Guiding Biomedical and Clinical Research
Questions of science policy, the organization of the scientific enterprise at the federal and state levels, the most promising paths to follow in pursuing specific areas of research inquiry, and research ethics are all subjects that have been studied by Institute of Medicine (IOM) committees.
EMBRYONIC STEM CELL RESEARCH
One of the most controversial issues facing the life sciences today centers on research involving the use of human embryonic stem cells (hES cells). These cells offer enormous promise for new medical interventions to prevent and treat disease, disability, and injuries. At the same time, the use of hES cells is one of the most polarizing issues to face medical research in decades. The limitation placed on federal funding of hES cell research has resulted in an anomalous situation where the majority of such research is being conducted using private or state-generated funds. A patchwork of federal regulations apply, most of which were not developed with such research in mind. Further, there are gaps in how well current regulations cover hES cell research. It is vital that a comprehensive and consistent approach be adopted and followed by all research institutions to ensure the integrity of this research and to avoid misadventures that further delay the acceptance and regularization of this important science.

In order to fill this void, the National Academies, with additional support from the Greenwall Foundation and the Ellison Medical Foundation, funded a study to define an approach and set of guidelines for the regulation of stem cell research. The study was conducted jointly by the IOM and the National Research Council’s (NRC) Board on Life Sciences. Guidelines for Human Embryonic Stem Cell Research (2005) recommends an oversight process which should assure that hES cell research is conducted in a responsible and ethically sensitive manner, and in compliance with all regulatory requirements pertaining to biomedical research in general. The guidelines cover how hES cells are obtained, stored, distributed, and used. All institutions conducting research with hES cells are urged to estab-
Guidelines for Human Embryonic Stem Cell Research (2005) recommends an oversight process which should assure that hES cell research is conducted in a responsible and ethically sensitive manner, and in compliance with all regulatory requirements pertaining to biomedical research in general.
lish oversight committees to ensure that the new guidelines will be followed. As more private entities and individual states move into hES cell research, and in light of the special concerns that such research presents, a national body should be established to assess periodically the adequacy of the guidelines and to provide a forum for a continuing discussion of hES cell research.
RESEARCH INVOLVING CHILDREN
Biomedical research has saved or improved the lives of countless children in the United States and worldwide. Still, many pediatricians and other observers have argued that infants, children, and adolescents have not shared equally with adults in advances in biomedicine. Congress and various government agencies have acted in recent years to expand research involving children. At the same time, concerns remain about the adequacy of current systems for protecting children who participate in research projects.
The Best Pharmaceuticals for Children Act of 2002 charged the IOM with reviewing current regulations and research efforts and recommending desirable practices for clinical research involving children. Ethical Conduct of Clinical Research Involving Children (2004) concludes that it is possible to strike an appropriate balance in protecting children and adolescents who take part in clinical research while helping all children reap more benefits from biomedical science. Developing an effective and adequately funded system for protecting all human research participants is a must, but safeguarding children requires extra attention and resources because they lack the maturity, and usually the legal right, to consent to experiments on their own behalf.
Developing an effective and adequately funded system for protecting all human research participants is a must, but safeguarding children requires extra attention and resources because they lack the maturity, and usually the legal right, to consent to experiments on their own behalf.
Existing federal rules to protect children from risky or unethical clinical research should be extended to cover all pediatric research in both the public and private sectors. Currently, the rules apply primarily to studies that are supported by the Department of Health and Human Services (DHHS) or regulated by the Food and Drug Administration (FDA), although many research institu-
|
BOX 5.2
SOURCES: Adapted from Children’s Hospital of Philadelphia, 2002; Children’s Hospital Boston, no date; and ECRI, 2002. Questions Parents May Want to Ask When Considering Their Child’s Participation in Clinical Research. SOURCE: Ethical Conduct of Clinical Research Involving Children, p. 178. |
tions voluntarily apply them to all of their studies. The government also should offer better guidance—and in more accessible formats—to clinical researchers and institutional review boards (IRBs) to help them interpret federal rules, which are more restrictive for children than adults. IRBs are responsible for approving human research, and they should be more thorough and explicit in judging whether research involving children meets the highest ethical and scientific standards. Parents and, when appropriate, children should be allowed sufficient time for questions and explanations of the research. In order to help fill gaps in information about how well current federal regulations are working in protecting children, the DHHS should develop and carry out a plan for collecting and reporting data on pediatric research and its oversight. In addition, national and local professional organizations and research institutions should voluntarily promote quality-improvement efforts in the design, review, and conduct of clinical research involving children.
THE FUTURE OF THE NATIONAL INSTITUTES OF HEALTH
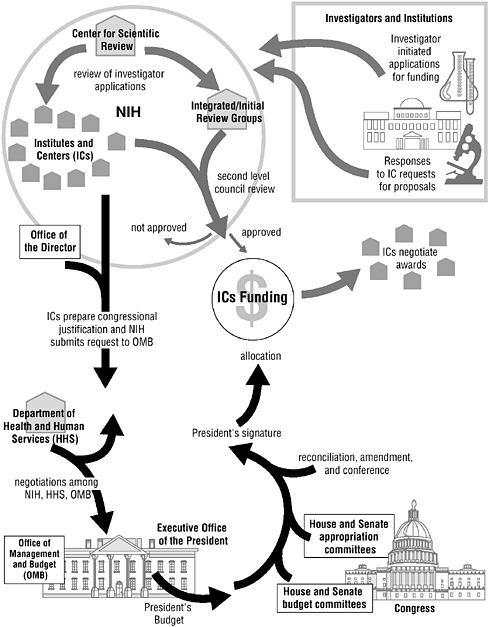
The National Institutes of Health (NIH) is the primary driver of biomedical research in the United States, and globally. Because the structure and quality of scientific decision-making at the NIH has such a large impact on the productivity of the research enterprise, it is a recurring subject of debate in the research community and in Congress. Two recent IOM studies requested by Congress have addressed these issues and made recommendations to improve the administrative efficiency and scientific focus of the NIH.

Enhancing the Vitality of the National Institutes of Health: Organizational Change to Meet New Challenges (2003), issued jointly by the IOM and the NRC’s Board on Life Sciences, agrees that significant changes are needed and provides a blueprint for strengthening the agency’s operations. In particular, it recommends modifications to improve funding procedures for innovative interdisciplinary research that reflects strategic objectives and cuts across all of the institutes and centers. One fruitful area for such “trans-NIH” research is obesity, which is associated with health problems (such as heart disease, diabetes, and arthritis) that concern multiple institutes. The NIH also should establish a special projects program, under the director’s authority, to fund cutting-edge research that is risky but offers a high potential for payoff in terms of cures and new treatments. The report does not recommend major reorganization of the NIH, noting the inevitable turmoil that radical restructuring creates within an organization. Instead, it calls for a congressional process for reviewing specific proposals for change in the NIH structure.
[The report] recommends modifications to improve funding procedures for innovative interdisciplinary research that reflects strategic objectives and cuts across all of the institutes and centers.
The second study addresses the contentious question of how to apportion funding within the NIH between investigator-initiated grants (RO1s) and support for specialized extramural centers located in universities, medical centers, and other nonprofit research institutions. Currently, approximately 9 percent of the NIH budget goes for the support of extramural centers. The centers also receive a substantial number of NIH awards to support individual investigators, group projects, research training, and other research-related efforts. The NIH supports such centers as a means of encouraging basic,
clinical, and population-based research or scientific problems not adequately addressed solely by RO1 grants. The centers are popular with Congress, patient advocacy groups, and the public because they can bring focus, visibility, and more funding to research on specific diseases and conditions. In order to bring greater order to the process by which funds are allocated, Congress asked the IOM to provide guidance in deciding which diseases and research areas warrant additional financial support by the establishment of new centers and which are adequately supported by present arrangements. NIH Extramural Center Programs: Criteria for Initiation and Evaluation (2004) offers recommendations for improving the classification and tracking of center programs, improving the decision processes, setting criteria for establishing new centers, resolving disagreements, and evaluating the performance of center programs more regularly and systematically. The report concludes by noting that recent changes in the nature of biomedical research have opened the possibilities for understanding complex biological systems through collaborations among multiple investigators in different fields and at different institutions and through the assembly of large-scale research infrastructures and databases. These changes will support the broader moves toward expanded use of centers and other mechanisms that support collaborative research by multidisciplinary teams.
In addition to advising on the general structure and method of conducting research at the NIH, the IOM also is involved in reviewing specific fields of research for the NIH, the Department of Defense (DoD), and state governments.
One such review considered the question of how the NIH should design its research strategy to study complementary and alternative medicine (CAM). U.S. consumers are increasingly using complementary and alternative medicine, including such products and practices as herbal remedies, acupuncture, and naturopathy, instead of or in addition to conventional medicine. Yet much remains unknown about CAM therapies, and there have been few scientific studies to determine how well they actually work. Facing such knowledge gaps, the NIH and the Agency for Healthcare Research and Quality turned to the IOM for help.
Complementary and Alternative Medicine in the United States (2005) reviews what is known about these therapies and offers a comprehensive set of strategies that the NIH can use to evaluate them. The core message is that health care should strive to be both comprehensive and evidence based, and that conventional medical treatments and complementary and alternative medical treatments should be held to the same standards for demonstrating
clinical effectiveness. The same general research principles should be followed in evaluating both types of treatments, although innovative methods to test some CAM therapies may have to be devised. Given that funding for research is limited, the report outlines criteria to help in determining which CAM therapies to prioritize for study. The report notes, in particular, the escalating popularity of dietary supplements as well as the lack of consistency and quality in these products, which are important components of several CAM approaches. Product inconsistency hinders health professionals’ abilities to guide patients on the use of supplements and hinders researchers’ abilities to study them. Thus, Congress should work with stakeholders to amend the current system for regulating supplements in order to improve quality control and consumer protections and to create incentives for research on the efficacy of these products. As a foundation for improving the performance of both CAM and conventional practitioners, the report calls for expanded education. CAM practitioners should receive more training in research principles and methods, while all doctors, nurses, and other health care providers should learn about CAM therapies as a regular part of their professional training.
…health care should strive to be both comprehensive and evidence based, and that conventional medical treatments and complementary and alternative medical treatments should be held to the same standards for demonstrating clinical effectiveness.
The DoD turned to the IOM for guidance about the design of a research strategy to study prion disease. The 1985 outbreak of mad cow disease in the United Kingdom generated global awareness of a new class of neurodegenerative diseases called transmissible spongiform encephalopathies (TSEs), which appear to be caused by infectious agents called prions. Identified in 1982, prions are an abnormally shaped form of a normal mammalian protein. There is no treatment for TSEs, which uniformly prove fatal, nor is there a fail-safe method of diagnosing TSEs or detecting prions during the lengthy period in which they incubate in the body. Because of the large numbers of service men and women stationed in countries where TSEs have emerged, the DoD launched the National Prion Research Program in 2002 and asked the IOM to provide a research agenda for the first round of grants.
Advancing Prion Science: Guidance for the National Prion Research Program (2004) provides that agenda. It recommends a number of strategies for achieving a diagnostic test that is rapid, sensitive, and specific enough to detect minute amounts of prions without producing false positives. One approach is to develop novel methods and reagents that detect or bind to prions, an advance
that could lead not only to better diagnostics but also to new methods of treating and even preventing TSEs. Another approach is to identify easier-to-spot surrogate markers that indicate the presence of prions or TSEs. The report stresses that funding should be expanded for basic research, as it will be virtually impossible to devise useful diagnostic tests without a better understanding of the structural and functional properties of prions and the pathogenesis of TSEs.
…funding should be expanded for basic research, as it will be virtually impossible to devise useful diagnostic tests without a better understanding of the structural and functional properties of prions and the pathogenesis of transmissible spongiform encephalopathies.
The state of New York asked for the IOM’s help in structuring its efforts aimed at improving the understanding and treatment of spinal cord injuries. The nation is aware of the heroic efforts of the late Christopher Reeve to encourage research that would find a cure for spinal cord injuries. It is less well known that New York and several other states have set aside funds to support research to that end. New York approached the IOM for assistance in defining a research strategy and setting priorities for its investments in this area. An estimated 11,000 people nationwide suffer spinal cord injuries each year, and some 247,000 people are living with a spinal cord injury. In recent decades, advances in neuroscience have widely expanded the horizons of potential therapies for spinal cord injuries. What once was dogma—that the central nervous system cannot regenerate—has been dismissed, and this newly discovered potential for regeneration and repair has opened up numerous therapeutic targets and opportunities.
Spinal Cord Injury: Progress, Promise, and Priorities (2005) surveys the current status of spinal cord injury research, examines research and infrastructure needs, and provides recommendations for advancing and accelerating progress in the treatment of spinal cord injuries. Particular attention is given to translational research, which focuses on transferring basic discoveries in the laboratory into clinical trials and ultimately into practice. Efforts should focus on three areas: increasing knowledge of basic neurobiology and therapeutic approaches, emphasizing and coordinating translational multidisciplinary research and clinical trials, and strengthening the research infrastructure and enhancing the training of scientists and physicians working in this field. As keys
to accelerating progress, the NIH should establish a National Spinal Cord Injury Research Network, which would support the work of an expanded cadre of researchers and connect individual researchers and research programs, and it should establish 5 to 7 Centers of Excellence that receive adequate resources to sustain multidisciplinary basic, clinical, and translational research on spinal cord injuries. The report also offers specific recommendations for the New York Spinal Cord Injury Research Board, which requested the study.
Efforts should focus on three areas: increasing knowledge of basic neurobiology and therapeutic approaches, emphasizing and coordinating translational multidisciplinary research and clinical trials, and strengthening the research infrastructure and enhancing the training of scientists and physicians working in this field.