Process Review of Lignocellulose Biochemical Conversion to Fuel Ethanol
BRUCE S. DIEN
National Center for Agricultural Utilization Research
U.S. Department of Agriculture
Peoria, Illinois
The United States is in transition. Each year we use 140 billion gallons of gasoline to fuel our cars. Over 50 percent is imported, and transportation accounts for 50 percent of U.S. greenhouse gas emissions. Increasingly worrisome headlines related to energy security and economic interests and strident warnings from climate scientists all suggest the time has come to reduce oil usage and its related CO2 emissions.
Currently three routes are available to reduce imported oil and greenhouse gas emissions associated with transportation: conserve, switch to plug-in hybrids, and rely on renewable biofuels. Quite likely all three will be needed to reduce gasoline consumption in a meaningful manner. Coal liquefaction dates back to World War II and can reduce or even eliminate oil imports but will increase, possibly even double, CO2 emissions.
Renewable fuels are defined as liquid fuels produced from biomass. The concept is simple; plants recycle the CO2 released from combustion and the plants are in turn harvested and converted to fuels. It is actually more complex because fossil fuels are used to plant, grow, harvest, and process the biomass into fuels. Despite this, lifecycle analysis indicates biofuels, especially from lignocellulose biomass, can greatly reduce CO2 emissions and very efficiently reduce net gasoline usage (Farrell et al., 2006).
Major sources of biofuels are ethanol and to a much lesser extent biodiesel. Last year 5 billion gallons of ethanol (95 percent from corn) was produced, and production is expected to grow to 10 billion gallons in the next few years
(Westcott, 2007). After that any further growth in biofuel production will need to rely on lignocellulosic feedstocks because of competing demands for corn from food, industrial, and export uses (Westcott, 2007).
Lignocellulose includes agricultural residues, forest industry wastes, and (potentially) perennial energy crops. Agricultural residues include corn stover (e.g., stalks and cobs) and wheat straw. Forest industry wastes include lumber scraps, pulping waste, as well as urban-generated cellulosic wastes. Perennial energy crops include warm season grasses (e.g., switchgrass) and fast growing trees (e.g., loblolly pine and poplar hybrids). Currently no crops are grown for energy, but warm season grasses are grown for forage, and trees, of course, are grown for pulping and to make lumber. Estimated availability of each is in the hundreds-of-million-ton range (Figure 1) and all summed together could theoretically meet 20 percent of our total liquid transportation fuels by 2017 (Perlack et al., 2005).
Recently the U.S. Department of Energy announced that it would help fund six commercialization efforts for converting biomass to biofuels, most of which are related to production of ethanol. While ethanol is the leading candidate for a renewably generated liquid fuel, there are other alternatives, some dating back nearly as far as ethanol. These include butanol produced by acetone-butanol-ethanol (ABE) fermentation of biomass, synthetic gasoline gasification produced by gasifying biomass to syngas followed by Fisher-Tropsch reformation, ethanol
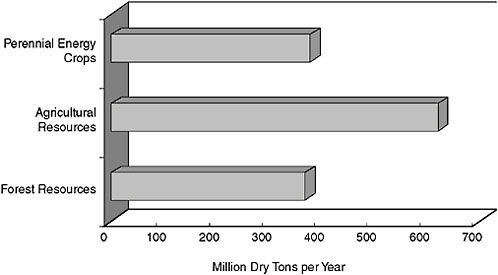
FIGURE 1 U.S. land can supply 1.3 billion tons of biomass for biofuels and still meet other needs. Source: Adapted from Perlack et al., 2005.
produced by fermentation of biomass-derived syngas, and biodiesel produced from algae grown in huge saltwater ponds. Algae are the exception in relying on a new “crop” and are also by far the earliest in development. Each is being actively pursued for commercialization; limited space prevents further discussion of these alternatives.
CHEMICAL COMPOSITION OF BIOMASS AND THEORETICAL ETHANOL YIELDS
Carbohydrates are the only portion of the plant that can be fermented to ethanol. In fibrous biomass, carbohydrates are mostly present in the plant cell walls and are in the form of cellulose and hemicellulose. Cellulose can be converted to glucose and hemicellulose to a mixture of sugars, the composition of which varies with the source of biomass. Herbaceous hemicellulose contains mostly xylose and significant amounts of arabinose and glucoronic acids (Dien et al., 2005). Other minor sugars include galactose and ribose. While glucose is a hexose and has six carbons, arabinose and xylose contain five carbons and are termed “pentoses.” The major significance of this is that distillers’ yeast (Saccharomyces) cannot ferment pentose sugars. This precludes the use of commercial yeast in converting lignocellulose to ethanol.
Plants are approximately 60 percent wt./wt. carbohydrates, of which hemicellulose accounts for about a third (Wiselogel et al., 1996). When fermented to ethanol, one CO2 is produced for each ethanol, so the theoretical yield for neutral sugars is 0.51 g of ethanol per 1 g of sugar. For herbaceous biomass the theoretical yield of ethanol is 100-110 gal. per dry ton; which compares with 124 gal. per dry ton for corn (for conversion calculator see www1.eere.energy.gov/biomass/ethanol_yield_calculator.html). As a practical matter most experts use conversion factors of 60-90 gal. of ethanol per dry ton of biomass, with the lower range having been demonstrated and the upper limit extrapolated from current research.
BIOCHEMICAL CONVERSION
Many processes have been conceptualized for converting fibrous biomass to ethanol. All have common aspects, so I will first discuss a conceptual design for a dilute acid pretreatment (Figure 2) (Aden et al., 2002). Dry biomass that arrives at the facility is first cleaned and milled. The biomass is mixed with a dilute mineral acid solution to a solids consistency of 30-40 percent wt./wt. The biomass is conveyed to a steam explosion reactor where it is heated to 180-220°C for 0.5-5.0 minutes before being quickly (and explosively) cooled by the sudden release of the reactor pressure (Schell et al., 2003). Treating in this manner physically reduces particle sizes and changes the consistency of the product from a damp fiber to sludge. It also breaks down the plant cell wall, removing the hemicellulose to the syrup, displacing the lignin, and swelling the tightly (highly crystalline)
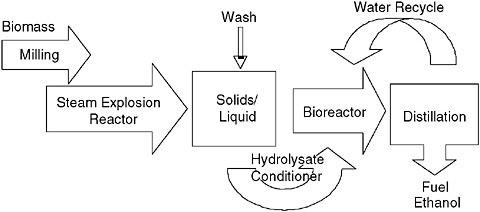
FIGURE 2 Conceptual flow diagram of process for converting lignocellulose to ethanol (see text for details).
arrayed cellulose fibers. To recap, following the steam explosion the solids consist largely of lignin and cellulose, and the syrup contains most of the hemicellulose carbohydrates, water extractables, a little glucose, and minor amounts of released lignin products.
Following pretreatment the solids are recovered and washed, possibly using a press. The syrup and wash water are mixed and the residual sulfuric acid neutralized by adding lime. Pretreatment produces a wide variety of soluble side products, some of which are quite toxic to microbes. Therefore, the syrup often needs to be conditioned to reduce its toxicity prior to fermentation. Following this the syrup is remixed with the solids. At this point the biomass is too thick to ferment directly, so enzymes are added to thin the slurry and to begin saccharifying the cellulose to glucose. For current commercial enzymes the temperature is held at 50-55°C for 18-24 hours. Next, the biocatalyst is added, which begins to ferment the released sugars to ethanol. The fermentation temperature will generally be lower than 50°C, but its specific set point will depend upon the choice of microorganism. At the same time the fermentation is occurring the enzymes continue to release sugars for fermentation. As mentioned above, a special microbe needs to be used that is capable of fermenting the pentose sugars in addition to the glucose. A number of microbes are now available that ferment either xylose or both xylose and L-arabinose in addition to glucose. The fermentation could theoretically last up to 7 days, but is usually ended after 3 days. The ethanol is stripped out of the beer, distilled, and finished by passing through a molecular sieve to remove the last of the water. The solids (e.g., largely lignin) are recovered from the stillage by centrifugation and combusted to generate steam for the overall process. The recovered liquid is (hopefully) treated and recycled in the process.
There are many process variations for converting biomass to ethanol. In a simultaneous saccharification and fermentation (SSF), the microbe is added with the enzyme (Takagi et al., 1977; Emert and Katzen, 1980). The key advantage of co-adding them is that the microbe ferments glucose immediately to ethanol, thereby avoiding any buildup of glucose in the culture. Maintaining a low glucose concentration has the advantage of avoiding end-product inhibition of the enzyme and helps minimize the risk for contamination. Fermentations are run as open processes, and contamination is always a concern. Detailed technoeconomic models for SSF of poplar wood and corn stover have been developed by the National Renewable Energy Laboratory (Aden et al., 2002; Wooley et al., 1999). Enzymes are a major cost of processing biomass to ethanol. If microbes were used that produce some of their own enzymes, it would be possible to eliminate a large expense item. Using microbes that produce their own carbohydrolytic enzymes is the central theme of consolidated bioprocessing (CBP) (Den Haan et al., 2007; Katahira et al., 2006; Lynd et al., 2005). An alternative to SSF would be to completely hydrolyze the carbohydrates and remove the solids prior to fermentation, which is referred to as SHF. This is the process used by Iogen Corp. for its demonstration plant (Tolan, 1999). It has the advantages of making the fermentation faster because it is not enzyme limited, eliminates solids from the bioreactor, supplies a cleaner burning lignin, and may (theoretically) allow for recovering and recycling of enzymes. However, end-product inhibition of the cellulases is a major concern.
UNIT OPERATIONS
The major processing steps for converting biomass to ethanol are pretreatment, enzymatic saccharification, fermentation, and recovery. Lignocellulose contains primarily structural carbohydrates, which are highly resistant to enzymatic conversion to monosaccharides. Thermochemical pretreatment is needed to deconstruct the cell wall structure, allowing enzymes access to the carbohydrate polymers. The cell wall has been compared with reinforced concrete, where hemicellulose is the concrete, lignin the hydrophobic sealant, and cellulose microfibrils are the reinforcing bars (Bidlack et al., 1992). Specifically, pretreatment is needed to reduce particle size, dissolve the xylan, displace the lignin, and create broken ends in and swell the cellulose microfibers. There are numerous pretreatments available, some of which are summarized in Figure 3 (reviews: Dien et al., 2005; Mosier et al., 2005).
There are three major categories of enzymes for converting pretreated biomass into fermentable sugars: cellulases, xylanases along with auxiliary enzymes for debranching xylan, and ligninases. A list of these enzymes is presented in Table 1.
Cellulases are by far the most important, because they are used to convert
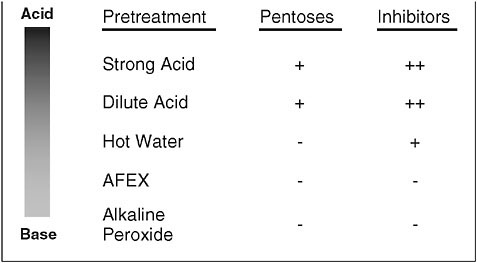
FIGURE 3 Selected pretreatments for lignocellulose. Strong acid and dilute acid have the advantage of producing monosaccharides while the neutral/alkaline pretreatments only solublized xylan. However, the acidic pretreatments also produce more inhibitors that are problematic for fermentation.
TABLE 1 Biomass-Related Enzymes
|
Cellulases endo-1,4-β-D-glucanase (EC-3.2.1.4), exo-1,4-β-glucanase (exocellobiohydrolase, EC-3.2.1.91) and β-D-glucosidase (β-D-glucoside glucanhydrolase, EC-3.2.1.21) |
|
Xylanases endo-1,4-β-D-xylanase, EC-3.2.1.8), β-xylosidase (EC-3.2.1.37) |
|
Xylan Debranching Enzymes α-L-arabinofuranosidase (EC-3.2.1.55), β-glucuronidase (EC-3.2.1.31), acetylxylan esterase (EC-3.1.1.72), feruloyl esterase (EC-3.1.1.73), p-coumaroyl esterase (EC-3.1.173), others |
|
Ligninases Lignin peroxidase (LiP, EC-1.11.1.7), manganese peroxidase (MnP, EC-1.11.1.13) and laccase (EC-1.10.3.2) |
cellulose into glucose (Zhang and Lynd, 2004). Dilute acid pretreatment converts the hemicellulose carbohydrates directly to monosaccharides and therefore only requires cellulase blends; commercial blends containing xylanase activity can in some cases improve conversion efficiency. Other pretreatments will solubilize the xylan but require additional enzymes (hemicellulases) (Saha, 2003) to saccharify
it completely to fermentable sugars. Ligninases have not been widely applied to biomass bioconversion as yet.
The bioethanol industry is dependent upon S. cerevisiae for fermentation of glucose. Unfortunately S. cerevisiae does not ferment pentose sugars, and these sugars are too abundant in lignocellulose to ignore. The bacterium Zymomonas mobilis also selectively produces ethanol and has been offered as a substitute for S. cerevisiae. But like Saccharomyces, Z. mobilis does not ferment pentoses. Therefore, researchers have had to depend upon molecular methods for developing new biocatalysts for converting pentoses (and especially xylose) into ethanol. Two approaches have been taken to solve this problem: (1) engineering S. cerevisiae and Z. mobilis to ferment xylose and in the case of Z. mobilis also arabinose or (2) engineering Gram-negative bacteria that use a wide variety of sugars to selectively produce ethanol under anaerobic conditions. There has been considerable work on using the latter strategy to develop Gram-positive bacteria, but while progress is being made, full success has been elusive. Table 2 reviews the microorganisms available for fermenting xylose (Dien et al., 2003; Jeffries and Jin, 2004).
TABLE 2 Comparison of Batch Fermentations with Xylose for Ethanologenic Strains
|
Strain |
Host |
Xylose (g/l) |
Max. EtOH (g/l) |
EtOH Effa(%) |
Reference |
|
E. coli |
K011b |
90 |
41.0 |
89 |
Yomano et al. (1998) |
|
|
FBR5 |
95 |
41.5 |
90 |
Dien et al. (2000) |
|
|
LY01 |
140 |
63.2 |
88 |
Yomano et al. (1998) |
|
K. oxytoca |
M5A1(pLOI555) |
100 |
46.0 |
95 |
Ohta et al. (1991) |
|
Z. mobilis |
CP4:pZB5 |
60 |
23.0 |
94 |
Lawford et al. (1999) |
|
S. cerevisiae |
TMB 3400c |
50 |
13.3 |
67 |
Karhumaa et al. (2007) |
|
|
RWB 218c |
20 |
8.4 |
85 |
Kuyper et al. (2005) |
|
|
RE700A(pKDR)d |
45 |
19.0 |
74 |
Sedlak and Ho (2004) |
|
aEthanol efficiency: % yield of theoretical based upon 51 g ethanol per 100 g of xylose present. bOn 140 g/l xylose, strain K011 produced 59.5 g/l ethanol in 120 hr (Yomano et al., 1998). cCultured on mineral medium. dCultured on rich medium, estimated from Fig. 8 of Sedlak and Ho (2004). |
|||||
FUTURE TRENDS
Ethanol production from wood dates back before World War II. Modern technology has allowed the potential of much higher yields with a smaller environmental footprint. However, challenges remain, and further research will be needed to make lingocellulosic ethanol cost-competitive. Efforts will continue toward producing more robust pentose-fermenting microorganisms with higher productivity and more efficient, less-expensive enzymes. More work will also be directed at understanding the cell wall and the sources of biomass recalcitrance. Simultaneously there should be increased efforts to engineer plants for easier conversion to sugars (e.g., less lignin or altered cell wall structures) or that produce some of the enzymes needed for breaking down cell walls in situ. The U.S. Department of Energy has recently announced that it will fund three institutions for 5 years with this goal in mind (http://www.genomicsgtl.energy.gov/centers/).
In an attempt to jump start a lignocellulose ethanol industry the U.S. Department of Energy has announced that it will fund six commercial efforts for up to a total of $385 million (news release at http://www.energy.gov/news/4827.htm). Abengoa Bioenergy Biomass, Poet Companies, and Iogen Biorefinery will focus on biochemical conversion of herbaceous biomasses, including corn cobs and fiber, switchgrass, and wheat straw. Alico Inc. will use a hybrid process whereby the biomass is converted to syngas, and the syngas is then fermented to ethanol. Range Fuels will apply a strictly thermochemical approach. Blue Fire Ethanol Inc. will utilize the Arkenol process, which relies on strong acid hydrolysis (http://www.bluefireethanol.com). It is hoped by those working in the field that the combination of strong political and industrial interests will help to unlock lignocellulose as a commercially successful feedstock for ethanol.
REFERENCES
Aden, A., M. Ruth, K. Ibsen, J. Jerchura, K. Neeves, J. Sheehan, B. Wallace, L. Montague, A. Slayton, and J. Lukas. 2002. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover. Rep. NREL/TP-510-32438. Golden, CO: National Renewable Energy Laboratory.
Bidlack, J., M. Malone, and R. Benson. 1992. Molecular structure and component integration of secondary cell walls in plants. Proceedings of the Oklahoma Academy of Sciences 72:51-56.
Den Haan, R., J. E. McBride, D. C. L. Grange, L. R. Lynd, and W. H. Van Zyl. 2007. Functional expression of cellobiohydrolases in Saccharomyces cerevisiae towards one-step conversion of cellulose to ethanol. Enzyme and Microbial Technology 40:1291-1299.
Dien, B. S., M. A. Cotta, and T. W. Jeffries. 2003. Bacteria engineered for fuel ethanol production: Current status. Applied Microbiology and Biotechnology 63:258-266.
Dien, B. S., L. Iten, and C. D. Skory. 2005. Converting herbaceous energy crops to bioethanol: A review with emphasis on pretreatment processes. Pp. 1-11 in Handbook of Industrial Biocatalysis. Boca Raton, FL: Taylor and Francis Group.
Dien, B. S., N. Nichols, P. J. O’Bryan, and R. J. Bothast. 2000. Development of new ethanologenic escherichia coli strains for fermentation of lignocellulosic biomass. Applied Biochemistry and Biotechnology 84(6):181-196.
Emert, G. H., and R. Katzen. 1980. Gulf’s cellulose-to-ethanol process. CHEMTECH 10:610-615.
Farrell, A. E., R. J. Plevin, B. T. Turner, A. D. Jones, M. O’Hare, and D. M. Kammen. 2006. Ethanol can contribute to energy and environmental goals. Science 311:506-508.
Jeffries, T. W., and Y. S. Jin. 2004. Metabolic engineering for improved fermentation of pentoses by yeasts. Applied Microbiology and Biotechnology 63:495-509.
Karhumaa, K., R. Sanchez, L. B. Hahn-Hagerdal, and M. F. Gorwa-Grauslund. 2007. Comparison of the xylose reductase-xylitol dehydrogenase and the xylose isomerase pathways for xylose fermentation by recombinant Saccharomyces cerevisiae. Microbial Cell Factories 6:5.
Katahira, S., A. Mizuike, H. Fukuda, and A. Kondo. 2006. Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Applied Microbiology and Biotechnology 72:1136-1143.
Kuyper, M., M. P. Hartog, M. J. Toirkens, M. J. H. Almering, A. A. Winkler, J. P. van Dijken, and J. T. Pronk. 2005. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Research 5:399-409.
Lawford, H. G., J. D. Rousseau, A. Mohagheghi, J. D. McMillan. 1999. Fermentation performance characteristics of a prehydrolyzateadapted xylose-fermenting recombinant Zymomonas in batch and continuous fermentations. Applied Biochemistry and Biotechnology 77:191-204.
Lynd, L. R., W. H. Van Zyl, J. E. McBride, and M. Laser. 2005. Consolidated bioprocessing of cellulosic biomass: An update. Current Opinion in Biotechnology 16:577-583.
Mosier, N., C. Wyman, B. Dale, R. Elander, Y. Lee, M. Holtzapple, and M. Ladisch. 2005. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology 96:673-686.
Ohta, K., D. S. Beall, J. P. Mejia, K. T. Shanmugam, and L. O. Ingram. 1991. Metabolic engineering of klebsiella oxytoca M5A1 for ethanol production from xylose and glucose. Applied and Environmental Microbiology 57:2810-2815.
Perlack, R. D., L. L. Wright, A. F. Turhollow, R. L. Graham, B. J. Strokes, and D. C. Erbach. 2005. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply. Report DOE/GO-102995-2135. Washington, DC: U.S. Department of Energy and U.S. Department of Agriculture.
Saha, B. C. 2003. Hemicellulose conversion. Journal of Industrial Microbiology and Biotechnology 30:279-291.
Schell, D. J., J. Farmer, M. Newman, and J. D. McMillan. 2003. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor: Investigation of yields, kinetics, and enzymatic digestibilities of solids. Applied Biochemistry and Biotechnology - Part A Enzyme Engineering and Biotechnology 108(1-3):69-86.
Sedlak, M., and N. W. Y. Ho. 2004. Production of ethanol from cellulosic biomass hydrolysates using genetically engineered Saccharomyces yeast capable of cofermenting glucose and xylose. Applied Biochemistry and Biotechnology 113-116:403-416.
Takagi, M., S. Abe, G. H. Suzuki, G. H. Emert, and N. Yata. 1977. A method for production of alcohol direct from cellulose using cellulase and yeast. Pp. 55-571 in Proceedings of the Bioconversion Symposium. New Delhi: Indian Institute of Technology.
Tolan, J. S. 1999. Alcohol production from cellulosic biomass: The iogen process, a model system in operation. Pp. 117-127 in The Alcohol Textbook Third Edition. Nottingham, UK: Nottingham University Press.
Westcott, P. C. 2007. Ethanol Expansion in the United States. Rep. FDS-07D-01. Washington, DC: U.S. Department of Agriculture.
Wiselogel, A., S. Tyson, and D. Johnson. 1996. Biomass feedstock resources and composition. Pp. 105-119 in Handbook on Bioethanol: Production and Utilization. Washington, DC: Taylor & Francis Inc.
Wooley, R., M. Ruth, J. Sheehan, K. Ibsen, H. Majdeski, and A. Galvez. 1999. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis Current and Future Scenarios. Rep. TP-580-26157. Golden, CO: National Renewable Energy Lab.
Yomano, L. P., S. W. York, and L. O. Ingram. 1998. Isolation and characterization of ethanol-tolerant mutants of Escherichia coli KO11 for fuel ethanol production. Journal of Industrial Microbiology and Biotechnology 20:132-138.
Zhang, Y. H. P., and L. R. Lynd. 2004. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnology and Bioengineering 88:797-824.










