines or appropriate chemoprophylactic agents. Infectious diseases continue to contribute substantially to morbidity during deployments, as shown in Figure 1-1.
The severity of the threat to military operations from infectious diseases has been recognized since the beginning of the science of microbiology and has prompted a substantial body of military research on the subject and many advances in public health. A better understanding of many infectious agents and their mechanisms of transmission have come from careful studies of epidemics in military populations and from research done by military epidemiologists and microbiologists. Two examples are Sir Ronald Ross’s studies on the role of the
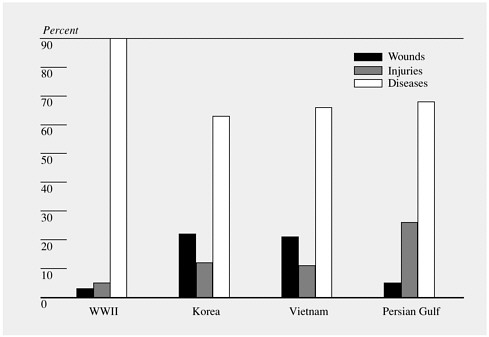
FIGURE 1-1 U.S. Army hospital admissions during war. SOURCE: NIC (2000).
Anopheles mosquito in the transmission of malaria and Walter Reed’s observations on the role of the Aedes aegypti mosquito as a vector for the spread of yellow fever. Another example is offered by the classic studies of infectious and serum hepatitis (due to hepatitis A and hepatitis B viruses, respectively) that were carried out by the U.S. Army during World War II and that clearly delineated the separate and unrelated nature of these infectious agents and the diseases that they caused (Paul and Gardner, 1960).
Immunization has long served as a key mode of prevention of infections in military populations. General George Washington ordered the first systematic immunization effort among American forces when he directed the variolation of Revolutionary War soldiers serving in the Continental Army to protect them from smallpox (Bayne-Jones, 1968). Table 1-1 summarizes important advances in the control of militarily important infectious diseases that have resulted in part or in whole from the activities of the Army Medical Department. The list is a veritable history of public health advances and testifies to the key role that military scientists and epidemiologists have played not only in keeping soldiers healthy but also in contributing to improvements in the general public health.
Infectious Disease Threats During Recent Deployments of U.S. Military Forces
U.S. troops must be prepared to be deployed anywhere in the world, often on very short notice, whether it is for actual combat, for a training exercise, or to serve as peacekeepers. Given the political instabilities in many parts of the world, U.S. warfighters must be ready to be deployed into environments where the risk of exposure to infectious diseases may be significant. Deployments occur in areas with widely different climates and very different ecological and demographic settings, including, within just the past 10 years, the Caribbean, the Middle East, South-Central Asia, and the Western Pacific. As this report is being drafted, U.S. warfighters are deployed in Afghanistan and are being sent in increasing numbers to the Philippines, neither of which would have readily been predicted as a location for deployment at the time that this study was commissioned. Predicting the nature and magnitude of infectious disease risks in advance of deployments may not always be possible, but maintaining a high degree of awareness is mandatory, given the lessons of history and the clear benefit-to-cost ratio. Table 1-2 summarizes the scope of infectious disease risks that U.S. troops have faced during deployments since 1900.
U.S. forces will face these risks, as well as new ones, as long as they are deployed into unfamiliar environments. Global military disease surveillance activities must continue to furnish information about these risks so that preventive strategies can be developed in the event of deployment (Ognibene, 1987).
TABLE 1-1 Historical Highlights in the Control of U.S. Military Infectious Diseases by Vaccines
|
Year |
Event |
|
1777 |
Members of Continental Army inoculated with the variola virus to prevent smallpox |
|
1812 |
Cowpox immunization replaced variolation for prevention of smallpox in troops |
|
1909 |
Typhoid vaccine developed |
|
1927 |
Chloroform-treated single-dose rabies vaccine for dogs developed through work done in the Philippines |
|
1940s |
Dengue virus types 1 and 2 isolated; first experiments begun with dengue vaccine |
|
1940s |
Tetanus toxoid and diphtheria toxoid shown to be highly effective in preventing wound-induced tetanus and diphtheria infections |
|
1941 |
Armed Forces Epidemiological Board established; commissions established to deal with influenza, hepatitis, encephalitis, and other diseases that threatened the war effort; vaccine-related activities included conducting research and providing immunization policy advice |
|
1942 |
Influenza vaccine developed and used for mass immunization of military forces |
|
1942 |
Yellow fever vaccine used in large numbers of military personnel; hepatitis B virus contamination of serum causes a large common-source outbreak of jaundice |
|
1944 |
Smallpox vaccine licensed |
|
1944 |
Troops stationed in Okinawa, Japan, immunized against Japanese encephalitis |
|
1950s |
Discovery that adenovirus types 3, 4, and 7 cause most cases of acute respiratory diseases in recruits; adenovirus vaccine research and development initiated |
|
1950s |
Anthrax vaccine developed |
|
1960s |
Outbreaks of meningococcal meningitis on military posts stimulated the study of meningococcal infection and the development of vaccines against meningococcal groups A, C, Y, and W-135 |
|
1960s |
Plague vaccine proven effective in Vietnam |
|
1960s |
Malaria vaccine program initiated (protection from bite of radiated mosquitoes shown) |
|
Year |
Event |
|
1965–1969 |
INDs* filed for vaccines against Venezuelan equine encephalitis, tularemia, eastern equine encephalitis, and Rift Valley fever |
|
1970s |
Development and testing of an oral typhoid vaccine |
|
1970s |
Prototype vaccines against Russian spring-summer encephalitis and tick-borne encephalitis made at Walter Reed Army Institute of Research (WRAIR) |
|
1970s |
Live attenuated dengue virus vaccine strains developed; INDs filed |
|
1970 |
Anthrax vaccine licensed |
|
1972–1975 |
INDs filed for Q fever vaccine and live attenuated Venezuelan equine encephalitis virus vaccine |
|
1980 |
Adenovirus vaccines licensed for use in military populations, leading to nearly complete control of epidemic respiratory diseases in recruits |
|
1984–1986 |
INDs filed for vaccines against western equine encephalitis, Argentine hemorrhagic fever, Venezuelan equine encephalitis, and chikungunya virus |
|
1985 |
Efficacy of Japanese encephalitis vaccine demonstrated in Thailand; licensure application coordinated by U.S. Army Medical Materiel Development Activity; license granted by Food and Drug Administration |
|
1985–1986 |
Hepatitis A vaccine developed and tested by WRAIR |
|
1986 |
WRAIR classification of human immunodeficiency virus infections published |
|
1987 |
Manufacturing technology for hepatitis A vaccine transferred from WRAIR to a commercial manufacturer; vaccine licensed in 1995 |
|
1991 |
IND filed for Rift Valley fever vaccine |
|
1996 |
Recombinant circumsporozoite malaria vaccine developed by the U.S. Army and an industrial partner shown to be protective in human volunteers |
|
1997 |
First successful vaccine against Shigella developed, produced, and tested |
|
1998 |
First DNA vaccine against malaria administered to humans |
|
* An investigational new drug (IND) application is filed when a product is ready for human testing. Specific regulations govern the use of IND products (Investigational new drug application [IND]. 21 CFR § 312.20–312.21, subpart B [2001]; also see discussion in Chapter 3). SOURCE: Modified from Hoke (2000a). |
|
TABLE 1-2 Major Infectious Disease Threats for Which There Were No Licensed Vaccines at the Time of Deployments and Overseas Exercises
|
Place |
Year |
Infectious Disease Threat |
|
Bosnia |
1996 |
Diarrhea, hemorrhagic fever renal syndrome, mycoplasma infection, tick-borne encephalitis |
|
Haiti |
1994 |
Dengue, malaria |
|
Somalia |
1993 |
Dengue, diarrhea, malaria |
|
Botswana |
1992 |
African tick typhus, malaria |
|
Saudi Arabia, Kuwait, and Iraq |
1990–1991 |
Botulism,* diarrhea, enterotoxigenic Escherichia coli infection, leishmaniasis, sandfly fever |
|
Egypt |
1983 |
Diarrhea |
|
Lebanon |
1982 |
Diarrhea |
|
Vietnam |
1959–1975 |
Dengue, diarrhea, hepatitis, Japanese encephalitis, leptospirosis, malaria, melioidosis, murine typhus, scrub typhus, sexually transmitted diseases (STDs; especially gonorrhea) |
|
Lebanon |
1958 |
Diarrhea |
|
Korea |
1950–1953 |
Hepatitis, Japanese encephalitis, Korean hemorrhagic fever, malaria, STDs (especially gonorrhea) |
|
World War II, Pacific |
1941–1946 |
Dengue, diarrhea, filariasis, Japanese encephalitis, malaria, meningitis, schistosomiasis, scrub typhus, STDs |
|
World War II, North Africa |
1940–1941 |
Diarrhea, hepatitis, malaria, meningitis, sandfly fever |
|
World War I |
1917–1918 |
Diarrhea, influenza, meningitis, pneumonia, tetanus, typhus, wound infections |
|
Cuba |
1900 |
Malaria, typhoid, yellow fever |
|
*The available product was not licensed and was administered as an investigational new drug. SOURCE: Modified from Hoke (2000a). |
||
SETTING PRIORITIES FOR MILITARY MEDICAL RESEARCH THROUGH THE TWENTIETH CENTURY
Since the end of the Cold War, the nature of U.S. military operations has changed. The troop deployments of today are smaller, faster, more diverse, and more diffuse, and they entail more frequent endeavors than military engagements of yore. This shift in activity has been accompanied by a change in operating strategy, including adoption by DoD of a fundamental Force Health Protection (FHP) tenet. Central to FHP is the concept that “the most valuable, most complex weapons system the U.S. military will ever field are its soldiers, sailors, airmen, and marines. These human weapon systems require lifecycle support and maintenance. . .” (JSLD, 1999, p. 2). Preventive medicine is a key component of FHP. Vaccination is, in turn, a key component of infectious disease prevention.
DoD interest in infectious disease prevention has been reinforced by Presidential Decision Directive NSTC (National Science and Technology Council)-7, which calls for DoD involvement in stepped-up U.S. efforts to address emerging infectious diseases (NSTC, 1996) and by National Intelligence Council (NIC) recognition that infectious diseases pose a threat to national security. “New and reemerging infectious diseases will pose a rising global health threat and will complicate U.S. and global security over the next 20 years,” NIC concludes in its January 2000 National Intelligence Estimate. “These diseases will endanger U.S. citizens at home and abroad, threaten U.S. armed forces deployed overseas, and exacerbate social and political instability in key countries and regions in which the United States has significant interests” (NIC, 2000, p. 5). Similar sentiments are echoed by many (IOM, 1992; Kassalow, 2001; Kelley, 1999). DoD’s responsibility for the protection of military and civilian populations alike compels its interest in infectious disease prevention and, by extension, vaccines.
History
Although many things have changed during the more than century-long history of the Army Medical Department’s research and development efforts, the Army Medical Department’s goal has stayed remarkably constant: highly focused research and product development efforts designed to mitigate the impacts of infectious diseases on military operations. In 1893, Army Surgeon General George M. Sternberg established the Army Medical School, now the Walter Reed Army Institute of Research, which has since served as a center for the Army’s medical research efforts (Engelman and Joy, 1975). At the end of the nineteenth and early in the twentieth century, for example, the department addressed the infectious disease threats that caused the greatest numbers of casualties during the Civil and Spanish–American Wars. It was not difficult for Sternberg, Reed, and colleagues to know which diseases they should focus on: the well-recorded
burden of typhoid fever, yellow fever, malaria, dengue, and diarrhea on military operations and medical care systems had made the priorities obvious.
As late as the Vietnam War, the surgeons general of the armed services used similar data—material that emerged from the military health care system and the records of the influence of infectious diseases on the effectiveness of military units—in setting priorities. In coming to their decisions, the surgeons general regularly relied on advice from the Armed Forces Epidemiological Board, a group of civilian experts who, for decades, considerably influenced both disease prevention policies and military medical research priorities.
Not surprisingly, research needs have differed from war to war. World War II generated intense research and development efforts on a wide range of infectious diseases. In contrast, the Korean War generated an upsurge in more focused research on malaria, arboviruses, and hemorrhagic fevers. The Vietnam War experience resulted in focused attention on malaria, viral hepatitis, dengue, scrub typhus, murine typhus, leptospirosis, bacterial diarrheas, and plague.
How Current Priorities Emerge
Ironically, the considerable success of these efforts has complicated the management of military medical research and development efforts to control infectious diseases. For example, many infectious disease threats of the past are no longer as dangerous as they once were. In the most recent deployments, military preventive medicine measures such as the provision of safe water and food and the use of vaccines, chemoprophylaxis, and vector control measures—along with favorable combat conditions—have kept the numbers of casualties from infectious diseases low. Therefore, decision makers often must rely on estimates of the potential of newly emerging infectious diseases, the extent of emerging microbial resistance to chemoprophylatic agents, and the regionally important illnesses for which epidemiologic information may be incomplete and for which proven vaccines or medical countermeasures do not exist.
At the same time, funding decisions and the administrative processes by which priorities are set must wend their way through increasingly complex layers of bureaucracy. This process is described in detail in Chapter 2.
Despite historic successes, in recent years DoD vaccine acquisition efforts have at times been troubled. This is best exemplified by the loss of the availability of adenovirus, plague, and anthrax vaccines. Although the circumstances contributing to the loss of the availability of each vaccine differ, each case illustrates the vulnerabilities inherent in the vaccine acquisition system.
In the 1960s and 1970s widespread adenovirus infections, especially those due to serotypes 4 and 7, plagued the armed forces basic training facilities throughout each winter-spring respiratory virus season, resulting in major morbidity and some mortality, overtaxed and overcrowded hospital facilities, and the loss of significant amounts of time from basic training as a result of recurrent
explosive outbreaks. As a result, military research efforts were directed toward the development of serotype-specific vaccines. These vaccines were shown to be highly effective in trials in the 1960s and early 1970s (Edmondson et al., 1966; Top et al., 1971) and became licensed in 1980. Administration of these oral, live encapsulated adenovirus type 4 and 7 vaccines to recruits on the first day of their arrival at a base rendered the outbreaks a thing of the past. After 25 years of successful use, discussions between DoD and the manufacturer failed to produce an agreement concerning improvements to the manufacturing facility that were required by the Food and Drug Administration (FDA). The sole manufacturer of the adenovirus vaccines stopped producing them in 1996, and the stock was totally depleted by mid-1999. Subsequently, adenovirus illness reemerged as a major cause of illness and hospitalization among new trainees (Gray et al., 1999; McNeill et al., 1999; Sanchez et al., 2001). Virus studies in 1999 and 2000 revealed that 82 percent of the infections were again due to types 4 and 7. Thousands of trainees have been affected, and as a result, many recruits must repeat their training because of time lost due to illness (Gray et al., 2000). Three basic training facilities found their infirmary and hospital facilities overwhelmed and were forced to seek other accommodations for trainees requiring inpatient care. The deaths of at least two previously healthy recruits have been attributed to vaccine-preventable adenovirus infections (CDC, 2001). This committee issued a letter report to the Commanding General of the U.S. Army Medical Research and Materiel Command on November 6, 2000, to urge action to restore the availability and production of adenovirus vaccines (IOM, 2000a). The letter report is reprinted as Appendix A to this report.
The availability of the plague vaccine has also been interrupted. Plague vaccine, first manufactured in the United States by Miles Inc. in 1942 (IOM, 1993), has mostly military but some commercial applications. In 1990, Greer Laboratories took over production of the vaccine (AFEB, 1999). In a September 22, 1997 warning letter to Greer Laboratories, FDA outlined several significant deviations from FDA production guidelines in the manufacture of the company’s plague vaccine (FDA, 1997c). Greer Laboratories discontinued the vaccine in 1998 because “FDA requirements for further testing and validation of the product could not be financially justified, and DoD was not able to fund further studies” (Greer Laboratories, 2001). Currently, plague vaccine is not available to protect U.S. forces.
The anthrax vaccine, adsorbed, also was available in only limited supply to the U.S. military due to regulatory compliance issues. The license to manufacture the vaccine was granted to one manufacturer, the Michigan Department of Public Health, in 1970. Ownership of the facility was transferred to Michigan Biologics Products Institute (MBPI) in 1995 and in 1998 the facility was sold to BioPort. Bioport retains the sole license to manufacture the anthrax vaccine. In March 1997, FDA issued MBPI a Notice of Intent to Revoke after routine inspection of the manufacturing facility by FDA in November 1996 revealed “significant devia-
tions from the Food, Drug, and Cosmetic Act, FDA’s regulations and the standards of MBPI’s license” for the manufacture of blood-derived products and bacterial vaccines (FDA, 1997a; Zoon, 2000, p. 12). Although production of the vaccine had resumed in 1999 (IOM, 2002), BioPort had not been able to release any new lots of the vaccine without further inspections and official FDA approval, significantly restricting the availability of the vaccine to the U.S. military. BioPort upgraded its facilities to comply with FDA standards and on December 27, 2001 and January 31, 2002, respectively, FDA approved a license supplement for the renovations to BioPort’s facility and an additional supplement for the contractor-operated filling site (BioPort Corporation, 2002; IOM, 2002). The approval of the two license supplements has made the vaccine available—once again—to the U.S. military.
ABOUT THIS REPORT
In April 2000, the Institute of Medicine (IOM) of the National Academies convened an expert committee to advise the U.S. Army Medical Research and Materiel Command on the management of research and development efforts related to naturally occurring infectious disease threats to members of the U.S. military, in particular, the acquisition of vaccines to prevent these diseases. This report is the final product of that group, the Committee on a Strategy for Minimizing the Impact of Naturally Occurring Infectious Diseases of Military Importance: Vaccine Issues in the U.S. Military.
The charge to the committee was as follows:
The committee will analyze available information, hold workshops and make specific recommendations on both technical and policy aspects regarding the Department of Defense vaccine strategy to combat infectious diseases. The issues include: (1) reviewing the problem of the naturally occurring infectious diseases threat to military operations; (2)defining and prioritizing the diseases of relevance to the U.S. military; (3) determining the status of vaccines available to protect military personnel; (4) examining the Military Infectious Diseases Research Program (MIDRP), with particular emphasis on current disease priorities, vaccine product development, and the role of the MIDRP not only within the framework of the overall Military Acquisition model, but also among other Federal government infectious disease programs; (5) reviewing the roles, if any, that the MIDRP should play in the licensure, manufacture, and distribution of vaccines against diseases of military importance, in the context of current interrelationships within DoD and among other federal agencies, industry, and university research activities; and (6)developing recommendations for a comprehensive strategy and doctrine that MIDRP and DoD could adopt to best use their resources to contribute toward the goal of effective development, licensure, production, stockpiling, distribution, and use of vaccines against naturally occurring diseases of military importance. Other issues regarding vaccine strategies against infectious diseases are likely to be brought to the attention of the committee by the DoD.
The IOM committee met six times, holding open sessions at its first five meetings and hearing presentations from military personnel, those familiar with
the vaccine industry, and infectious disease and vaccine experts. The committee used those briefings, its review of background material, and its members’ experiences and expertise in its deliberations. As the committee began its work, it made two interpretive decisions about the charge it had been given.
On the basis of their experiences before they became members of the committee, committee members believed that DoD’s current administrative separation of research and development efforts related to vaccines against naturally occurring infectious diseases and vaccines against biological agents that may be weaponized was scientifically—and likely organizationally—unsound. The challenges of vaccine-related research and development are similar for vaccines against both natural and weaponized infectious agents. Moreover, many of the agents both occur naturally and can be used as biological weapons, and vaccination remains the preferred type of medical defense against both types of threats. Thus, although this report was initially intended to address only naturally occurring infectious disease threats, because vaccine policy concerns related to biodefense are inseparable from those dealing with naturally occurring infectious disease threats, in this report, when pertinent, the committee has touched on issues addressing the acquisition of vaccines against biological agents that may be weaponized.
In addition, the committee has interpreted the charge’s reference to “defining and prioritizing the diseases of relevance to the U.S. military” as a request to address how DoD might approach the issue of prioritization rather than a request for the committee to offer a list of specific threats, diseases, or needed vaccine products.
Report Organization
This report, presented in four chapters, began with an historical overview of the influence of naturally occurring infectious diseases on U.S. military operations and the research that has been conducted in response to these threats. In Chapter 2, the committee describes the current role of the U.S. Army Medical Research and Materiel Command in infectious disease-related research and development and vaccine acquisition, including the committee’s understanding of how current priorities emerge and the organizational context within which MIDRP operates within DoD. Chapter 3 describes current naturally occurring infectious disease threats and the available vaccine countermeasures. In Chapter 4, the committee presents it recommendations in the context of its view of the limitations imposed by the current structure for managing infectious disease-related research and development and vaccine acquisition within DoD.











