11
Drinking Water Standards for Fluoride
The U.S. Environmental Protection Agency (EPA) has three standards for fluoride in drinking water: a maximum-contaminant-level goal (MCLG), a maximum contaminant level (MCL), and a secondary maximum contaminant level (SMCL). In this chapter, the committee reviews the MCLG and SMCL for fluoride, the two nonenforceable standards, for their scientific basis and adequacy for protecting the public from adverse effects. First, an overview of current procedures for establishing exposure standards is provided, and risk assessment issues that have developed since the original MCLG and SMCL for fluoride were established are discussed.
CURRENT METHODS FOR SETTING STANDARDS FOR DRINKING WATER
To establish MCLGs for drinking water, EPA reviews studies of health effects of individual contaminants and uses the information to calculate an exposure level at which no known or anticipated adverse health effects would occur with an adequate margin of safety. MCLGs consider only public health and not the limits of detection or treatment technology, so they may be set at concentrations that water systems cannot achieve.
Noncarcinogenic Contaminants
For noncarcinogenic chemicals, the MCLG is based on the reference dose, which is defined as an estimate (with uncertainty spanning perhaps an order of magnitude or greater) of a daily dose to the human population
(including susceptible subpopulations) that is likely to have no appreciable risk of deleterious health effects during a lifetime. The reference dose characterizes exposure conditions that are unlikely to cause noncancer health effects, which are typically assumed to have a threshold dose above which adverse health effects would be expected to occur.
Traditionally, reference doses are determined by identifying the most sensitive health effects that are relevant to the human, selecting a no-observed-adverse-effect level (NOAEL) or a lowest-observed-adverse-effect level (LOAEL), and dividing the NOAEL or LOAEL by one or more uncertainty factors to provide a margin of safety. Uncertainty factors are applied to address uncertainties with using experimental animal data for human effects (interspecies differences) to account for variable susceptibilities in the human population (intraspecies differences), to adjust for differences between the LOAEL and NOAEL when a LOAEL is used instead of a NOAEL (LOAEL-to-NOAEL extrapolation), to account for uncertainties with predicting chronic exposure effects on the basis of subchronic exposure studies (subchronic to chronic extrapolation), and to address uncertainties when the database on the chemical is inadequate. Sometimes a modifying factor is used to account for additional uncertainty not addressed by the standard uncertainty factors.
Typically, uncertainty factors are assigned values ranging from 1 to 10. If information about a factor is sparse and uncertainty is high, a default value of 10 is generally used. If information is available, the uncertainty factor might be reduced to 1. For an uncertainty factor that falls between 1 and 10, a factor of 3 is typically assigned, because 3 is the approximate logarithmic mean of 1 and 10, and it is assumed that the uncertainty factor is distributed lognormally (EPA 1994). To calculate a reference dose, the NOAEL or LOAEL is divided by the product of the uncertainty factors. EPA typically uses a maximum of 3,000 for the product of four uncertainty factors that individually are greater than 1 and a maximum of 10,000 with five uncertainty factors (Dourson 1994).
More recently, the benchmark dose is being used as the starting point for calculating reference doses. The benchmark dose is a dose with a specified low level of excess health risk, generally in the range of 1% to 10%, which can be estimated from data with little or no extrapolation outside the experimental dose range. Specifically, the benchmark dose is derived by modeling the data in the observed experimental range, selecting an incidence level within or near the observed range (e.g., the effective dose producing a 10% increased incidence of response), and determining the upper confidence limit on the model. To account for experimental variation, a lower confidence limit or uncertainty factors on the benchmark dose are used to ensure that the specified excess risk is not likely to be exceeded.
To derive an MCLG, the reference dose is multiplied by a typical adult
body weight of 70 kg and divided by an assumed daily water consumption of 2 L to yield a drinking water equivalent level. That level is multiplied by a percentage of the total daily exposure contributed by drinking water (usually 20%) to calculate the MCLG. EPA then uses the MCLG to set an enforceable standard (the MCL). The MCL is set as close to the MCLG as feasible.
Carcinogenic Contaminants
EPA sets MCLGs of zero for contaminants that are known or probable human carcinogens. For chemicals judged to be possibly carcinogenic to humans, EPA has recently begun applying an uncertainty factor between 1 and 10 to the reference dose derived from noncancer health effects to determine some exposure standards, such as certain ambient water-quality criteria (EPA 2000d). EPA stipulates that the water concentrations estimated to result in 1 × 10−6 to 1 × 10−5 excess cancer risks should also be compared with the reference dose.
NEW RISK ASSESSMENT CONSIDERATIONS
Since the fluoride MCLG and SMCL were originally issued, there have been a number of developments in risk assessment. A few of those issues were described above in the discussion of current risk assessment practices (e.g., use of benchmark dose). Below, a few specific issues relevant to the committee’s review of the drinking water standards for fluoride are discussed, including advances in carcinogenicity assessment, relative source contribution, special considerations for children, and explicit treatment of uncertainty and variability.
Carcinogenicity Assessment
In 2005, EPA issued its new Guidelines for Carcinogen Risk Assessment (EPA 2005a) as a replacement for its 1986 guidelines (EPA 1986). The revised guidelines were issued partly to address changes in the understanding of the variety of ways in which carcinogens can operate. For example, the guidelines provide a framework that allows all relevant biological information to be incorporated and the flexibility to consider future scientific advances.
The guidelines provide several options for constructing the dose-response relationship, in contrast to the single default dose-response relationship of the 1986 cancer guidelines. Biologically based extrapolation is the preferred approach for quantifying risk. It involves extrapolating from animals to humans based on a similar underlying mode of action. However,
in the absence of data on the parameters used in such models, the guidelines allow for alternative quantitative methods. In the default approaches, response data are modeled in the range of observation and then the point of departure or the range of extrapolation below the range of observation is determined. In addition to modeling tumor data, other kinds of responses are modeled if they are considered measures of carcinogenic risk. Three default approaches—linear, nonlinear, and both—are provided. Curve fitting in the observed range provides the effective dose corresponding to the lower 95% limit on a dose associated with a low level of response (usually in the range of 1% to 10%). That dose is then used as a point of departure for extrapolating the origin as the linear default or for a margin of exposure as the nonlinear default.
Other modifications of interest in the new guidelines include the following:
-
All biological information and not just tumor findings is considered in the hazard-assessment phase of risk assessment.
-
Mode of action is emphasized to reduce the uncertainty in describing the likelihood of harm and in determining the dose-response approaches.
-
A weight-of-evidence narrative replaces the 1986 alphanumeric classification categories. The narrative describes the key evidence, potential modes of action, conditions of hazard expression, and key default options used.
-
Direction is provided on how the overall conclusion and the confidence about risk are presented and a call is made for assumptions and uncertainties to be clearly explained.
Relative Source Contribution
EPA has developed a relative source contribution policy for assessing total human exposure to a contaminant. Under this policy, nonwater sources of exposure are considered in development of the reference dose. The percentage of total exposure typically accounted for by drinking water is applied to the reference dose to determine the maximum amount of the reference dose “apportioned” to drinking water reflected by the MCLG value. In the drinking water program, the MCLG cannot account for more than 80% or for less than 20% of the reference dose (EPA 2000d). Typically, a conservative approach is used by applying a relative source contribution factor of 20% to the reference dose when exposure data are inadequate. It is assumed that the major portion (80%) of the total exposure comes from other sources, such as the diet. This policy contrasts with past “subtraction” methods of determining relative source contributions, in which
sources of exposure other than drinking water were subtracted from the reference dose.
In EPA’s Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health, a process called the exposure decision tree (Figure 11-1) is proposed as another means for determining relative source
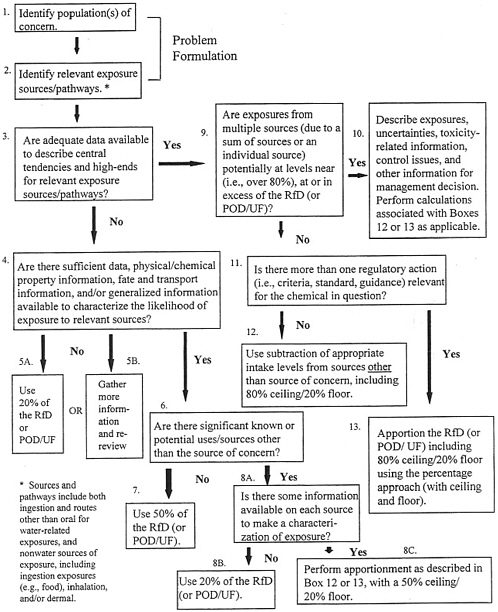
FIGURE 11-1 Exposure Decision Tree for Defining Proposed Reference Dose Apportionment. SOURCE: EPA 2000d. Abbreviations: POD, point of departure; UF, uncertainty factor
contributions (EPA 2000d). This method considers the adequacy of available exposure data, levels of exposure, relevant sources/media of exposure, and regulatory agendas. The exposure decision tree approach offers flexibility in the reference dose apportionment among sources of exposure and uses chemical information (e.g., chemical and physical properties, uses of the chemical, environmental fate and transformation, likelihood of occurrence in various media) when monitoring data are inadequate. The process also allows for use of either the subtraction or the percentage method to account for other exposures, depending on whether one or more health-based criterion is relevant for the chemical in question. The subtraction method can be used when only one criterion is relevant to a chemical. In those cases, other sources of exposure can be considered “background” and can be subtracted from the reference dose (EPA 2000d).
Risk to Children
In 1996, EPA’s Office of the Administrator issued Environmental Health Threats to Children (EPA 1996b) and set an agenda that called for considering children’s risks in all EPA actions. Children are considered a special subpopulation because their health risks can differ from those of adults as a result of their immature physiology, metabolism, and differing levels of exposure due to factors such as greater food consumption per unit of body weight and outdoor play activities. Different levels of exposure for children are typically considered in risk assessments, but the underlying toxicity database often does not specifically address effects on children. Such limitations in toxicity data are typically addressed by applying uncertainty factors to protect susceptible populations. In 2005, EPA issued special guidance for assessing susceptibility to carcinogens during early life stages (EPA 2005b).
FLUORIDE STANDARDS
Maximum-Contaminant-Level Goal
In 1986, EPA established an MCLG for fluoride of 4 mg/L to protect against “crippling” (clinical stage III) skeletal fluorosis. At that time, a reference dose for fluoride was not available, and the MCLG was calculated from a LOAEL of 20 mg/day estimated from case studies (Moller and Gudjonsson 1932), the assumption that adult water intake is 2 L per day, and the application of a safety factor of 2.5. EPA selected the safety factor to establish an MCLG that was in agreement with a recommendation from the U.S. Surgeon General (see Chapter 1).
The committee considered three toxicity end points for which there were sufficient relevant data for assessing the adequacy of the MCLG for
fluoride to protect public health: severe enamel fluorosis, skeletal fluorosis, and bone fractures.
Severe Enamel Fluorosis
In the past, moderate to severe forms of enamel fluorosis were considered to be aesthetically displeasing but not adverse to health, largely because there was no direct evidence that moderate-to-severe enamel fluorosis, as observed in the United States, had resulted in tooth loss, loss of tooth function, or psychological problems. In reviewing the collective evidence, the committee considered moderate and severe forms of the condition separately. Severe enamel fluorosis is characterized by enamel loss and pitting. This damage compromises enamel’s protective barrier and can make the teeth more susceptible to environmental stresses and to caries formation because it allows bacteria, plaque, and food particles to become entrapped in the enamel. Caries is dental decay caused by bacterial infection. When the infection goes unchecked, cavities may form that can cause toothache and tooth sensitivity to temperature and sweets. If cavities are untreated, the infection can lead to abscess, destruction of bone, and spread of the infection to other parts of the body (USDHHS 2000). While increased risk of caries has not been firmly established, the majority of the committee found that destruction of the enamel and the clinical practice of treating the condition even in the absence of caries provide additional lines of evidence for concluding that severe enamel fluorosis is an adverse health effect. Severe enamel fluorosis occurs at an appreciable frequency, approximately 10% on average, among children in U.S. communities with water fluoride concentrations at or near the current MCLG of 4 mg/L. Thus, the committee concludes that the MCLG of 4 mg/L is not protective against severe enamel fluorosis.
Two of the 12 members of the committee did not agree that severe enamel fluorosis should now be considered an adverse health effect. They agreed that it is an adverse dental effect but found that no new evidence has emerged to suggest a link between severe enamel fluorosis, as experienced in the United States, and a person’s ability to function. They judged that demonstration of enamel defects alone from fluorosis is not sufficient to change the prevailing opinion that severe enamel fluorosis is an adverse cosmetic effect. Despite their disagreement on characterization of the condition, these two members concurred with the committee’s conclusion that the MCLG should prevent the occurrence of this unwanted condition.
Strong evidence exits that the prevalence of severe enamel fluorosis is nearly zero at water fluoride concentrations to below 2 mg/L. For example, Horowitz et al. (1972) found that partial defluorination of drinking water from 6.7 mg/L to slightly below 2 mg/L prevented severe enamel fluorosis. Moderate forms of enamel fluorosis decreased from 42% to 3%.
Skeletal Fluorosis
Skeletal fluorosis is a bone and joint condition associated with prolonged exposure to high concentrations of fluoride. Fluoride increases bone density and appears to exacerbate the growth of osteophytes in the bone and joints, which leads to the radiological characteristics of the condition and associated pain. Crippling skeletal fluorosis (or clinical stage III) is the current basis of EPA’s MCLG. The term crippling historically has been used to describe alterations in bone architecture and calcification of tissues that progress to the degree that they limit an individual’s range of motion.
The committee judges that stage II skeletal fluorosis (the stage before mobility is significantly affected) should also be considered an adverse health effect. This stage is characterized by chronic joint pain, arthritic symptoms, slightly calcified ligaments, increased osteosclerosis/cancellous bones, and possibly osteoporosis of long bones (PHS 1991). No new studies and few clinical cases of skeletal fluorosis in healthy U.S. populations have been reported in recent decades. To determine whether EPA’s MCLG protects the general public from stage II and stage III skeletal fluorosis, the committee compared pharmacokinetic predictions of bone-fluoride concentrations and historical data on iliac-crest bone-fluoride concentrations associated with the different stages of skeletal fluorosis. It found that bone-fluoride concentrations estimated to be achieved from lifetime exposure to fluoride at 4 mg/L (10,000 to 12,000 milligrams per kilogram [mg/kg] ash) fall within or exceed the ranges historically associated with stage II and stage III skeletal fluorosis (4,300 to 9,200 gm/kg ash and 4,200 to 12,700 mg/kg ash, respectively). This suggests that the MCLG might not protect all individuals from the adverse stages of the condition. However, stage III skeletal fluorosis appears to be a rare condition in the United States, and the existing epidemiologic evidence is insufficient for determining whether stage II skeletal fluorosis is occurring in U.S. residents. Thus, before any conclusions can be drawn, more research is needed to clarify the relationship between fluoride ingestion, fluoride concentrations in bone, and stage of skeletal fluorosis.
Bone Fractures
The database on fluoride’s effects on bone fractures has expanded since the earlier National Research Council (NRC) review. A number of observational studies have compared bone fracture rates between populations exposed to different concentrations of fluoride in drinking water. The committee focused its review on studies involving exposure to fluoride near or within the range of 2 to 4 mg/L. Several strong studies (Sowers et al. 1991; Kurttio et al. 1999; Li et al. 2001) indicated an increased risk of bone fracture, and the results of other studies (Sowers et al. 1986; Alarcón-Herrera et
al. 2001) were qualitatively consistent with that finding. The one study using serum fluoride concentrations found no appreciable relationship to fractures (Sowers et al. 2005). Because serum fluoride concentrations may not be a good measure of bone fluoride concentrations or long-term exposure, the ability to show an association might have been diminished.
A larger database on clinical trials of fluoride as an osteoporosis treatment was also reviewed. A meta-analysis of randomized clinical trials of fluoride reported an elevated risk of new nonvertebral fractures (1.85, 95% CI = 1.36, 2.50) and a slightly decreased risk of vertebral fractures (0.90, 95% CI = 0.71, 1.14) after 4 years (Haguenauer et al. 2000). An increased risk of bone fracture was found among those studies. Although the doses of fluoride were higher in the clinical trials than were experienced by people drinking water with fluoride at 4 mg/L, the length of exposure was shorter. Although comparison of these sets of data involves several assumptions, the ranges of estimated concentrations of bone fluoride were similar in the clinical trials (5,400 to 12,000 mg/kg ash) and observational studies (6,200 to >1,000 mg/kg ash). Pharmacokinetic modeling indicates that these concentrations of fluoride in bone could result from lifetime exposure to fluoride at 4 mg/L in drinking water.
Fracture risk and bone strength have been studied in animal models. The studies have shown that fluoride increases bone mass but results about its effect on the strength of bone are conflicting. Some investigators have reported a biphasic effect on bone strength (Beary 1969; Rich and Feist 1970; Turner et al. 1992), with lower concentrations of fluoride increasing strength and higher concentrations reducing it, but others have not found this effect (Turner et al. 1995). The weight of the evidence from laboratory studies indicates that, although fluoride might increase bone volume, strength per unit volume is lower. Studies of rats indicate that bone strength begins to decline when fluoride in bone ash reaches the range of 6,000 to 7,000 mg/kg (Turner et al. 1992). Studies in rabbits have shown that fluoride might decrease bone strength by altering the structural integrity of the bone microarchitecture (Turner et al. 1997; Chachra et al. 1999). However, more research is needed to address uncertainties associated with extrapolating animal data on bone strength and fractures to humans.
Overall, there was consensus among the committee that there is scientific evidence that under certain conditions fluoride can weaken bone and increase the risk of fractures. The majority of the committee concluded that lifetime exposure to fluoride at drinking water concentrations of 4 mg/L or higher is likely to increase fracture rates in the population, compared with exposure to 1 mg/L, particularly in some demographic subgroups that are prone to accumulate fluoride in their bones (e.g., people with renal disease). However, 3 of the 12 members judged that the evidence only supported a conclusion that the MCLG might not be protective against bone fracture.
These members judge that more evidence is needed that bone fractures occur at an appreciable frequency in human populations exposed to fluoride at 4 mg/L before drawing a conclusion that the MCLG is likely to be not protective.
Secondary Maximum Contaminant Level
EPA established an SMCL of 2 mg/L on the basis of cosmetically “objectionable” enamel fluorosis, defined as discoloration and/or pitting of teeth. The SMCL was selected to prevent objectionable enamel fluorosis in a significant portion of the population. EPA reviewed data on the prevalence of moderate and severe enamel fluorosis and found that, at a fluoride concentration of 2 mg/L in drinking water, the prevalence of moderate fluorosis ranged from 4% to 15% and that severe cases were observed at concentrations above 2.5 mg/L. Because of the anticaries properties of fluoride, EPA judged 2 mg/L to be an adequate upper-boundary guideline to limit the occurrence of objectionable enamel fluorosis and provide some anticaries benefit. The SMCL is not a recommendation to add fluoride to drinking water. The SMCL is a guideline for naturally occurring fluoride to be used by the states for reducing the occurrence and severity of enamel fluorosis, a condition considered by EPA to be a cosmetic condition. If fluoride in a community water system exceeds the SMCL but not the regulatory MCL, a notice about the potential risk of enamel fluorosis must be sent to all customers served by the system. The committee evaluated the SMCL only in terms of its protection against adverse cosmetic and health effects, including enamel fluorosis, skeletal fluorosis, and bone fracture. Prevention of caries was not evaluated.
Enamel Fluorosis
The committee considers moderate enamel fluorosis to be a cosmetic effect, because the available data are inadequate for categorizing the moderate form as adverse to health on the basis of structural or psychological effects. There are no studies since 1993 to assess the prevalence of enamel fluorosis at 2 mg/L, but previous reports have shown a distinct increase (approximately 15%) in moderate enamel fluorosis around 2 mg/L. Thus, the SMCL will not completely prevent the occurrence of moderate enamel fluorosis. As noted above, SMCL was intended to reduce the severity and occurrence of the condition to 15% or less of the exposed population. The available data indicates that less than 15% of children would experience moderate enamel fluorosis of aesthetic concern (discoloration of the front teeth). However, the degree to which moderate enamel fluorosis might go
beyond a cosmetic effect to create an adverse psychological effect or an adverse effect on social functioning is not known.
While a few cases of severe enamel fluorosis occasionally have been reported in populations exposed at 2 mg/L, it appears that other sources of exposure to fluoride or other factors contributed to the condition. For example, similar rates of severe enamel fluorosis were reported in populations exposed to negligible amounts of fluoride in drinking water and in populations exposed at 2 mg/L (Selwitz et al. 1995; Kumar and Swango 1999; Nowjack-Raymer et al. 1995). Thus, the committee concludes that the SMCL of 2 mg/L adequately protects the public from the most severe stage of the condition (enamel pitting).
Skeletal Fluorosis
Few new data are available on skeletal fluorosis in populations exposed to fluoride in drinking water at 2 mg/L. Thus, the committee’s evaluation was based on new estimates of the accumulation of fluoride into bone (iliac crest/pelvis) at that concentration (on average 4,000 to 5,000 mg/kg ash) and historical information on stage II skeletal fluorosis (4,300 to 9,200 mg/kg ash). A comparison of the bone concentrations indicates that lifetime exposure at the SMCL could lead to bone fluoride concentrations that historically have been associated with stage II skeletal fluorosis. However, as noted above, the existing epidemiologic evidence is insufficient for determining whether stage II skeletal fluorosis is occurring in U.S. residents, so no quantitative conclusions could be made about risks or safety at 2-mg/L exposures.
Bone Fracture
There were few studies to assess bone fracture risk in populations exposed to fluoride at 2 mg/L in drinking water. The best available study was from Finland, which provided data that suggested an increased rate of hip fracture in populations exposed to fluoride at >1.5 mg/L (Kurttio et al. 1999). However, this study alone is not sufficient to base judgment of fracture risk for people exposed to fluoride at 2 mg/L in drinking water. Thus, no quantitative conclusions could be drawn about fracture risk or safety at the SMCL.
Susceptible Subpopulations
Populations in need of special consideration when determining the MCLG and SMCL for fluoride include those at risk because their exposure to fluoride is greater than that of the average person or because they are
particularly vulnerable to the effects of fluoride. The first category includes people who consume much larger volumes of water than assumed by EPA, such as athletes and outdoor workers, who consume large volumes of water to replace fluids lost because of strenuous activity, and people with medical conditions that cause them to consume excessive amounts of water (e.g., diabetes insipidus). Individuals who consume well over 2 L of water per day will accumulate more fluoride and reach critical bone concentrations before the average water drinker exposed to the same concentration of fluoride in drinking water. In Chapter 2, it was estimated that for high-water-intake individuals, drinking water would contribute 92% to 98% of the exposure to fluoride at 4 mg/L and 86% to 96% at 2 mg/L. Another consideration is individuals who are exposed to other significant sources of fluoride, such as occupational, industrial, and therapeutic sources.
There are also environmental, metabolic, and disease conditions that cause more fluoride to be retained in the body. For example, fluoride retention might be affected by environments or conditions that chronically affect urinary pH, including diet, drugs, altitude, and certain diseases (e.g., chronic obstructive pulmonary disease) (reviewed by Whitford 1996). It is also affected by renal function, because renal excretion is the primary route of fluoride elimination. Age and health status can affect renal excretion. Individuals with renal disease are of particular concern because their ability to excrete fluoride can be seriously inhibited, causing greater uptake of fluoride into their bones. However, the available data are insufficient to provide quantitative estimates of the differences between healthy individuals and people with renal disease.
Another category of individuals in need of special consideration includes those who are particularly susceptible or vulnerable to the effects of fluoride. For example, children are vulnerable for developing enamel fluorosis, because the condition occurs only when there is exposure while teeth are being formed (the pre-eruption stages). Thus, children up to the age of 8 are the susceptible subpopulation of concern for that end point. The elderly are another population of concern because of their long-term accumulation of fluoride into their bones. There are also medical conditions that can make people more susceptible to the effects of fluoride.
Relative Source Contribution
At the time the MCLG was established for fluoride, a reference dose was not available and the MCLG was calculated directly from available data rather than as an apportioned part of the reference dose. In Chapter 2, the committee shows that at 4 mg/L, drinking water is the primary contributor to total fluoride exposure, ranging from 72% to 94% for average-water-intake individuals and from 92% to 98% for high-water-intake individuals.
At 2 mg/L, drinking water contributes 57% to 90% for average-water-intake individuals and 86% to 96% for high-water-intake individuals. Thus, it is important that future revisions to the MCLG take into consideration that water is a significant, and sometimes the most significant, source of exposure to fluoride.
FINDINGS AND RECOMMENDATIONS
Maximum-Contaminant-Level Goal
In light of the collective evidence on various health end points and total exposure to fluoride, the committee concludes that EPA’s MCLG of 4 mg/L should be lowered. Lowering the MCLG will prevent children from developing severe enamel fluorosis and will reduce the lifetime accumulation of fluoride into bone that the majority of the committee concluded is likely to put individuals at increased risk of bone fracture and possibly skeletal fluorosis, which are particular concerns for subpopulations that are prone to accumulating fluoride in their bone.
Recommendation: To develop an MCLG that is protective of severe enamel fluorosis, clinical stage II skeletal fluorosis, and bone fractures, EPA should update the risk assessment of fluoride to include new data on health risks and better estimates of total exposure (relative source contribution) in individuals and to use current approaches to quantifying risk, considering susceptible subpopulations, and characterizing uncertainties and variability.
Secondary Maximum Contaminant Level
The prevalence of severe enamel fluorosis is very low (near zero) at fluoride concentrations below 2 mg/L. However, from a cosmetic standpoint, the SMCL does not completely prevent the occurrence of moderate enamel fluorosis. EPA has indicated that the SMCL was intended to reduce the severity and occurrence of the condition to 15% or less of the exposed population. The available data indicates that fewer than 15% of children would experience moderate enamel fluorosis of aesthetic concern (discoloration of the front teeth). However, the degree to which moderate enamel fluorosis might go beyond a cosmetic effect to create an adverse psychological effect or an adverse effect on social functioning is not known.
Recommendations: Additional studies, including longitudinal studies, of the prevalence and severity of enamel fluorosis should be done in U.S. communities with fluoride concentrations greater than
1 mg/L. These studies should focus on moderate and severe enamel fluorosis in relation to caries and in relation to psychological, behavioral, and social effects among affected children, among their parents, and among affected children after they become adults.
To better define the aesthetics of enamel fluorosis, methods should be developed and validated to objectively assess enamel fluorosis. Staining and mottling of the anterior teeth should be distinguished from staining of the posterior teeth so that aesthetic consequences can be more easily assessed.














