APPENDIX C
Trichloroethylene Metabolism
Knowledge of trichloroethylene metabolism is critical for determining susceptibility, target organ specificity, and gender and species differences and for extrapolating animal data to humans. Lash et al. (2000a) provided a comprehensive overview of the state of knowledge on trichloroethylene metabolism, and key aspects of that review are summarized in this appendix.
Liver is the major site of trichloroethylene metabolism, which occurs mainly through the oxidative and glutathione-dependent pathways. Most of the focus on the oxidative pathway has been on the liver, which has the highest activities of any tissue of the various isoforms of cytochrome P-450s (CYPs). CYP-mediated metabolites of trichloroethylene have been directly associated with liver injury. Trichloroethylene is metabolized primarily by CYP2E1 to a trichloroethylene oxide intermediate, which spontaneously rearranges to chloral. Chloral is further metabolized to trichloroethanol (except in lungs), trichloroethanol glucuronide, and trichloroacetic acid. Minor metabolites include carbon dioxide, dichloroacetic acid, oxalic acid, and N-(hydroxyacetyl)aminoethanol. The glutathione-dependent pathway yields glutathione conjugate, S-(1,2-dichlorovinyl)glutathione, which occurs predominantly in the liver and can also occur in extrahepatic tissues, but additional biotransformation of S-(1,2-dichlorovinyl)glutathione takes place in the kidney. Processing of S-(1,2-dichlorovinyl)glutathione through aminoacid-conserving mechanisms yields highly reactive S-(1,2-dichlorovinyl)thiol (alpha-chloroenethiolate) through the action of β-lyase and causes renal injury. Other minor metabolites of S-(1,2-dichlorovinyl)-L-cysteine may also contribute to renal injury.
ABSORPTION AND DISTRIBUTION
There are three types of exposures to consider for humans and laboratory animals: inhalation, oral, and dermal. Exposure is usually either from trichloroethylene vapor or from trichloroethylene in drinking water. In either form, trichloroethylene is rapidly and extensively absorbed through the lungs and gastrointestinal tract, and less so dermally. Absorbed trichloroethylene is then distributed to different target organs (e.g., lungs, liver, kidneys, nervous system) via the circulatory system. Trichloroethylene readily equilibrates from the circulation into richly perfused tissues, with reported partition coefficients for liver:blood or richly perfused tissue:blood for male rats of approximately 1.2 (Dallas et al. 1991; Fisher et al. 1991). Most trichloroethylene taken into the body is metabolized, but it can also be eliminated via exhalation.
Species differences exist in the fraction of administered dose of trichloroethylene that becomes available for conversion to toxic metabolites in the target organs because of differences in blood flow and overall metabolic rate. For example, blood concentrations of the three metabolites of trichloroethylene—chloral hydrate, trichloroethanol, and trichloroacetic acid— over time after administration of an oral dose of trichloroethylene at 1,000 mg/kg to male Osborne-Mendel rats and male B6C3F1 mice were markedly higher in mice than in rats, whereas concentrations for trichloroethylene were higher in rats than in mice, indicating more rapid metabolism and elimination of trichloroethylene (Prout et al. 1985). Similarly, higher peak plasma concentrations of trichloroacetic acid, the metabolite thought to be primarily responsible for liver effects (Bull 2000), were found in male and female mice than in male and female rats. These observations suggest species differences in susceptibility to the toxic effects of trichloroethylene.
PHARMACOKINETICS
Orally administered trichloroethylene is readily absorbed into the systemic circulation. In rats dosed with [36Cl]trichloroethylene by stomach tube (60 mg/kg), 90% to 95% of the radiolabel was recovered in expired air and urine (Daniel 1963). Administration of a range of doses of labeled trichloroethylene (10-2,000 mg/kg) to rats and mice yielded peak blood concentrations in 1 hour in mice and in 3 hours in rats (Dekant et al. 1984; Prout et al. 1985). Using classic pharmacokinetic analysis, D’Souza et al. (1985) reported that oral and intravenous bioavailability of trichloroethylene was 60% to 90% in nonfasted rats and greater than 90% in fasted rats. Peak blood concentrations occurred between 6 and 10 minutes and blood concentrations were two to three times higher in the fasted rats than in the nonfasted rats. Lee et al. (1996) showed that elimination of low doses
of trichloroethylene by metabolism was inversely related to dose and was nonlinear, suggesting that trace amounts of trichloroethylene in the drinking water might not enter the systemic circulation.
Trichloroethylene enters the systemic circulation rapidly after inhalation exposure (Fisher et al. 1991). Peak blood concentrations of trichloroethylene after exposure to trichloroethylene vapors are achieved in 1-2 hours in mice exposed at 100-750 parts per million (ppm), in 4-6 hours in rats exposed at 500-600 ppm, and in 8-12 hours in humans exposed at 100 ppm (Prout et al. 1985; Allen and Fisher 1993). Dermal absorption from exposure to trichloroethylene vapor is negligible, although direct skin contact with trichloroethylene could lead to significant absorption. Dermal absorption of dilute aqueous solutions of trichloroethylene in hairless guinea pigs indicates that significant absorption occurs (Bogen et al. 1992).
Trichloroethylene is metabolized rapidly in the systemic circulation. Physiologically based pharmacokinetic models of rodents and humans show that rodents have a greater capacity to metabolize trichloroethylene than humans (Lash et al. 2000a). Michaelis-Menten affinity constants (Km) for rodents and humans were estimated to be low (0.25-1.5 mg/L), reflecting a high substrate affinity.
Trichloroacetic acid has a much shorter plasma half-life in rodents than in humans (see Table C-1). The half-life of free trichloroethanol in blood is less than that in plasma for trichloroacetic acid in rodents and humans. The half-life of free trichloroethanol in blood is about 12 hours in humans exposed to trichloroethylene by inhalation (50 ppm) (Muller et al. 1972) and 3 hours in mice after oral intubation (1,200 mg/kg) (Abbas and Fisher
TABLE C-1 Plasma Half-life of Trichloroacetic Acid
|
Route |
Administered Dose/Concentration |
Species (sex) |
Half-Life (h) |
Reference |
|
Intravenous injection |
5-6 mg/kg TCA |
Rat (male) Rat (female) |
12 7 |
Fisher et al. 1991 |
|
Intraperitoneal injection |
5-10 mg/kg TCA |
Mice (male) Mice (female) |
7 3 |
Fisher et al. 1991 |
|
Inhalation |
42-889 ppm TCA |
Mice (male) Mice (female) |
16 7 |
Fisher et al. 1991 |
|
|
500-600 ppm TCA |
Rat (male and female) |
15 |
Fisher et al. 1989 |
|
|
50 or 100 ppm TCE |
Human |
86-99 |
Fisher et al. 1998 |
|
ABBREVIATIONS: TCA, trichloroacetic acid; TCE, trichloroethylene. |
||||
1997). Chloral hydrate and trichloroethanol glucuronide are readily measured in the blood of exposed mice (Abbas and Fisher 1997) but not in humans (Fisher et al. 1998).
Pathways of Metabolism
Although many enzymes that catalyze specific steps of the metabolic pathway are widely distributed, a range of activities of specific isozymes are found in different target tissues as well as in a given tissue in males and females from various species.
The first step in trichloroethylene metabolism (Figure C-1) is either conjugation with glutathione or oxidation by CYPs. The oxidative pathway is the major pathway for trichloroethylene metabolism, which occurs primarily in the liver, although different amounts and isoforms of CYP are present in most tissues.
Trichloroethylene oxide metabolite is formed as a result of the action of CYPs, primarily CYP2E1. However, Miller and Guengerich (1982, 1983) and Cai and Guengerich (2001) concluded that trichloroethylene epoxide is not an intermediate in the formation of chloral and chloral hydrate. Therefore, trichloroethylene epoxide cannot be the intermediate responsible for irreversible binding to protein and DNA. Stable lysine adducts were formed in proteins following reaction with trichloroethylene oxide. N(6)-Formyllysine, N(6)-(dichloroacetyl)lysine, and N(6)-glyoxyllysine were formed, with the ratio being influenced by the particular protein (Cai and Guengerich 2000). The majority of the protein adducts (~80%) formed had a collective half-life of only an hour (Cai and Guengerich 2001). These studies also indicate that rat CYP2B1 is more likely to oxidize trichloroethylene to form trichloroethylene oxide and protein lysine adducts than human CYP2E1. The difference is thought to result from the influence of the protein on chloride migration in an enzyme reaction. Trichloroethylene oxide forms adducts with proteins and 2′-deoxyguanosine but not with the other three nucleosides found in DNA (Cai and Guengerich 2001). Approximately 2% of trichloroethylene oxide is adducted with 2′-deoxyguanosine. During the reaction of trichloroethylene oxide with a synthetic 8-mer oligonucleotide, these adducts were short lived having a half-life of only 30 minutes at a of pH 8.5, suggesting the transient nature of these adducts formed from the reaction of trichloroethylene oxide with macromolecules. Green and Prout (1985) also concluded that there was little evidence to support the formation of an epoxide intermediate in the oxidative metabolism of trichloroethylene in rat and mouse liver microsomes. However, Forkert and co-workers (Dowsley et al. 1996; Forkert 1999; Forkert et al. 1999) provided substantial evidence that a compound similar to trichloroethylene, 1,1- dichloroethylene, is metabolized by CYPs to an epoxide intermediate. Table C-2 provides Km
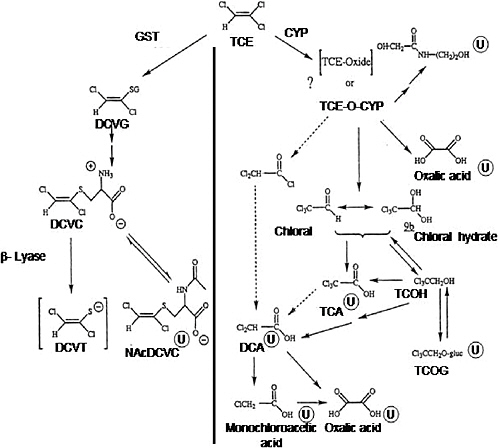
FIGURE C-1 Metabolism of trichloroethylene. Metabolites marked with ![]() are known urinary metabolites. Arrows with broken lines indicate other possible steps in forming DCA. Abbreviations: CYP, cytochrome P-450; DCA, dichloroacetic acid; DCVC, S-(1,2-dichlorovinyl)-L-cysteine; DCVG, S-(1,2dichlorovinyl)glutathione; DCVT, S-(1,2-dichlorovinyl)thiol; GST, glutathione S-transferase; NAcDCVC, N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine; TCA, trichloroacetic acid; TCE, trichloroethylene; TCE-O-CYP, trichloroethyleneoxide-cytochrome P-450 complex; TCOH, trichloroethanol; TCOG, trichloroethanol glucuronide.
are known urinary metabolites. Arrows with broken lines indicate other possible steps in forming DCA. Abbreviations: CYP, cytochrome P-450; DCA, dichloroacetic acid; DCVC, S-(1,2-dichlorovinyl)-L-cysteine; DCVG, S-(1,2dichlorovinyl)glutathione; DCVT, S-(1,2-dichlorovinyl)thiol; GST, glutathione S-transferase; NAcDCVC, N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine; TCA, trichloroacetic acid; TCE, trichloroethylene; TCE-O-CYP, trichloroethyleneoxide-cytochrome P-450 complex; TCOH, trichloroethanol; TCOG, trichloroethanol glucuronide.
SOURCE: Adapted from Lash et al. 2000a.
and Vmax values for oxidative metabolism of trichloroethylene in mice, rats, and human microsomal incubations.
Four CYP isoforms are thought to play a role in trichloroethylene metabolism: CYP1A1/2, CYP2B1/2, CYP2C11/6, and CYP2E1 (Guengerich and Shimada1991; Nakajima et al. 1993; Lash et al. 2000a). CYP2E1 is the major form with the highest affinity for trichloroethylene (Guengerich
TABLE C-2 Kinetic Constants for Total Oxidative Metabolite Formation from Trichloroethylenea
and Shimada 1991), although the relative roles of the different isoforms can vary depending on physiologic state and the presence of other drugs or inducing agents.
After formation of the trichloroethylene-oxide-CYP, chloral hydrate is the metabolite produced. Because chloral hydrate is rapidly converted to other compounds in the liver, this metabolite is unlikely to be a major contributor to hepatotoxicity or hepatocarcinogenicity. In the lung, however, chloral is the metabolite found, primarily in Clara cells. Oxalic acid, N-(hydroxyacetyl)aminoethanol, and dichloroacetic acid also might be formed from the trichloroethylene-oxide-CYP intermediate (Figure C-1).
Chloral hydrate is further metabolized to either trichloroethanol or trichloroacetic acid, both of which can be further oxidized to dichloroacetic acid. Some formation of dichloroacetic acid directly from trichloroacetic acid has been observed (Abbas et al. 1996). Trichloroethanol also undergoes glucuronidation to form trichloroethanol glucuronide, which can also undergo enterohepatic recirculation and regenerate trichloroethanol.
Chloral hydrate reduction to trichloroethanol has been reported to be inhibited by ethanol, which suggests that this reaction is catalyzed by alcohol dehydrogenase (Muller et al. 1975; Larson and Bull 1989). However, Ni et al. (1996) suggested that metabolism of chloral hydrate to trichloroethanol and trichloroacetic acid is catalyzed primarily by CYP2E1. Furthermore, Shultz and Weiner (1979) found that a human lymphoblastoid cell line expressing CYP2E1 metabolized chloral hydrate to mutagenic metabolites, whereas a cell line lacking CYP2E1 expression was inactive in chloral hydrate metabolism (Shultz and Weiner 1979). The proposed pathway for the formation of the mutagenic metabolites appears to be similar in the transfected human lymphoblastoid cell line and in mouse liver microsomes. Both alcohol dehydrogenase and CYP2E1 are likely involved in trichloroethanol formation in mouse liver (Larson and Bull 1989; Lipscomb et al. 1996). Lipscomb et al. (1996) suggested that, at higher substrate concentrations, a lower-affinity enzyme becomes largely responsible for chloral hydrate reduction. However, similar findings have not been demonstrated in rat or human liver cytosol.
Trichloroacetic and Dichloroacetic Acids
Trichloroacetic acid is produced by oxidation of either chloral hydrate or trichloroethanol. Chloral hydrate oxidation is thought to be catalyzed by an aldehyde oxidase, whereas trichloroethanol oxidation is catalyzed predominantly by CYP (Ni et al. 1996). Subsequent reactions of chloral hydrate occur rapidly in the liver, producing trichloroethanol and trichloroacetic acid. This is consistent with trichloroacetic acid being derived from both chloral hydrate and trichloroethanol oxidation (Green and Prout 1985; Dekant et al. 1986a; Larson and Bull 1992a ; Templin et al. 1995).
There are marked differences in chloral hydrate metabolism to trichloroethanol and trichloroacetic acid in liver and blood of rats, mice, and humans (Lipscomb et al. 1996). Kinetic parameters at physiologically obtainable concentrations of chloral hydrate (in the range of 50 µM) showed that chloral hydrate is cleared from human blood through hepatic metabolism at approximately 60% of the rate observed in rodents. Thus, larger amounts of chloral hydrate might be present in human blood and tissues than in rodents after a given exposure to trichloroethylene.
Dichloroacetic acid formation (Figure C-1), particularly in humans, has been a controversial issue. This metabolite was detected in the urine of rats and mice treated with trichloroacetic acid and in blood of mice treated with trichloroethylene (Larson and Bull 1992a,b; Templin et al. 1993). However, problems with analytic methodologies appear to have led to overestimation of dichloroacetic acid formation (Ketcha et al. 1996). It appears that, in the presence of strong acids, some of the trichloroacetic acid in whole blood can undergo nonenzymatic conversion to dichloroacetic acid, leading to overestimation of dichloroacetic acid formation. Templin et al. (1995) found measurable concentrations of dichloroacetic acid in blood from mice but not in rats or dogs. Henderson et al. (1997) identified dichloroacetic acid in children treated therapeutically with chloral hydrate, but whether dichloroacetic acid is formed under nonclinical exposure situations is unclear.
Other Oxidative End Products
Dichloroacetic acid is further metabolized to other species, including oxalic acid, monochloroacetic acid, glycolic acid, and glyoxylic acid (Lash et al. 2000a; Saghir and Schultz 2002). Metabolism (or excretion) of dichloroacetic acid appears to be rapid, as its half-life is much shorter than that of trichloroacetic acid in rats and mice (Larson and Bull 1992a). Degradation of dichloroacetic acid appears to be CYP independent (Tong et al. 1998; Saghir and Schultz 2002). As noted by Lash et al. (2000a), the significance of these metabolites in trichloroethylene-induced toxicity and carcinogenesis is likely to be quantitatively minor.
TISSUE DISTRIBUTION OF OXIDATIVE METABOLISM
The liver is the most important site of oxidative metabolism of trichloroethylene because of its size, abundance of enzymes, and high blood flow. Oxidative metabolism also occurs in the kidneys but at much lower rates than in the liver (Lash et al. 2000a). Interorgan metabolism can affect the biotransformation of trichloroethylene metabolites and target organ toxicity. For example, trichloroethanol glucuronide formed in the liver and excreted into bile can return to the liver by enterohepatic circulation, where it might be hydrolyzed back to trichloroethanol and metabolized to trichloroacetic acid or dichloroacetic acid. Trichloroacetic acid is the major circulating metabolite of trichloroethylene. Concentrations of this metabolite in the blood of mice show a biphasic pattern (Prout et al. 1985), consistent with enterohepatic circulation of trichloroethanol. Renal-hepatic circulation is responsible for excretion of metabolites in the urine, such as trichloroacetic acid and trichloroethanol or trichloroethanol glucuronide.
SEX- AND SPECIES-DEPENDENT OXIDATIVE METABOLISM
Trichloroethylene metabolism differs considerably among species (Lipscomb et al. 1997; Verma and Rana 2003). Blood concentrations of the metabolites trichloroethanol, chloral hydrate, and trichloroacetic acid were found to be severalfold higher in B6C3F1 mice than in Osborne-Mendel rats after acute exposure. Larson and Bull (1992a) reported that metabolism of trichloroethylene to trichloroacetic acid was much higher in mice than in rats, leading to higher blood concentrations of trichloroacetic acid in mice. Rates of oxidative metabolism of trichloroethylene in liver microsomes of male B6C3F1 mice were 2- to 3-fold higher, depending on the dose, than in those from male Wistar rats (Nakajima et al. 1993). Kinetic parameters for total oxidative metabolite formation in liver microsomes from trichloroethylene-treated male B6C3F1 mice, F344 rats, and humans are shown in Table C-2, which indicates that the kinetics are biphasic in liver microsomes from rats and humans but monophasic in liver microsomes from mice. Oxidative metabolism rates were 2- to 2.5-fold faster for high-affinity than for low-affinity processes in rats and humans. Although catalytic rates in humans are lower than in other species, the activity in humans exhibits the same efficiency as that in rats and mice. The rat appears to be a better model than the rabbit for studying human CYP2E1 expression and its role in metabolism of xenobiotics (Wrighton and Stevens 1992; Lipscomb et al. 1998).
Sex-dependent differences in susceptibility to trichloroethylene-induced toxicity and carcinogenicity have been reported, but few studies of metabolic differences between sexes have been conducted. One study that investigated the influence of sex, age, and pregnancy on the expression and regulation of CYP2E1 and CYP2C1I in Wistar rats found no sex-dependent differences but reported a 3-fold decrease in trichloroethylene metabolism to chloral hydrate between the ages of 3 and 18 weeks and a 2-fold decrease during pregnancy (Nakajima et al. 1992b).
GLUTATHIONE-DEPENDENT METABOLISM
The other possible fate of trichloroethylene besides oxidative metabolism is conjugation with glutathione, which is catalyzed by glutathione S-transferases (Figure C-1). Trichloroethylene is metabolized in rats and mice to S-(1,2-dichlorovinyl)glutathione both in vivo and in isolated liver microsomes: S-(1,2-dichlorovinyl)glutathione appears in bile and N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine appears in the urine after exposure to trichloroethylene (Stenner et al. 1997, 1998). Glutathione conjugation of trichloroethylene occurs at rates that are generally severalfold slower than the CYP-catalyzed oxidation reactions. Conversion to the cysteine conju-
gate is a critical step for subsequent formation of cytotoxic or carcinogenic metabolites.
Metabolism of S-(1,2-Dichlorovinyl)glutathione to S-(1,2-Dichlorovinyl)-L-cysteine
S-(1,2-Dichlorovinyl)glutathione is metabolized by γ-glutamyltransferase to the cysteinylglycine conjugate S-(l,2-dichlorovinyl)-L-cysteinylglycine and then to S-(1,2-dichlorovinyl)-L-cysteine (Lash et al. 1988). The requirement of γ-glutamyltransferase for hydrolysis of S-(1,2-dichlorovinyl)gluta thione was demonstrated with acivicin, a potent and irreversible inhibitor of γ-glutamyltransferase, and with a cosubstrate of the enzyme (Elfarra and Anders 1984; Elfarra et al. 1986). Similarly, inhibitors of dipeptidase and ß-lyase activity prevented or greatly diminished cytotoxicity in vitro or nephrotoxicity in vivo of chemicals that occur before the inhibited step (Elfarra and Anders 1984; Elfarra et al. 1986; Lash and Anders 1986).
S-(1,2-Dichlorovinyl)-L-cysteine is metabolized further by multiple enzymes to yield detoxification products that are excreted and reactive species that are associated with nephrotoxicity and possibly associated with nephrocarcinogenicity.
Metabolism of S-(1,2-Dichlorovinyl)-L-cysteine to N-Acetyl-S-(1,2-dichlorovinyl)-L-cysteine
S-(1,2-Dichlorovinyl)-L-cysteine is N-acetylated by a cysteine S-conjugate N-acetyltransferase to form the mercapturate metabolite N-acetyl-S(1,2-dichlorovinyl)-L-cysteine (Duffel and Jacoby 1982). This metabolite has been detected in the urine of rats (Commandeur and Vermeulen 1990; Larson and Bull 1992a; Birner et al. 1993), mice (Birner et al. 1993), and humans (Commandeur and Vermeulen 1990; Birner et al. 1993: Brüning et al. 1998) exposed to trichloroethylene. The cysteine conjugate S-(1,2dichlorovinyl)-L-cysteine can be regenerated when N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine is deacetylated intracellularly. Thus, the relative rates of N-acetylation and deacetylation determine the fraction of the mercapturate that can produce toxic metabolites. In vitro studies indicate that S-(1,2dichlorovinyl)-L-cysteine is rapidly transported into the tubules and is converted to N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine or reactive species covalently bound to cellular proteins (Zhang and Stevens 1989). A larger proportion of S-(1,2-dichlorovinyl)-L-cysteine was bound than N-acetyl-S(1,2-dichlorovinyl)-L-cysteine, suggesting that deacetylation of N-acetyl-S(1,2-dichlorovinyl)-L-cysteine is relatively slow. Metabolism in the liver also contributes to circulating concentrations of S-(1,2-dichlorovinyl)-L-cysteine
and N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine. S-(1,2-Dichlorovinyl)glutath ione excreted into the bile is converted to S-(1,2-dichlorovinyl)-L-cysteine, and some is converted to N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine in the liver and then excreted into the plasma and translocated to the kidneys.
S-(1, 2-Dichlorovinyl)-L-cysteine is metabolized by cytosolic and mitochondrial forms of the enzyme cysteine-S-conjugate β-lyase (Anders et al. 1987; Darnerud et al. 1991; Eyre et al. 1995b; Koechel et al. 1991) (Figure C-1). In the rat kidney, β-lyase exists as a cytosolic glutamine transaminase K isoform (Stevens et al. 1986), while in the liver the kynureninase form of this enzyme is more predominant (Stevens 1985). Similar isoforms of β-lyase have also been reported in mitochondrial fractions of brain tissue (Cooper 2004). Renal metabolism of S-(1, 2-dichlorovinyl)-L-cysteine via cysteine conjugate β-lyase yields a reactive thiol, S-(1, 2-dichlorovinyl)thiol (Figure C-1). This thiol converts to reactive species that alkylate and form covalent cellular nucleophiles, including proteins (Dekant et al. 1988). Net activation rates of trichloroethylene by the β-lyase pathway were greater in mice than in rats (Eyre et al. 1995a,b). The formation of additional products from trichloroethylene S-conjugates is described in Chapter 3, including acetylated derivatives and products that are independent of β-lyase activity.
Glutathione-Dependent Metabolism: Tissue, Sex, and Species Differences
The liver and kidneys are the major sites of glutathione conjugation. Because trichloroethylene undergoes glutathione conjugation and is translocated to the kidneys for further metabolism, most glutathione conjugation is thought to occur in the liver. Data on the formation of S-(1,2-dichlorovi nyl)glutathione from trichloroethylene in liver and kidney cytosol and microsomes of rats and mice are shown in Table C-3. Glutathione conjugation in rats is higher in males than in females. However, measurements of the subcellular fractions found no detectable S-(1,2-dichlorovinyl)glutathione in the liver microsomes of male rats but 0.6 nmol per mg of protein in those of female rats (Lash et al. 2000a). In mice, metabolism rates were generally faster in males than in females and were higher than in rats. Higher rates of adduct formation (Eyre et al. 1995a,b) and glutathione conjugation (Lash et al. 2000a) of trichloroethylene occur in the mouse kidney.
HUMAN STUDIES
Metabolism of trichloroethylene appears to be substantially less efficient in humans than in rodents (Lash et al. 2000a). Few of the major metabolites of trichloroethylene have been characterized pharmacokinetically in hu-
TABLE C-3 Summary of Metabolism of Trichloroethylene by Glutathione Conjugation in Kidney and Liver Subcellular Fractions from Male and Female F344 Rats and B6C3F1 Micea
|
|
S-(1,2-Dichlorovinyl)glutathione Formation (nmol/mg of protein or 106 cells per 60 min) |
|
|
Subcellular fractions |
Male |
Female |
|
Rat kidney cells |
0.48 ± 0.02 0 |
.65 ± 0.15 |
|
Rat hepatocytes |
9.70 ± 0.29b |
2.67 ± 0.69 |
|
Rat kidney cytosol |
0.32 ± 0.02d |
|
|
Rat kidney microsomes |
0.61 ± 0.06 |
|
|
Rat liver cytosol |
7.30 ± 2.80c |
4.86 ± 0.14d |
|
Rat liver microsomes |
10.3 ± 2.8c |
7.24 ± 0.24 |
|
Mouse kidney cytosol |
5.60 ± 0.24d |
3.70 ± 0.48d |
|
Mouse kidney microsomes |
5.47 ± 1.41b |
16.7 ± 4.7 |
|
Mouse liver cytosol |
24.5 ± 2.4d |
21.7 ± 0.9 |
|
Mouse liver microsomes |
40.0 ± 3.1b |
25.6 ± 0.8 |
|
aResults are means ± standard error of measurements from three separate cell or tissue preparations incubated with trichloroethylene at 2 mM, with glutathione at 5 mM for 60 min. S-(1,2-Dichlorovinyl)glutathione formation was measured after derivatization of acid extracts with iodoacetate and 1-fluoro-2,4-dinitrobenzene, separation by ion-exchange gradient highperformance liquid chromatography on an amine column using a methanol-acetate mobile phase and detection of N-dinitrophenyl S-(1,2-dichlorovinyl)glutathione at 365 nm. bSignificantly different (P < 0.05) from S-(1,2-dichlorovinyl)glutathione formation in same species and tissue sample in females. cSignificantly different (P < 0.05) from S-(1,2-dichlorovinyl)glutathione formation in corresponding sample in mice of same sex. dSignificantly different (P < 0.05) from S-(1,2-dichlorovinyl)glutathione formation in microsomes from same sex, species, and tissue. ABBREVIATIONS: ND, not detectable; limit of detection was 0.05 nmol per mg protein or 106 cells. SOURCE: Lash et al. 1998. |
||
mans. Fisher et al. (1998) reported that the plasma half-life of trichloroacetic acid in humans ranges from 86 to 99 hours after short-term inhalation of trichloroethylene at 50 or 100 ppm. Oral studies of trichloroethanol (10 mg/kg) and chloral hydrate (15 mg/kg) indicate a plasma half-life of 63 to 65 hours for the metabolite trichloroacetic acid. The plasma half-life for trichloroacetic acid was 51 hours after exposure to trichloroacetic acid alone (3 mg/kg) (Allen and Fisher 1993). Chloral hydrate, oxalic acid, N-(hydro xyacetyl)aminoethanol, and dichloroacetic acid have been recovered in the urine of humans exposed to trichloroethylene. The glucuronidation product of trichloroethanol has been recovered in the urine of humans exposed to
trichloroethylene. Trichloroethanol glucuronide can undergo enterohepatic recirculation and regenerate trichloroethanol. There are marked differences in chloral hydrate metabolism to trichloroethanol and trichloroacetic acid in human liver and blood (Lipscomb et al. 1996). Chloral hydrate clearance from human blood through hepatic metabolism is 40% lower than in rodents, suggesting that there may be larger amounts of chloral hydrate in human blood and tissues than in those of rodents.
Lipscomb et al. (1997) reported considerable variability in CYP-catalyzed oxidation of trichloroethylene in human liver microsomes. Individual samples seemed to cluster into three groups with Km values of 16.7 ± 2.5, 30.9 ± 3.3, and 51.1 ± 3.8 µM. Km and Vmax values in the groups did not differ among ethnic groups, but the Km value in females (21.9 ± 3.5 µM) was significantly lower than in males (33.1 ± 3.5 µM).
Table C-4 shows Km values for CYP1A2, CYP2E1, and CYP3A4, the three isoforms known to catalyze trichloroethylene metabolism in human liver microsomes. CYP1A2 activity was lowest in the low Km group, and CYP2E1 activity was highest in the high Km group. There were no significant differences in CYP3A4 activity among the groups (Lash et al. 2000a). CYP2E1 accounts for more than 60% of total microsomal metabolism (Lipscomb et al. 1997, 2003), which indicates that the capacity of humans to metabolize trichloroethylene varies considerably and that factors that alter P-450 activity, particularly CYP2E1 activity, can alter susceptibility to trichloroethylene-induced toxicity. Nakajima et al. (1992b) also reported significant variation in oxidative metabolism of trichloroethylene as a function of physiologic state. The contributions of CYP1A2 and CYP3A4 are low compared with that of CYP2E1 (Shimada et al. 1994).
TABLE C-4 Evaluation of Selective CYP-Mediated Metabolism in Human Liver Microsomes Expressing Different Km Values for Trichloroethylene Metabolism
SPECIES DIFFERENCES
CYP-Dependent Metabolism of Trichloroethylene
In vitro data on metabolite parameters for the oxidative metabolism of trichloroethylene are presented in Table C-5. The data show that humans are less capable than rodents of metabolizing trichloroethylene and chloral hydrate. Formation of chloral hydrate is approximately 20% less in humans than in mice. Dichloroacetic acid formation was not demonstrated in any tissue fraction.
Chloral Hydrate Formation
Humans metabolize trichloroethylene to chloral hydrate at a slower rate than rats or mice (Table C-6) (Lash et al. 2000a). Kinetic parameters in individual human liver samples varied considerably (Km values of 16-56 µM and Vmax values of 490-3,455 pmol/min/mg) (Lipscomb et al. 1997). No correlation was found between Km and Vmax values among the individual samples.
A concentration-dependent inhibition of CYP2E1 activity was found in human liver microsomes from low, mid, and high Km individuals. Inhi-
TABLE C-5 Comparison of Metabolic Parameters for Oxidative Metabolism of Trichloroethylene Determined in Vitro
|
Metabolic Step |
Mouse |
Rat |
Human |
|
Trichloroethylene to chloral hydrate |
|
|
|
|
Km (µM trichloroethylene) |
35.4 |
55.5 |
24.6 |
|
Vmax (nmol/min/mg of microsomal protein) |
5,425 |
4826 |
1440 |
|
Chloral hydrate to trichloroethanol |
|
|
|
|
Km (mM chloral hydrate) |
0.12 (low affinity) 0.51 (high affinity) |
0.52 |
1.34 |
|
Vmax (nmol/min/mg of supernatant protein) |
6.3 (low affinity) 6.1(high affinity) |
24.3 |
34.7 |
|
Vmax/Km |
52.5 (low affinity) 12.0 (high affinity) |
46.7 |
25.9 |
|
Chloral hydrate to trichloroacetic acid |
|
|
|
|
Km (mM chloral hydrate) |
3.5 |
6.4 |
23.9 |
|
Vmax (nmol/min/mg of supernatant protein) |
10.6 |
4.0 |
65.2 |
|
Vmax/Km |
3.03 |
0.24 |
2.73 |
|
Dichloroacetic acid degradation |
|
|
|
|
Km (mM dichloroacetic acid) |
0.350 |
0.280 |
0.071 |
|
Vmax (nmol/min/mg of cytosolic protein) |
13.1 |
11.6 |
0.37 |
|
Vmax/Km |
37.4 |
41.4 |
5.2 |
|
SOURCE: Lash et al. 2000a. |
|||
TABLE C-6 Overall Kinetics of Trichloroethylene Metabolism to Chloral Hydrate and Trichloroethanol
bition paralleled the Km for trichloroethylene metabolism in microsomal incubations, suggesting that CYP2E1 contributes more to trichloroethylene metabolism in the high Km sample than in the lower Km samples (Lash et al. 2000a).
Trichloroethanol and Trichloroacetic Acid Formation
The formation of trichloroethanol from chloral hydrate is substantially greater (10- to 200-fold) than the formation of trichloroacetic acid in rodents and humans (Lash et al. 2000a). Formations of trichloroacetic acid in mice and humans were similar, whereas it was approximately 10fold lower in rats. Thus, trichloroethanol formation will predominate over trichloroacetic acid formation in the liver of these species. It appears that the mice might have two enzymes responsible for forming trichloroethanol from chloral hydrate in liver, whereas rats and humans have a single enzyme. Pravecek et al. (1996) found that at least one mouse cytosolic enzyme responsible for trichloroethanol formation becomes inhibited at high chloral hydrate concentrations.
When hepatic concentrations of chloral hydrate are low (below 0.5 mM), it is preferentially metabolized to trichloroethanol (Sellers et al. 1978; Dekant et al. 1986a; Larson and Bull 1992a). Lumpkin et al. (2003) found that plasma binding of trichloroacetic acid is higher in humans than in rats and mice. Greater plasma binding in humans would be expected to increase the residence time of trichloroacetic acid but to decrease its availability for hepatic uptake in humans.
Chloral Hydrate Metabolism by Blood
In vitro studies of blood metabolism of chloral hydrate show that more trichloroethanol than trichloroacetic acid is produced (Sellers et al. 1972).
More trichloroethanol was produced in rat and mouse blood than in human blood. However, trichloroacetic acid production in lysed human blood was significantly greater than production in rat blood and slightly higher than in mouse blood. Erythrocytes were the site of trichloroacetic acid production. Plasma is the primary site of trichloroethanol production by blood.
Dichloroacetic Acid Metabolism
Dichloroacetic acid is a trichloroethylene metabolite found in rodents but is generally not identifiable in humans. Dichloroacetic acid degradation occurs primarily in rat hepatic cytosol (Lipscomb et al. 1995). Km values for dichloroacetic acid were 350, 280, and 71 µM and Vmax values were 13.1, 11.6, and 0.37 nmol/min/mg of protein in mice, rats, and humans, respectively (Lipscomb et al. 1995). Clearance values indicate that degradation of dichloroacetic acid in liver cytosol is less efficient in humans than in rodents.
Glutathione-Dependent Metabolism of Trichloroethylene
Glutathione conjugation of trichloroethylene is much slower than CYPcatalyzed oxidation reactions. Lash et al. (1999) exposed human volunteers (n = 21) to trichloroethylene vapors at 50, 60, or 100 ppm for 4 hours and measured metabolites produced by the glutathione pathway in blood and urine samples. S-(1,2-Dichlorovinyl)glutathione was detected in blood within 30 minutes after exposure was completed, and it persisted in the blood for up to 12 hours. Concentrations of S-(1,2-dichlorovinyl)glutathione were higher in males than in females. Formation of S-(1,2-dichlorovinyl)glutathi one is the initial step in the generation of nephrotoxic metabolites and does not directly correlate with it because subsequent detoxification reactions, such as mercapturate formation, can still occur (see Chapter 3 for specific details).
The rate of glutathione conjugation has been found to vary by as much as 3.4-fold in liver cytosol obtained from 9 males and 11 females and by as much as 8.5-fold in microsomes from 5 males and 15 females (Lipscomb et al. 2003). No significant sex-dependent differences in mean activity values were found, but the degree of variation was greater in microsomes from females (7.4-fold) than in males (4.1-fold). Although the overall degree of variation in glutathione S-transferase activity is significantly less than that observed with P-450 activity, it could be another factor to account for in a human health risk assessment of trichloroethylene.
















