Raman Microscopy in the Identification of Pigments on Manuscripts and Other Artwork
Robin J. H. Clark
Christopher Ingold Laboratories
University College London
London
The identification of pigments on manuscripts, paintings, enamels, ceramics, icons, polychromes, and papyri is critical in finding solutions to problems of restoration, conservation, dating, and authentication in artwork, and many techniques (molecular and elemental) have been used for this purpose. Raman microscopy has emerged, thanks to recent advances in optics and detectors, as perhaps the most suitable of these techniques on account of its high spatial (≤ 1 µm) and spectral (≤ 1 cm−1) resolution, its specificity, its excellent sensitivity by way of charge coupled device (CCD) detectors, and the fact that many artifacts may be analyzed in situ. Long-needed links between the arts and the sciences in this area are now rapidly being developed.
The Raman effect was first detected in 1928 and rapidly became, and then remained for the following 20 years, the basis of the key technique for providing vibrational information on molecules in all states of matter, and of ions and lattice structures (Raman and Krishnan, 1928). However, the technique was then very slow, as it involved the use of a mercury arc as radiation source and photographic plates for detection. With the introduction in the 1960s of lasers as monochromatic polarized light beams of high irradiance and of semiconductors (e.g., GaAs) as detectors, the ability to detect weak Raman signals from materials of all kinds increased markedly. The technique came to be applied to a wide variety of chemical problems and increasingly to those amenable to analysis with a microsampling configuration. In the past 25 years, and especially in the last decade, the technique has been increasingly applied with great effect to the analysis of micrometre-sized particles using optical microscopes interfaced with existing spectrometer systems. These developments have revolutionized the usage of the
technique, which is seen to have many advantages over infrared (IR) microscopy and other techniques for phase identification.
The intrinsic weakness of the Raman effect (Long, 2002) in the absence of resonance effects (Clark and Dines, 1986), and the moderate sensitivity of even semiconductors or multichannel intensified diode arrays as detectors were still not ideal features of Raman microscopy for many applications. However, the introduction of CCD detectors over the past decade has made it feasible to detect and identify even poor Raman scatterers of micrometre dimensions and, by change of excitation line, even many fluorescent materials. These technical advances have opened up many new areas to which Raman microscopy could make a major contribution, pigment identification being one of these. It is now recognized that the technique combines the attributes of high reproducibility and high sensitivity with that of being nondestructive. Moreover, the technique can be applied in situ, an important consideration for manuscript study. It also has high spatial resolution (≤ 1 µm) and high spectral resolution (≤ 1 cm−1), features that are of obvious value for establishing the composition of pigment mixtures and even of binders on works of art.
Conservators and restorers need to be concerned with pigment identification for at least four reasons.
-
To decide whether (a) all restoration should be carried out with the original pigment and not with alternatives of similar hue; this is important since some alternatives might be liable to react with contiguous pigments with deleterious visual effects or (b) restoration with a different pigment might be desirable owing to instability of the original one or because it is more desirable to restore with carefully documented and easily identifiable nonoriginal pigment, possibly modern.
-
To identify any degradation products of pigments and to suggest possible treatments whereby degradation processes may be prevented, arrested or reversed.
-
It is increasingly likely that auction houses will be required to assess scientifically any works of art that they intend to offer for sale. One way of checking for obvious forgeries is to establish the palette and to check that no pigments of inappropriate dates of first manufacture or usage are present. Such analysis is extremely important in view of the immense prices—often > $1 million—for which medieval manuscripts and codices can currently be sold, usually without any scientific validation of the pigments present.
-
It is essential that conclusions on artwork as to the date, school, artist, etc., be drawn not only on paleographical, philological, and stylistic grounds but also on scientific grounds based upon an experimentally established palette. The arts community has been in general slow to accept this point.
This article begins with a brief comment on the range of pigments traditionally used to illuminate artifacts throughout time, and then moves on to a discus-
sion of various case histories in which knowledge of pigments present and of their degradation products has proved to be of interest to both the arts and the science communities.
THE PIGMENTS
The colors of inorganic pigments arise in most cases from ligand field, charge transfer or intervalence charge transfer transitions, and in the case of metals—commonly for silver or gold—from specular reflectance (Clark, 1995, 2002; Jorgensen, 1962). The depth of color of inorganic pigments is related both to the molar decadic absorption coefficients of electronic transitions in the visible region of the spectrum and to the dimensions of the pigment particles. These dimensions affect the balance between diffuse reflectance, which is controlled by the absorption coefficients and bandwidths, and specular reflectance, which is controlled by complementary factors (Clark, 1964). Early artists were well aware of these effects in practice, and with a restricted palette could often achieve a wide range of hues by selective control of particle size.
Listings of standard inorganic pigments are given in reviews (Clark, 1995, 2002), books (Mayer, 1972; Feller, 1986; Roy, 1993; FitzHugh, 1997; Thompson, 1956; Gettens and Stout, 1966; Wehlte, 1975), and proprietary literature. By way of illustration, the commonly used blue inorganic pigments are listed in Table 1, together with their chemical names, formulas, provenance, and an indication as to the nature of the electronic transitions principally responsible for the blue color in each case. Of course, the identification of blue pigments alone would only be of restricted value for dating purposes, and so extensive studies of all the pigments on a work of art are essential in order for it to be possible to estimate the date of production of any particular piece.
Many different organic dyes have been extracted from plants through the ages for use on illuminations of all sorts, notably indigo from woad for blue, alizarin from madder for red, weld from the weld plant (related to mignonette) for yellow, crocetin from saffron, and gamboge from gum resin—both also for yellow. In addition, organic dyes were extracted from marine life (e.g., Tyrian purple [6,6'-dibromoindigo] from mollusks and sepia from cuttlefish); from animal life (e.g., Indian yellow from cow urine); from insects (e.g., carmine from cochineal or kermes beetles); and others from lichens (Clark, 1995). Since the isolation by W. H. Perkin in 1856 of mauveine, the first synthetic dye, many hundreds of other organic dyes have been synthesized, and this has greatly extended the nature of the palette to which artists have had access. The main scientific concerns in the examination of a work of art are
-
to identify each pigment, its crystal structure and, if possible, its place of origin and to identify the pigment medium;
-
to assess whether it is feasible to restore any degradation to the paintwork
TABLE 1 Commonly Used Blue Inorganic Pigments
|
Pigment |
Chemical Name |
Formula |
Datea |
Transitionb |
|
Azurite |
Basic copper(II) carbonate |
2CuCO3.Cu(OH)2 |
min. |
LF |
|
Cerulean blue |
Cobalt(II) stannate |
CoO.nSnO2 |
1821 |
LF |
|
Chinese blue |
Barium copper(II) silicate |
BaCuSi4O10 |
ca 480 BC |
LF |
|
Cobalt blue |
Cobalt(II)-doped alumina glass |
CoO.nAl2O3 |
ca 1550 Ming dynasty |
LF |
|
Egyptian blue |
Calcium copper(II) silicate |
CaCuSi4O10 |
ca 3100 BC |
LF |
|
Fluorite (and antonozite) |
Calcium fluoride (purple) |
CaF2 |
min. |
Trapped electrons? |
|
Lazurite (from lapis lazuli) |
Sodalite + sulfur radical anions |
Na8[Al6Si6O24]Sn |
min. 1828 |
CT S3−, S2− |
|
Manganese blue |
Barium manganate(V) sulfate |
Ba(MnO4)2 + BaSO4 |
1907 |
LF |
|
Maya blue |
Palygorskite/indigo/nano-material |
Mg5(Si,Al)8O20(OH)2.8H2O, etc. |
Mayan |
Mie scatteringc |
|
Phthalocyanine blue/Winsor blue |
Copper(II) phthalocyanine |
Cu(C32H16N8) |
1936 |
π-π*d |
|
Posnjakite |
Basic copper(II) sulfate |
CuSO4.3Cu(OH)2.H2O |
min. |
LF |
|
Prussian blue |
Iron(III) hexa-cyanoferrate |
Fe4[Fe(CN)6]3.14-16H2O |
1704 |
IVCT Fe(II)/Fe(III) |
|
Smalt |
Cobalt(II) silicate |
CoO.nSiO2 |
Earlier than |
1500 LF |
|
Vanadium blue |
Vanadium(IV)-doped zircon |
ZrSiO4(V(IV)) |
1950? |
LF |
|
Verdigris |
Basic copper(II) acetate |
2Cu(O2CCH3)2.Cu(OH)2 |
Corrosion product |
LF |
|
Vivianite |
Iron(II,III)- phosphate |
Fe3P2O8.8H2O |
min. |
IVCT Fe(II)/Fe(III) |
|
aThe pigment is specified to be a mineral (min.) and/or the date of its first manufacture is listed. bLF = ligand field; CT = charge transfer; IVCT = intervalence charge transfer transition. cThe origin of the color is uncertain. (José-Yacamán M., L. Rendon, J. Arenas, and M. C. Serra Puche. 1996. Science 273:223-227.) dπ-π* = electric-dipole-allowed charge transfer transition of the phthalocyanine ring system. |
||||
-
or fabric, given the nature of the chemical processes likely to be involved;
-
to consider whether knowledge of the identity of the pigments present on a work of art would give an indication as to date of production and hence the authenticity, school, and/or artist; and
-
to identify the correct measures needed to preserve a work of art from the effects of heat, light, and gaseous pollutants, and from contiguous or underlying pigments, dyes, or inks.
THE RAMAN MICROSCOPE
In a Raman microscope the incident laser beam is brought to a focus by the objective onto each different pigment grain in turn on the manuscript under study (see Figure 1). The Raman scattering is collected by the same objective and then directed by a beamsplitter in the optical path to the monochromator and the detector. Although the use of a beamsplitter reduces the overall efficiency of the system, as does the use of a pinhole as a spatial filter, both devices restrict the amount of unwanted scattered light collected from outside the focus of the laser beam. The benefit of a pinhole is that it ensures a good confocal arrangement, thereby providing spatial resolution as a function of sample depth.
The overall efficiency of detection of the Raman signal from the older Raman spectrometers was still relatively poor in the 1980s, because it was based on double
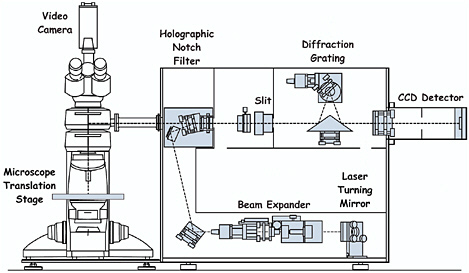
FIGURE 1 Schematic representation of a Raman microscope (Renishaw RM 1000), which employs notch filter assemblies and a CCD detector.
or triple spectrometers with a large number of optical surfaces and on multichannel intensified diode arrays, which are relatively insensitive detectors, albeit better than photographic plates. However, two devices have improved matters greatly: two-dimensional CCDs with up to 80 percent quantum efficiency of detection and holographic notch filters, which are wavelength specific and have the property of blocking out the unwanted Rayleigh scattering. These filters possess a cutoff that readily permits approach to within 50 cm−1 or less of the excitation line. This has the consequence that monochromators with the high dispersion previously required to filter out the Rayleigh line can be replaced by a spectrograph system with a single grating only, leading to much greater efficiency of throughput. The introduction of notch filters eliminates the need for separate pinholes in the optical path. The benefits of CCD detector/notch filter spectrographs are that (1) low-powered air-cooled lasers can be used, which both reduce the costs of purchase and operation and make the entire system portable (since elaborate and fixed water-cooling systems for the lasers are not required); (2) the spectrographs are much lighter and much easier to realign than earlier double or triple grating systems; and (3) the time required to acquire significant data is greatly reduced, in some cases even to seconds. Such spectrographs can also be used for remote Raman microscopy in which a probe head assembly both delivers the excitation beam to the sample and collects the scattered radiation from the sample by means of fiber optics. They may be appropriate for the study of heavy items that need support from a specially designed cradle, notably large codices that cannot fit safely onto the microscope stage, or for archaeological studies of murals, cave paintings, etc.
It is now also possible to collect data taken from many different sample points on an inhomogeneous surface to produce a Raman spectral map. The method involves direct two-dimensional imaging of an inhomogeneous surface by analyzing one or more of the Raman bands characteristic of a given component on the surface. It offers intriguing further opportunities for the study of very small (approximately 1 mm2) areas of artwork on stamps, maps, and writing (so as to be able to follow iron gall ink diffusion, paper damage, etc.), and in many other fields (e.g., that of identification of the precise whereabouts and proportions of active ingredients in pharmaceutical tablets).
It is usually desirable to have a wide range of excitation lines (frequency ν0) available in order to search for the most enhanced Raman spectrum, bearing in mind the often opposing effects on the scattering intensity of ν4 (the fourth power of the frequency of the scattered light), absorption, resonance, and possible photochemical and/or thermal degradation of the sample. Fluorescence from certain materials is often best avoided by use of a Nd/YAG (yttrium aluminum garnet) laser operating at 1064 nm, albeit with significant loss in spatial resolution and scattering intensity. By use of equipment such as that described above, libraries of Raman spectra of common inorganic, mineral and earth pigments, and organic pigments and dyes have been compiled (Griffith, 1987; Bell et al., 1997; Burgio
TABLE 2 Comparison of Different Techniques for Pigment Identification
|
Technique |
Compound specificity and type of information |
Sensitivity |
|
Raman |
excellent, molecular |
gooda |
|
IR |
excellent, molecular |
fair |
|
PLM |
fair, molecular |
fairb |
|
UV/VIS |
poor, molecular |
good |
|
LIBS |
good, elemental |
excellent |
|
XRF |
good, elemental |
goodc |
|
XPS |
good, elemental |
goode |
|
PIXE/PIGE |
good, elemental |
excellentg |
|
SEM/EDX |
good, elemental |
goode |
|
XRD |
excellent, molecular |
fairb |
|
Raman, visible laser Raman microscopy; IR, mid-infrared reflectance microscopy; PLM, polarised light microscopy; UV/VIS, ultraviolet/visible reflectance spectroscopy or fibre optic reflectance spectroscopy (FORS); LIBS, laser-induced breakdown spectroscopy; SEM/EDX, scanning electron microscopy with Be-windowed energy dispersive X-ray detection; XRF, X-ray fluorescence spectroscopy; XPS, X-ray photoelectron spectroscopy, also called electron spectroscopy for chemical analysis (ESCA); PIXE/PIGE, external beam proton-induced X-ray emission/proton-induced γ-ray emission; XRD, powder X-ray diffraction. aSensitivity can be excellent under resonance conditions. bOnly possible with crystalline materials. cOnly atoms with atomic number Z ≥14 (Si) without an evacuated sample chamber. |
||
and Clark, 2001; Bikiaris et al., 2000; Vandenabeele et al., 2000), as well as of some pigment media (binders, gums, resins, etc.), and these are now widely available for reference purposes.
Many other techniques have been and are used for pigment identification, and estimates of their strengths and weaknesses vis-à-vis Raman microscopy have been given (Cilberto and Spoto, 2000; Pollard and Heron, 1996; Brundle et al., 1992; Bousfield, 1992; Smith and Clark, 2002b) and are summarized in Table 2. Raman microscopy is considered by many to be the best single technique for this purpose and is extremely effective when used in conjunction with other complementary techniques, such as polarized light microscopy (PLM), infrared microscopy (IR), X-ray fluorescence (XRF), X-ray diffraction (XRD), particle-induced X-ray emission (PIXE), or laser-induced breakdown spectroscopy (LIBS). Further techniques such as laser-induced fluorescence (LIF) have occasionally been used, as have nuclear ones, such as nuclear reaction analysis (NRA) and Rutherford back scattering (RBS), at laboratories in which a cyclotron source is available.
Studies of Western and Eastern manuscripts, painting cross-sections, ceramics, papyri, icons, polychromes, and other artifacts (stamps, coins, etc.) as well as degradation and corrosion products are now discussed with particular reference
|
Immunity to interference |
Spatial resolution |
In situ analysis |
Portable |
|
good |
excellent (< 1 µm) |
yes |
yes |
|
poor |
good (~ 20 µm) |
yes |
yes |
|
excellent |
good (~ 10 µm) |
no |
yes |
|
fair |
good (~ 10 µm) |
yes |
yes |
|
good |
good (~ 20 µm) |
yes |
no |
|
good |
faird (~ 1 mm) |
yes |
yes |
|
good |
fairf (~ 1 mm) |
yes |
no |
|
good |
fair (< 1 mm) |
yes |
no |
|
good |
excellent (< 1 µm) |
noh |
no |
|
good |
fair (< 0.1 mm) |
no |
no |
|
dX-ray escape depth varies from nm to mm dimensions depending on the material and the element being detected. Can lead to loss of spatial resolution and to interference from sub-surface layers. eAll atoms with Z ≥ 5 (B). fDepth of sample is normally confined to several nanometres. gAll atoms with Z ≥ 11(Na). A proton beam (via PIXE) generates less bremsstrahlung background radiation than other X-ray techniques, providing greater sensitivity. PIXE can also be modified with different detectors to perform nuclear reaction analysis (NRA) and Rutherford back-scattering (RBS) studies. hEnvironmental SEM can analyse small, intact artefacts. |
|||
to those carried out in London. Raman microscopy also has wide application to many other fields of study, as discussed by Corset et al. (1989) and Turrell and Corset (1996).
WESTERN AND EASTERN MANUSCRIPTS
The Anglo-Saxon manuscripts in the British Library, one of the world’s foremost collections, have been well studied from a paleographical standpoint but only slightly in respect of the materials used and the methods followed in their construction. Conclusive identification allows the correct materials to be used for the conservation of any object. Most of the pigments present on a large number of Western and Eastern manuscripts have now been identified at University College London, the more important items being cited below.
Western Manuscripts
Early Raman studies of a Paris Bible of ca 1275 in Latin rapidly revealed the ease with which most inorganic pigments may be distinguished, even with Raman microscope systems of a 1980s design (Best et al., 1992, 1993). Spectra could
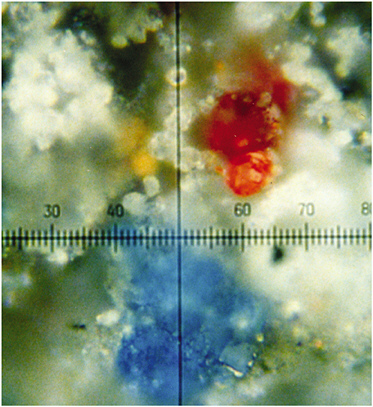
FIGURE 2 Magnified (100 times) portion of a dark grey-black part of an illuminated letter R on a German choir book (sixteenth century) showing grains of different pigments, all identifiable by Raman microscopy (Best et al., 1992). Reproduced with permission, Elsevier.
readily be obtained from a pigment grain of 1-2 µm diameter, even when adjacent to other grains of different composition (see Figure 2), this is not an uncommon situation in artwork, since many artists choose to mix pigments in order to obtain shades of color not otherwise available. Other studies established the palettes of Latin manuscripts (Burgio et al., 1997a), a German choir book (Burgio et al., 1997b), German manuscripts (Burgio et al., 1997b), and illuminated plates from the Flora Danica (Burgio et al., 1999a). Of particular interest is the Skard copy of the Icelandic Book of Law, ca 1360, which was shown to have been richly illuminated, albeit not with either of the lead pigments commonly used in Europe at that time (i.e., white lead [2PbCO3.Pb(OH)2] and red lead [Pb3O4] [Best et al., 1995]) but with bone white (Ca3PO4) and vermilion (HgS)/red ochre (Fe2O3), respectively, possibly owing to the lack of lead ores in Iceland. Extensive studies have now been carried out on many manuscripts and codices in the British Library, the Victoria and Albert Museum, the Museum of London, the Beinecke

FIGURE 3 Illumination on the prologue page of the King George III version of the Gutenberg Bible held in the British Library (Chaplin et al., 2002a, 2005). Reproduced with permission, Wiley and the American Chemical Society.
Library, and elsewhere, the more recent work having the benefit of detection by CCD of the Raman scattered light from the pigments.
One of the most notable of the recent studies is that in which the palettes of seven different Gutenberg Bibles were established (i.e., the King George III in the British Library, the ones at Eton College [Windsor] and Lambeth Palace [London], and two in France and two in Germany [Chaplin et al., 2002a, 2005]). These are brilliantly illuminated codices, the red, green, blue, white, and black pigments on the King George III Bible consisting of vermilion (HgS), lead tin yellow Type I (Pb2SnO4), carbon black, azurite (2CuCO3.Cu(OH)2), malachite (CuCO3. Cu(OH)2), verdigris (approximately 2Cu(CH3CO2)2.Cu(OH)2), chalk (CaCO3), gypsum (CaSO4.2H2O), and lead white (2PbCO3.Pb(OH)2), in agreement with instructions given in the accompanying model book (a situation that is far from being always the case). The illuminations on the British Library and
Eton College Bibles are similar to one another, consisting of interwoven flora and fauna around the columns of printed text (see Figure 3). Those on the Lambeth Palace Bible differ for the major illuminations, which consist of geometric patterns in blue, white, and gold but are similar for the minor ones.
Also recently completed has been a detailed Raman study (Brown and Clark, 2004a) of the Lindisfarne Gospels (valued at perhaps $40 million), which represent to many the pinnacle of artistic achievement of manuscript illumination. Considered to have been created around 715 by Eadfrith in honor of St. Cuthbert, who was bishop of Lindisfarne in Northumbria during the period 685-687, the major pages display fantastic complexity of zoomorphic and interlace ornament, by contrast to the simplicity of the evangelist portraits. The most important result of the early pigment analyses of the Gospels by light microscopy was thought to be the apparent identification (Roosen-Runge and Werner, 1960) of lazurite (along with some indigo) on the evangelist portraits of St. Mark, f. 93v (folio page 93, verso) and St. Luke, f. 139, a result which would indicate the earliest known usage of lazurite on an Anglo-Saxon manuscript. Since the presence of non-indigenous materials on a work of art of known origin is regarded as indicating that a trade route between the source of the material and the place of construction of the work existed at its date of construction, the existence is implied at that very early date of a trade route to Northumbria from the Badakshan mines in Afghanistan, then the only source of lazurite. The Gospels were recognised even in ca 715 to be very prestigious, and so there is little doubt that the most impressive and expensive blue pigment would have been used thereon, if available. However, Raman studies (Brown and Clark, 2004a) led to the identification of indigo alone, both on f. 93v as well as on f. 139 (see Figure 4), there being no evidence at all for lazurite. This suggests that trade in lazurite to Northumbria was most unlikely to have begun by the early eighth century. Several other substantial studies of Anglo-Saxon (Brown and Clark, 2004b,c) and Carolingian (Clark and van der Weerd, 2004) manuscripts have also recently been published.
Eastern Manuscripts
Studies quickly revealed that the palette of Eastern manuscripts was not in general greatly different from that of Western ones, except in respect to some plant and animal extracts (e.g., Indian yellow [euxanthic acid, MgC19H16O11.5H2O, from cow urine]). Included in early studies were ones on various Persian manuscripts: “Anatomy of the Body,” a nineteenth-century copy of an earlier manuscript, and “Poetry in Praise,” sixteenth century (Ciomartan and Clark, 1996); three very rare sixteenth-century copies of the Qazwini manuscript “Wonders of Creation and Oddities of Existence,” a late thirteenth-century encyclopedic work in Arabic but of Indian style (Clark and Gibbs, 1998a); a Qu’ran section, Iran or Central Asia, thirteenth century, eastern Kufic script (Clark and Huxley, 1996); a Byzantine/Syriac Gospel lectionary, Iraq, thirteenth
century (Clark and Gibbs, 1998b); manuscript and textile fragments from Dunhuang, northwest China, tenth century (Clark et al., 1997a); Thai, Javanese, Korean, Chinese, and Uighur manuscripts (Burgio et al, 1999b); and a precious sixteenth-century Turkish manuscript (Hurev U Sirin) (Jurado-Lopez et al., 2004).
The serious and widespread blackening of the white areas on the valuable lectionary referred to above (Clark and Gibbs, 1998b) is considered to arise from the conversion of white lead, either alone or in admixture with colored pigments, into black lead(II) sulfide by hydrogen sulfide or other sulfur-containing species. Hydrogen sulfide could arise from atmospheric pollutants, from bacteria, from degradation of adjacent pigments, or from pigments on the reverse side of the page containing the illumination in question. This blackening is a widespread problem in artwork, the reversal of which has posed as many questions as answers. Treatment of lead(II) sulfide with alkaline hydrogen peroxide generates lead(II) sulfate, which is white, and this superficially at least, appears to solve the problem. Whether the resulting pigment will remain permanently attached to the manuscript has yet to be established.
The photochemical conversion of the red pigment realgar (As4S4) to yellow pararealgar (also As4S4) by light optimally in the wavelength range 530-560 nm is of great interest. The conversion occurs naturally in sunlight and was apparently recognized in Mesopotamia even as early as 1220, when pararealgar was applied to the above lectionary as a yellow pigment in addition to, and in distinctly different places from, the much more common yellow pigment orpiment, As2S3 (Clark and Gibbs, 1998b).
PAINTINGS
It may be possible to remove samples from watercolor or oil paintings in such a way that the lacunae are not discernible to the naked eye, a procedure that is rarely if ever permitted for manuscripts. Alternatively, the pigment might be able to be sampled from under the frame of a painting or, in the case of codices, from offsets transferred to the opposite page. A further possibility is to sample pigment cross-sections, a procedure with the advantages of requiring neither the removal of the work from its permanent location nor, equally importantly, the removal of delicate and valuable scientific equipment from a laboratory to a library. Studies of cross-sections are of considerable importance for paintings and icons when depth profile analyses are required.
Many Raman studies on pigments removed from paintings have now been made in order to complement those made by infrared spectroscopy and other techniques. For example, studies of Titian and Veronese paintings at the National Gallery in London have allowed the characterization of two distinct types of lead tin yellow, Type I, Pb2SnO4, and Type II, PbSn0.76Si0.24O3, which was shown to have a defect pyrochlore structure (Clark et al., 1995). These two pigments, in use
FIGURE 5 Painting “Young Woman Seated at a Virginal” under study by Raman microscopy to identify the pigments present, and leading to evidence consistent with its attribution to Vermeer, ca 1670 (Burgio et al., 2005). Reprinted with permission, American Chemical Society.
at different periods of history, have very similar colors, but they are readily distinguishable by their Raman spectra.
Some paintings are sufficiently small that they may be examined in situ under a Raman microscope. Figure 5 shows a painting—considered by art historians to be from the late 17th century and Dutch—of a young woman with red ribbons in her hair, a pearl necklace, and a cream-coloured skirt beneath a yellow shawl. The pigments (vermilion, lazurite, and lead tin yellow Type I, particularly the last two) identified by Raman microscopy and other techniques are consistent with the attribution of this painting to Vermeer. In consequence of these identifications and other considerations, when it was auctioned at Sotheby’s on July 8, 2004, it realised US$30 million (Burgio et al., 2005).
CERAMICS
The effective use of Raman microscopy in the study of ceramics was demonstrated in 1997 on the glaze from buried fragments of medieval faience/majolica excavated from the abandoned village of Castel Fiorentino in southern Italy. The studies showed for the first time that lapis lazuli is stable at the firing temperature of the glaze and that it had been used as a pigment in Italian glaze; the brown/ black pigment used was manganese(IV) dioxide (the latter identified at the time by photoelectron spectroscopy) (Clark et al., 1997b,c). Red-brown shards of medieval pottery from many sites in Italy have, not surprisingly, been identified to be pigmented with iron oxides and yellow shards with hydrated iron(III) oxides (Clark and Curri, 1998). Raman studies of many different ochres used in wall paintings have also been carried out (Bikiaris et al., 2000).
A Raman study (Clark and Gibbs, 1997) of the pigments on Egyptian faience of the XVIIIth dynasty (ca 1350 BC) recovered in 1891 from the Nile Valley by Sir William Flinders Petrie and held at the Petrie Museum, University College London, has revealed that the red shards are pigmented with red ochre/red earth and the brilliant yellow shards with lead antimony yellow (Pb2Sb2O7), a very early synthetic pigment dating back to ca 1500 BC (see Figure 6). The latter gives a very clear and distinctive Raman spectrum that is identical to that of a contemporary sample of this pigment. Similar studies of shards from an ancient (4300-2800 BC)

FIGURE 6 Lotus leaf shard from El Amarna; the pigment used is lead antimony yellow, Pb2Sb2O7 (Clark and Gibbs, 1997). Reproduced with permission, Wiley.
site in Xishan, Henan, China, indicate that anatase, hematite, and magnetite were among the pigments used to decorate pottery found at that site (Zuo et al., 1999).
Raman studies have been made by Colomban et al. (2001) of the palettes characteristic of the Sèvres Factory, one relating to colored glazes/enamels on bisque (1050 °C) and the other on moufle (850 °C) painting colors. Other recent studies by the same group relate to Vietnamese porcelain and celadon glazes (Liem et al., 2002; Faurel et al., 2003). It is now clear that Raman microscopy is a very valuable technique for compositional and provenance studies on ceramics from sites of archaeological interest (van der Weerd et al., 2004b), this area having recently been reviewed by Smith and Clark (2004).
PAPYRI
Egyptian papyri supposedly dating from the thirteenth to the first centuries BC and brought to London for auction were recently shown to be illuminated not only with mineral pigments but also with the modern pigments phthalocyanine blue (1935) and green (1936), a Hansa yellow (ca 1950), ultramarine blue (1828)—the synthetic form of lazurite, red organic lakes (probably β-napthols, ca 1939), and synthetic anatase (1923) (Burgio and Clark, 2000). Moreover, the pigments had been painted directly onto the papyri, with no intervening ground layer of mineral pigments. The papyri are clearly modern. An authentic papyrus from the Petrie Museum at University College London had no modern pigments on its illuminations, only carbon, orpiment (As2S3), malachite, and Egyptian blue (CaCuSi4O10), the earliest synthetic pigment (ca 3000 BC). Such discoveries highlight the urgent need for proper scientific evaluation of items offered for sale or auction; indeed purchasers increasingly expect this prior to purchase.
ICONS AND POLYCHROMES
The combined application of Raman spectroscopy and LIBS has proved to be very effective for the identification of pigments at different depths below the surfaces of icons and polychromes. LIBS is an atomic emission technique in which an intense nanosecond laser pulse onto the surface of the sample results in the formation of plasma which, upon being allowed to cool, emits radiation characteristic of the elements present. The technique has high sensitivity and selectivity, and only a minute amount of material is consumed during each pulse (Anglos et al., 1997). Successive pulses probe deeper into the artwork, so that depth profiling becomes possible. A continuous-wave laser beam can be used as a Raman probe at each depth and so complementary molecular information can also be obtained.
Recent combined LIBS and Raman studies of a nineteenth-century Russian icon (see Figure 7) have revealed the identities of the pigments found in the upper layers to be white lead, zinc oxide (ZnO, for repair purposes), vermilion, and red earth, etc., all above a silver foil. Below this was found the white ground consisting

FIGURE 7 Nineteenth-century Russian icon of St. Nicholas held in Greece on which studies by LIBS and Raman microscopy enabled depth profile analyses of the pigments to be made (Burgio et al., 2000). Reproduced with permission, Society for Applied Spectroscopy.
mainly of gypsum immediately above the wood (Burgio et al., 2000). Similar studies of a rococo polychrome, a fragment from a gilded altarpiece in a church in Escatrón in Spain, has been carried out (Castillejo et al., 2000), and these reveal the power of the combined application of the two techniques for stratigraphic analyses of the pigmentation on artwork. An extensive study of cross-sections from two post-Byzantine icons from Chalkidiki, Greece, have revealed the current state of preservation of these icons, any damage thereto, and details of the pigments and materials used in the original paintings and in their overpaintings (Daniilia et al., 2002). Similar details are revealed in cross-sections from a monastic habit on the icon of St. Athanasios the Athonite (see Figure 8).
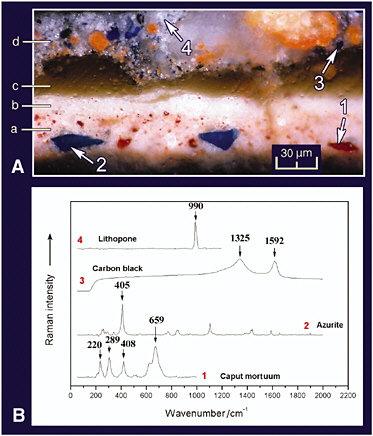
FIGURE 8 A. Cross-section of a monastic habit painted on a Greek icon; photography with a microscope in reflected light. B. Spectra of pigments taken with a Raman microscope. Identification: (a) underlayer: caput mortuum, lead white, azurite, red lake, and yellow ochre; (b) highlight: lead white and grains of caput mortuum; (c) varnish; (d) overpainting: ultramarine blue, minium, lithopone, and carbon black (Daniilia et al., 2002). Reproduced with permission, Wiley.
PHILATELY
Raman microscopy has recently been shown to have potential for establishing whether postage stamps are authentic or forgeries by way of offering an effective, rapid, and nondestructive way of identifying the pigments and dyes used in the inks, paper, and cancel marks. Thus the rare and valuable Hawaiian Missionary stamps (1851) from the Tapling Collection at the British Library were shown to have been printed using Prussian blue, Fe4[Fe(CN)6]3.14-16H2O, as the blue pig-
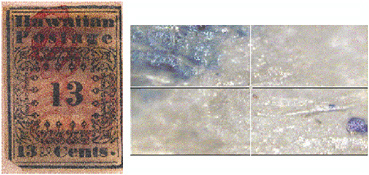
FIGURE 9 Hawaiian Missionary stamp (left) of 1851 showing (right) the blue printing (Prussian blue, Fe4[Fe(CN)6]3.14-16H2O) on the surface of the stamp (top left) and particles of ultramarine blue (bottom right) within the paper fibers (Chaplin et al., 2002b). Reproduced with permission, Wiley.
ment (see Figure 9). In addition, the paper fibers of the stamps were shown to have ultramarine blue particles interspersed between them, to act as an optical brightener. Distinctions between genuine and forged or reproduction stamps can be drawn on the basis of the pigments used (Chaplin et al., 2002b).
Similar studies of the earliest Mauritian stamps (1847) have been carried out, notably on an extremely rare one penny (1d, orange-red, used) stamp, a rare 2d (blue, unused) stamp, as well as on a reproduction stamp (1905), early forgeries, and Britannia-type stamps (1858-1862), in order to identify the pigments used. For the Britannia-type stamps the pigments used were red lead (Pb3O4) on the 1d stamp, Prussian blue on the 2d stamp, chrome green—a mixture of Prussian blue and chrome yellow (PbCrO4)—on the 4d stamp, and vermilion (HgS) on the 6d (orange) stamp. The technique has great potential for expertising, i.e. distinguishing between, genuine and forged or reproduction, stamps (Chaplin et al., 2004).
WALL PAINTINGS
Many studies have now been carried out on the pigments used to illuminate colored frescos, whose palettes are much more restricted than those of manuscripts and paintings. By contrast with scriptoria, libraries, and museum collections, where pigment degradation has usually only been slight, wall paintings and frescos often show obvious signs of overexposure to environmental extremes of temperature and humidity. Moreover, the effects of microbial, fungal, and lichen colonization on exposed frescos can be severe (Perez et al., 1999). Many more Raman studies of the pigments and pigment degradation products on frescos and on wall paintings in caves are likely to be made with the effective development of mobile Raman systems (Clark and Gibbs, 1998a).
PIGMENT DEGRADATION PROBLEMS
Manuscripts are subject to problems arising from the degradation of pigments, particularly those that are copper-, arsenic-, or lead-based. Degradation products of pigments can be identified by Raman microscopy, enabling each such process to be revealed and its progress monitored.
Verdigris is an umbrella term used for any green or blue corrosion product resulting from the action of atmospheric agents on copper, in particular acetic acid and sometimes formic acid. It can be regarded as a disfiguring product of the corrosion of objects made of copper alloys or as a pigment made deliberately by corrosion of copper or by the conversion of a copper compound. Moreover, it dissolves in many oils and resins to form copper resinates that can themselves be dissolved in size or gelatin to form copper proteinates (Scott et al., 2001). Raman microscopy has recently been applied to the problem of characterizing these chemically very similar compounds.
Many black minerals are used, or may have been used, as pigments at different periods of time, notably carbon black, chromite (FeCr2O4), covellite (CuS), galena (PbS), ilmenite (FeTiO3), magnetite (Fe3O4), plattnerite (PbO2), pyrolusite (MnO2), tenorite (CuO), and silver glance (Ag2S). Both galena and plattnerite may develop on artwork as degradation products of other lead-containing pigments, notably white lead, the net result being a gross disfigurement of the manuscript or painting. The identification may now be carried out using modern Raman microscopes and excitation lines of low power, both directly and by way of the recognition of their oxidation products (i.e., PbO.PbSO4, 3PbO.PbSO4, and 4PbO.PbSO4) (Giovannoni et al., 1990; Burgio et al., 2001). Reversal of black PbS to white PbSO4 is one option sometimes used for restoration of the intended effects of the artist, but this procedure is by no means fully accepted by conservators. Detailed Raman studies have also been carried out on millimetre-sized single crystals of PbS, a model material for quantum dot research, under resonance Raman conditions, and the phonon modes identified and assigned (Smith et al., 2002b).
In Figure 10 a detail of the illumination (a set of dividers) in volume 1, f. 33v, of the Jamnitzer Manuscript reveals that severe degradation of lead white to lead sulfide can readily be demonstrated in situ by Raman microscopy, most particularly in the highlights (now black) (Smith et al., 2002a). Copper pigments, such as azurite, likewise rapidly degrade to covellite in the presence of gaseous H2S (Smith and Clark, 2002a), as is also easily shown by Raman microscopy (see Figure 11).
Raman microscopy may also be applied to the study of the corrosion of metals (Martens et al., 2003), including the main encrustments on ancient bronzes derived from copper, tin, and lead. Thus bronze artifacts from Chinese tombs of the Eastern Han dynasty (25-220) have been shown to include among their corrosion products Cu2O (cuprite), CuCO3.Cu(OH)2, PbO, PbCO3, and PbSO4 (McCann et al., 1999). Two-dimensional mapping provided information on the
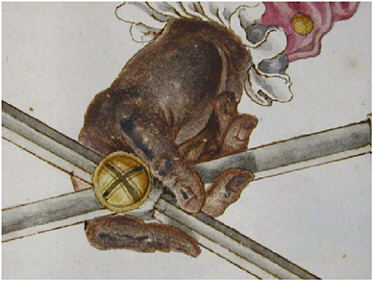
FIGURE 10 Detail of the illumination in vol. 1, f. 33v, of the Jamnitzer manuscript at the Victoria and Albert Museum, London, in which the original highlights of lead white have degraded to lead sulfide (black) (Smith et al., 2002a). Reproduced with permission, International Institute for Conservation.
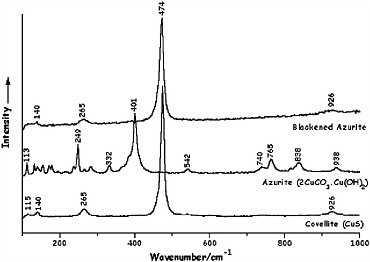
FIGURE 11 Raman spectra of covellite, CuS, azurite, 2CuCO3.Cu(OH)2, and blackened azurite following brief exposure of the azurite to H2S vapor (Smith and Clark, 2002a). Reproduced with permission, Elsevier.
spatial extent of each corrosion product. Many extensions to this work are being planned with the vast collections of degraded metals in museums in order to understand the nature and causes of the degradation. The most recent studies of this sort have led to the Raman-based identification of iron oxide impurities (magnetite, iron-deficient magnetite, and hematite) in early industrial-scale processed platinum (e.g., in the platinum metal constituting the three-ruble Russian coin of 1837) (van der Weerd et al., 2004a).
Modern Raman spectrometers may now be capable of identifying iron gallotannate inks on manuscripts, a notoriously difficult task. Recent studies on the Vinland Map (Beinecke Library, Yale University) have shown that the fragmented black ink lines that define the map consist of carbon black rather that iron gallotannate and that the yellow-brown background to the lines, but not elsewhere, contains nearly pure anatase (TiO2) (Brown and Clark, 2002). This material had earlier been shown to have a particle size (approximately 0.15 µm) and particle size distribution that is characteristic of the synthetic ca 1920 product (McCrone, 1988). The Raman results thus confirm that the map dates from the twentieth not the early fifteenth century, and thus that it is not pre-Columbian; this is in complete agreement with the analysis of Towe (1990).
CONCLUSION
Raman microscopy is now established to be a key technique for the identification of pigments on works of art, largely because of its high spatial and spectral resolution, excellent sensitivity and specificity, and because it can be applied to an object in situ. Difficulties may arise on occasions with certain organic pigments, supports, and binders that fluoresce, that are photosensitive, or that fail to yield a Raman spectrum owing to their small particle size, high dilution, or poor scattering efficiency. The use of other techniques in conjunction with Raman microscopy then becomes essential in order to effect full pigment characterization. Remote laser Raman microscopy (Clark and Gibbs, 1998a) will increasingly be used for the study of objects unable to be moved from their place of exhibition. The whole area is one in which the arts and the sciences can coordinate with great effect.
ACKNOWLEDGEMENTS
The author is most grateful to the members of his group in this field, most recently Drs. K. L. Brown, L. Burgio, T. D. Chaplin, S. Firth, A. Jurado-Lopez, G. D. Smith, and J. van der Weerd, and to Renishaw PLC, the Engineering and Physical Sciences Research Council, the European Union, and the British Library for their support of this research.
REFERENCES
Anglos, D., S. Couris, and C. Fotakis. 1997. Applied Spectroscopy 51:1025-1030.
Bell, I. M., R. J. H. Clark, and P. J. Gibbs. 1997. Spectrochimica Acta Part A 53:2159-2179.
Best, S. P., R. J. H. Clark, and R. Withnall. 1992. Endeavour, New Series 16:66-73.
Best, S. P., R. J. H. Clark, M. A. M. Daniels, and R. Withnall. 1993. Chemistry in Britain 118-122.
Best, S. P., R. J. H. Clark, M. A. M. Daniels, C. A. Porter, and R. Withnall. 1995. Studies in Conservation 40:31-40.
Bikiaris, D., Sister Daniilia, S. Sotiropoulou, O. Katsimbiri, E. Pavlidou, A. P. Moutsatsou, and Y. Chryssoulakis. 2000. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 56:3-18.
Bousfield, B. 1992. Surface Preparation and Microscopy of Materials. Chichester, U.K.: Wiley.
Brown, K. L., and R. J. H. Clark. 2002. Analytical Chemistry 74:3658-3661.
Brown, K. L., and R. J. H. Clark. 2004a. Journal of Raman Spectroscopy 35:4-12.
Brown, K. L., and R. J. H. Clark. 2004b. Journal of Raman Spectroscopy 35:181-189.
Brown, K. L., and R. J. H. Clark. 2004c. Journal of Raman Spectroscopy 35:217-223.
Brundle, C. R., C. A. Evans, and S. Wilson. 1992. Encyclopaedia of Materials Characterization. Boston: Butterworth-Heinemann.
Burgio, L., and R. J. H. Clark. 2000. Journal of Raman Spectroscopy 31:395-401.
Burgio, L., and R. J. H. Clark. 2001. Spectrochimica Acta Part A 57:1491-1521.
Burgio, L., D. Ciomartan, and R. J. H. Clark. 1997a. Journal of Raman Spectroscopy 28:79-83.
Burgio, L., D. Ciomartan, and R. J. H. Clark. 1997b. Journal of Molecular Structure 405:1-11.
Burgio, L., R. J. H. Clark, and H. Toftlund. 1999a. Acta Chemica Scandinavica 53:181-187.
Burgio, L., R. J. H. Clark, and P. J. Gibbs. 1999b. Journal of Raman Spectroscopy 30:181-184.
Burgio, L., R. J. H. Clark, T. Stratoudaki, D. Anglos, and M. Doulgeridis. 2000. Applied Spectroscopy 54:463-470.
Burgio, L., R. J. H. Clark, and S. Firth. 2001. Analyst 126:222-227.
Burgio, L., R. J. H. Clark, L. Sheldon, and G. D. Smith, 2005. Analytical Chemistry 77:1261-1267.
Castillejo, M., M. Martin, D. Silva, T. Stratoudaki, D. Anglos, L. Burgio, and R. J. H. Clark. 2000. Journal of Molecular Structure 550:191-198.
Chaplin, T. D., R. J. H. Clark, D. Jacobs, K. Jensen, and G. D. Smith. 2002a. In Raman Spectroscopy, eds. J. Mink, G. Jalovszky, and G. Kereszbury, pp. 823-824. Chichester, U.K.: Wiley.
Chaplin, T. D., R. J. H. Clark, D. Jacobs, K. Jensen, and G. D. Smith. 2005. Analytical Chemistry 77.
Chaplin, T. D., R. J. H. Clark, and D. R. Beech. 2002b. Journal of Raman Spectroscopy 33:424-428.
Chaplin, T. D., A. Jurado-Lopez, R. J. H. Clark, and D. R. Beech. 2004. Journal of Raman Spectroscopy 35:600-604.
Cilberto, E., and G. Spoto, eds. 2000. Modern Analytical Methods in Art and Archaeology. New York: Wiley.
Ciomartan, D., and R. J. H. Clark. 1996. Journal of the Brazilian Chemical Society 7:395-402.
Clark, R. J. H. 1964. Journal of Chemical Education 41:488-492.
Clark, R. J. H. 1995. Chemical Society Reviews 24:187-196.
Clark, R. J. H. 2002. In Handbook of Vibrational Spectroscopy, eds. J. M. Chalmers and P. R. Griffiths, pp. 2977-2992. Chichester, U.K.: Wiley.
Clark, R. J. H., and M. L. Curri. 1998. Journal of Molecular Structure 440:105-111.
Clark, R. J. H., and T. J. Dines. 1986. Angewandte Chemie-International Edition in English 25:131-158.
Clark, R. J. H., and P. J. Gibbs. 1997. Journal of Raman Spectroscopy 28:99-103.
Clark, R. J. H., and P. J. Gibbs. 1998a. Journal of Archaeological Science 25:621-629.
Clark, R. J. H., and P. J. Gibbs. 1998b. Analytical Chemistry 70:99A-104A.
Clark, R. J. H, and K. Huxley. 1996. Science and Technology for Cultural Heritage 5:95-101.
Clark, R. J. H., and J. van der Weerd. 2004. Journal of Raman Spectroscopy 35:279-283.
Clark, R. J. H., L. Cridland, B. M. Kariuki, K. D. M. Harris, and R. Withnall. 1995. Journal of the Chemical Society, Dalton Transactions 2577-2582.
Clark, R. J. H., P. J. Gibbs, K. R. Seddon, N. M. Brovenko, and Y. A. Petrosyan. 1997a. Journal of Raman Spectroscopy 28:91-94.
Clark, R. J. H., M. L. Curri, and C. Laganara, 1997b. Spectrochimica Acta Part A 53:597-603.
Clark, R. J. H., M. L. Curri, G. S. Henshaw, and C. Laganara. 1997c. Journal of Raman Spectroscopy 28:105-109.
Colomban, P., G. Sagon, and X. Faurel. 2001. Journal of Raman Spectroscopy 32:351-360.
Corset, J., P. Dhamelincourt, and J. Barbillat. 1989. Chemistry in Britain 612-616.
Daniilia, Sister, D. Bikiaris, P. Gavala, R. J. H. Clark, and Y. Chryssoulakis. 2002. Journal of Raman Spectroscopy 33:807-814.
Faurel, X., A. Vanderperre, and P. Colomban. 2003. Journal of Raman Spectroscopy 34:290-294.
Feller, R. L., ed. 1986. Artists’ Pigments, vol. 1. Cambridge: Cambridge University Press.
FitzHugh, E. W., ed. 1997. Artists’ Pigments, vol. 3. Oxford: Oxford University Press.
Gettens, R. J., and G. L. Stout. 1966. Painting Materials. New York: Dover.
Giovannoni, S., M. Matteini, and S. Moles. 1990. Studies in Conservation 35:21-25.
Griffith, W. P. 1987. In Advances in Spectroscopy, vol. 14, eds. R. J. H. Clark and R. E. Hester, pp. 119-186. Chichester, U.K.: Wiley.
Jorgensen, C. K. 1962. Absorption Spectra and Chemical Bonding. Oxford: Pergamon.
Jurado-Lopez, A., O. Demko, R. J. H. Clark, and D. Jacobs. 2004. Journal of Raman Spectroscopy 35:119-124.
Liem, N. Q., N. T. Thanh, and P. Colomban. 2002. Journal of Raman Spectroscopy 33:287-294.
Long, D. A. 2002. The Raman Effect. Chichester, U.K.: Wiley.
Martens, W., R. L. Frost, J. T. Kloprogge, and P. A. Williams. 2003. Journal of Raman Spectroscopy 34:145-151.
Mayer, R. 1972. The Artist’s Handbook. London: Faber and Faber.
McCann, L. I., K. Trentleman, T. Possley, and B. Golding. 1999. Journal of Raman Spectroscopy 30:121-132.
McCrone, W. C. 1988. Analytical Chemistry 60:1009-1018.
Perez, F. R., H. G. M. Edwards, A. Rivas, and L. Drummond. 1999. Journal of Raman Spectroscopy 30:301-305.
Pollard, A. M., and C. Heron. 1996. Archaeological Chemistry. Cambridge: Royal Society of Chemistry.
Raman, C. V., and K. S. Krishnan. 1928. Nature 121:501.
Roosen-Runge, H., and A. E. A. Werner. 1960. In Evangeliorum Quattuor Codex Lindisfarnensis, vol. 2, ed. T. D. Kendrick, pp. 263-272. Lausanne: Urs Gras.
Roy, A., ed. 1993. Artists’ Pigments, vol. 2. Oxford: Oxford University Press.
Scott, D. A., Y. Taniguchi, and E. Koseto. 2001. Reviews in Conservation 2:73-91.
Smith, G. D., and R. J. H. Clark. 2002a. Journal of Cultural Heritage 3:101-105.
Smith, G. D., and R. J. H. Clark. 2002b. Reviews in Conservation 2:92-106.
Smith, G. D., and R. J. H. Clark. 2004. Journal of Archaeological Science 31:1137-1160.
Smith, G. D., A. Derbyshire, and R. J. H. Clark. 2002a. Studies in Conservation 47:250-257.
Smith, G. D., S. Firth, R. J. H. Clark, and M. Cardona. 2002b. Journal of Applied Physics 92:4375-4380.
Thompson, D. V. 1956. The Materials and Techniques of Painting. New York: Dover.
Towe, K. M. 1990. Accounts of Chemical Research 23:84-87.
Turrell, G., and J. Corset, eds. 1996. Raman Microscopy: Developments and Applications. London: Academic Press.
van der Weerd, J., T. Rehren, S. Firth, and R. J. H. Clark. 2004a. Materials Characterisation 53: 63-70.
van der Weerd, J., G. D. Smith, S. Firth, and R. J. H. Clark. 2004b. Journal of Archaeological Science 31:1429-1437.
Vandenabeele, P., L. Moens, H. G. M. Edwards, and R. Dams. 2000. Journal of Raman Spectroscopy 31:509-517.
Wehlte, K. 1975. The Materials and Techniques of Painting. New York: Van Nostrand Reinhold.
Zuo, J., C. Xu, C. Wang, and Z. Yushi. 1999. Journal of Raman Spectroscopy 30:1053-1055.

























