3
Interfaces
INTRODUCTION
Materials chemistry and engineering are interdisciplinary by nature, and have given rise to many of the synthetic materials that were commonly used over the course of the twentieth century. Advances in the development of new materials often result from work on a specific or perceived need in a host of different areas. As a result, materials chemistry and engineering is closely linked to physics, biology, medicine, metallurgy, ceramic engineering, along with many other fields. Work at the interfaces between these disciplines is some of the most exciting and challenging scientific inquiry today and raises expectations of significant technological impact in the years to come. This chapter provides some illustrative, but by no means exhaustive, examples of the impact of materials chemistry on different disciplines and endeavors, as well as some thoughts on areas in which future technological impact is likely.
MATERIALS CHEMISTRY AND MEDICINE
Many decades of work at the interface between materials chemistry and medicine has brought innumerable advances that are now taken for granted. Developing materials that can be implanted in the body and remain for many years without adverse effects requires understanding of the biological processes that occur around the material and reactions that may occur once implanted in the body, especially if they can have harmful consequences. For example, this type of detailed knowledge has led to the development of special-purpose metal alloys and polymer coatings to prevent the body from rejecting prosthetic bone replacements.
Many other new materials are also used in medical applications where they must adhere to bone, mimic color, flex like natural tissues, and/or keep their form under conditions of use. Today these implantable materials are largely passive and provide structural integrity, such as a hip replacement.
In addition to these implantable materials, materials chemistry and engineering has also had a significant impact on separations technologies used in medicine. Examples of these applications include hemodialyzers, blood oxygenators, leukofilters, intravenous filters, apheresis filters, and diagnostic assays.
One area in which the use of new materials for separations is particularly important is in the development of new biocatalysts. The development of low-cost bioseparations systems has been the focus of much work in the effort to make them competitive with classic organic and inorganic catalysts. Another example is the use of “PS Gel,” a resin used to obtain high-purity blood serum. This material allows for more efficient and accurate clinical testing than is possible using conventional separation techniques.
Developments in materials chemistry and engineering have led to significant improvements in drug delivery systems. Biocompatible polymeric materials have been developed that allow for the controlled delivery of drugs, proteins, and genes. Copolymer networks are being developed that form a mesh-like structure and are potential delivery systems for drugs. By varying the monomers that make up these copolymers, it may be possible to tailor the dosage and time over which these drugs are delivered to the body.
Materials research in the biomedical field has included extensive work on new materials for medical diagnostics, particularly medical sensors. Strained-layer semiconductor superlattices allow scientists to tailor the electrical and optical properties to design materials and devices with targeted properties. These enable the development of new lasers with potential applications to medical diagnostics. Novel polymeric systems are also utilized in devices, instruments, or implants for medical diagnostics.
The interface between materials chemistry, engineering, biology, and medicine presents a number of challenges that must be overcome. One overarching issue is the integration of biocompatible materials into living systems. This includes areas such as bone scaffolding, artificial organs, and tissue engineering. Research on biocompatible materials will emphasize an active role in sensing and responding to stimuli in such areas as the development of neuroprostheses or synthetic muscles. Such functions are likely to be achieved only by highly heterogeneous materials. In the future, research in the chemical sciences related to materials may include such esoteric areas as in situ drug production, nanocellular systems, and human integrated computing. It is almost certain that as research in the biomedical field advances, better sensors that take advantage of the latest developments in new materials will be essential. Success in any of these areas will require detailed understanding of the interactions between the material and the human body, knowledge of the chemistry between the various constituents of
the heterogeneous composite, and understanding of how to control the properties of the composite to produce the desired response.
STRUCTURAL MATERIALS
The role of chemistry is so embedded in structural materials that it is almost taken for granted. An example is in the differences in properties of iron and steel alloys. Materials in modern cars and airplanes, including polymers and polymer composites, make them safer, lighter, and more fuel-efficient than their predecessors as a result of advances in materials synthesis and processing. Progress in the development of structural materials takes many forms, including research, inexpensive production methods, fire resistant materials, materials that are easily recycled, and the incorporation of existing materials in new environments. In all of these areas, significant contributions have been made by chemistry and engineering.
Coatings on structural materials, whether to inhibit corrosion, protect, beautify, or serve some other purpose, are the products of all chemical sciences. So, too, is the science behind the adhesion of these coatings to the base material.
Over the past several decades, composite materials that typically involve the intricate mixing of different materials (metals, ceramics, and/or polymers) in a controlled manner, have come into use as structural materials due to their unusual combinations of properties—for example, immense strength or toughness and light weight. The identification of appropriate combinations of materials in a composite and the optimization of the processing conditions needed to give optimal properties require a detailed understanding of the chemical processes that govern the synthesis route.
In the future, structural materials will incorporate sensing, reporting, and even healing functions into the body of the material. A likely area of development to produce these materials will be multicomponent materials that combine properties of both plastics and ceramics. In addition, sensing materials almost certainly will require tailoring of material properties on a scale that has yet to be achieved for the large amounts of material required for most structural applications. Widely dissimilar materials may have to be controllably integrated, which is an area of research that is well within the domain of the chemical sciences.
INFORMATION TECHNOLOGY AND COMMUNICATIONS
The tremendous advances in computer, information, and communications technologies made over the last few decades have made profound changes in everyday life. The tremendous role that materials chemistry has had in enabling these changes is less obvious. The modern computer chip fabrication facility utilizes chemical processes in manufacturing. The deposition on and removal of material from silicon wafers during processing along with the control and mea
surement of impurities to incredibly low levels all require tremendous understanding of the many chemical processes involved.
The transmission of light through long stretches of optical fiber has historically been limited to two wavelength regions in the infrared spectrum. These two regions are separated by a region in which trace amounts of hydroxyl incorporated into the fiber during fabrication absorb light and prevent its long-distance transmission. Recent advances in controlling the chemical processes at work during fiber fabrication have reduced the amount of hydroxyl in the fiber to the point where long-distance transmission of these formerly unavailable wavelengths of light is now possible. This is a boon to multiplex technology, which uses many closely spaced wavelengths and requires high-quality fiber with properties that are substantially independent of wavelength.
Two exciting future directions in information technology and communications will rely heavily on materials chemistry for success. The first of these is the migration of electronic and optical functions that historically have been performed in inorganic materials (e.g., semiconductors, silica-based fibers) to newly developing organic materials. Prototypes utilizing organic semiconductors in applications such as electronic paper are favorable indications that these materials may have a significant commercial application (Sidebar 3.1).
The second direction is in the development of the control of photons via photonic lattices analogous to that for electrons and holes (Sidebar 3.2). This requires control of materials structure at the length scale of the wavelength of the light to be controlled.
NATIONAL SECURITY
One of the areas of particular concern in national security is the aging and reliability of materials found in systems ranging from weapons to air, land, and sea transportation or combat vehicles. As these various components of our national security apparatus remain in service long beyond their original design life, concern increases about subtle changes in their chemical and structural nature. In order to understand these changes and their implications, it is critical to have the analytical tools and chemical knowledge that allow us to understand the chemical reactions that take place under realistic service conditions. This knowledge in turn gives confidence in the integrity of the system and allows predictive determination of when component replacement or retirement is needed. Future directions may include the development of materials or material systems that report their condition or even repair themselves. Such developments will require the tailoring of materials on a molecular scale.
ENVIRONMENT
Achieving improved quality of life and achieving improved environmental
|
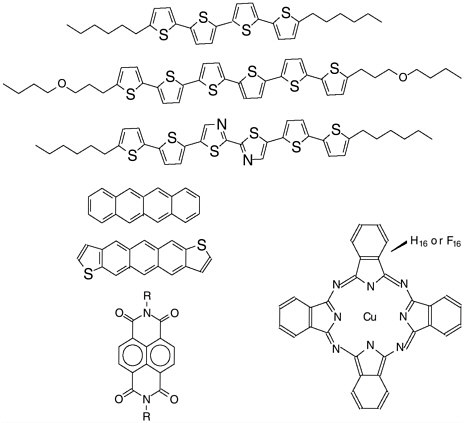
SIDEBAR 3.1 Organic Materials Synthesis: Inspiration and Driver of Organic Semiconductor Devicesa Organic semiconductor development is a highly multidisciplinary effort, encompassing specialists ranging from synthetic chemists to electrical engineers. A main driving force for studying organic-based electronic devices is the potential lower fabrication cost compared to silicon. Organic transistors might be useful in a variety of applications, including large-area displays, memories, sensors, and identification tags, where the low cost is of paramount importance.b Other potential applications are less cost-driven. For example, the use of organic materials increases the opportunity to integrate an electronic device covalently with molecules or biomolecules. Organic materials require only moderate temperatures during processing. This allows the use of applications integrating components or materials of limited thermal stability. Organic materials also enable the production of semiconducting devices on flexible substrates or in nonconventional or nonplanar geometries. An organic semiconductor film, which generally consists of a crystalline film of conjugated aromatic monomers, oligomers, or polymers (Figure 3.1), can be incorporated into a field-effect transistor, a device that enables charge to flow across a channel in a controlled manner. An applied voltage controls the amount of charge that can travel through this channel and can turn the charge flow on or off. Optimizing the mobility— the speed with which a charge will move in a given electric field—of this semiconductor material is one way to enhance the performance of this kind of device. In order to control the properties (on-off ratio, mobility) and hence the function of organic semiconducting devices, the properties of the films formed by the organic molecules must be controlled. Molecular design and synthesis have played vital roles in the emergence of this technology. A variety of aromatic ring systems and sequences of aromatic rings have been used as the active cores of semiconducting film-forming molecules. Furthermore, an almost limitless array of side chains distinguished by length or functionality can be appended to these cores. The figure below presents some representative examples of organic semiconductor molecular structures. Self-assembly properties, charge carrier energy levels, and environmental robustness are some of the properties that can be optimized through variation of the molecular structure. There are many other aspects to the chemistry of organic semiconductors. The film’s substrate can be modified to ease deposition, minimize current leakage, and even provide additional function such as charge storage. The film formation process must be controlled in order to obtain morphologies that allow a charge to be transferred easily among |
|
SIDEBAR 3.2 The Race for the Photonic Chipa Three-dimensional photonic crystals (PCs) with complete photonic band gaps at optical telecommunication wavelengths have generated much interest in the last few years (Figure 3.2). Devices founded on this new class of materials have the potential to revolutionize future photonic technologies. This kind of research may pave the way to future information technologies based on optically integrated, highly compact microphotonic crystal devices, chips, computers, and telecommunication systems.b One approach to moving these synthetic three-dimensional Si-PCs to envisioned photonic technologies hinges on the ability to conduct wafer-scale patterning of single-crystal silica colloidal crystal templates called opal-patterned chips. These templates enable the chemical replication and coupling of photonic crystal lattices—inverse opals—in high-refractive-index semiconductors such as silicon and germanium, to optical waveguides to create photonic crystal devices. In this context, recent and exciting research involves the discovery of the opal-patterned chip. The chip is composed of single-crystal micron-scale features of silica colloidal—opals—that have controlled thickness, area, topography, and orientation and are embedded within an oriented single-crystal silicon wafer. Production of this chip is a straightforward, rapid, and reproducible chemical procedure that is easy to integrate into existing chip fabrication facilities, which makes it amenable to mass production. Opal-patterned chips may provide an enabling technology for engineering photonic crystal lattices, photonic band structures, designed functional defects, and internal light sources in three-dimensional PCs that have complete photonic band gaps operating around 1.5 micrometers. |
quality are inherently in conflict in current society. In order to reach both goals simultaneously, dramatic progress will be required on many fronts, including materials chemistry. Catalytic conversion, which allows the conversion of environmentally harmful chemicals in exhaust streams to relatively benign ones, has already had a dramatic impact on local environmental quality while enabling us to increase our reliance on internal combustion engines. Life-cycle engineering, in which the waste stream is minimized from the production of an initial material to the eventual reclamation and recycling of the product, is becoming widespread in Europe and is attracting increased interest throughout the world. Putting this concept into practice requires materials chemistry at every step. This involves the selection and design of environmentally benign materials, the development of environmentally friendly materials processing methods, and the disassembly of materials into new products or harmless waste.
 FIGURE 3.2 Three-dimensional photonic crystals. These advances in three-dimensional photonic crystals, if reduced to practice, could pave the way to an amalgamation of microphotonic crystal devices with optical waveguides on chips for future optically integrated photonic circuits, computer, and telecommunication systems.
|
AGRICULTURE AND FOOD SERVICES
The current productivity of our agricultural enterprise would not be possible without the developments in fertilization, pesticides, and herbicides made by the chemical sciences. The unprecedented safety of our food supply is largely due to refrigeration (enabled by Freon© and its more environmentally benign successors), clean food-processing conditions, and the development of new generations of packaging materials and technologies. Future developments may include the incorporation of sensors into packaging materials to indicate spoilage or unsafe storage conditions. Developing materials for such sensors and integrating them into packages, will involve the tailoring of materials at the molecular level to achieve the desired combination(s) of properties.
ART AND LITERATURE
Works of art and literature are usually designed to endure for generations if not millennia. Unfortunately, chemical changes in the paper, paint, canvas, film, or stone over the centuries damaged some works. The chemical sciences have contributed to art and literature by allowing the stabilization or restoration of great works of the past. Chemistry has also contributed to the formulation of archival quality supplies for these “traditional” media. The variety of media available is exploding with the growth of electronic and optical media and communication. One new medium is the flat-panel display, which is becoming ubiquitous in the home, workplace, and even outdoors as its cost drops. Liquid crystals, the materials at the heart of many of these displays, are the creation of materials chemists. The molecules in a liquid crystal display tend to align with neighboring molecules even though they are in a liquid state and do not have the long-range translational order of a solid. The chemical sciences are an integral part of the search for the electronic, optical, and/or magnetic archival media of the future.
SUMMARY AND FINDINGS
The nature of the interaction between chemistry and other sciences is driven by the nature of the problem. For example, the problem of developing protein templates draws on the ability of chemists to perform complex syntheses on many different scales. The chemical sciences have had significant impact on advances at the scientific interface, including research into the superparamagnetic effect that has led to a higher storage density as well as advances in micro- and nanofabrication that have enabled the development of new materials.
Finding: Self-diagnosing, self-repairing, multifunctional materials would be of great value for applications in structural components, military equipment, and materials integrated into living systems.
Finding: Progress in materials chemistry faces a series of scientific and technical challenges. These include understanding interfacial science related to materials, multiscale modeling and prediction, and controlled synthesis and controlled assembly.
However, these challenges also show opportunities where skills and expertise could be developed, and where education can be made available for those entering the field.
As the examples in this chapter show, materials chemistry is a central part of many technologies that we take for granted. It will also play a critical role in developments that are poised to improve our lives in the not-too-distant future.