10
Climatic Significance of Lake and Evaporite Deposits
HANS P.EUGSTER
The Johns Hopkins University
INTRODUCTION
Lake deposits and evaporites are sensitive indicators of local climatic conditions and changes in climate—lakes because they are ephemeral and owe their existence to a delicate balance between hydrology, sedimentation, and tectonics and evaporites because they represent extreme stages of desiccation. The very aspects that make these deposits valuable for our purposes are also responsible for some of the difficulties encountered in extracting climatic inferences. Few lake deposits are large enough to have attracted the general attention of geologists, notable exceptions being Devonian Lake Orcadie (Old Red Sandstone), Triassic Lake Lockatong, and Eocene Lakes Gosiute and Uinta (Green River Formation). The lacustrine record in the geologic column has yet to be tapped, although a fine summary of paleolimnology in North America has been provided (Bradley, 1963).
Evaporite deposits, especially of marine derivation, have been studied extensively, if not exhaustively, in part because of their economic significance (see recent summaries by Dean and Schreiber, 1978; Holser, 1979). Evaporite minerals respond readily to postdepositional changes. Because depositional and postdepositional processes form a continuum, mineral textures and sedimentary structures must be interpreted with great care.
LAKE DEPOSITS
The study of ancient lake deposits has been helped immeasurably by limnologists, sedimentologists, and geochemists, who make observations on active lakes, and by geologists, who have concerned themselves with Pleistocene lakes and their Holocene residues. Paleoclimatic information may be contained in the nature of the basin itself as well as in the material that filled it. Every lake deposit is testimony to at least two important environmental changes, one initiating lacustrine deposition and the other terminating it. Climatic factors may be involved in either or both events. For instance, glacial lakes often form during a warming trend, and they can be recognized from the shape of the basins and the fact that many are dammed by moraines. Tectonic events unrelated to overall climatic change may lead to steepened hydraulic gradients and increased precipitation in the watershed and hence earlier filling of the
basin. Choking with sediment probably is the most common death of lakes, but lakes can also dry up. In that case, a clear record is usually left in the sediment in the form of an evaporitic terminal stage.
Reliable reconstruction of depositional environments of lake sediments, a prerequisite for extracting climatic inferences, depends on a whole array of geologic, geochemical, and geophysical tools. Most of our experiences have been gained from Holocene-Pleistocene settings (see e.g., Gilbert, 1890; Hansen, 1961; Jones, 1965; Neev and Emery, 1967; Anderson and Kirkland, 1969; Brunskill, 1969; Ludlam, 1969; Irion, 1970, 1973; Müller et al., 1972; Degens et al., 1973; Eardley et al., 1973; Dean and Gorham, 1976; Eugster and Hardie, 1976, 1978; Friedman et al., 1976; Hardie et al., 1978; Jones and Bowser, 1978; Kelts and Hsü, 1978; Lerman, 1978; Matter and Tucker, 1978; Müller and Wagner, 1978; Smith, 1978, 1979; Stoessel and Hay, 1978; Stoffers and Hecky, 1978; Cerling, 1979; Yuretich, 1979; Bradbury and Whiteside, 1980; Eugster, 1980; McKenzie et al., 1981; Sims et al., 1981; Spencer et al., 1981a, 1981b; Smith et al., in press). Once the stratigraphic time lines have been established for a particular basin, using traditional approaches as well as radiogenic dating, tephrochronology, paleomagnetics, amino acid racemization, and other methods, facies maps are constructed. Information on sediment type, sedimentary structures, mineralogy, isotopic
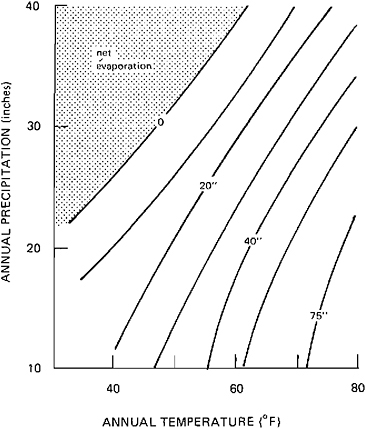
FIGURE 10.1 Correlation between annual precipitation, net evaporation (evaporation from lake surface-precipitation on lake surface), and mean annual temperature for closed-basin lakes. From Langbein (1961).
compositions, biota (such as ostracodes, diatoms, algae, molluscs, and fish), and pore-water chemistry are combined in a detailed reconstruction of the depositional environments and their evolution in space and time. Comparisons with adjacent basins may then make it possible to separate local changes due to hydrology or tectonics from regional climatic trends.
Such studies are difficult at best and are most likely to succeed in Holocene-Pleistocene basins, where one moment in time can be studied directly. For older deposits there often exists doubt whether they are in fact lacustrine or not. Such deposits can usually be identified as such from lithologies, sedimentary structures, and fossil content. Associated rock units formed immediately preceding or contemporaneously with lake deposits may also be good indicators. For instance, the Green River Formation is defined as a lens of lacustrine deposits following and interbedded with the unquestionably continental and largely fluviatile Wasatch formation. Among evaporites, lacustrine deposits can most readily be identified if their chemistry was alkaline, an evolutionary path not accessible to seawater derivatives (Hardie and Eugster, 1970).
The mere existence of lakes in a particular locality contains little climatic information, because lakes can exist from arctic to tropical environments. Futhermore, many lakes are formed by nonclimatic events, including tectonic graben lakes, oxbow lakes (channel migration), volcanic lakes, and landslide lakes. Most useful will be glacial lakes and evaporitic lakes. We will focus on the latter; glacial deposits are treated elsewhere (Chapter 6). Geologic and geochemical work on saline lakes has been summarized recently (Eugster and Hardie, 1978; Hardie et al., 1978; Eugster, 1980).
SALINE LAKES
Saline lakes form in hydrologically closed basins under conditions where evaporation exceeds inflow. They respond to climatic changes, and hence their deposits contain excellent records of such changes. Most published studies have dealt with Pleistocene-Holocene examples such as Searles Lake (Smith, 1979), Lake Bonneville-Great Salt Lake (Eardley et al., 1973), or Lake Lisan-Dead Sea (Begin et al., 1974).
A classic study of climatic parameters that define closed-basin lakes was presented by Langbein (1961). Figure 10.1 shows the interrelations between temperature, precipitation, and net evaporation. In response to changes in the inflow-evaporation balance, closed takes go through cycles with respect to lake level, areal extent, volume, and salinity; such cycles are reflected in their deposits in a variety of ways. Transgressive-regressive events may be recognized by sedimentary structures and changes in lithologies. Good examples are the depositional cycles described by Eugster and Hardie (1975) from the Wilkins Peak Member of the Green River formation. Transgression is indicated by flat-pebble conglomerates that are usually followed by oil shales, indicating a higher lake stand. Oil shales gradually give way to mudflat facies rocks in response to falling lake levels. Extreme regression is indicated by evaporite accumulation. In reading this record we must be conscious of the many ways in which the inflow-evaporation
balance can be effected: tectonic changes, hydrology, and climate all playing their part.
In fresh lakes, the mineralogy of the sediments is usually inherited from the bedrock lithologies of the watershed (Jones and Bowser, 1978). In contrast, progressive desiccation of a closed basin brings with it profound changes in the chemistry of the waters and hence also the chemical sediments (Eugster and Jones, 1979). Some of these changes may be useful as indicators of the onset of arid conditions even when evaporite deposition never took place. Perhaps the most useful minerals are the alkaline earth carbonates calcite, aragonite, and dolomite. In general, because CaCO3 precipitation is related to evaporative concentration, increasing salinity increases the Mg/Ca ratio of the water. In response, the principal carbonate precipitate may change from low-Mg calcite to high-Mg calcite and eventually aragonite. Good examples have been described by Müller from a variety of Holocene and Pleistocene lakes (for example, Lake Balaton, Müller and Wagner, 1978). Great Salt Lake, Utah, near the Holocene-Pleistocene boundary experienced a drop of 100 m in less than 2000 yr, a drop recorded in the carbonate mineralogy as well as in the oxygen isotopes of the carbonates (McKenzie et al., 1981; Spencer et al., 1981a, 1981b).
An excellent record of arid conditions may also be left in the authigenic mineral assemblages, because such minerals form by reaction of intestitial brine with volcanogenic or chemical sediments. Best known are the zeolite sequences of alkaline lake deposits (Surdam and Sheppard, 1978). Increasing salinity is reflected in the sequence smectite-zeolite-anaclime-K-feldspar, with the dominant zeolites being phillipsite, clinoptilolite, erionite, and chabazite. Such sequences may be displayed laterally or vertically. Because high-pH brines are necessary, zeolites do not form in Cl or SO4-dominated bases. Saline, alkaline conditions are also indicated by the presence of Magadi-type cherts, that is, cherts that have formed from a sodium silicate precursor, such as magadiite.
The climatic information to be extracted from saline lake deposits must be calibrated in comparison with active salt lakes. Considering such diverse settings as Great Salt Lake (Utah), Death Valley (California), Dead Sea (Asia), Lake Chad (Africa), Lake Uyuni (Bolivia), Lake Vanda (Antarctica), Tso-Kar Lake (Lhadak), it becomes clear that saline lakes do not give independent information on latitude, temperature, precipitation, and other climatic parameters, but they do tell us that in these localities evaporation exceeds inflow. Evaporation is experienced at the lake level, but inflow is a complex product of hydrologic processes in the entire watershed. Mean parameters, such as mean annual humidity, are not safe guides, because a rainy season can be compensated for by an intense dry period. Also, we realize that salt lakes do not occur in the most extreme deserts for lack of inflow. Ideal settings are down-faulted valley flows in the rain shadow of mountains that act as snow catch.
There is no doubt that the most valuable climatic information contained in lake deposits relates to short-term temporal changes and to local differences between adjacent basins. This is most clearly documented in the Eocene Green River deposits of Utah, Colorado, and Wyoming.
THE GREEN RIVER FORMATION
Paleolimnology, paleoclimatology, and the Green River formation are inextricably linked, as demonstrated through the work of Bradley (1929, 1931, 1948, 1963), who combined a wealth of information to deduce the climate of the region at the time of deposition. Bradley was forced to oversimplify and generalize in deducing a Green River climate. Paleotopographic arguments fixed the basin floors at 1000 ft. Using the pine-spruce distribution of the Southern Appalachians, mountain crests were fixed at a minimum elevation of 6000–8000 ft. Several lines of evidence yielded mean annual temperatures of 67°F. From Langbein’s (1961) formulas, Bradley calculated an annual precipitation of 34 in. at the lake level for a just-full stage and an average of 38 in. for the whole watershed. During times of overflow, precipitation must have been higher. During evaporite deposition (Wilkins Peak time), mean annual precipitation was estimated at 24 in. The large and varied flora, which contained cypress and palm trees (see, for example, Brown, 1934, MacGinitie, 1969), persuaded Bradley to call the overall climate warm-temperate like that of the present Gulf Coast states.
Recent work on the Green River formation has led to some-what different conclusions (Eugster and Hardie, 1975, 1978; Surdam and Stanley, 1979, 1980). We have focused more on the differences between basins, on the temporal changes, and on the complexity of the environments. In modifying Bradley’s conclusions, we have relied heavily on sedimentological arguments and on our experience with active continental evaporites.
The Eocene Green River formation was deposited in at least three basins, which were occupied by Lakes Uinta and Gosiute and Fossil Lake (see Figure 10.2). The Uinta and Green River (Gosiute) Basins, separated by the Uinta Mountains, were very large, with wide, flat floors. Their principal sediments are oil shales, which are rocks rich in carbonates and kerogen. Although it is clear that Lake Gosiute at times drained into the Uinta Basin (Bradley, 1963; Surdam and Stanley, 1979, 1980), we believe that there is good sedimentological evidence that both basins were hydrologically closed throughout most of their lives. Shallow, oil-shale producing lakes were surrounded by playa fringes, where carbonate mud was produced, modified, transported, and redeposited (see Smoot, 1978). Lake levels fluctuated extensively, and waters varied from brackish to saline. The basins south of the Uinta Mountains contained lakes for a much longer time (at least 12 million years) than did the Green River Basin (at least 5 million years). In fact, Lake Gosiute not only was of shorter duration, but during part of its life it was more ephemeral in nature. At least 25 separate episodes have been recorded during Wilkins Peak time, where the lake essentially dried up and became a salt pan. Salt deposition also occurred in Lake Uinta to the south, but less frequently and on a more localized scale. During much of its existence, Lake Uinta was a permanent lake with oil shale one of its principal deposits. Although the stratigraphic correlations and age dates are still in doubt (O’Neill et al., 1981), it seems clear from the summary of Wolfbauer (1971) that evaporites frequently were deposited in the north while oil shales formed in the
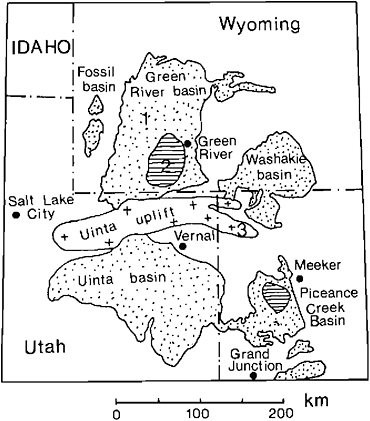
FIGURE 10.2 Sedimentary deposits of Eocene Lakes Uinta (Uinta and Piceance Creek Basins) and Gosiute (Green River and Washakie Basins) and Fossil Lake (Fossil Basin). 1, Green River Formation; 2, bedded salts; 3, basement. From Eugster and Hardie (1978).
south. Such local differences in the evaporation-inflow balance are not surprising and may be due to a number of factors. However, O’Neill et al. (1981) conclude that the “high stands of Lakes Gosiute and Uinta were essentially synchronous and therefore were likely controlled by climatic rather than tectonic factors.” Surdam and Stanley (1980), in a comparison of the drainage basins and sedimentation histories of the two basins, take the opposite position; “Basin filling and enlargement of the drainage system were probably a consequence of tectonic activity and stability of the basins and adjacent uplifts, although climatic conditions that increased sediment yield and runoff in the hydrographic basins also could have hastened their filling. However, it is difficult to explain patterns of evaporite minerals, oil shale, mudstone, and sandstone formed in Lakes Gosiute and Uinta if climate was the dominant factor.”
While Lakes Gosiute and Uinta went through their evolutionary changes, the much smaller Fossil Lake to the west (Figure 10.2) was caught between the ridges of the Thrust Belt. Fed perhaps by a precursor of the Snake River, this lake was more like a tub, narrow, long, and probably deeper (most of the time). Its sediments are the classic varve limestones often taken as prototype Green River rocks, which contain the famous, exquisitely preserved fish fossils. We now know that these rocks owe their existence to special circumstances that have to do as much with hydrology and sedimentation as with climate. No sizable accumulations of evaporites or oil shales occurred, probably because the lake was deeper and sediment-choked. Yet there is a clear record of several periods of desiccation or near desiccation, with trees or bushes growing on the bed of the lake.
This brief summary of three contrasting basins should make it obvious how difficult it is to speak of a Green River climate. Nevertheless, I do not agree that the present Gulf Coast climate provides a valid analog. The overall conditions were much drier, and I believe that the significance of the subtropical flora has been overemphasized. The nature of the sediments indicates a dry, continental climate with evaporation exceeding inflow most of the time. Bradley was aware of the evidence indicating such an environment; in speaking of Ephedra pollen (Bradley, 1963, pp. 639–640) he notes that “If its Eocene forebears lived in the same parched environment as the Ephedra of the Rocky Mountain region of today, it is odd that it should show up in the sediments of the earliest stage (Luman) of Gosiute Lake. It also occurs, as would be expected, in the deposits of the later saline stage. The presence of Ephedra pollen agrees with an observation by R.W.Brown (1934, pp. 280–281) that certain thick, coraceous leaves in the Green River flora indicate hot, dry summers like those of southern California.”
Southeastern California probably provides a much better climatic comparison. Though none are very large, the region contains many hydrologically closed basins, with waters ranging from brackish to very saline. Palm trees grow wherever there is enough water.
MARINE EVAPORITES
Marine evaporites have traditionally been useful climatic indicators because of their bulk and obvious cyclicity. Although they occur throughout the geologic column from the Precambrian onward, there are definite centers of evaporite depositions in time and space (see Kozary et al., 1968), for example, during the Devonian in North America, the Permian and the Triassic in Europe, and the Jurassic in North America and Miocene in Europe. As has been pointed out often, no large-scale evaporites are forming at present anywhere on Earth. There seems to be no lack of evaporitic environments, but the necessary depositional conditions do not coincide. Again, the presence of evaporites is due as much to tectonic as climatic factors.
Onset of evaporitic conditions can often be recognized from underlying and laterally contiguous sediments. Biogenic limestones and reef deposits are common precursors, indicating that tropical to subtropical climates prevailed. Other organism-rich sediments such as oil shales record the increasing preservation, if not productivity, of organic matter in penesaline environments. Laterally associated with evaporite deposition, continental sediments such as red beds, arkoses, and conglomerates may accumulate in down-faulted basins.
Although there must be a much better correlation between marine evaporites and latitude than there is between continental evaporites and latitude, there is no intrinsic reason why marine evaporites could not form at high latitudes, except for the fact that evaporation rates are so much lower. It should be possible to recognize such deposits by the presence of mirabilite, Na2SO4·10H2O, a typical precipitate formed by chilling
of brines having the composition of concentrated seawater such as those of the Great Salt Lake of Utah.
For the present discussion it is useful to separate marine evaporites into three groups: (1) those dominated by gypsum and anhydrite, (2) those dominated by halite, and (3) potash-bearing deposits. By far the largest number of fossil deposits belong to the gypsum-anhydrite group, and these deposits are best understood because there are good Holocene analogs. Sabkha settings such as those of the Persian Gulf (Purser, 1973) are usually invoked for these deposits, which often consist of CaMg carbonate-gypsum laminites. Typical examples and criteria for recognizing them have been described from the Miocene of Sicily (Hardie and Eugster, 1971), and a depositional model has been suggested by Eugster and Hardie (1978). To produce such deposits, seawater has to be concentrated at least three but not more than ten times. As pointed out by Kinsman (1976), the average humidity must vary from 98 to 76 percent. Transgressive-regressive cycles are common at this stage, in response to the interplay between climatic and tectonic forces. Excellent examples have been described from the Permian of northern Italy (Bosellini and Hardie, 1973) and the Permian of Texas and New Mexico (Anderson et al., 1972). Subsidence is essential for continued deposition in shallow basins like those envisaged for group 1 evaporites, but each step down may lead to transgression across the bar that protects the basin from the open sea. Similarly, subsidence must be slow enough so that sedimentation is able to keep pace and the basin never becomes deep.
In contrast, there is increasing evidence that the thick halite-dominated accumulation (group 2) so characteristic of the saline giants (Hsü, 1972) probably formed in deep basins (see Gill, 1977; Hardie et al., 1978; Briggs et al., 1980; Harvie et al., 1980). Brine bodies several hundreds of meters deep may accumulate as a consequence of increased subsidence coupled with more intense evaporation. Such a body, though of different composition, exists now in the Dead Sea. An appropriate hydrologic model to account for the sequence of progressively evaporated mineral assemblages has been proposed by Harvie et al. (1980). Evaporation for halite sequences to form must be from 10- to 20-fold seawater concentration. This may or may not signal increasing aridity, depending on whether or not a hydrologic system for preconcentration is available.
Most marine evaporites stop at the halite stage, and the final concentration products (group 3 evaporites) are either not formed or not preserved. These products are the K-Mg salts, so important for economic reasons and also as climatic indicators. Minerals such as polyhalite [K2MgCa2(SO4)4·2H2O], epsomite (MgSO4·7H2O), carnallite (KMgCl3·6H2O), and kieserite (MgSO4·H2O) require such extreme desiccation that it is difficult to envisage an appropriate near-marine climatic setting. This has been pointed out by Kinsman (1976), who attempted to correlate mean annual humidity with stage and intensity of evaporation. The activity of H2O, aH2O, of a brine is a direct measure of the humidity of the air in equilibrium with such a brine, as
where ![]() is the water-vapor pressure and RH is the relative humidity in percent. Calculated aH2O values for seawater concentrates and minerals with which they are in equilibrium are shown in Figure 10.3 (see Eugster et al., 1980). Potash deposits do not form until aH2O falls below 0.7, the Mg sulfates epsomite and kieserite require values below 0.6, and carnallite 0.45, that is, an average humidity of less than 45 percent is required for carnallite to accumulate, a condition difficult to imagine in a marine setting.
is the water-vapor pressure and RH is the relative humidity in percent. Calculated aH2O values for seawater concentrates and minerals with which they are in equilibrium are shown in Figure 10.3 (see Eugster et al., 1980). Potash deposits do not form until aH2O falls below 0.7, the Mg sulfates epsomite and kieserite require values below 0.6, and carnallite 0.45, that is, an average humidity of less than 45 percent is required for carnallite to accumulate, a condition difficult to imagine in a marine setting.
For the deposition of group 3 evaporites we favor a depositional model, termed the playa model. To reach this stage, the deep brine basin filled up with evaporites; that is, subsidence did not keep pace with sedimentation. In the resulting flat, broad basin, now under the influence of extreme aridity, fractional dissolution and recycling of the most soluble salts will occur, as observed in continental playas (Eugster and Jones, 1979). In consequence, the most soluble minerals will accumulate in the hydrographic center as a bedded deposit. These minerals are quite hygroscopic, and they can be preserved only
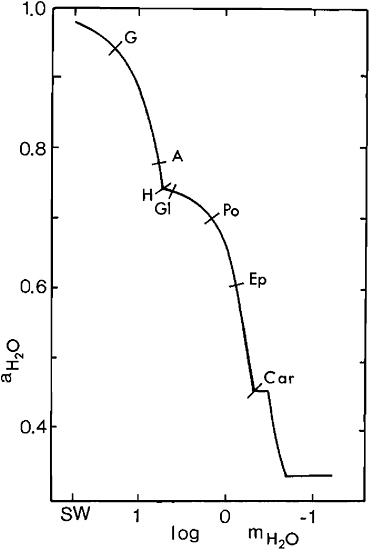
FIGURE 10.3 Activity of H2O, aH2O, of seawater concentrates. Evaporation begins with 55.5 moles of seawater (SW) and proceeds to the right. First appearances of precipitates are noted. G, gypsum; A, anhydrite; H, halite; Gl, glauberite; Po, polyhalite; Ep, epsomite; Car, carnallite. From Eugster et al. (1980).
by extreme conditions of aridity. The appropriate situation can perhaps be created by combining the hydrologic setting of the Persian Gulf with the climate of the coast of Chile.
There is one final group of evaporites, often considered to be of marine parentage, that require still further evaporation. These are the deposits that contain tachyhydrite (CaMgCl4· 6H2O) (see, for example, Wardlaw, 1972; Szatmari et al., 1979). However, as pointed out by Hardie (1979), such deposits may in fact not be directly related to seawater. Nevertheless, where they did form and were preserved, climatic conditions must have been unimaginably severe.
CONCLUSIONS
Lake deposits are sensitive indicators of paleoclimatic changes. Particularly useful are glacial and saline lakes. The latter are not related to latitude and form when evaporation exceeds inflow, recording a complex balance between climate, hydrology, and tectonics. Changes in depositional conditions are indicated by lithologies, sedimentary structures, and mineralogy and often result in transgressive-regressive cycles. Local differences may override general climatic conditions. This is illustrated by a reinterpretation of the Eocene Green River climate—a climate exemplified by southeastern California is preferred to that of the Gulf Coast states.
Climatic interpretation of marine evaporites depends on the special hydrologic requirements. Three stages are distinquished, with each stage dominated in turn by Ca sulfates, halite, and K-Mg salts. The bulk of marine evaporites belong to stage 1. A sabkha setting is a good depositional model. Sea-water can be concentrated up to tenfold before halite precipitates. Thick halite deposits may accumulate in a deep stratified basin such as that suggested by Harvie et al. (1989). For the final stage, we prefer the playa model, which requires relative humidities of less than 60–45 percent. Final products are hygroscopic, and it is not easy to reconcile their requirements for extreme aridity with a marine setting.
This brief summary should make it clear how difficult it is to derive unequivocal information about paleoclimates from sedimentary deposits, because of the delicate interplay between climatic, tectonic, and hydrologic forces. In order to arrive at valid climatic conclusions, all the evidence must be reviewed, including paleontologic and sedimentologic aspects. We must calibrate and sharpen our tools on the sediments of currently active lakes and evaporitic environments.
REFERENCES
Anderson, R.Y., and D.W.Kirkland (1969). Paleoecology of an early Pleistocene lake on the high plains of Texas, Geol. Soc. Am. Mem. 113, 211 pp.
Anderson, R.Y., W.E.Dean, D.W.Kirkland, and H.I.Snide, (1972). Permian Castile varved evaporite sequence, West Texas and New Mexico, Geol. Soc. Am. Bull. 83, 59–86.
Begin, A.B., A.Ehrlich, and Y.Nathan (1974). Lake Lisan, the Pleistocene precursor of the Dead Sea, Geol. Surv. Israel Bull. 63, 30 pp.
Bosellini, A., and L.A.Hardie (1973). Depositional theme of a marginal marine evaporite, Sedimentology 20, 5–27.
Bradbury, J.P., and M.C.Whiteside (1980). Paleolimnology of two lakes in the Klutlan glacier region, Yukon Territory, Canada, Quat. Res. 4, 149–168.
Bradley, W.H. (1929). The varves and climate of the Green River epoch, U.S. Geol. Surv. Prof. Pap. 158-E, 87–110.
Bradley, W.H. (1931). Origin and microfossils of the oil shale of the Green River Formation of Colorado and Utah, U.S. Geol. Surv. Prof. Pap. 168, 58 pp.
Bradley, W.H. (1948). Limnology and the Eocene lakes of the Rocky Mountain region, Geol. Soc. Am. Bull. 59, 635–648.
Bradley, W.II. (1963). Paleolimnology, in Limnology in North America, D.G.Frey, ed., U. of Wisconsin Press, Madison, Wisc., pp. 621–652.
Briggs, L.I., D.Gill, D.F.Briggs, and R.D.Elmore (1980). Transition from open marine to evaporite deposition in the Silurian Michigan Basin, in Hypersaline Brines and Evaporitic Environments, A.Nissenbaum, ed., Developments in Sedimentology 28, Elsevier, Amsterdam, pp. 253–270.
Brown, R.W. (1934). The recognizable species of the Green River flora, U.S. Geol. Surv. Prof. Pap. 185-C, pp. 45–68.
Brunskill, G.J. (1969). Fayetteville Green Lake, New York, II. Precipitation and sedimentation of calcite in a meromictic lake with laminated sediments, Limnol. Oceanogr. 14, 830–847.
Cerling, T.E. (1979). Paleochemistry of Plio-Pleistocene Lake Turkana, Kenya, Paleogeogr. Paleoclimatol. Paleoecol. 27, 247–285.
Dean, W.E., and E.Gorham (1976). Major chemical and mineral components of profundal surface sediments in Minnesota lakes, Limnol. Oceanogr. 21, 259–284.
Dean, W.E., and B.C.Schreiber, eds. (1978). Marine Evaporites, Soc. Econ. Paleontol. Mineral. Short Course Notes, Oklahoma City, 188 pp.
Degens, E.T., R.P.Von Herzen, H.K.Wong, and H.W.Tannasch (1973). Structure, chemistry and biology of an East Africa Rift Lake, Geol. Rundsch. 62, 245–276.
Eardley, A.J., R.T.Shuey, V.Gvosdetsky, W.P.Nash, M.D. Picard, D.C.Grey, and G.J.Kukla (1973). Lake cycles in the Bonneville basin, Utah, Geol. Soc. Am. Bull. 84, 211–216.
Eugster, H.P. (1980). Lake Magadi, Kenya, and its precursors, in Hypersaline Brines and Evaporite Environments, A.Nissenbaum, ed., Developments in Sedimentology 28, Elsevier, Amsterdam, pp. 195–232.
Eugster, H.P., and L.A.Hardie (1975). Sedimentation in an ancient playa-lake complex: The Wilkins Peak member of the Green River Formation of Wyoming, Geol. Soc. Am. Bull. 86, 319–334.
Eugster, H.P., and L.A.Hardie (1976). Some further thoughts on the depositional environment of the Solfifera Series of Sicily, Mem. Geol. Soc. Italy 16, 29–38.
Eugster, H.P., and L.A.Hardie (1978). Saline lakes, in Lakes—Chemistry, Geology, Physics, A.Lerman, ed., Springer-Verlag, New York, pp. 237–293.
Eugster, H.P., and B.F.Jones (1979). Behavior of major solutes during closed-basin brine evolution, Am. J. Sci. 279, 609–631.
Eugster, H.P., C.E.Harvie, and J.H.Weare (1980). Mineral equilibria in the six-component sea water system, Na-K-Mg-Ca-SO4-Cl-H2O at 25°C, Geochim. Cosmochim. Acta 44, 1335–1347.
Friedman, I., G.I.Smith, and K.Hardcastle (1976). Studies of Quaternary saline lakes—II. Isotopic and compositional changes during desiccation of the brines in Owens Lake, California 1969–71, Geochim. Cosmochim. Acta 40, 501–511.
Gilbert, G.K. (1890). Lake Bonneville, U.S. Geol. Surv. Monogr. 1, 438 pp.
Gill, D. (1977). Saline A-1 sabkha cycles and the late Silurian paleogeography of the Michigan Basin, J. Sed. Petrol. 47, 979–1017.
Hansen, K. (1961). Lake types and lake sediments, Verh. Internat. Verein Limnol. 14, 121–145.
Hardie, L.A. (1979). Evaporites: Marine or non-marine? Geol. Soc. Am. Abstr. Programs 1979, 368.
Hardie, L.A., and H.P.Eugster (1970). The evolution of closed-basin brines, Mineral. Soc. Am. Spec. Publ. 3, 273–290.
Hardie, L.A., and H.P.Eugster (1971). The depositional environment of marine evaporites: A case for shallow, elastic accumulation, Sedimentology 16, 187–220.
Hardie, L.A., J.P.Smoot, and H.P.Eugster (1978). Saline lakes and their deposits: A sedimentological approach, Int. Assoc. Sedimentol. Spec. Publ. 2, 7–41.
Harvie, C.E., J.H.Weare, L.A.Hardie, and H.P.Eugster (1980). Evaporation of sea water: Calculated mineral sequences, Science 208, 498–500.
Holser, W.T. (1979). Mineralogy of evaporites, in Marine Minerals, R.B.Burns, ed., Min. Soc. Am. Short Course Notes 6, pp. 211–294.
Hsü, K.H. (1972). Origin of saline giants: A critical review after the discovery of the Mediterranean evaporite, Earth Sci. Rev. 8, 371–396.
Irion, G. (1970). Mineralogisch-sedimentpetrographische und geochemische untersuchungen am Tuz Gölö (Salzee), Türkei, Chem. Erde 29, 163–226.
Irion, G. (1973). Die anatolischen Salzeen, ihr Chemismus und die Entstehung ihrer chemischen Sedimente, Arch. Hydrobiol. 71, 517–557.
Jones, B.F. (1965). The hydrology and mineralogy of Deep Springs Lake, Inyo County, California, U.S. Geol. Surv. Prof. Pap. 502-A, 56 pp.
Jones, B.F., and C.J.Bowser (1978). The mineralogy and related chemistry of lake sediments, in Lakes—Chemistry, Geology, Physics, A.Lerman, ed., Springer-Verlag, New York, pp. 179–227.
Kelts, K., and K.J.Hsü (1978). Freshwater carbonate sedimentation, in Lakes—Chemistry, Geology, Physics, A.Lerman, ed., Springer-Verlag, New York, pp. 295–323.
Kinsman, D.J.J. (1976). Evaporites: Relative humidity control of primary mineral facies, J. Sed. Petrol. 46, 273–279.
Kozary, M.T., J.C.Dunlap, and W.E.Humphrey (1968). Incidence of saline deposits in geologic time, in Saline Deposits, R.B.Mattox, ed., Geol. Soc. Am. Spec. Pap. 88, pp. 43–57.
Langbein, W.B. (1961). The salinity and hydrology of closed lakes, U.S. Geol. Surv. Prof. Pap. 412, 1–20.
Lerman, A., ed. (1978). Lakes—Chemistry, Geology, Physics, Springer-Verlag, New York, 363 pp.
Ludlum, S.D. (1969). Fayetteville Green Lake, New York III. The laminated sediments, Limnol. Oceanogr. 14, 848–861.
MacGinitie, H.D. (1969). The Eocene Green River flora of northwestern Colorado and northeastern Utah, U. Calif. Publ. Geol. Sci. 83, 203 pp.
Matter, A., and M.E.Tucker, eds. (1978). Modern and Ancient Lake Sediments, Int. Assoc. Sedimentol. Spec. Publ. 2, 290 pp.
McKenzie, T., K.Kelts, J.Pika, H.P.Eugster, R.C.Spencer, B.F. Jones, M.J.Baedeker, S.L.Rettig, M.B.Goldhaber, and C.J. Bowser (1981). Oxygen-18 composition of Great Salt Lake sediments: An indicator of lake level fluctuations (abstract), American Association of Petroleum Geologists Meeting, San Francisco.
Müller, G., and F.Wagner (1978). Holocene carbonate evolution in Lake Balaton (Hungary); A response to climate and impact of man, in Modern and Ancient Lake Sediments, A.Matter and M.E. Tucker, eds., Internat. Assoc. Sedimentol. Spec. Publ. 2, pp. 57–81.
Müller, G., G.Irion, and U.Förstner (1972). Formation and diagenesis of inorganic Ca-Mg Carbonates in the lacustrine environment, Naturwissenschaften 59, 158–164.
Neev, D., and K.O.Emery (1967). The Dead Sea—depositional processes and environments of evaporites, Israel Geol. Surv. Bull. 41, 147 pp.
O’Neill, W.A., J.F.Sutter, and K.O.Stanley (1981). Sedimentation history of rich oil-shale sequences in Eocene Lakes Gosiute and Uinta, Wyoming and Utah, Geol. Soc. Am., Rocky Mountain Sec. Abstr. Programs.
Purser, B.H., ed. (1973). The Persian Gulf, Springer-Verlag, New York, 471 pp.
Sims, T.D., D.P.Adam, and M.J.Rymer (1981). Late Pleistocene stratigraphy and palynology of Clear Lake, Lake County, California, U.S. Geol. Surv. Prof. Pap. 1041.
Smith, G.I., ed. (1978). Climate variation and its effect on our land and water, U.S. Geol. Surv. Circ. 776-B, 52 pp.
Smith, G.I. (1979). Subsurface stratigraphy and geochemistry of late Quaternary evaporites, Searles Lake, California, U.S. Geol. Surv. Prof. Pap. 1043, 130 pp.
Smith, G.I., V.J.Barczak, G.F.Moulton, and J.C.Liddicoat (in press). Core KM-3, a surface-to-bedrock record of late Cenozoic sedimentation in Searles Valley, California, U.S. Geol. Surv. Prof. Pap.
Smoot, J.P. (1978). Origin of the carbonate sediments in the Wilkins Peak Member of the lacustrine Green River Formation (Eocene), Wyoming, U.S.A., in Modern and Ancient Lake Sediments, A. Matter and M.E.Tucker, eds., Internat. Assoc. Sedimentol. Spec. Publ. 2, pp. 109–127.
Spencer, R.C., H.P.Eugster, K.Kelb, J.McKenzie, B.F.Jones, M.J.Baedeker, S.L.Rettig, M.B.Goldhaber, and E.J.Bowser (1981a). Late Pleistocene and Holocene sedimentary history of Great Salt Lake, Utah (abstract), American Association of Petroleum Geologists meeting, San Francisco.
Spencer, R.C., H.P.Eugster, B.F.Jones, M.J.Baedeker, S.L. Rettig, M.B.Goldhaber, C.J.Bowser, K.Keltig, and J.McKenzie (1981b). Mineralogy and pore fluid geochemistry of Great Salt Lake, Utah (abstract), American Association of Petroleum Geologists meeting, San Francisco.
Stoessel, R.K., and R.L.Hay (1978). The geochemical origin of sepiolite and kerolite at Amboseli, Kenya, Contrib. Mineral. Petrol. 65, 255–267.
Stoffers, P., and R.E.Hecky (1978). Late Pleistocene-Holocene evolution of the Kivu-Tanjanyika basins, in Modern and Ancient Lake Sediments, A.Matter and M.E.Tucker, eds., Internat. Assoc. Sedimentol. Spec. Publ. 2, pp. 43–44.
Surdam, R.C., and R.A.Sheppard (1978). Zeolites in saline, alkaline-lake deposits, in Natural Zeolites, Occurrence, Properties, Use, L.B.Sand and F.A.Mumpton, ed., Pergamon, Oxford, pp. 145–174.
Surdam, R.C., and K.O.Stanley (1979). Lacustrine sedimentation during the culminating phase of Eocene Lake Gosiute, Wyoming, Geol. Soc. Am. Bull. 90, 93–110.
Surdam, R.C., and K.O.Stanley (1980). Effects of changes in drain-age-basin boundaries on sedimentation in Eocene Lakes Gosiute and Uinta of Wyoming, Utah, and Colorado, Geology 8, 135–139.
Szatmari, P., R.S.Carvalho, and I.A.Simoes (1979). A comparison of evaporites facies in the late Paleozoic Amazon and the Middle Cretaceous South Atlantic salt basins, Econ. Geol. 74, 432–447.
Wardlaw, N.C. (1972). Unusual marine evaporites with salts of calcium and magnesium chloride in Cretaceous basins of Sergipe, Brazil, Econ. Geol. 67, 156–168.
Wolfbauer, C.A. (1971). Geologic framework of the Green River Formation in Wyoming, Contrib. Geol. U. Wyoming 10, 3–8.
Yuretich, R.F. (1979). Modern sediments and sedimentary processes in Lake Rudolf (Lake Turkana) eastern Rift Valley, Kenya, Sedimentology 26, 313–331.







