Workshop Summary
INTRODUCTION
At the request of the Alzheimer’s Association, the Institute of Medicine’s (IOM’s) Forum on Neuroscience and Nervous System Disorders planned and hosted a 2-hour public workshop at the 2010 International Conference on Alzheimer’s Disease (ICAD).1 Held in Honolulu, HI, on July 12, 2010, the session was designed to explore future opportunities to leverage the Alzheimer’s Disease Neuroimaging Initiative (ADNI), with particular focus on the second phase of the ADNI project, ADNI 2. Panelists from industry, academia, and government examined the key elements of success of the research partnerships established as a result of ADNI, and contemplated the establishment of future multisector efforts to explore central nervous system developmental and neurodegenerative disorders. This report is limited to a review of workshop speaker presentations and commentary by panelists.
BACKGROUND
Alzheimer’s disease (AD) is a progressive and fatal disease of the brain that destroys brain cells, resulting in memory loss and problems with cog-
nition. Nearly 5.3 million Americans are currently living with AD, which impacts their work and social lives. However, AD is by no means a disease that only impacts Americans. In 2006 the global prevalence of AD was 26.6 million. By 2050 an estimated 1 in 85 individuals could be living with the disease (Brookmeyer et al., 2007; United Nations, 2007). Although a great deal of research and funding by both the public and private sectors are being invested to understand the pathophysiology of AD, no cure has been found.
Valley of Death
Translational research in the neurosciences is advancing as a result of sophisticated tools and the availability of solid data, but the burden of disease remains great. For translational research in general, an ongoing challenge is bridging the “valley of death,” the gap between federally funded early research and innovation and product development that is generally conducted by commercial entities. Later stage product development presents numerous challenges: costs are steep and failure rates are high, and pharmaceutical and medical device companies increasingly are becoming risk averse.
The neurosciences, like many other biomedical fields, lack a consistent interface between basic academic research and private-sector product research and development. A cultural gap separates these two worlds. Academic researchers are often uncertain about what exactly the pharmaceutical and diagnostic developers are seeking, and how academic researchers can best contribute to a productive partnership. The result of this disconnect can be stagnation of innovation and misdirected resources and efforts.
Alzheimer’s Disease Neuroimaging Initiative
These barriers have been highlighted as concerns in the development of biomarkers and therapies for Alzheimer’s disease. To begin to address these challenges, in 2004 the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the U.S. Food and Drug Administration (FDA), 20 private-sector corporations,2 and the phil-
anthropic community came together to establish ADNI.3 A primary goal of this ongoing $67 million, precompetitive public–private partnership is to develop validated biomarkers for AD clinical trials that could assess disease progression and predict clinical outcome. As the name implies, the emphasis of the initiative is to identify such biomarkers through neuroimaging, but also through biomarkers in blood and cerebrospinal fluid (CSF).
A key component of ADNI is the publicly available dataset for analyzing a host of potential biomarkers over the course of disease. Clinical and imaging data are available from more than 800 participants, including elderly controls, participants with mild cognitive impairment (MCI), and patients with Alzheimer’s disease, collected by 57 participating sites across the United States and Canada. To date, ADNI data have been accessed by more than 1,500 investigators worldwide. ADNI is considered to be a model public–private partnership that leverages the strengths of each sector and shares the associated risks. Because of its success, it is has been a model that is now being used to address other neurodegenerative disorders.
The first phase of ADNI is scheduled to conclude in late 2010. A second phase, ADNI 2, has now been funded and will be based on a similar public–private partnership model. The overall goals and research objectives of ADNI 2 include following the cognitively normal and MCI subjects from ADNI 1 and adding more groups of these subjects, along with early AD and early MCI cohorts. All subjects will receive structural magnetic resonance imaging (MRI) and amyloid positron emission tomography (PET) imaging as well as a lumbar puncture for collection of CSF. As more research has been performed on AD, it has become increasingly clear that by the time individuals exhibit symptoms of AD, a great deal of damage already has been done to the brain. The foundation for this damage begins years earlier, before individuals are symptomatic. Cellular, tissue, and organ damage then follows (Figures 1 and 2). As will be discussed throughout this summary, workshop participants highlighted the need to gain a deeper understanding of the pathophysiological changes that occur in early and presymptomatic individuals. ADNI 2 will expand the cohorts to include an earlier phase of MCI.
Worldwide Alzheimer’s Disease Neuroimaging Initiative
Reflecting the global burden of AD, a number of efforts are being directed around the world, including in Europe (E-ADNI), Japan (J-ADNI), and Australia (AIBL). Argentina, China, Korea, and Thailand are among the other countries planning research in this area. Because so many efforts
|
3 |
Further information on ADNI can be found at the project’s two websites: www.adni-info.org and www.loni.ucla.edu/ADNI. |
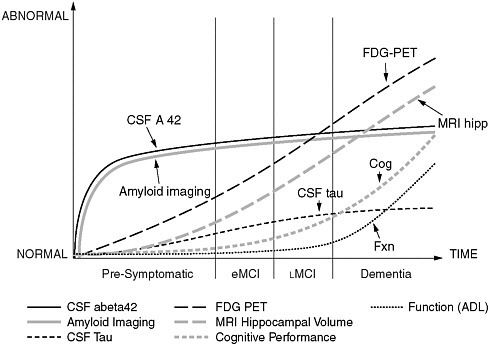
FIGURE 1 Known, dynamic parameters of Alzheimer’s disease that can be captured with advanced imaging techniques could be used for algorithmic modeling of the pathophysiology of the disease.
SOURCE: Jack et al. (2010). Jack et al. (2010).
are being made, coordination of the science is extremely important. The Alzheimer’s Association, through the World Wide Alzheimer’s Disease Neuroimaging Initiative (WW-ADNI), is planning to standardize the methods used for conducting the science to help ensure the data from all international sites can be readily compiled and analyzed.
SESSION OVERVIEW
The session was co-chaired by William Potter, cochair emeritus of the Neuroscience Steering Committee of the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium and formerly vice president of Translational Neuroscience at Merck & Co., Inc., and William Thies, vice president of Medical and Scientific Relations at the National Alzheimer’s Association.
Opening the session with an overview of initiatives and issues, Potter set the stage for a discussion of whether participants perceived value in a broader ADNI-like effort encompassing a range of neurodegenerative dis-
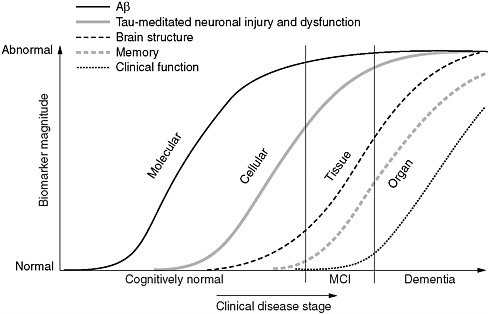
FIGURE 2 Maximizing the value of the Alzheimer’s Disease Neuroimaging Initiative. Reverse translation approach to development of diagnostics and preventative medicine for Alzheimer’s disease as envisioned for J-ADNI 2.
eases, or whether efforts and resources should be targeted toward expanding the depth of focus on Alzheimer’s disease. He said the global efforts present an opportunity for coordination and collaboration across national and regional databases.
Potter mentioned several other public–private research initiatives focused on neurodegenerative diseases, such as those of the Michael J. Fox Foundation for Parkinson’s Disease Research, the Coalition Against Major Diseases (overseen by the Critical Path Institute, and which has a primary focus on AD), the Fast Forward initiative of the Multiple Sclerosis Society, the ALS Association, the Huntington’s Dieasese Society of America, and the International Retinal Research Foundation. Examples of public–private partnerships that address multiple neurological disorders include the new degenerative dementia-focused Competence Network of the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung), and the Innovative Medicines Initiative (IMI) in the European Union (EU).
Some of the common (or potentially common) elements across the various initiatives, Potter said, include
-
Imaging tools to study brain structure and function (e.g., MRI; fluorodeoxyglucose PET; functional MRI; and electroencephalography/magnetoencephalography);
-
Data (potential for standard elements and format, common storage and retrieval);
-
Tissue samples (CSF and blood for analytes and genetic analysis); and
-
Informatics (tools for extracting information from complex data).
Other elements are disease specific in nature, including the following:
-
Study population (access to patients is generally via institutional medical specialty; the population may be influenced by the technologies available at the facility; and participant willingness to consent to a full range of procedures may vary);
-
Staff (specialized caregivers may be familiar with a limited range of tools; relationships between caregivers and investigators are important); and
-
Prevalence and course of the disease (these factors impact the number of centers, duration of a study, and frequency of measures necessary to achieve an adequate, informative study sample).
With these commonalities and differences in mind, Potter posed the question of whether a worldwide neurodegenerative disease effort should be considered. Such an effort would potentially include frontotemporal dementia (FTD), vascular dementia, amyotrophic lateral sclerosis (ALS), multiple sclerosis, and rarer diseases, and would align with the Alzheimer’s and Parkinson’s disease initiatives. He noted, however, that the North American ADNI may not be an applicable model for such an effort due to regional differences.
Later in the workshop, participants further discussed the potential of expanding the ADNI partnership to include other dementias. They noted that two commonalities allowed ADNI to advance: (1) champions for the cause were present in both academia and industry, and (2) scientists agreed about the need for large science. These two commonalities may not currently exist for other dementias, so expansion may be premature. Instead, as will be discussed in the final section of this report, a need was identified to expand the worldwide ADNI efforts to include larger cohorts of individuals that help to identify sensitive biomarkers (which may include genetics, behavioral, physiological, and biochemical biomarkers) by monitoring the progression of “presymptomatic AD” (familial and sporadic) individuals.
A worldwide initiative would help to increase subject accrual, Potter noted, and could be used to disseminate knowledge and tools to more rap-
idly develop and test treatments. It could also demonstrate replication (or the lack thereof) across populations. Conversely, the absence of timely processes to implement new method and data standards internationally could impact the success of a worldwide effort. In addition, political aspects are associated with funding, investigator and institution selection, and control and ownership of data. Local caregiving practices, human research regulations, and data storage are also factors in a global initiative.
Ultimately, the success of any initiative depends on engaging all of the relevant stakeholders, including patients and disease advocacy groups; politicians and health authorities; academic and industry scientists; pharmaceutical business leadership; and scientific organizations (e.g., ICAD, IOM, FNIH Biomarkers Consortium, IMI).
KEY ELEMENTS OF ADNI: WHAT LESSONS CAN BE LEARNED FOR MOVING FORWARD?
The National Institutes of Health (NIH) Perspective
Neil Buckholtz, chief of the Dementias of Aging Branch of the Division of Neuroscience at the NIH’s National Institute on Aging, explained that the usual clinical and neuropsychological outcome measures cannot distinguish between a symptomatic benefit and a disease-modifying effect of a therapeutic product. ADNI grew out of the need to provide a tool to evaluate whether a drug has an actual disease-modifying effect in Alzheimer’s disease. Researchers believed that the effect of a drug on the pathophysiological mechanisms of AD in the brain could be evaluated in clinical trials by using neuroimaging and fluid biomarkers.
Development of the neuroimaging initiative began in 2002, with planning meetings involving the NIH, the FDA, academia, and industry. In July 2003, a meeting was held under the auspices of the FNIH4 that resulted in a solicitation of applications, and funding of ADNI began in September 2004.
ADNI’s goals, Buckholtz said, include
-
Establishment of a database of clinical, neuropsychological, neuroimaging, fluid biomarker, and genetic data to facilitate identification of the best markers for following disease progression and monitoring treatment response;
-
Determination of optimal methods for image and biomarker data acquisition, processing, and distribution in a multisite context;
-
Validation of imaging and fluid biomarker data by correlation with clinical and neuropsychological data; and
-
Rapid public access to all data and facilitation of access to samples.
ADNI was designed as a longitudinal, multisite observational study following participants with MCI, mild Alzheimer’s disease, and cognitively normal elderly controls. Participants receive a variety of cognitive/neuropsychological, neuroimaging (e.g., MRI, PET), and blood and CSF evaluations approximately every 6 months for 4 years, Buckholtz said. In addition, DNA and immortalized cell lines are obtained from all participants, and a Genome-Wide Association Studies (GWAS) has been completed.
As described above, ADNI is structured as a public–private partnership, with the private partners coordinated through the FNIH. ADNI has a number of “cores” (including administrative, publications, biostatistics, informatics, PET, MRI, clinical, biomarker, and neuropathology cores) that are involved in the collection of various types of data at the 57 ADNI clinical sites in the United States and Canada.
Total funding for ADNI was more than $65 million, with about $40 million from the NIH (primarily from the National Institute on Aging, with contributions from the NIBIB and other NIH Institutes) and $25 million from industry and other private partners (through the FNIH). An important and novel aspect of ADNI, Buckholtz noted, is that all of the data, raw and processed, are available immediately on a public database to all qualified investigators who register and agree to the ADNI Data Use Agreement. No individual receives any sort of special access. All quality-assured MRI and PET images are housed in a central repository at the Laboratory of Neuroimaging (LONI) at the University of California–Los Angeles that is linked to a clinical database at the University of California–San Diego. A provision is made for sample sharing, and requests are considered by a Resource Allocation Review Committee.
More than 150 publications have been based on ADNI data, Buckholtz said, focusing on a variety of research issues, including: which measures or combinations of measures are best for assessing progression from MCI to Alzheimer’s disease, and from normal cognition to MCI; which measures are most sensitive to change over time; and which measures are associated in either a positive or negative direction with each other. ADNI data also have been analyzed for power calculations to determine the sample sizes needed to detect a change in clinical trials. The results of these analyses clearly suggest that fewer subjects would be needed if, for example,
various MRI measures were used as outcome measures than if clinical/neuropsychological outcomes were used, he explained. These types of biomarkers are starting to be incorporated into academic and industry clinical trials. How such biomarkers change in response to an intervention, relative to change in the clinical/neuropsychological outcome measures, will be an important finding. Using GWAS data in combination with MRI-defined brain areas, associations have been demonstrated in ADNI participants between certain genes and various aspects of the structural MRI measures. ADNI has developed a heuristic model of the progression of various biomarkers, cognition, and function, from the presymptomatic stage to early and late MCI and into AD (Figures 2 and 3). The model will be tested as more longitudinal data are collected and analyzed.
In summary, Buckholtz concluded, ADNI has been successful in:
-
Standardizing the collection of imaging and fluid biomarkers in a multisite setting;
-
Studying the relationships among biomarkers and their trajectories over time to understand the underlying pathophysiology of the transition from normal cognitive aging to MCI to Alzheimer’s disease;
-
Facilitating the incorporation of biomarkers into clinical trials for MCI and Alzheimer’s disease;
-
Providing a public database that can be used by scientists all over the world; and
-
Facilitating collaborations among academia, industry, governments, nonprofits, and regulatory agencies worldwide.
ADNI may be a useful model for other diseases, such as Parkinson’s disease, FTD, or atherosclerosis.
ADNI-GO
ADNI-Grand Opportunity (ADNI-GO), an extension of the original ADNI, was funded in 2009 from NIH American Recovery and Reinvestment Act funds. The goal of ADNI-GO is to recruit 200 additional participants who have been diagnosed with early MCI. In addition, ADNI-GO will continue to follow the normal control and later MCI subjects from the original ADNI cohort. A new PET amyloid imaging ligand, called AV-45, will be used to image brain beta-amyloid in all subjects.
As ADNI comes to a close, an application has been submitted to the NIH for ADNI 2, which, if funded, would enroll and assess additional early and late MCI, normal control, and mild AD subjects, as well as follow the original and ADNI-GO cohorts for an additional 5 years.
Japanese ADNI
As Japan’s largest public research and development management organization, the New Energy and Industrial Technology Development Organization (NEDO) promotes technology development that will enhance Japan’s industrial competitiveness and resolve energy and global environmental issues, explained Ikuhisa Sawada, project coordinator in the Biotechnology and Medical Technology Development Department of NEDO. J-ADNI is a national project under the NEDO Translational Research Promotion Project. Funded through 2011, J-ADNI receives support from NEDO, the Ministry of Health, Labor and Welfare, and Japanese and international pharmaceutical companies, at a rate equivalent to about $5 million per year.
Under the supervision of the Cabinet-level Council of Science and Technology Policy, three related ministries are making a cooperative effort to accelerate the translation of research into practical medical applications: (1) the Ministry of Education, Culture, Sports, Science and Technology, overseeing coordination, support, and training programs for translational research universities and centers; (2) the Ministry of Economy, Trade and Industry, which includes NEDO and oversees programs to assist bioindustry in the development of innovative medical technologies through translational research; and (3) the Ministry of Health, Labor and Welfare, which oversees the infrastructure for clinical research at national centers and hospitals.
The mission of J-ADNI is the development of biomarkers of Alzheimer’s disease in elderly patients. A major goal of the study is to establish and validate MRI and PET imaging and measure biomarkers in CSF and blood. As predictors of the disease, these biomarkers would assist pharmaceutical companies in the development of effective drug treatments. Specifically, it is a 5-year longitudinal observational research study involving 600 volunteers (300 with MCI, 150 with AD, and 150 healthy controls). J-ADNI involves 38 clinical sites throughout Japan. The program has three cores: clinical, imaging, and biomarker, as well as consortiums of commercial partners (including pharmaceutical, medical imaging instrument, and diagnostics company consortiums). Consistent with the goals of WW-ADNI, Sawada noted that many of the tests in the clinical test battery used in J-ADNI are compatible with the North American ADNI test battery, and the study protocols were designed to allow for possible clinical data exchange with the U.S. initiative.
J-ADNI 2
Using data obtained from J-ADNI, the next phase, J-ADNI 2, like J-ADNI 2, like, like ADNI-GO, will shift its participant cohort to the presymptomatic and early, will shift its participant cohort to the presymptomatic and early MCI stages of AD, focusing on identification of diagnostics and preven-
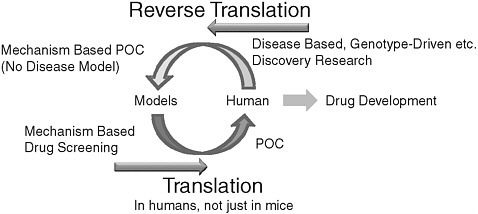
FIGURE 3 Model of Alzheimer’s disease progression
SOURCE: Jack et al. (2010).
tion of disease progression using a reverse translational research approach (Figure 3). J-ADNI 2 will again be part of the national health innovation program, under the planned Technology Development for Integration of Diagnostics and Therapy Project. NEDO will focus on technology development for reverse translational research, such as new imaging modalities, personal genomics, microdosing, and induced pluripotent stem cell technology.
The current proposal for J-ADNI 2 as outlined by Sawada calls for an additional 5-year project on early MCI diagnostics, to be conducted in a collaborative manner with WW-ADNI. The clinical network for early MCI would include 38 clinical sites plus the Tokyo University Center. Multiple-imaging modalities will be used for assessment of early MCI, including MRI volumetric change, VSRAD (Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease), PET Tau imaging, and functional MRI. There is an interest in genotype-driven research, possibly including whole-genome sequencing of beta-amyloid-positive cognitively normal individuals. J-ADNI 2 would include blood and CSF sampling, and an improved cognitive test battery for early MCI. J-ADNI 2 is at the planning stage and aiming to start during Fiscal Year 2011.
European ADNI
Giovanni B. Frisoni, vice scientific director at IRCCS Centro San Giovanni di Dio FBF and a cofounder of the National Center for Alzheimer’s and Mental Diseases in Brescia, Italy, described E-ADNI as a biomarker discovery project; a technological platform; a standard for multicenter
studies in AD and related disorders; and an opportunity for global scientific cooperation. E-ADNI is really an integrated multiproject effort including, among others, a pilot E-ADNI study begun in 2006 by the European Alzheimer’s Disease Consortium to assess the feasibility of adopting the ADNI platform, and several projects funded under the European Commission’s Seventh Framework Program for Research (EC FP7), including the research infrastructure programs neuGrid and outGrid, and the IMI PharmaCOG project. At least two of these projects, he said, would lend themselves well to a U.S.–EU cooperative effort.
IMI is the European Commission’s research plan to foster drug development by promoting public–private partnerships between academia and other public research entities, and the pharmaceutical industry. Frisoni noted that 15 projects have been funded already, including research on AD, cancer, diabetes, pain, depression, schizophrenia, respiratory diseases, and basic pharmacology.
Virtual Physiological Human
One of these projects descends directly from WW-ADNI and aims to develop a physiological hypermodel of AD. The EU, Frisoni said, is at the forefront of research in the modeling of physiological systems in health and disease with the Virtual Physiological Human (VPH) Initiative. The methodological and technological framework of VPH will enable the investigation of the human body as a single complex system through mathematical modeling. The goal is to enable personalized healthcare solutions, early diagnostics and predictive medicine, and an understanding of diseases across molecular, cellular, tissue, and organ levels.
One example of a successful VPH program, Frisoni said, is the euHeart initiative, described as “integrated cardiac care using patient-specific cardiovascular modeling.” With 17 partners (including 6 companies, 6 universities, and 5 clinics), the goal is to develop and validate computational models of the heart to improve diagnosis, treatment planning, interventions, and design of implantable devices.
The pathophysiology of AD, Frisoni noted, also lends itself particularly well to algorithmic modeling of the known dynamic changes at the molecular, cellular, tissue, and organ levels that can be captured with advanced imaging methods (Figure 1). AD might be summarized in a hypermodel, where dynamic changes at the different physiological levels in the three-dimensional space are captured in a single high-order equation over the entire course of disease (asymptomatic to symptomatic phases).
neuGRID
Another ADNI-related initiative that lends itself to more international cooperation and coordination, Frisoni said, descends from the currently ongoing neuGRID and outGRID efforts to develop a global e-infrastructure for computational imaging. The European Commission is devoting significant resources to develop the necessary scientific e-infrastructures for accessing and sharing resources and tools. Horizontal layers (pan-European, and in the first two cases, global infrastructures) are used for communications, resource sharing, computing, and data repositories (OpenAIRE, Driver). Spanning across these broad layers are individual initiatives for use by specific scientific communities (i.e., research infrastructures). This e-infrastructure provides a means for global cooperation, Frisoni said.
neuGRID is one such e-infrastructure, a virtual imaging laboratory housing data, sophisticated algorithms, and computational power that scientists will access from their own sites. Similar, although not identical, initiatives are ongoing in the United States and Canada, Frisoni noted, with the Laboratory of Neuroimaging, UCLA (LONI) and the Canadian Brain Imaging Research Network (CBRAIN), respectively. Another initiative, outGRID, aims to make these three imaging infrastructures interoperable, leading to a unique, global computational infrastructure that will allow scientists worldwide to advance the science on neurological disorders.
In conclusion, Frisoni proposed two actions. The first is an EU–U.S. partnership to develop a general physiological framework for modeling neurodegenerative diseases. The United States, he noted, has the largest collection of data, and the EU has expertise in modeling. The second proposed action is an EU–U.S. partnership to develop a global computational infrastructure to advance integrated imaging analysis and foster global science on neurodegenerative conditions.
Australian Imaging Biomarkers and Lifestyle Flagship Study
In late 2005, Australia’s national science agency, the Commonwealth Science and Industrial Research Organization (CSIRO), identified the need to address the growing problem of Alzheimer’s disease, said Christopher Rowe, director of the Department of Nuclear Medicine and Center for PET, and professorial fellow at the University of Melbourne–Austin Health. To this end, CSIRO formed partnerships with the University of Melbourne–Edith Cowan University (Perth), the Mental Health Research Institute (Melbourne), and Neurosciences Australia Ltd., resulting in the Australian Imaging Biomarkers and Lifestyle Flagship Study of Aging (AIBL). Additional collaborations were formed to facilitate access to required expertise
and technology (e.g., Austin Health Center, for the ability to conduct Pittsburgh compound B [PiB] PET imaging).
As a Preventative Health Flagship Research program, AIBL receives $1 million per year in financial support from CSIRO, and in-kind support from partner institutions. Later, additional contributions for specific projects were obtained from Pfizer for blood biomarker analysis costs; the Alzheimer’s Association for costs associated with data release via the ADNI/LONI website; the Alzheimer’s Drug Discovery Foundation to cover 3 years of PiB/MRI imaging; and an anonymous foundation for genetic analysis and extended amyloid imaging. Additional funding was also obtained from the National Health and Medical Research Council.
The primary aims of AIBL are to do the following:
-
Develop tests for earlier diagnosis of AD;
-
Identify diet and lifestyle factors that may influence the development of AD for testing in future clinical trials; and
-
Increase understanding of the processes that lead to the development of AD.
To facilitate this prospective research into aging and AD, AIBL has assembled a large cohort of 1,100 individuals from three populations: healthy (60 percent), MCI (20 percent), and AD (20 percent) populations. The populations would be followed over an extended period of time.
Organizationally, AIBL is composed of four streams (neuroimaging, clinical and cognitive, biomarkers, and lifestyle), which are overseen by a management committee. The neuroimaging stream is conducting PiB-PET and MRI scans in 287 study participants. The clinical and cognitive stream collects information on medical history, medications, and demography, and conducts a comprehensive range of cognitive assessments. The biomarkers stream conducts genotyping and comprehensive clinical blood pathology. Blood samples are also fractionated and stored for future assessment. Finally, the lifestyle stream collects information regarding participant lifestyles, such as detailed dietary and exercise information, objective activity measures, and body composition scans (Dual Energy X-Ray Absorptiometry).
Imaging and the neuropsychology battery for AIBL were designed to allow for comparison with ADNI data (e.g., Logical Memory, MRI sequences, PiB scan acquisition). Rowe noted that ADNI advice was sought in developing these measures. AIBL researchers were invited to present at ADNI meetings, and subsequently to informally join the worldwide ADNI umbrella. Rowe highlighted mutual advantages of an ADNI–AIBL collaboration, including information and idea sharing, access to U.S. funding sources, and cross-validation of findings.
An 18-month follow-up of the 1,111 participants in the AIBL baseline cohort was completed in March 2010. Rowe noted that after exclusion of non-returns and deaths, there were 968 participants at the 18-month mark. Of the 287 participants in the imaging cohort, 227 remained enrolled at follow-up. Rowe provided data highlights (Ellis et al., 2009; Rowe et al., 2010).
With regard to biomarker discovery, Rowe noted that plasma beta-amyloid measures are not robust. However, an analysis has identified candidate biomarkers with moderate diagnostic accuracy. Plasma apolipo-protein E (apoE) is lower in Alzheimer’s disease and inversely correlated with beta-amyloid load in the PiB-PET subset. ApoE levels were also significantly lower among participants homozygous for the ApoE 4 allele. Results of a GWAS of the PiB-PET cohort performed in collaboration with Harvard University are pending, he said.
With regard to lifestyle, both total and higher intensity physical activity have been shown to be associated with lower insulin, lower triglycerides, and higher levels of high-density lipoproteins. Higher levels of intense physical activity is associated with better performance in assessments targeting working memory, attention, verbal and spatial learning and recall, and executive functioning.
In the next phase of AIBL, AIBL II, Rowe said that neuroimaging will be extended to all participants. CSF collection will increase (but will not be compulsory), and a new high-risk healthy elderly and MCI cohort of 200 will be collected. A genetics stream and a data analysis/statistics core also will be added.
Industry Perspective
Howard Feldman, vice president and therapeutic area head of Neuroscience Global Clinical Research at Bristol-Myers Squibb (BMS), began by discussing key factors that were critical for ensuring the success of ADNI. These factors included a mature science based with a testable hypothesis; strategic investment; partnerships designed to share the risk; leveraging the strengths of both the public and private sectors. ADNI is valuable to industry partners as it provides clinical data from which to evaluate the longitudinal course of the disease, effect sizes on outcome measures, effects of varying covariates, and the use of acetylcholinesterase inhibitors (AChEIs). In addition, ADNI provides industry with data on biomarkers, facilitates scientific collaborations and publications and, most importantly, enables universal real-time data sharing.
BMS, like many other companies, has cross-functional working groups using ADNI data, as well as collaborations with external investigators on biomarkers. BMS has leveraged ADNI to build internal support
for, and confidence in, the company’s large investment in AD product development.
Feldman described ongoing work at BMS on prodromal Alzheimer’s disease. In collaboration with an international working group, BMS framed out the conceptual basis for revised research criteria for the diagnosis of Alzheimer’s disease, built heavily on the lessons learned from studies of MCI (Dubois et al., 2007). The new criteria are based on the core clinical feature of a hippocampal pattern of memory loss reflecting the earliest and most typical stages of disease, supported by at least one or more AD biomarkers reflecting either molecular neuropathology or downstream brain effects (presence of medial temporal lobe atrophy; abnormal CSF biomarker; specific pattern on functional neuroimaging with PET; or proven AD autosomal dominant mutation within the immediate family).
Moving from development of this new criteria to a clinical program in prodromal AD, Feldman noted that BMS is establishing the cut-off points for clinical and biomarker criteria, and addressing the validation and qualification of biomarkers. Feldman described a simulation of a prodromal Alzheimer’s trial that was presented at the 2010 American Academy of Neurology meeting, and drew attention to a poster being presented at the 2010 ICAD using retrospective ADNI data to investigate CSF cut-off points for predicting progression to AD from MCI (Burns et al., 2010). Using the new research diagnostic criteria, BMS is now recruiting patients for the first prodromal AD clinical trial, a Phase II, multicenter, double-blind, placebo-controlled study of an investigational BMS drug.5
Looking forward, Feldman posed several points for consideration. Does the path to success in AD involve progress in related neurodegenerative diseases? For example, will studying non-AD tauopathies like Pick’s disease and cortico-basal ganglionic degeneration facilitate success in AD? Will examination of neurodegeneration mechanisms be a potential pathway to success in AD? Will results generated through large-scale investments in other neurodegenerative diseases (FTD, ALS) prove beneficial to AD research? Is the value of examining healthy aging underestimated? How do we take into account the individual and their unique risk (e.g., comorbidities and genetics)?
Feldman noted that ADNI was able to take advantage of the science being uniquely primed for success, with decades of research progress in AD, and advances in biomarkers that were ready to apply. Other neuro-degenerative disorders are now also at or near that stage, he said. The real value of ADNI, Feldman said, is in being able to fully access data, samples, and collaborations. The clinical observational cohort is also key. Overall, there is tremendous value in assessing and coordinating the data, and
|
5 |
See NCT00890890 at http://www.clinicaltrials.gov. |
ADNI is a model for public–private partnerships in neurological disease research.
Intellectual Property
Steven Paul, formerly the executive vice president of science and technology at Eli Lilly and Company, noted that intellectual property (IP) and patent issues are substantial due to the extended time frame of AD clinical trials. The prodromal nature of the disease and need to expand studies into presymptomatic intervals especially compounds the challenge. IP protections (e.g., patents) have limited lifespans. Therefore, it is especially difficult for the private sector to develop a business case to perform clinical trials that will take many more years to complete because in the end they will be left with less patent protection once a potential drug hits the market. In addition, IP protection for many compounds have expired or will soon. As a result companies have no incentive to go back and redo clinical trials of off-patent products in presymptomatic individuals. Therefore, without changes in IP rules it will be difficult for companies to invest in the development of treatments for neurodegenerative disorders, such as AD.
MOVING FORWARD: OPPORTUNITIES AND FUTURE SYNERGIES FOR ADNI 2
The end of the workshop featured a panel discussion by the workshop presenters. Panelists reviewed a number of opportunities to leverage existing ADNI efforts to expand ADNI 2. In summary, panelists believe the greatest need in AD clinical research is for longitudinal “pre-symptomatic” cohort studies. It is clear that the pathophysiology of AD leads to brain damage well before the onset of symptoms (see Figures 1 and 3). Therefore, data need to be collected and analyzed from individuals who are cognitively normal, as well as those with MCI and moderate AD. Panelists expressed the need for more information technology tools to analyze the datasets. This information will provide opportunities for initiation of large-scale “preventive” trials in normal controls.
One aim of ADNI 2 is to increase subject enrollment in all categories, including elderly controls and early amnestic MCI. Data and samples collected from these cohorts have the potential to help with diagnostic test development. Accurate and early diagnosis of AD is a critical component in both treatment and prevention and will require diagnostic tests that are both sensitive and specific. In addition, these data may identify novel biomarkers. Limited studies of a single biomarker or panel of biomarkers may allow for focused evaluation; however, drug development based on a finite number of biomarkers may not result in drug approval if there is no
evidence of cognitive improvement especially if those biomarkers are, in actuality, surrogate markers.
The information e-infrastructure described by Frisoni would seem appropriate for any project, and ultimately could serve the needs of researchers of all neurodegenerative conditions to accumulate and share relevant data around the globe.
Panelists noted their concerns that resources may still be constrained, and broadening ADNI efforts beyond AD could create competition for funding. The sentiment was that there is a need to push for targeted funding of a longitudinal study of a presymptomatic AD cohort, and to identify novel ways to engage industry and incentivize long-term investment in study of the prodromal cohort.


















