2
Nuclear Medicine
This chapter provides an overview of the field of nuclear medicine for readers who are not familiar with the discipline. It includes a description of the history and major discoveries in this field, the challenges of conducting nuclear medicine research, and the foreseeable new technologies and opportunities for personalizing health care that could result from aggressive development of the field.
Nuclear medicine is a highly multi-disciplinary specialty that develops and uses instrumentation and radiopharmaceuticals to study physiological processes and non-invasively diagnose, stage,1 and treat diseases. A radiopharmaceutical is either a radionuclide alone, such as iodine-131 (Sidebar 2.1) or a radionuclide that is attached to a carrier molecule (a drug, protein, or peptide) or particle, which when introduced into the body by injection, swallowing, or inhalation accumulates in the organ or tissue of interest. In a nuclear medicine scan, a radiopharmaceutical is administered to the patient, and an imaging instrument that detects radiation is used to show biochemical changes in the body. Nuclear medicine imaging (Sidebar 2.2), in contrast to imaging techniques that mainly show anatomy (e.g., conventional ultrasound, computed tomography [CT], or magnetic resonance imaging [MRI]), can provide important quantitative functional information about normal tissues or disease conditions in living subjects. For treatment, highly targeted radiopharmaceuticals (Sidebar 2.3) may be used to deposit lethal radiation at tumor sites.
Nuclear medicine has been developed over the past 50 years through a
unique partnership among the national laboratories, academia, and industry (Section 2.1). They have collaborated to develop:
-
nuclear reactors and particle accelerators that produce radionuclides;
-
chemical processes to synthesize radiopharmaceuticals that can be used for imaging and treatment; and
-
instruments that can detect radiation emitted from the radionuclides that accumulate in the human body.
According to data from the Center for Medicare and Medicaid Services (CMS), nuclear medicine plays an essential role in medical specialties from cardiology to oncology to neurology and psychiatry and is a $1.7 billion industry. The Society of Nuclear Medicine estimates that 20 million nuclear
|
SIDEBAR 2.2 Nuclear Medicine Imaging Positron emission tomography (PET) is a nuclear medicine imaging technique that exploits the unique decay physics of positron-emitting radionuclides (Sidebar 2.9) and produces a three-dimensional image of radionuclide distribution. For example, the radiopharmaceutical fluorine-18-fluorodeoxyglucose (FDG) is a form of sugar labeled with a radionuclide [fluorine-18] that is imaged using PET. This imaging technique, which is commonly known as FDG-PET, detects differences between cancer and normal cells in the consumption of glucose. Cancer cells, particularly those from aggressive tumors, proliferate more rapidly than normal cells and consume considerably larger amounts of glucose. Not only can tumor sites be pinpointed through the detection of increased FDG consumption, but differences in FDG consumption in tissues can be detected. However, FDG may be taken up by other lesions, such as infectious foci, and not just tumors, so the diagnostic specificity of FDG-PET is limited. In the future, the network of cyclotron/radiopharmacies that are now focused exclusively on making FDG are well positioned to provide distribution of other fluorine-18-labeled radiopharmaceuticals to regional hospitals as these are developed and approved for clinical use. In addition, development and regional deployment of lower cost radionuclide-producing machines may make other radiopharmaceuticals based on radionuclides with shorter half-lives such as carbon-11 more widely available. Single photon emission computed tomography (SPECT) is another common nuclear medicine imaging device. SPECT uses gamma cameras to obtain three-dimensional images. To acquire SPECT images, the gamma camera is rotated around the patient and multiple images from multiple angles are obtained. A computer can then reconstruct the images. Radiopharmaceuticals used for SPECT are labeled with gamma-emitting radionuclides such as technetium-99m, iodine-123, and thallium-201. SPECT is used extensively to study cardiac health (e.g., blood flow to the heart through myocardial perfusion imaging) and to image blood flow to the brain. PET and SPECT each have distinct advantages and disadvantages that make them useful for detecting certain conditions. Each technique uses different properties of radioactive elements in creating an image. For example, one of the advantages of SPECT compared with PET is that more than one radiotracer can be used at a time. In addition, the longer half-life of radionuclides used with SPECT makes this imaging procedure more readily available to the medical community at large. However, PET images have higher sensitivity than SPECT images by a factor of 2 to 3 and use radiopharmaceuticals that provide more physiological information. |
|
SIDEBAR 2.3 Targeted Radionuclide Therapy Targeted radionuclide therapy is a form of treatment that delivers therapeutic doses of radiation to malignant tumors by administering a molecule that is labeled with a radionuclide. The radiotherapeutic agent is made of two components: the radionuclide and the carrier that is used to seek out the tumor cells. Molecular carriers that can be used include, but are not limited to, peptides that seek their corresponding receptors on cells, and monoclonal antibodies that seek out antigens that are similarly expressed on the cells, as shown in the figure. 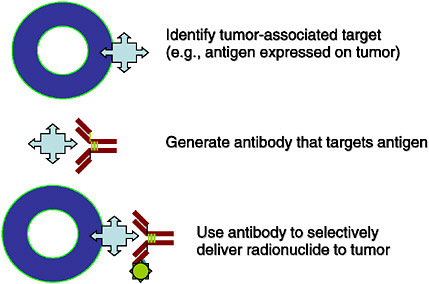 FIGURE Schematic of a tumor cell expressing targets for a radiotherapeutic agent. SOURCE: Courtesy of Michael Zalutsky, Duke University. The radionuclide that is attached to the carrier molecule can be chosen for specific characteristics, such as type of radiation decay (e.g., alpha-emitter, beta-emitter), radiation range, and half-life. It is this modular nature, where the two components can be varied like Lego® pieces to match characteristics specific to the tumor that makes targeted radionuclide therapy an attractive approach to cancer treatment (Zalutsky 2003). To date, two antibody radiopharmaceuticals have been approved by the FDA (yttrium-90-ibritumomab tiuxetan and iodine-131-tositumomab) for the treatment of lymphoma. |
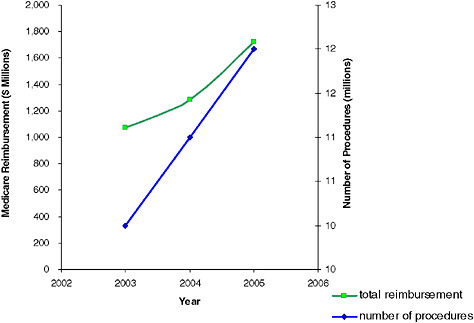
FIGURE 2.1 Number of nuclear medicine procedures that were approved for reimbursement by the Center for Medicare and Medicaid Services and total reimbursement for 2003–2005. SOURCE: Data provided by CMS.
medicine procedures are performed annually in the United States, of which 12 million are procedures approved for and reimbursed by CMS. Figure 2.1 illustrates the number of nuclear medicine procedures approved and the total payment reimbursed by the CMS in the United States in 2003, 2004, and 2005. Based on data from CMS, the use of positron emission tomography (PET) is growing faster than the use of any other imaging modality. From 2000 to 2005, the average annual growth rate in the volume of PET and PET/CT procedures was 80 percent compared with 9 percent for non-PET nuclear medicine procedures, 11 percent for CT, and 13 percent for MRI (ACR 2007). The use of nuclear medicine procedures will likely continue to rise in the future (Table 2.1).
More importantly, the use of nuclear medicine procedures has improved patient care in many ways. Nuclear imaging allows physicians to cost-effectively obtain medical information that would otherwise be unavailable or would require more invasive procedures, such as surgery or biopsy. For example, FDG-PET imaging has been estimated to save almost $400,000 per 100 patients when compared to surgery to assess for the presence of malignancy in indeterminate lung lesions as seen on CT (NLM 1998). This
TABLE 2.1 Procedures per Medicare Fee-for-Service Beneficiary, by Imaging Modality
|
|
|
|
Average Annual Growth Rate (%) |
Share of All Imaging (%) |
|
|
|
2000 |
2005 |
2000 |
2005 |
|
|
All |
3.83 |
4.99 |
5 |
100 |
100 |
|
CT |
0.35 |
0.57 |
11 |
9 |
10 |
|
MRI |
0.10 |
0.19 |
13 |
3 |
3 |
|
Nuclear Medicine |
|
|
|
|
|
|
Non-PET |
0.21 |
0.33 |
9 |
5 |
6 |
|
PET and PET/CT |
0.00 |
0.01 |
80 |
0.02 |
0.05 |
|
Ultrasound |
0.84 |
1.14 |
6 |
22 |
22 |
|
Interventional |
0.17 |
0.26 |
8 |
5 |
5 |
|
Mammography |
0.21 |
0.33 |
9 |
6 |
6 |
|
X-ray, excluding mammography |
1.94 |
2.14 |
2 |
51 |
49 |
|
Other |
0.01 |
0.03 |
37 |
0.2 |
0.2 |
|
SOURCE: Data from American College of Radiology Research Department. |
|||||
procedure, which has been in use for over 25 years, is also used to diagnose and stage esophageal cancer and non-small-cell lung cancer; to stage melanoma and colorectal cancers; and to monitor treatment response in lymphoma and locally advanced and metastatic breast cancer. FDG-PET has also had a considerable impact in detecting distant metastases and metastatic disease in lymph nodes that appear normal on CT scan (e.g., in lymphoma) (Kelloff et al. 2005).
2.1
SIGNIFICANT DISCOVERIES
The modern era of nuclear medicine is an outgrowth of the charge to the Atomic Energy Commission (AEC) “to exploit nuclear energy to promote human health” (Atoms for Peace Program). For more than 50 years, the AEC and later the Department of Energy (DOE) have supported high-risk research and development of nuclear medicine technology and have supplied radionuclides to the research community including physicists, chemists, engineers, computer scientists, biologists, and physicians. One of the earliest applications of nuclear medicine was the use of radioactive iodine to treat thyroid cancer. It also was used to measure thyroid function, diagnose thyroid disease, and treat hyperthyroidism, a condition where the thyroid gland produces excess amounts of thyroid hormones. The significant discoveries in nuclear medicine were made possible by advancements in the basic understanding of biological processes, chemistry, physics, and
computer technology. Sidebar 2.4 lists the major breakthroughs resulting from past federal investment in nuclear medicine research.
2.2
FRONTIERS IN NUCLEAR MEDICINE
The output over the past 50 years, as documented in the preceding section, has been extensive. Although nuclear medicine already contributes to biomedical research and disease management, its promise is only beginning to be realized in areas such as neuroscience, drug development, preventive health care, and other aspects of medicine (Sidebar 2.5). Examples of advances that may be possible from continued multi-disciplinary research and development are discussed in the sections below. The first section (2.2.1) describes the various ways in which nuclear medicine can contribute to personalized health care. The second section (2.2.2) is devoted to the technologies currently under development that could enable advances in the field of nuclear medicine.
2.2.1
Opportunities in Personalizing Health Care
The knowledge gained and the tools developed during the course of the Human Genome Project2 in addition to several decades of focused biomedical research are revolutionizing medicine. For example, thousands of genetic changes with known biological functions have been discovered and the number will grow as low-cost, next-generation genome analysis technologies are applied. This information will allow one to predict an individual’s risk for disease, detect diseases earlier, predict disease outcome, and identify more effective treatments that will further personalize health care.
Disease Detection and Treatment Response
Omic3 analyses are revealing differences in DNA, RNA, and protein expression between patients with cancer or heart disease and healthy subjects that can be detected in their blood, urine, feces, and sputum. Current tests are now approaching sensitivity levels that will allow detection of disease at subclinical levels, which is especially important for cancer management. Detection of subclinical disease demands the development of imaging procedures that can accurately pinpoint the location of the diseased tissue so
|
2 |
The Human Genome Project was a 13-year international effort to determine the DNA sequence of human beings. It was initially conceived, proposed, and initiated by the DOE’s Office of Biological and Environmental Research (DOE-OBER). The full sequence was completed in April 2003. |
|
3 |
Omic is an all-encompassing term used to describe comprehensive analyses of molecular or cellular characteristics. Genomics, for example, describes molecular assessment of the entire genome, and proteomics refers to measurement of the proteins found in cells and tissues. |
|
SIDEBAR 2.4 Chronology of Significant Discoveries from Past Federal Funding 1930s E.O. Lawrence at the UC Radiation Laboratory (later to become the Lawrence Berkeley National Laboratory) develops the cyclotron that will produce the first medically useful radionuclides, including iodine-131, thallium-201, technetium-99m, carbon-14, and gallium-67. 1940s The first reactor-produced radionuclides for medical research are made at Oak Ridge National Laboratory (ORNL); these included phosphorous-32, iron-52, and chromium-51. Carbon-11 was first produced and used in biological studies at the University of California at Berkeley by Martin Kamen and colleagues. 1950s Benedict Cassen at the University of California at Los Angeles (UCLA) invents the first automated scanner to image the thyroid gland after administering radioiodine to patients. Hal Anger invents the stationary gamma camera (now know as the Anger camera) at the UC Radiation Laboratory. The molybdenum-99/technetium-99m generator is developed at Brookhaven National Laboratory (BNL) by Powell Richards. Today, technetium-99m is used in over 70 percent of nuclear medicine procedures worldwide (Nuclear Energy Agency 2000). David Kuhl at the University of Pennsylvania constructs the prototype that will eventually lead to today’s SPECT and CT scanners. 1960s Scientists at ORNL discover the affinity of gallium-67 for soft-tissue tumors. This radionuclide has been used to image lymphomas, lung cancer, and brain tumors. Hot atom chemistrya work by Alfred Wolf, Michael Welch, and other scientists lays the groundwork for what will become radiopharmaceutical chemistry. William Eckelman and Powell Richards developed instant technetium kits. 1970s The efficient production of thallium-201 is developed by scientists at BNL. This procedure is still used today to assess reduced blood flow or tissue damage to the heart. |
|
PET scanners that will later be successfully commercialized are developed by Michael Phelps, Edward Hoffman, and Michel Ter-Pogossian at Washington University based on earlier work by Gordon Brownell at the Massachusetts Institute of Technology (MIT) and James Robertson at BNL. Fluorine-18-FDG, a positron-emitting compound, is synthesized by chemists at BNL. Scientists at the University of Pennsylvania and at the NIH use fluorine-18-FDG to image glucose metabolism in the human brain. 1980s A new radiopharmaceutical, iodine-131 m-Iodine-benzyl-guanidine (I-131 MIBG), is developed by Donald Wieland for the diagnosis and treatment of rare childhood cancers. Michael Welch of Washington University and John Katzenellenbogen of the University of Illinois develop the first PET radiotracer used to image tumors expressing the estrogen receptor. Scientists at Harvard Medical School and MIT develop technetium-99m-methoxyisobutylnitrile, an agent to measure blood flow to the heart muscle (used in myocardial perfusion scans). Chemists at national laboratories and federally supported academic laboratories developed methods to synthesize high-specific-activity C-11- and F-18-labeled compounds for imaging neurotransmitter and other physiological activities, laying the foundation for modern molecular imaging. 1990s A high-resolution PET scanner designed to image small laboratory animals (i.e., microPET) is developed at UCLA by Simon Cherry. Scientists at ORNL develop the rhenium-188 generator, which provides hospitals with a ready source of isotopes to treat bone pain in cancer patients. Radionuclides (scandium-47, copper-67, samarium-153, rhenium-188, and gold-199) used in therapeutic nuclear medicine procedures are developed by scientists at multiple national laboratories. Radiolabeled antibodies are developed for therapy (see Sidebar 2.3). Advances are made in the application of alpha-particle emitters for therapy. SOURCE: DOE 2001. |
|
SIDEBAR 2.5 What is Personalized Medicine? Personalized medicine refers to the tailoring of strategies to detect, treat, and prevent disease based on an individual’s molecular characteristics (see examples below). Physicians already practice a form of personalized medicine by using diagnostic tests to choose treatment options; however, the wealth of knowledge that is emerging is helping physicians individualize treatment for each patient with greater precision (i.e., identify patients most likely to respond to a given treatment). Two examples, the second of which is specific to nuclear medicine, are provided to illustrate this concept. Example 1: Trastuzumab (Herceptin®) as Treatment for Breast Cancer Breast cancer is the most common form of cancer in women, after non-melanoma skin cancer and lung cancer, and approximately 180,000 new cases are diagnosed each year (CDC 2006). There are different types of breast cancer, and they are largely classified and treated on the basis of anatomy (i.e., which cells in the breast turn into cancer). More recently, with advances in the molecular characterization of the disease, oncologists now recognize that the subtypes of breast cancer are separate diseases that require different biologically based therapies. One type of breast cancer overexpresses the human epidermal growth factor receptor 2 (HER2) receptor. An estimated 25 percent of breast cancer tumors are HER2-positive and these tumors tend to grow and spread more quickly compared with breast cancer tumors that are HER2-negative (Herceptin 2007). Trastuzumab, which is more commonly known under the trade name Herceptin®, is a monoclonal antibody that is designed to target breast tumors that express HER2. It is thought that trastuzumab stops the cancer cells from growing and dividing, and results from a large international clinical trial showed that the patients who received trastuzumab and chemotherapy were half as likely to have a recurrence of breast cancer as those who received chemotherapy alone (Piccart-Gebhard et al. 2005). This drug offers no benefit to patients whose breast cancer tumors do not overexpress HER2. By differentiating the tumors based on molecular differences and targeting these differences, more effective treatment can be delivered to the patient. Example 2: Monitoring Response to Cancer Treatment with FDG-PET Imaging FDG-PET is widely used in oncology to diagnose, stage, and restagea cancer. It is also used to detect residual cancer and to monitor the reduction in tumor |
that it can be treated with a minimally invasive and, often, image-guided approach. The most exciting area in imaging today and going forward is molecular imaging through various imaging technologies from the laboratory setting to clinical research and practice. Molecular imaging has become a scientific discipline in its own right, as well as a growing practice in medicine. MRI and optical imaging, as well as nuclear medicine imaging, can be used for molecular targeting. The three approaches differ in sensitivity:
|
volume in response to therapy in cancer. Although its use for monitoring response is only reimbursed by Medicare for certain applications in the management of breast cancer, clinical trials in non-small-cell lung cancer, lymphoma, and esophageal cancer have shown that FDG-PET imaging can predict patient response to treatment. The figure below shows images taken in a lymphoma patient before and after treatment with the radioactively labeled anticancer agent, Zevalin. The tumor shows intense FDG uptake in the image taken before initiation of treatment. In contrast, the image taken after treatment shows a marked decrease in FDG uptake, indicating a favorable response to therapy. FDG-PET has the potential to improve patient care by allowing treatments with approved medicines to be selected to maximize individual patient response (Kelloff et al. 2005, Webber 2005).
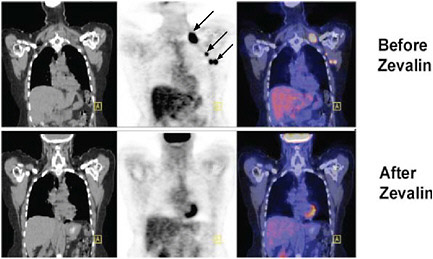 FIGURE Monitoring response to treatment using FDG-PET in a lymphoma patient treated with Zevalin. SOURCE: courtesy of Peter Conti, University of Southern California. |
MRI probes can be detected at micromolar concentrations, optical probes at picomolar concentrations, and nuclear probes at nanomolar concentrations. All of these probes can be chemically attached or encapsulated to target specific tissue receptor sites or may be attached to a moiety that preferentially accumulates in a region of interest. Nuclear photons, unlike optical ones, can escape from the body and thus can be used for more deeply seated targets (Weissleder 2006). It is therefore likely that nuclear
medicine procedures can be developed to provide the precise information required to pinpoint the location of subclinical disease for minimally invasive treatment. This will require high-specific-activity radiotracers targeted to specific molecular markers as well as imaging devices with greater resolution and sensitivity.
“Omic” approaches are not only revealing previously unsuspected disease, but are also identifying subtypes of disease. The existence of disease subtypes may, in part, explain why similar clinical diagnoses often result in substantially different outcomes in different individuals. Nuclear medicine may contribute to the management of such diseases by providing information about individual responses to therapy. As illustrated in Sidebar 2.5, FDG-PET can be used to monitor responses of individual tumors to treatment by demonstrating whether there has been a change in FDG uptake. Such monitoring allows earlier determination of the effectiveness of approved treatments in individual patients and, if necessary, enables the patient to start an alternative treatment sooner. It also may facilitate evaluation of the effectiveness of experimental medicines, thereby speeding their entry into clinical practice while reducing cost. Other current as well as next-generation nuclear medicine procedures will similarly accelerate the delivery of personalized care to the patient.
Physiological Assessment
Nuclear medicine imaging will enable functional investigations of numerous aspects of normal and abnormal physiologies. These include, but are not limited to, neurotransmitter activity, chemical determinants of behavior, neurodegeneration, immune response, remodeling of heart tissue, and bone metabolism. Imaging of specific carbon-11-labeled agents to assess brain function is possible, but increasingly, fluorine-18-labeled compounds such as FDG, fluorine-18-dihydroxyphenylalanine, and fluorine-18-labeled fallypride4 are being used to assess brain degeneration and cognitive function. Improvements in imaging instruments that have greater spatial and temporal resolution and radiotracers with high specific activities will allow more precise non-invasive assessment of these physiological functions. Ideally, next-generation procedures will use “natural” radionuclides such as carbon-11 that do not change the chemical properties of the tracer. In many cases, this will require imaging of low concentrations of proteins
|
4 |
Fallypride is a chemical compound (i.e., benzamide) that binds specifically to the dopamine-2 and dopamine-3 receptors in the brain. Dopamine receptors have a role in processes such as motor and learning. Dysfunction in these receptors has been associated with a variety of neurological disorders, such as Parkinson’s disease, schizophrenia, attention-deficit hyperactivity disorder, and drug dependence. |
using radiotracers with short half-lives that have high specific activity. To achieve this, new technologies will need to be developed that can produce these short-half-life radionuclides cost-effectively at many sites distributed around the country (Section 2.2.2). Currently, they are available in research settings where a cyclotron and chemists are nearby.
Drug Development
The Pharmaceutical Research and Manufacturers of America (PHRMA 2006) reported for 2006 that 646 medicines were under development for cancer. This wealth of targets and therapeutic agents bodes well for individualized disease management. However, capitalizing on these developments requires years of work and considerable financial commitment. Using current approaches, the cost to bring a new drug to market is now estimated to be between $0.8 billion (DiMasi et al. 2003) and $1.7 billion (Mullin 2003) with a substantial risk of failure (Nunn 2006). In part, this is because only one in five drugs that enter clinical trials actually proceeds to an approval stage (Wierenga and Eaton 2007), and many drugs fail in the late stages of clinical testing (i.e., phase II or III), after a considerable amount of money has been spent. Moreover, the time line for bringing a new drug to clinical use takes, on average, 12 years (Wierenga and Eaton 2007).
The time and expense required to bring a drug to market may be reduced by using nuclear medicine imaging technologies to identify which drugs should advance from animal to human studies, reveal mechanisms of drug action, evaluate drug distribution to target tissue; establish the drug occupancy of receptor sites; assess the actions of new agents on specific molecular targets or pathways; and determine appropriate dose range and regimen (Eckelman 2003). It has been estimated that the use of PET during Phase I studies (Sidebar 2.6) could save upward of $235 million in research and drug development costs (Phelps 2006) for each successful drug. It is anticipated that the drug development process will be facilitated by the availability of molecularly targeted radiopharmaceuticals, high-resolution PET/CT and PET/MRI imaging machines, and image quantification software. With this in mind, the Oncology Biomarker Qualification Initiative, through cooperation among big Pharma, the Food and Drug Administration (FDA), the National Cancer Institute (NCI), and CMS, has begun to foster qualification of molecular imaging endpoints (FDA 2006b). The overall approach will bring together the strengths of these three agencies and the pharmaceutical industry to determine the optimum use of biomarkers to evaluate treatment response. Clearly, radiotracers are likely to be excellent biomarkers. For example, the NCI has begun the development of task forces to plan joint trials based on PET/CT with the goal of qualifying FDG as a biomarker in non-small-cell lung cancer and in lymphoma.
|
SIDEBAR 2.6 Introduction to Clinical Trials A clinical trial is a research study conducted in human volunteers that is designed to answer specific questions. There are different types of clinical trials, such as treatment, prevention, diagnostic, and screening trials, each of which answers a different question. Treatment trials are the most common, and clinical trials are conducted in phases, where each phase has a different purpose. Phase I trials: An experimental drug or treatment is given to a small group of patient volunteers (usually between 20 and 80) to evaluate its safety, determine a safe dosage range, and identify side effects. Phase II trials: The experimental drug is given to a larger group of patient volunteers (typically 100 to 300) to determine whether it is effective and to further evaluate its safety. Phase III trials: The effectiveness of the experimental drug is confirmed when compared to commonly used treatments in large groups of patient volunteers. Sample size largely depends on the anticipated effect size from the treatments being compared and the size needed to detect a difference. Typically, phase III trials have several hundred to several thousand patient volunteers. Side effects and safety in patients continue to be monitored. Phase IV trials (also known as post-marketing studies): These studies are conducted to collect additional information on the drug’s risks and benefits that may not have emerged during the previous studies. SOURCE: NIH 2006. |
Furthermore, the value of molecular imaging in drug discovery and development has been recognized by big Pharma with nearly 65 percent of drugs losing their patent protection by 2010, which represents a $70 billion loss in revenue per year. Merck, Glaxo, Pfizer, Bristol-Myers-Squibb, Genentech, and Johnson and Johnson are among the companies with active in-house or collaborative programs for conducting radiotracer imaging as a guide to drug discovery and development. The types of studies being conducted relate to both pharmacokinetics, through labeling of drugs of interest, and pharmacodynamics, using molecular imaging for key processes (e.g., glycolysis, proliferation, and hypoxia) fundamental to oncology and other medical specialties, as ways to observe the effects of drugs in vivo. In some of the larger programs, such as those of Merck and Glaxo, the staff is measured in the dozens, and includes nuclear medicine physicians, medicinal chemists, kineticists, radiochemists, pharmacologists, and imaging technicians.
Imaging development is often in the context of the broad capabilities
of molecular imaging and may include magnetic resonance and spectroscopy, bioluminescence and fluorescence imaging, but nuclear imaging plays a prominent role. In part, this is because of major advances in enabling technology, such as microPET and microSPECT (single positron emission computed tomography), with resolution in the 1-mm range that is suitable for experiments and small laboratory animals. New molecules under development are often chemically designed for easy radiolabeling to facilitate the production of representative radiotracers for pharmacological bioavailability and pharmacodynamic studies. Radiotracers developed in this way are often initially studied in animals, with straightforward pharmacology studies seamlessly translated to human volunteers using PET/CT high-resolution imaging.
Also, in the past few years, medical imaging instrument companies have teamed up with radiopharmaceutical development groups, within both industry and academia, to foster radiopharmaceutical development for the rapidly growing market in PET/CT and SPECT/CT. General Electric acquired Amersham, a large radiopharmaceutical company. Siemens acquired CTI, including PET-NET, which is a network of radiopharmacies involved in distributing positron emitters and single photon emitters to nuclear medicine practitioners within hospitals. Phillips has developed numerous collaborations with academic institutions in both Europe and the United States in molecular imaging.
In companies that develop neuroleptic drugs, there has been a heavy emphasis on pharmacokinetic and pharmacodynamic studies directed at evaluating saturation of key receptors in vivo. These studies are being done because of the recognition that doses that are greater than those required to saturate the target neuroreceptor simply result in more neurotoxicity without beneficial effects. For example, the dopamine D2 receptor binding agent carbon-11-racalopride may be used as a radiotracer for the dopamine D2 receptor, and a novel new drug intended for use in schizophrenia, with high affinity for the D2 receptor, may be used in conjunction with the radiotracer to find optimal dosage regimens in humans that will just saturate the dopamine D2 receptor, as shown by the displacement of carbon-11-racalopride. In this way, the optimal biologic dosage may be used without running the risk of binding to collateral receptor targets in the brain and producing undesirable toxicity.
Targeted Radiotherapeutics
Therapeutic nuclear medicine procedures are now used to treat thyroid cancer and other thyroid disorders, relieve pain from bone metastases, or
|
SIDEBAR 2.7 Scientific Fields Expanding Nuclear Medicine Capabilities Nanotechnology is a broad scientific field that creates and uses materials and devices that are so small they are measured in nanometers. One nanometer is one-billionth of a meter. Although application of this technology is still limited, it is expected to change the computer industry and medical practice (NNI 2007). Materials science is an inter-disciplinary field comprising applied physics, chemistry, and engineering that studies the physical properties of matter and its applications. Microfluidics is a multi-disciplinary field that studies how fluids behave at microliter and nanoliter volumes and stimulates the design of systems in which small volumes of fluids are used to provide automated sample processing, synthesis, separation, and measurements in devices commonly described by the term “lab-on-a-chip” (see Chapter 6). For example, microfluidics is used in DNA analysis. |
treat blood disorders such as lymphoma and polycythemia vera.5 Research programs and clinical trials are currently underway to address the utility of molecularly targeted radionuclide therapies in treating rheumatoid arthritis, degenerative joint diseases, heart disease, non-small-cell lung cancer, colon cancer, prostate cancer, pancreatic cancer, ovarian cancer, meningitis, and AIDS. However, this is only the beginning. Information from large-scale omic analyses stimulated by the Cancer Genome Atlas Project6 and from focused molecular studies being conducted throughout the scientific community may reveal many new tumor-specific molecules through which therapeutic doses of radiation can potentially be delivered. New carrier molecules exploiting nanotechnologies and other advances in materials science (Sidebar 2.7) may further increase the efficacy of targeted radiotherapeutics. It is envisioned that treatments that are specific to a patient will be developed. For example, depending on the characteristics of a tumor, a variety of radionuclides, carrier molecules, and molecular targets (see Sidebar 2.3) could be used to maximize treatment efficacy and minimize normal tissue toxicity. Targeted radionuclide therapy is further discussed in Chapter 7.
|
5 |
Polycythemia vera is a blood disorder where there is an overproduction of red blood cells, white blood cells, and platelets. Patients with this disorder are prone to developing clots that can result in strokes or heart attacks. |
|
6 |
The Cancer Genome Atlas Project is an interdisciplinary program established and administered by the NCI (NCI 2007a) and the National Human Genome Research Institute. Its goal is to comprehensively measure changes in DNA sequence, genome copy number, allelotype, gene expression, and methylation in normal and cancer cells in order to identify abnormalities that influence cancer genesis and progression. |
2.2.2
Enabling Technologies
The future of nuclear medicine depends on the development of enabling technologies in several areas. These include technologies that will cost-effectively increase access to radionuclides, miniaturize the chemical process that will make it possible to produce multiple different radiopharmaceuticals to meet pre-clinical and clinical demands, and increase the speed and resolution of SPECT, PET, and combined-modality imaging.
Compact Devices to Generate Radionuclides with Short Half-Lives
One of the principal obstacles to realizing the full potential of nuclear medicine in advancing medical science and patient care is the limited accessibility of radionuclides with short half-lives (i.e., less than 30 minutes). Although these radionuclides have numerous advantages (Sidebar 2.8),
|
SIDEBAR 2.8 Advantages of Radionuclides with Short Half-Lives Carbon, nitrogen, oxygen, and fluorine are common elements found in biologically active molecules and pharmaceuticals. The use of the radionuclides carbon-11, nitrogen-13, and oxygen-15 as replacements for non-radioactive carbon, nitrogen, and oxygen provides radioactive compounds with the exact same chemical and biological properties as the non-radioactive compounds. As carbon is abundant in biologically active molecules, the replacement of carbon-12 by carbon-11 provides a convenient way to produce many tracers and drugs where the properties of the parent carbon-12 molecule are well-established. There are many examples of carbon-11 compounds that have been developed. They have been used to study carbohydrate and lipid metabolism, receptors and enzymes in the brain, and drug pharmacokinetics.a Carbon-11 (half-life = 20 min), nitrogen-13 (half-life =10 min) and oxygen-15 (half-life = 2 min) are all positron emitters with short half-lives. Short-lived radionuclides have several advantages. The absorbed radiation dose to the patient being studied is generally less than with a longer-lived tracer, allowing more tracer to be injected. In turn, the higher amount of tracer increases the signal that the imaging instrument can detect. Furthermore, several studies may be performed on the same patient on the same day since the tracer radioactivity decays quickly. Despite their versatility, their short half-lives limit their use to institutions that are near facilities that can rapidly synthesize and purify radiotracer compounds so that imaging studies can be completed before the radioactivity decays. |
their use dictates that imaging be performed near the facility in which the radionuclide is produced. The current supply is from a few cyclotrons that are primarily used to produce fluorine-18. The initial investment for such a cyclotron is $2 million, with an additional $0.5 million needed for renovation and installation. At a minimum, another $0.8 million is needed to cover annual operating costs,7 assuming no major repairs are needed (personal communication, Thomas Budinger, Lawrence Berkeley National Laboratory, July 2, 2007). Consideration could be given to developing a low-cost, low-maintenance accelerator that would be in the category of a tabletop instrument. Some design specifications for a compact generator that might be developed include a miniature linear proton accelerator using modern engineering and new target designs, acceleration of helium-3 atoms into a primary target doped with deuterium to produce 15 MeV protons, photonuclear-based isotope production, and laser-stimulated proton production. Shielding and minimization of external radiation would have to be incorporated into its design.
Nanotechnology and Microfluidics
Remarkable advances are now being made in materials science and microfluidics that provide unique opportunities in nuclear medicine. Miniaturization of radiochemical production systems has the potential to improve reaction yields, increase cost-effectiveness, and expand access to the products to more users. These smaller devices in combination with compact radionuclide generators may facilitate the production of multiple radiopharmaceuticals to meet the pre-clinical and clinical demands of researchers and physicians. The miniaturization of the chemistry will make it possible to reduce radiation shielding requirements and further simplify the required infrastructure for preparing the radiopharmaceuticals. New chemical reagents, such as polymer-supported precursors, may also be used to produce cleaner, higher specific-activity tracers. Increased specific activity and decreased impurities will assist with FDA approval of tracers.
Scintillator8 Crystals and Semiconductors
Both PET and SPECT depend on multi-element radiation detectors to produce anatomic images of radionuclide distribution (Sidebar 2.9). The images achieved in current instruments are degraded by radiation scattering
|
7 |
Annual operating costs include salaries for a full-time cyclotron technician, a full-time radiopharmacist, a half-time radiochemist, supplies, service contracts, and overhead. |
|
8 |
A scintillator is a substance that absorbs energy of charged particle radiation or gamma radiation and then releases this energy through fluorescence. |
within the body.9 Detection of this scatter can be decreased substantially by improving the energy resolution of multi-element radiation detectors used in imaging. This can be accomplished by developing detectors that have increasing radiation detection efficiency (photoelectric absorption), short timing resolution, good energy resolution, high luminosity, and low dead time. Next-generation fast, high-efficiency scintillators are now being developed to support homeland security applications that are intended to have this optimum combination of detector properties. These new detector materials will substantially improve nuclear medicine imaging when incorporated into next-generation PET and SPECT devices.
Combined-Modality Imaging (PET/CT, PET/MRI, and SPECT/CT)
CT, MRI, and PET all provide complementary “views” of normal and diseased tissues, with PET offering quantitative functional information and MRI and CT scans providing high-resolution anatomical information. The power of combined-modality imaging will increase dramatically as molecularly targeted radiotracers with high specific activity are developed and as the sensitivity and resolution of PET increase to allow for high-resolution, temporal imaging. The simple methods developed in the 1970s for image reconstruction in microscopy are no longer sufficient for reconstructing images taken with PET and SPECT. The images are reconstructed using iterative procedures that take into account the relationship between the image space and the detector or projection space. The connection between the two is known as the system matrix. In modern imaging technologies, the system matrix becomes quite large, and currently, the computational speed and memory of commercial computers are inadequate. Full realization of the potential of combined-modality imaging will emerge as computational techniques are developed to manage noise (i.e., increase the signal-to-background ratio) and improve segmentation,10 feature recognition, and multimodality image registration.11
|
9 |
Scattering is a physical process in which particles are deflected from their paths through interactions with other particles. |
|
10 |
Image segmentation is a procedure that allows for unwanted structures in the image to be removed. |
|
11 |
Data taken from a given patient at different points in time or from different angles need to be transformed so comparisons can be made. Image registration is the process by which the different measurements are integrated. |
|
SIDEBAR 2.9 Introduction to the Physics of PET A radiotracer that is labeled with a positron-emitting radionuclide, such as fluorine-18, is injected into the patient undergoing a PET scan. The radionuclides then decay, emitting positrons. The resulting positrons subsequently annihilate on contact with electrons within the body (Figure 1). Each annihilation event produces two photons traveling in opposite directions that are detected by the detectors surrounding the patient. If this detection occurs within a certain time, it is considered to have come from the same annihilation event and is “coincident” (Figure 2). 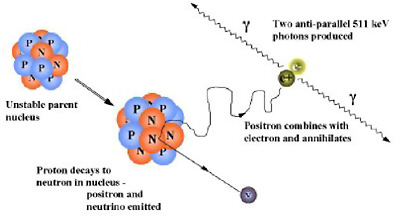 FIGURE 1 Positron emission and annihilation. 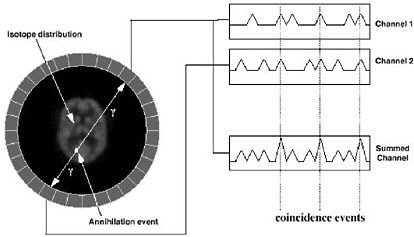 FIGURE 2 Schematic of coincidence event detection. |
|
In PET, there are four types of coincidence events: true, scattered, random, and multiple. Figure 3 illustrates the first three. A coincidence event is assigned to a line of response (LOR). In this way, positional information is gained from the radiation that is detected. 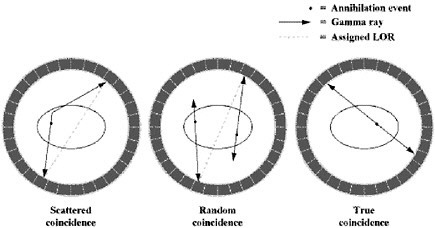 FIGURE 3 Types of coincidences. Time-of-flight means that for each annihilation event the precise time that each of the coincident photons is detected is noted and the difference in arrival time is calculated. Since the closer photon will arrive at the detector first, calculating the difference in arrival time helps determine the location of the annihilation event between the two detectors. Theoretically, perfect time-of-flight information would eliminate the need to reconstruct images. However, even the addition of imperfect time-of-flight information reduces noise and improves the image by approximating the location of the annihilation event. This improvement in image quality is particularly useful in large patients (Badawi 1999, Karp 2006). |
2.3
COMPLEXITIES OF NUCLEAR MEDICINE PRACTICE AND RESEARCH
The field of nuclear medicine is multi-disciplinary, and successful development and delivery of these potentially life-saving procedures to patients involves specialists from clinical fields such as radiology, nuclear medicine, cardiology, oncology, psychiatry, infectious disease, surgery, and endocrinology, collaborating with imaging specialists, engineers, computer scientists, physicists, chemists, and molecular biologists.
As described above, the developments in nuclear medicine over the past 50 years have been extensive and the future is bright. However, for the field to flourish, there are scientific, regulatory, and financial obstacles that need to be addressed. Section 2.3.1 describes the basic science research challenges; Section 2.3.2 summarizes clinical research challenges; Section 2.3.3 summarizes the regulatory hurdles and explores the costs and economics of the field; and Section 2.3.4 discusses radiation exposure from nuclear medicine procedures and its relative safety.
2.3.1
Basic Science Research Challenges
Most of the significant developments in nuclear medicine during the past 50 years have leveraged the substantial engineering and physical sciences infrastructure that was developed to support research in nuclear physics, neutron science, and nuclear power technologies, which included, but were not limited to nuclear power production and nuclear propulsion. For example, the nuclear reactors used to produce radionuclides were developed primarily in support of research directed toward nuclear power generation. Similarly, cyclotrons used to produce medical radionuclides were developed using technologies that were originally invented to support particle physics research. This is also true for the scintillator detectors and electronics used in PET imaging. In addition, these technological advancements were the result of work spanning several decades. Long-term investments that could sustain multi-disciplinary teams were necessary to allow for the time needed to develop new concepts using insights from the fields of physics, chemistry, and materials science; to identify areas of biology or medicine where these concepts might be applied; and to develop practical devices or reagents that could be tested in the biological or clinical setting.
Today, the substantial nuclear physics research infrastructure that spawned many developments in nuclear medicine has diminished considerably. It has been offset to some extent by research infrastructure being developed to support efforts in biofuels12 research, homeland security, and
nanotechnology. However, the financial support needed to sustain research in nuclear physics and these new fields to advance nuclear medicine has been reduced, and the funding that remains is generally awarded to small teams of scientists for short durations. As a consequence, research teams focus predominantly on short-term proof-of-principle experiments and not on sustained development of practical instruments or radiotracer chemistry methods. This change in research focus has substantially impeded the development of next-generation technologies in nuclear medicine that could potentially allow more personalized health care.
2.3.2
Clinical Research Challenges
Beyond the need for greater inter-disciplinary cooperation, the field of nuclear medicine faces other challenges that are unique to it. Investigators face regulatory hurdles in the investigational new drug (IND) application process13 (explored further in Section 2.3.3), limited isotope availability (Chapter 5), and a shortage of expertise in radiopharmaceutical chemistry, radiopharmacy (Chapter 8), and image acquisition and interpretation. All of these are barriers to bringing novel imaging agents, radio-therapeutics, and devices to the clinical environment.
Assuming that animal studies of a new diagnostic or therapeutic radiopharmaceutical produce encouraging results and that studies in humans can begin, the field is currently limited by a lack of standardization and coordination in clinical trials. In the field of targeted radionuclide therapy, for example, there are currently too many individual clinical trials enrolling too few patients and treating them in widely varying ways. Similarly, clinical trials for diagnostic imaging suffer from different clinical centers using different imaging platforms. This has led to a lack of uniformity in how data are acquired, handled, stored, and interpreted. Building on the experience of cooperative groups, such as the American College of Radiology Imaging Network (ACRIN 2007) and the U.S. FDA, it is important that investigational approaches be standardized so that data can be compared across clinical trials and translated into clinical practice.
2.3.3
Regulatory Hurdles and Costs
Regulatory requirements pose additional hurdles to translational research and clinical investigations. In the United States, all pharmacologic
|
13 |
During drug development, promising candidates are selected using in vitro models. Candidates that are not rejected are then tested in in vivo animal models. After toxicological data have been collected and basic safety tests have been performed in animals, an IND must be submitted to the FDA before testing in human subjects can begin in a phase I study (see Sidebar 2.6). |
agents, including diagnostic radiopharmaceuticals and radiotherapeutics, undergo regulatory oversight by the FDA. However, radiopharmaceuticals face additional scrutiny and have unique regulatory and approval pathways. At times, the requirements, such as extensive toxicology testing, have been unpredictable, posing considerable financial burdens that are frequently beyond the means of investigators in academia. There are concerns that these regulatory hurdles have impeded translational research to the extent that some major clinical investigations have migrated from the United States to other countries with less demanding requirements.
For example, obtaining an IND to permit clinical evaluation of a promising targeted radiotherapeutic agent requires toxicology data and information on pharmacokinetics and dosimetry of the radiopharmaceutical (FDA 2005). Furthermore, radiochemistry methods frequently need significant modification and optimization in order to be able to reliably supply the labeled drug at the activity levels needed for patient treatment. Because this is generally not hypothesis-driven research, it is difficult to obtain grant support for it. Unlike the Development of Clinical Imaging Drugs and Enhancers program (NCI 2007b), which can help offset the costs of some of these studies for imaging agents, there is no analogous mechanism available for targeted radiotherapeutics.
In an effort to reduce some of the regulatory hurdles, the FDA issued guidance to the research community on the exploratory Investigational New Drug (eIND)14 process (FDA 2006a). The stated goal of eIND studies is to reduce the time and resources expended on candidate products that are unlikely to succeed. Although the introduction of eINDs has aided in bringing new radiotracers, it is too early to determine whether the stated goal will be achieved.
An academic research base is necessary to allow industry to rise to the challenge of delivering novel technology for future clinical use. For clinical trials to be successful, industry must be engaged and see a clear pathway for economic success. Industry will not develop the technology necessary to deploy the next generation of genome-based medicines unless the scientific and economic rationale has been identified by academia.
|
14 |
In its March 2004 Critical Path Report, the FDA stated that new tools were needed to distinguish earlier in the drug development process which candidates hold promise and which ones do not. Because only 8 percent of new medical compounds entering phase I testing reach the market, the FDA established eIND studies as a way of trying to reduce the time and cost of drug development. In an eIND study, the goal is to verify results observed in experimental models in humans and determine pharmacological properties rather than determine dose-limiting toxicities. Because eIND studies present fewer potential risks than do traditional phase I studies, they may reduce the number of human subjects and resources needed to identify promising drugs. |
2.3.4
Radiation Exposure and Safety
There has been greater awareness recently that medical imaging procedures, especially interventional, CT, and nuclear medicine tests, contribute significantly to the annual collective radiation dose (AMA 2006, NCRP 2007, Amis et al. 2007). In the past 25 years, medical radiation exposure has risen from about 15 percent to greater than 50 percent of the total annual exposures to the U.S. population. For the currently estimated medical collective dose of 930,000 person-Sieverts (Sv), 23 percent derives from nuclear medical procedures, of which 85 percent are cardiac examinations. The individual (effective) dose for a rest and exercise cardiac study using a technetium-99m-labeled agent is ~10mSv and is estimated to produce an approximate radiation-induced cancer risk of 0.05 percent in a naturally healthy individual against a background cancer rate of approximately 40 percent (NRC 2006). Given the age and infirmity of the group undergoing cardiac studies, this risk estimate is undoubtedly overestimated. Nonetheless, indications for such procedures need to be deemed to have a medical benefit and continued efforts must be made to reduce the absorbed radiation dose without sacrificing diagnostic accuracy. In 2006, the American Medical Association House of Delegates adopted a directive, in collaboration with specialty societies and interested stakeholders, “(a) to examine the feasibility of monitoring and quantifying the cumulative radiation exposure sustained by individual patients in medical settings; and (b) to discuss methods to educate physicians and the public on the appropriate use and risks of low linear energy transfer radiation in order to reduce unnecessary exposure in the medical setting.”
The increasing use of FDG-PET scanning, which provides approximately the same absorbed dose to patients as cardiac studies, deserves similar considerations as well as raises some additional concerns given the higher energy of annihilation (positron-producing) photons. Shielding and other measures need to be employed in order to protect radiation personnel, families, and bystanders (Madsen et al. 2006) and need to be incorporated into contemporary clinical and preparatory facilities.
Radiopharmaceuticals are administered in small mass amounts, generally nanomoles, so as to follow the tracer principle. Consequently, imaging agents have little or no pharmacologic effect. Furthermore, the tracer activities employed in nuclear medicine diagnostic procedures result in radiation doses well below the threshold for any acute (deterministic) radiation toxicity. The incidence of misadministration has been extremely low in the past (NCRP 1991). With the promulgation of even stricter rules of the Joint Commission on Accreditation of Healthcare Organizations on monitoring of all prescribed substances to patients, new measures aimed to lessen
patient, staff, and family radiation exposures to offset the trend set by the increasing number of diagnostic procedures is to be expected.
2.4
CONCLUSION
We have arrived at a crossroads in nuclear medicine. Further development of the field will likely contribute substantially to the development of personalized medicine by (1) providing more efficient and lower cost strategies to bring new drugs to market; (2) developing new and more effective treatments for cancer and cardiovascular disease; (3) improving understanding of abnormal physiological conditions; and (4) developing new, effective anticancer drugs. Moreover, new developments in accelerator engineering, computer science, materials science, chemistry, and nanotechnology suggest that a new generation of nuclear medicine instruments and radiopharmaceuticals can now be made that will be less expensive, more widely available, and more precise. Although there are challenges ahead, by investing in the infrastructure of radionuclide production; committing to train and nurture the next generation of nuclear medicine researchers, technicians, and clinicians; and developing a program that will sustain nuclear medicine research, we will all reap the benefits of better health care.


























