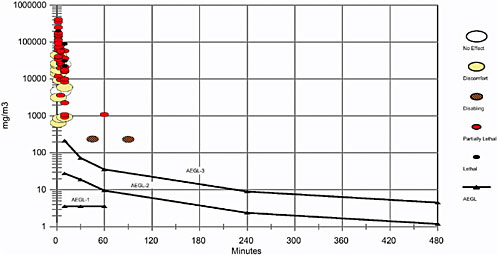
5
Uranium Hexafluoride1
Acute Exposure Guideline Levels
SUMMARY
Uranium hexafluoride (UF6) is a volatile solid. It is one of the most highly soluble industrial uranium compounds and, when airborne, hydrolyzes immediately on contact with water to form hydrofluoric acid (HF) and uranyl fluoride (UO2F2) as follows:
Thus, an inhalation exposure to UF6 is actually an inhalation exposure to a mixture of fluorides. The HF component may produce pulmonary irritation, corrosion, or edema, and the uranium component may produce renal injury.
If death does not occur as a result of HF exposure, renal effects from the uranium moiety might occur (Just 1984).
In the absence of relevant chemical-specific data for deriving the lowest acute exposure guideline level (AEGL) values for UF6, modifications of the AEGL-1 values for HF were used. The use of HF as a surrogate for UF6 was deemed appropriate for the development of AEGL-1 values, which are based on irritation symptoms, because it is likely that HF, a hydrolysis product, is responsible for those low-level effects. The HF AEGL-1 values were based on the threshold for pulmonary inflammation in healthy human adults (Lund et al. 1999). Because a maximum of 4 moles (mol) of HF are produced for every mole of UF6 hydrolyzed, a stoichiometric adjustment factor of 4 was applied to the HF AEGL-1 values to approximate AEGL-1 values for UF6; the AEGL-1 values for UF6 are constant across time up to 1 hour (h) because the HF AEGL-1 values were held constant across time. AEGL-1 values for UF6 were derived for only the 10-minute (min), 30-min, and 1-h time points because derivation of 4- and 8-h values resulted in AEGL-1 values greater than the 4- and 8-h AEGL-2 values calculated for UF6. That would be inconsistent with the total database.
The AEGL-2 values were based on renal pathology in dogs exposed to UF6 at 192 milligrams per cubic meter (mg/m3) for 30 min (Morrow et al. 1982). An uncertainty factor (UF) of 3 was used to extrapolate from animals to humans, and a UF of 3 was also applied to account for sensitive individuals (total UF=10). This total UF of 10 is considered sufficient because the use of a larger total UF would yield AEGL-2 values below or approaching the AEGL-1 values, which are considered no-observed-effect levels and were based on a threshold for inflammation in humans. Furthermore, humans were exposed to HF repeatedly at up to 8 parts per million (ppm) with only slight nasal irritation; that is stoichiometrically equivalent to a UF6 exposure at 28.8 mg/m3, a concentration equivalent to the 10-min AEGL-2. The concentration-exposure time relationship for many irritant and systemically acting vapors and gases may be described by Cn×t=k (C =concentration, t=time, and k is a constant), where the exponent n ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain protective AEGL values in the absence of an empirically derived, chemical-specific scaling exponent, temporal scaling was performed using n=3 when extrapolating to shorter time points and n=1 when extrapolating to longer time points. (Although a chemical-specific exponent of 0.66 was derived from a rat lethality study in which the end point was pulmonary edema, the default values were used for time-scaling AEGL-2 values, because the end points for AEGL-2 [renal toxicity] and for death [pulmonary edema] involve different mechanisms of action.)
The AEGL-3 was based on an estimated 1-h lethality threshold in rats (one-third the LC50 [concentration lethal to 50% of subjects] of 365 mg/m3) (Leach et al 1984). That approach is considered appropriate due to the steepness (n=0.66) of the concentration-response curve for lethality in rats exposed to UF6. An intraspecies UF of 3 was applied and is considered sufficient because the steep concentration-response curve for lethality implies little intra-individual variability. A UF of 3 was also applied for interspecies variability (total UF=10). An application of the full interspecies UF of 10 would result in AEGL-3 values that are inconsistent with the overall data set. For example, assuming the production of 4 mol of HF from the hydrolysis of 1 mol of UF6 (1 mg/m3=0.0695 ppm), exposure at the proposed AEGL-3 values would be equivalent to exposure to HF at 60 ppm for 10-min, 20 ppm for 30-min, 10 ppm for 1-h, 2.5 ppm for 4-h, and 1.3 ppm for 8-h. No effects on respiratory parameters were noted in healthy humans after exposure to HF at 6.4 ppm for 1-h or after repeated exposures of up to 8.1 ppm (Lund et al. 1999; Largent 1960, 1961). Although this scenario does not account for the uranium portion of the exposure, given the steepness of the concentration-response curve it is unlikely that people would experience life-threatening effects at these concentrations. Therefore, an interspecies UF of 3 is justified. The value was then scaled to the 10- and 30-min and 4- and 8-h time points using C1×t=k. An exponent of 0.66 was derived from rat lethality data collected from exposures ranging from 2 min to 1 h duration in the key study. The exponent was rounded to 1.0 for extrapolation because the data used to derive the exponent were limited (from one study) and the derived n was below the range of a normal dose-response curve.
The calculated AEGLs are listed in Table 5–1.
1. INTRODUCTION
UF6 is a volatile solid. It may present both chemical and radiological hazards. It is one of the most highly soluble industrial uranium compounds and, when airborne, hydrolyzes rapidly on contact with water to form hydrofluoric acid (HF) and uranyl fluoride (UO2F2) as follows:
Thus, an inhalation exposure to UF6 is actually an inhalation exposure to a mixture of fluorides. Chemical toxicity may involve pulmonary irritation, corrosion, or edema from the HF component and/or renal injury from the
TABLE 5–1 Summary of Proposed AEGLs for Uranium Hexafluoride (mg/m3)
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
End Point (Reference) |
|
AEGL-1 (Nondisabling) |
3.6 |
3.6 |
3.6 |
NR |
NR |
Modification of hydrogen fluoride AEGL-1 values (EPA 2001) |
|
AEGL-2 (Disabling) |
28 |
19 |
9.6 |
2.4 |
1.2 |
Renal tubular pathology in dogs (Morrow et al. 1982) |
|
AEGL-3 (Lethal) |
216 |
72 |
36 |
9.0 |
4.5 |
Estimated 1-h NOEL for death in the rat (Leach et al. 1984) |
|
Abbreviations: mg/m3, milligrams per cubic meter; NOEL, no-observed-effect level; NR, not recommended. |
||||||
uranium component (Fisher et al. 1991). There is also evidence to suggest that the fluoride ion might contribute to renal toxicity after repeated exposure (G.Rusch, Honeywell Inc., personal commun., Nov. 24, 1999). As concentration decreases and duration increases, the effects of HF are reduced, and the effects of the uranium component may increase (Spiegel 1949). Thus, if death does not occur from HF exposure, renal effects from the uranium moiety may occur (Just 1984).
In addition, UF6 emits alpha, beta, and gamma radiation (Allied Signal 1994). The predominant uranium isotope found in nature, 238U, is not fissionable, and consequently, for uranium to be used as a fuel in nuclear reactors, the ratio of 235U to 238U is increased from 0.72% to 2–4% (enriched) by the gaseous diffusion process. UF6 is produced by fluorinating processed uranium oxide ore (U3O8), and is used in the uranium enrichment process. The UF6 stream containing both 235U and 238U is divided into separate streams. One is enriched, and the other is depleted (ATSDR 1997).
The predominant concern from acute exposure to soluble uranium compounds is chemical toxicity. However, depending on the degree of enrichment, radiologic toxicity might also be important. However, it is difficult to quantitate the radiological hazard without knowing the precise degree of enrichment (ATSDR 1997).
The physicochemical properties of UF6 are presented in Table 5–2.
TABLE 5–2 Physical and Chemical Data for Uranium Hexafluoride
|
Parameter |
Value |
Reference |
|
Common name |
Uranium hexafluoride |
ATSDR 1997 |
|
Synonyms |
Uranium fluoride (fissile); UN2977 |
ATSDR 1997 |
|
CAS registry no. |
7783–81–5 |
ATSDR 1997 |
|
Chemical formula |
UF6 |
ATSDR 1997 |
|
Molecular weight |
352.02 |
ATSDR 1997 |
|
Physical state |
Colorless solid |
ATSDR 1997 |
|
Odor |
Odorless |
AIHA 1995 |
|
Vapor pressure |
115 mm Hg at 25°C |
ATSDR 1997 |
|
Density |
4.68 g/cm2 at 21°C |
ATSDR 1997 |
|
Triple (melting) point |
64.05°C at 760 mmHg |
AIHA 1995 |
|
Boiling point |
56.2°C |
ATSDR 1997 |
|
Solubility |
Soluble in carbon tetrachloride and chloroform; hydrolyzes in cold water, alcohol, and ether |
AIHA 1995 |
|
Conversion factors in air |
1 ppm=14.4 mg/m3 (in dry air) 1 mg/m3=0.0695 ppm (in dry air) 1 mg of UF6=0.676 mg of U 1 mg of UO2F2=0.767 mg of U (based on a mixture of 235U and 238U) |
AIHA 1995 |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
2.1.1. Case Reports
On September 2, 1944, an accidental UF6 release occurred at a Manhattan Engineering District pilot plant located at the Philadelphia naval shipyard (Kathren and Moore 1986). A weld ruptured in a 2.4 m×0.2 m cylinder containing gaseous UF6, causing the cylinder to act as a rocket. It traveled 164 m, tore out pipes and fittings in its path, and released an estimated 182 kg of UF6. The released UF6 and steam produced a dense cloud of UF6
and hydrolysis products that enveloped the area within 91.4 m of the rupture site. The cloud rapidly dispersed and exposure duration was estimated to be 17 seconds. Twenty workers were exposed at varying degrees, depending on their location during the rupture. Two people, both in a direct line with the cylinder, died—one died 10–16 min after exposure, and the other died 70 min after exposure. Three people were seriously injured, 12 were hospitalized for observation, and three were without symptoms. The seriously injured individuals experienced chemical conjunctivitis with edema, chemical erosion of the cornea (resulting in temporary blindness), first-, second-, and third-degree chemical burns, nausea and vomiting, chemical bronchitis, pulmonary edema, and/or shock. Blood counts suggested hemoconcentration in one seriously injured individual, and albumin and casts were observed in the urine. Another seriously injured individual had an essentially normal blood profile; however, albuminuria and urine casts were noted. The seriously injured workers completely recovered within 3 weeks (wk) of the accident. Two of the seriously injured workers were re-examined 38 years (y) after the accident for deposition and potential long-term effects of uranium exposure. The initial lung deposition was estimated to be 40–50 mg of uranium on the basis of fragmentary urinary excretion data obtained shortly after the accident, and the initial long-term bone deposition was estimated at 410 µg (5.2 Bq) in the worker exposed at the highest concentration. That would result in a 40-y dose-equivalent to the bone of approximately 200 mrem. The 38-y follow-up medical and health physics exams of two of the seriously injured workers revealed no detectable uranium deposition and no findings attributable to uranium exposure (Kathren and Moore 1986; Moore and Kathren 1985).
Another accident occurred on January 4, 1986, at a uranium conversion facility in Gore, Oklahoma (Nuclear Regulatory Commission 1986). A 12,700 kg overloaded cylinder containing approximately 13,500 kg of UF6 ruptured when it was being heated in a steam chest to remove excess UF6. The release lasted for approximately 40 min. A dense white cloud formed and was pushed by the wind. It quickly engulfed the process building (the facility’s main structure) and formed a plume expanding to the south-southeast. When the UF6 left the ruptured cylinder, not all of it reacted immediately to form UO2F2 and HF; pieces of solid UF6 were scattered widely around the steam chest area. Any UF6 remaining when the area was sprayed down reacted with the water and was likely captured in the water spray. It was estimated that approximately half of the released UF6 was washed into an emergency pond. Therefore, approximately 6,700 kg contributed to the noxious white cloud. When completely reacted with water,
6,700 kg of UF6 forms 5,900 kg of UO2F2 and 1,500 kg of HF. Peak 10-min UO2F2 concentrations ranged from 0.011 mg/m3 to 8.8 mg/m3, and peak 10-min HF concentrations ranged from 0.008 mg/m3 to 2.4 mg/m3. One-hour average uranium concentrations ranged from <0.052 µg/m3 to 20,000 µg/m3. It was assumed that 4.55×106 g of uranium was released into the atmosphere in a 45 min period. The isotopic composition was 1.49 Ci of 234U, 0.07 Ci of 235U, and 1.49 Ci of 238U.
There were 42 workers at the plant site when the accident occurred. Seven of the workers (contract workers) were in a trailer well away from and upwind of the release point. One worker (an operator in a scrubber building 50 ft from the steam chest) died from pulmonary edema produced by HF inhalation within a few hours of the exposure. Another was treated for skin irritation and burns from HF exposure, and 21 others were examined at a hospital and kept overnight for observation. Four of the 21 workers were released the following morning, 14 were kept more than 1 day (d) and were given oral sodium bicarbonate to enhance uranium excretion, and three were transferred to another hospital for observation and treatment of potential lung damage from HF exposure. Of the eight employees who returned to spray water on the fumes, one was in the group transferred to another hospital with serious respiratory symptoms, and five were hospitalized for more than 1 d (including the individual with HF burns). Only two of those workers were released on the day of the accident.
Local off-site residents were also asked to report to a hospital for examination. One resident was kept overnight for observation, but no medical treatment was deemed necessary. Urine samples were collected from all on-site workers and from 100 off-site members of the public for uranium bioassay and urinalysis (osmolality, creatinine, protein, glucose, LDH, albumin, β-microglobulin, and n-acetyl-β-glucosaminidase). Initial uranium concentrations in the urine of employees (noncontract workers) ranged from <2 µg/L to 11,000 µg/L; however, those values might not be indicative of the total amount of uranium excreted, because the total sample volumes were less than 1 L. Levels in the urine among the contracted employees ranged from <2 µg/L to 2,600 µg/L. The initial mean for all workers was 2,165 µg/L. The initial mean for those that returned to the scene of the release to wash down the area was 2,898 µg/L, and the initial mean for the contract workers in the trailer was 456 µg/L. These data were used to calculate the following estimated uranium intakes. The noncontracted employees had mean estimated intakes of 0.26 mg to 27.63 mg, and contracted employees had intakes ranging from 0.02 mg to 7.73 mg. The average for all workers was 6.5 mg. Urinalysis values showed elevated glucose
levels in five workers, increased β-microglobulin levels in nine other workers, and increased n-acetyl-β-glucosaminidase in seven workers (three of whom also had increased β-microglobulin and one of whom showed increased glucose). However, there was no correlation between estimated uranium intake and urinalysis values, and those data are further confounded by the fact that several workers were diagnosed with urinary tract infections and/or diabetes (both incidental to the accident) during their medical examinations. Therefore, no conclusions can be drawn concerning possible renal damage from the UF6 exposure.
The uranium bioassay data for the 100 off-site individuals suggest estimated intakes of 0.1 mg to 0.9 mg of uranium. Effective total-body dose equivalents and doses to organs were estimated assuming a 1-h exposure to the plume. The hypothetically maximally exposed off-site individual would have received a total-body dose of 6.5 millirem (mrem), 43.3 mrem to the kidneys, 202.5 mrem to the bone, and 2.5 mrem to the lungs. The nearest residents would have received a total-body dose of 2.2 mrem, 14.6 mrem to the kidneys, 68.2 mrem to the bone, and 0.8 mrem to the lungs. The maximally exposed worker would have received 46 mrem to the total body and 1,400 mrem to the bone. The background radiation total-body dose for persons living in the Gore, Oklahoma, area is 106 mrem/y. Thus, for maximally exposed off-site persons, residents, and even for exposed workers, the doses are too small to produce any acute health effects from radiation. The likelihood of long-term (carcinogenic) effects is discussed in Section 2.5.
2.2. Nonlethal Toxicity
2.2.1. Case Reports
Both lethal and nonlethal and effects from UF6 exposure were identified in two accident reports (Nuclear Regulatory Commission 1986; Kathren and Moore 1986). Those case reports are described in Section 2.1.1.
2.3. Developmental and Reproductive Toxicity
No information concerning developmental and reproductive toxicity in humans following acute inhalation exposure to UF6 was located.
2.4. Genotoxicity
Genotoxicity studies regarding acute human exposure to UF6 were not available.
2.5. Carcinogenicity
No information concerning carcinogenicity in humans following acute inhalation exposure to UF6 was located. Because UF6 is radioactive, it could potentially damage DNA and lead to cancer; however, without knowing the precise degree of enrichment, it is difficult to quantitate the potential radiologic hazard. Lung cancers observed in uranium mine workers are believed to be the result of chronic radon exposure (ATSDR 1997).
A 38-y follow-up examination of two workers seriously injured from an accidental acute UF6 exposure revealed no findings (carcinogenic or noncarcinogenic) attributable to uranium exposure (Moore and Kathren 1985).
The estimated lifetime cancer risk from the Gore, Oklahoma, accident was calculated for maximally exposed off-site individuals and nearby residents assuming a 1-h exposure to the plume and additional exposure by inhalation of resuspended material (annual inhalation) and ingestion of potentially contaminated vegetables, meat, and milk (annual ingestion) (Nuclear Regulatory Commission 1986). The assessment took into account the specific activities of the uranium isotopes. The calculated cancer fatality risks were extremely small—4.3×10−7 to the maximally exposed individual and 1.4×10−7 to a typical nearby resident.
2.6. Summary
Case reports from human accidental exposures to UF6 indicate that acute toxicity is chemical, not radiologic, in nature and is due to the hydrolysis products, hydrogen fluoride (HF) and uranyl fluoride (UO2F2). At high concentrations, death from HF-induced pulmonary edema is observed. Severe ocular injury; skin burns; and ocular, mucous membrane, and respiratory irritation are also attributable to HF. Kidney damage attributable to UO2F2, was also suggested from urinalysis data. No developmental and reproductive or genotoxicity data were available. The carcinogenic hazard
from radiation exposure is negligible compared with the chemical toxicity from acute inhalation exposure to UF6.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
Groups of 20 rats (strain and gender not specified) were exposed to UF6 at 942, 1,083, or 2,284 mg/m3 for 10 min and observed for up to 30 d (Spiegel 1949). Animals were exposed in a 4-ft3 chamber made of Monel metal that was set 3 ft above the floor. Small portable cages were placed in two layers within the chamber. UF6 was introduced into the chamber as a continuous stream. Six 1-min samples were taken during the 10 min period and analyzed for concentration using a ferrocyanide acetic acid chemical method. Upon removal from the chamber, all animals were gasping for breath; severe irritation of the nasal passages and conjunctivitis was also observed and persisted for a period of several hours. No rats died during the exposure; mortality during the 30 d after exposure was 10%, 30%, and 75%, respectively, for the 942-, 1,083-, or 2,284-mg/m3 exposure groups (Table 5–3). The maximum incidence of mortality was observed from 5 d to 8 d post-exposure, with few animals dying after day 10. Additional rats sacrificed in a daily serial study after the termination of exposure exhibited severe damage to the renal cortical tubules. This renal damage peaked between 5 and 8 d after exposure, and then regenerative processes started. Mild, inflammatory pulmonary changes were observed in rats sacrificed 1–4 d after exposure. One rat that died on day 5 exhibited severe lung injury.
In 46 single exposures, Leach et al. (1984) exposed groups of 10 male Long-Evans rats (200–250 g) to concentrations of UF6 ranging from 651 mg/m3 to 409,275 mg/m3 for 2, 5, or 10 min. Two forms of UF6 were used in the experiments; the first was prepared from natural uranium and the second was enriched to 94% 235U. Exposures were nose-only in a polyethylene and stainless steel chamber. The UF6 was metered from a heated gas cylinder into the inlet air system of the exposure unit. Before introduction into the exposure chamber, the concentrated vapor was mixed with clean compressed air maintained at a relative humidity of 50–95%. The UF6 was drawn into the chamber and past the individual test animals’ noses. The chamber airflow was maintained at 16–28 L/min at a temperature of 22–
TABLE 5–3 Mortality After 10-Min Uranium Hexafluoride Exposure (%)
|
|
Rat |
Mouse |
Guinea Pig |
|||
|
|
Mortality |
Mortality |
Mortality |
|||
|
Concentration (mg/m3) |
During Exposure |
During 30 d After |
During Exposure |
During 30 d After |
During Exposure |
During 30 d After |
|
942 |
0 |
10 |
0 |
20 |
0 |
13 |
|
1,083 |
0 |
30 |
0 |
70 |
0 |
20 |
|
2,284 |
0 |
75 |
30 |
95 |
27 |
40 |
|
Source: Data from Spiegel et al. 1949. |
||||||
26°C. During exposure, a colorimetric, cascade impactor method was used to monitor uranium concentration, and fluoride ion was measured with a fluoride ion-specific electrode. Immediately after exposure, the animals were removed from the restraining tubes and their heads were washed with aqueous detergent to remove deposited uranium and HF. Selected animals were whole-body counted for radioactivity. Surviving animals were returned to individual metabolism cages and held for up to 14 d. Urine and feces were collected daily, and when enriched uranium was used, excreta was measured for uranium content by gamma counting. The assessment of renal injury was monitored (only in exposures with 0–20% mortality) by measuring daily excretory changes in urine volume, protein, glucose, and selected enzymes (NAG, GGT, AP, LDH, AST, ALT).
Mortality data are presented in Tables 5–4, 5–5, and 5–6. Of the rats that died, 25% died during exposure or within 48 h of exposure, 59% died between days 3 and 7, and 17% died between days 8 and 14. The study authors attributed deaths occurring within 48 h of exposure to HF toxicity. Deaths occurring after 48 h were attributed to renal (uranium) toxicity or a combination of uranium and HF toxicity. Calculated rat LC50 values were 117,515 mg/m3, 57,100 mg/m3, and 17,751 mg/m3 for the 2-, 5-, and 10-min time points, respectively. The mean percentages of absorbed uranium dose found in urine, bone, kidneys, and lungs were 13–61%, 28–79%, 3–9%, and 0–6%, respectively. Cumulative uranium excretion over 6 d in the feces ranged from 59% to 81% of the inhaled dose. With a soluble uranium compound such as UF6, one might have expected predominately urinary excretion (especially given that fecal excretion typically accounts for less than 1% of excretion of soluble uranium compounds after inhalation expo-
TABLE 5–4 Rat Mortality and Renal Injury Indicators After 2-Min Uranium Hexafluoride Exposure
|
UF6 Concentration (mg/m3) |
Mortality at 14-d Post-Exposure |
Renal Injury Indicators of Surviving Animals |
|
651 |
0/10 |
P, G, PO |
|
3,151 |
0/10 |
P, G, PO |
|
11,925a |
1/10 |
P, G, PO |
|
14,216a |
0/10 |
P, G, PO |
|
17,899 |
0/10 |
P, G, PO |
|
24,689 |
0/10 |
P, G, PO, AP, LDH |
|
44,615 |
0/10 |
P, G, PO |
|
60,533 |
1/10 |
P, G, PO |
|
98,979 |
2/10 |
P, G, PO |
|
116,050 |
3/10 |
P, G, PO |
|
140,533 |
7/10 |
— |
|
153,107 |
6/10 |
— |
|
172,411 |
2/10 |
P, G, PO |
|
251,479 |
8/10 |
— |
|
352,663 |
9/10 |
— |
|
409,275 |
9/10 |
— |
|
aEnriched UF6 (94% 235U), other exposures were natural uranium. Abbreviations: AP, alkaline phosphatase; G, glucosuria; LDH, lactate dehydrogenase; P, proteinuria; PO, polyuria. Source: Data from Leach et al. 1984. |
||
sure); however, it is likely that the rats trapped the particles in the nasopharyngeal region and swallowed them. Urine bioassays indicated renal injury at all exposure durations (Tables 5–4, 5–5, and 5–6). Proteinuria and glucosuria were greatest 4–5 d following exposure and tended to disappear during the second week. Urine volumes reached their maximum on days 5 and 6 post-exposure. Histopathologic examination of kidneys (14-d post-exposure) showed renal lesions at all concentrations except for the two lowest 2-min exposures. Regeneration of tubular epithelium was apparent, and many tubules were dilated and lined with flattened abnormal epithelium. The lungs of surviving rats examined 14-d post-exposure showed no
TABLE 5–5 Rat Mortality and Renal Injury Indicators After 5-Min Exposure
|
UF6 Concentration (mg/m3) |
Mortality at 14-d Post-Exposure |
Renal Injury Indicators of Surviving Animals |
|
859 |
0/10 |
P, G, PO, UP |
|
3,669 |
1/10 |
P, G, PO |
|
4,675a |
0/10 |
— |
|
9,260a |
0/10 |
PO |
|
9,571a |
1/10 |
P, G, PO |
|
20,000 |
1/10 |
P, G, PO |
|
25,178a |
0/10 |
— |
|
25,562 |
1/10 |
PO |
|
26,686 |
6/10 |
— |
|
26,938a |
4/10 |
— |
|
30,429 |
8/10 |
— |
|
37,899 |
8/10 |
— |
|
39,260a |
1/10 |
P, G, PO |
|
41,731a |
6/10 |
— |
|
54,940 |
1/10 |
PO |
|
65,237 |
5/10 |
— |
|
81,731 |
8/10 |
— |
|
aEnriched UF6 (94% 235U), other exposures were natural uranium. Abbreviations: G, glucosuria; P, proteinuria; PO, polyuria; UP, five urinary enzymes (GGT, LDH, AP, LAP, NAG). Source: Data from Leach et al. 1984. |
||
treatment-related effects. However, animals that died during or shortly after exposure had congestion, acute inflammation, and focal degeneration of the upper respiratory tract. The tracheas, bronchi, and lungs exhibited acute inflammation with epithelial degeneration, acute bronchial inflammation, and acute pulmonary edema and inflammation, respectively.
Leach et al. (1984) also reported a 1-h rat LC50 of 1,095 mg/m3. No further information was presented, although it is assumed that exposure conditions were similar to those used in the 2-min, 5-min, and 10-min studies.
TABLE 5–6 Rat Mortality and Renal Injury Indicators After 10-Min Exposure
|
UF6 Concentration (mg/m3) |
Mortality at 14-d Post-Exposure |
Renal Injury Indicators of Surviving Animals |
|
932a |
0/10 |
P, G, PO, NAG |
|
5,947 |
0/10 |
P, G, PO |
|
8,388a |
1/10 |
P, G, PO, NAG |
|
10,059 |
2/10 |
P, G, PO |
|
10,074 |
1/10 |
P, G, PO |
|
16,006 |
3/10 |
P, G, PO |
|
16,435 |
1/10 |
P, G, PO |
|
17,766 |
8/10 |
— |
|
21,464 |
6/10 |
PO |
|
22,855 |
10/10 |
— |
|
31,302 |
10/10 |
— |
|
36,568 |
8/10 |
— |
|
57,899 |
7/10 |
PO |
|
89,334 |
10/10 |
— |
|
aEnriched UF6 (94% 235U), other exposures were natural uranium. Abbreviations: G, glucosuria; NAG, n-acetyl glucosamine; P, proteinuria; PO, polyuria. Source: Data from Leach et al. 1984. |
||
3.1.2. Mice
Groups of 20 mice (strain and gender not specified) were exposed to UF6 at 942, 1,083, or 2,284 mg/m3 for 10 min and observed for up to 30 d (Spiegel 1949). Animals were exposed, in 4-ft3 chamber made of Monel metal that was set 3 ft above the floor. Small portable cages were placed in two layers within the chamber. The UF6 was introduced into the chamber as a continuous stream. Six 1-min samples were taken during the 10-min period and were analyzed for concentration using a ferrocyanide acetic acid chemical method. Upon removal from the chamber, all animals were gasping for breath; severe irritation of the nasal passages, conjunctivitis, and closed, encrusted eyes were also observed and persisted in survivors for a period of several hours. Six mice exposed at the highest concentration died
during the exposure period; mortality during the 30 d after exposure was 20%, 70%, and 95%, respectively, for the 942-, 1,083-, or 2,284-mg/m3 exposure groups (Table 5–3). The maximum incidence of mortality was observed from day 5 through day 8 post-exposure, with few animals dying after day 10.
3.1.3. Guinea Pigs
Groups of 15 guinea pigs (strain and gender not specified) were exposed to UF6 at 942, 1,083, or 2,284 mg/m3 for 10 min and observed for up to 30 d (Spiegel 1949). Animals were exposed in a 4-ft3 chamber made of Monel metal that was set 3 ft above the floor. Small portable cages were placed in two layers within the chamber. The UF6 was introduced into the chamber as a continuous stream. Six 1-min samples were taken during the 10-min period and analyzed for concentration using a ferrocyanide acetic acid chemical method. Upon removal from the chamber, all animals were gasping for breath; severe irritation of the nasal passages and conjunctivitis were also observed and persisted in survivors for a period of several hours. Four guinea pigs in the high-concentration group died during the exposure period; mortality during the 30 d after exposure was 13%, 20%, and 40%, respectively, for the 942-, 1,083-, and 2,284-mg/m3 exposure groups (Table 5–3). The maximum incidence of mortality was observed from day 5 through day 8 post-exposure, and few animals died after day 10.
In 14 single exposures, Leach et al. (1984) exposed groups of six male Hartley guinea pigs (approximately 350 g) to concentrations of UF6 ranging from 26,642 mg/m3 to 203,580 mg/m3 for 2 min. Two forms of UF6 were used in the experiments; the first was prepared from natural uranium and the second was enriched to 94% 235U. Exposures were nose-only in a polyethylene and stainless steel chamber. The UF6 was metered from a heated gas cylinder into the inlet air system of the exposure unit. Before introduction into the exposure chamber, the concentrated vapor was mixed with clean compressed air maintained at a relative humidity of 50–95%. The UF6 was drawn into the chamber and past the individual test animals noses. The chamber airflow was maintained at 16–28 L/min at a temperature of 22–26°C. During exposure, a colorimetric cascade impactor method was used to monitor uranium concentration, and fluoride ion was measured with a fluoride ion-specific electrode. Immediately after exposure, the animals were removed from the restraining tubes and their heads were washed with aqueous detergent to remove deposited uranium and HF. Selected animals
were whole-body counted for radioactivity. Surviving animals were returned to individual metabolism cages and held for up to 14 d. Urine and feces were collected daily, and when enriched uranium was used, excreta were measured for uranium content by gamma counting. The assessment of renal injury was monitored (only in exposures with 0–20% mortality) by measuring daily excretory changes in urine volume, protein, glucose, and selected enzymes (NAG, GGT, AP, LDH, AST, ALT). Mortality data are presented in Table 5–7. Of the guinea pigs that died, 64% died during exposure or within 48 h of exposure; 31% died between days 3 and 7; and 6% died between days 8 and 14. Deaths occurring within 48 h of exposure were attributed to HF toxicity. Deaths occurring after 48 h were attributed to renal (uranium) toxicity or a combination of uranium and HF toxicity. The calculated guinea pig 2-min LC50 value was 91,864 mg/m3 (Table 5–8). The mean percent of absorbed uranium dose found in urine, bone, kidneys, and lungs was 56–59%, 34–43%, 2–5%, and 0–5%, respectively. Cumulative uranium excretion over 6 d in the feces was approximately 59% of the inhaled dose. With a soluble uranium compound such as UF6, one might have expected predominately urinary excretion; however, it is likely that the guinea pigs trapped the particles in the nasopharyngeal region and swallowed them. Urine bioassays indicated renal injury at all exposure concentrations (Table 5–7).
3.2. Nonlethal Toxicity
3.2.1. Dogs
Morrow et al. (1982) exposed two young adult female beagle dogs (6–10 kg) to UF6 at 192–284 mg/m3 for 30 min to 1 h. The dogs had been anesthetized with intravenous pentobarbital sodium and placed into individual body plethysmographs with their heads exteriorized through a neck seal. Volume meters were connected to the plethysmograph and the dogs were intubated with a cuffed endotracheal tube. The chambers used in the recent studies of Leach et al. (1984) were also used for this dog study; however, in place of the rat/guinea pig holders being connected to the chamber, the dog’s endotracheal tube was connected using a stopper. One dog was necropsied 6 d post-exposure, and the other 16 d post-exposure. No lung pathology was observed. Renal pathology showed tubular regeneration typified by increased mitotic figures and development of flattened tubular epithelium. Residual manifestations of tubular dilation were observed in the dog sacrificed 16 d after exposure.
TABLE 5–7 Guinea Pig Mortality and Renal Injury Indicators After 2-Min Exposure
|
UF6 Concentration (mg/m3) |
Mortality at 14-d Post-Exposure |
Renal Injury Indicators of Surviving Animals |
|
26,642 |
0/6 |
P, G, PO |
|
34,083a |
2/6 |
PO |
|
39,896 |
1/6 |
P, G, PO, NAG |
|
40,459 |
1/6 |
PO |
|
60,429a |
4/6 |
— |
|
61,317 |
3/6 |
P, G, PO |
|
73,092 |
3/6 |
PO |
|
96,376 |
1/6 |
PO |
|
98,979 |
3/6 |
P, G, PO |
|
112,944 |
3/6 |
PO |
|
140,533 |
5/6 |
— |
|
153,107 |
3/6 |
P, G, PO |
|
156,464 |
4/6 |
— |
|
203,580 |
6/6 |
— |
|
aEnriched UF6 (94% 235U), other exposures were natural U. Sources: G, glucosuria; NAG, n-acetyl glucosamine; P, proteinuria; PO, polyuria. Source: Data from Leach et al. 1984. |
||
In this same study, 17 dogs were similarly exposed to UO2F2 at 200–270 mg/m3 for 30 min to 2.5 h and sacrificed from 1 d to 19 d post-exposure. No lung damage was noted in these dogs. Between 1 d and 3 d, kidneys
TABLE 5–8 Calculated LC50 Values for Animals Exposed to Uranium Hexafluoride
|
Species |
Exposure Time (min) |
LC50 (mg/m3) |
|
Rat |
2 |
177,515 |
|
Rat |
5 |
57,100 |
|
Rat |
10 |
17,751 |
|
Rat |
60 |
1,095 |
|
Guinea Pig |
2 |
91,864 |
|
Source: Data from Leach et al. 1984. |
||
showed widely scattered necrosis of segments of the deep convoluted tubules and the straight portions of the corticomedullary junctions extending toward the mid-cortex. The tubules contained hyaline material and proteinaceous casts, and some collecting tubules contained calcified debris. The tubular epithelial cells were pale and were often anucleated and denuded. After 3 d, evidence of tubular regeneration was rare, but after 6 d, it was seen in all dogs, especially at lower concentrations. In animals sacrificed 14 d and 19 d after exposure, tubular dilation and atrophy were present.
3.3. Developmental and Reproductive Toxicity
No studies were located concerning developmental and reproductive toxicity from acute inhalation of UF6 in animals.
3.4. Genotoxicity
No genotoxicity studies were located concerning UF6 acute inhalation exposures.
3.5. Carcinogenicity
Inhalation of UF6 at 0.05 mg/m3 for 1 y caused mild renal tubular effects in one of 13 dogs and one of 141 rats. Mild atrophic changes were observed in “most dogs and in some rats and rabbits.” No neoplastic effects were noted (Spiegel 1949), and no control data were presented.
3.6. Summary
Rats and guinea pigs exposed to UF6 for 2–10 min showed upper and lower respiratory tract irritation, pulmonary edema, and renal lesions. Those effects are the results of the hydrolysis products of UF HF is responsible for the respiratory effects, and uranium is responsible for the renal pathology. Dogs administered UF6 intratracheally showed no respiratory or pulmonary effects but showed renal effects from uranium. Lethality data suggest that rats are more resistant to UF6-induced lethality than are guinea
pigs (Table 5–8). No reproductive and developmental data or genotoxicity data were located. There was no evidence of carcinogenicity in dogs that inhaled 0.05 mg/m3 for 1 y.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
UF6 and its hydrolysis products, hydrogen fluoride (HF) and uranyl fluoride (UO2F2), are all soluble and may be absorbed from the lung after inhalation exposure.
UO2F2 is rapidly absorbed from the lung into the bloodstream as the UO22+ ion behaves like calcium and complexes with serum proteins and bicarbonate. The uranyl ion forms biscarbonate, UO2(CO3)3−, and citrate complexes in plasma. The UO22+ also binds to red blood cells. As the UO22+ ion passes through the kidney and is filtered from blood, it dissociates within the tubular filtrate and recombines with cell surface ligands. Removal of the uranyl ion from the kidneys is described by a two-component exponential model where 92–95% is excreted in the urine with a half-time of 2–6 d and the remainder is excreted with a half-time of 30–340 d. While most of the uranium absorbed into the blood is excreted in urine, there is some generalized systemic deposition in soft tissues, primarily the liver, and in the bone, where it replaces calcium. Deposition rates in bone correlate with bone growth and remodeling rates. Recirculating UO22+ from bone goes to the kidneys. Long-term retention of uranium in bone is described by a two-compartment model, where 90% of uranium is cleared in a half-time of 20–300 d and the remainder is cleared in a half-time of 3,700–5,000 d (Fisher et al. 1991).
HF, the other UF6 hydrolysis product, is highly soluble in water and is readily absorbed in the upper respiratory tract at lower concentrations. However, at very high concentrations HF may reach the lungs. The relatively low dissociation constant (3.5×10−4) allows the non-ionized compound to penetrate the skin, respiratory system, or gastrointestinal system and form a reservoir of fluoride ions that bind calcium and magnesium, forming insoluble salts (Bertolini 1992). Fluoride ion is readily absorbed into the blood stream and is carried to all organs of the body in proportion to their vascularity and the concentration in the blood; equilibrium across biological membranes is rapid (Perry et al. 1994). Significant deposition occurs in the bone, where the fluoride ion substitutes for the hydroxyl group
of hydroxyapatite, the principal mineral component of bone. In humans, chronic exposure to elevated levels of fluoride or HF has produced osteofluorosis. Elimination is through the kidneys.
4.2. Mechanism of Toxicity
The mechanism of toxicity of UF6 involves both chemical and radiologic components. The chemical toxicity is due to the two hydrolysis products, UO2F2 and HF. Uranium metal has five oxidation states; however, only the +4 and +6 are stable enough to be of practical importance. The +6 forms the uranyl ion, which forms the water soluble compounds and is an important species of uranium in body fluids (ATSDR 1999).
Nephrotoxicity is the most sensitive indicator of toxicity from acute high-level exposure to uranium in mammals. The kidney effects are manifested histologically as glomerular and tubular wall degeneration, and ultrastructural examination indicates damage to endothelial cells in the glomerulus, such as loss of cell processes and decreased density of the endothelial fenestrae. Loss of the brush border, cellular vacolization, and necrosis may be noted in the terminal segments of the proximal convoluted tubules. This process may lead to the disruption of tubular resorption of solutes and to a decreased filtration rate of the glomerulus. Indicators of uranium-induced nephrotoxicity include excessive urinary excretion of protein, glucose, enzymes, catalase, or alkaline phosphatase.
Bicarbonate activity in the kidneys has been suggested as a possible mechanism for uranium-induced renal toxicity. Uranium is usually combined with bicarbonate or a plasma protein in the blood. When uranium is released from the bicarbonate in the kidneys it is free to form complexes with phosphate ligands and protein in the tubular wall, which may result in damage. However, the uranium is not tightly bound and is thus released within days. Within a week post-exposure, the uranium is cleared from the kidneys, and the tubules start to regenerate. Another proposed mechanism of uranium-induced renal toxicity involves inhibition of sodium transport-dependent and transport-independent ATP utilization and mitochondrial oxidative phosphorylation in the proximal tubule (ATSDR 1999).
There is also evidence to suggest that the fluoride ion might contribute to renal toxicity, especially after repeated exposures. Renal cortex degeneration and necrosis was noted in rats exposed to fluoride (as HF) at 24 mg/m3 for 6 h/d, 6 d/wk for 30 d; however, no renal effects were observed in rats similarly exposed at 7 mg/m3 (Stokinger 1949). Renal tubular necrosis and congestion were reported in rats and guinea pigs exposed to HF (unreported
concentration and duration) (Machle et al. 1934). Degeneration and necrosis was noted in the convoluted tubules of rabbits exposed to fluoride (as HF) at 14 mg/m3 for 6–7 h/d for 50 d (Machle and Kitzmiller 1935). The relevance of HF inhalation exposure to kidney toxicity in humans is questionable, because the occupational exposure data suggest that HF is not nephrotixic in humans (ATSDR 1993).
The available studies indicate that HF is a severe irritant to the skin, eyes, and respiratory tract. It is particularly irritating to the anterior nasal passages where, depending on species and concentration, it appears to be effectively scrubbed from the inhaled air. Effective deposition in the anterior nasal passages may be attributed to the high solubility and reactivity of HF. Penetration into the lungs results in pulmonary hemorrhage and edema and may result in death. Although renal and hepatic changes have been observed in animal studies, serious systemic effects are unlikely to occur from an acute exposure.
UF6 emits alpha, beta, and gamma radiation and, thus, may interact with and damage DNA and chromosomes. If not repaired, that damage may eventually lead to cancer. The amount of radiation emitted is dependent on the degree of enrichment of the UF6. In the case of acute inhalation exposures to the soluble UF6, short-term radiotoxicity would be negligible compared with chemical toxicity, even in the case of highly enriched UF6 (Just and Elmer 1984). The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR 1993) has concluded that naturally occurring and depleted uranium, although radioactive, present primarily a toxicologic, rather than radiologic hazard, because the hypothetical radiologic toxicity predicted in skeletal tissues has not been observed in humans or experimental animals.
4.3. Susceptible Populations
People with impaired renal function (chemical toxicity of uranium), fetuses and developing neonates (radiologic hazard), and asthmatic individuals (HF toxicity) might be especially susceptible to UF6 toxicity.
4.4. Species Variability
Acute inhalation studies suggest that, with regard to UF6-induced lethality, mice are most sensitive, rats are moderately sensitive, and guinea pigs are most resistant (Spiegel 1949; Leach et al. 1984). In a subchronic study,
TABLE 5–9 Mortality for Animals Exposed to Uranium Hexafluoridea (%)
|
Concentration (mg/m3) |
Guinea Pigs |
Dogs |
Rats |
Mice |
Rabbits |
|
0.3 |
0 |
0 |
5 |
5 |
0 |
|
3 |
5 |
20 |
0 |
92 |
80 |
|
20 |
45 |
40 |
75 |
100 |
100 |
|
aAnimals were exposed for 6 h/d for 30 d. Source: Data from Spiegel 1949. |
|||||
Spiegel (1949) exposed adult mice, rats, guinea pigs, rabbits, and dogs to UF6 at 0.3–20 mg/m3 for 6 h/d for 30 d (Table 5–9, above). With regard to lethality in adult animals, guinea pigs and dogs were least susceptible, rats were moderately susceptible, and mice and rabbits were highly susceptible. However, those relative species sensitivities must be viewed with caution, because firm concentration-response relationships were not established (5% of rats died at 0.3 mg/m3, but 0% died at 3 mg/m3). With regard to body weight (Table 5–10), the greatest weight loss in the subchronic study was observed in rabbits, followed by guinea pigs; dogs and rats showed similar weight loss patterns.
4.5. Temporal Extrapolation
The concentration-exposure time relationship for many irritant and systemically acting vapors and gases has been described by Cn×t=k, where the exponent, n, typically ranges from 0.8 to 3.5 (ten Berge et al. 1986). When the rat 2–60 min LC50 values from Leach et al. (1984) are considered in the derivation of the exponent, a value of 0.66 is obtained (Figure 5–1).
4.6. Other Information
In a previous attempt to assess the toxicity of UF6 and its hydrolysis products to humans, four toxicologists were asked to use the available animal data to calculate levels for human health effects (Just and Elmer 1984; Just 1984). The four individuals independently derived values using four different approaches. The group then convened and reached a set of con-
TABLE 5–10 Percent Weight Change in Animals Exposed to Uranium Hexafluoridea
|
Concentration (mg/m3) |
Guinea Pigs |
Dogs |
Rats |
Rabbits |
|
0.3 |
+11 |
+9 |
+3 |
0 |
|
3 |
+1 |
−12 |
−8 |
−12 |
|
20 |
−13 |
−3 |
−6 |
−21 |
|
0.075b |
+11 |
−2 |
+70 |
— |
|
0.3b |
— |
+10 |
+75 |
+3 |
|
aAnimals were exposed for 6 h/d for 30 d. bVery young, growing animals. |
||||
sensus values for 1-h exposures. Assumptions used in the derivation were as follows: a 70-kg man, a resting respiration rate of 7.5 L/min, and an absorption factor of 0.43. The ICRP (International Commission on Radiological Protection) method was used to model absorbed uranium to the inhalation exposure concentration. The derived estimates are shown in Table 5–11.
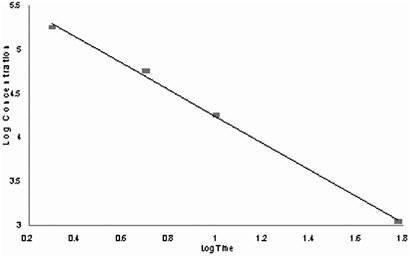
FIGURE 5–1 Best fit concentration-time curve.
TABLE 5–11 Human Toxicity Estimates for a 1-h Inhalation Exposure to Uranium Hexafluoride
|
Effect |
Concentration (mg/m3) |
|
No effect |
<9.6 |
|
Mild health effects |
9.6–18.5 |
|
Renal injury |
18.5 |
|
10% lethal |
352 |
|
50% lethal |
862 |
|
Sources: data from Just and Elmer 1984; Just 1984. |
|
5. RATIONALE AND PROPOSED AEGL-1
5.1. Human Data Relevant to AEGL-1
No human exposure data consistent with the definition of AEGL-1 were available for UF6. However, the work of Just and Elmer (1984), which estimates a 1-h no-observed-effect level (NOEL) of <9.6 mg/m3, supports the derivation of the AEGL-1 values presented below.
5.2. Animal Data Relevant to AEGL-1
Effects observed from inhalation exposure to UF6 in experimental animals were more severe than those defined by AEGL-1.
5.3. Derivation of AEGL-1
In the absence of relevant chemical-specific data for the derivation of AEGL-1 values, modifications of the AEGL-1 values for HF were used to derive the AEGL-1 values for UF6. The use of HF as a surrogate for UF6 was deemed appropriate for the development of AEGL-1 values because it is likely that HF, a hydrolysis product of UF6, is responsible for the low level irritation effects of UF6 relevant to the AEGL-1 definition. The HF AEGL-1 values were based on the threshold for pulmonary inflammation in healthy human adults (Lund et al. 1999). Because a maximum of 4 mol of HF are produced for every mole of UF6 hydrolyzed, a stoichiometric adjustment factor of 4 was applied to the HF AEGL-1 values to approxi-
mate AEGL-1 values for UF6; the AEGL-1 values for UF6 are constant across time up to 1 h because the HF AEGL-1 values were held constant across time. AEGL-1 values were derived for only the 10-min, 30-min, and 1-h time points, because derivation of the 4- and 8-h AEGL-1s resulted in values greater than the 4- and 8-h AEGL-2s for UF6, which would be inconsistent with the total database. The AEGL-1 values for UF6 are presented in Table 5–12, and the calculations for those AEGL-1 values are presented in Appendix A. The relationship between AEGL-1 and animal exposures is shown in Figure 5–2.
The values are supported by the estimated 1-h human NOEL of <9.6 mg/m3 (Just and Elmer 1984; Just 1984). Applying an uncertainty factor (UF) of 3 for sensitive individuals, a value of 3.2 mg/m3 is obtained, which is in close agreement with the values derived for AEGL-1.
6. RATIONALE AND PROPOSED AEGL-2
6.1. Human Data Relevant to AEGL-2
Case reports describing accidental human exposures to UF6 that led to effects consistent with the definition of AEGL-2 exist. However, because of the lack of reliable concentration and duration parameters, those data are not appropriate for derivation of AEGL-2 values.
6.2. Animal Data Relevant to AEGL-2
Two dogs exposed to UF6 at 192–284 mg/m3 for 30 min to 1 h exhibited renal tubular pathology 6 d and 16 d post-exposure (Morrow et al. 1982).
6.3. Derivation of AEGL-2
The renal pathology observed in dogs was used as the basis for AEGL-2 values. The lowest concentration (192 mg/m3) and the shortest exposure
TABLE 5–12 AEGL-1 Values for Uranium Hexafluoride (mg/m3)
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.6 |
3.6 |
3.6 |
NR |
NR |
|
Abbreviation: NR, not recommended. |
||||
TABLE 5–13 AEGL-2 Values for Uranium Hexafluoride (mg/m3)
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
28 |
19 |
9.6 |
2.4 |
1.2 |
time (30 min) were used in the derivation. Although only one dog was exposed at each treatment level, the use of this data is supported by the Morrow et al. (1982) study in which 17 dogs exposed to UO2F2 at 200–270 mg/m3 for 30 min to 2.5 h exhibited similar renal pathology. Although the UO2F2 is not the title compound, it is the hydrolysis product of UF6 likely responsible for the renal effects. A UF of 3 was used to extrapolate from animals to humans, and a UF of 3 was also applied to account for sensitive individuals (total UF=10). This total UF is considered sufficient because the use of a larger total UF would yield AEGL-2 values below or approaching the AEGL-1 values, which for HF were considered to be no-observed-effect levels and were based on a threshold for inflammation in humans (HF study). Also, humans were exposed repeatedly to HF at up to 8 ppm with only slight nasal irritation; that concentration is stoichiometrically equivalent to UF at 28.8 mg/m3. The concentration-exposure time relationship for many irritant and systemically acting vapors and gases may be described by Cn×t=k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). To obtain protective AEGL values in the absence of an empirically derived, chemical-specific scaling exponent, temporal scaling was performed using n=3 when extrapolating to shorter time points and n=1 when extrapolating to longer time points. (Although a chemical-specific exponent of 0.66 was derived from rat lethality data, the default values were utilized for time-scaling AEGL-2 values because the end points for AEGL-2 [renal toxicity] and for death [pulmonary edema] involve different mechanisms of action.) The AEGL-2 values for UF6 are presented in Table 5–13, above, and the calculations for those AEGL-2 values are presented in Appendix A. The relationship between AEGL-2 and animal exposures is shown in Figure 5–2.
7. RATIONALE AND PROPOSED AEGL-3
7.1. Human Data Relevant to AEGL-3
Deaths from accidental exposure to UF6 were reported in two case reports. However, because of the lack of reliable concentration and dura-
tion parameters, those data are not appropriate for derivation of AEGL-3 values.
7.2. Animal Data Relevant to AEGL-3
Well-conducted LC50 studies are available in rats, mice, and guinea pigs (Spiegel 1949; Leach et al. 1984). However, the majority of those studies used exposure durations of 2-, 5-, or 10-min and are, thus, inappropriate (too short) for extrapolating to the longer AEGL time periods. The exception is a 1-h rat LC50 study (Leach et al. 1984).
7.3. Derivation of AEGL-3
An estimated 1-h lethality threshold (one-third the LC50) in the rat is 365 mg/m3. That value was used as the basis for deriving the AEGL-3 values. This approach is considered appropriate due to the steepness (n=0.66) of the concentration-response curve for lethality in rats exposed to UF6. An intraspecies species UF of 3 was applied and is considered sufficient because the steep concentration-response curve for lethality implies little intra-individual variability. A UF of 3 was also applied for interspecies variability (total UF=10). Application of the full interspecies UF of 10 would result in AEGL-3 values inconsistent with the overall data set. For example, assuming the production of 4 mol of HF from the hydrolysis of 1 mol of UF6, exposure at the proposed AEGL-3 values would be equivalent to exposure to HF at 60 ppm for 10 min, 20 ppm for 30 min, 10 ppm for 1 h, 2.5 ppm for 4 h, and 1.3 ppm for 8 h. No effects on respiratory parameters were noted in healthy humans after exposure to HF at 6.4 ppm for 1 h or after repeated exposures of up to 8.1 ppm (Lund et al. 1999; Largent 1960, 1961). Although this scenario does not take into account the uranium portion of the exposure, given the steepness of the concentration-response curve, it is unlikely that one would experience life-threatening effects at these concentrations. Therefore, an interspecies UF of 3 is justified. The value was then scaled to the 10-min, 30-min, 4-h, and 8-h time points using C1×t=k. An exponent value of n=0.66 was derived from rat lethality data from experiments ranging in duration from 2 min to 1 h in the key study. The exponent was rounded to 1.0 for extrapolation because the data used to derive the exponent were limited (one study) and the derived n is below the range of a normal dose-response curve. The AEGL-3 values for
TABLE 5–14 AEGL-3 Values for Uranium Hexafluoride (mg/m3)
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
216 |
72 |
36 |
9.0 |
4.5 |
UF6 are presented in Table 5–14 (above), and the calculations for those AEGL-3 values are presented in Appendix A. The relationship between AEGL-3 and animal exposures is shown in Figure 5–2.
8. SUMMARY OF PROPOSED AEGLS
8.1. AEGL Values and Toxicity End Points
The derived AEGL values for various levels of effects and durations of exposure are summarized in Table 5–15. Because no data consistent with the definition of AEGL-1 were available for the title compound, it was necessary to modify HF AEGL-1 values to approximate AEGL-1 values for UF6. Renal effects in dogs were used as the basis for AEGL-2. An estimate of the concentration causing no deaths in rats was used for the AEGL-3.
8.2. Other Exposure Criteria
The established exposure criteria for UF6 or soluble uranium compounds are presented in Table 5–16.
FIGURE 5–15 Summary of Proposed AEGLs (mg/m3)
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (Nondisabling) |
3.6 |
3.6 |
3.6 |
NR |
NR |
|
AEGL-2 (Disabling) |
28 |
19 |
9.6 |
2.4 |
1.2 |
|
AEGL-3 (Lethal) |
216 |
72 |
36 |
9.0 |
4.5 |
|
Abbreviation: NR, not recommended. |
|||||
TABLE 5–16 Extant Standards and Guidelines for Uranium Hexafluoride (mg/m3)
|
|
Exposure Duration |
||||
|
Guideline |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 |
3.6 |
3.6 |
3.6 |
NR |
NR |
|
AEGL-2 |
28 |
19 |
9.6 |
2.4 |
1.2 |
|
AEGL-3 |
216 |
72 |
36 |
9.0 |
4.5 |
|
ERPG-1a |
5 |
|
|||
|
ERPG-2a |
15 |
||||
|
ERPG-3a |
30 |
||||
|
NIOSH IDLHb |
10 (soluble compounds as uranium) (UF6 at 14.8 mg/m3) |
|
|||
|
NIOSH TWAc |
|
0.05 (soluble compounds as uranium) (UF6 at 0.07 mg/m3) |
|||
|
OSHA TWAd |
0.05 (soluble compounds as uranium) (UF6 at 0.07 mg/m3) |
||||
|
ACGIH TLV-TWAe |
0.2 (soluble compounds as uranium) (UF6 at 0.30 mg/m3) |
||||
|
ACGIH TLV-STELf |
0.6 (soluble compounds as uranium) (UF6 at 0.89 mg/m3) |
|
|||
|
aERPG (emergency response planning guidelines of the American Industrial Hygiene Association) (AIHA 1991). The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing symptoms other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The ERPG-1 for uranium hexafluoride is based on an estimated no-effect exposure level and irritation on conversion to HF. The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-2 for uranium hexafluoride is based on renal injury. The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for uranium hexafluoride is based on lethality data. |
|||||
8.3. Data Quality and Research Needs
Data appropriate for AEGL-1 derivation were not available, necessitating the use of HF AEGL-1 values to approximate AEGL-1 values for UF6. Data appropriate for derivation of AEGL-2 values were limited to one study using two dogs. Data appropriate for derivation of AEGL-3 values were limited to one rat LC50 study with little reported experimental detail.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists, Inc). 1999. Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (BEIs). Cincinnati, OH: ACGIH.
AIHA (American Industrial Hygiene Association). 1995. Emergency Response Planning Guidelines for Uranium Hexafluoride. Fairfax, VA: AIHA.
ATSDR (Agency for Toxic Substances and Disease Registry). 1993. Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorines. U.S. Department of Health and Human Services, Washington, DC. April 1993.
ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Uranium. U.S. Department of Health and Human Services, Washington, DC. September 1999.
EPA (U.S. Environmental Protection Agency). 2001. Acute exposure guideline levels for hydrogen fluoride [interim draft 3, 7/2001].
Fisher, D.R., K.L.Kathren, and M.J.Swint. 1991. Modified biokinetic model for uranium from analysis of acute exposure to uranium hexafluoride. Health Phys. 60:335–342.
Just, R.A. 1984. Report on Toxicological Studies Concerning Exposures to UF6 and UF6 Hydrolysis Products. Oak Ridge Gaseous Diffusion Plant, operated by Martin Marietta Energy Systems, Inc., for the U.S. Department of Energy, Washington, DC.
Just, R.A., and V.S.Elmer. 1984. Generic Report on Health Effects for the U.S. Gaseous Diffusion Plants. Oak Ridge Gaseous Diffusion Plant, Oak Ridge, TN, operated by Martim Marietta Energy Systems, Inc., for the U.S. Department of Energy, Washington, DC.
Kathren, R.L. and R.H.Moore. 1986. Acute accidental inhalation of U: A 38-year follow-up. Health Phys. 51:609–619.
Largent, E.J. 1960. The metabolism of fluorides in man. AMA Arch. Ind. Health 21:318–323.
Largent, E.J. 1961. Fluorosis: The Health Aspects of Fluorine Compounds. Columbus, OH: Ohio State University Press. Pp. 34–39, 43–48.
Leach, L.J., R.M.Gelein, B.J.Panner, C.L.Yulie, C.C.Cox, M.M.Balys, and P.M. Rolchigo. 1984. Acute Toxicity of the Hydrolysis Products of Uranium Hexafluoride (UF6) when Inhaled by the Rat and Guinea Pig. Final Report (K/SUB/81–9039/3). Rochester, NY: University of Rochester Medical Center.
Lund, K., M.Refsnes, T.Sandstrom, P.Sostrand, P.Schwarze, J.Boe, and J. Kongerud. 1999. Increased CD3 positive cells in bronchoalveolar lavage fluid after hydrogen fluoride inhalation. Scand. J. Work Environ. Health 25:326–334.
Machle, W., and K.Kitzmiller. 1935. The effects of the inhalation of hydrogen fluoride: II. The response following exposure to low concentrations. J. Ind. Hyg. 17:223–229.
Machle, W., F.Thamann, K.Kitzmiller, et al. 1934. The effects of the inhalation of hydrogen fluoride: I. The response following exposure to high concentrations. J. Ind. Hyg. 16:129–145.
Moore, R.H., and R.L.Kathren. 1985. A World War II uranium hexafluoride inhalation event with pulmonary implications for today. J. Occup. Med. 27:753–756.
Morrow, P., R.Gelein, H.Beiter, J.Scott, J.Picano, and C.Yulie. 1982. Inhalation and intraveneous studies of UF6 and UO2F2 in dogs. Health Phys. 43:859–873.
NIOSH (National Institute of Occupational Safety and Health). 1997. NIOSH Pocket Guide to Chemical Hazards. U.S. Department of Health and Human Services, Washington, DC.
Nuclear Regulatory Commission. 1986. Assessment of the Public Health Impact from the Accidental Release of UF6 at the Sequoyah Fuels Corporation Facility at Gore, Oklahoma. NUREG-1189, Vols. 1, 2. U.S. Nuclear Regulatory Commission, Rockville, MD.
Spiegel, C.J. 1949. Uranium Hexafluoride. Pp. 532–547 in Pharmacology and Toxicology of Uranium Compounds. New York, NY: McGraw-Hill Book Company.
Stokinger, H.E. 1949. Toxicity following inhalation of fluorine and hydrogen fluoride. Pp. 1021–1057 in Pharmacology and Toxicology of Uranium Compounds, C.Voegtlin and H.C.Hodge, eds. New York, NY: McGraw Hill.
ten Berge, W.F., A.Zwart, and L.M.Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mat. 13:301–309.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). 1993. Sources and Effects of Ionizing Radiation. New York: United Nations.
APPENDIX A
Derivation of AEGL Values
|
Derivation of AEGL-1 Values |
|
|
Key study: |
Based on HF AEGL-1 values (Lund et al. 1999) |
|
10-min, 30-min, and 1-h HF AEGL-1: |
1.0 ppm |
|
Stoichiometric adjustment factor: |
4, because 4 mol of HF are produced from hydrolysis of 1 mol of UF6. |
|
10-min, 30-min, and 1-h AEGL-1: |
AEGL=1.0 ppm÷4=0.25 ppm; AEGL=0.25 ppm×14.4=3.6 mg/m3 |
|
Derivation of AEGL-2 Values |
|
|
Key study: |
Morrow et al., 1982 |
|
Toxicity end points: |
Renal pathology in dogs. |
|
Time-scaling: |
10-min and 30-min C3×t=k (192 mg/m3)3×0.5 h=3,538,944 mg/m3·h |
|
|
1-h, 4-h, and 8-h C1×t=k (192 mg/m3)1×0.5 h=96 mg/m3·h |
|
Uncertainty factors: |
3 for interspecies variability 3 for intraspecies variability |
|
10-min AEGL-2: |
C3×0.167 h=3,538,944 mg/m3·h C3=21191281 mg/m3 C=276.7 mg/m3 10-min AEGL-2=276.7/10=27.7 mg/m3 |
|
30-min AEGL-2: |
C3×0.5 h=3,538,944 mg/m3·h C3=7,077,888 mg/m3 C=192 mg/m3 30-min AEGL-2=192/10=19.2 mg/m3 |
|
1-h AEGL-2: |
C1×1 h=96 mg/m3·h C1=96 mg/m3 C=96 mg/m3 1-h AEGL-2=96/10=9.6 mg/m3 |
|
4-h AEGL-2: |
C1×4 h=96 mg/m3·h C1=24 mg/m3 C=24 mg/m3 4-h AEGL-2=24/10=2.4 mg/m3 |
|
8-h AEGL-2: |
C1×8 h=96 mg/m3·h C1=12 mg/m3 C=12 mg/m3 8-h AEGL-2=12/10=1.2 mg/m3 |
|
Derivation of AEGL-3 Values |
|
|
Key study: |
Leach et al. 1984 |
|
Toxicity end point: |
Estimated 1-h threshold for death in rats (one-third the LC50) |
|
Time-scaling: |
C1×t=k (365) 1×1 h=365 mg/m3·h |
|
Uncertainty factors: |
3 for interspecies variability 3 for intraspecies variability |
|
10-min AEGL-3: |
C1×0.167 h=365 mg/m3·h C=2,165.6 mg/m3 10-min AEGL-3=2,165.6/10=216 mg/m3 |
|
30-min AEGL-3: |
C1×0.5 h=365 mg/m3·h C=730 mg/m3 30-min AEGL-3=730/10=73 mg/m3 |
|
1-h AEGL-3: |
C1×1 h=365 mg/m3·h C1=49 mg/m3·h C=364 mg/m3 1-h AEGL-3=364/10=36.4 mg/m3 |
|
4-h AEGL-3: |
C1×4 h=365 mg/m3·h C=90 mg/m3 4-h AEGL-3=90/10=9.0 mg/m3 |
|
8-h AEGL-3: |
C1×8 h=365 mg/m3·h C=45.6 mg/m3 8-h AEGL-3=45.6/10=4.5 mg/m3 |
APPENDIX B
ACUTE EXPOSURE GUIDELINE LEVELS FOR URANIUM HEXAFLUORIDE (CAS No. 7783–81–5)
DERIVATION SUMMARY
|
AEGL-1 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
3.6 mg/m3 |
3.6 mg/m3 |
3.6 mg/m3 |
NR |
NR |
|
Key Reference: |
EPA (U.S. Environmental Protection Agency). 2001. Acute exposure guideline levels for hydrogen fluoride [interim draft 3, 7/2001]. (Lund et al. 1999. Increased CD3 positive cells in bronchoalveolar lavage fluid after hydrogen fluoride inhalation. Scand. J. Work. Environ. Health 25:326–334.) |
|||
|
Test species/strain/number: 20 healthy male volunteers |
||||
|
Exposure route/concentrations/durations: Inhalation, average concentrations at 0.2–0.7 ppm (n=9), 0.85–2.9 ppm (n=7), and 3.0–9.3 ppm (n=7). |
||||
|
Effects: 0.2–0.7 ppm: No to low sensory irritation; no change in FVC, FEV1; no inflammatory response in bronchoalveolar lavage fluid (BAL). 0.85–2.9 ppm: No to low sensory irritation; no change in FVC, FEV1; increase in the percentage of CD3 cells and myeloperoxidase in bronchial portion of BAL; no increases in neutrophils, eosinophils, protein, or methyl histamine in BAL. 3.0–6.3 ppm: Low sensory irritation; no change in FVC, FEV1; increase in the percentage of CD3 cells and myeloperoxidase in bronchial portion of BAL; no increases in neutrophils, eosinophils, protein, or methyl histamine in BAL. |
||||
|
End point/concentration/rationale: The subthreshold concentration for inflammation at 3 ppm (0.85–2.9 ppm) for 1 h, which was without sensory irritation, was chosen as the basis for the AEGL-1. |
||||
|
Uncertainty factors/rationale: |
||||
|
Total uncertainty factor: 3 |
||||
|
Interspecies: Not applicable since human subjects were the test species. |
||||
|
Intraspecies: 3. The subjects were healthy adult males. The concentration used is far below tested concentrations that did not cause symptoms of bronchial constriction in healthy adults (ranges up to 6.3 ppm [Lund et al. 1997] and 8.1 ppm [Largent 1960, 1961]). |
||||
|
Stoichiometric adjustment factor: 4. A maximum of 4 mol of HF may be produced by hydrolysis from 1 mol of UF6 |
||||
|
Animal to human dosimetric adjustment: Not applicable, human data used |
||||
|
Time-scaling: Not applied. AEGL-1 values for HF were calculated by adjusting the 1-h concentration of 3 ppm by an UF of 3. Because the response would be similar at shorter exposure durations, the 10- and 30-minute values were set equal to the 1-h concentration. The UF6 AEGL-1 values were derived by applying a modifying factor of 4 to the HF AEGL values, because a maximum of 4 mol of HF are produced for every mole of UF6 hydrolyzed. AEGL-1 values for UF6 were derived only for the 10-min, 30-min, and 1-h time points, because derivation of 4- and 8-h values results in AEGL-1 values greater than the 4- and 8-h AEGL-2s, which would be inconsistent with the total database. |
||||
|
Data quality: Data appropriate for AEGL-1 derivation were not available for UF6. Therefore, AEGL-1 values for UF6 were approximated from the AEGL-1 values for HF, a known hydrolysis product and a likely source of respiratory irritation. For each mole of UF6, 4 mol of HF may be produced by hydrolysis. Thus, the HF AEGL-1 was divided by a factor of 4 to approximate an AEGL-1 for UF6. |
|
AEGL-2 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
28 mg/m3 |
19 mg/m3 |
9.6 mg/m3 |
2.4 mg/m3 |
1.2 mg/m3 |
|
Key reference: |
Morrow et al. 1982. Inhalation and intravenous studies of UF6 and UO2F2 in dogs. Health Physics. 43:859–873. |
|||
|
Test species/strain/number: 1 female beagle dog per concentration |
||||
|
Exposure route/concentration/durations: Inhalation, 192–284 mg/m3 for 30 min to 1 h. |
||||
|
Effects: At 192 mg/m3, kidney pathology; at 284 mg/m3, kidney pathology |
||||
|
End point/concentration/rationale: 192 mg/m3 for 30 min, LOEL for kidney pathology |
||||
|
Uncertainty factors/rationale: |
||||
|
Total uncertainty factor: 10 This total UF is considered sufficient because the use of a larger total UF would yield AEGL-2 values below or approaching the AEGL-1 values, which are considered to be no-observed-effect levels and were based on a threshold for inflammation in humans (HF study). Also, humans were exposed to HF repeatedly at up to 8 ppm with only slight nasal irritation; that concentration is stoichiometrically equivalent to UF6 at 28.8 mg/m3. |
||||
|
Interspecies: 3 |
||||
|
Intraspecies: 3 |
||||
|
Modifying factor: NA |
||||
|
Animal to human dosimetric adjustment: None, insufficient data |
||||
|
Time-scaling: To obtain conservative and protective AEGL values in the absence of an empirically derived, chemical-specific scaling exponent, temporal scaling was performed using the Cn×t=k equation where n=3 when extrapolating to shorter time points and n=1 when extrapolating to longer time points. (Although a chemical-specific exponent of 0.66 was derived from rat lethality data, the default values were utilized for time-scaling AEGL-2 values because the end points for AEGL-2 [renal toxicity] and for death [pulmonary edema] involve different mechanisms of action). |
||||
|
Data quality: Although only one dog was exposed at each treatment level, the use of that data is supported by the fact that Morrow et al. (1982) similarly exposed 17 dogs to UO2F2 at 200–270 mg/m3 for 30 min to 2.5 h and observed similar renal pathology. Although the UO2F2 is not the title compound, it is the hydrolysis product of UF6 responsible for the renal effects. |
||||
|
AEGL-3 |
||||
|
10 min |
30 min |
1 h |
4 h |
8 h |
|
216 mg/m3 |
72 mg/m3 |
36 mg/m3 |
9.0 mg/m3 |
4.5 mg/m3 |
|
Key reference: |
Leach et al. 1984. Acute toxicity of the hydrolysis products of uranium hexafluoride (UF6) when inhaled by the rat and guinea pig. Final Report. (K/SUB/81–9039/3). University of Rochester Medical Center. Rochester, NY. |
|||
|
Test species/strain/gender/number: Long Evans rats, males |
||||
|
Exposure route/concentrations/durations: Inhalation, 1 h |
||||
|
Effects: LC50, 1,095 mg/m3 |
||||
|
End point/concentration/rationale: Estimated 1-h threshold for death (one-third LC50), 365 mg/m3 |
||||
|
Uncertainty factors/rationale: |
||||
|
Total uncertainty factor: 10 |
||||
|
Interspecies: 3. An application of the full interspecies UF of 10 would result in AEGL-3 values that are inconsistent with the overall data set. For example, assuming the production of 4 mol of HF from the hydrolysis of 1 mol of UF6, exposure at the proposed AEGL-3 values would be equivalent to exposure to HF at 60 ppm for 10 min, 20 ppm for 30 min, 10 ppm for 1 h, 2.5 ppm for 4 h, and 1.3 ppm for 8 h. No effects on respiratory parameters were noted in healthy humans after exposure to HF at 6.4 ppm for 1 h or after repeated exposures at up to 8.1 ppm (Lund et al. 1999; Largent 1960, 1961). Although this scenario does not take into account the uranium portion of the exposure, given the steepness of the concentration-response curve, it is unlikely that one would experience life-threatening effects at these concentrations. |
||||
|
Intraspecies: 3. The steep concentration-response curve implies limited intra-individual variability. |
||||
|
Modifying factor: Not applicable |
||||
|
Animal to human dosimetric adjustment: Insufficient data |
||||
|
Time-scaling: Cn×t=k where n=1 on the basis of regression analysis of rat lethality data from experiments ranging in duration from 2 min to 1 h. Calculated value of n=0.66 was rounded to n=1 because limited data were used to derive n and the calculated value is below the range of a normal dose-response curve (Leach et al. 1984). |
||||
APPENDIX C
Time-Scaling Calculation and Key Study Description for Hydrogen Fluoride AEGL-1 (EPA 2001)
|
Key study: |
Lund et al. 1997, 1999 |
|
Toxicity end point: |
Biomarkers of exposure during 1-h exposure of human subjects at several ranges of concentrations. |
|
Time-scaling: |
Not applied |
|
Uncertainty factors: |
3 for differences in human sensitivity (effects are unlikely to differ among individuals). This concentration should be protective of asthmatic individuals because it is below average (2 ppm) and ranges of concentrations (up to 8.1 ppm) (Largent 1960, 1961) that produced slight to mild irritation in healthy adult male subjects. |
|
Calculations: |
The 3 ppm concentration was divided by an intraspecies UF of 3. The resulting concentration, 1 ppm, was used for the 10- and 30-min and 1-h, 4-h, and 8-h AEGL-1. |
Summary of Key Study and Rationale Used in the Hydrogen Fluoride AEGL-1 Derivation (EPA 2001)
The AEGL-1 was based on a concentration of 3 ppm (range, 0.85–2.93 ppm) for 1 h, which was the threshold for pulmonary inflammation as evidenced by an increase in the percentages of several inflammatory parameters, such as CD3 cells and myeloperoxidase, in the bronchoalveolar lavage fluid of 20 healthy adult human subjects (Lund et al. 1999). There were no increases in neutrophils, eosinophils, protein, or methyl histamine at this or the next higher average exposure concentration of 4.7 ppm (range, 3.05–
6.34 ppm). There were no changes in lung function and no to minor increased symptoms of irritation at this concentration (Lund et al. 1997). Although healthy adults were tested, several individuals had increased immune factors, indicating atopy. The 3 ppm concentration was divided by an intraspecies UF of 3 because the response to an irritant gas is not expected to differ more than 3-fold among individuals, including susceptible individuals such as asthmatic people. The 1 ppm concentration was used for the 10-min, 30-min, and 1-h exposure durations. The 1 ppm concentration was reduced by a factor of 2 for the 4- and 8-h exposure durations. Because there were no effects on respiratory parameters of healthy adults at concentrations that ranged up to 6.34 ppm in this study and up to 8.1 ppm with repeated exposures in a supporting study (Largent 1960, 1961), the calculated AEGL-1 values will probably be protective of the asthmatic population.