1
Introduction
Mammography is currently the primary tool for detecting breast cancer at an early stage when it is most curable. When coupled with appropriate treatment, early detection can significantly reduce breast cancer mortality. Randomized clinical trials have shown that screening mammography can reduce breast cancer specific mortality by approximately 20 to 30 percent (reviewed by IOM, 2001, 2005). One evaluation of modern service screening in Sweden suggests mortality reductions as high as 40 to 50 percent are possible among women who actually are screened. Since about 1990, breast cancer mortality has been declining slowly but steadily in the United States (National Center for Health Statistics, 2004), and screening mammography, along with improved therapy, has been credited with reducing the number of breast cancer deaths in the United States and other countries (Peto et al, 2000; Duffy et al, 2002; Anttila et al, 2002; Jatoi and Miller, 2003; Beckett et al., 2003; Kobayashi, 2004; Coburn et al., 2004). But in order to maximize the potential benefits of mammography, high standards of quality assurance are necessary. Many factors contribute to the quality of mammography, including structural features such as the equipment used, the knowledge and skills of the staff providing the services, and the organization of service delivery at a given facility. Many of these factors are regulated by the Food and Drug Administration (FDA) under the Mammography Quality Standards Act (MQSA).
A study by the U.S. Government Accountability Office (GAO, formerly known as the General Accounting Office) found that the technical quality of mammography has increased since MQSA was enacted (GAO, 1998), and mammography facilities and personnel are to be commended for the efforts they have taken to meet the requirements of MQSA and to improve quality. Nonetheless, questions still remain regarding the impact of MQSA on access to mammography services as well as the impact on health outcomes for women who undergo mammography screening. With regard to the former, concerns have been raised about whether the additional workload and costs associated with meeting all the requirements of MQSA may be a disincentive for facilities to offer mammography services or a disincentive for medical personnel to enter or remain in the field. With regard to the latter, it is unknown whether the implementation of MQSA regulations has led to improved accuracy of mammographic interpretation, a crucial element of screening and diagnosis that is essential for reducing breast cancer morbidity and mortality. However, interpretive performance has been found to be variable in the United States.
The goal of this study was to examine the current practice of mammography and breast cancer detection, with a focus on MQSA oversight, and to identify areas in need of improvement. This report recommends strategies for achieving continued progress in assuring mammography quality, including additions, deletions, and changes to MQSA regulations, as well as approaches that do not fall within the purview of MQSA. These proposed strategies are based on careful consideration of the potential for feasibility and acceptability to patients and providers, and the available evidence to support them. The Committee stresses that the recommendations are interconnected, and that implementing
the entire set is critical for achieving the objective of further improving the effectiveness of breast cancer detection. In particular, adopting supportive elements in conjunction with additional regulatory requirements will be essential to sustain access to breast imaging services. Increasing regulation without providing financial and other support could not only fail to improve quality but could also result in decreased access. In addition, although this report was intended to inform the next reauthorization of MQSA, which is now projected for 2007, most of these recommendations could and should be implemented immediately. Indeed, adoption of many of the recommendations is long overdue.
A BRIEF HISTORY OF MQSA
The adoption and use of X-ray mammography increased greatly during the 1980s (Bassett et al., 1993; Lerner, 2001; IOM, 2001). As a result, mammography was included in the 1985 Nationwide Evaluation of X-Ray Trends (NEXT) study, organized by FDA and the Conference of Radiation Control Program Directors. That study determined that mammography facilities across the country varied widely with regard to image quality and radiation dose.
To combat the problem of poor mammography quality, the American College of Radiology (ACR) established the Mammography Accreditation Program in 1987, at the behest of the American Cancer Society. Although this was a critical first step toward improving mammography quality, the ACR program was voluntary; by 1992, only 7,246 facilities out of an estimated 11,000 had applied for accreditation, and many of these were motivated by awareness that MQSA was about to become law. Of those that had applied for accreditation, only 4,662 were fully accredited (Barr, 2004). In addition, the lack of onsite inspections potentially allowed substandard facilities to obtain ACR accreditation. Despite these limitations, the voluntary program resulted in some significant improvements, including improvements in quality control practices of medical physicists and radio logic technologists (Hendrick et al., 1998).
During this same time period, states began to pass legislation requiring health insurance coverage of mammography, and many stipulated quality assurance requirements as well. By 1993, 41 states and the District of Columbia had either passed legislation or established regulations addressing the quality of mammography (Smith and D’Orsi, 2004). In 1990, the first federal regulations of mammography quality went into effect via the Breast and Cervical Cancer Mortality Prevention Act, which aimed to increase access to mammograms for low-income women. Participating state facilities had to be ACR accredited, certified by the Health Care Financing Administration (now the Centers for Medicare and Medicaid Services), and use the ACR’s Breast Imaging Reporting and Data System (Barr, 2004). In addition, the Omnibus Budget Reconciliation Act of 1990 extended Medicare coverage to mammography facilities meeting certain standards (Houn et al., 1995). However, oversight was minimal at best. Clinical images were not evaluated for quality, and facilities merely claimed to meet the given standards (Barr, 2004).
In 1991 and 1992, the Senate Committee on Labor and Human Resources1 discussed the quality of mammography programs across the country as part of a larger hearing on breast cancer. It was noted that the “patchwork of Federal, State, and private stan-
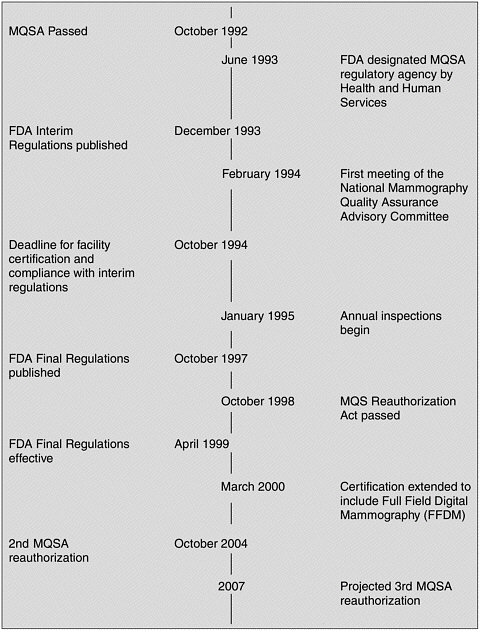
dards” regarding screening mammography were “confused and inadequate and point to the need for comprehensive regulation” (U.S. Senate, 1992). As a result, Congress passed the Mammography Quality Standards Act of 1992 (Figure 1–1). The Act presented a general framework for ensuring national quality standards for all facilities performing mammography, except those operated by the Department of Veterans Affairs. (The Department of Veterans Affairs requires accreditation, certification, and inspection for its mammography facilities, similar to MQSA.) The Secretary of Health and Human Services (HHS) was charged with establishing quality standards, determining accreditation and certification criteria, overseeing inspections, measuring compliance, directing enforcement, creating an advisory committee, and promoting education.
FDA was granted authority by the Secretary of HHS in June 1993 to implement and regulate the Act’s provisions. MQSA required all mammography facilities to comply with the regulations by October 1, 1994 (21 C.F.R. § 900). In order to meet that deadline, FDA published interim regulations based largely on the ACR’s voluntary accreditation program standards. On October 28, 1997, FDA published MQSA final regulations, which became effective in April 1999 (FDA, 2002a).
MQSA regulations include several key components (see Chapter 3 for more detail). First, FDA established national quality standards. Mammography personnel, including interpreting physicians, radiologic technologists, and medical physicists, are required to meet initial and continuing education and experience requirements. Only equipment specifically designed for mammography can be used. Documentation of the daily, weekly, quarterly, semiannual, and annual quality control tests performed at the facility must be retained for FDA annual inspections. Mammography equipment must have equipment evaluations and annual surveys by qualified medical physicists, although equipment used solely for interventional procedures is exempt from the regulations.
FDA regulations also require every mammography facility to obtain accreditation, become certified, and undergo annual inspection. FDA-approved accreditation bodies (ACR or states) review clinical and phantom images from every facility once every 3 years to monitor compliance with quality standards. Annual inspections are carried out either by FDA inspectors or state inspectors contracted by FDA. Facilities undergoing inspection are required to pay fees to cover the costs for these inspections
Finally, federal grant money was earmarked for research entities examining incidence rates, methods of detection, and diagnostic procedures for breast cancer (Ballard-Barbash et al., 1997). Efforts to measure participation rates in screening mammography and the effectiveness of screening programs in the United States also receive funding (FDA, 2002a).
MQSA was set to expire shortly after the final FDA regulations came into effect. Studies undertaken by GAO led to the conclusion that MQSA had had a positive effect on mammography quality. Between 1995 and 1997, the severity of violations at mammography facilities steadily decreased, and the proportion of facilities without violations jumped from approximately 30 percent in 1995 to 55 percent in 1997. In addition, GAO concluded that MQSA had not inadvertently limited access to mammography (GAO, 1998). A total of 163 facilities closed between 1994 and 1997, but the majority of these were either low-volume or low-quality providers. In a few cases, closure was due to consolidation with another practice (GAO, 1998).
In response to these findings, Congress passed the Mammography Quality Standards Reauthorization Act on October 9, 1998. The 1998 Act reflected several changes from the Act of 1992. For example, facilities were required to provide their patients with a written letter summarizing the results of the mammogram in lay terms. In addition, the original mammography films were to be provided to the patient on request.
Currently, nearly 70 percent of facilities pass inspection with no violations, and generally only about 2 percent of issued citations are for the most serious level of violations. GAO and FDA have both concluded that MQSA has significantly improved the quality of mammography over the past decade. Nonetheless, a 2001 FDA inspection survey found that inspections could be more efficient and inspectors more consistent (FDA, 2002b). In addition, there has been continuing concern about the quality of mammography interpretation. As described in Chapter 2, the available evidence indicates that interpretive performance is quite variable. There are also lingering concerns that the costs and workload associated with meeting MQSA requirements might lead to facility closures, with a subsequent reduction in patient access. Although a more recent study by GAO concluded that adequate access to mammography services exists, it also reported evidence of a decline in the number of radiologists and radio logic technologists entering the field of mammography in its April 2002 report (GAO, 2002). MQSA was reauthorized a second time in the fall of 2004, without major changes.2
COMMITTEE CHARGE
In preparation for the next MQSA reauthorization (originally anticipated in 2005, but now expected in 2007), Congress requested a study from the Institute of Medicine (IOM) to address remaining issues of concern regarding the quality and availability of mammography. In particular, the IOM Committee on Improving Mammography Quality Standards was charged with the task of proposing changes that could ensure and improve the accuracy of image interpretation while still ensuring adequate access to quality mammography services in the United States (Box 1-1).
METHODS
In addition to reviewing the available literature, the Committee obtained novel data and information from several sources. Data from two recent surveys by the ACR and the Society of Breast Imaging were used to evaluate the current status of the breast imaging workforce and available services. Detailed descriptions of the survey methods and analysis can be found in Appendixes A and B. The Committee also had access to workforce data collected by the American Society of Radiologic Technologists. Additionally, in an effort to predict the potential effects of present trends and possible changes in MQSA on future access to mammography, the Committee commissioned the Center for Workforce Studies at the State University of New York School of Public Health in Albany to model the supply and demand for interpreting physicians and radiologic technologists working in mammography (see Appendix C for methodological details). Staff at FDA, as well as the mammography accrediting bodies, were also very responsive to the
|
BOX 1–1 The Labor-Health and Human Services (HHS) Appropriations Conference Report and the Omnibus Bill (H.R. 2673) requested an Institute of Medicine study that would provide information, analysis, and recommendations to inform the projected Mammography Quality Standards Act (MQSA) reauthorization. In a series of meetings and a workshop, a committee will review MQSA and recommend provisions to make improvements in areas of identified concern to Congress. Specifically, the committee will consider interpretation skills assessment as a possible tool to improve physician reading of mammograms and will also consider how the annual medical outcomes audit required under MQSA regulations could be used to improve mammographic quality and interpretation. The committee will examine:
|
Committee’s questions and provided valuable information and data, including files containing the names of all interpreting physicians listed on inspection reports. Input was also sought and obtained from experts in the field and interested individuals and institutions. The recommendations put forth in this report represent Committee consensus that was developed through review and discussion of the above information sources.
FRAMEWORK OF THE REPORT
This report builds on two previous IOM studies on early breast cancer detection and mammography: Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer (2001) and Saving Women’s Lives: Strategies for Improving Breast Cancer Detection and Diagnosis (2005).
Chapter 2 describes the challenges associated with interpreting mammograms and measuring interpretive performance, and makes suggestions for how to ensure and improve the quality of mammographic interpretation.
Chapter 3 provides an overview of the regulation of mammography under MQSA, and suggests a variety of changes to the current regulations, inspections, and enforcement to streamline the process, reduce redundancy, clarify the intent, and address new technologies.
Chapter 4 characterizes the current mammography workforce and describes the challenges to maintaining women’s access to quality mammography services in the future. Strategies to ensure an adequate breast imaging workforce are recommended.
Chapter 5 suggests additional measures that could be taken to optimize the early detection of breast cancer, including quality improvement strategies for other methods of breast imaging.
REFERENCES
Anttila A, Koskela J, Hakama M. 2002. Programme sensitivity and effectiveness of mammography service screening in Helsinki, Finland. Journal of Medical Screening 9(4):153–158.
Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA, Barlow WE, Geller BM, Kerlikowske K, Edwards BK, Lynch CF, Urban N, Chrvala CA, Key CR, Poplack SP, Worden JK, Kessler LG. 1997. Breast Cancer Surveillance Consortium: A national mammography screening and outcomes database. American Journal of Roentgenology 169(4):1001–1008.
Barr H. 2004 (July 6). The Mammography Quality Standards Act (MQSA). Presentation at the meeting of the Institute of Medicine Committee on Improving Mammography Quality Standards, Washington, DC.
Bassett LW, Gold RH, Kimme-Smith C. 1993. History of the technical development of mammography. In: Haus AG, Yaffe MJ, eds. Syllabus: A Categorical Course in Physics: Technical Aspects of Breast Imaging. 2nd ed. Chicago, IL: Radiological Society of North America. Pp. 9–20.
Beckett JR, Kotre CJ, Michaelson JS. 2003. Analysis of benefit: Risk ratio and mortality reduction for the UK Breast Screening Programme. British Journal of Radiology 76(905):309–320.
Coburn NG, Chung MA, Fulton J, Cady B. 2004. Decreased breast cancer tumor size, stage, and mortality in Rhode Island: An example of a well-screened population. Cancer Control 11(4):222–230.
Duffy SW, Tabar L, Chen HH, Holmqvist M, Yen MF, Abdsalah S, Epstein B, Frodis E, Ljungberg E, Hedborg-Melander C, Sundbom A, Tholin M, Wiege M, Akerlund A, Wu HM, Tung TS, Chiu YH, Chiu CP, Huang CC, Smith RA, Rosen M, Stenbeck M, Holmberg L. 2002. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 95(3):458–469.
FDA (U.S. Food and Drug Administration). 2002a. Mammography Quality Standards Act Regulations. [Online]. Available: http://www.fda.gov/CDRH/MAMMOGRAPHY/frmamcom2.html [accessed July 30, 2004].
FDA. 2002b. MQSA Facility Satisfaction Survey (First Segment). [Online]. Available: http://www.fda.gov/cdrh/mammography/facsatissurvey.html [accessed August 18, 2004].
GAO (U.S. Government Accountability Office). 1998. Mammography Quality Standards Act: X-Ray Quality Improved, Access Unaffected, but Impact on Health Outcomes Unknown. GAO/T-HEHS-98–164. Washington, DC: GAO.
GAO. 2002. Mammography: Capacity Generally Exists to Deliver Services. GAO-02–532. Washington, DC: GAO.
Hendrick RE, Chrvala CA, Plott CM, Cutter GR, Jessop NW, Wilcox-Buchalla P. 1998. Improvement in mammography quality control: 1987–1995. Radiology 207(3):663–668.
Houn F, Franke KA, Elliott ML, Finder CA, Burkhart RL, Fischer R. 1995. The Mammography Quality Standards Act of 1992: History and process. Food & Drug Law Journal 50(4):485–492.
IOM (Institute of Medicine). 2001. Mammography and Beyond: Developing Technologies for the Early Detection of Breast Cancer. Washington, DC: National Academy Press.
IOM. 2005. Saving Women’s Lives: Strategies for Improving Breast Cancer Detection and Diagnosis. Washington, DC: The National Academies Press.
Jatoi I, Miller AB. 2003. Why is breast-cancer mortality declining? Lancet Oncology 4(4):251–254.
Kobayashi S. 2004. What caused the decline in breast cancer mortality in the United Kingdom? Breast Cancer 11(2):156–159.
Lerner BH. 2001. To See Today with the Eyes of Tomorrow: A History of Screening Mammography. [Online]. Available: http://www.iom.edu/file.asp?id=12775 [accessed February 22, 2005].
National Center for Health Statistics. 2004. Health, United States, 2004 with Chartbook on Trends in the Health of Americans. DHHS Publication No. 2004–1232. Hyattsville, MD: National Center for Health Statistics.
Peto R, Boreham J, Clarke M, Davies C, Beral V. 2000. UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet 355(9217):1822.
Smith RA, D’Orsi C. 2004. Screening for breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, eds. Diseases of the Breast. New York: Lippincott Williams & Wilkins. Pp. 103–130.
U.S. Senate, Committee on Labor and Human Resources. 1992. Senate Report 102–448: Mammography Quality Standards Act of 1992. 102nd Cong., 2nd Sess. October 1, 1992.