Appendix G
Hydrogen Production Technologies: Additional Discussion
This appendix discusses in more detail the technologies that can be used to produce hydrogen and which are addressed in Chapter 8. Cost analyses for them are presented in Chapter 5. In this appendix, the committee addresses the following technologies: (1) reforming of natural gas to hydrogen, (2) conversion of coal to hydrogen, (3) nuclear energy to produce hydrogen, (4) electrolysis, (5) wind energy to produce hydrogen, (6) production of hydrogen from biomass, and (7) production of hydrogen from solar energy. The following major sections—one for each of the technologies—include a brief description of the current technology; possible improvements for future technology; refer to Chapter 5 and Appendix E (which presents spreadsheet data from the committee’s cost analyses), where applicable, for the current and possible future costs, CO2 emissions, and energy efficiencies; note the potential advantages and disadvantages of using the technology for hydrogen production; and comment on the Department of Energy’s (DOE’s) research, development, and demonstration (RD&D) plan for hydrogen.
In general, in developing estimates about future possible technologies, the committee systematically adopted an optimistic posture. The estimates are meant to represent what possibly could be achieved with concerted research and development (R&D). But the committee is not predicting that the requisite R&D will be pursued, nor is it predicting that these technical advances necessarily will be achieved, even with a concerted R&D program. Estimates were made of what might be achieved with appropriate R&D.
The state of development referred to as “possible future” technologies is based on technological improvements that may be achieved if the appropriate research and development are successful. These improvements are not guaranteed; rather, they may be the result of successful R&D programs. And they may require significant technological breakthroughs. Generally, these possible future technologies are available at a significantly lower cost than are the “current technologies” using the same feedstocks.
HYDROGEN FROM NATURAL GAS
Compared with other fossil fuels, natural gas is a cost-effective feed for making hydrogen, in part because it is widely available, is easy to handle, and has a high hydrogen-to-carbon ratio, which minimizes the formation of by-product CO2. However, as pointed out elsewhere in this report, natural gas is already imported as liquefied natural gas (LNG)1 into the United States today, and imports are projected to increase. Thus, increased use of natural gas for a hydrogen economy would only increase imports further. As a result, the committee considers natural gas to be a transitional fuel for distributed generation units, not a long-range fuel for central station plants for the hydrogen economy.
Production Techniques
The primary ways in which natural gas, mostly methane, is converted to hydrogen involve reaction with either steam (steam reforming), oxygen (partial oxidation), or both in sequence (autothermal reforming). The overall reactions are shown below:
CH4 + 2H2O → CO2 + 4H2
CH4 + O2 → CO2 + 2H2
In practice, gas mixtures containing carbon monoxide (CO) as well as carbon dioxide (CO2) and unconverted methane (CH4) are produced and require further processing. The reaction of CO with steam (water-gas shift) over a catalyst produces additional hydrogen and CO2, and after purification, high-purity hydrogen (H2) is recovered. In most cases,
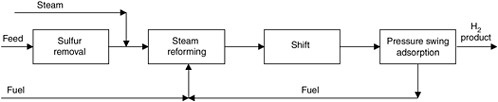
FIGURE G-1 Schematic representation of the steam methane reforming process.
CO2 is vented to the atmosphere today, but there are options for capturing it for subsequent sequestration.
Worldwide production of hydrogen is about 41 million tons per year (ORNL, 2003). Since over 80 percent of this production is accomplished by steam methane reforming (SMR), this method is discussed first.
Steam Methane Reforming
Steam methane reforming involves four basic steps (see Figure G-1). Natural gas is first catalytically treated with hydrogen to remove sulfur compounds. It is then reformed by mixing it with steam and passing it over a nickel-on-alumina catalyst, making CO and hydrogen. This step is followed by catalytic water-gas shift to convert the CO to hydrogen and CO2. Finally, the hydrogen gas is purified with pressure swing adsorption (PSA). The reject stream from PSA forms a portion of the fuel that is burned in the reformer to supply the needed heat energy. Therefore, CO2 contained in the PSA reject gas is currently vented with the flue gas. If the CO2 were to be sequestered, a separations process would be added to capture it.
The reforming reactions are as follows:
CH4 + H2O → CO + 3H2
CO + H2O → CO2 + H2 (water-gas-shift reaction)
Overall: CH4 + 2 H2O → CO2 + 4H2
The reaction of natural gas with steam to form CO and H2 requires a large amount of heat (206 kJ/mol methane). In current commercial practice, this heat is added using fired furnaces containing tubular reactors filled with catalyst.
Partial Oxidation
Partial oxidation (POX) of natural gas with oxygen is carried out in a high-pressure, refractory-lined reactor. The ratio of oxygen to carbon is carefully controlled to maximize the yield of CO and H2 while maintaining an acceptable level of CO2 and residual methane and minimizing the formation of soot. Downstream equipment is provided to remove the large amount of heat generated by the oxidation reaction, shift the CO to H2, remove CO2, which could be sequestered, and purify the hydrogen product. Of course, this process requires a source of oxygen, which is usually provided by including an air separation plant. Alternatively, air can be used instead of oxygen and product hydrogen recovered from nitrogen and other gases using palladium diffusion. POX can also be carried out in the presence of an oxidation catalyst, and in this case is called catalytic partial oxidation.
Autothermal Reforming
As already indicated, SMR is highly endothermic, and tubular reactors are used commercially to achieve the heat input required. When oxygen and steam are used in the conversion and are combined with SMR in autothemal reforming (ATR), the heat input required can be achieved by the partial combustion of methane. The reformer consists of a ceramic-lined reactor with a combustion zone and a subsequent fixed-bed catalytic SMR zone. Heat generated in the combustion zone is directly transferred to the catalytic zone by the flowing reaction gas mixture, thus providing the heat needed for the endothermic reforming reaction. As will be discussed, ATR is used today primarily for very large conversion units. There are several other design concepts that combine direct oxygen injection and catalytic conversion, including secondary reforming.
It has been suggested that methane conversion to hydrogen and elemental carbon might also be an attractive route, but the committee believes that this is unlikely. Such an approach would generate a large amount of carbon by-product,2 and less than 60 percent of the combined heats of combustion of the hydrogen and carbon products is associated with the hydrogen. For this approach to become a viable alternative, uses for large amounts of carbon must be found.
Natural Gas Conversion Today
Steam methane reforming is widely used worldwide to generate both synthesis gas and hydrogen. The gas produced is
used to make chemicals such as ammonia and methanol, to refine petroleum, metals, and electronic materials, and to process food components. More than 32 million tons per year (t/yr) H2 (80 million kg/day) are produced using natural gas SMR. Hydrogen is also made today using partial oxidation and ATR.
The vast commercial experience based on this manufacturing capacity has led to many improvements in the technology, reducing costs and increasing efficiency. Perhaps the most important element is the tubular reactor in which the SMR reaction takes place. Progress has led to higher tube wall temperatures, better control of carbon formation, and feedstock flexibility.3 This progress in turn has led to lower steam-to-carbon ratios and improved efficiency. The water-gas-shift unit has also been improved, and now one-step shift can be employed to replace the former two-step operation at different temperatures. Finally, purification of the hydrogen product has been simplified by using PSA to remove methane, carbon oxides, and trace impurities in a single step. While designs today do not generally include CO2 capture, technology is currently available to accomplish this. Using a commercial selective absorption process, CO2 could be recovered for subsequent sequestration.
Progress has also been made in designing and building larger SMR plants. Currently, single-train commercial plants of up to 480,000 kg H2 per day (200 million standard cubic feet per day [scf/d]) are being built, and even larger plants can be constructed using multiple trains. Units as small as 300 kg/day are also being built.4 In many cases, the units built are one of a kind, with specific features to meet the requirements of a site, application, or customer. At least one company is fabricating commercial SMR hydrogen plants as small as 300 kg/day using components of fixed design, one of the elements of mass production.5
Partial oxidation utilizing natural gas is fully developed and used commercially. In most cases today, commercial units use feeds of lower value than natural gas, such as coal, coke, petroleum residues, or other by-products, because of economics. However, natural gas is a preferred feed for POX from a technical standpoint and can be used to generate hydrogen where competitive.
Oxygen-blown ATR with natural gas is used today in very large units that generate a mixture of CO and H2 for the Fischer-Tropsch process or methanol synthesis. This is attractive in part because the units can produce the hydrogen-to-carbon monoxide ratio needed in the synthesis step. Since the heat of reaction is added by combustion with oxygen, the catalyst can be incorporated as a fixed bed that can be scaled up to achieve further benefits of larger plant size in both the ATR and the oxygen plant that is required. ATR also offers benefits when CO2 capture is included. This is because the optimum separation technology for this design recovers CO2 at 3 atmospheres (atm), thus reducing the cost of compression to pipeline pressure (75 atm).
In summary, all three processes (SMR, POX, and ATR) are mature technologies today for the conversion of natural gas to hydrogen. SMR is less costly except in very large units, where ATR has an advantage. SMR is also somewhat more efficient when the energy for air separation is included. POX has the advantage of being applicable to lower-quality feeds such as petroleum coke, but this is not directly relevant to natural gas conversion.
Future Natural Gas Conversion Plants
Given the current interest in possibilities for a hydrogen economy and the current commercial need for hydrogen, significant effort is being focused on improving natural gas conversion to hydrogen. Improved catalysts and materials of construction, process simplification, new separations processes, and reactor concepts that could improve the integration of steam reforming and partial oxidation are being investigated. Catalytic partial oxidation is also under consideration. Since steam reforming and partial oxidation are mature technologies, the primary opportunities for improvement involve developing designs for specific applications that are cost-effective and efficient.
Several thousand distributed generators will be needed for the hydrogen economy, and it should be possible to lower the cost of these generators significantly through mass production of a generation “appliance.” Such appliances may be further improved by tailoring the design to the fueling application. For example, hydrogen would likely be stored at roughly 400 atm, and to the extent that the conversion reactor pressure can be increased, hydrogen compression costs would be reduced and efficiency improved. For distributed generators incorporating POX or ATR, suitable cost-effective methods for hydrogen purification need to be developed. Alternatively, in such cases there are potentially attractive opportunities to recover the oxygen needed with membranes and thus to lower the cost.
Other concepts are also in the exploratory research stage. These involve new or modified ways of providing the endothermic heat of steam reforming or utilizing the heat of reaction in partial oxidation.
New, lower-cost designs for distributed generation probably can be advanced to the commercial prototype stage in the next 5 to 7 years. Some of these improvements could be applicable to large plants.
Economics
The committee undertook cost studies as described elsewhere (in Chapter 5 and Appendix E) to identify the areas
TABLE G-1 Economics of Conversion of Natural Gas to Hydrogen
|
|
Plant Size (kilograms of hydrogen per stream day [SD]) and Case |
|||||
|
|
1,200,000a |
24,000b |
480c |
|||
|
|
Current |
Possible Future |
Current |
Possible Future |
Current |
Possible Future |
|
Investment (no sequestration), $/kg/SD |
411 |
297 |
897 |
713 |
3847 |
2001d |
|
Investment (with sequestration), $/kg/SDe |
520 |
355 |
1219 |
961 |
— |
— |
|
Total H2 cost (no sequestration), $/kg |
1.03f |
0.92f |
1.38 f |
1.21 f |
3.51g |
2.33g |
|
Total H2 cost (with sequestration), $/kge |
1.22 f |
1.02 f |
1.67 f |
1.46 f |
— |
— |
|
CO2 emissions (no sequestration), kg/kg H2 |
9.22 |
8.75 |
9.83 |
9.12 |
12.1 |
10.3 |
|
CO2 emissions (with sequestration), kg/kg H2 |
1.53 |
1.30 |
1.71 |
1.53 |
— |
— |
|
Overall thermal efficiency (no sequestration), %h |
72.3a |
77.9a |
46.1 |
53.1 |
55.5 |
65.2 |
|
61.1 |
68.2 |
43.4 |
49.0 |
— |
— |
|
|
aIncludes compression of product hydrogen to pipeline pressure of 75 atm. bIncludes liquefaction of H2 prior to transport. cIncludes compression of H2 to 400 atm for storage/fueling vehicles. dIncludes estimated benefits of mass production. eIncludes capture and compression of CO2 to 135 atm for pipeline transport to sequestration site. fBased on natural gas at $4.50/million Btu. gBased on natural gas at $6.50/million Btu. hBased on lower heating values for natural gas and hydrogen; includes hydrogen generation, purification, and compression, and energy imported from offsite, as well as distribution and dispensing. |
||||||
that could have the greatest impact on the introduction of hydrogen fuel. For hydrogen production from natural gas, plant sizes of 1,200,000 kg per stream day (kg/SD), 24,000 kg/SD, and 480 kg/SD were studied (see Table G-1).6 For each plant size, a current case representing what can be done today with modern technology and a future case representing what might be possible in the future were included. The possible future case for the 480 kg/SD plant includes the estimated benefits of mass production. For the two larger plants, options were included to capture CO2 and to compress it to pipeline pressure (75 atm) for sequestration offsite. Capture was not included for the smallest plant, since the cost for collection of CO2 from distributed plants was considered to be prohibitive, in that forecourt sequestration of CO2 added $4.40/kg H2 to the cost (DiPietro, 1997).
As shown in Table G-1, current investments vary with plant size, from $411 to $3847/kg/SD as size is decreased from 1.2 million to 480 kg/SD. While improved technology visualized in the possible future cases lowers investment by 20 to 48 percent, plant size has a more pronounced effect (see Figure G-2). For the two larger plants, CO2 capture increases investment by 22 to 35 percent.
As illustrated in Figure G-3, hydrogen cost7 in the largest plant with no CO2 capture is $1.03/kg of hydrogen with current technology and $0.92/kg with future technology. This cost increases to $1.38/kg and $1.21/kg in a midsize plant, and to $3.51/kg and $2.33/kg in the smallest plant. CO2 capture adds 11 to 21 percent, depending on the case. Table G-1 shows overall thermal efficiency8 for the largest plant to be 72.3 to 77.9 percent without CO2 capture (for current and possible future technology, respectively), and 61.1 to 68.2 percent with CO2 capture (for current and possible future technology, respectively). Efficiency for the smallest plant is 55.5 to 65.2 percent.9 Without capture, the CO2 emissions are 8.8 to 12.1 kg CO2 per kilogram hydrogen. Capture lowers these emissions to 1.3 to 1.7 kg CO2 per kilogram of
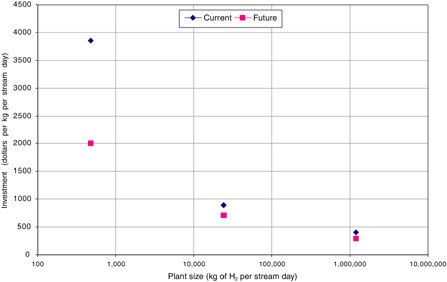
FIGURE G-2 Estimated investment costs for current and possible future hydrogen plants (with no carbon sequestration) of three sizes.
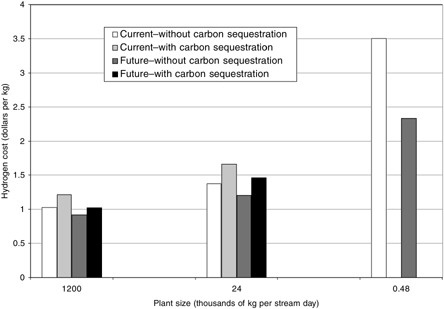
FIGURE G-3 Estimated costs for conversion of natural gas to hydrogen in plants of three sizes, current and possible future cases, with and without sequestration of CO2.
hydrogen. Emissions and thermal efficiency estimates include the effects of generating the required electricity offsite in state-of-the-art power generation facilities with 65 percent efficiency with 0.32 kg CO2/kWh of electricity.
The DOE states that its goal by 2010 is to reduce the cost of the distributed production of hydrogen from natural gas and/or liquid fuels to $1.50/kg (delivered, untaxed, without sequestration) at the pump, based on a natural gas price of $4/million Btu (DOE, 2003a). The committee’s analysis indicates that this goal will be very difficult to achieve for the distributed-size hydrogen plants and will likely require additional time. The possible future case for distributed generation, which already incorporates the estimated benefits of mass production of SMR units, yields a hydrogen cost of $1.88/kg with $4/million Btu of natural gas. Achievement of the DOE goal would require additional thermal efficiency improvements and investment reductions. The goal could be met if, for example, the SMR thermal efficiency were further increased from 70 to 80 percent (excluding the compression of product hydrogen to storage pressure) and the SMR investment was cut by 35 percent, assuming that the benefits of mass production have been appropriately included. The committee did not study the likelihood of achieving these additional improvements. It is also important to note that the committee’s cost estimates are based on the assumption that distributed generators operate throughout the year at 90 percent of design capacity. As a consequence, units would have to operate at or near design capacity 24 hours per day, or else the actual cost of hydrogen from such units would be higher than calculated.10 Achieving a 90 percent capacity factor would require careful integration of the design rate of the hydrogen generator, hourly demand variations at fueling stations, and onsite storage capability.
The committee believes that there is considerable uncertainty regarding the future cost of hydrogen from small hydrogen plants. This uncertainty is further increased by the need for high reliability and safe operation with infrequent attention from relatively unskilled operators (i.e., customers and station attendants). In the committee’s view, the DOE program should address these issues on a priority basis, as discussed below.
Hydrogen cost using steam methane reforming is sensitive to the price of natural gas, as shown in Figure G-4. Based on current technology cases, an increase in natural gas price from $2.50 to $6.50/million Btu increases hydrogen cost by 97 percent in a 1.2 million kg/SD plant and by 68 percent in a 24,000 kg/SD unit. For the 480 kg/SD unit, an increase from $4.50 to $8.50/million Btu raises hydrogen cost by 28 percent. These numbers highlight the importance of focusing research on improving efficiency in addition to reducing investment.
TABLE G-2 U.S. Natural Gas Consumption and Methane Emissions from Operations, 1990 and 2000
|
Consumption/Emissions |
1990 |
2000 |
|
Natural gas consumption (Tcf)a |
18.7 |
22.6 |
|
Methane emissions (Gg)b |
5772 |
5541 |
|
aSee EPA (2002). bU.S. Department of Energy, Energy Information Sheets, “Natural Gas Consumption,” May 12, 2003, Washington, D.C. |
||
Other Environmental Impacts
Natural gas is lost to the atmosphere during the production, processing, transmission, storage, and distribution of hydrogen. Since methane, the major component of natural gas, has a global warming potential of 23,11 this matter deserves discussion.
Methane is produced primarily in biological systems through the natural decomposition of organic waste. Methane emissions include those from the cultivation of agricultural land and the decomposition of animal wastes. The Environmental Protection Agency (EPA) estimates that 70 percent of methane emissions result from human activities and the balance from natural processes.12 Less than 20 percent of total global emissions of methane are related to fossil fuels, including natural gas operations (IPCC, 1995). The EPA reports that 19 percent of the anthropogenic emissions of methane in 2000 came from natural gas operations, and 25 percent of that came from distribution of natural gas within cities, primarily to individual users (EPA, 2002).
Perhaps the most compelling statistic is that between 1990 and 2000, methane emissions from natural gas operations decreased even though natural gas consumption increased (Table G-2). Clearly, improvements are being made to reduce losses from natural gas operations. For example, the EPA says that a voluntary program with industry, the Natural Gas STAR Program,13 has reduced methane emissions by 216 billion cubic feet (Bcf) since its inception in 1993.
As already pointed out, the advent of hydrogen-powered cars would increase natural gas consumption significantly.
|
10 |
Based on the committee’s model, a reduction of on-stream time from 90 to 70 percent would increase the cost of hydrogen in a 480 kg/SD unit by 11 to 15 percent. |
|
11 |
The Intergovernmental Panel on Climate Change (IPCC) has defined global warming potential as follows: “An index, describing the radiative characteristics of well mixed greenhouse gases, that represents the combined effect of the differing times these gases remain in the atmosphere and their relative effectiveness in absorbing outgoing infrared radiation. This index approximates the time-integrated warming effect of a unit mass of a given greenhouse gas in today’s atmosphere, relative to that of carbon dioxide” (IPCC, 2001a). |
|
12 |
See Environmental Protection Agency (EPA), “Current and Future Methane Emissions from Natural Sources.” Available online at http://www.epa.gov/ghginfo/reports/curr.htm. Accessed December 10, 2003. |
|
13 |
Information on the U.S. Environmental Protection Agency’s STAR Program is available online at http://www.epa.gov.gasstar/. Accessed November 15, 2003. |
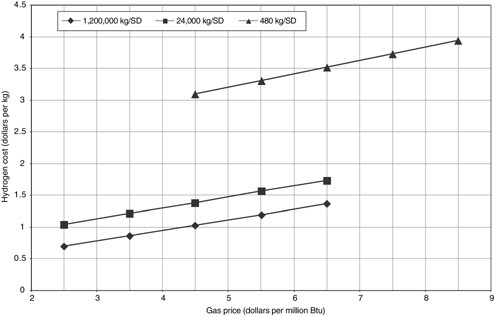
FIGURE G-4 Estimated effects of the price of natural gas on the cost of hydrogen at plants of three sizes using steam methane reforming. Costs based on current technology. NOTE: SD = stream day.
However, this increase would not necessarily increase losses from the natural gas system.
Advantages and Disadvantages
There are several advantages to generating hydrogen from natural gas. Feedstock availability is quite widespread, since an extensive pipeline distribution system for natural gas already exists in the United States and natural gas is available in most populated areas of the country. Further, there is extensive commercial experience, and natural-gas-to-hydrogen conversion technology is widely used commercially throughout the world and is at an advanced stage of optimization in large plants. If centralized, large-scale natural gas conversion plants are built, CO2 can be captured for subsequent sequestration, although its separation and capture are probably not economically feasible with small, distributed hydrogen generators. Furthermore, the committee believes that small-scale reformers at fueling stations are one of the technologies most likely to be implemented in the transition period if policies are put in place to stimulate a transition to hydrogen for light-duty vehicles.
The primary disadvantages of using natural gas are that it is a nonrenewable, limited resource, and increasing amounts are projected to be imported in the future to meet U.S. market needs—which runs counter to the DOE’s goal of improving national security. Also, natural gas prices are volatile and are very sensitive to seasonal demand. Over the past 12 months, for example, the price has varied from $2.70 to more than $9.50/million Btu,14 and there has been an upward trend in the U.S. wellhead gas price since 1998. This variability becomes even more important given that SMR economics are sensitive to natural gas price.
Research Needs and the Department of Energy Program
Distributed generation of hydrogen from natural gas in fueling facilities could be the lowest-cost option for hydrogen production during the transition. However, the future cost of this option is uncertain, given the technical and engineering uncertainties and special requirements that demand priority attention in the DOE program, as it is advanced by contract research organizations.
Distributed generation of hydrogen as envisioned has never before been achieved because of two particular requirements: (1) the mass production of the thousands of generating units, incorporating the latest technology improve-
|
14 |
See NYMEX Henry-Hub NATURAL GAS PRICE, available online at http://www.oilnergy.com/1gnymex.htm#year. Accessed December 10, 2003. |
ments, needed to meet demand, minimize cost, and improve efficiency; and (2) unit designs and operating procedures that ensure the reliable and safe operation of these appliances with only periodic surveillance by relatively unskilled personnel (station attendants and consumers). Currently, there is a market for such units in the merchant industrial sector, which accounts for about 12 percent of the total hydrogen market in the United States (ORNL, 2003). It is clear that the DOE must provide the impetus for the program.
In contrast, centralized generation of hydrogen in one-of-a-kind, medium-sized and large plants is widely practiced, and as a result there is extensive commercial experience in this area. Given the commercial market for hydrogen, the committee believes that suppliers will continue to search for ways to improve the technology and make it even more competitive for medium- and large-scale plants.
Publications from the DOE hydrogen program indicate that the program on distributed generation will include demonstration of a “low-cost, small-footprint plant” (DOE, 2003a, b). However, it is not clear whether the program gives priority to distributed generation or includes an effort to demonstrate the benefits of and specific designs for mass production in the specified time frame of the program. The needed designs would involve concomitant engineering that would create designs for manufacturing engineering, to guide research and to prepare for mass production of the appliance, and would also develop a system design for a typical fueling facility, including the generation appliance, compression, high-pressure storage incorporating the latest storage technology, and dispensers. With today’s technology, such ancillary systems cost 30 percent as much as the reformer. The committee believes that these costs can be reduced by over 50 percent and that efficiency can be improved through system integration and the incorporation of the latest technology. Compression and high-pressure storage are examples of systems in which significant improvements are expected.
The DOE hydrogen program is positioned to stimulate the development of newer concepts, such as membrane separation coupled with chemical conversion, and this seems appropriate to the committee. However, most of the effort in this area appears directed toward POX and ATR. The committee believes that SMR could be the preferred process for this application, and that it should also be pursued in parallel with the effort involving POX and ATR.
HYDROGEN FROM COAL
This section presents the basics of making hydrogen from coal in large, central station plants. The viability of this option is contingent on demand for hydrogen large enough to support an associated distribution system, a large resource base, competitive uses of coal, the environmental impacts of production and transportation, and the technologies and the associated costs for converting coal into hydrogen.
Many of the issues and technologies associated with making hydrogen from coal are similar to those of making power from coal. These subjects are closely linked and should be considered in concert—particularly with respect to clean coal technologies. These technologies will be required for making hydrogen, and they also offer the best opportunity for low-cost, high-efficiency, and low-emission power production. The lowest-cost hydrogen coal plants are likely to be ones that coproduce power and hydrogen.15
Coal is a viable option for making hydrogen in large, central station plants when the demand for hydrogen becomes sufficient to support an associated, large distribution system. The United States has enough coal to make hydrogen far into the future. A substantial coal infrastructure already exists, commercial technologies for converting coal to hydrogen are available from several licensors, the cost of hydrogen from coal is among the lowest available, and technology improvements are identified that should reach future DOE cost targets. The major consideration is that, because of the high carbon content in coal, the CO2 emissions from making hydrogen from coal are larger than those from any other conversion technology for making hydrogen. This underscores the need to develop carbon sequestration techniques that can handle very large amounts of CO2 before the widespread implementation of coal to make hydrogen should occur.
Coal Transportation
If coal is to be a major source for future hydrogen production, the infrastructure for delivering it to the future hydrogen plants will need to be expanded enough to handle these future requirements. Based on the assumptions used by the committee, the current production and delivery infrastructure capacity would need to be increased by 11 percent to meet the 2030 hydrogen demand, and by 57 percent to meet the 2050 hydrogen demand. Coal is a viable option for making hydrogen in large, central station plants when the demand for hydrogen becomes large enough to support an associated transport, storage, and distribution system.
Most bulk coal transportation is by rail, with trucks used for local transport. For reasons of economics, most of the world’s coal consumption is in power plants located nearby coal mines, which minimizes the necessity for long-distance transportation. More than 60 percent of the coal used for power generation worldwide is consumed within 50 km of the mine site. In the United States, the average distance that coal is shipped by rail is farther, at about 800 miles. That distance has increased in recent years owing to the move toward greater use of coals with lower sulfur content (found mainly in the West) to meet sulfur oxide emissions standards in plants located mainly in the South and the East. As coal is currently shipped over great distances in the United States,
delivery to broad geographic areas should not be a barrier to the use of coal to make hydrogen for at least the next 30 years, since demand will not be much different from current trends.
Environmental Impacts of Coal Consumption and Transportation
Using more coal to produce hydrogen will have a number of environmental consequences. Coal mining itself causes numerous environmental issues, ranging from widespread land disturbance, soil erosion, dust, biodiversity impacts, waste piles, and so forth, to subsidence and abandoned mine workings. Once coal has been extracted, it needs to be moved from the mine to the power plant or other place of use.
The main pollutants resulting from conventional combustion of coal are sulfur oxides (SOx), nitrogen oxides (NOx), particulates, CO2, and mercury (Hg). SOx is dealt with through lower-sulfur-content coal as well as flue gas desulfurization (FGD). Approximately 30 percent of U.S. coal power generating equipment had some sort of FGD or SOx reduction technology at the end of 1999, according to data gathered by DOE’s Energy Information Administration.16 Newer processes for power generation, such as integrated gasification combined cycle power generation, which involves a conversion rather than a combustion process, is more effective at reducing criteria pollutants than existing pollution control technologies are (East-West Center, 2000).
Potentially the most significant future issue for coal combustion is CO2 emissions, since on a net energy basis coal combustion produces 80 percent more CO2 than the combustion of natural gas does, and 20 percent more than does residual fuel oil, which is the most widely used other fuel for power generation (EIA [2001], Table B1). Likewise, the CO2 emissions associated with making hydrogen from coal will be larger than those for making hydrogen from natural gas. Using currently available technology, the CO2 emissions are about 19 kg CO2 per kilogram of hydrogen produced, compared with approximately 10 kg CO2 per kilogram of hydrogen manufactured from natural gas.
Atmospheric emissions from coal-fired generating plants are of concern to various bodies—national (criteria pollutants [CO, particulates,17 O3, NO2, SO2, and Pb], are defined and regulated by the EPA under the National Ambient Air Quality Standards) and international (greenhouse gases, considered under the UN Framework Convention on Climate Change, are mainly CO2, CH4, N2O, hydrofluorocarbons, perfluorocarbons, and SF6). Since the 1970s, the U.S. electricity industry has made considerable progress in reducing SO2, NO2, and particulate emissions, despite a large increase in coal consumption, through the use of FGD, filtration, electrostatic precipitators, and selective catalytic reduction (SCR). To the extent that new emission control technologies can be applied to existing plants and that new generating technologies can be used, further progress is expected in overall emissions reductions (Ness et al., 1999).
Current Coal Technologies
Conventional coal-fired power generation uses a combustion boiler that heats water to make steam, which is used to drive an expansion steam turbine and generator. Various designs of coal combustion boilers exist, the most modern and efficient of which use pulverized coal and produce supercritical (high-pressure/high-temperature) steam. Overall efficiencies are typically in the 36 to 40 percent range. Although a staple for power generation for decades, this conventional combustion technique is not suitable for making hydrogen. Hydrogen-making technologies employ a conversion process rather than a combustion process. These conversion processes, such as gasification, are suitable for making power and/or hydrogen.
Clean Coal Technologies
Clean coal technologies use alternative ways of converting coal so as to reduce plant emissions and increase plant thermal efficiency, leading to an overall cost of electricity that is lower than the cost for electricity from conventional plants. Systems under development include low-emission boiler systems (LEBSs), high-performance power systems (HIPPSs), integrated gasification combined cycle (IGCC), and pressurized fluidized-bed combustion (PFBC) (Ness et al., 1999). The goal is to attain thermal efficiencies in the 55 to 60 percent range (higher heating value [HHV]) (Ness et al., 1999). With the exception of the IGCC systems, all of the others rely on increasingly sophisticated emissions control systems; IGCC uses a different conversion system to reduce emissions at the outset. It is this gasification technology that is best suited to making hydrogen from coal.
Gasification Technology
Gasification systems typically involve partial oxidation of the coal with oxygen and steam in a high-temperature and elevated-pressure reactor. The short-duration reaction proceeds in a highly reducing atmosphere that creates a synthesis gas, a mix of predominantly CO and H2 with some steam and CO2. This syngas can be further shifted to increase H2 yield. The gas can be cleaned in conventional ways to recover elemental sulfur (or make sulfuric acid), and a high-concentration CO2 stream can be easily isolated and sent for
disposal. The use of high temperature and pressure and oxygen minimizes NOx production. The slag and ash that is drawn off from the bottom of the reactor encapsulate heavy metals in an inert, vitreous material, which currently is used for road fill. The high temperature also eliminates any production of organic materials, and more than 90 percent of the mercury is removed in syngas processing. Syngas produced from current gasification plants is used in a variety of applications, often with multiple applications from a single facility. These applications include syngas used as feedstock for chemicals and fertilizers, syngas converted to hydrogen used for hydro-processing in refineries, production, generation of electricity by burning the syngas in a gas turbine, and additional heat recovery steam generation using a combined cycle configuration.
There are currently at least 111 operating gasification plants running on a variety of feedstocks. These include residual oils from refining crude oil, petroleum coke, and to a lesser extent, coal. The syngas that is generated has typically been used for subsequent chemicals manufacture; making power from IGCC systems is a more recent innovation, successfully demonstrated in the mid-1980s and commercially operated since the mid-1990s. Gasification is, therefore, a well-proven commercial process technology, and several companies offer licenses for its use.
Oxygen-Blown Versus Air-Blown Gasification
Gasification plants exist that use either air-blown or oxygen-blown designs. Air-blown designs save the capital cost and operating expense of air separation units, but the dilution of the combustion products with nitrogen makes the separation of CO2, in particular, a much more expensive exercise. In addition, the extra inert nitrogen volume going through the plant increases vessel sizes significantly and increases the cost of downstream equipment. Oxygen-blown designs do not introduce the additional nitrogen, so once the sulfur compounds have been removed from the syngas, what is left is a high-purity stream of CO2 that can be more easily and cheaply separated. Because of the need to consider CO2 capture and sequestration for future hydrogen generation plants, only oxygen-blown designs are feasible for consideration.
Estimated Costs of Hydrogen Production and Carbon Dioxide Emissions
Most gasification plants produce syngas for chemical production, and often for steam. IGCC plants then burn the syngas to produce power. The flexibility to polygenerate multiple products to suit a given situation is one of the strengths of the gasification system. Thus, relatively few gasification plants are dedicated to producing hydrogen only (or indeed any other single product). The future large-scale hydrogen generation plant will likely also generate some amounts of power because of the advantages provided through polygeneration. It is necessary therefore to preface any remarks concerning the costs of producing only hydrogen or the costs of sequestering CO2 with this caveat.
All of the technology needed to produce hydrogen from coal is commercially proven and in operation today, and designs already exist for hydrogen and power coproduction facilities. However, technology advances currently in development will continue to drive down the costs and increase the efficiency of these facilities. Hydrogen-from-coal plants combine a number of technologies including oxygen supply, gasification, CO shift, sulfur removal, and gas turbine technologies. All of these technology areas have advances under development that will significantly improve the plant’s capital and operating costs and thermal efficiency. Examples of these pending technology advances include Ion Transport Membrane (ITM) technology for air separation (oxygen supply); advances in gasifier technology (feedstock preparation, conversion, availability); warm gas cleanup; advanced gas turbines for both syngas and hydrogen; CO2 capture technology advances; new, lower-cost sulfur-removal technology; and slag-handling improvements.
It is estimated that today a gasification plant producing hydrogen only would be able to deliver hydrogen to the plant gate at a cost of about $0.96/kg H2 with no CO2 sequestration. If CO2 capture were also required, it would cost $1.03/ kg H2. This pricing reflects costs for producing hydrogen from very large, central station plants at which hydrogen will be distributed through pipelines. In these plants a single gasifier can produce more than 100 million scf/day H2. It is envisioned that a typical installation would include two to three gasifiers.
The economics of making hydrogen from coal is somewhat different from that for making it from other fossil fuels, in that the capital costs needed per kilogram of produced hydrogen are larger for coal plants, but the raw material costs per kilogram of produced hydrogen are lower. Coal is inexpensive, but the coal gasification plant is expensive. If the coal price is changed by 25 percent, the hydrogen cost is changed by only $0.05/kg. If the cost of the plant is changed by 25 percent, the hydrogen cost is changed by $0.16/kg. This should lead to a very stable cost of hydrogen production that can be lowered through future improvements in technology.
In addition to the CO2 produced from making the electricity consumed in producing hydrogen, CO2 emissions result from the carbon in the coal. The emissions depend on the type and quality of coal, but for typical Western coal with 2 percent sulfur and 12,000 Btu/dry lb, approximately 18.8 kg CO2 are emitted per kilogram of hydrogen produced. With a CO2 capture system in place, it is estimated that this figure could be reduced by as much as 80 to 90 percent, the exact amount depending on capital efficiency and cost-benefit analysis. Although the economics of hydrogen production from coal does vary somewhat with the quality of coal being gasified, essentially any coal can be gasified to produce hy-
drogen. Coals with ash content greater than 30 percent are already being gasified. The main effects of coal-quality variance on hydrogen production are the amount of by-products produced (primarily slag and elemental sulfur) and the capital cost, which would be affected mostly by the amount of additional inert material in the coal that has to be handled. For a gasification plant producing maximum hydrogen from coal, the variance in potential feed coal quality is estimated to produce a variance of less than 15 percent in the amount of CO2 generated per ton of hydrogen produced. The lower-quality coals generate lower amounts of CO2 per ton of hydrogen. Other effects of coal quality are less significant.
Research and Development Needs
In terms of its stage of development, coal gasification is a less mature commercial process than coal combustion processes and other hydrogen generation processes using other fossil fuels, especially in the aspects of capturing CO2 and providing flexibility in hydrogen and electricity production. In that sense the potential for improvement through technology development is significant. The main issues are capital cost and reliability (the latter is usually addressed through including standby equipment). Both are major reasons why IGCC technology has not been widely adopted for power generation, which is a very competitive business. The flexibility to vary between hydrogen production and power production will cost extra capital, which has to be recovered.
For the commercial processes available from several different licensors, the R&D needs should be directed at capital cost reduction, standardization of plant design and execution concept, gas cooler designs, process integration, oxygen plant optimization, and acid gas removal technology. The potential efficiency and capital cost improvements in these areas could combine to lower the overall cost of hydrogen from coal by about 10 to 15 percent from today’s costs. Since many parts of the coal-to-hydrogen process are the same as for coal-to-power processes, similar improvements in power costs from IGCC should be possible. These areas are improvements to existing technology, so they should be able to be achieved in the near term.
The potential also exists for new technologies to make larger improvements in the efficiency and cost of making hydrogen from coal. For new gasification technologies, the best opportunities for R&D appear to be for new reactor designs (entrained bed gasification), improved gas separation (hot gas separation), and purification techniques. These technologies, and the concept of integrating them with one another, are in very early development phases and will require longer-term development to verify the true potential and to reach commercial readiness. Recent studies have indicated that the combined potential of these new technologies could lower the cost of making hydrogen from coal by about 25 percent.
Future Costs
Evolutionary improvements in current technology can lower the cost of hydrogen from coal from the estimated $0.96/kg to about $0.90/kg. The evolution of future costs will be a function of the number of units constructed over time, since each subsequent plant gives an additional opportunity to apply the experience derived from prior plants, as well as economies of scale for process unit production.
The introduction of new technologies can lower costs even further. New gasification technologies along with new syngas cleanup and separation technologies hold potential for further improving efficiencies and lowering the costs of producing hydrogen to about $0.71/kg (see Chapter 5 and Appendix E). Separating and capturing CO2 will increase these costs to $0.77/kg.
Department of Energy Programs for Coal to Hydrogen
The DOE programs for making hydrogen from coal reside in the Office of Fossil Energy and are related to programs to make electricity from coal. The overall goal of the Hydrogen from Coal Program is to have an operational, zero-emissions, coal-fueled facility in 2015 that coproduces hydrogen and electricity with 60 percent overall efficiency (DOE, 2003c). Major milestones for reaching this goal include these:
-
2006—Advanced hydrogen separation technology, including membranes tolerant of trace contaminants, identified;
-
2011—Hydrogen modules for coal gasification combined-cycle coproduction facility demonstrated; and
-
2015— Zero-emission, coal-based plant producing hydrogen and electric power (with sequestration) that reduces the cost of hydrogen by 25 percent compared with the cost at current coal-based plants demonstrated.
To reach these milestones, R&D activities within the Hydrogen from Coal Program are focused on the development of novel processes that include these:
-
Advanced water-gas-shift reactors using sulfur-tolerant catalysts,
-
Novel membranes for hydrogen separation from CO2,
-
Technology concepts that combine hydrogen separation and water-gas shift, and
-
Fewer-step designs to separate impurities from hydrogen.
Associated coal gasification R&D programs in which success is dependent on efficiency improvements and lower cost include these:
-
Advanced ITM technology for oxygen separation from air,
-
Advanced cleaning of raw synthesis gas,
-
Improvements in gasifier design, and
-
CO2 capture and sequestration technology.
Summary
The United States has enough coal to make all of the hydrogen that the economy will need for a very long time, a substantial coal infrastructure already exists, commercial technologies for converting coal to hydrogen are available from several licensors, the cost of hydrogen from coal is among the lowest available, and technology improvements are identified to reach the future DOE cost targets. As such, coal is a viable option for making hydrogen in large, central station plants when the demand for hydrogen becomes large enough to support an associated distribution system.
The key to the efficient and clean manufacture of hydrogen from coal is to gasify the coal first, to produce a synthesis gas—a mixture of hydrogen and CO—and then to further process the CO with water to produce additional hydrogen and CO2.
Combinations of coal gasifiers and gas cleanup processes have been built, tested, and used to produce electric power in the integrated gasification combined cycle (IGCC) process. While IGCC power plants have been built and operated on a commercial scale, further process improvements to lower costs and to improve reliability are both possible and desirable. Accordingly, a number of years ago the DOE initiated a related R&D program called Vision 21, which is up and running and has been reviewed by the National Research Council, most recently in early 2003 (NRC, 2003b). Major aspects of this program will be applicable to making hydrogen from coal and will lead to more efficient and lower-cost hydrogen production designs.
Making hydrogen from coal produces a large amount of CO2 as a by-product. At present, the United States does not restrict the emissions of CO2 from any sources, but it is possible that such restrictions might be invoked in the future. Because of the possible effects of CO2 on global climate change, the government has accelerated R&D aimed at reducing or eliminating CO2 emissions from energy-producing systems, one of these being coal-fueled systems. A part of the Department of Energy’s hydrogen program is aimed at developing safe and economic methods of sequestering CO2 in a variety of underground geologic formations. Indeed, a sequestration R&D program was initiated in the department’s Office of Fossil Energy a number of years ago and is now supported at a significant level. The new coal-based power systems being developed under the DOE’s Vision 21 program are aimed at coupling power plant with sequestration systems.
Beyond the Vision 21 program, the DOE recently announced its intention to proceed with a large, coal-to-electricity-and-hydrogen verification plant with coupled sequestration. This plant, called FutureGen, is now in the early stages of detailed planning. In addition to demonstrating coproduction of electricity and hydrogen with sequestration, the system is intended to act as a large-scale testbed for innovative new technologies aimed at reducing systems costs.
HYDROGEN FROM NUCLEAR ENERGY
Nuclear Power Technology Today
The United States derived about 20 percent of its electricity from nuclear energy in 2002 (EIA, 2003). While no nuclear power plants have been ordered in the United States since 1975, the orders prior to that date resulted in the 103 power reactors operating today. With a total capacity of nearly 100 gigawatts electric (GWe), they constitute about 13 percent of the installed U.S. electric generation capacity. Since their operating costs are relatively low, the existing plants tend to be part of the base load for their owner companies, and their output has been increasing since the late 1980s. The current U.S. plants use water as the coolant and neutron moderator, and they rely on the steam Rankine cycle as the thermal-to-electrical power conversion cycle. Nearly 65 percent of these light-water reactors (LWRs) are of the pressurized-water reactor (PWR) type, and 35 percent are of the boiling-water reactor (BWR) type. The LWR technology has dominated the reactor market and constitutes about 80 percent of the nearly 440 operating plants in the world today. Different technologies have been deployed in Great Britain, which depends mostly on gas-cooled reactors (GCRs) and advanced gas-cooled reactors (AGRs) cooled by CO2 but using an indirect steam power cycle. Canada, India, and a few other countries operate heavy-water reactors (HWRs), also with an indirect steam power cycle.
Other reactor technology options were tested in several countries. These include helium-cooled high-temperature gas-cooled reactors (HTGRs) and sodium-cooled fast reactors (SFRs). However, the operation of these plants did not spur wider market penetration. The HTGR has a significant technical base due to the experience gained from power plants in the United States and Germany and the more recent, smaller test reactors in Japan and China. Coupling gas-cooled reactors to a direct or indirect gas turbine Brayton power cycle can yield thermal efficiencies much higher than the 33 percent of current LWRs. However, there is no experience in gas-turbine powered nuclear plants, since the U.S. and German HTGR plants use an indirect steam Rankine cycle for electricity production.
While the LWR technology dominates the global nuclear energy market, the fuel cycle technology has not had similar unanimity. The United States, Spain, Sweden, and several other countries opted for a once-through uranium-based fuel cycle, in which the used fuel is destined for a geologic repository for highly radioactive waste, after a storage period of a decade or more. France, Germany, Japan, and Russia, among other countries, have preferred to extract and recycle the fissile material in the spent fuel to increase the energy
derived from the fuel and to reduce the volume and toxicity of the waste that will be disposed of in geologic repositories. The U.S. approach is less costly while the supply of inexpensive uranium lasts—which at the current rate of consumption should be for at least 50 to 100 years. When additional fuel material is needed, chemical reprocessing of the used fuel to recycle it can extend the fuel availability to thousands of years—that is when fast reactors, such as the sodium-cooled reactors, would become desirable.
Nuclear Power Technology in the Future
In the past 20 years, several advanced versions of the LWR, collectively called advanced LWRs (ALWRs), have been designed, but only one type has been built: the advanced boiling-water reactor (ABWR), which was built in Japan. These reactors are generally known today as Generation III reactors. Some of these designs have been certified as safe by the Nuclear Regulatory Commission (USNRC), but no orders have materialized for them in the United States. New versions of light-water reactors are now under review for safety certification by the USNRC.
Two versions of the HTGR are being designed by international consortia. One is led by the South African utility, ESKOM, with a direct helium gas turbine power cycle. This reactor builds on the German experience with circulating graphite pebbles containing ceramic-coated oxide fuel microparticles. The fuel is designed to be robust for the temperature range of operation and accidents. The ability of the microparticle fuel to reach very high burnup induced another consortium (Framatome ANP, General Atomics [GA], and Russian collaborators) to design a plutonium consumption reactor. In this case, the microparticles will be housed in stationary graphite blocks, typical of the earlier GA-designed HTGRs in the United States.
In 2002, several reactor concepts were selected by an international team representing 10 countries as promising technologies that should be further explored for availability beyond 2025; these technologies are collectively known as the Generation IV reactors. The goals for the new reactor systems are to improve the economics, safety, waste characteristics, and security of the reactors and the fuel cycle. The emphasis in the development was given to the following six concepts:
-
Very high temperature reactor (VHTR), a version of the HTGR;
-
Supercritical water reactor (SCWR), with a direct power cycle;
-
Fast gas-cooled reactor (FGR), with a direct helium or CO2 gas turbine power cycle;
-
Heavy metal (lead alloy)-cooled reactor (HMCR), with an indirect power cycle;
-
Sodium-cooled reactor (SCR), with an indirect steam power cycle; and
-
Molten salt-cooled reactor (MSR), with a fluid fuel and an indirect power cycle.
It is notable that all Generation IV reactors aim to operate at higher coolant temperatures than those of the LWRs, thereby increasing the efficiency of thermal-to-electrical-energy conversion. The main characteristics of some of the reactors mentioned here and others are given in Table G-3.
Proposed Technologies for Hydrogen Production
Hydrogen can be produced using current reactor technology for electricity for electrolysis (water splitting). Potentially more efficient hydrogen production may be attained by significantly raising the water temperature before splitting the molecules using thermochemistry or electrolysis. Such approaches require temperatures in the range of 700°C to 1000°C. Current water-cooled reactors produce temperatures under 350°C, and cannot be used for such purposes. However, the coolants from several advanced reactor concepts do reach such high temperatures and may be coupled to thermochemical plants (Brown et al., 2003; Doctor et al., 2002; and Forsberg, 2003). A recent experimental helium-cooled reactor at the Japan Atomic Energy Research Institute (JAERI) was built specifically with the goal of hydrogen production. Its desired coolant maximum temperature is 900°C. It started operation in 1999 and is still undergoing testing of its fuels and its operations at lower temperatures.
Another possibility for producing hydrogen is the use of nuclear heat to provide the energy needed for heating in the steam methane reforming (SMR) process, as suggested recently by the Electric Power Research Institute (EPRI) (Sandell, 2003). That too requires high temperatures, above 700°C, for efficiency. Therefore, it must be coupled to a high-temperature reactor. This process reduces but does not eliminate the CO2 emissions associated with conventional SMR. It also reduces the amount of natural gas required for hydrogen production.
TABLE G-3 Nuclear Reactor Options and Their Power Cycle Efficiency
|
Current and Advanced Reactor Type |
Toutlet (°C) |
ηth (%) |
|
Current light-water reactor (LWR) |
280–320 |
32–34 |
|
Advanced light-water reactor (ALWR) |
285–330 |
32–35 |
|
Supercritical water-cooled reactor (SCWR)a |
400–600 |
38–45 |
|
He high-temperature graphite reactor (HTGR) |
850–950 |
42–48 |
|
Supercritical CO2 advanced gas reactor (S-AGR) |
650–750 |
46–51 |
|
Molten salt-cooled reactor (AHTR)b |
750–1000 |
NE |
|
Heavy metal (lead alloy)-cooled reactor (HMCR)a |
540–650 |
NE |
|
NOTE: NE = not evaluated. aOne of the Generation IV reactors. bThe fuel resembles that of an HTGR but with a salt coolant. |
||
The various options for nuclear hydrogen production are given in Table G-4. The basic chemistry, projected efficiency, established experience, and other related issues for each technology option are now briefly addressed.
High-Temperature Electrolysis of Steam
The electrical energy demand in the electrolysis process decreases with increasing water (or steam) temperature. While the demand for heat energy is increased, the decrease in the electrical energy demand improves the overall thermal-to-hydrogen heat conversion efficiency. Higher temperatures also help lower the cathodic and anodic overvoltages. Therefore, it is possible to increase the current density at higher temperatures, which yields a significant increase in the process efficiency. Thus, the high-temperature electrolysis of stream (HTES) is advantageous from both thermodynamic and kinetic standpoints. The electrodes of the HTES unit can be made of ceramic materials, which avoids corrosion problems, though hydrogen embrittlement might still be a problem for electrode durability. High-pressure operation would also be preferable, in order to reduce the size of the chemical units and transmission lines.
The HTES process is potentially advantageous when coupled to high-efficiency power cycles and can consequently yield high overall thermal-to-hydrogen energy efficiency. The efficiency of hydrogen production via coupling of HTES to either of two high-temperature nuclear reactors is given in Figure G-5 (Yildiz and Kazimi, 2003). One reactor is the gas turbine modular high-temperature reactor (GT-MHR) (LaBar, 2002). The second is an advanced gas-cooled reactor (AGR) coupled to a direct supercritical CO2 power cycle. The cycle was originally proposed for fast reactors (Dostal et al., 2002). The supercritical AGR (S-AGR), also referred to as the S-CO2, necessitates upgrading the AGR design pressure from the current 4 megapascals (MPa) to about 20 MPa, which has not been attempted before in a concrete containment. A reference HTES design called HOTELLY (high-operating-temperature electrolysis) (Doenitz et al., 1988) is chosen as the basis for this example.
Implementation of the GT-MHR-HTES at the temperature of 850°C for the near term appears possible, while achieving temperatures of 950°C and higher might be expected for the years 2025 and beyond. Similarly, for the S-AGR-HTES, the near-term and far-term goals may be 650°C
TABLE G-4 An Overview of Nuclear Hydrogen Production Options
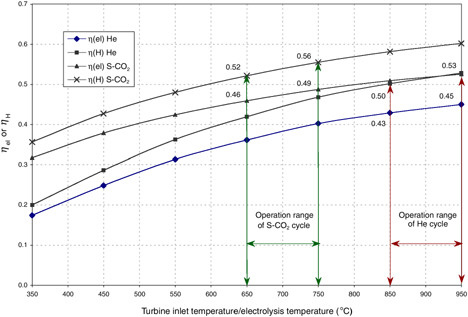
FIGURE G-5 Power cycle net efficiency (ηel) and thermal-to-hydrogen efficiency (ηH) for the gas turbine modular helium reactor (He) high-temperature electrolysis of steam (HTES) and the supercritical CO2 (S-CO2) advanced gas-cooled reactor HTES technologies. SOURCE: Yildiz and Kazimi (2003).
and 750°C, respectively. The thermal energy (MJ) needed to produce 1 kg H2 is presented in Figure G-6.18
Nuclear reactors coupled to HTES are capital-intensive technologies, due to both the nuclear plant and the electrolysis plant. The development of economical and durable HTES unit materials, which can be similar to those of the solid oxide fuel cell materials, can contribute to cost reduction. The development of improved HTES units with low electrode overvoltage at lower temperatures can enable their use with lower-temperature and thereby lower-cost nuclear plants. Improved HTES cell designs are currently being investigated at Lawrence Livermore National Laboratory (Pham, 2000) and Idaho National Engineering and Environmental Laboratory (Herring, 2002). In addition, attaining high power cycle efficiency at the nuclear plant with relatively low temperatures can contribute to cost reduction. Finally, development of economic high-temperature radiation-resistant graphite or ceramic-coated graphite materials for the nuclear plant is needed.
Thermochemical Water Splitting
A recent screening of several hundred possible reactions (Besenbruch et al., 2000) has identified two candidate thermochemical cycles that have the highest commercialization potential, with high efficiency and practical applicability to nuclear heat sources. These are the sulfur-iodine (SI) and calcium-bromine-iron (Ca-Br) cycles. The S-I cycle is being investigated by General Atomics and JAERI. The Ca-Br cycle, which is sometimes called UT-3 to honor its origin at the University of Tokyo, is being investigated by JAERI. Argonne National Laboratory (ANL) is currently working on achieving thermochemical water-splitting processes at lower temperatures than the SI and Ca-Br cycles. ANL has identified the copper-chlorine (Cu-Cl) thermochemical cycle for this purpose (Doctor et al., 2002).
Sulfur-Iodine Cycle and Other Sulfur Cycles The SI cycle has been proposed in several forms. (The SI cycle and other
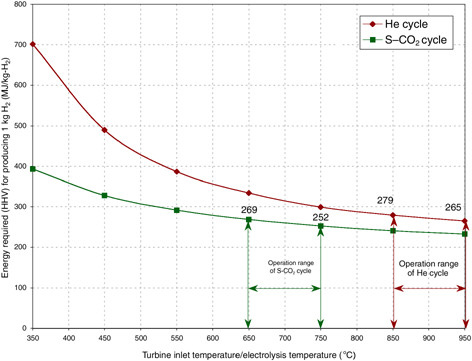
FIGURE G-6 The energy needs for hydrogen production by the gas turbine modular helium reactor (He cycle) high-temperature electrolysis of steam (HTES) and the supercritical CO2 (S-CO2 cycle) advanced gas-cooled reactor HTES technologies. NOTE: HHV = higher heating value.
sulfur cycles are depicted schematically in Figure G-7.) The most promising form consists of the following three chemical reactions, which yield the dissociation of water (Brown et al., 2003):
A hybrid sulfur-based process does not require iodine and has the same high-temperature step as sulfur iodine but a single electrochemical low-temperature step that forms sulfuric acid. That electrolysis step makes sulfuric acid at very low voltage (power). The low-voltage electrolysis step (low power compared with electrolysis of water) may allow much larger scale-up of the electrochemical cells. (High-voltage systems have high internal heat generation rates that often limit the scale-up of a single cell.) The efficiency of this process is about the same as that of the SI process, but is influenced by the efficiency of the electrical power cycle. It is one of only four processes for which a fully integrated process has been demonstrated in a hood. It is the only process for which a full conceptual design report for a full-scale facility has been developed. Lastly, like the SI process, it has the potential for major improvements.
The SI cycle requires high operating temperatures but offers the opportunity for high-efficiency conversion of heat to hydrogen energy, ηH, as shown in Figure G-8. The SI cycle can be coupled to the modular high-temperature reactor (MHR) (a version of the HTGR) (LaBar, 2002). This reactor consists of 600 megawatt-thermal (MWth) modules, which are cooled by helium gas, with high coolant exit temperatures that can provide the necessary heat to the SI reactions. The coupling of the MHR and SI cycle, MHR-SI, provides a large-scale, centralized production of hydrogen.
The MHR-SI is a capital-intensive technology. Future cost reduction can be achieved from high efficiency by devising materials that can withstand higher temperatures. Reactor materials that are temperature-, irradiation- and corrosion-resistant would be needed. Also, possible reduc-
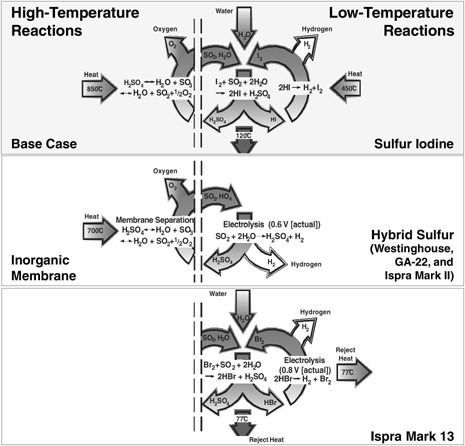
FIGURE G-7 Depiction of the most promising sulfur thermochemical cycles for water splitting. Courtesy of Charles Forsberg, Oak Ridge National Laboratory.
tion in the capital cost may result from improved catalytic materials and higher hydrogen production capacity in each facility.
Calcium-Bromine-Iron Cycle The calcium-bromine-iron (Ca-Br, or UT-3) cycle involves solid-gas interactions that may facilitate the reagent-product separations, as opposed to the all-fluid interactions in the SI cycle, but it will introduce the problems of solids handling, support, and attrition. This process is formed of the following reactions (Doctor et al., 2002):
The thermodynamics of these reactions have been found favorable. However, the hydrogen production efficiency of the process is limited to about 40 percent, owing to the melting point of Ca-Br2 at 760°C (Schultz et al., 2002).
Other Cycles Argonne National Laboratory’s Chemical Engineering Division is studying other cycles like the copper-chlorine thermochemical cycle. The energy efficiency of the process is projected to be 40 to 45 percent (ANL, 2003). This work is currently being investigated only by ANL, at a bench-scale R&D level, and no pilot demonstra-
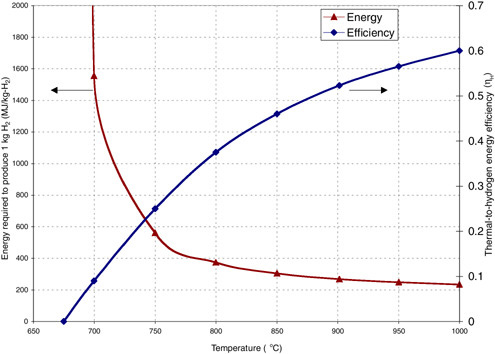
FIGURE G-8 Estimated thermal-to-hydrogen efficiency (ηH) of the sulfur-iodine (SI) process and thermal energy required to produce a kilogram of hydrogen from the modular high-temperature reactor-SI technology. SOURCE: Brown et al. (2003).
tions have been undertaken. One of the main advantages of this process is that construction materials and corrosion-resistance are more tractable at 500°C than at higher temperatures. Another advantage is that, owing to its relatively low operating temperature, it can become compatible with several current and advanced nuclear reactor technologies.
Steam Methane Reforming
Steam methane reforming (SMR) is currently the main commercial technology for hydrogen production in the United States. The SMR process requires high temperature, and the most common means of providing the heat for the process is through the burning of natural gas in the reforming furnaces, as described in the section “Hydrogen from Natural Gas,” earlier in this appendix.
The SMR process can be coupled to a high-temperature helium-cooled reactor, such as the MHR. The MHR can function as the heat source operating at about 850°C, to replace the natural gas burning. The high operating temperature can enable the process to take place at about 80 percent efficiency. This approach (which might be called N [nuclear]-SMR) reduces the CO2 emissions to the atmosphere by large quantities. Elimination of the natural-gas-burning furnace in this process reduces the CH4 consumption by about 40 percent (Spath et al., 2000), which is parallel to the amount of CO2 emission reduction.
Cost of Nuclear Hydrogen Production Plants
The cost of hydrogen produced by electricity generated from existing nuclear power through water electrolysis is equivalent to using the electricity supplied by the grid for hydrogen production. Today this cost is about a factor of 3 higher than what is achievable by conventional SMR, with natural gas prices at $4.5/million Btu, even when the cost of hydrogen distribution is taken into account. The improved power-cycle efficiencies of the advanced nuclear power plants may bring this cost differential in the future down to a factor of 1.5.
The cost of hydrogen production using the MHR-SMR option is dependent on the cost of natural gas feedstock to the reforming process. However, the cost from MHR-SMR is less sensitive to the cost of natural gas than is conventional
SMR. Hydrogen production cost estimates by EPRI (Sandell et al., 2003) and by the Massachusetts Institute of Technology (Yildiz and Kazim, 2003) indicate that this approach may be competitive with conventional SMR if the natural gas prices go above $6/million Btu. However, their analyses did not include any taxes or fees for CO2 production.
The cost of hydrogen production by the nth-of-a-kind of MHR using the SI process was assessed by Brown et al. (2003). The authors considered the cost of producing 800 t H2 per day using heat from four units of 600 MWth, each producing a coolant at 850°C and having an overall efficiency of 42 percent. Starting with an overnight cost of $470/ MWth for the nuclear electric plant, adding a heat exchanger, and replacing the electric generation capacity with a thermochemical plant, the total plant capital cost was found to be about $750/MWth. (A recent review of the costs of nuclear power at recent plants—built in the past 10 years in Korea, Finland, and Japan—finds the overnight costs of plants to be in the range of $530 to $800/MWth [Deutch and Moniz, 2003].) The cost of running the MHR nuclear plant is estimated to be $93.9 million per year and the hydrogen plant to be $50.7 million per year. This resulted in the cost of hydrogen production being about $1.50/kg. However, it is possible to argue that future developments could facilitate reaching higher efficiency in the conversion of the nuclear thermal energy into hydrogen production. Furthermore, larger numbers of units in one place could lead to lower costs; thus, larger plants could be associated with lower plant and operating costs. Using optimistic assumptions about advances in nuclear plant construction and thermochemical plant efficiency, the cost of a 1200 t/day MHR-SI hydrogen plant may be assumed to reach a level of $600/MWth as the technology matures. Including the usual contingency and permitting costs could add about one-third to this cost, thus leading to an effective plant cost estimate of $800/MWth and, assuming a 3-year construction time, the hydrogen production cost would be about $1.60/kg.
Advantages of Nuclear Energy Use for Hydrogen Production
-
Long-term domestic source. Nuclear fuel will be available for a long time in the future, both domestically and worldwide. Its price is not subject to global geopolitical pressures.
-
Carbon implications. If nuclear energy is used in the short term as the heat source in the SMR process, the result would be to reduce CO2 emissions by nearly 40 percent. If one of the water-splitting processes is used, whether via a thermochemical process or an electrolysis approach, there will be no CO or CO2 emissions.
-
Efficiency of the overall process. In comparison with several other sources of hydrogen, the capability of attaining overall thermal-to-hydrogen energy efficiency in excess of 50 percent values by future technologies (e.g., the N-SMR, HTES, SI, and possibly other paths) is one of the advantages of nuclear energy use in hydrogen production. The higher the temperatures that can be achieved for the reactors, the higher their efficiencies.
-
Environmental implication. There are no polluting emissions, or toxic gas, or particulate releases due to nuclear energy use for water splitting as the means for hydrogen production. N-SMR will have CO2 emissions. The water-splitting processes coupled to high-temperature reactors assume complete recycling of all reactants. The volume of waste from the nuclear reactor cycle, while highly radioactive, is confined to small quantities compared with that from several other sources of energy, but it will have high levels of concentrated radioactivity.
Disadvantages of Nuclear Energy Use for Hydrogen Production
-
Efficiency of the conventional electrolysis process. Even though it is a proven and clean technology, the low efficiency of low-temperature electrolysis makes the process uneconomic.
-
Capital cost. Both the new nuclear reactor plants and the hydrogen plants coupled to the nuclear plants are capital-intensive. While the operating costs will be low owing to the expected high thermal efficiencies, the economics of the whole process may be disadvantageous. Capital and life-cycle costs remain high, and plant designs are in need of simplification. Enabling shorter periods of construction and increased factory-based manufacturing of components will also reduce the cost of the plants.
-
Nuclear waste. The nuclear waste disposal scheme remains to be finalized. The Yucca Mountain project in Nevada has made good advances recently, and when licensed it can provide a destination for the spent fuel accumulating at the plant sites. The development of a closed fuel cycle that involves the extraction and use of the fissile contents from the irradiated fuel would reduce the long-lived radioactivity associated with the waste to be sent to the repository.
-
Proliferation. Nuclear-fuel-cycle operations leave open the possibility of improper access to fissile material through theft or diversion. Proliferation can be addressed through near-term measures designed to improve the proliferation-resistance of current nuclear reactor operations and through long-term research to explore proliferation-resistant designs (PCAST, 1999).
-
Public concerns and permitting needs. There is a public perception that nuclear energy and its emissions during normal operations increase radiation risks. There is also some fear of widespread devastation in case of accidents. These concerns would be reduced by the continued safe operation of existing plants and increased safety margins in new plants. In addition, the recent concerns about terrorism may add to the public fear of nuclear plants. The concerns of the public have led in the past to prolonged permitting periods for nuclear plants. Thus, the permitting of commercial
-
nuclear energy may pose a barrier to any expansion of this technology.
Research and Development Needs for Economic Hydrogen Production Using Nuclear Energy
-
A high priority should be given to the development of high-temperature reactors that can provide coolants at temperatures higher than 800°C. This objective seems most readily achievable using the helium-cooled gas reactor technology of HTGRs. The ability of the reactor’s structural materials to operate for a long time at temperatures between 800°C and 1000°C needs to be established. The R&D program should include the following:
-
Qualification of particle fuel materials to operate at the desired high temperatures,
-
Qualification of the irradiation properties of graphite and other structural materials at the desired range of temperatures, and
-
Operation and control of the helium power cycle at very high temperatures.
-
-
The efficiency of thermochemical schemes to accomplish water splitting without any CO2 emissions should be examined at a laboratory scale for the promising cycles, such as the SI cycles. Materials compatibility issues and catalysts to enhance the reaction at lower temperatures should be pursued. Reasonably-sized demonstration plants using the integrated process should be pursued in a few years for the most promising scheme.
-
Development of the high-temperature steam electrolysis process should be pursued. The issues of materials durability, reduction of overvoltages, effects of the operating pressure, and separation of gas products in an efficient and safe manner should be investigated.
-
Development of a supercritical CO2 cycle should be given a high priority. It can be directly used with a CO2-cooled reactor such as the AGR, or indirectly used with the other reactors such as an HTGR. It can be the bottoming cycle for a high-temperature reactor, whose coolant would supply heat at higher temperatures to a thermochemical plant. Demonstration of the thermal conversion efficiency for a moderate-size turbine and compressor (in the MWe range) is needed to validate the cycle thermodynamics.
-
The safety issues of coupling the nuclear island to the hydrogen-producing chemical island need to be examined in order to establish the guidelines necessary for avoiding accident propagation from one island to the other. Such guidelines would be needed even if the first application of nuclear hydrogen production was based on the nuclear-assisted SMR approach.
Comments on the Department of Energy Program
The DOE nuclear hydrogen program is being pursued in two streams: one for reactor technology development and one for the chemical processes. The DOE’s total program for reactor development was not reviewed by the committee, but it is understood that the DOE is pursuing the development of several versions of high-temperature reactors and is giving priority to the gas-cooled reactor options. This priority is compatible with the reactors suitable for hydrogen production. However, the molten salt-cooled graphite reactor may be a variant missing in the DOE program.
The DOE R&D program related to hydrogen from nuclear energy includes the chemical processes as well as the high-temperature electrolysis path. A balanced approach is wise in order to benefit from high-efficiency electricity generation at lower temperatures than appear to be required for the thermochemical processes. A systems analysis of the electrolysis approach is needed in order to determine the impact of the more efficient distributed generation capability. The electrolyzer units can use materials similar to those of fuel cells that operate at high temperatures, and a synergistic materials program may be possible. Finally, both electrolysis and thermochemistry are potentially applicable to the use of solar energy for hydrogen production.
The overall size of the hydrogen plant R&D appears modest at this point, seeking $2 million in new funds under the Hydrogen Fuel Initiative in FY 2004, in addition to the $2 million being spent through the Nuclear Energy Research Initiative. That level might be appropriate for laboratory-level investigations of one option. Covering several options in both the thermochemistry and high-temperature electrolysis properly requires a level of $10 million to $20 million per year for 4 or 5 years before reaching a conclusion on the best approach for a large (100 MWth) demonstration plant based on the most promising option.
The current R&D portfolio does not allow for “out of the box” thinking. It needs to encourage exploratory basic research involving other approaches, such as methods of enhancing hydrogen production by radiolysis or photolysis in properly designed radiation sources.
Summary
Hydrogen can be produced from current nuclear reactors using electrolysis of water. More efficient hydrogen production may be attained by thermochemical splitting of water or electrolysis of high-temperature steam. Another possibility is the use of nuclear energy as the source of heat for steam methane reforming (SMR). The water-splitting approach releases no CO2. Efficient water-splitting processes and nuclear-SMR all require temperatures well above 700°C. Current water-cooled reactors produce temperatures under 350°C, and cannot be used for efficient hydrogen production. Advanced reactors, such as gas-cooled reactors, involve coolants that can achieve the required high temperatures.
As indicated, the DOE’s total program for reactor development was not reviewed by the committee, but it is understood to include high-temperature reactors, with focus on
gas-cooled reactor options. This priority is compatible with the reactors suitable for hydrogen production. The DOE R&D program on the chemical processes for nuclear hydrogen production appears to favor thermochemical processing over the high-temperature electrolysis path. A more balanced approach would be wiser in order to make use of potentially high efficiency electricity generation at lower temperatures than are required for thermochemical processes. Furthermore, the electrolyzer units can use materials similar to those for fuel cells that operate at high temperatures, and a synergistic materials program may be possible.
The research budget for the hydrogen technology part of the Department of Energy’s nuclear hydrogen program is at the level of $4 million for FY 2004, which appears to be modest. The examination of several options for promising cycles, including the process kinetics, the material’s ability to withstand the aggressive chemistry and temperatures, the separation of fluids, and the overall efficiency of the systems involved, requires a significantly higher level of funding for a few years, until the most promising process is selected for demonstration. Advances made in the thermochemical cycles or in high-temperature electrolysis are of benefit to hydrogen production using other fuel sources, such as solar energy. A portfolio of advancing near-term technologies needs to be maintained while innovative approaches are being examined.
The research portfolio should also include safety aspects of integrating the nuclear reactor with the chemical plant for hydrogen production. This aspect of the program is an important ingredient in establishing guidelines for the designs to avoid the potential for accident propagation. The involvement of industry in assessing the practicality and cost of the technology that might be selected for development in order to ensure the highest economic potential should be emphasized.
HYDROGEN FROM ELECTROLYSIS
Two basic options exist for producing hydrogen. One way is to separate the hydrogen from hydrocarbons through processes referred to as reforming or fuel processing. The second way to make hydrogen is from water, using the process of electrolysis to dissociate water into its separate hydrogen and oxygen constituents. Electrolysis technologies that have been in use for decades both dissociate water and capture oxygen and/or hydrogen, primarily to meet industrial chemical needs. Electrolysis has also played a critical role in life support (oxygen replenishment) in space and submarine ap plications over the past several decades.
Importance of Electrolysis
Making hydrogen through electrolysis generally consumes considerably more energy per unit of hydrogen produced than does making hydrogen from hydrocarbons. Nonetheless, electrolysis is of interest as a potential source of hydrogen energy for several reasons. First, water (and the hydrogen it contains) is more abundant than hydrocarbons are. Depletion and geopolitical concerns for water are in general far less serious than are those for hydrocarbons. Further, there are geographical regions in the nation and around the world where hydrocarbons (especially natural gas, the predominant source of hydrogen reformation) are simply not available; hydrogen from water may be the only practical means of providing hydrogen in such settings.
Second, the net energy costs of making hydrogen through electrolysis must be viewed in an economic context. Electrolysis can be a means of converting low-cost Btus (e.g., coal) into much-higher-value Btus if the result is to replace gasoline or other transport fuels.
Third (and as is discussed further in the analysis that follows), electrolysis is seen as a potentially cost-effective means of producing hydrogen on a distributed scale and at costs appropriate to meet the challenges of supplying the hydrogen needs of the early generations of fuel cell vehicles. Electrolyzers are compact and can realistically be situated at existing fueling stations.
Fourth, electrolysis presents a path to hydrogen production from renewably generated electrical power. From an energy perspective, electrolysis is literally a way to transform electricity into fuel. Electrolysis is thus the means of linking renewably generated power to transport fuels markets. Currently, renewable solar, wind, and hydro power, by themselves, produce only electricity.
And finally, electrolyzers operating in tandem with power-generating devices (including fuel cells) present a new architecture for markets related to distributed energy storage. Various electrolyzer makers are developing products that can make hydrogen when primary electricity is available, and then store and use that hydrogen for subsequent regeneration into electricity as needed. For example, several firms are involved with developing backup power devices that operate in the 1 to 20 kilowatt (kW) range for up to 24 hours, well beyond the capability of conventional batteries. This same concept is being applied directly to renewable sources, creating the means to produce power-on-demand from inherently intermittent renewables. And finally, electrolysis may play a role in regenerative braking on vehicles. Electrolyzers and hydrogen have the appropriate scale and functionality to become part of the distributed generation marketplace as the cost of electrolyzers comes down over time.
Technology Options
Current electrolysis technologies fall into two basic categories: (1) solid polymer (which provides for a solid electrolyte) and (2) liquid electrolyte, most commonly potassium hydroxide (KOH). In both technologies, water is introduced into the reaction environment and subjected to an electrical current that causes dissociation; the resulting hydrogen and oxygen atoms are then put through an ionic transfer mecha-
nism that causes the hydrogen and oxygen to accumulate in separate physical streams.
Solid polymer, or proton exchange, membranes were developed at General Electric and other companies in the 1950s and 1960s to support the U.S. space program. A proton exchange membrane (PEM) electrolyzer is literally a PEM fuel cell operating in reverse mode. When water is introduced to the PEM electrolyzer cell, hydrogen ions are drawn into and through the membrane, where they recombine with electrons to form hydrogen atoms. Oxygen gas remains behind in the water. As this water is recirculated, oxygen accumulates in a separation tank and can then be removed from the system. Hydrogen gas is separately channeled from the cell stack and captured.
Liquid electrolyte systems typically utilize a caustic solution to perform the functions analogous to those of a PEM electrolyzer. In such systems, oxygen ions migrate through the electrolytic material, leaving hydrogen gas dissolved in the water stream. This hydrogen is readily extracted from the water when directed into a separating chamber.
KOH systems have historically been used in larger-scale applications than PEM systems. Electrolyzer Corporation of Canada (now Stuart Energy) and the electrolyzer division of Norsk Hydro have built relatively large plants (100 kg/hour and larger) to meet fertilizer production needs in locations around the globe where natural gas is not available to provide hydrogen for the process.
The all-inclusive costs of hydrogen from PEM and KOH systems today are roughly comparable. Reaction efficiency tends to be higher for KOH systems because the ionic resistance of the liquid electrolyte is lower than the resistance of current PEM membranes. But the reaction efficiency advantage of KOH systems over PEM systems is offset by higher purification and compression requirements, especially at small scale (1 to 5 kg/hour).
Today’s Electrolysis Markets
Chemical and Niche Energy Applications
Electrolyzers are today commercially viable only in selected industrial gas applications (excepting various noncommercial military and aerospace applications). Commercial applications include the previously mentioned remote fertilizer market in which natural gas feedstock is not available. The other major commercial market for electrolysis today is the distributed, or “merchant,” industrial hydrogen market. This merchant market involves hydrogen delivered by truck in various containers. Large containers are referred to as tube trailers. An industrial gas company will deliver a full tube trailer to a customer and take the empty trailer back for refilling. Customers with smaller-scale requirements are served by cylinders that are delivered by truck and literally installed by hand.
In general, the smaller the quantities of hydrogen required by a customer, the higher will be the all-inclusive delivered cost. Tube trailer customers (e.g., semiconductor, glass, or specialty metals manufacturers) pay in the range of $3.00/ 100 scf, or about $12/kg. Cylinder customers (e.g., laboratories, research facilities, and smaller manufacturing concerns) pay at least twice the tube trailer price. The value of hydrogen in distributed chemical markets today is much higher than the value of hydrogen if it were to be used as fuel. The price of hydrogen will need to be in the $2.00/kg range to compete with conventional fuels for transportation.
It will take significant cost-reduction and efficiency improvements for electrolytic hydrogen to compete in vehicle fueling markets. Nonetheless, a number of stationary energy-related applications for electrolytic hydrogen are beginning to materialize. These smaller but higher-value energy applications merit the DOE’s attention and support as a means of advancing the practical development of hydrogen from electrolysis for future, larger-scale fueling markets.
Off-Grid Renewables Applications
Power-on-demand from inherently intermittent renewables is another interesting application for electrolysis. Offgrid, renewable-based systems need electricity at night or when the wind doesn’t blow. The value difference between electricity when available and when needed is often great enough to merit the utilization of batteries to fill this gap. In circumstances in which the amount and duration of stored energy becomes relatively large in relation to battery functionality, an electrolyzer-hydrogen regenerative system may prove a lower-cost solution, ultimately enabling greater use of renewables for meeting off-grid energy needs.
Current Electrolyzer Technology and Fueling Costs
The cost of hydrogen from electrolysis is dominated by two factors: (1) the cost of electricity and (2) capital-cost recovery for the system. A third cost factor—operation and maintenance expenses (O&M)—adds perhaps 3 to 5 percent to total annual costs. The electrochemical efficiency of the unit, coupled with the price of electricity, determine the variable cost. The total capital cost of the electrolyzer unit, including compression, storage, and dispensing equipment, is the basis of fixed-cost recovery.
Electrochemical Efficiency
Proton exchange membranes, whether operating in electrolysis mode or fuel cell mode, have the property of higher efficiency at lower current density. There is a 1:1 relationship in electrolysis between the rate of hydrogen production and current applied to the system.
The energy required in the theoretical efficiency limit of any water electrolysis process is 39.4 kWh per kilogram. PEM electrolyzers operating at low current density can approach this efficiency limit. However, the quantities of hy-
drogen produced at low current density are small, resulting in very high capital costs per unit of hydrogen produced. As shown in Figure G-9, cell stack efficiencies drop to 75 percent when current densities rise into the range of 1000 amps per square foot (ASF). As previously stated, the electrochemical efficiency of KOH systems is higher over a broader range of current densities, but this higher reaction efficiency is offset at least in part by higher compression and purification costs, as well as by higher costs associated with managing the liquid electrolyte itself.
The committee believes that current technology is capable of producing an electrolyzer-based fueling facility having the capacity to produce 480 kg/day, or 20 kg/hour. This plant would be capable of fueling 120 cars per day, assuming an average purchase of 4 kg per car. A plant of this scale would of necessity today be a KOH system, but with additional development, PEM technology should be capable of providing systems of comparable scale.
Electrolyzer systems of this scale should be capable of operating with an overall efficiency of 63.5 percent lower heating value [LHV], including all parasitic loads other than compression. The electrolyzer is assumed to be able to generate hydrogen at an internal pressure in the 150 psi range; supplementary compression will be required to raise the pressure to automotive fueling pressures in the 7000 psi (400 atm) range. The electrical requirement associated with compression is assumed at 2.3 kW/kg/hour, adding about 5 percent to the plant’s electrical consumption and bringing overall efficiency down to about 59 percent.
Equipment Costs
Regarding capital cost recovery, the cost of the 480 kg/ day system, excluding compression and dispensing, is assumed at $1000/kW input. The cost of the complete fueling system is summarized in Table G-5.
The total cost of a system at this scale would be about $2.5 million. It is anticipated that electrolysis technology scales with an 85 percent factor, so smaller-scale systems, with somewhat higher unit costs, are entirely feasible. For example, a facility with half the fueling capability (60 cars per day) would cost about $1.25 million, plus a 15 percent scaling factor. The scalability of electrolysis is one of the important factors relating to its likely use in early-stage fuel cell vehicle adoption. The electrochemical efficiency of electrolysis is essentially independent of scale.
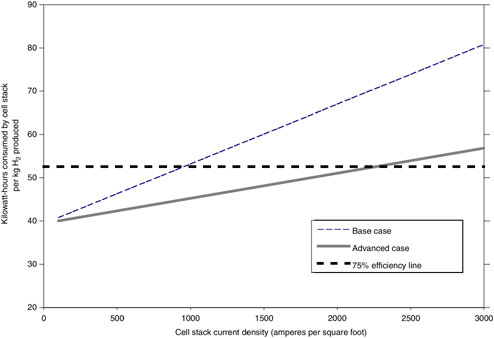
FIGURE G-9 Electrolysis cell stack energy consumption as a function of cell stack current density.
TABLE G-5 Capital Costs of Current Electrolysis Fueler Producing 480 Kilograms of Hydrogen per Day
|
|
Unit Cost ($) |
Total Cost ($ millions) |
|
Electrolyzer unit |
1,000/kW |
1.17 |
|
Hydrogen compressor |
3,000/kW |
0.16 |
|
Hydrogen storage |
100/gal. |
0.24 |
|
Hydrogen dispenser |
15,000/unit |
0.02 |
|
Total process units |
|
1.59 |
|
General facilities |
20% |
0.32 |
|
Engineering, permitting, start-up |
10% |
0.16 |
|
Contingencies |
10% |
0.16 |
|
Working capital and miscellaneous |
5% |
0.08 |
|
Total capital |
|
2.31 |
|
Siting factor (110% of Gulf Coast) |
|
0.23 |
|
Total |
|
2.54 |
|
NOTE: See Table E-37 in Appendix E in this report. |
||
All-Inclusive Cost of Hydrogen Fuel from Electrolysis
The total cost of electrolytic hydrogen from currently available technology is summarized in Table G-6. This table assumes a 14 percent capital cost-recovery factor, and presents the total cost (variable, capital, and O&M) associated with the assumed fueling facility. The delivered cost of grid electricity is assumed at 7 cents/kWh. Total costs are in the range of $6.50/kg.
Future Electrolysis Technology Enhancements
Among the research priorities that can improve the efficiency and/or reduce the cost of future electrolysis fueling devices are the following:
Efficiency-Enhancing Objectives
-
Reducing the ionic resistance of the membrane. New membranes will be thinner and will incorporate improved ion-conducting formulations that lower the resistance of the membrane and cause more of the electrical energy delivered to the membrane to be translated into hydrogen chemical energy and less into heat. In alkaline (KOH) systems, ionic resistance tends to be less than in proton exchange membrane systems, but KOH systems tend to have more complex materials handling and pressurization regimes.
-
Reducing other (parasitic) system energy losses. A variety of parasitic loads, such as power conditioning, can be reduced through system redesign and optimization. Power conditioning is one area of efficiency loss; current systems lose as much as 10 percent electrical efficiency with currently available inverters. These losses will be reduced by half or more with new inverters redesigned to meet the specific needs of electrolyzers. Power supply companies will
TABLE G-6 All-Inclusive Cost of Hydrogen from Current Electrolysis Fueling Technology
|
|
Cost per Year per Station ($ million) |
Cost per Kilogram ($) |
|
Nonfuel variable operation and maintenance (1% of capital) |
0.025 |
0.16 |
|
Electricity (7 cents/kWh) |
0.605 |
3.84 |
|
Variable operating costs |
0.630 |
4.00 |
|
Fixed operating costs (2%/year of capital) |
0.051 |
0.32 |
|
Capital charges (14%/year of capital) |
0.354 |
2.24 |
|
Total cost |
1.035 |
6.56 |
-
need to see enough market assurance before those redesigns will be forthcoming.
Other cost reductions can come from optimizing an array of components and the overall operating system. Volume manufacturing and pricing are also important cost factors.
In calling out the efficiency costs of alternating current/direct current (ac/dc) power conversion, one advantage of renewable power becomes worthy of note. Renewables generate dc power that can be applied to the dc-using electrolyzer cell stack without inversion. This incremental efficiency advantage associated with renewables may become material as the cost of power from renewables continues to drop.
-
Reducing current density. Conversion efficiencies are a function of electric current density, so the substitution of more electrolyte or more cell surface area has the impact of reducing overall power requirements per unit of hydrogen produced. Improved catalyst deposition technology will also lower the amount and cost of materials per unit of hydrogen production. Operating system redesign for optimization is another area of cost reduction opportunity.
Technology advances will be required to get to efficiencies beyond the current level. One area that promises to improve efficiency is higher temperature, which has the effect of lowering the ionic resistance within the cell environment.
-
Higher temperatures. PEM technologies typically operate at low temperatures (below 100°C) because of membrane durability limitations. Higher-temperature proton exchange membranes are in development; these should be able to tolerate significantly higher temperatures and thereby deliver higher efficiencies.
Cost Reduction Objectives
The committee believes that PEM electrolysis is subject to the same basic cost reduction drivers as those for fuel cells. Cost breakthroughs in (1) catalyst formulation and loading, (2) bipolar plate/flow field, (3) membrane expense and durability, (4) volume manufacturing of subsystems and modules by third parties, (5) overall design simplifications, and (6) scale economies (within limits) all promise to lower
the cost per unit of production. The committee finds it plausible that electrolyzer capital costs can fall by a factor of 8—from $1000 per kW in the near term to $125 per kW over the next 15 to 20 years, contingent on similar cost reductions occurring in fuel cells. This reduction seems attainable when considered against the claims by fuel cell developers that they can bring the cost of fuel cells to $50/kW from today’s nearly $5000/kW prices.
Advanced Future Electrolysis Technologies
The committee was presented with the view that technologies beyond PEM may offer higher overall efficiency by going to significantly higher temperatures and design concepts. Solid oxide fuel cell technology operates at much higher temperatures than PEM technology does, and so it may be a source of advanced electrolyzer performance going forward. Efficiencies moving toward 95 percent may be possible with solid oxide. But solid oxide systems operating at 500°C to 1000°C are probably at least 5 and perhaps 10 years in the future.
Solid oxide systems, because of their thermal management needs, may be confined to systems of significantly larger scale than PEM systems. Solid oxide electrolyzers may be scalable down to gas station duty, but that remains to be proven. Clearly, PEM systems can scale appropriately for distributed refueling duty.
Electrolysis/Oxidation Hybrids Still further advances in electrolysis technology, such as have been conceived at Lawrence Livermore National Laboratory, involve solid oxide electrolyzer/hydrocarbon hybrids. The hybrid concept involves enhancing the efficiency of the already-high-temperature electrolysis process by using the oxidation of natural gas as a means of intensifying the migration of oxygen ions through the electrolyte and thereby reducing the effective amount of electric energy required to transport the oxygen ion. The concept appears to offer the potential for significantly improved net electrochemical efficiency. However, the concept relies on a number of technical breakthroughs in harnessing solid oxide technology and ultimately requires a separate stream of methane or another combustible fuel supply in addition to water and electricity.
Future Electrolytic Hydrogen Fuel Costs
The committee’s assessment of electrolysis improvements focused on PEM-based technologies rather than on advanced concepts. The effect is to offer a view of futures that are based on today’s technology and do not rely on new technological breakthroughs that, should they occur, would only enhance the cost and performance picture.
Overall, improvements in electrolyzer performance will come from three advancements: (1) improved electrochemical efficiency—efficiency gains from 63.5 percent system efficiency to 75 percent system efficiency (LHV) could be attainable; (2) system costs—as stated above, the system capital costs may be reduced by a factor of 8, from $1000/ kW to $125/kW, driven largely by the same cost factors that must be addressed by fuel cell developers if there is to be any meaningful penetration by fuel cells into the transportation marketplace; and (3) compressor performance and cost are seen to be improving as a result of a variety of emerging hydrogen energy alternatives, all of which depend on taking hydrogen to significantly higher energy densities than can today be attained with only hydrogen compression.
The resulting impact of technology development on the future cost of hydrogen from electrolysis is summarized in Table G-7. Variable costs (electricity) fall as a result of improved electrochemical efficiency. The biggest change comes from the large drop in capital costs, which translates directly into lower capital cost per unit of production. This, along with lower compression costs, results in reduced all-inclusive costs of hydrogen from $6.58/kg using current technology to $3.94/kg as a result of future improvements.
Sensitivity to Electricity Costs
Figure G-10 illustrates the considerable sensitivity of the cost of hydrogen from electrolysis to the price of input electricity. Each 1 cent reduction in the price of electricity reduces the cost of electrolytic hydrogen fuel by 53 cents/kg, or more than 8 percent per penny. Effective utilization of electrolysis as a fueling option will involve the cooperation of utilities and rate-making bodies.
Environmental Impacts of Electrolysis
The environmental impact of the use of electrolysis to produce hydrogen depends on the source of electricity. The
TABLE G-7 Cost of Hydrogen from Future Electrolysis Fueling Technology
|
Capital Cost |
Unit Cost ($) |
Cost per Station ($ million) |
|
Electrolyzer |
125/kW |
0.13 |
|
Compressor |
1,500/kW |
0.03 |
|
Storage |
75/gal |
0.19 |
|
Dispenser |
10,000/unit |
0.01 |
|
Other |
|
0.17 |
|
Total capital (with a 1.1 siting factor) |
|
0.57 |
|
Cost |
|
$/kg |
|
Nonfuel variable cost |
|
0.04 |
|
Electricity |
|
3.31 |
|
Fixed operating costs |
|
0.07 |
|
Capital charges |
|
0.51 |
|
Total |
|
3.93 |
|
NOTE: See Table E-38 in Appendix E in this report. |
||
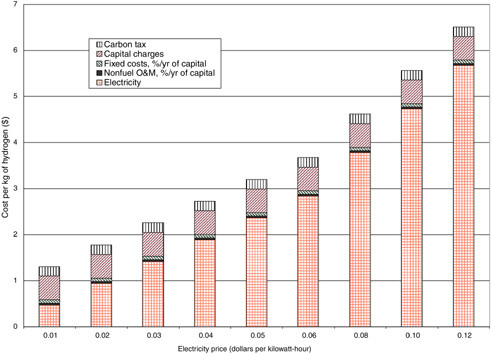
FIGURE G-10 Sensitivity of the cost of hydrogen from distributed electrolysis to the price of input electricity.
electrolysis process produces little if any CO2 or other greenhouse gas emissions per se. Electrolyzers contain no combustion devices, and the only input to the process other than electricity is pure water.
However, there does exist a relationship between emissions and electrolysis. Any pollution associated with electricity consumed by the electrolyzer needs to be taken into account. As stated previously, one fundamental appeal of electrolysis is that it creates a path for converting renewable power into fuel. But the low capacity factors of renewables (other than geothermal and hydro power) make an all-renewables case very difficult on an economic basis. Electricity from nuclear plants is also non-emitting on a greenhouse gas emissions basis, but the outlook for additional nuclear plants is uncertain at best.
Power from the grid is assumed to derive from the grid’s average generating mix. With today’s grid mix, about 17.6 kg CO2 are emitted per kilogram of hydrogen. As the portfolio of energy resources utilized to supply electric power evolves, the amount of CO2 emitted to produce 1 kg H2 could either increase or decrease.
Electrolysis as an Early-Stage Transitional Hydrogen Fuel Source
Electrolysis may be particularly well suited to meeting the early-stage fueling needs of a fuel cell vehicle market. Electrolyzers scale down reasonably well; the efficiency of the electrolysis reaction is independent of the size of the cell or cell stacks involved. And the balance of plant costs in an electrolyzer are also fairly scalable.
The compact size of electrolyzers makes them suitable to be placed at or near existing fueling stations. And finally, electrolyzers can utilize existing water and electricity infrastructures to a considerable extent, obviating the need for a new pipeline or surface hydrogen transport infrastructure that would be required of larger, central station hydrogen production technologies.
Summary
Electrolytic hydrogen production is an existing technology that serves a high-value industrial chemicals-based mar-
ket today. The key to adapting this technology to meet energy-related applications in the future is cost reduction and performance enhancement. The Department of Energy has already identified several technology objectives relating to electrolytic hydrogen production.
Hydrogen can be made from renewable sources, enabling a perfectly sustainable energy path. The falling cost of renewable energy resources and the improving cost and efficiency outlook for electrolysis contribute to the prospect that renewably sourced electrolytic hydrogen may be competitive with other hydrogen supply in at least some instances.
Electrolyzers typically operate from grid-quality power, so a new variety of power control and conditioning equipment needs to be developed in order for electrolyzers to operate efficiently from renewable sources. The prospect exists for good efficiency in converting renewable power to hydrogen, insofar as electrolyzers require direct current and renewables generate direct current, so there are no losses associated with ac/dc conversion.
HYDROGEN PRODUCED FROM WIND ENERGY
Introduction
The production of hydrogen from renewable energy sources is often stated as the long-term goal of a mature hydrogen economy (Turner, 1999). As such the development of cost-effective renewable technologies should clearly be a priority in the hydrogen program, especially since considerable progress is required before these technologies reach the levels of productivity and economic viability needed to compete effectively with the traditional alternatives. Thus, basic renewables research needs to be expanded and the development of renewable hydrogen production systems accelerated.
Of all the renewables currently on the drawing boards, in the near to medium term, wind arguably has the highest potential as an excellent source for producing pollution-free hydrogen, using the electricity generated by the wind turbines to electrolyze water into hydrogen and oxygen. The issues for its successful development and deployment are threefold: (1) further reducing the cost of wind turbine technology and the cost of the electricity generated by wind, (2) reducing the cost of electrolyzers, and (3) optimizing the wind turbine-electrolyzer with hydrogen storage system. This section discusses current costs and projections for future costs of electricity produced by wind energy and then looks at the cost of producing hydrogen using an integrated wind turbine-electrolyzer system. (Discussion of electrolyzer technology is presented in the section “Hydrogen from Electrolysis.”) This section focuses on wind energy systems that would be deployed on a distributed scale.
Status of Wind Energy in the World Today
While wind energy has been one of humanity’s primary energy sources for transporting goods, milling grain, and pumping water for several millennia, its use as an energy source began to decline as industrialization took place in Europe and then in America. The decline was at first gradual as the use of petroleum and coal, both cheaper and more reliable energy sources, became widespread, and then it fell more sharply as power transmission lines were extended into most rural areas of industrialized countries. The oil crises of the 1970s, however, triggered renewed interest in wind energy technology for grid-connected electricity production, water pumping, and power supply in remote areas, promoting the industry’s rebirth. By 2002, grid-connected wind power in operation surpassed 31,000 MW worldwide (see Figure G-11).
In the early 1980s, the United States accounted for 95 percent of the world’s installed wind energy capacity (see Figure G-11). The U.S. share has since dropped to 15 percent in 2002. Other countries dramatically increased their capacity starting in the mid-1990s, while the U.S. capacity essentially stagnated until 1999, when more than 600 MW in new capacity were installed in a rush to beat an expiring production tax credit for utility-scale projects. This credit has since been extended through December 31, 2003. In 2001 and 2002, the total installed wind capacity doubled in the United States, and in 2003 it was expected to increase another 25 percent, to more than 6000 MW, with installations of 1400 to 1600 MW of new wind power (AWEA, 2003).
The decline in the U.S. capacity world share can be explained by a combination of economic factors and changes in government-sponsored support programs that impeded the development of new capacity. The U.S. wind industry was born in 1981 in the aftermath of the world oil crises of 1973–1974 and 1978–1979. Wind energy was not cost-competitive with fossil fuel energy, but federal legislation guaranteed a market for wind-generated power and offered generous tax credits to developers of wind energy. However, 1986 marked the beginning of the slowdown in U.S. wind energy development. The availability of relatively cheap oil and natural gas and improvements in gas generating technology, coupled with the expiration of federal tax credits at the end of 1985, meant that wind energy remained significantly more costly than fossil fuels. The tax credit incentives had been more effective in building capacity than in maintaining productivity, and as a consequence electricity generation from wind did not grow as rapidly as initially anticipated. This trend appears to have reversed itself in the past 5 years, with more than a 22 percent annual increase in installed generating capacity since 1998, despite the recent problems permeating the electric utility industry. This recent growth, coupled with progressive state policies—30 states have installed wind capacity—the continuing extension of the federal wind energy production tax credit, and maturing wind turbine technology, appears to have signaled a rebirth for the industry in the United States.
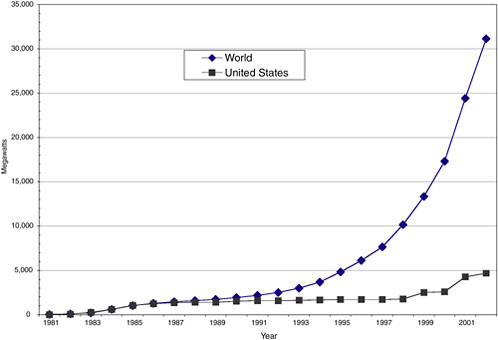
FIGURE G-11 Wind generating capacity, 1981–2002, world and U.S. totals. SOURCES: AWEA (2003), Worldwatch Institute (1999), and WEA (2000).
Potential for Wind Energy: Technical and Resource Availability
The main technical parameter determining the economic success of a wind turbine system is its annual energy output, which in turn is determined by parameters such as average wind speed, statistical wind speed distribution, distribution of occurring wind directions, turbulence intensities, and roughness of the surrounding terrain. Of these, the most important and sensitive parameter is the wind speed, which increases exponentially with height above the ground; the power in the wind is proportional to the third power of the momentary wind speed. As accurate meteorological measurements and wind energy maps (as shown in Figure G-12) become more commonly available, wind project developers can more reliably assess the long-term economic performance of wind farms.
Estimates show that U.S. wind resources could provide more than 10 trillion kWh (Deyette et al., 2003; Elliott and Schwartz, 1993), which includes land areas with wind class 3 or above (corresponds to wind speeds greater than 7 meters per second [m/s] [15.7 mph] at a height of 50 m), within 20 miles of existing transmission lines, and excludes all urban and environmentally sensitive areas. This is over 4 times the total electricity currently generated in the United States. In the DOE’s Hydrogen Posture Plan (DOE, 2003a), wind availability is estimated to be 3250 GW, equivalent to the above value for a capacity factor of 35 percent. In 2002, installed wind capacity was about 5 GW generating 12.16 billion kWh, corresponding to a capacity factor of 29 percent (EIA, 2003).
There has been a gradual growth of the unit size of commercial machines since the mid-1970s. In the mid-1970s the typical size of a wind turbine was 30 kW. By 1998, the largest units installed had a capacity of 1.65 MW, while turbines with an installed power of 2 MW have now been introduced into the market with over 3 MW machines being developed. The trend toward larger machines is driven by the demand side of the market to utilize economies of scale and to reduce visual impact on the landscape per unit of installed power, and by the expectation that the offshore potential will be growing.
Recent technical advances have made wind turbines more controllable and grid-compatible and have reduced the number of components, making them more reliable and robust. The technology is likely to continue to improve. Such im-
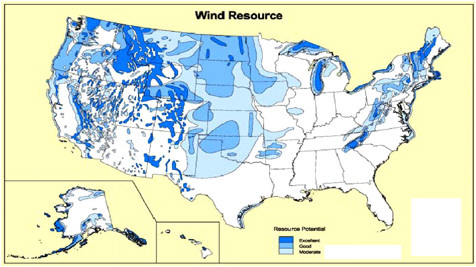
FIGURE G-12 Hydrogen from wind power availability. SOURCE: U.S. Department of Energy, National Renewable Energy Laboratory.
provements include enhanced performance at variable wind speeds, thereby capturing the maximum amount of wind according to local wind conditions, and better grid-compatibility. These advancements can occur through better turbine design and optimization of rotor blades, more efficient power electronic controls and drive trains, and better materials. Furthermore, economies of scale and automated production may also continue to reduce costs (Corey et al., 1999).
Economics of Wind Energy
Larger turbines, more efficient manufacturing, and careful siting of wind machines have brought the installed capital cost of wind turbines down from more than $2500/kW in the early 1980s to less then $1000/kW today at the best wind sites. However, on-stream capacity factor for wind is generally in the range of 30 to 40 percent, which raises the effective cost. While this decrease is due primarily to improvements in wind turbine technology, it is also a result of the general increase in wind farm size, which benefits from economies of scale, as fixed costs can be spread over a larger generating capacity. As a result, wind energy is currently one of the most cost-competitive renewable energy technologies, and in some places it is beginning to compete with new fossil fuel generation (Reeves, 2003).
Worldwide, the cost of generating electricity from wind has fallen by more than 80 percent, from about 38 cents/ kWh in the early 1980s to a current range for good wind sites located across the United States of 4 to 7 cents/kWh,19 with average capacity factors of close to 30 percent. The current federal production tax credit of 1.8 cents/kWh for wind-generated electricity lowers this cost to below 3 cents/kWh at the best wind sites. This is an order-of-magnitude decrease in cost in two decades. Analysts generally forecast that costs will continue to drop significantly as the technology improves further and the market grows around the world (Corey et al., 1999), though some do not (for example, the EIA). In the committee’s analysis, for possible future technologies it is assumed that the cost of electricity generated using wind turbines decreases to 4 cents/kWh (including transmission costs). This assumption is based on a wind tur-
|
19 |
Cost of electricity (COE) estimates from the National Renewable Energy Laboratory (NREL), Lawrence Berkeley National Laboratory (LBNL), Northern Power, and GE regarding the current cost of wind-generated electricity excluding the federal production tax credit (PTC) subsidy of 1.8 cents/ kWh. NREL: Personal communication with Lee Fingersh: 3.2 to 5 cents/ kWh today, depending on location; August 2003. See the web site http://www.eere.energy.gov/wind/web.html. Accessed December 10, 2003. LBNL: Personal communication with Ryan Wiser: Wind prices are about 4.3 to 5.3 cents/kWh throughout the Midwest, 5.8 cents/kWh in the Mid-Atlantic, around 5.8 to 6.8 cents/kWh in California, and perhaps 4.3 to 5.8 cents in the Northwest; August 2003. Northern Power: 4 to 6 cents/kWh for wind farms greater than 50 MW located at good wind sites, while for one or two turbines located at a marginal wind site, prices can be as high as 8 to 12 cents/kWh or higher. Dan Reicher, Northern Power Systems, “Hydrogen: Opportunities and Challenges,” presentation to the committee, June 2003. |
bine capital cost of $500/kW, total capital costs of $745/kW, and a capacity factor of 40 percent.20 The expectation is that wind turbine design will be refined and economies of scale will accrue. While these values can be considered optimistic (e.g., by the EIA), others predict even lower values, given successful technology advancement and supportive policy conditions (Bailie et al., 2003; Corey et al., 1999; WEA, 2000). In the future, cost reduction can occur with multiple advancements: further improvements in turbine design and optimization of rotor blades, more-efficient power controls and drive trains, and improvements in materials. The improvements in materials are expected to facilitate increased turbine height, leading to better access to the higher-energy wind resources available at these greater heights. The desire of new U.S. vendors to participate in wind energy markets will increase competition, leading to an overall optimization and lower cost of the wind turbine system.
Wind technology does not have fuel requirements as do coal, gas, and petroleum generating technologies. However, both the equipment costs and the costs of accommodating special characteristics such as intermittence, resource variability, competing demands for land use, and transmission and distribution availability can add substantially to the costs of generating electricity from wind. For wind resources to be useful for electricity generation and/or hydrogen production, the site must (1) have sufficiently powerful winds, (2) be located near existing distribution networks, and (3) be economically competitive with respect to alternative energy sources. While the technical potential of wind power to fulfill the need for energy services is substantial, the economic potential of wind energy will remain dependent on the cost of wind turbine systems as well as the economics of alternative options.
Hydrogen Production by Electrolysis from Wind Power
Hydrogen production from wind power and electrolysis is a particularly interesting proposition since, as just discussed, among renewable sources, wind power is economically the most competitive, with electricity prices at 4 to 5 cents/kWh at the best wind sites (without subsidies). This means that wind power can generate hydrogen at lower costs than those for any of the other renewable options available today.
In the committee’s analysis, it considered wind deployed on a distributed scale, thus bypassing the extra costs and requirements of hydrogen distribution. Since hydrogen from wind energy can be produced close to where it will be used, there is a clear role for it to play in the early years of hydrogen infrastructure development, especially as the committee believes that a hydrogen economy is most likely, at least initially, to develop in a distributed manner.
For distributed wind-electrolysis-hydrogen generation systems, it is estimated that by using today’s technologies hydrogen can be produced at good wind sites (class 4 and above) without a production tax credit for approximately $6.64/kg H2, using grid electricity as backup for when the wind is not blowing. The committee’s analysis considers a system that uses the grid as backup to alleviate the capital underutilization of the electrolyzer with a wind capacity factor of 30 percent. It assumes an average cost of electricity generated by wind of 6 cents/kWh (including transmission costs), while the cost of grid electricity is pegged at 7 cents/kWh, a typical commercial rate. This hybrid hydrogen production system has pros and cons. It reduces the cost of producing the hydrogen, which without grid backup would otherwise be $10.69/kg H2, but it also incurs CO2 emissions from what would otherwise be an emission-free hydrogen production system. The CO2 emissions are a product of using grid electricity; they are 3.35 kg C per kilogram of hydrogen.
In the future the wind-electrolysis-hydrogen system could be substantially optimized. The wind turbine technology could improve, reducing the cost of electricity to 4 cents/kWh with an increased capacity factor of 40 percent, as discussed previously, and the electrolyzer could also come down substantially in cost and could increase in efficiency (see the discussion in the section “Hydrogen from Electrolysis”). The combination of the increase in capacity factor and the reduction in the capital cost of the electrolyzer and cost of wind-generated electricity results in eliminating the need for using grid electricity (price still pegged at 7 cents/kWh) as a backup. The wind machines and the electrolyzer are assumed to be made large enough that sufficient hydrogen can be generated during the 40 percent of the time that the wind turbines are assumed to provide electricity. Due to the assumed reductions in the cost of the electrolyzer and the cost of wind-turbine-generated electricity, this option is now less costly than using a smaller electrolyzer and purchasing grid-supplied electricity when the wind turbine is not generating electricity. Hydrogen produced in this manner from wind with no grid backup is estimated to cost $2.85/kg H2, while for the alternative system with grid backup it is $3.38/kg H2. Furthermore, there is now the added advantage of a hydrogen production system that is CO2-emission free. The results of the committee’s analysis are summarized in Table G-8.
Wind-electrolysis-hydrogen production systems are currently far from optimized. For example, the design of wind turbines has to date been geared toward electricity production, not hydrogen. To optimize for better hydrogen production, integrated power control systems between the wind turbine and electrolyzer need to be analyzed, as should hydrogen storage tailored to the wind turbine design. Furthermore, there is the potential to design a system that can coproduce electricity and hydrogen from wind. Under the right circumstances this could be more cost-effective and
TABLE G-8 Results from Analysis Calculating Cost and Emissions of Hydrogen Production from Wind Energy
|
|
Current Technology |
Future Technology |
||
|
|
With Grid Backup |
No Grid Backup |
With Grid Backup |
No Grid Backup |
|
Average cost of electricity (cents/kWh) |
6 |
6 |
4 |
4 |
|
Wind turbine capacity factor (%) |
30 |
30 |
40 |
40 |
|
Hydrogen ($/kg) |
6.64 |
10.69 |
3.38 |
2.86 |
|
Carbon emissions (kg C/kg H2) |
3.35 |
0 |
2.48 |
0 |
provide broader system utility, thereby facilitating wind hydrogen system deployment (Fingersh, 2003).
Electricity systems have evolved so that they can now deliver power to consumers with high efficiency through a highly integrated system that aggregates supply and demand. Wind power benefits from this level of aggregation in that system. Numerous utility studies have indicated that wind can readily be absorbed into an integrated network until the wind capacity accounts for about 20 percent of maximum demand. Beyond this, changes to operational practice would likely be needed. Practical experience, as wind penetrates to higher levels, will continue to provide a better understanding of these system integration issues. The degree to which grid compatibility and integration play into future hydrogen production from wind needs to be better understood.
Advantages and Disadvantages
There are obvious environmental advantages to hydrogen produced from wind power. It does not generate solid, radioactive, or hazardous wastes; it does not require water; and it is essentially emission free, producing no CO2 or criteria pollutants, such as NOx and SO2. In addition, it is a domestic source of energy. Thus, it addresses the main concerns that are motivating the current drive toward a hydrogen economy—environmental quality and energy security. But wind power is not problem free.
Environmental Issues
Wind energy, although considered an environmentally sound energy option, does have several negative environmental aspects connected to its use. These include acoustic noise, visual impact on the landscape, impact on bird life, shadows caused by the rotors, and electromagnetic interference influencing the reception of radio, TV, and radar signals. In practice, the noise and visual impacts appear to cause the most problems for siting projects. Noise issues have been reduced by progress in aero-acoustic research providing design tools and blade configurations that have successfully made blades considerably quieter. With careful siting, the impact on bird life appears to be a relatively minor problem. Avoiding habitats of endangered species and major migration routes in the siting of wind farms can for the most part eliminate this problem.
A growing and often intractable problem involves land use issues, particularly the “not in my backyard” phenomenon (i.e., NIMBY). In densely populated countries where the best sites on land are occupied, there is increasing public resistance, making it impossible to realize projects at acceptable cost. This is one of the main reasons that countries such as Denmark and the Netherlands are concentrating on offshore projects, despite the fact that technically and economically they are expected to be less favorable than good land sites are. In countries such as the United Kingdom and Sweden, offshore projects are being planned not because of scarcity of suitable land sites but because preserving the landscape is such an important national value—though there is now also growing resistance to offshore wind projects for the same reason, as seen for a recently proposed wind project off Cape Cod in the United States.
Technical Issues
Wind energy has some technical advantages, in addition to being both a clean and secure energy source, as compared with conventional fossil fuel generation and even some other renewable energy sources. First, it is modular: that is, the generating capacity of wind farms can easily be expanded, since new turbines can be quickly manufactured and installed; this is not the case for either coal-fired or nuclear power plants. Furthermore, a repair to one wind turbine does not affect the power production of all the others. Second, the energy generated by wind turbines can pay for the materials used to make them in as little time as 3 to 4 months for good wind sites (AWEA, 2003).
Despite these advantages, wind’s biggest drawback continues to be its intermittence and mismatch with demand, an issue both for electricity generation and hydrogen production (Johansson, 1993). The best wind site locations are often not in close proximity to populations with the greatest energy needs, as in the U.S. Midwest; this problem makes such sites potentially impractical for onsite hydrogen production, owing to the high costs of storage and long-distance hydrogen distribution. On the other hand, if hydrogen storage and distribution were to become more cost-effective, potentially large quantities of relatively cheap hydrogen could be produced at remote, high-quality wind sites and distributed around the country.
Conclusions
Wind energy has some very clear advantages as a source of hydrogen. It fulfills the two main motivations that are propel-
ling the current push toward a hydrogen economy, namely, reducing CO2 emissions and reducing the need for hydrocarbon imports. In addition, it is the most affordable renewable technology deployed today, with expectations that costs will continue to decline. Since renewable technologies effectively address two of the major public benefits of a move to a hydrogen energy system, and wind energy is the closest to practical utilization with the technical potential to produce a sizable percentage of future hydrogen, it deserves continued, focused attention in the DOE’s hydrogen program.
Although wind technology is the most commercially developed of the renewable technologies, it still faces many barriers to deployment as a hydrogen production system. There is a need to develop optimized wind-to-hydrogen systems. Partnerships with industry are essential in identifying the R&D needed to help advance these systems to the next level.
Department of Energy’s Multi-Year Research, Development, and Demonstration Plan
There is little mention of hydrogen production from wind throughout the entire June 2003 draft of “Hydrogen, Fuel Cells and Infrastructure Technologies Program: Multi-Year Research, Development and Demonstration Plan” (DOE, 2003b) or in the July 2003 Hydrogen Posture Plan: An Integrated Research, Development, and Demonstration Plan (DOE, 2003a). An RD&D plan for hydrogen production from wind power needs to be developed and integrated into the overall hydrogen strategic RD&D plan.
Summary
Energy security and environmental quality, including reduction of CO2 emissions, are strong factors motivating a hydrogen economy. These goals can both be fulfilled by wind-hydrogen systems. Thus, wind has the potential to play a significant role in a future hydrogen economy, both during the transition and in the long term. Since wind is currently the renewable technology that is most developed and lowest cost, wind-electrolysis-hydrogen systems merit serious attention.
Wind-electrolysis-hydrogen systems have yet to be fully optimized. There are integration opportunities and issues with respect to wind machines and electrolyzers and hydrogen storage that need to be explored. For example, coproduction of electricity and hydrogen can potentially reduce costs and increase the function of the wind-hydrogen system. This could facilitate the development of wind energy systems that are more cost-effective and have broader utility, thereby assisting their development and deployment.
HYDROGEN PRODUCTION FROM BIOMASS AND BY PHOTOBIOLOGICAL PROCESSES
Two basic avenues for molecular hydrogen production by biological processes are currently being considered: (1) via photosynthetically produced biomass followed by subsequent thermochemical processing, and (2) via direct photobiological processes without biomass as intermediate. The first process is well known and intensely researched, while the second is still in the early research stage. These processes have in common the capturing and conversion of solar energy into chemical energy mediated by photosynthetic processes. In both cases, solar energy serves ultimately as the primary energy source for the production of molecular hydrogen by biological processes. In contrast to processes using fossil fuels as primary energy sources, biological processes do not involve net production of CO2.
Efficiency of Photosynthetic Biomass Production
In photosynthesis as carried out by plants, cyanobacteria, and microalgae, solar energy is converted into biomass in commonly occurring ecosystems at an overall thermodynamic efficiency of about 0.4 percent (see Figure G-13; Hall and Rao, 1999). This low efficiency is due to the molecular properties of the photosynthetic and biochemical machinery, as well as to the ecological and physical-chemical properties of the environment. Of the incident light energy, only about 50 percent is photosynthetically useful. This light energy is used at an efficiency of about 70 percent by the photosynthetic reaction center and is converted into chemical energy, which is converted further into glucose as the primary CO2 fixation end product at an efficiency of about 30 percent. Of this energy, about 40 percent is lost due to dark respiration. Because of the photo inhibition effect and the nonoptimal conditions in nature, a further significant loss in efficiency is observed when growing plants in natural ecosystems. Therefore, the energy content of common biomass collected from natural ecosystems contains only on the order of 0.4 percent of the primary incoming energy (see Figure G-13). Although higher yields (in the 1 to 5 percent range) have been reported for some crops (e.g., sugarcane), the theoretical maximal efficiency is about 11 percent.
Generally, two types of biomass resources can be considered in the discussion on renewable energy feedstock: (1) primary biomass, such as energy crops, including switchgrass, poplar, and willow, and (2) biomass residues (primary when derived from wood or processed agricultural biomass; secondary when derived from food or fiber processing by-products, or animal waste; and tertiary when derived from urban residues).21
Biomass Availability
Today about 4 percent of total energy use in the United States is based on the use of biomass, mainly in the form of
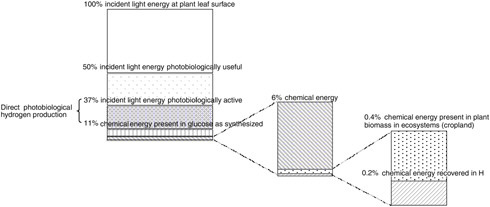
FIGURE G-13 Efficiency of biological conversion of solar energy (adapted from Hall and Rao, 1999).
forest residues. At a cost of $30 to $40/t, available biomass can be estimated to be between 220 million and 335 million dry tons per year.22 This biomass consists mainly of urban residues, sludge, energy crop, and wood and agricultural residues. A significant fraction of this biomass, especially forest residues, is already used by industry or in other competing processes, such as energy generation directly. However, if all of this theoretically available biomass could be converted to hydrogen, the annually available amount would be on the order of 17 million to 26 million t H2. As Figure 6-3 indicates, in an all-fuel-cell-vehicle scenario in the year 2050, 112 million t H2 would be required annually. Considering this demand and the competing demands for other uses of biomass, the currently available biomass is insufficient to satisfy the entire demand in a hydrogen economy, and new sources for biomass production would need to be considered.
Primary biomass in the form of energy crops is expected to have the quantitatively most significant impact on hydrogen production for use as transportation fuel by 2050.23 Estimates of energy that can potentially be derived from energy crops to produce biomass by 2050 range between 45 and 250 exajoules (EJ) per year. Bioenergy crops are currently not produced as dedicated bioenergy feedstock in the United States. Therefore, crop yields, management practices, and associated costs are based on agricultural models rather than on empirical data (Milne et al., 2002; de la Torre Ugarte et al., 2003; Walsh et al., 2000).
Land Use for Additional Biomass Production
In the most aggressive scenario for a hydrogen economy as considered in Chapter 6, a land area between 280,000 and 650,000 square miles is required to grow energy crops in order to support 100 percent of a hydrogen economy. The magnitude for this demand on land becomes apparent when comparing these numbers with the currently used cropland area of 545,000 square miles in the United States. Consequently, bioenergy crop production would require a significant redistribution of the land currently dedicated to food crop production and/or the development of a new land source from the U.S. Department of Agriculture’s (USDA’s) Conservation Reserve Program (CRP).
Although bioenergy crops can be grown in all regions of the United States, regional variability in productivity, rainfall conditions, and management practices limit energy crop farming to states in the Midwest, South, Southeast, and East (see Figure G-14) (Milne et al., 2002; de la Torre Ugarte et al., 2003; Walsh et al., 2000). Considering all cropland used for agriculture, as well as cropland in the CRP, in pasture and idle cropland, de la Torre Ugarte et al. (2003) considered two management scenarios for profitable bioenergy crop production: one to achieve high biomass production (production management scenario, or PMS), and another to achieve high levels of wildlife diversity (wildlife management scenario, or WMS). The production management scenario would annually produce about 188 million tons of dry biomass, which would be equivalent to 15 million tons of H2, requiring 41.8 million acres of cropland, of which about
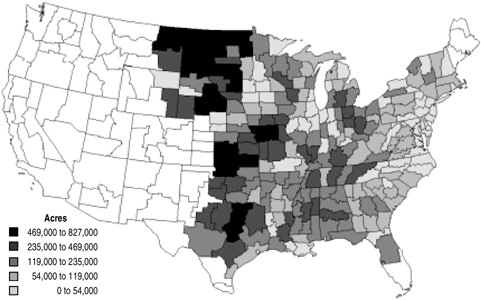
FIGURE G-14 Geographic distribution of projected bioenergy crop plantings on all acres in 2008 in the production management scenario (after Walsh et al., 2000).
56 percent would be from currently used cropland, 30 per cent from the CRP, and 13 percent from idle cropland and pasture. The crop would be exclusively switchgrass. In the wildlife management scenario, 96 million dry tons (dt) of biomass (equivalent to 7.6 million t H2) would be produced on 19.4 million acres of cropland, of which about 53 percent would be from currently used cropland, 42 percent from the CRP, and 4 percent from idle cropland and pasture. Land from the CRP would become a significant source for farming biomass crops. The CRP sets aside environmentally sensitive acres under 10- to 15-year contracts. Appropriate management practices must be developed before CRP lands are used. Environmental ramifications of various management practices must be examined to ensure that there is no substantial loss of environmental benefits, including biodiversity and soil and water quality. It is conceivable that a farming scenario alternating between agricultural crops and bioenergy crops on existing agricultural and CRP lands could be developed; however, those unproven cases were not considered in this analysis.
Biomass Cost
Bioenergy crop production is considered profitable at $40/dt, and could compete with currently grown agricultural crops (TIAX LLC, 2003; Milne et al., 2002). Based on assumed yields, management practices, and input costs, switchgrass is the least-expensive bioenergy crop to produce on a per dry ton basis. Production costs (farm gate costs) for switchgrass are estimated to range from $30/dt to $40/dt, depending on the management scenarios (WMS versus PMS) (de la Torre Ugarte et al., 2003). Adding processing and delivery costs would result in an approximate delivered biomass price on the order of $40 to $50/dt, respectively. Using these feedstock costs as well as current and projected gasifier efficiencies (50 percent versus 70 percent) in the committee’s analysis, the future costs per kilogram of hydrogen produced from biomass and delivered at the vehicle is about $3.60 (scenario MS Bio-F; see Figure 5-4 in Chapter 5). In this scenario, a reduction in biomass cost was assumed to be achieved by increasing the crop yield per hectare by 50 percent, which presents significant technical challenges.
The profitability of bioenergy crop farming will vary with given field and soil types (Milne et al., 2002). Notably, the price per dry ton of bioenergy crop is predicted to increase with the total biomass produced. A shift of cropland use from traditional agricultural crops to bioenergy crops will also result in higher prices for traditional crops. Because of land ownership, management, and crop establishment, biomass production by energy crop production will be more expensive than using residue biomass. Also, regional variation in the availability of residue biomass, such as in woody areas in the northeastern United States, could make hydrogen pro-
duction from biomass competitive in such regions in the short term. However, such operations would be restricted to selected regions in the United States, and, in a long-term sustainable scenario, would require biomass production at the same rate as its consumption. The committee considered it to be unlikely that such localized operations would contribute significantly to the nation’s H2 supply. Therefore, such cases were not considered further in this analysis, nor were fertilizer costs and the energy required to produce, harvest, and transport biomass.
Environmental Impact of Biomass Used for Hydrogen Production
In the overall process of biomass production and gasification, no net CO2 is generated, except for the CO2 released from fossil fuels used for (1) harvesting and transportation of biomass, (2) operation of the gasification systems, and (3) electricity, as well as for (4) production and delivery of fertilizers in an advanced biomass system. Biomass handling alone is estimated to consume about 25 percent of the total capital costs of operation of a midsize biomass gasification plant. Furthermore, biomass production requires, in addition to land (see above), about 1000 to 3000 t of water per ton of biomass, as well as nutrients in the form of nitrogen (ammonia), phosphorus (phosphate), sulfur, and trace metals. Profitable future hydrogen production from biomass will require energy crops with increased growth yields, which translates into increased need for fertilizers, energy for production of fertilizers, and potentially water. As is the case with the production of food crops, erosion, nutrient depletion of the soil, and altered water use practices could result in potentially significant environmental impacts as a consequence of farming activities. These effects need to be carefully considered.
Technologies for Hydrogen Production from Biomass
Current Technologies
Current technologies for converting biomass into molecular hydrogen include gasification/pyrolysis of biomass coupled to subsequent steam reformation24 (Milne et al., 2002; Spath et al., 2000). The main conversion processes are (1) indirectly heated gasification, (2) oxygen-blown gasification, and (3) pyrolysis, as well as (4) biological gasification (anaerobic fermentation). Biomass gasification has been demonstrated at a scale of 100 tons of biomass per day.25 Only a small, 10 kg/day of H2 pilot biomass plant is in operation, and no empirical data on the operation, performance, and economics of a full-scale biomass-to-hydrogen plant are available.26 The thermodynamic efficiencies of these processes are currently around 50 percent. Considering the low energy content of biomass, between 0.2 percent and 0.4 percent of the total available solar energy is converted to molecular hydrogen.
Biomass gasifiers are designed to operate at low pressure and are limited to midsize-scale operations, owing to the heterogeneity of biomass, its localized production, and the relatively high costs of gathering and transporting biomass. Therefore, current biomass gasification plants are associated inherently with unit capital costs that are at least five times as high as those for coal gasification (see Figure 5-2 in Chapter 5) and operate at lower efficiency.
Coproduction (biorefinery) of, for example, phenolic adhesives, polymers, waxes, and other products with hydrogen production from biomass, is being discussed in the context of plant designs to improve the overall economics of biomass-to-hydrogen conversion27 (Milne et al., 2002). The technical and economic viability of such coproduction plants is unproven and was not considered in this analysis.
Several major technical challenges of biomass gasification/pyrolysis exist and include variable efficiencies, tar production, and catalyst attrition28 (Milne et al., 2002). Moisture content as well as the relative composition and heterogeneity of biomass can result in significant deactivation of the catalyst. Recent fundamental research has identified a new, potentially inexpensive class of catalysts for aqueous-phase reforming of biomass-derived polyalcohols (Huber et al., 2003). In contrast to residue biomass, the use of bioenergy crops as biomass for gasification is advantageous, as its composition and moisture content are predictable, and the gasification process can be optimized for the corresponding crop.
Using anaerobic fermentation to convert biomass into hydrogen, a maximum of about 67 percent of the energy content (e.g., of glucose) can be recovered in hydrogen theoretically (calculated after Thauer et al., 1977). Considering the currently known fermentation pathways, a practical efficiency of biomass conversion to hydrogen by fermentation is between 15 and 33 percent (4 mol H2/mol glucose), although this is only possible at low hydrogen partial pressure. However, more efficient fermentation pathways could be conceived and would require significant bioengineering ef-
forts. These values compare with a biomass gasification efficiency of around 50 percent. The impurity of the hydrogen from biomass may be of concern, as fuel cell operations require relatively high-grade quality.
Economic Analysis of Current and Future Biomass-to-Hydrogen Conversion
In the past, and through funding support by the DOE, the process of biomass gasification has received most attention. Gasification technology using biomass, typically wood residues as feedstock, was adapted from coal gasification, and a few small-scale prototypes of biomass gasification plants have been built. Thus, the committee considered only the economics of biomass gasification. However, no midsize gasification facility exists to date that converts biomass to hydrogen, and no empirical data are available on the operation, performance, and economics of a midsize biomass-to-hydrogen plant, as assumed in the economic model. The assumptions made for the committee’s analysis of current technology consist of modular combinations adopted from existing technical units for coal gasification (shell gasifier, air separation unit, traditional shift), without considering the variability in chemical composition and moisture content of typical biomass. An overall gasification efficiency of 50 percent is assumed. Furthermore, the committee assumed a scenario in which 100 percent of the H2 demand would need to be met by biomass-derived hydrogen, acknowledging that in a possible future scenario, a mix of different primary energy sources is more likely. As the relative proportion of such mixes of primary feedstock is unknown, the committee considered the simplified case.
Estimation of the economics of future technology for biomass-to-hydrogen conversion using gasification is more problematic and much more uncertain because of the necessary extrapolations. The committee made the following assumptions for a midsize plant: (1) advanced biomass gasifiers can be developed and will use newly developed technology, such as fluidized catalytic cracking; (2) biomass gasifiers can be modified to produce a CO and H2 syngas, as does coal gasification; (3) biomass gasification will operate at an overall efficiency of about 70 percent; and (4) through genetic engineering and other breeding methods, the growth yield of switchgrass can be increased by 50 percent. The committee also assumed that the future biomass is derived from bioenergy crops at a price of $50/dt, as opposed to coming from less expensive biomass residues, although it is possible that a mixture of bioenergy crops and residues could be used for future gasifications. With these assumptions, the current price per kilogram of hydrogen delivered at the vehicle of $7.04 (see Figure 5-2) could be reduced in the future to about $3.60. As can be see in Figure 5-4, two factors contribute to the high price: the high capital charges for gasification and the high biomass costs.
Photobiological Hydrogen Production
In recent years, fundamental research on hydrogen production by photosynthetic organisms has received significant attention. In photosynthesis, water is oxidized photo-biologically to molecular oxygen and hydrogen in order to satisfy the organism’s need to build biomass from CO2. This notion has prompted the idea of reengineering this process to release those equivalents as molecular hydrogen directly. Such direct production of molecular hydrogen is probably thermodynamically the most efficient use of solar energy in biological hydrogen production (theoretically about 10 percent to 30 percent), because it circumvents inefficiencies in the biochemical steps involved in biomass production, as well as those involved in biomass conversion to hydrogen (see Figure G-13). The photosynthetic formation of molecular hydrogen from water is thermodynamically feasible even at high hydrogen partial pressure. However, such biological capability does not occur in any known organism; thus, it will require substantial metabolic engineering using new approaches in molecular biotechnology. In a variation of this approach, electron flow from the photosynthetic reaction center could be coupled to nitrogenase, which also releases H2. Another mode of hydrogen production, discussed in context of photosynthetic H2 production, is dark fermentation mediated by photosynthetic microorganisms. In all cases, the reducing equivalents for producing hydrogen are derived from water, which is abundant and inexpensive. It is unclear to what extent the DOE is providing substantial funds for such research.
Technologies Competing for Land Surface Area
Since the primary energy of all biological processes for hydrogen production is renewable solar energy, all other technologies using solar energy, including photovoltaic and other newer processes, such as thin-film technology, are competing for (land) surface area. Wind energy is indirectly solar energy. Currently, the solar-to-electrical conversion efficiency of newer photoelectric processes is 15 to 18 percent, compared with 0.4 percent for bulk biomass formation, and about 10 percent, potentially, for direct hydrogen production by photosynthetic organisms. Because solar energy harvesting technologies are competing for land use among each other and with other societal activities, such as farming, housing, and recreation, the overall efficiency of a solar energy conversion process will be a key determinant for its economic viability.
Advantages and Disadvantages of Using Biomass and Photobiological Systems for Molecular Hydrogen Production
Hydrogen production from biomass is an attractive technology, as the primary energy is solar (i.e., “renewable”), with
no net CO2 being released (except for transport). Notably, when coupled to CO2 capture and sequestration on a larger technical scale, this technology might be the most important means to achieve a net reduction of atmospheric CO2 (see Chapter 6, Figures 6-9 and 6-10). Furthermore, different forms of biomass (bioenergy crops, residues including municipal waste, etc.) could be used in different combinations.
The current concept of biomass-to-hydrogen conversion has several limitations. Biomass conversion to hydrogen is intrinsically inefficient, and only a small percentage of solar energy is converted into hydrogen. Moreover, in order to contribute significantly to a hydrogen economy, the quantity of biomass that needs to be available necessitates the farming of bioenergy crops. However, bioenergy crops obtained by farming will be intrinsically expensive. Residue biomass is less expensive but more variable and heterogeneous in composition, thus making the gasification process less efficient. In addition, significant costs are associated with the collection and transportation of dispersed, low-energy-density bioenergy crops and residues. Most importantly, large-scale biomass production also would pose significant demand on land, nutrient supply, water, and the associated energy for increased biomass production. The environmental impact of significant energy crop farming is unclear, but it can be assumed to be similar to that in crop farming and include soil erosion, significant water and fertilizer demand, eutrophication of downstream waters, and impact on biological diversity. Biomass production is also sensitive to seasonal variability as well as to vagaries of weather and to diseases, with significant demands regarding the storage of biomass in order to compensate for the anticipated fluctuations. The public acceptance of growing and using potentially genetically engineered, high-yield energy crops is also unclear. In addition, competing uses of biomass for purposes other than hydrogen production will also control the price of biomass. Overall, it appears that hydrogen production from farmed and agriculture-type biomass by gasification/pyrolysis will only be marginally economical and competitive.
Biomass gasification could play a significant role in meeting the DOE’s goal of greenhouse gas mitigation. It is likely that both in the transition phase to a hydrogen economy and in the steady state, a significant fraction of hydrogen might be derived from domestically abundant coal. In co-firing applications with coal, biomass can provide up to 15 percent of the total energy input of the fuel mixture. The DOE could address greenhouse gas mitigation by co-firing biomass with coal to offset the losses of carbon dioxide to the atmosphere that are inherent in coal combustion processes (even with the best-engineered capture and storage of carbon). Since growth of biomass fixes atmospheric carbon, its combustion leads to no net addition of atmospheric CO2 even if vented. Thus, co-firing of biomass with coal in an efficient coal gasification process, affording the opportunity for capture and storage of CO2, could lead to a net reduction of atmospheric CO2. The co-firing fuel mixture, being dilute in biomass, places lower demands on biomass feedstock. Thus cheaper, though less plentiful, biomass residue could supplant bioenergy crops as feedstock. Using residue biomass would also have a much less significant impact on the environment than would farming of bioenergy crops.
Photobiological hydrogen production is a significantly more efficient process and requires nutrients to a lesser extent than does biomass-to-hydrogen conversion. The objective is to engineer a (micro)organism that catalyzes the light-mediated cleavage of water with the concomitant production of hydrogen at high rates and high thermodynamic efficiency. This process does not take place in naturally occurring organisms at an appreciable rate or scale. While this approach has much potential, there are also major challenges. Substantial bioengineering efforts have to be undertaken to engineer microorganisms with a robust metabolic pathway, including improved kinetics for hydrogen production and efficiencies in light energy conversion and hydrogen production, before a pilot-scale photobiological system could be evaluated. This requires long-term, fundamental research at a significant funding level. Also, inexpensive, large-scale reactor systems need to be designed that minimize the susceptibility of the reactor system to biological contamination. In addition, the public perception of the use and possible concerns over the potential “escape” of genetically engineered microorganisms need to be addressed.
The Department of Energy’s Research and Development Program
According to the June 2003 draft of “Hydrogen, Fuel Cells and Infrastructure Technologies Program: Multi-Year Research, Development and Demonstration Plan” (DOE, 2003b), DOE’s Office of Energy Efficiency and Renewable Energy program has set technical targets for the years 2005, 2010, and 2015 to reduce costs for biomass gasification/ pyrolysis and subsequent steam reforming. Specific goals include the reduction of costs for (1) biomass feedstock, (2) gasification operation (including efficiency), (3) steam reforming, and (4) hydrogen gas purification. Although no specific budget amounts were reported (except at a very high level of aggregation), major funding for R&D of hydrogen production from biomass is apparently in improving gasification/pyrolysis processes. The goals are quite ambitious. The committee’s economic analysis (Chapter 5) shows that gasification and the availability of large quantities of inexpensive biomass are major economic barriers for hydrogen derived from biomass. Although listed in the draft report, the EERE program seems to support photobiological hydrogen research, but specific funding levels are unclear. The DOE’s R&D targets for increasing the utilization efficiency of absorbed light and hydrogen production are extremely ambitious, and it is unclear how realistic they are. It appears that if such molecular projects are funded, they are for small amounts.
Summary
The committee’s analysis indicates the following:
-
Considering the assumptions for future technology, biomass-to-hydrogen conversion is unlikely to produce hydrogen at a competitive price, even when compared with hydrogen generated from distributed natural gas.
-
The environmental impact of growing significant quantities of biomass as energy crops, including engineered, high-yield crops, will most likely place significant strains on natural resources, including water, soil, land availability, and biodiversity.
-
Because of the inherently high cost for collecting and transporting biomass, a biomass gasification plant will be limited in size, will not make full use of the economics of scale, and will be limited to certain geographic regions in the United States.
-
Biomass-to-hydrogen conversion is a thermodynamically inefficient path for using solar energy.
-
The use of biomass (residues), when co-fired (e.g., with coal) and coupled to subsequent carbon sequestration, might be an important technical option for achieving zero emission and, potentially, a net reduction of atmospheric CO2.
-
Photobiological hydrogen production is a theoretically more efficient process, but significant fundamental molecular research is needed to identify and improve the limiting factors in order to evaluate fully this approach for hydrogen production.
HYDROGEN FROM SOLAR ENERGY
Introduction
It has been estimated that solar energy has the potential of meeting the energy demand of the human race well into the future.29 One of the methods of recovering solar energy is through the use of photovoltaic (PV) cells. Upon illumina-tion with sunlight, PV cells generate electric energy. Commercial PV modules are available for a wide range of applications. However, they represent a miniscule contribution to U.S. electric power production. The current cost of electricity from a PV module is 6 to 10 times the cost of electricity from coal or natural gas. Therefore, if PV electricity were to be used to make hydrogen, the cost would be significantly higher than if fossil fuels were used. The key for solar energy to be used on a large scale for electricity or hydrogen production is cost reduction. This would require a number of advancements in the current technology.
Current State of Technology
Approximately 85 percent of the current commercial PV modules are based on single-crystal or polycrystalline silicon. The single-crystal or polycrystalline silicon cells are generally of the dimension of 10 to 15 centimeter (cm) (Archer and Hill, 2001). They are either circular or rectangular. In a module, a number of cells are soldered together. Each cell is capable of providing a maximum output of 0.6 volt (V), with the total module output approaching 20 V. The output current of each cell in bright sunlight is generally in the range of to 2 to 5 amps. The single-crystal silicon cells are made from wafers obtained by continuous wire sawing of single-crystal ingots grown by the Czochralski process. Similarly, a large portion of the polycrystalline silicon cells are made from ingots obtained by directional solidification of silicon within a mold. The wafer thickness is generally in the range of 250 to 400 microns. It is worth noting that nearly half of the silicon is wasted as “kerf” loss during cutting. Polycrystalline silicon cells are also made from silicon sheet or ribbon grown by other techniques (Archer and Hill, 2001). This process avoids the cost associated with cutting silicon ingots into wafers. The silicon wafers or ribbons are then further processed to develop p-n junctions and wire contacts. The array of cells is laminated using glass and transparent polymer, called ethylvinylacetate (EVA), to provide the final PV module. The modules are known to have long lifetime (10- to 25-year warranty from manufacturers). The current technology gives about 18 percent cell efficiency and 15 percent module efficiency.30
A second type of PV technology is based on deposition of thin films. PV cells are prepared by deposition of amorphous as well as microcrystalline silicon from a variety of techniques, including plasma-enhanced chemical vapor deposition, hot wire chemical vapor deposition, and so on. Polycrystalline thin-film compounds based on group II-VI of the periodic table, such as cadmium telluride (CdTe), and group I-III-VI ternary mixtures such as copper-indium-diselenide (CIS), have been used to make thin-film solar cells (Ullal et al., 2002). The thickness of deposited layers is much less than 1 micron. As compared with crystalline silicon solar cells, the thin-film technology potentially has a number of significant advantages in manufacturing: (1) lower consumption of materials; (2) fewer processing steps; (3) automation of processing steps; (4) integrated, monolithic circuit design leading to elimination of the assembly of individual solar cells into final modules; and (5) fast roll-to-roll deposition (Wieting, 2002). It has been estimated that for crystalline silicon solar cells, the complete process involves more than two dozen separate steps to prepare and process ingots, wafers, cells, and circuit assemblies before a module is complete (Wieting, 2002). On the other hand, thin-film module
production requires only half as many process steps, with simplified materials handling.
Thin-film technology appears to hold greater promise for cost reduction, which has led to research by several laboratories over the past two or three decades. Some of the results in efficiency improvement of small laboratory research-size cells, typically of the size of 1 cm2, are shown in Figure G-15. Research cell efficiencies as high as 21.5 percent for copper-indium (gallium)-diselenide (CIGS) are reported (Ullal et al., 2002). Similarly, high efficiency of 16.5 percent has been reported for CdTe research cells. Amorphous silicon is deposited by using silane (SiH4) and hydrogen mixtures. In laboratory-scale cells of amorphous silicon, the highest efficiencies obtained are about 12 percent.
One big challenge for thin-film solar cells is to overcome the large drop in efficiency from the laboratory-scale cell to that of a real module. For example, commercial modules of CdTe and CIGS have efficiencies in the range of 7 percent to 12 percent (as compared with laboratory-scale cell efficiencies of 16.5 percent and 21.5 percent). Similarly, commercial amorphous silicon modules have efficiencies less than 10 percent (Shah et al., 1999). The drop in efficiency as cell size is increased is substantial. Attempts are being made to increase the efficiency of amorphous and microcrystalline silicon cells by making dual and triple junction cells (Yang et al., 1997). This change leads to multiple layers, each having a different optimum band gap. However, the deposition of multiple layers increases the processing steps and therefore the cost. A final note is that amorphous silicon modules, when exposed to sunlight, undergo light-induced degradation, operating thereafter at a lower, stabilized efficiency (Shah et al. 1999; Staebler and Wronski, 1977).
In spite of its promise, the thin-film technology has been unable to reduce the cost of solar modules, owing to low deposition rates that have led to low capital utilization of expensive machines. The yields and throughputs have been low. These plants need better inline controls. In recent times, owing to manufacturing problems, some corporations have shut down their thin-film manufacturing facilities. Clearly, easier and faster deposition techniques leading to reproducible results are needed. Also, deposition techniques that would not result in a substantial drop in efficiency from laboratory scale to module scale are required.
Today there is no one clear “winner technology.” More than a dozen firms produce solar modules. Even the largest of these firms do not have world-class, large-scale produc-
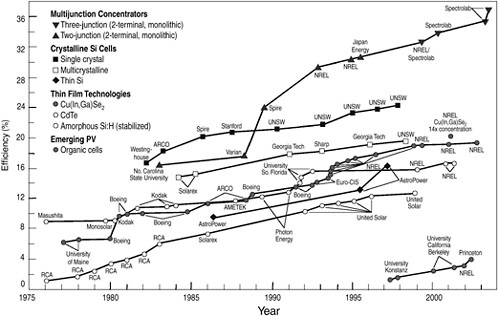
FIGURE G-15 Best research cell efficiencies for multijunction concentrator, thin-film, crystalline silicon, and emerging photovoltaic technologies. SOURCE: National Renewable Energy Laboratory.
tion facilities (greater than 100 MWp worth of solar modules per year). This size limitation does not allow the economy-of-scale benefits for the solar cell production. Many companies use multiple technologies. The current cost of solar modules is in the range of $3 to $6 per peak watt (Wp). For solar cells to be competitive with the conventional technologies for electricity production, the module cost must come down below $1/Wp. Table G-9 provides cost estimates of producing electricity as well as hydrogen calculated by the committee. In the current scenario, with a favorable, installed cost of about $3.285/Wp, the electricity cost is estimated to be about $0.319/kWh (scenario Dist PV-C of Chapter 5). For a futuristic case with all of the expected technology and production advances, the anticipated installed cost of $1.011/ Wp provides electricity cost of $0.098/kWh (scenario Dist PV-F, Table E-49 in Appendix E). While this target is attractive for electricity generation, it does not produce hydrogen at a competitive cost.
Energy is consumed in the manufacture of solar modules. It has been estimated by NREL that for a crystalline silicon module, the payback period of energy is about 4 years. For an amorphous silicon module this period is currently about 2 years, with the expectation that it will eventually be less than 1 year.
Future Technology
Photovoltaic Cells
Various developments are likely to improve the economic competitiveness of solar technology, especially for thin-film technology. The current research on microcrystalline silicon deposition techniques is leading to higher efficiencies. Techniques leading to higher deposition rates at moderate pressures are being developed (Schroeder, 2003). Better barrier materials to eliminate moisture ingress in the thin-film modules will prolong the module life span. Robust deposition techniques will increase the yield from a given type of equip
TABLE G-9 Estimated Cost of Hydrogen Production for Solar Cases
|
Case |
Installed Cost ($/kW) |
Electricity Cost ($/kWh) |
Hydrogen Cost with Electrolyzer ($/kg) |
|
Current (Dist PV-C) |
3285 |
0.319 |
28.19 (Dist PV Ele-C) |
|
Future (Dist PV-F) |
1011 |
0.098 |
6.18 (Dist PV Ele-F) |
|
NOTE: See Appendix E for definition of the symbols for the solar technology cases. See also Tables E-48 and E-49 of Appendix E. |
|||
ment. Inline detection and control methods will help to reduce the cost. Some of this advancement will require creative tools and methods.
The committee believes that installed costs of roughly $1/ Wp are attainable. Material costs are quite low, but substrate material, expensive coating equipment, low utilization of equipment, and labor-intensive technology lead to high overall costs. It is expected that in the next decade or two, improvements in these areas have a potential to bring the cost much below $1/Wp. World-class plants with economies of scale will further contribute to the lowering of cost. For crystalline-silicon-wafer-based technology, the raw material costs by themselves are almost $1/Wp. However, improvements in operating efficiency, the cost of raw materials, and reduced usage of certain materials are expected to bring overall cost in the neighborhood of $1/Wp.
A concept that has been proposed is the dye-sensitized solar cell, also known as the Grätzel cell (O’Regan and Grätzel, 1991). A dye is incorporated in a porous inorganic matrix such as TiO2, and a liquid electrolyte is used for positive charge transport. Photons are absorbed by the dye, and electrons are injected from the dye into n-type titania nanoparticles. The nanoparticles of titania are fused together and carry electrons to a conducting electrode. The dye gets its electron from the electrolyte, and the positive ion of the electrolyte moves to the other electrode (Grätzel, 2001). This type of cell has a potential to be low-cost. However, the current efficiencies are quite low, and the stability of the cell in sunlight is very poor. Research is needed to improve performance at both fronts.
Another area of intense research is that on the integration of organic and inorganic materials at the nanometer scale into hybrid solar cells. The current advancement in conductive polymers and the use of such polymers in electronic devices and displays provides the impetus for optimism. The nano-sized particles or rods of the suitable inorganic materials are embedded in the conductive organic polymer matrix. Once again, the research is in the early phase and the current efficiencies are quite low. However, the production of solar cells based either solely on conductive polymers or on hybrids with inorganic materials has a large potential to provide low-cost solar cells. It is hoped that one would be able to cast thin-film solar cells of such materials at a high speed, resulting in low cost.
Regarding production costs, all of the technologies discussed so far convert solar energy into electricity and use the electricity to generate hydrogen through the electrolysis of water. Since PV cells produce dc currents, the electric power can be directly used for electrolysis. As discussed in the section above on electrolyzers, considerable cost reductions are anticipated, which will lower the cost of hydrogen from solar cells. These cost reductions will be particularly valuable for solar cell electricity because the low usage factor associated with PV modules also contributes to the low usage of electrolyzers. This contributes heavily to the cost of hydro-
gen produced. For example, in the committee’s analysis of costs discussed in Chapter 5 (summarized in Table G-9), for the future optimistic case the cost of hydrogen is calculated to be $6.18/kg (Dist PV Ele-F). For this case, the cost of the installed PV panels, including all of the general facilities, is estimated to be $1.011/Wp, and is used in conjunction with an electrolyzer that is assumed to take advantage of all of the advancements made in the fuel cell. The PV part is responsible for $4.64/kg, and the electrolyzer is $1.54/kg. Compared with this, the cost of hydrogen from a future central coal plant at the dispensing station is estimated to be $1.63/ kg with carbon tax (CS Coal-F). This cost implies that for a PV-electrolyzer to compete in the future with a coal plant, either the cost of PV modules must be reduced by an order of magnitude or the electrolyzer cost must drop substantially from $125/kW. A factor contributing to this is the low utilization of the electrolyzer capital. It has been proposed to use electricity from the grid to run the electrolyzer when solar electricity is unavailable. This use increases the time on-stream for the electrolyzer; however, in the long term, for solar to play a dominant role in the hydrogen economy, it cannot rely on power from the grid to supplement equipment utilization. Therefore, while electricity at $0.098/kWh from a PV module can be quite attractive for distributive applications where electricity is directly used, its use in conjunction with electrolysis to produce hydrogen is certainly not competitive with the projected cost of hydrogen from coal.
Direct Production
Research is being done to create photoelectrochemical cells for the direct production of hydrogen (Grätzel, 2001).31 In this method, light is converted to electrical and chemical energy. The technical challenge stems from the fact that energy from two photons is needed to split one water molecule. A solid inorganic oxide electrode is used to absorb photons and provide oxygen and electrons. The electrons flow through an external circuit to a metal electrode, and hydrogen is liberated at this electrode. The candidate inorganic oxides are SrTiO3, KTaO3, TiO2, SnO2, and Fe2O3. When successful, such a method holds promise of directly providing low-cost hydrogen from solar energy.
Regarding production costs, it seems that a photoelectrochemical device in which all of the functions of photon absorption and water splitting are combined in the same equipment may have better potential for hydrogen production at reasonable costs. However, it is instructive to do a quick “back of the envelope” analysis for the acceptable cost by such a system. It is assumed that cost per peak watt for a photoelectrochemical device is the same as that for the possible future PV modules (see Table E-48 of Appendix E.) It is further assumed that this energy is recovered as hydrogen rather than as electricity. Therefore, a recovery of 39.4 kWh translates into a kilogram of hydrogen. This implies that 4729 kWe worth of solar plant in the Dist PV-F spreadsheet will produce about 576 kg/day of hydrogen (assuming an annual capacity factor of 20 percent). At the total cost of $0.813 million per year, this gives $3.87/kg of hydrogen! This cost is still too high when compared with that of hydrogen from coal or natural gas plants. It implies that photoelectrochemical devices should recover hydrogen at an energy equivalent of $0.4 to $0.5/Wp. This cost challenge is similar to that for electricity production from the solar cells.
Advantages and Disadvantages of Solar Energy
Solar energy holds the promise of being inexhaustible. If harnessed, it can meet all of the energy needed in the foreseeable future. It is clean and environmentally friendly. It converts solar energy into hydrogen without the emission of any greenhouse gas. Because of its distributed nature of power production, it contributes to the national security.
There are certain challenges associated with the use of solar energy. The intermittent nature of sunshine, on both a daily and a seasonal basis, presents a number of challenges. A backup system, or a storage system for electricity/hydrogen, is needed for the periods when sunshine is not available and power demand exists. Furthermore, this intermittent availability means that four to six times more solar modules have to be installed than the peak watt rating would dictate. This intermittency also implies that a significant decrease in the module cost is required. Another challenge is to ensure that no toxic materials are discharged during the fabrication of solar cells and over the complete life cycle of the cell. Such questions have been raised in the context of cadmium-containing solar cells, and public perception in such cases will play a key role.
Challenges and Research and Development Needs
Large-scale use of solar energy for hydrogen economy will require research and development efforts on multiple fronts. In the short term, there is a need to reduce the cost of thin-film solar cells. This reduction will require the development of silicon deposition techniques that are robust and provide high throughput rates. New deposition techniques at moderate pressures with microcrystalline silicon structures for higher efficiencies are needed. Inline detection and control and the development of better roll-to-roll coating processes can lead to reductions in the manufacturing costs. Increased automation will also contribute to the decreased cost. Issues related to a large decrease in efficiency from small laboratory samples to the module level should be addressed. In the short run, thin-film deposition methods can potentially gain from a fresh look at the overall process from the laboratory scale to the manufacturing scale. The research in
this area is expensive. Some additional centers for such research in academia with industrial alliances could be beneficial. It will be necessary to collect multifunctional teams from different engineering disciplines for such studies.
In the midterm to long term, organic-polymer-based solar cells hold promise for mass production at low cost. They have an appeal for being cast as thin films at very high speeds using known polymer film casting techniques. Currently, the efficiency of such a system is quite low (in the neighborhood of 3 to 4 percent or lower), and stability in sunlight is poor. However, owing to the tremendous development in conducting polymers and other electronics-related applications, it is anticipated that research in such an area has a high potential for success.
Similarly, the search for a stable dye material and better electrolyte material in dye-sensitized cells (Grätzel cells) has a potential to lead to lower-cost solar cells. There is a need to increase the stable efficiency of such cells; a stable efficiency of about 10 percent could be quite useful.
In the long run, the success of directly splitting water molecules by using photons is quite attractive. Research in this area could be very fruitful.
Department of Energy Programs for Solar Energy to Hydrogen
The current DOE target for photoelectrochemical hydrogen production in 2015 is $5/kg H2 at the plant gate. Even if this target is met, solar hydrogen is unlikely to be competitive. Therefore, beyond 2015 a much more aggressive cost target for hydrogen production by photoelectrochemical methods is needed.
Since photoelectrochemical hydrogen production is in an embryonic stage, a parallel effort to reduce the cost of electricity production from PV modules must be made. A substantial reduction in PV module cost (lower than $1/Wp), coupled with a similar reduction in electrolyzer costs (much below $125/kW at a reasonably high efficiency of about 70 percent based on lower heating value), could provide hydrogen at reasonable cost. In the long run, considering the environmental issues associated with fossil fuels and considering the limitless supply of solar energy, this has a potential to be quite attractive. This option will be especially attractive if advances in battery technology are unable to substantially increase the electricity storage density (based on mass of battery) and greatly reduce the cost of batteries. Therefore, it is recommended that thin-film technologies and other emerging PV technologies that hold the promise for cost reduction be aggressively pursued. As stated earlier, it means that more efficient and robust methods for thin-film coating must be developed. Organic-polymer-based solar cells should also be funded. There is tremendous development underway in conducting polymers for light-emitting diodes and other display technologies. The potential of these materials for solar cell PVs must be actively explored.
Summary
All of the current methods and the projected technologies of producing hydrogen from solar energy are much more expensive (greater than a factor of 3) when compared with hydrogen production from coal or natural gas plants. This is due partly to the lower annual utilization factor of about 20 percent (as compared with say, wind, at 30 to 40 percent). This high cost puts enormous pressure on the need to reduce the cost of a solar energy recovery device. While an expected future installed module cost of about $1/Wp is very attractive for electricity generation and deserves a strong research effort in its own right, this cost fails to provide hydrogen at a competitive value. The raw material cost for crystalline silicon-wafer-based technologies is a large fraction of the $1/Wp value and is therefore less likely to provide hydrogen economically. On the other hand, thin-film technologies do not use much raw material in thin films themselves but require tremendous progress in the deposition technology. There is a need for a robust deposition method that would have a potential to reduce cost much below $1/Wp. Emerging polymer-based technologies have a potential to provide low-cost devices to harness solar energy. It is apparent that there is no one method of harnessing solar energy that is clearly preferable. However, it appears possible that new concepts may emerge that would be competitive. The benefits of such developments would be very substantial.
In the future, as the cost of the fuel cell approaches $50 per kilowatt, the cost of an electrolytic cell to electrolyze water is also expected to approach a low number (about $125/kW). With such low-cost electrolyzer units, the electricity cost of about $0.02 to $0.03/kWh is expected to result in a competitive hydrogen cost. It is also estimated that for a photoelectrochemical method to compete, its cost must approach $0.04 to $0.05/kWh. The order-of-magnitude reductions in cost for both hydrogen processes are similar.










































