3
Mobile Animals and Disease
OVERVIEW
As discussed in the previous chapter, trade in livestock, poultry, and animal products precipitated the emergence of several important zoonotic diseases, including H5N1 influenza and bovine spongiform encephalopathy (BSE). The essays collected in this chapter consider additional mobile animals, such as pets, wildlife, research animals, and insect vectors (with and without their various hosts) as factors in infectious disease emergence. In addition to introducing diseases to new animal and human populations, some of these animals are changing ecosystems in ways that alter the transmission dynamics of infectious diseases.
The first paper, by workshop speaker Nina Marano and colleagues of the Centers for Disease Control and Prevention (CDC), describes regulatory procedures designed to reduce the threat of zoonotic diseases to the United States. The CDC is one of four government agencies that regulate the importation of animals based on their risk for zoonotic disease; the others are the Department of Homeland Security (Customs and Border Protection), the Department of Agriculture (Animal and Plant Health Inspection Service), and the Department of the Interior (Fish and Wildlife Service). Marano et al. review the CDC’s animal regulations, including those that were developed in response to such noteworthy events as an Ebola outbreak among research animals in a government primate research facility in Reston, Virginia; the emergence of monkeypox in pet prairie dogs; the detection of zoonotic viruses in bushmeat; and the presence of highly pathogenic avian influenza in imported birds.
Until recently, the CDC’s regulatory actions to address disease threats from imported animals have been largely reactive, species-specific, and pathogen-
specific, the authors state. Now the agency—much like the Food and Drug Administration (FDA) as described by Acheson in the previous chapter—is engaged in developing a “risk based, proactive approach to preventing the importation of animals and vectors that pose a zoonotic disease risk,” according to Marano et al. This effort, which they describe in some detail, focuses on the systematic and targeted surveillance of high-risk animals, animal products, and vectors in their countries of origin.
Rapid expansion of trade and transportation during the Industrial Revolution resulted in the global proliferation of mosquito-borne diseases, such as dengue and chikungunya. Thanks to today’s globalized economy, these and other vector-borne diseases—once considered well-controlled in industrialized countries—are poised for resurgence, while others, such as West Nile viral fever and chikungunya, have significantly expanded their geographic range. In his contribution to this chapter, workshop speaker Paul Reiter, of Institut Pasteur, examines the role of human activities in the dispersal of several important insect vectors (such as the mosquito species that transmit malaria and yellow fever to humans) and of vector-borne diseases of both humans and animals, including chikungunya, West Nile viral fever, Rift Valley fever, and bluetongue. He also predicts future range expansions for certain vectors and vector-borne diseases; for example, he expects that Aedes gambiae, “perhaps [the] most effective malaria vector on earth,” will migrate northward out of its native home in sub-Saharan Africa, and also across the Atlantic to South America.
Reiter, who captured the first specimen of the mosquito species Aedes albopictus in the United States in 1983, and who subsequently discovered that this Asian native had been distributed globally in shipments of used tires, observes that, while “it is not difficult to survey a species once it has been detected, it is much more difficult to detect new introductions when they occur, particularly when cargoes are imported in locked containers.” Therefore, he concludes, “with a few exceptions—e.g., the enforcement of vaccination requirements—we must expect the continued establishment of new exotic species as an inevitable consequence of modern transportation technology.”
Might it be possible to prevent the emergence of infectious diseases by anticipating and blocking the movements of pathogens into new ecosystems? This question is posed by speaker Andy Dobson of Princeton University and Sarah Cleaveland of the University of Glasgow in this chapter’s final essay. Through a detailed examination of the circumstances that led up to the emergence of Nipah virus in Malaysia, the authors provide a number of insights into how other “novel” pathogens are likely to emerge, and they suggest a series of general questions that must be answered in order to predict and prevent future outbreaks of emerging infectious diseases.
To quantify the risk presented by a novel microbe to a potential host, Dobson and Cleaveland explain, information must be gathered and assessed at each of several stages in the development of an epidemic, from characterizing the back-
ground of all potential pathogens to analyzing transmission dynamics among novel hosts. “Ultimately the only way we can quantify the risk of novel microbes to humans (and domestic livestock) is to create a huge phylogeny of all pathogens and their hosts,” they write. “We then need to examine the pathology of closely related pathogens, in their reservoir hosts and other host species they infect and examine the factors that modify virulence and transmissibility.” Such an effort “will require considerable capacity-building in areas that are woefully underfunded,” they acknowledge.
PUBLIC HEALTH IMPACT OF GLOBAL TRADE IN ANIMALS
Nina N. Marano, D.V.M., M.P.H.,1 G. Gale Galland, D.V.M., M.S.,1 Jesse D. Blanton, M.S.,2 Charles E. Rupprecht, D.V.M., Ph.D.,2 James N. Mills, Ph.D.,2 Heather Bair-Brake, D.V.M., M.P.H.,1 Betsy Schroeder, M.P.H.,1 Martin S. Cetron, M.D.1
Centers for Disease Control and Prevention
Introduction
Zoonoses are diseases that are transmissible from animals to people. The prevention and management of zoonoses in humans pose unique considerations for surveillance and detection of these diseases and require acknowledgment of the role of animals in disease transmission. Wildlife and animals intended for the pet trade can serve as hosts for a variety of well-known and emerging zoonotic pathogens. The Centers for Disease Control and Prevention’s (CDC’s) regulations exist to prevent the importation of animals and animal by-products that pose a risk to public health. However, globalization of the food supply, consumer goods, and live animals—combined with human behaviors and preferences for the exotic—are ever-growing risk factors for translocation to the United States of zoonotic diseases from parts of the world where they are endemic (or exist in a reservoir state) (Smith et al., 2009). This paper describes the CDC’s regulatory framework for mitigating response to the introduction of zoonotic diseases, which has traditionally been reactive. The challenges of the twenty-first century call for a more proactive approach rooted in a risk-based strategy to prevent the introduction of animals and vectors that pose a risk to public health.
CDC’s Animal Regulations: Mitigating Public Health Threats
Under Section 361 of the Public Health Service Act3 (42 USC § 264), the CDC is responsible for regulations to prevent the introduction, transmission, and spread of communicable diseases from foreign countries into the United States. The CDC currently regulates the importation of nonhuman primates, dogs and cats, small turtles, African rodents, civets, and Asian birds to prevent the entry of zoonotic diseases and also regulates the importation of etiologic agents, hosts, and vectors (HHS, 2001).
Nonhuman Primates
Nonhuman primates (NHPs), particularly those recently captured in the wild, may harbor agents in their blood or other body tissues that are infectious to humans. Persons working in temporary and long-term animal holding facilities and individuals involved in transporting animals (e.g., cargo handlers and inspectors) are especially at risk for infection. NHPs are a potential source of pathogens that can cause severe or fatal disease in humans, including filoviruses, hepatitis, herpes B virus, rabies, tuberculosis, and parasitic infections (NRC, 2003). Some cynomolgus, African green, and rhesus monkeys imported into the United States have been previously demonstrated to be infected with Ebola Reston virus (CDC, 1990). An epidemiologic link between hepatitis A infections in NHPs, especially chimpanzees, and their caretakers has been demonstrated (Robertson, 2001). Herpes B virus is a zoonotic agent that naturally infects only macaque monkeys causing mild illness or no illness but can cause fatal encephalomyelitis in humans. Previously reported fatal cases of herpes B virus disease in humans have been caused by animal bites, scratches, or mucous membrane contact with infected materials (Cohen et al., 2002). NHPs, especially macaques, are highly susceptible to tuberculosis and rabies and most are imported from areas of the world with a high prevalence of these diseases in humans and animals (CDC, 1993). NHPs may also be a source of flaviviruses (e.g., yellow fever virus), which may be transmitted to humans by mosquitoes that have previously fed on an infected NHP (Mansfield and King, 1998); transmission of yellow fever to humans in NHP research work has also occurred (Richardson, 1987). Quarantine requirements for imported NHPs are designed to reduce these infectious disease risks. Since October 10, 1975, the CDC, through 42 CFR § 71.53, has prohibited the importation of NHPs except for scientific, educational, or exhibition purposes. Under this regulation, NHP importers are required to register with the CDC and this registra-
tion must be renewed every two years. NHPs are required to be held in quarantine for a minimum of 31 days following entry into the United States. This regulation also requires registered importers to maintain records on imported NHPs and to immediately report illness suspected of being communicable to humans. Imported NHPs and the offspring of imported NHPs may not be maintained as pets, a hobby, or as an avocation with occasional display to the general public. Additional requirements for importers of NHPs were developed and implemented in response to specific public health threats. On January 19, 1990, the CDC published interim guidelines for handling NHPs during transit and quarantine in response to identification of Ebola virus (Reston strain) in NHPs imported from the Philippines (CDC, 1990). In April 1990, there was confirmation of Ebola virus infection in four NHP caretakers, and serologic findings suggested that cynomolgus, African green, and rhesus monkeys posed a risk for human filovirus infection. As a result of these findings, the CDC placed additional restrictions and permit requirements for importers wishing to import these species.
Dogs
The CDC restricts the importation of dogs primarily to prevent the entry of rabies (CDC, 2003a). Rabies is a lyssavirus that causes a fatal encephalitis in mammals. In the United States, widespread mandatory vaccination of dogs has eliminated the canine variant of rabies and dramatically reduced the number of human cases (Velasco-Villa et al., 2008). However, canine rabies virus variants continue to be imported via unvaccinated dogs from areas where rabies is enzootic, such as Asia, Africa, the Middle East, and parts of Latin America. Globally, canine variants are responsible for most of the estimated 55,000 human rabies deaths worldwide each year (HHS, 2001; WHO, 2009). Since May 2004, there have been at least four documented instances of dogs being imported to the United States from rabies enzootic areas that subsequently were diagnosed with rabies, necessitating extensive public health investigations to identify persons at risk of exposure and in need of post-exposure prophylaxis (PEP), as shown in Table 3-1 (CDC, 2008b). “In May 2004, an unvaccinated puppy was flown from Puerto Rico to Massachusetts as part of an animal rescue program. The day after arrival, the puppy exhibited neurologic signs, was euthanized, and was subsequently confirmed to have rabies” (CDC, 2008b), with a variant identified as enzootic to dogs and mongoose from Puerto Rico. Among 11 people evaluated, 6 persons were recommended to receive PEP because of potential exposure (personal communication, Frederic Cantor, Massachusetts Department of Public Health, June 20, 2004; CDC, 2008b).
“In June 2004, an unvaccinated puppy adopted by a U.S. resident in Thailand was confirmed to have rabies by the California Department of Public Health” (CDC, 2008b), and a dog rabies virus variant identified as enzootic to Thailand. Of 40 persons interviewed for potential rabies exposure, 12 received PEP (personal communication, Ben Sun, California Department of Public Health, August 16,
TABLE 3-1 Importations of Rabid Dogs to the Continental United States, 2004-2008
|
Month/Year |
No. of Dogs with Rabies/No. of Animals in Shipment |
Territory or Country of Origin |
No. of persons receiving PEP/No. of persons interviewed |
|
May 2004a |
1/6 |
Puerto Rico |
6/11 |
|
June 2004b |
1/1 |
Thailand |
12/40 |
|
March 2007c |
1/2 |
India |
8/20 |
|
June 2008d |
1/24 |
Iraq |
13/38 |
|
Based on data from: aMassachusetts Department of Public Health. bCalifornia Department of Public Health. cAlaska Department of Health and Social Services. dNew Jersey Department of Health and Senior Services. |
|||
2004; CDC, 2008b). “In March 2007, a puppy was adopted by a U.S. veterinarian while volunteering in India…. The puppy was flown in cargo to Seattle, Washington then adopted by another veterinarian in Juneau, Alaska, where it was flown seven days after arrival” (CDC, 2008b). The puppy exhibited neurologic signs and was confirmed to have rabies by the Alaska Department of Health and Social Services, with a dog rabies virus variant identified as enzootic to India. Of 20 persons interviewed for potential rabies exposure, eight received PEP (Castrodale et al., 2008). Most recently in June 2008, a shipment of 24 dogs and 2 cats arrived in the United States from Iraq as part of an international animal rescue operation. Subsequently, an 11-month-old dog from this group became ill; rabies was confirmed and the virus was determined to be a rabies virus variant associated with dogs in the Middle East. During the public health investigation, 13 of 28 persons were identified with potential exposure of sufficient magnitude to initiate PEP (personal communication, Faye Sorhage, New Jersey Department of Health and Senior Services, July 1, 2008).
In all four of these cases, the rabies viruses were identified as exotic variants circulating in dogs and terrestrial wildlife in the animal’s country or region of origin, and were associated with human fatalities.
Besides the threat of human and domestic animal exposure and the direct public health, veterinary, and economic consequences associated with PEP, particularly during times when supplies of rabies biologics are less than ideal, such events serve to underline the fragility of the canine rabies virus-free status in the United States posed by such introductions. The introduction of canine rabies, and its potential to become enzootic again in domestic animals or wildlife, would increase the demand for prophylaxis and exacerbate fragile supplies of rabies vaccines and immune globulins. Moreover, other lyssaviruses besides rabies virus persist in the Old World. The danger of importation posed by these agents is greatly magnified because current human and veterinary rabies vaccines do not
cross-protect against lyssaviruses from other phylogroups and no pan-lyssavirus vaccines are on the horizon for serious commercial development.
Since canine variants of rabies remain a very serious health threat in many other countries, preventing the entry of potentially infected dogs into the United States is a critical public health priority. CDC requires dogs entering the United States to be vaccinated for rabies or, if they are not vaccinated, that the importer agree to have the dog vaccinated and confined for 30 days after rabies vaccination to allow for acquisition of vaccine-induced immunity (HHS, 2001). The CDC is currently considering amending its regulations to institute further requirements for entry of dogs and other pet animals to the United States to prevent importation of rabies.
Etiologic Agents, Hosts, and Vectors
Under Section 71.54 of the Public Health Service Act (Foreign Quarantine4) the CDC also regulates etiologic agents, hosts, and vectors (2003b). This regulation means that a person may not import into the United States, or distribute after importation, any etiologic agent or any arthropod or other animal host or vector of human disease, or any exotic living arthropod or other animal capable of being a host or vector of human disease unless accompanied by a permit issued by the director. “All live bats require an import permit from the CDC and the U.S. Department of Interior’s Fish and Wildlife Services, and may not be imported as pets” (CDC, 2008c; see also HHS and CDC, 2003a). We are particularly concerned about bats as reservoirs for infectious agents, as we recognize that Marburg virus is clearly associated with a species of bat called Rousettus aegyptiacus, at least in Uganda, and one or more other species are almost surely associated with Ebola virus (Calisher et al., 2006). In addition, bats are known to be the keystone reservoirs for viruses such as rabies virus, other lyssaviruses related to rabies, and henipaviruses and have most recently been identified as the reservoir for severe acute respiratory syndrome (SARS) coronavirus (Cui et al., 2007). Any living insect or other arthropod that is known or suspected of containing an etiologic agent (human pathogen) requires a CDC import permit, and vector snail species capable of transmitting a human pathogen require a permit as well (CDC, 2008c; HHS, 2001).
CDC limits imports of small turtles; those with a shell length of less than four inches may not be imported for any commercial purpose (CDC, 2008d; HHS and CDC, 2003b). “This rule was implemented in 1975 after it was discovered that small turtles frequently transmitted Salmonella to humans, particularly young children” (CDC, 2008d; see also HHS, 2001).
Zoonotic pathogens are important not only because of the known illnesses they cause—which can move to new parts of the world—but also because of new human diseases that can arise from animal sources. In 2003, an outbreak of SARS in humans spread worldwide, and the initial transmission to humans was linked to infected civets sold for food in [Chinese wet markets]. The emergence of SARS in humans following exposure to wild animals is an example of how a previously unrecognized zoonotic disease can quickly cause unexpected illness in human populations. (CDC, 2007b)
In 2003, the CDC issued an order to ban the importation of civets because of concerns at the time that these animals were involved in the transmission of SARS coronavirus to humans (CDC, 2004a).
Birds
Since 1997, and to the present, the outbreaks of avian influenza H5N1 in birds and humans are a prime example of how globalization of the food supply affects public and animal health. In November 1997, the Hong Kong Special Administrative Region Department of Health5 detected new cases of a human illness caused by an avian influenza H5N1 virus.
By late December, the total number of confirmed new cases had climbed to 17, of which 5 were fatal…. Except for one doubtful unconfirmed case, all illnesses or laboratory evidence of infection was in patients who had been near live chickens (e.g., in market places) in the days before onset of illness, which suggested direct transmission of virus from chickens to human rather than person-to-person spread…. Because these cases occurred at the beginning of the usual influenza season in Hong Kong, public health officials were concerned that human [influenza] strains might cocirculate with avian influenza strains to generate human and avian reassortant viruses with [the] capacity for efficient person-to-person spread.
[In December 1997,] veterinary authorities began to slaughter all 1.6 million chickens present in wholesale facilities or vendors within Hong Kong, and importation of chickens from neighboring areas was stopped. Subsequently, no more human cases caused by avian influenza virus were detected. (Snacken et al., 1999)
Highly pathogenic avian influenza (HPAI) H5N1 in poultry and wild birds reemerged in Asia in 2003 and has become established as a veterinary and human health threat throughout the world, presenting challenges for control due to the widespread geographic areas and large numbers of poultry that are affected.
|
5 |
See http://www.who.int/mediacentre/factsheets/avian_influenza/en/#history (accessed July 13, 2009). |
Because birds imported into the United States from countries with HPAI H5N1 could pose a risk for human infection or spread of virus to U.S. birds, in 2004 the CDC issued emergency orders to ban the importation of birds and bird products from specific countries with HPAI H5N1. These orders mirrored similar regulatory actions taken by the U.S. Department of Agriculture Animal and Plant Health Inspection Service (USDA/APHIS) to prevent the importation of birds with HPAI H5N1 (CDC, 2007a). On January 21, 2008, the CDC published a notice in the Federal Register seeking public comment on a proposal to rescind its bird embargoes (CDC, 2004b). In 2004, when HPAI H5N1 was first recognized as a threat, CDC took emergency action to ban the importation of birds and thus prevent the disease from entering the United States.
Since that time, partnerships with public health and agricultural agencies around the world have increased the capacity for surveillance and communication about emerging outbreaks of HPAI [H5N1]…. All the bird embargoes currently in force under USDA regulations will remain in force. (CDC, 2009)
CDC continues to work closely with USDA, the World Health Organization, the World Animal Health Organization, the Food and Agriculture Organization, and individual ministries of health to monitor the situation regarding HPAI [H5N1 abroad] to ensure that the threat to human health is being adequately addressed through animal control measures. If necessary, CDC can take measures to control a human health threat based upon its authority to prevent the introduction, transmission, or spread of communicable diseases from foreign countries into the United States. (CDC, 2009)
Rodents
The emergence of human monkeypox in the Western Hemisphere in May and June 2003 is a vivid reminder of why we are, and should continue to be, concerned about the importation of wild animals into the United States. Monkey-pox is a zoonotic disease endemic to Central and West Africa. African rodents are considered to be the natural hosts of the virus which, in humans, causes rashes similar to smallpox, fever, chills, and headache (CDC, 2004c; Khodakevich et al., 1988). Human infections during the 2003 outbreak were traced back and were determined to have resulted from contact with pet prairie dogs that contracted monkeypox from diseased African rodents imported for the commercial pet trade (CDC, 2003; Hutson et al., 2007; Reed et al., 2004) (Figure 3-1). The shipment of mammals imported from Ghana contained more than six species and a total of 762 African rodents, some of which were confirmed to be infected with monkey-pox. The monkeypox outbreak resulted in 72 human cases, with 37 of those cases being laboratory-confirmed (CDC, 2003). Most patients had direct or close contact with the infected prairie dogs, including 28 children at a day care center and veterinary clinic staff (Reynolds et al., 2007).
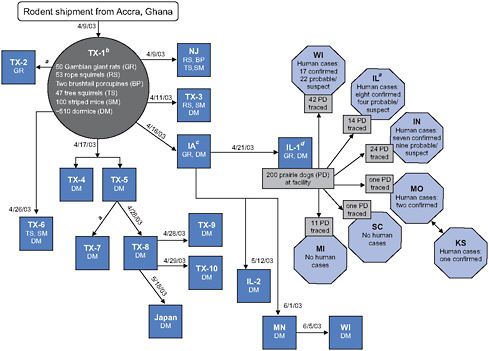
FIGURE 3-1 Movement of imported African rodents to animal distributors and distribution of prairie dogs from an animal distributor associated with human cases of monkeypox, 11 states, as of July 8, 2003: Illinois (IL), Indiana (IN), Iowa (IA), Kansas (KS), Michigan (MI), Minnesota (MN), Missouri (MO), New Jersey (NJ), South Carolina (SC), Texas (TX), and Wisconsin (WI). Japan is included among sites having received shipments of rodents implicated in this outbreak. Does not include one probable human case from Ohio; investigation is ongoing. Includes two persons who were employees at IL-1.
aDate of shipment unknown.
bIdentified as distributor C in MMWR 2003; 52:561-564.
cIdentified as distributor D in MMWR 2003; 52:561-564.
dIdentified as distributor B in MMWR 2003; 52:561-564.
SOURCE: CDC (2003).
On June 11, 2003, the CDC and the Food and Drug Administration (FDA) pursuant to 42 CFR § 70.2 and 21 CFR § 1240.30, respectively, issued a joint order prohibiting, until further notice, the transportation or offering of transportation in interstate commerce, or the sale, offering for sale, or offering for any other type of commercial or public distribution, including release into the environment, of prairie dogs and the six implicated species of African rodents (FDA, 2003; Gerberding and McClellan, 2003). In addition, pursuant to 42 CFR § 71.32(b), the CDC implemented an immediate embargo on the importation of all rodents (order Rodentia) from Africa. This emergency order was superseded on November 4,
2003, when the two agencies issued an interim final rule creating two complementary regulations restricting both domestic trade and importation, intended to prevent the further introduction, establishment, and spread of the monkeypox virus in the United States.
We are also concerned about rodents that originate outside of Africa, from other parts of the world such as Asia, Europe, and South America. We recently conducted an analysis of the numbers and origins of rodents imported to the United States since our African rodent ban was instituted in 2003. We analyzed data from the U.S. Fish and Wildlife Service’s Law Enforcement Management Information System (LEMIS) database, which records the entry of wildlife species to the United States. Since 2003, our ban has effectively limited legal importation of African rodents; the number of different rodent species entering the United States has decreased by 31 percent (Table 3-2). This decrease appears to be due to the restrictions on importation of African-origin rodents.
However, the commercial pet market has found a new niche in rodents from other parts of the world, as the number of rodents from Asia, Europe, and South America has increased by 223 percent. Rodents harbor hantaviruses, [resulting in] more than 100,000 hospitalized cases of hemorrhagic fevers in Europe and Asia (McKee et al., 1991). Rodents are also associated with rickettsial diseases. (CDC, 2008e)
Scrub typhus and murine typhus cause hundreds of thousands of cases annually; up to 50 percent of some human populations in Asia have antibodies to R. typhus (Azad, 1990). Outbreaks of Salmonella Typhimurium (CDC, 2005) and lymphocytic choriomeningitis (CDC, 2008a) have been associated with pet rodents in recent years. Since they are easier to care for than a dog or cat, these “pocket pets” are considered good choices for children. Because children interact with their pets in a closer and more intimate manner than they do with other animals, they may be at a heightened risk of infection. Table 3-3 provides a listing of pathogens in rodents that meet the following qualifications: they are zoonotic; nonindigenous; capable of causing significant human illness; and, if vector-borne, the vector is present in the United States (Acha and Szyfres, 2003; Eremeeva and Dasch, 2008; Heymann, 2008; Hugh-Jones et al., 1995). Rodents, once established, have several traits that make them ideal hosts for zoonotic diseases. They reproduce rapidly, and, unlike many other species of larger wild mammals, can be found in our gardens, storage buildings, and homes.
Insectivorous Mammals
Another potential concern for CDC may be insectivorous mammals, as there is some new evidence for hantaviruses being associated with shrews. We do not know whether these shrew-associated hantaviruses are human pathogens, and
TABLE 3-2 Numbers of Individual Rodents and Rodent Species Imported into the United States Pre-CDC African Rodent Ban (1999-2003) and Post-Ban (2004-2006)
|
|
1999-2003 |
2004-2006 |
% Change |
|
Rodents |
53,068 |
171,421 |
+223 |
|
Species |
77 |
53 |
−31 |
|
SOURCE: Department of Interior, U.S. Fish and Wildlife Service LEMIS. |
|||
TABLE 3-3 Some Important Rodent-Borne Zoonotic Pathogens and Their Hostsa
|
Pathogen |
Host species |
Disease |
|
Viruses |
||
|
Cowpox virus |
Apodemus, Myodes |
Cowpox |
|
Monkeypox virus |
Rodents |
Monkeypox |
|
Omsk hemorrhagic fever virus |
Rodents |
Omsk hemorrhagic fever |
|
Kyasanur forest disease virus |
Rodents |
Kyasanur forest disease |
|
Arenaviruses |
||
|
Flexal virus |
Unidentified rodent |
Hemorrhagic fever |
|
Guanarito virus |
Zygodontomys brevicauda |
Venezuelan hemorrhagic fever |
|
Junín virus |
Calomys musculinus |
Argentine hemorrhagic fever |
|
Lassa virus |
Mastomys natalensis |
Lassa fever |
|
Sabiá virus |
Unidentified rodent |
Brazilian hemorrhagic fever |
|
Chapare virus |
Unidentified rodent |
|
|
Hantaviruses |
||
|
Amur virus |
Apodemus peninsulae |
HFRS |
|
Dobrava-Belgrade virus |
Apodemus flavicollis |
HFRS |
|
Hantaan virus |
Apodemus agrarius |
HFRS |
|
Muju virus |
Myodes regulus |
HFRS |
|
Puumala virus |
Myodes glareolus |
HFRS |
|
Saaremaa virus |
Apodemus agrarius |
HFRS |
|
Seoul virus |
Rattus norvegicus |
HFRS |
|
Thailand virus |
Bandicota indica |
HFRS |
|
Andes virus |
Oligoryzomys longicaudatus |
HPS |
|
Araraquara virus |
Necromys lasiurus |
HPS |
|
Bermejo virus |
Oligoryzomys flavescens |
HPS |
|
Castelo dos Sonhos virus |
Unidentified rodent |
HPS |
|
Central Plata virus |
Oligoryzomys flavescens |
HPS |
|
Choclo virus |
Oligoryzomys fulvescens |
HPS |
|
Juquitiba virus |
Oligoryzomys nigripes |
HPS |
|
Laguna Negra virus |
Calomys laucha |
HPS |
|
Lechiguanas virus |
Oligoryzomys flavescens |
HPS |
|
Oran virus |
Oligoryzomys chacoensis |
HPS |
humans rarely have contact with shrews. However, that could change rapidly if someone decided to import shrews as pets (Song et al., 2007).
Animal Products
CDC’s regulations also prohibit the importation of products that originate from the animals we regulate. Rodents, bats, NHPs, and other mammals serve as a food source called bushmeat in other parts of the world, especially in parts of West Africa. The Bushmeat Crisis Task Force estimates that approximately 15,000 pounds of meat harvested from African wildlife is illegally imported into the United States each month (Goldman, 2007). Bushmeat may be derived from any species of wildlife, including rodents, bats, antelope, and NHPs. It is an important source of food and it is highly desired among many African expatriates. Bushmeat may enter the United States through large-scale vendors seeking
a commercial sale or may be carried in piece by piece by immigrants seeking a “taste of home.” Many methods have been used to disguise bushmeat: wrapping luggage in plastic to hide the odor, burying bushmeat under legal smoked fish, or mailing bushmeat via FedEx or DHL. Regardless of how it is brought in, unregulated overharvesting of wildlife for food, fur, or fiber has negative implications on conservation efforts and is a potentially dangerous source of disease for humans and animals in the United States.
Discussion
Challenges
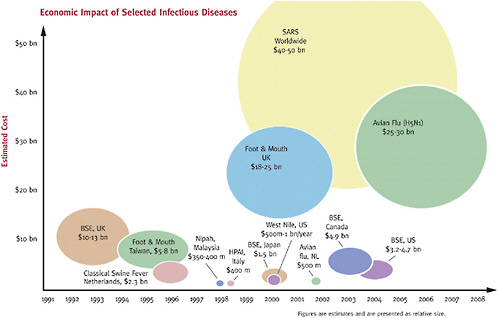
There are many challenges facing public health to effectively control zoonotic diseases related to movement of animals across international borders. At the CDC, the public health response to SARS involved participation by 866 employees including deployments to 10 foreign countries and 19 domestic ports of entry. The number of person-days during the SARS response equaled 46,714 (Posid et al., 2005). In addition, the impacts of a globally emerging zoonotic disease are far reaching and extend well beyond the public health realm. Worldwide, the economic impact of SARS was estimated to be $30-50 billion, largely due to its impact on tourism and thus the global economy, as shown in Figure 3-2 (Newcomb, 2003).
The monkeypox outbreak illustrates the possibility of animals as sources of human infections and the special risk associated with keeping wild animals as pets. During the monkeypox outbreak our investigators could not identify many potentially infected animals because no accurate records were available to trace their movements. The importation of wildlife poses a health risk because shipments often involve a high volume of animals, most of which are wild-caught and not captive-raised. “Many shipments also include different species comingled and/or kept in close proximity in confined spaces—conditions ideal for the transmission of disease. For most species, there is no screening for the presence of infectious diseases prior to shipment, and no holding or testing is required on entry into the United States” (Humane Society, 2009), which creates an opportunity for the widespread exposure of humans to pathogens these animals could be harboring. “High mortality rates among some animals, such as rodents, are common, and current U.S. [statutes and] regulations do not require importers to have [diagnostic necropsies] performed to determine whether the mortality is from a [pathogen] that could have an adverse effect on public health” (Pet Relocation, 2007).
We are further challenged by the fact that currently no single agency has the lead for implementing animal import regulations at ports of entry. Thus, depending on whether the import is classified as a food item or a product, livestock, wildlife, or endangered species, different agencies including the Department of
Homeland Security, the FDA, the Department of the Interior, the USDA, and the Department of Health of Human Services are called in to handle the situation. Additionally, federal agencies have variable amounts of resources at points of entry; thus, regulations are not applied consistently and comprehensively everywhere.
The regulatory approach to controlling zoonotic diseases creates opportunities for further mitigation but also leaves gaps in public health protection. In September 2008, the FDA lifted its portion of the ban on interstate movement of prairie dogs because the agency had determined through a risk-assessment process that the virus implicated in the 2003 outbreak no longer persisted in the environment. However, because prairie dogs are vectors for other zoonotic diseases, including tularemia and plague, the ban on interstate movement limited the possibility of human exposure to other diseases via widespread sale and adoption of prairie dogs as pets, and also limited the possibility of prairie dogs being exported to other countries. Until 2003, it was estimated that several thousand prairie dogs were sold within the United States as pets annually, and it was reported that the United States exported approximately15,000 prairie dogs as pets to other countries. As of 2003, Japan was the main importer of U.S. prairie dogs, but it had placed a ban on prairie dog importation in March of that year. Tularemia and plague, in addition to causing an estimated 200 natural human infections in the United States each year, are also listed as CDC Category A bioterrorism agents;6 thus, exportation of animal vectors of these diseases may be viewed under the International Health Regulations (2005) as a threat to international health and safety.
Potential Solutions
Regulatory approaches
CDC believes a number of approaches could further limit the transmission of zoonotic diseases. Potential solutions to this problem include screening animals with reliable laboratory tests, [vaccinating or] treating the animals empirically for known diseases, or quarantining the animals upon entry into the United States for the duration of an incubation period or duration of transmissibility. Many of those solutions, however, are currently not feasible [either as part of
pre-departure or post-arrival protocols,] or practical to employ on the large volume of imported animals. In addition, the control measures cannot prevent new or emerging pathogens or infections for which no laboratory tests or no empiric treatments exist, when practical experiences regarding a species’ susceptibility are lacking, when incubation periods are unknown, or when the infections are subclinical. In these instances, import restrictions of a wider range of species than currently regulated could be the only effective means of preventing the introduction of exotic infections into this country.
In May 2006, CDC hosted a public meeting on the subject of infectious disease threats associated with the importation and trade of exotic animals. Stakeholders, [including the National Association of State Public Health Veterinarians, the Wildlife Conservation Society, and the American Veterinary Medical Association] submitted a variety of positions and views to the public meeting. Of the 22 statements received for consideration, 7 indicated a measure of support for increased restrictions on the importation and sale of exotic species, while 15 expressed support for alternatives to regulatory or legal restrictions, or opposition to possible restrictions. (HHS and CDC, 2007a)
On July 31, 2007, the CDC published an Advance Notice of Proposed Rulemaking (ANPRM) to begin the process of revising our animal importation regulations (CDC, 2007b). This ANPRM was intended to solicit public comment and feedback on the issue of animal importation to determine the need for further rulemaking. We received more than 800 comments from our ANPRM posting and we are currently in the process of reviewing these comments to assist in new rulemaking.
The CDC’s current approach to controlling zoonotic disease threats has been to issue emergency orders or rules prohibiting importation of implicated animals. These actions are usually reactive—taken after an outbreak occurs rather than to proactively prevent outbreaks from known high-risk animals. This approach appears insufficient to prevent the introduction of many zoonotic diseases, especially given the high volume and speed of globalized trade in animal species and their byproducts. For public health purposes we need a risk-based, proactive approach to preventing the importation of animals and vectors that pose a zoonotic disease risk. The risk-based approach should include systematic and targeted surveillance of high-risk animals and animal products and vectors in the countries of origin. Emphasis should be placed on restricting the importation of animals and vectors of diseases not already present in the United States.
To effectively restrict importation of these vectors we must build the capacity of existing systems to accurately identify and track imported animal species and quantity of shipments. A recent analysis of the U.S. Fish and Wildlife Service LEMIS database indicated that the United States imported more than 1.1 billion live animals from 2000 to 2004. Of these, only 17 percent were species native to the United States. Only 27 percent of shipments were identified taxonomically lower than the family level, making it impossible to assess the diversity of ani-
mals imported or calculate the risk of nonnative species or pathogen introduction (Jenkins et al., 2007).
In 2008, legislation was introduced to Congress entitled the Non-Native Wildlife Invasion Prevention Act (U.S. Congress, House, 2008). This act required the Secretary of the Interior to formulate regulations establishing a process for assessing the risk of all nonnative wildlife species proposed for importation into the United States, other than those included in a list of approved species established under the act. Factors that must be considered at a minimum included the identity of the organism to the species level, its geographic source, and the likelihood of spread and harm to groups of species or habitats. The bill received considerable feedback from groups supporting it and from those opposed to it. Although the bill did not pass in the most recent legislative session, it is hoped that elements of the bill can be retained and modified to further mitigate the risks posed by the importation of animals to the United States that will protect public health and the environment.
Educational Approaches
Regulatory approaches may reduce the supply of animals, but we also need to educate the public to reduce demand. For example, with bushmeat, we need to educate bushmeat importers and consumers about the laws against and potential health risks involved with hunting, transport, and consumption of bushmeat. Though there have been extensive studies of African wildlife covering both conservation and disease outbreaks, little work has been done to understand the social reasons behind the importation of bushmeat into the United States and to effectively target the expatriate population. To understand the desire for bush-meat and be able to create prevention materials, the CDC is partnering with the Bushmeat Crisis Task Force (BCTF) and Zoo Atlanta to conduct focus groups among African expatriates. Preliminary information gathered during focus group sessions held by BCTF in New York City found that African immigrants crave African wildlife because of its perceived wholesomeness and often do not understand why bushmeat is prohibited from entering the United States. When results of these ongoing studies are compiled, the CDC, together with its partners, will develop an educational program regarding the consumption and illegal importation of bushmeat into the United States. The program will have material focusing on conservation and the potential health hazards of consuming bushmeat, as well as the regulations surrounding its importation.
Educational strategies have already been implemented, but need to be expanded, to inform the public about the risks of zoonotic diseases. Recent zoonotic transmissions of infectious diseases from pets, such as tularemia, salmonellosis, and lymphocytic choriomeningitis from pet hamsters, have served as opportunities to educate the public about safe handling of animals. Pet retailers have been and can continue to be valuable partners in this effort. Guidance
published by the American Academy of Pediatrics, the CDC, and the National Association of State Public Health Veterinarians (CDC, 2007c; National Association of State Public Health Veterinarians, 2007; Pickering et al., 2008) also remind the public of the dangers of contact with any wildlife, whether imported or domestic.
A MOLLUSC ON THE LEG OF A BEETLE: HUMAN ACTIVITIES AND THE GLOBAL DISPERSAL OF VECTORS AND VECTOR-BORNE PATHOGENS
Paul Reiter, Ph.D.7
Institut Pasteur
Charles Darwin’s last published article was a letter to Nature in which he described a specimen of Dytiscus marginalis—a water beetle common in Britain and much of Europe—that had been captured with a minute mollusc attached to its middle leg (Darwin, 1882). The beetle and its passenger had been sent to him by W. D. Crick,8 an amateur naturalist who, like Darwin, was intrigued by mechanisms for dispersal.
Darwin was keenly interested because he recognized that dispersal—and its antithesis, isolation—are key to the biogeography and evolution of species. He observed that dispersal takes many forms, but that passive dispersal—dispersal that takes advantage of the activities of other species or of movements of the physical environment such as wind or ocean currents9—was of outstanding importance. This review considers the dispersal of vectors and vector-borne pathogens by the activities of humankind.
Malaria
The principal parasites that cause malaria are strictly human pathogens, so their geographic range is determined by the presence of humans. There is a widespread misconception that the disease is strictly “tropical,” yet until the mid-nineteenth century, it was common as far north as central Sweden, Siberia, and the northern United States (Reiter, 2008a). In the past 150 years, the factors that have contributed to the reduction of its range are a reversal of the expansion that occurred with the development of agricultural settlements and the geographic expansion of humankind.
Molecular studies of the diversity of Plasmodium falciparum give strong evidence that it originated in Africa and advanced into Eurasia with the spread of
|
7 |
Unit of Insects and Infectious Diseases, 25-28 rue du Dr Roux 75015 Paris, France. E-mail: paul.reiter@pasteur.fr. |
|
8 |
Grandfather of Francis Crick. |
|
9 |
Or of movements of the physical environment. |
the human population, some 100,000 years ago (Carter and Mendis, 2002; Hume et al., 2003). Less attention has been paid to the other three species, but it is clear that transmission could not have occurred in Northern Europe until the retreat of the ice caps at the end of the last ice age. Whatever their origin, this passive dispersal was, of course, contingent on the presence of suitable vectors, which also had a changing geographic distribution.
In the New World, malaria has a much more recent history; it is unlikely that the disease was present before its introduction from Africa during the slave trade. African species of mosquitoes do not exist in the Americas,10 but several indigenous anophelines are highly effective malaria vectors. Importation was not restricted to parasites from the tropics. Until the late-nineteenth century malaria was a major cause of morbidity and mortality in farming communities in the upper Mississippi Valley (Ackerknecht, 1945), and there is good evidence that it arrived with peasant immigrants from Scandinavia, where P. vivax was endemic (Hulden and Heliovaara, 2005).
Aedes aegypti and Yellow Fever
Unlike human malaria, yellow fever is a zoonotic11 disease. It circulates among African primates in forested areas, transmitted by day-active mosquitoes of the genus Aedes (sub-genera Stegomyia and Diceromyia), which feed exclusively on primates. Humans who enter the forest, or live close to forested areas, are infected by the bites of infected mosquitoes. Outside the forest, inter-human transmission can continue if suitable vectors are present. Chief among these is Ae. aegypti, a species that is remarkable because it has adopted the peridomestic environment to great advantage.
In its natural habitat, Ae. aegypti breeds in tree-holes, plant axils, rock-holes, and other small items that hold water. In the peridomestic environment it remains strictly primatophilic,12 but freely lays its eggs in man-made containers. In villages close to enzootic transmission,13 water storage jars are usually the principal breeding sites; in cultures where water storage is not traditional, human-to-human transmission of yellow fever may not occur. In the modern peridomestic environment, Ae. aegypti—and Ae. albopictus (Figure 3-3)—a species with similar sylvatic origins—exploits other man-made articles that retain water such as discarded tires, buckets, saucers under flowerpots, and flower vases. Indeed, humans are literally the perfect host: they provide safe shelter, plentiful food, and abundant sites for procreation (Reiter, 2007).
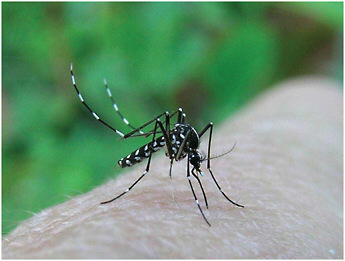
FIGURE 3-3 Ae. albopictus, the Asian tiger mosquito. In less than 30 years, this species—native to Asia from northern China, Korea, and Japan to the tropics—has become established, often common, in many countries in North and South America, Europe, Africa, and the Middle East. In 2006-2007, it was responsible for major epidemics of chikungunya virus on islands in the Indian Ocean, and for a small outbreak in Northern Italy. The principal “vector” for the mosquito has been a global trade in used tires.
SOURCE: Institut Pasteur.
From the seventeenth century onward, yellow fever was one of the most feared diseases, not only in Africa, but in much of the New World and in many European cities in the Old World. It was not uncommon for ships to arrive in port with dead or dying persons aboard, hence the yellow flag of quarantine. The principal source of this scourge was the transatlantic slave trade (Figure 3-4). Transmission was often active in the coastal slave-trading settlements and in the hinterland where the slaves were captured. The passage to the Americas from the west coast of Africa by boat under sail took four to six weeks. Given that viremia14 sufficient to infect mosquitoes does not usually last much more than a week, the virus could not have survived onboard without transmission en voyage. The critical factor, therefore, was the presence of the vector; prior to departure, tens of thousands of litres of drinking water were stowed below the lower decks in wooden casks. This water undoubtedly contained enough organic material to support rapid development of large numbers of Ae. aegypti larvae, particularly as the voyage progressed, so these casks must have been prolific breeding sites, with several generations of mosquito per voyage. The humid environment below deck was ideal for the adult mosquitoes, and the crew and the slaves were a copious source of blood.
Virus passed ashore in infected mosquitoes and humans. In the days before
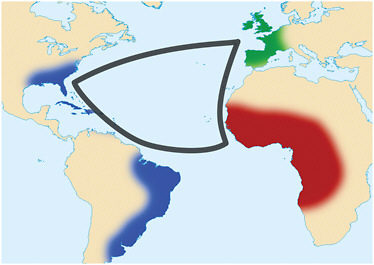
FIGURE 3-4 The transatlantic triangular trade. From the seventeenth to the nineteenth centuries, ships carried goods and supplies from Europe to Africa, for sale or barter for slaves, who were then transported to the New World, from where sugar, tobacco, and other produce was shipped to Europe. Slave ships carried as many as 900 captives; the large volume of freshwater required for their survival was stowed in barrels, and the water was inevitably infested with larvae of the African mosquito Aedes aegypti. Yellow fever, also native to Africa, was transmitted on board between humans by these mosquitoes. Devastating epidemics of yellow fever were a frequent event in the neotropics and subtropics, as well as in temperate regions of North America and Europe as far north as Boston and Dublin. Both the mosquito and the virus are now endemic/enzootic in the Americas.
SOURCE: Wikimedia Commons (2009).
piped water, water storage was obligatory, so the mosquitoes were abundant in seaports and inland. Inevitably, devastating epidemics, sometimes with tens of thousands of cases, were common in coastal cities in the Americas as far north as Boston. In Europe, major outbreaks occurred in many port cities from the Mediterranean to as far north as Brest, Bristol, Cardiff, and Dublin.
The disease continued to be a major cause of mortality in many temperate regions, long after prohibition of the slave trade. In 1870, for example, 120,000 panic-stricken people fled from Barcelona when thousands had contracted the disease after several vessels arrived from Cuba with fever onboard.15 In the United States, the great yellow fever epidemic of 1878-1879 made its way northward from Louisiana by river traffic on the Mississippi, with high mortality at every port of call. Despite advance warning, the authorities in Memphis, Tennessee, were reluctant to prevent the docking of river traffic. There were an estimated
19,500 cases, and the fleeing population carried the virus to the interior parts of the country far removed from the river. In all, there were an estimated 100,000 cases with an associated 10 percent mortality. Memphis (temporarily) lost its city charter and never regained its status as the capital of the southern states. Indeed, in the year that followed, there were calls to raze the city to the ground to prevent future epidemic disasters (Bloom, 1993).
The implication of Ae. aegypti as a vector of yellow fever by Carlos Finlay in Havana, Cuba, and experimental confirmation by Walter Reed, led to major sanitation campaigns and an end to major urban transmission in most of the Americas. In the 1920s, however, it became clear that the virus had become established in an enzootic cycle in the forests of Mexico, and Central and South America, transmitted by New World mosquitoes of the genera Sabethes and Haemagogus (Reiter, 2008b). In this circumstance, there is no prospect of eradication from the hemisphere. Epizootics16 are repeatedly reported in the South American rainforest, and there are small numbers of human cases every year. Sustained control of urban Ae. aegypti has rarely been achieved and never sustained (Reiter and Gubler, 1997), but a safe and effective vaccine is available. Few countries, however, have a well-organized vaccination program, so there is an ever-present danger that a massive urban epidemic will occur if the virus is introduced to the many burgeoning cities where Ae. aegypti is common.
Curiously, yellow fever has never been reported from any part of Asia. There is no apparent reason for this: endemic Ae. aegypti are certainly capable of transmission, and conditions in Asian cities appear as ideal for yellow fever transmission as they are for several other arboviral17 diseases, notably dengue and chikungunya. It may have been simply a matter of chance that it was never introduced.
Aedes albopictus
Americas
In June 1983, a single adult specimen of Aedes (Stegomyia) albopictus (Skuse), a mosquito native to Asia, was captured during studies of mosquitoes in Memphis, Tennessee (Reiter and Darsie, 1984). The species had been recorded as far west as Hawaii, but never in the Western Hemisphere. It is a vector of dengue and chikungunya in urban areas, albeit considered less effective than Ae. aegypti (Reiter et al., 2006). Speculation on how it was introduced into the continental United States drew attention to a major innovation in the transportation industry—containerization—and it was suggested that this new technology would lead to further introductions of medically important insects.
In 1985, Ae. albopictus was detected in Harris County, Texas, which includes the city of Houston. Surveillance by the local authorities revealed that it was widespread and common throughout the area, and had become a major nuisance species. Discarded used tires—abundant in many areas—were infested with the species, and its distribution within the county indicated that it could have been present for several years prior to detection. Investigations in early 1986 revealed that, since the 1960s, there had been an extensive and rapidly growing national and international trade in used tires. Millions were being imported annually from all over the world to destinations throughout the United States (Reiter, 1987). It appeared likely that the new species had been imported in such tires, perhaps from Japan, the world’s largest exporter. Discarded used tires provided abundant breeding habitats for these mosquitoes. The used tire trade was not restricted to Japan and the United States. Virtually every country in the world was importing and/or exporting used tires. Tires are an awkward item to handle, so it was evident that this trade could not have been practical without the advent of containerization.
In the same year, a survey of 12 states—Alabama, Arkansas, Florida, Georgia, Illinois, Indiana, Louisiana, Mississippi, Missouri, Ohio, Tennessee, and Texas—revealed that 48 out of 57 counties surveyed (84 percent) were positive for the species. An alert from the Pan American Health Organization (PAHO) prompted national authorities in Brazil to examine specimens of an Aedes species that had been awaiting identification. These also proved to be Ae. albopictus. By the end of July 1985, infestations had been detected in 63 municipalities in three Brazilian states. Meanwhile, an exhaustive inspection of 22,000 used tires arriving from Japan revealed that 25 percent contained water (Craven et al., 1988) and five species of mosquitoes were identified, including Ae. albopictus and three other exotics.
A study of cold-hardiness and the photoperiod required for onset of winter diapause18 in Ae. albopictus gave further evidence that U.S. infestations had originated in northern Asia, perhaps South Korea or Japan (Hawley et al., 1987). Interestingly, Houston was at the minimum latitude for infestations at that time. South Texas, Florida, and Mexico were unaffected, perhaps because maximum day length was too short to terminate winter diapause.
Thus, by late 1986, it was apparent that the species was widely established in the United States and Brazil. It seemed likely, moreover, that the species had been present in both countries for a number of years prior to detection. It was also apparent that domestic interstate traffic in used tires was a major factor in continued dispersal of the insect within both countries. Despite its widespread presence and abundance, there was considerable pressure on the U.S. government to prevent further introductions—mainly on the grounds that this would limit the genetic variation of the newly established population—and to prevent the
|
18 |
A period of physiologically controlled dormancy in insects (http://www.ipm.ucdavis.edu/PMG/glossary.html, accessed June 23, 2009). |
introduction of exotic viruses. A federal regulation was implemented whereby all used tires arriving in the United States from Japan, Korea, Taiwan, Hong Kong, Thailand, and other Asian countries where Ae. albopictus was known to occur should be certified as dry, clean, and free from insects. All noncompliant cargoes were to be fumigated with (highly toxic) methyl bromide, or treated with a pressurized spray of detergent/water solution at 88°C, or by steam cleaning.
Inspections and treatments were highly labor-intensive and unlikely to succeed. The maximum charge for noncompliance that could be levied per container was $1,000, hardly a deterrent when staff from the Division of Quarantine of the CDC could only make cursory inspection of at best 10 percent of all cargoes arriving at a few selected seaports (Figures 3-5A and 3-5B). After several years it was apparent that the effort was merely cosmetic, and the regulations were quietly withdrawn.
The federal experience with Ae. albopictus and used tire imports underlines four problems that are probably insurmountable:
-
Containers are often packed tightly to the roof, so inspection is highly labor-intensive and fumigation is of doubtful efficacy unless their contents are unloaded.
-
In port, containers are handled with speed and efficiency; delays for inspection are neither practical nor acceptable to the shippers.
-
Containers are designed to be delivered by truck, rail, or barge— unopened—directly to the customer. This is of paramount importance: in the past, cargoes could be inspected piece-by-piece as they were being unloaded at the dock-side. With containers, this step has been eliminated, so attempts to detect vectors at the port of entry have become largely irrelevant.
-
Under international law, imports are generally the responsibility of the importer.
In summary, although it is not difficult to survey a species once it has been detected, it is much more difficult to detect new introductions when they occur, particularly when cargoes are imported in locked containers. It is unrealistic to expect authorities to establish routine surveillance for imported species that have not been detected in the past, particularly when potential infestation sites may be anywhere on a whole continent.
Europe
In retrospect, after the initial detection of the species in Memphis, Tennessee, it was learned that Ae. albopictus had been present in Albania for at least 10 years and was a major nuisance in many areas (Adhami and Reiter, 1998). At that time, the country was politically isolated, a virtual enclave in the Balkans, with little or

FIGURE 3-5 Containerization. Approximately 90 percent of non-bulk cargo worldwide moves by containers stacked on transport ships. More than 20 million such containers make over 200 million voyages per year; some ships can carry more than 14,500 units. The speed, efficiency, and convenience of this form of transport enable cargoes to be delivered directly from the ship to their destination with minimum delay at the dockside. In consequence, it has become totally impractical to make routine inspections, either at the port of arrival or at the point where the container is finally opened.
SOURCE: Fotosearch, LLC.
no contact with the outside world except China. Since that time, Ae. albopictus has been detected at least once in 16 countries and is considered present and expanding its range in Albania, Croatia, France, Greece, Monaco, Montenegro, Italy, San Marino, Slovenia, Spain, and Vatican City.19 Italy is by far the most widely infested, and in many places, infestation rates are remarkably high; in some parts of Rome, including important tourist sites, biting rates are intolerably high for at least five months of the year. As with those mosquitoes that have become established in the United States, the European strains are adapted to survive northern winters. If winter temperatures define the limits of distribution, there is little reason to believe that infestations will not move northward, perhaps as far north as Scandinavia.20 There are also reports that the species is established in the Bekaa Valley, on the Lebanon/Syria border (anonymous source to the author). Here again, it is not unlikely that the species will eventually move eastward into central Asia. Finally, Ae. albopictus is common in urban areas and in rubber plantations in Cameroon, Gabon, and southeast Nigeria. It has been implicated in an outbreak of chikungunya in Gabon and there are fears that it may become a significant vector of yellow fever.
Italian entomologists have traced at least one of their Ae. albopictus infestations to imports of used tires from Atlanta, Georgia21 (Dalla Pozza et al., 1994). Thus, in the space of a few decades, an alien species has exploited a chain of modern transport that has brought it from Asia to the Americas and thence to Europe.
Dengue, Chikungunya, and the Passenger Aircraft
Dengue (DEN) is caused by a virus closely related to yellow fever; both are in the Japanese encephalitis subgroup of the family Flaviviridae. It is generally accepted that it originated in Asian forests, transmitted between monkeys by primatophilic Aedes mosquitoes, although at least one serotype22 circulates in a sylvatic cycle in West Africa (Diallo et al., 2005). The first major epidemic of what is considered to have been dengue was recorded in Philadelphia in 1780 and was concurrent with documented epidemics in Indonesia, India, Persia, Arabia,
Egypt, and Spain (Gubler, 1998). Epidemics were common in North America and in Europe until the mid-twentieth century. Indeed, one of the largest epidemics on record occurred in Greece in 1927-1928 with an estimated 1 million cases and 1,000 deaths (Rosen, 1986).23 As with yellow fever, the principal peridomestic vector is Ae. aegypti, Ae. albopictus is generally considered a “secondary” vector because it does not feed exclusively on humans, but significant epidemics have occurred in regions where Ae. aegypti is not present (Coulanges et al., 1979).
Dengue is now the most important mosquito-borne viral disease affecting humans. Its global distribution is comparable to that of malaria, and an estimated 2.5 billion people live in areas at risk for epidemic transmission. It is above all an urban disease, and it thrives in the crowded cities of the tropics, where homes are often so close together and Ae. aegypti breeding sites so abundant that urbanizations can be regarded as a single unit—a factory for the vector and the virus. Unlike yellow fever, DEN exists in four distinct serotypes so in theory a person can suffer four infections before becoming immune to the disease. Serosurveys24 reveal that by the age of 15 up to 80 percent of children in cities such as Bangkok and Kuala Lumpur have been infected by at least one serotype. In cities where populations exceed several million, all four serotypes may be in circulation simultaneously, though peaks of transmission may be asynchronous, with timing dominated by local history of transmission25 (Gubler, 2004).
Chikungunya (CHIK), like yellow fever and DEN, is a primatophilic virus (family Togaviridae) that is enzootic in African (and perhaps Asian) forests and transmitted by primatophilic mosquitoes. Although not generally life threatening, symptoms include arthritic joint pain that can persist for months and even years. Both DEN and CHIK present remarkable examples of the worldwide dispersal of arboviruses by a new vector, the passenger aircraft. Thousands of imported cases of DEN are reported every year in Europe and the United States, many in tourists returning from the tropics.
Serotype and sequence data of viruses isolated in widely separated countries confirm frequent intercontinental movement of the viruses. Best documented are successive exports of Asian strains of dengue virus (DENV) to the New World. In the first half of the twentieth century, DENV-2 was the only serotype in circulation. This changed in 1963, when an Asian strain of DENV-3 appeared in Puerto Rico and spread rapidly southward through the Antilles to South America. An Asian strain of DENV-1 appeared in 1977, followed by DENV-4 in 1981, and
a new strain of DENV-3 in 1994 (Effler et al., 2005; Gubler, 2005; Imrie et al., 2006; Neff et al., 1967).
A more recent example is the pandemic of chikungunya virus (CHIKV) that was first apparent in 2004 in Mombassa and Lamu (Chretien et al., 2007), on the Kenya coast, and subsequently appeared in a succession of small islands in the western Indian Ocean—the Comoros, Mayotte, Mauritius, Reunion, and the Seychelles—undoubtedly introduced by infected air passengers (Charrel et al., 2007). A massive epidemic followed in India, with estimates of at least 6.9 million cases (Mavalankar et al., 2007), and swept eastward to Southeast Asia, Indonesia, and the Philippines, where high rates of transmission continue at the time of writing (June 2009).
The most graphic event was the appearance of the virus in June 2007 in two contiguous villages in the Emilia-Romagna region of the province of Ravenna, in northwest Italy.26 The introduction was traced to a single traveler who arrived from India on June 21, developed fever on the afternoon of June 23, and triggered 205 infections in the region between early July and late September (Rezza et al., 2007). The vector, Ae. albopictus, was superabundant in the area. As had occurred with yellow fever in the Americas, transmission of CHIKV was by an exotic mosquito matched with an exotic virus.
There is evidence that the strain of CHIKV involved in the pandemic may have a particular affinity for Ae. albopictus (Tsetsarkin et al., 2009; Vazeille et al., 2007), although Ae. aegypti was certainly involved in transmission as well. The high rate of transmission in India undoubtedly raised the likelihood of introduction into Europe, but such circumstances are likely to recur. Moreover, even if the species has an exceptional susceptibility for recent CHIKV strains, it has also been responsible for epidemics of DEN where Ae. aegypti is absent (Coulanges et al., 1979), so we may well see the return of transmission to Europe. Of course, if Ae. aegypti were to become reestablished on the continent, repetition of the DEN epidemics of the past would also be possible.
Viruses cannot hop over oceans, but, as with the expansion of malaria in paleolithic times, they travel in people; only the rate of movement has changed (Figure 3-6). In addition to air transport, of course, tens of millions of people travel on land, within cities, within countries, and between countries; a few minutes spent in a crowded railway station, say, in India, will leave no doubt that this is a powerful engine for dispersal. The only requirement is the presence of a competent vector at the destination. The history of yellow fever, DEN, and CHIK confirms that this is perfectly feasible.

FIGURE 3-6 An infected person can travel to virtually any airport destination in the world in less than 48 hours, far shorter than the period of incubation and infectivity of vector-borne infections. The air-passenger industry carries more than 2 billion passengers in more than 23,000 aircraft and 28 million scheduled flights to more than 3,700 airports worldwide. The growth of world air travel has averaged 5 percent per year for the past 30 years, and the current rate of increase is greatest in the emerging economies of the world, particularly India, China, Southeast Asia, and the Middle East (IATA, 2009).
SOURCE: Fotosearch, LLC.
West Nile Virus
West Nile virus (WNV) is by far the most widely distributed arbovirus in the world. It is classified in the family Flaviviridae, which also includes dengue and yellow fever, but it is transmitted in an avian cycle by mosquitoes—chiefly of the genus Culex—that primarily feed on birds. Mammals, including humans and horses, can also be infected, but are considered “dead end” hosts because viremia is generally too low to infect mosquitoes.
In its original range, WNV is enzootic throughout Africa, parts of Europe, Asia, and Australia, but it received little attention until 1999, when a strain circulating in the eastern Mediterranean appeared in the Bronx, New York (Gubler et al., 2000; Hayes et al., 2005). The epizootic that followed was spectacular and unprecedented: within five years, the virus appeared ubiquitous, sometimes common, in nearly all counties of all states east of the Rocky Mountains, as well as parts of western Nevada and southern California. Sizeable outbreaks were also observed
in six Canadian provinces. It is now widely established from Canada to Venezuela (Petersen and Hayes, 2008) and has also been confirmed in Argentina (Morales et al., 2006). In the United States to date (1999-2008), 28,943 clinical cases and 1,393 deaths have been reported in humans, and more than 27,000 cases in horses, with a case fatality rate of about 33 percent (CDC, 2009). Two-thirds of the U.S. horse population are now vaccinated, but no vaccine is available for humans.
In Eurasia, epizootics are rarely evident except when there are cases of neurologic disease in humans or horses. By contrast, in the United States, fatal infections have been recorded in more than 320 species of birds. The virus is highly infectious by the oral route and is shed in the oral cavity and in the feces (Komar et al., 2003). The American crow appears particularly vulnerable, probably because it is a scavenger. Mortality among raptors is also high, presumably because they feed on infected prey.
The contrast in pathogenicity between virus in the Old and the New World is indicative of a long association between the virus and its avian hosts in its original range. Indeed, even during major epizootics, mortality appears rare in two super-abundant urban species, the house sparrow (Passer domesticus) and the rock dove (Colomba livi); both are exotics imported from the Old World. In this context, there is a clear parallel with yellow fever; in Africa, its original range, infections in wild primates are generally asymptomatic, but in the Americas, the virus is lethal to monkeys. Local inhabitants recognize an epizootic when there is mass mortality among howler monkeys (Alouatta palliata): the forest goes silent.
In both cases, the introduction of an exotic zoonotic virus that is not pathogenic in its original range has had a catastrophic impact on the local fauna in its new habitat. This is an important point, because there are many examples of destructive impact after the introduction of exotic pathogens that enter zoonotic transmission. In the case of infections that can also affect humans, a high incidence of infection in the zoonotic cycle will raise the probability of human infections. This is precisely what has happened with WNV in the New World.
The mode of introduction of WNV to New York is unknown, but presumably, just as with DENV and CHIKV, it arrived in an infected vertebrate. Viremia in humans, horses, and other mammals is generally assumed insufficient to infect mosquitoes. It is far more likely that the virus was imported in caged wild birds; in the early 1990s, an estimated five million wild birds of more than 3,000 species were traded annually, with the United States as the world’s largest importer (Wildlife Extra, 2008).27 Caged wild birds are highly susceptible to stress, so direct flights and speed of transit is critical.28 On arrival, they are held indoors in quarantine but not necessarily isolated from local mosquitoes. The implications for the importation of WNV and other avian pathogens are clear.
|
27 |
The European Community banned the trade in 2007, but there is concern that this will only drive it underground (Cooney and Jepson, 2006). |
|
28 |
Details of the procedure are given in http://petrelocation.blogspot.com/2008/03/importing-birds-to-usa.html (accessed February 3, 2010). |
Veterinary Diseases
Modern transportation has also given unprecedented mobility to livestock. Millions of sheep and cattle are shipped between countries worldwide. Race-horses fly between international racetracks like executives to business meetings. Hundreds of millions of day-old chicks travel between countries as geographically separate as China and Nigeria. With these livestock, inevitably, come pathogens. In the past three years, examples include the following:
-
Trypanosoma evansi is a blood parasite that causes acute disease in camels and horses, and chronic disease in domestic livestock. In 2006, an outbreak occurred in dromedary camels exported to France from the Canary Islands, France (Desquesnes et al., 2008). Indigenous biting flies (probably Stomoxyx spp.) passed the parasite on to local sheep. Transmission by such flies is mechanical, so no species-specificity is involved.
-
Rift Valley fever virus is a zoonotic mosquito-borne pathogen that primarily infects livestock. It is readily transmitted to humans by handling infected tissues, and can be fatal. In 2007-2008, an outbreak occurred in the Comoros Islands and the French territory of Mayotte. The mode of entry is not known, but a major epidemic was under way in East Africa, and it is probable that the virus arrived in (illegally) imported infected animals or meat (Sissoko et al., 2009).
-
Bluetongue virus (BTV) is an orbivirus, transmitted by Culicoides sand-flies, that affects ruminants. It has long been a veterinary problem in the United States (up to and occasionally over the Canadian border), Africa, the Middle East, parts of Asia, and Australia. Prior to 1998, however, transmission in Europe had only been documented in Spain, but in the following six years, multiple outbreaks of six strains spread across 12 countries that included Turkey, the Balkans, Italy, and new regions of Spain. This astonishing proliferation into new territory included the islands of Sicily, Sardinia, Corsica, Majorca, and Menorca, and continued in 2006, when six serotypes of the virus suddenly appeared in the Netherlands, Belgium, Germany, and Luxembourg, and more recently in the Czech Republic, Britain, Norway, and Sweden. The mode of introduction remains an enigma, but transport of animals or materials contaminated with infected vectors is clearly implicated.
Conclusion
In 1988, the federal employees who had laboriously searched for mosquitoes in 79 container loads of Asian used tires (Craven et al., 1988) wrote:
Ae. albopictus has joined the housefly, the flour beetle, the cockroach, the Mediterranean fruit fly, the yellow fever mosquito and many other insects that have
vastly extended their range by virtue of their association with mankind. Time will tell whether Ae. albopictus also joins the list of exotic vectors that transmit human or animal pathogens.
Ae. albopictus has indeed joined the list, and it is inevitable that more vectors and vector-borne pathogens will follow, perhaps with serious consequences. For example:
-
An. gambiae is perhaps the most effective malaria vector on Earth. Like Ae. aegypti, it is closely associated with the peridomestic environment and is strictly primatophilic. At present, it only exists in sub-Saharan Africa, but a new trade route from Lagos to Algiers is nearly complete and three other highways are proposed. A significant malaria problem could arise if the species were to be established in North Africa. The same would apply if it were to be reintroduced to Brazil, or to other regions in Latin America.
-
Saint Louis encephalitis virus is a New World pathogen, closely related to WNV, with the same vectors (in the Americas), the same transmission cycles, and similar pathology. If exported to Eurasia or Africa, it could enter a local transmission cycle, transmitted by local vectors, with similar, perhaps catastrophic, impact on wildlife, human, and veterinary health.
-
Japanese encephalitis virus is an avian pathogen transmitted by ornithophilic mosquitoes that can cause severe illness and death in humans. In Asia, pigs serve as amplifying hosts because they develop high viremia and infect large numbers of mosquitoes; human disease is prevalent where people live in close proximity to pigs. If exported to Mexico or Central America, competent vectors such as Cx. quinquefasciatus could perpetuate transmission in the widespread communities where pigs are also abundant in the peridomestic environment.
In conclusion, with a few exceptions—such as the enforcement of vaccination requirements—we can expect the continued establishment of exotic species and pathogens as an inevitable consequence of modern transportation technology.
Final Remark
It is regrettable that in recent years, outbreaks of yellow fever, dengue, chikungunya, WNV, bluetongue, and other vector-borne pathogens, as well as the appearance of Ae. albopictus and Ae. aegypti in new regions, have all been attributed to anthropogenic climate change or “global warming.” Statements to this effect are not limited to lay persons. For example, after the chikungunya outbreak in Italy, a United Nations official stated: “We cannot be certain [sic] that this outbreak was caused by global warming, but at least we now know that the
Asian tiger (mosquito) can survive in northern Italy.” The statement, taken up by the world press on the eve of an international climate change conference, ignored the fact that the villages were on the site of what was once a malarious swamp, and that, for Ae. albopictus, the Mediterranean winter is surely preferable to the bitter cold of Beijing and Seoul. Another example is bluetongue: there is no doubt that the climate of Europe has been warming over the past 200 years, but summer temperatures in Norway and Sweden have yet to reach those of North Africa and southern Spain, and winter temperatures in The Netherlands and surrounding countries cannot be compared to those in Montana, Wyoming, or the Balkans.
In the opinion of the author, the issue is an important one. Global warming has become the defining moral and political issue of our age. Draconian measures are being implemented that will have enormous impact on the economies and well-being of people all over the world. Vector-borne diseases continue to feature high in the list of perceived dangers, despite attempts to rationalize the debate by specialists in the field (Gubler et al., 2001; Hay et al., 2002; Reiter et al., 2004; Shanks et al., 2002; Sumilo et al., 2007). This review is no place to debate the science or the politics of the issue, but it is surely important to maintain perspective on a phenomenon that is better explained by the attachment of a mollusk to the leg of a beetle.
Acknowledgments
This research was partially funded by EU grant GOCE-2003-010284 EDEN, and the paper is catalogued by the EDEN Steering Committee as EDEN0154 (http://www.eden-fp6project.net/). The contents of this publication are the responsibility of the authors and do not necessarily reflect the views of the European Commission.
PREDICTING AND PREVENTING EMERGENT DISEASE OUTBREAKS
Andrew Dobson, D.Phil.29
Princeton University
Sarah Cleaveland, Ph.D., D.V.M.30
University of Glasgow
In 1998, a mysterious new disease syndrome appeared in pigs in Malaysia; it was then diagnosed in pig handlers. The pathology in humans implied an encephalitis-like virus that could cause significant mortality. Initial work by
local animal health expert Paul Chua and scientists from Australia’s Commonwealth Scientific and Industrial Research Organization (CSIRO) suggested the disease was similar to Hendra virus, a pathogen that had recently emerged in Australia (Lam and Chua, 2002). As outbreaks began to occur at different locations throughout Malaysia, the authorities decided to eradicate all pigs from the Malaysian peninsula; this resulted in the slaughter of over a million pigs and the complete collapse of the entire Malaysian pork industry. The causative agent was identified as Nipah virus, a Paramyxoviridae quite closely related to Hendra virus and one that also uses pteropid fruit bats as its reservoir host (Philbey et al., 1998). A detailed examination of the circumstances that led up to the emergence of Nipah virus in Malaysia provides a number of important insights into how other “novel” pathogens are likely to emerge. It also suggests a number of broader questions that need to be addressed if we are to capitalize on a resurgence of interest in predicting and preventing emergent disease outbreaks.
The past 25 years have seen a steady stream of “new diseases” emerge and enter either the human population or populations of domestic livestock. A recent study suggests that we are seeing approximately two new viral pathogens emerge each year (Woolhouse and Gaunt, 2007; Woolhouse et al., 2008). A significant number of emerging pathogens are strains of bacteria that have evolved resistance to the drugs that have been used to keep their ancestors at bay over the past 50 years. Others are older pathogens that have resurged due to the reduced levels of immune-competence in patients suffering from HIV. A small number were not previously known to science—HIV would be the classic example here—but the past decade has also seen the emergence of several viral diseases including SARS, Nipah, and Hendra viruses, and chikungunya, causing significant epidemics in their new host-environment contexts (Weiss, 2001). There are still other pathogens that we thought were new when outbreaks first occurred, but these ultimately proved to be pathogens we had forgotten about (e.g., Hanta virus), particularly if their scientific records predated the contemporary web-based collations of knowledge about microbes (Daszak et al., 2000; Garrett, 1994; Morse, 1995; Murphy, 1994).
Understanding how to predict and prevent future outbreaks could be a hugely beneficial exercise. What would we need to do to achieve this goal? And what are the major constraints to achieving success in this endeavor?
Predicting and Preventing Future Outbreaks: Probability and Cost
If we are interested in attempting to quantify the risk of new pathogens “emerging” and infecting humans, or populations of livestock and the plants that are central to our food supply, then we need to subdivide the process of emergence into a number of discrete steps (Lloyd-Smith et al., 2009). We then need to quantify the probability that each step will proceed to the next one while
simultaneously estimating the cost of preventing this from happening. As with any risk analysis, our key goal is to examine the interaction between probability and cost that creates the risk associated with an event we may wish to prevent (Burgman, 2005). For example, the emergence of Nipah virus in Malaysia breaks into a number of key steps that are likely to occur in slightly modified form in other “emergent pathogens” (Figure 3-7). Let us briefly consider the probability and cost associated with each of these stages.
FIGURE 3-7 Relative risk of pathogen emergence. Stages in the emergence of a new pathogen at which we need to quantify the trade-off between probabilities of occurrence and cost of intervention that define “risk” of emergence. Stage 1 is transmission in the reservoir, which we assume is ongoing and occurs at least every year (during the mating season), if not every day. Stage 2 occurs when a novel host species acquires an infection.a Stage 3 occurs when successful chains of transmission are established in the human (or domestic livestock) population and individuals become seriously ill and may die.b
aOur biggest worry is that this is a human host, but costs are associated with novel infections in domestic livestock and even exotic species that may have value for tourism or as pollinators or predators of pests. Most of these crossover events will not lead to any further transmission and many will go undetected.
bMost emerging pathogens are only detected at this stage, when the cost of intervention and economic and human suffering impact can be highly significant.
SOURCE: Dobson (in review).
Stage One:
Background Transmission
The background stage carries the highest probability of occurrence, and the lowest risk of threat; paradoxically, it is likely the most expensive to study, with the smallest probability of detecting new “pathogens.” Every day pathogens that are potentially devastating to humans are transmitted harmlessly between individuals of their natural hosts, which we parochially term “reservoir host” species. This goes on in the forests of central Africa, South America, and the few remaining forests of Indonesia; it also continues in the savannas of East Africa, and within the communities of small mammals that inhabit the world’s tundra and mountain ranges. In most cases, the pathogens are unknown to science and have only a negligible impact on the fitness of their reservoir hosts; they have coexisted with them for millennia. A subset of the pathogens are potentially capable of crossing the species barrier from their reservoir host and either entering humans or passing into domestic livestock populations. However, we have very limited facilities for differentiating between the small proportion of these as-yet-undiscovered pathogen species that can be transmitted within humans (or livestock) and even fewer ways of quantifying their potential pathological impact.
At first glance our only way to quantify the threat posed by any of the large number of potentially novel pathogens detected in epidemiological “fishing expeditions” into the habitats listed above would be to extrapolate their properties from their similarity to pathogens that have already crossed the species barrier into humans. I personally find the Nipah and Hendra viruses very scary, because they are fairly closely related to measles, which is arguably the most successful and devastating of human pathogens. But should we be more worried about pathogens that are totally dissimilar to ones that have already crossed the species barrier? HIV is completely different from most other human pathogens, yet it is steadily creating one of the most devastating epidemics in human history.
Ultimately, the only way we can quantify the risk of novel microbes to humans (and domestic livestock) is to create a huge phylogeny of all pathogens and their hosts. We then need to examine the pathology of closely related pathogens, in their reservoir hosts and other host species they infect, and examine the factors that modify virulence and transmissibility. This is a nontrivial exercise. It is one to which genomics will only supply limited insights in the absence of controlled etiological data and exhaustive field surveys. Furthermore, it is a task whose scale is beyond any current level of funding. At present, we have only a rudimentary estimate of the number of species that share the planet with us (May, 1988, 1990) and an even less clear picture of how many pathogens and parasites utilize these species as hosts (Dobson et al., 2008). But as soon as we restrict our search for emergent pathogens to host species that are either close to humans in their evolutionary origins or live in close physical proximity to humans, we then risk missing the rare and unexpected event(s) that create a new epidemic. This is the ultimate “catch 22” of the fish and microchip quests to discover emergent
pathogens in habitats where we only have a limited understanding of what we are actually looking for.
Stage Two:
Crossover Transmission
The second stage in “successful” disease emergence occurs when a pathogen is transmitted between its reservoir host and a novel host species. This is both a rare event and one whose outcome is often missed. In most cases, the novel host’s immune system will overcome the pathogen’s arrival, or more commonly the pathogen will be unable to survive in the novel host species. The host may only suffer mild discomfort and we are only likely to detect the event if the host is human or if a domestic species monitored during routine slaughter is checked before entering the human food chain. Don Burke has called these events “viral chatter”31—they occur all the time, but only a tiny subset gives rise to cases where the virus (not forgetting bacteria, protozoa, fungi, prions, and the parasitic helminthes) is able to infect the host and reproduce in a way that leads to transmission to other members of the novel host species. In many cases, these initial “stuttering chains” of transmission will be broken by rapid intervention or, more often, because the new host is not a particularly good environment for the pathogen (for example, the index case may be an immunologically compromised host, whereas subsequent hosts with healthy immune systems can withstand infection).
Analysis of the risks involved at this stage need to focus on the circumstances that caused increased contact between the reservoir host and the novel host species. Changes in the environment are particularly important here. In the case of Nipah virus in Malaysia, for example, deforestation had removed the natural habitat of fruit bats. Their populations were increasingly concentrated in the remaining areas of habitat with fruit trees. Unfortunately, fruit trees were often left around pig farms as they provided shade for the pigpens and an additional source of revenue for pig farmers. Because of the weight constraints of flight, bats only partially eat fruit by sucking out the juices and then regurgitating the seeds and fruit pith below the fruit tree where they have been feeding (Dobson, 2005). The regurgitated fruit can then be eaten by pigs at a time when it is covered by saliva that may be contaminated by Nipah virus. Similar spatial mechanisms are likely to occur as the reservoir hosts of other pathogens become increasingly contained in the fragmented patches of natural habitat that remain when humans have converted the rest of the landscape into agricultural land, shopping malls, and golf courses. If the patches of land contain too few resources to support wildlife, the reservoir hosts may well develop ways of exploiting food resources that are more closely associated with humans, thus increasing the risk of disease transmission to humans.
Analogous effects occur during the build-up of mice populations in small forest fragments in the eastern United States; these help amplify the spread of Lyme disease (Ostfeld and LoGiudice, 2003). Considerably lower abundances of small mammals occur in large continuous forests where their abundance is reduced by predatory species that cannot survive on the more limited food resources in smaller forest fragments. Similarly, increased populations of domestic livestock will lead to increased contact between these species and remaining populations of wildlife. A potential disease transmission event can occur just as easily when a cow or sheep breaks through a fence and enters a wood or grassland nature reserve, as when an infected sparrow finds its way into a chicken barn.
Most of the novel disease events that occur in livestock will also go undetected and rapidly fade out. The tiny subset that does initiate an epidemic outbreak can usually be stopped by culling all infected hosts along with those in which they have been in contact. However, this requires significant vigilance on the part of animal health inspection services and also assumes that farmers will not try to sell or move their livestock before movement restriction orders are put in place. Unfortunately, this assumes all farmers are honest; in Malaysia (during the Nipah outbreak) and in the United Kingdom during the 2001 foot-and-mouth outbreak (Ferguson et al., 2001; Keeling et al., 2001), farmers illegally moved infected livestock into previously uncontaminated areas and initiated new outbreaks that considerably increased the spatial scale of the outbreak and the number of livestock that ultimately had to be destroyed. The economic theory of “crime and punishment” provides important insights here (Becker, 1968; Sutinen and Anderson, 1985); ultimately, detecting the pathogen at the earliest stage of the epidemic and detecting illegal movement of potentially infected livestock will be much more effective in minimizing the size of the epidemic outbreak than will retrospectively imposed large fines or long prison sentences on the small subset of farmers eventually found guilty of illegally moving livestock.
Stage Three:
Outbreak
Detecting infected individuals is crucial if the initial stuttering chains begin to give rise to a sustained epidemic. Two factors are crucial here: (1) the rate of transmission between infectious and susceptible hosts and (2) the ratio of the duration of time before symptoms appear to the duration of time before the hosts can transmit the disease (Fraser et al., 2004). If symptoms appear before transmission is efficient, then control of the outbreak can be achieved by isolation of infected individuals and their primary contacts, particularly when combined with relatively low transmission rates. This is primarily why it was relatively straightforward to contain the SARS outbreak (Anderson et al., 2004). In contrast, when symptoms do not appear until long after transmission has been established, the pathogen can spread and infect a significant number of hosts; HIV is the classic example.
Once an outbreak has been established and is spreading, the direct and indirect costs will start to increase faster than the exponential spread of the pathogen between hosts. Stories in the media have dynamics that are similar to epidemics; their transmission rates are much more efficient and their impact is often out of scale from the actual risk involved. The economic cost of the SARS epidemic in Southeast Asia was huge given the small number of people who actually became sick or died (McLean et al., 2005). The economic cost of the foot-and-mouth epidemic in the United Kingdom far exceeded the cost of all the cattle slaughtered due to the large indirect impact on tourism and movement restrictions in rural areas.
Searching for novel pathogens and understanding their potential threat and risk of crossing over will require considerable capacity-building in areas that are woefully underfunded. The world has less than 100 people trained to understand the ecology and population dynamics of infectious diseases; this is roughly comparable to the number of knee specialists in New Jersey. Ultimately, this lack of intellectual capacity at the population level within the National Institutes of Health (NIH) has led to the current underestimate of the scale and impact of the H1N1 influenza epidemic in the United States. Equally disconcerting is that the annual meeting of the Wildlife Disease Association attracts less than 400 people and more than 80 percent of them are interested graduate students who are training as veterinarians. The scariest and most intriguing thing about their annual meetings is that each seems to provide an example of a novel pathogen that no one has heard about before and that requires a paradigm shift in the thinking and of people working in the discipline. This year it was white-nose syndrome in bats; last year Tasmanian Devil facial tumors; the year before that, highly pathogenic avian influenza. It is hard to think of another scientific society where a major paradigm shift occurs on an annual basis.
Summary
The past 25 years have seen major advances in our understanding of the mathematical population dynamic processes that underlie the movement of pathogens within and between host species (Anderson and May, 1991; Grenfell and Dobson, 1995). Over the past 25 years, this whole intellectual enterprise has moved beyond its origins as an area of applied mathematics to one that provides central quantitative insights into public health response to infectious disease outbreaks (Smith et al., 2005). This quantitative ecological-based understanding is central to our understanding of emerging disease dynamics. Any discussion of these problems in the absence of a quantititative mathematical framework are arguably best left in the cocktail reception.
The spatial and temporal scales at which epidemic outbreaks occur requires the use of this mathematical machinery; attempting to understand disease dynamics in its absence is equivalent to attempting pathology without a microscope.
Unfortunately, levels of funding and training within the United States are considerably below adequate. It is thus likely to be an area where the United States will be increasingly dependent upon talent trained overseas. This is ironic because of the considerable pool of people in the United States trained in physics and mathematics who could make a huge contribution to this area, particularly when coupled into the U.S. domination of the world’s microcomputers, which will increasingly be needed to understand the dynamics of pathogens in populations and communities with complex spatial and temporal connections to each other.
Detecting and preventing new pathogens from entering populations of humans and domestic livestock is an important initiative that can potentially tell a lot about the diversity of pathogens that currently inhabit the planet. Even an initial survey of what is out there suggests that there are hundreds of thousands of potentially pathogenic microorganisms already sharing the planet with us. While this is disconcerting, it makes me totally unconcerned about novel ones that might arrive from other planets—the pathetically thin arguments for these “alien pathogens” strike me as little more than a further plea from the National Aeronautics and Space Administration (NASA) for some form of relevance to studies of life on Earth. But, if we are worried about the emergence of new pathogens, early detection will always be significantly less costly than later prevention. If nothing else, there are no indirect costs associated with early detection, and everything to be gained.
MARANO ET AL. REFERENCES
Acha, P. N., and B. Szyfres. 1987. Zoonoses and communicable diseases common to man and animals. Washington, DC: Pan American Health Organization and World Health Organization.
———. 2003. Zoonoses and communicable diseases common to man and animals. Washington, DC: Pan American Health Organization and World Health Organization.
Azad, A. F. 1990. Epidemiology of murine typhus. Annual Review of Entomology 35:553-569.
Calisher, C. H., J. E. Childs, H. E. Field, K. V. Holmes, and T. Schountz. 2006. Bats: important reservoir hosts of emerging viruses. Clinical Microbiology Reviews 19(3):531-545.
Castrodale, L., V. Walker, J. Baldwin, J. Hofmann, and C. Hanlon. 2008. Rabies in a puppy imported from India to the USA, March 2007. Zoonoses and Public Health 55(8-10):427-430.
CDC (Centers for Disease Control and Prevention). 1990. Update: Ebola-related filovirus infection in nonhuman primates and interim guidelines for handling nonhuman primates during transit and quarantine. Morbidity and Mortality Weekly Report 39(2):22-24, 29-30.
———. 1993. Tuberculosis in imported nonhuman primates—United States, June 1990-May 1993. Morbidity and Mortality Weekly Report 42(29):572-576.
———. 2003. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Morbidity and Mortality Weekly Report 52(27):642-646.
———. 2004a. Notice of embargo of civets, http://www.cdc.gov/ncidod/sars/pdf/order_civet_ban_011304.pdf (accessed April 8, 2009).
———. 2004b. Rescission of February 4, 2004, order and subsequent amendments prohibiting the importation of birds and bird products from specified countries, http://edocket.access.gpo.gov/2009/E9-1029.htm (accessed April 8, 2009).
———. 2004c. Updated interim case definition for human monkeypox, January 2004, http://www.cdc.gov/ncidod/monkeypox (accessed April 8, 2009).
———. 2005. Outbreak of multidrug-resistant Salmonella Typhimurium associated with rodents purchased at retail pet stores—United States, December 2003-October 2004. Morbidity and Mortality Weekly Report 54(17):429-433.
———. 2007a. Embargo of birds from specified countries, http://www.cdc.gov/flu/avian/outbreaks/embargo.htm (accessed April 8, 2009).
———. 2007b. Foreign quarantine regulations, proposed revision of HHS/CDC animal-importation regulations, 42 CFR Part 71.51. Federal Register 72(146), http://www.cdc.gov/ncidod/dq/pdf/anprm.pdf (accessed April 8, 2009).
———. 2007c. Healthy pets healthy people, http://www.cdc.gov/healthypets/ (accessed February 1, 2009).
———. 2008a. Brief report: lymphocytic choriomeningitis virus transmitted through solid organ transplantation—Massachusetts, 2008. Morbidity and Mortality Weekly Report 57(29):799-801.
———. 2008b. Rabies in a dog imported from Iraq—New Jersey, June 2008. Morbidity and Mortality Weekly Report 57(40):1076-1078.
———. 2008c. Etiologic agent import permit program, http://www.cdc.gov/od/eaipp/ (accessed October 20, 2009).
———. 2008d. Bringing an animal into the United States, http://www.cdc.gov/ncidod/dq/animal/dogs.htm (accessed October 20, 2009).
———. 2008e. Testimony of Nina Marano before the United States House of Representatives Natural Resources Subcommittee on Fisheries, Wildlife and Oceans, http://www.cdc.gov/Washington/testimony/2008/t20080626.htm (accessed October 20, 2009).
———. 2009. Frequently asked questions: information regarding CDC’s rescission of its embargo on birds and bird products from countries with highly pathogenic avian influenza (HPAI), http://198.246.98.21/ncidod/dq/laws_regs/fed_reg/bird/bird_faq.htm (accessed October 20, 2009).
Cohen, J. I., D. S. Davenport, J. A. Stewart, S. Deitchman, J. K. Hilliard, L. E. Chapman, and B Virus Working Group. 2002. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine Herpesvirus 1). Clinical Infectious Diseases 35(10):1191-1203.
Cui, J., N. Han, D. Streicker, G. Li, X. Tang, Z. Shi, Z. Hu, G. Zhao, A. Fontanet, Y. Guan, L. Wang, G. Jones, H. E. Field, P. Daszak, and S. Zhang. 2007. Evolutionary relationships between bat coronaviruses and their hosts. Emerging Infectious Diseases 13(10):1526-1532.
Eremeeva, M. E., and G. A. Dasch. 2008. Rickettsial infections. In CDC health information for international travel, edited by P. M. Arguin, P. E. Kozarsky, and C. Reed. Philadelphia, PA: Elsevier.
FDA (Food and Drug Administration). 2003. Control of communicable diseases. Federal Register 68(117), http://frwebgate6.access.gpo.gov/cgi-bin/PDFgate.cgi?WAISdocID=219538308835+2+2+0&WAISaction=retrieve (accessed April 8, 2009).
Gerberding, J. L., and M. B. McClellan. 2003. Joint order of the Centers for Disease Control and Prevention and the Food and Drug Administration, Department of Health and Human Services, http://www.premierinc.com/quality-safety/tools-services/safety/public-health/downloads/monkeypox_joint_order_ltr_06-11-03.pdf (accessed April 8, 2009).
Goldman, R. 2007 (March 15). Bushmeat: curse of the monkey’s paw. ABC News, http://abcnews.go.com/Health/Story?id=2952077&page=2 (accessed February 8, 2009).
Heymann, D. L. 2008. Control of communicable diseases manual. Washington, DC: American Public Health Association.
HHS (Department of Health and Human Services). 2001. Part 71: Foreign Quarantine. Code of Federal Regulations: Title 42–Public Health), http://www.access.gpo.gov/nara/cfr/waisidx_03/42cfr71_03.html (accessed April 8, 2009).
HHS and CDC. 2003a. Regulations on etiologic agents and vectors, http://edocket.access.gpo.gov/cfr_2003/octqtr/pdf/42cfr71.54.pdf (accessed July 1, 2009).
———. 2003b. Regulations on turtles, http://edocket.access.gpo.gov/cfr_2003/octqtr/pdf/42cfr71.52.pdf (accessed July 1, 2009).
———. 2007. Foreign quarantine regulations, proposed revision of HHS/CDC animal-importation regulations, http://edocket.access.gpo.gov/2007/E7-14623.htm (accessed October 20, 2009).
Hugh-Jones, M. E., W. T. Hubbert, and H. V. Hagstad. 1995. Zoonoses: recognition, control, and prevention. Ames, IA: Iowa State University Press.
Humane Society. 2009. Prohibit the importation of exotic pets to decrease the risk of zoonotic disease, http://www.hsus.org/wildlife/wildlife_news/exotic_pet_trade_and_disease.html# (accessed October 20, 2009).
Hutson, C. L., K. N. Lee, J. Abel, D. S. Carroll, J. M. Montgomery, V. A. Olson, Y. Li, W. Davidson, C. Hughes, M. Dillon, P. Spurlock, J. J. Kazmierczak, C. Austin, L. Miser, F. E. Sorhage, J. Howell, J. P. Davis, M. G. Reynolds, Z. Braden, K. L. Karem, I. K. Damon, and R. L. Regnery. 2007. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. American Journal of Tropical Medicine and Hygiene 76(4):757-767.
Jenkins, P., K. Genovese, and H. Ruffler. 2007. Broken screens: the regulation of live animal imports in the United States. Washington, DC: Defenders of Wildlife.
Khodakevich, L., Z. Jezek, and D. Messinger. 1988. Monkeypox virus: ecology and public health significance. Bulletin of the World Health Organization 66(6):747-752.
Mansfield, K., and N. King. 1998. Nonhuman primates in biomedical research. In Viral diseases, edited by B. T. Bennett, C. R. Abee, and R. Henrickson. San Diego, CA: Academic Press.
McKee, K. T., J. W. LeDuc, and C. J. Peters. 1991. Hantaviruses. In Textbook of human virology, edited by R. B. Belshe. St. Louis, MO: Mosby Yearbook.
National Association of State Public Health Veterinarians. 2007. Compendium of measures to prevent disease associated with animals in public settings, 2007, http://www.nasphv.org/documentsCompendiaAnimals.html (accessed February 1, 2009).
Newcomb, J. 2003. Biology and borders: SARS and the new economics of biosecurity. Bioera, http://www.bio-era.net/research/add_research_9.html (accessed April 8, 2009).
NRC (National Research Council). 2003. Occupational health and safety in the care and use of nonhuman primates. Washington, DC: The National Academies Press.
Pet Relocation. 2007. Importing pets to the USA—CDC proposes new regulations, http://www.petrelocation.com/importing-pets-usa-cdc-proposes-new-regulations (accessed October 20, 2009).
Pickering, L. K., N. Marano, J. A. Bocchini, and F. J. Angulo. 2008. Exposure to nontraditional pets at home and to animals in public settings: risks to children. Pediatrics 122(4):876-886.
Posid, J. M., S. M. Bruce, J. T. Guarnizo, M. L. Taylor, and B. W. Garza. 2005. SARS: mobilizing and maintaining a public health emergency response. Journal of Public Health Management Practice 11(3):208-215.
Reed, K. D., J. W. Melski, M. B. Graham, R. L. Regnery, M. J. Sotir, M. V. Wegner, J. J. Kazmierczak, E. J. Stratman, Y. Li, J. A. Fairley, G. R. Swain, V. A. Olson, E. K. Sargent, S. C. Kehl, M. A. Frace, R. Kline, S. L. Foldy, J. P. Davis, and I. K. Damon. 2004. The detection of monkeypox in humans in the western hemisphere. New England Journal of Medicine 350(4):342-350.
Reynolds, M. G., W. B. Davidson, A. T. Curns, C. S. Conover, G. Huhn, J. P. Davis, M. Wegner, D. R. Croft, A. Newman, N. N. Obiesie, G. R. Hansen, P. L. Hays, P. Pontones, B. Beard, R. Teclaw, J. F. Howell, Z. Braden, R. C. Holman, K. L. Karem, and I. K. Damon. 2007. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerging Infectious Diseases 13(9):1332-1339.
Richardson, J. H. 1987. Basic considerations in assessing and preventing occupational infections in personnel working with nonhuman primates. Journal of Medical Primatology 16(2):83-89.
Robertson, B. H. 2001. Viral hepatitis and primates: historical and molecular analysis of human and nonhuman primate hepatitis A, B, and the GB-related viruses. Journal of Viral Hepatitis 8(4):233-242.
Smith, K. F., M. Behrens, L. M. Schloegel, N. Marano, S. Burgiel, and P. Daszak. 2009. Ecology: reducing the risks of the wildlife trade. Science 324(5927):594-595.
Snacken, R., A. P. Kendal, L. R. Haaheim, and J. M. Wood. 1999. The next influenza pandemic: lessons from Hong Kong, 1997. Emerging Infectious Diseases 5(2):195-203.
Song, J. W., H. J. Kang, K. J. Song, T. T. Truong, S. N. Bennett, S. Arai, N. U. Truong, and R. Yanagihara. 2007. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerging Infectious Diseases 13(11):1784-1787.
U.S. Congress, House. 2008. The Nonnative Wildlife Invasion Prevention Act. H. R. 6311 (introduced). 100th Congress, 2nd session.
Velasco-Villa, A., S. A. Reeder, L. A. Orciari, P. A. Yager, R. Franka, J. D. Blanton, L. Zuckero, P. Hunt, E. H. Oertli, L. E. Robinson, and C. E. Rupprecht. 2008. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerging Infectious Diseases 14(12):1849-1854.
WHO (World Health Organization). 2009. Rabies–Bulletin–Europe. Rabies Information System of the WHO Collaborating Centre for Rabies Surveillance and Research, http://www.who-rabies-bulletin.org/Queries/Distribution.aspx (accessed April 8, 2009).
REITER REFERENCES
Ackerknecht, E. H. 1945. Malaria in the Upper Mississippi Valley. Baltimore: Johns Hopkins. P. 142.
Adhami, J., and P. Reiter. 1998. Introduction and establishment of Aedes (Stegomyia) albopictus Skuse (Diptera: Culicidae) in Albania. Journal of the American Mosquito Control Association 14(3):340-343.
Bloom, K. 1993. The Mississippi Valley‘s great yellow fever epidemic of 1878. Baton Rouge and London: Louisiana State University Press.
Carter, R., and K. N. Mendis. 2002. Evolutionary and historical aspects of the burden of malaria. Clinical Microbiology Reviews 15(4):564-594.
CDC (Centers for Disease Control and Prevention). 2009. West Nile virus, http://www.cdc.gov/ncidod/dvbid/westnile/index.htm (accessed June 23, 2009).
Charrel, R., X. de Lamballerie, and D. Raoult. 2007. Chikungunya outbreaks—the globalization of vectorborne diseases. New England Journal of Medicine 356(8):769-771.
Chretien, J. P., A. Anyamba, S. A. Bedno, R. F. Breiman, R. Sanq, K. Sergon, A. M. Powers, C. O. Onyango, J. Small, C. J. Tucker, and K. J. Linthicum. 2007. Drought-associated chikungunya emergence along coastal East Africa. American Journal of Tropical Medicine and Hygiene 76(3):405-407.
Cooney, R., and P. Jepson. 2006. The international wild bird trade: what’s wrong with blanket bans? Oryx 40(3):261-265.
Coulanges, P., Y. Clercy, F. X. Jousset, F. Rodhain, and C. Hannoun. 1979. Dengue at Reunion: isolation of a strain at the Pasteur Institute of Madagascar. Bulletin de la Société de Pathologie Exotique et de Ses Filiales 72(3):205-209.
Craven, R. B., D. A. Eliason, D. B. Francy, P. Reiter, E. G. Campos, W. L. Jakob, G. C. Smith, C. J. Bozzi, C. G. Moore, G. O. Maupin, and T. P. Monath. 1988. Importation of Aedes albopictus and other exotic mosquito species into the United States in used tires from Asia. Journal of the American Mosquito Control Association 4(2):138-142.
Dalla Pozza, G. L., R. Romi, and C. Severini. 1994. Source and spread of Aedes albopictus in the Veneto region of Italy. Journal of the American Mosquito Control Association 10(4):589-592.
Darwin, C. 1882. On the dispersal of freshwater bivalves. Nature (April 6):529-530.
Desquesnes, M., G. Bossard, D. Patrel, S. Herder, O. Patout, E. Lepetitcolin, S. Thevenon, D. Berthier, D. Pavlovic, R. Brugidou, P. Jacquiet, F. Schelcher, B. Faye, L. Touratier, and G. Cuny. 2008. First outbreak of Trypanosoma evansi in camels in metropolitan France. Veterinary Record 162(23):750-752.
Diallo, M., A. A. Sall, A. C. Moncayo, Y. Ba, Z. Fernandez, D. Ortiz, L. L. Coffey, C. Mathiot, R. B. Tesh, and S. C. Weaver. 2005. Potential role of sylvatic and domestic African mosquito species in dengue emergence. American Journal of Tropical Medicine and Hygiene 73(2):445-449.
Effler, P. V., L. Pang, P. Kitsutani, V. Vorndam, M. Nakata, T. Ayers, J. Elm, T. Tom, P. Reiter, J. G. Rigau-Perez, J. M. Hayes, K. Mills, M. Napier, G. G. Clark, D. J. Gubler, and Hawaii Dengue Outbreak Invesstigation Team. 2005. Dengue fever, Hawaii, 2001-2002. Emerging Infectious Diseases 11(5):742-749.
Gubler, D. 1998. Dengue and dengue hemorrhagic fever. Clinical Microbiology Reviews 11(3):480-496.
———. 2004. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comparative Immunology, Microbiology and Infectious Diseases 27:319-330.
———. 2005. The emergence of epidemic dengue fever and dengue hemorrhagic fever into the Americas: a case of failed public health policy. Pan American Journal of Public Health 17(4):221-224.
Gubler, D. J., G. L. Campbell, R. Nasci, N. Komar, L. Petersen, and J. T. Roehriq. 2000. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunology 13(4):469-475.
Gubler, D. J., P. Reiter, K. L. Ebi, W. Yap, R. Nasci, and J. A. Patz. 2001. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environmental Health Perspectives 109(Suppl 2):223-233.
Hawley, W. A., P. Reiter, R. S. Copeland, C. B. Pumpuni, and G. B. Craig, Jr. 1987. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 236(4805):1114-1116.
Hay, S. I., J. Cox, D. J. Rogers, S. E. Randolph, D. I. Stern, G. D. Shanks, M. F. Myers, and R. W. Snow. 2002. Climate change and the resurgence of malaria in the East African highlands. Nature 415(6874):905-909.
Hayes, E. B., N. Komar, R. S. Nasci, S. P. Montgomery, D. R. O’Leary, and G. L. Campbell. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerging Infectious Diseases 11(8):1167-1173.
Hulden, L., and K. Heliovaara. 2005. Endemic malaria: an “indoor” disease in northern Europe. Historical data analysed. Malaria Journal 4(1):19.
Hume, J. C., E. J. Lyons, and K. P. Day. 2003. Human migration, mosquitoes and the evolution of Plasmodium falciparum. Trends in Parasitology 19(3):144-149.
IATA (International Air Transportation Association). 2009. Fact sheet: world industry statistics, www.iata.org (accessed November 9, 2009).
Imrie, A., Z. Zhao, S. N. Bennett, P. Kitsutani, M. Laille, and P. Effler. 2006. Molecular epidemiology of dengue in the Pacific: introduction of two distinct strains of dengue virus type-1 [corrected] into Hawaii. Annals of Tropical Medicine and Parasitology 100(4):327-336.
Komar, N., S. Langevin, S. Hinten, N. Nemeth, E. Edwards, D. Hettler, B. Davis, R. Bowen, and M. Bunning. 2003. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infectious Diseases 9(3):311-322.
Mavalankar, D., P. Shastri, and P. Raman. 2007. Chikungunya epidemic in India: a major public-health disaster. Lancet Infectious Diseases 7(5):306-307.
Morales, M. A., M. Barrandequy, C. Fabbri, J. B. Garcia, A. Vissani, K. Trono, G. Gutierrez, S. Pigretti, H. Menchaca, N. Garrido, N. Taylor, F. Fernandez, S. Levis, and D. Enria. 2006. West Nile virus isolation from equines in Argentina, 2006. Emerging Infectious Diseases 12(10):1559-1561.
Neff, J., L. Morris, R. Gonzalez-Alcover, P. Coleman, and H. Negron. 1967. Dengue fever in a Puerto Rican community. American Journal of Epidemiology 86(1):162-184.
Petersen, L. R., and E. B. Hayes. 2008. West Nile virus in the Americas. Medical Clinics of North America 92(6):1307-1322, ix.
Reiter, P. 1987. The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. Journal of the American Mosquito Control Association 3(3):494-501.
———. 2007. Oviposition, dispersal and survival in Aedes aegypti; implications for the efficacy of control strategies. Vector Borne Zoonotic Diseases 7(2):261-273.
———. 2008a. Global warming and malaria: knowing the horse before hitching the cart. Malaria Journal 7(Suppl 1):S3.
———. 2008b. Climate change and mosquito-borne disease: knowing the horse before hitching the cart. Revue Scientifique et Technique (OIE) 27(2):383-398.
Reiter, P., and R. Darsie. 1984. Aedes albopictus in Memphis, Tennessee (USA): an achievement of modern transportation? Mosquito News 44:396-399.
Reiter, P., and D. J. Gubler. 1997. Surveillance and control of urban dengue vectors. In Dengue and dengue hemorrhagic fever, edited by D. J. Gubler and G. Kuno. New York: CAB International. Pp. 425-462.
Reiter, P., C. J. Thomas, P. M. Atkinson, S. T. Hay, S. E. Randolph, D. J. Rogers, G. D. Shanks, R. W. Snow, and A. Spielman. 2004. Global warming and malaria: a call for accuracy. Lancet Infectious Diseases 4(6):323-324.
Reiter, P., D. Fontenille, and C. Paupy. 2006. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infectious Diseases 6(8):463-464.
Rezza, G., L. Nicoletti, R. Angelini, R. Romi, A. C. Finarelli, M. Panning, P. Cordioli, C. Fortuna, S. Boros, F. Magurano, G. Silvi, P. Angelini, M. Dottori, M. G. Ciufolini, G. C. Majori, A. Cassone, and CHIKV Study Group. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370(9602):1840-1846.
Rosen, L. 1986. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. American Journal of Tropical Medicine and Hygiene 35(3):642-653.
Scholte, E. J., E. Dijkstra, H. Blok, A. De Vries, W. Takken, A. Hofhuis, M. Koopmans, A. De Boer, and C. B. Reusken. 2008. Accidental importation of the mosquito Aedes albopictus into the Netherlands: a survey of mosquito distribution and the presence of dengue virus. Medical and Veterinary Entomology 22(4):352-358.
Shanks, G. D., S. I. Hay, D. I. Stern, K. Biomndo, and R. W. Snow. 2002. Meteorologic influences on Plasmodium falciparum malaria in the Highland Tea Estates of Kericho, western Kenya. Emerging Infectious Disease 8(12):1404-1408.
Sissoko, D., C. Giry, P. Gabrie, A. Tarantola, F. Pettinelli, L. Collet, E. D’Ortenzio, P. Renault, and V. Pierre. 2009. Rift Valley fever, Mayotte, 2007-2008. Emerging Infectious Diseases 15(4): 568-570.
Sumilo, D., L. Asokliene, A. Bormane, V. Vasilenko, I. Golovljova, and S. E. Randolph. 2007. Climate change cannot explain the upsurge of tick-borne encephalitis in the Baltics. PLoS ONE 2(6):e500.
Tsetsarkin, K. A., C. E. McGee, S. M. Volk, D. L. Vanlandingham, S. C. Weaver, and S. Higgs. 2009. Epistatic roles of E2 glycoprotein mutations in adaption of chikungunya virus to Aedes albopictus and Ae. aegypti mosquitoes. PLoS One 4(8):e6835.
Vazeille, M., S. Moutailler, D. Coudrier, C. Rousseaux, H. Khun, M. Huerre, J. Thiria, J. S. Dehecq, D. Fontenille, I. Schuffenecker, P. Despres, and A. B. Failloux. 2007. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One 2(11):e1168.
Wikimedia Commons. 2009. Triangular trade, http://en.wikipedia.org/wiki/File:Triangular_trade.png (accessed November 9, 2009).
Wildlife Extra. 2008. EU bans wild bird imports, http://www.wildlifeextra.com/go/news/import-ban.html#cr (accessed June 23, 2009).
DOBSON AND CLEAVELAND REFERENCES
Anderson, R. M., and R. M. May. 1991. Infectious diseases of humans. Dynamics and control. Oxford, UK: Oxford University Press.
Anderson, R. M., C. Fraser, A. C. Ghani, C. A. Donnelly, S. Riley, N. M. Ferguson, G. M. Leung, T. H. Lam, and A. J. Hedley. 2004. Epidemiology, transmission dynamics and control of SARS: the 2002-2003 epidemic. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 359(1447):1091-1105.
Becker, G. S. 1968. Crime and punishment: an economic approach. Journal of Political Economy 76(2):169-217.
Burgman, M. 2005. Risks and decisions for conservation and environmental management. Cambridge, UK: Cambridge University Press.
Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287(5452):443-449.
Dobson, A. P. 2005. Virology. What links bats to emerging infectious diseases? Science 310(5748): 628-629.
Dobson, A. P., K. D. Lafferty, A. M Kuris, R. F. Hechinger, and W. Jetz. 2008. Homage to Linnaeus: how many parasites? How many hosts? Proceedings of the National Academy of Sciences 105(Suppl 1):11482-11489.
Ferguson, N. M., C. S. Donnelly, and R. M. Anderson. 2001. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science 292(5519):1155-1160.
Fraser, C., S. Riley, R. M. Anderson, and N. M. Ferguson. 2004. Factors that make an infectious disease outbreak controllable. Proceedings of the National Academy of Sciences 101(16):6146-6151.
Garrett, L. 1994. The coming plague. Newly emerging diseases in a world out of balance. New York: Farrar, Straus, and Giroux.
Grenfell, B. T., and A. P. Dobson. 1995. Ecology of infectious diseases in natural populations. Cambridge, UK: Cambridge University Press.
Keeling, M. J., M. E. J. Woolhouse, D. J. Shaw, L. Matthews, M. Chase-Topping, D. T. Haydon, S. J. Cornell, J. Kappey, J. Wilesmith, and B. T. Grenfell, 2001. Dynamics of the UK foot-and-mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science 294(5543):813-817.
Lam, S. K., and K. B. Chua. 2002. Nipah virus encephalitis outbreak in Malaysia. Clinical Infectious Diseases 34(Suppl 2):S48-S51.
Lloyd-Smith, J. O., D. George, K. M. Pepin, V. E. C. Pitzer, J. R. C., Pulliam, A. P. Dobson, P. J. Hudson, and B. T. Grenfell. 2009. Epidemic dynamics at the human-animal interface. Science (in press).
May, R. M. 1988. How many species are there on earth? Science 241(4872):1441.
May, R. M., and R. J. H. Beverton. 1990. How many species? Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 330(1257):293-304.
McLean, A. R., R. M. May, J. Pattison, and R. A. Weiss. 2005. SARS. A case study in emerging infections. Oxford: Oxford University Press.
Morse, S. S. 1995. Factors in the emergence of infectious diseases. Emerging Infectious Diseases 1(1):7-15.
Murphy, F. A. 1994. New, emerging, and reemerging infectious diseases. Advances in Virus Research 43:1-52.
Ostfeld, R. O., and K. L. LoGiudice. 2003. Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84(6):1421-1427.
Philbey, A. W., P. D. Kirkland, A. D. Ross, R. J. Davis, A. B. Gleeson, R. J. Love, P. W. Daniels, A. R. Gould, and A. D. Hyatt. 1998. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerging Infectious Diseases 4(2):269-271.
Smith, K. F., A. P. Dobson, F. E. McKenzie, L. A. Real, D. L. Smith, and M. L. Wilson. 2005. Ecological theory to enhance infectious disease control and public health policy. Frontiers in Ecology 3(1):29-37.
Sutinen, J. G., and P. Andersen. 1985. The economics of fisheries law enforcement. Land Economics 61(14):387-397.
Weiss, R. A. 2001. The Leeuwenhoek Lecture 2001. Animal origins of human infectious disease. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 356(1410):957-977.
Woolhouse, M., and E. Gaunt. 2007. Ecological origins of novel human pathogens. Critical Reviews in Microbiology 33(4):1-12.
Woolhouse, M. E. J., R. Howey, E. Gaunt, L. Reilly, M. Chase-Topping, and N. Savill. 2008. Temporal trends in the discovery of human viruses. Proceedings of the Royal Society of London Series B, Biological Sciences 275(1647):2111-2115.