Engineering Biomimetic Peptides for Targeted Drug Delivery
EFROSINI KOKKOLI
University of Minnesota
Targeted drug delivery, the ability to deliver a drug to a specific site of disease, is the leading frontier in the pursuit of better strategies for selectively treating diseases with minimal side effects. One promising class of targeted-delivery vehicles are peptide-functionalized nanovectors. Biomimetic peptide-targeting ligands (peptides that mimic cell-binding domains of proteins) can be readily designed to bind selectively to a target (e.g., an adhesion receptor on the surface of a cell) with high affinity and specificity. Even more important, biomimetic peptides are accessible by chemical synthesis and relatively compact compared to antibodies and full proteins.
PEPTIDE-FUNCTIONALIZED LIPOSOMES
Liposomes are the most extensively studied drug-transport systems to date. A number of non-targeted liposome delivery systems are already FDA approved and are being used in a clinical setting. Liposomes range in diameter from approximately 50 nm to microns, although diameters of 100 to 200 nm are often desirable for drug delivery.
“Stealth” liposomes, often referred to as sterically stabilized liposomes, have short polyethylene glycol (PEG) polymer strands attached to a fraction of hydrophilic lipid headgroups. These PEG chains form a polymer brush on the surface of the liposome that, through steric repulsion, resists protein adhesion, and therefore clearance by the reticuloendothelial system (RES). An ongoing area of liposome drug-delivery research involves conjugating ligands, such as peptides, onto “stealth” liposomes to confer active as well as passive targeting capabilities on these drug carriers.
Like liposomes, polymeric drug-delivery vectors also encapsulate their cargo and shield it from degradation and clearance from the body. In addition, many of the same peptide-targeting ligands are being conjugated to polymeric delivery vehicles. However, an inherent conflict of design for most targeted-delivery nanovectors is that the surface is typically coated with a polymer brush to inhibit protein adhesion, and therefore clearance by RES, while at the same time ligands are installed on the surface to promote targeted adhesion.
Peptides have many of the same advantages as targeting ligands: they are small molecules; they can be efficiently chemically synthesized; they can achieve high specificity; and they are easily integrated into liposomes as peptide-amphiphiles (Tu et al., 2004). Peptide ligands can be designed to mimic protein-binding sites, or they can be identified, by phage display and other selection techniques, from large peptide libraries.
Today there are a multitude of peptide ligands for a wide range of target receptors; each of which exhibits different levels of binding specificity and affinity. Liposomes have been functionalized with different peptides: SP5-52, a peptide that binds to tumor vasculature; the basic fibroblast growth factor peptide (bFGFp), which specifically binds to FGFR-expressing cells, such as melanoma, breast cancer, and prostate cancer cells; the pentapeptide YIGSR, derived from the glycoprotein laminin, which has been shown to bind with high affinity to the laminin receptor over-expressed in human tumor cells; the NGR and APRGP peptides, which have been used as potential targeting moieties against tumor vasculature; and others (Pangburn et al., 2009).
A different strategy for delivering therapeutic loads to target cells is to use peptide sequences derived from protein transduction domains (PTDs), also called cell-penetrating peptides (CPPs). PTDs are short peptide sequences that mediate translocation across the cell membrane (Torchilin, 2008). Examples of PTDs include the Antennapedia peptide (Antp), the HIV-TAT (transactivator of transcription) peptide, poly-arginine peptides, and penetratin (Breunig et al., 2008). Cell uptake by PTD peptides appears to bypass the endocytic pathway, and there are different theories about the mechanism of CPP-mediated uptake (Torchilin, 2008).
The TAT peptide derived from HIV-TAT is a frequently used CPP (Torchilin, 2008) that has been conjugated to liposomes to improve the intercellular delivery of therapeutic loads (Kale and Torchilin 2007; Marty et al., 2004; Oba et al., 2007; Torchilin et al., 2003; Tseng et al., 2002). For example, Kale and Torchilin formulated a stealth liposomal delivery system with TAT conjugated on the surface of the particles. The liposomes were delivered to the tumor sites by the EPR effect and lost their PEG coating in the low pH tumor environment thus exposing the underlying TAT peptides, which were then able to mediate transport into the tumor cells (Kale and Torchilin, 2007).
Another class of targeting peptides are fusogenic peptides. The capacity of fuso-
genic peptides of natural (e.g., N-terminus of hemagglutinin subunit HA-2 of influenza virus) or synthetic (e.g., WEAALAEALAEALAEHLAEALAEALEALAA (GALA) or WEAKLAKALAKALAKHLAKALAKALKACEA (KALA)) origin has been exploited for the endosomal/lysosomal escape of several drug-delivery systems (Li et al., 2004; Plank et al., 1998). Fusogenic peptides assume a random coil structure at pH 7. Acidification triggers a conformational transition that allows their subsequent interaction with the lipid membrane, resulting in pore formation or the induction of membrane fusion and/or lysis (Breunig et al., 2008). The incorporation of synthetic membrane-active peptides into delivery systems has been found to improve the intracellular delivery of drugs, such as oligonucleotides, peptides, and plasmid DNA (Breunig et al., 2008).
Liposomes Functionalized with Collagen-Mimetic Peptides
Collagen-mimetic peptides have been developed to target the CD44 receptor, which is over-expressed in many tumor cells, and nanoparticles functionalized with the collagen-mimetic peptide ligands are endocytosed after the ligand binds to the CD44 receptor (Jiang et al., 2002; Tammi et al., 2001). Specifically, CD44 in metastatic melanoma is in the chondroitin sulfate proteoglycan (CSPG) modified form (Naor et al., 2002). CD44/CSPG receptors bind to a specific amino acid sequence from type IV collagen α1(IV)1263-1277 (GVKGDKGNPGWPGAP), called IV-H1 (Chelberg et al., 1990; Fields et al., 1993; Lauer-Fields et al., 2003). More important, binding is highly dependant on the triple-helical structure of the sequence and the modified (CSPG) form of CD44 (Fields et al., 1993; Lauer-Fields et al., 2003; Malkar et al., 2002).
The IV-H1 peptide sequence was functionalized with different dialkyl tails to create collagen-like peptide-amphiphiles. Results showed that, although the IV-H1 peptide did not exhibit any positive ellipticity similar to a polyPro II helix, the peptide-amphiphiles investigated were all in triple-helical conformations (Yu et al., 1996). Moreover, the triple-helical peptide-amphiphiles were very stable. In this example, the self-assembly of the hydrophobic tails of the peptide-amphiphiles align the peptide strands and induce and/or stabilize the three-dimensional structure of the peptide headgroup into triple helices, giving rise to protein-like molecular architectures (Figure 1).
Previous studies have shown that a peptide-amphiphile with a peptide headgroup [(GP-Hyp)4-GVKGDKGNPGWPGAP-(GP-Hyp)4-NH2] mimics the ™1(IV1263-1277 sequence in structure and function and specifically binds to CD44/CSPG (Lauer-Fields et al., 2003; Yu et al., 1996, 1998, 1999). When this peptide-amphiphile was incorporated into a stealth liposome and targeted to M14#5 metastatic melanoma cells, it promoted specific ligand/receptor interactions. Non-targeted liposomes showed no binding (Rezler et al., 2007).
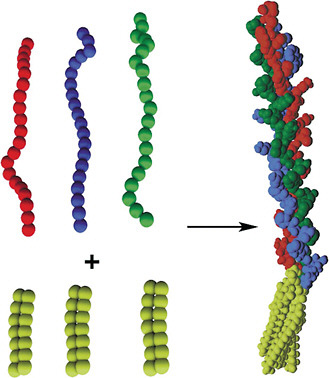
FIGURE 1 Structure of a peptide-amphiphile with triple-helical protein-like molecular architecture. Long-chain dialkyl ester lipid tails (top left) are connected to linear peptide chains (bottom left). The tails associate by hydrophobic interactions, inducing and/or stabilizing the 3-D structure of the peptide headgroup (right). Triple-helical molecular architecture is stabilized in the peptide-amphiphile. Color figure available online at http://www.nap.edu/catalog.php?record_id=13043. Source: Tirrell et al., 2002. Reprinted with permission from Elsevier.
PR_b-Functionalized Liposomes
Peptide ligands based on the tripeptide RGD (Arg-Gly-Asp) sequence are widely used in targeting research. The RGD sequence, located in the 10th type III repeat of the fibronectin molecule, which was originally identified as a cell-binding site in the extracellular matrix protein fibronectin, has been used as a targeting moiety on numerous occasions. Although RGD has been used with some success as a targeting moiety against integrins, it does not have the same adhesive properties as native fibronectin (Akiyama et al., 1995; Garcia et al., 2002; Yang et al., 2001).
A synergy amino acid sequence, Pro-His-Ser-Arg-Asn (PHSRN), located in the 9th type III repeat of fibronectin (Figure 2), has been shown to improve bind-
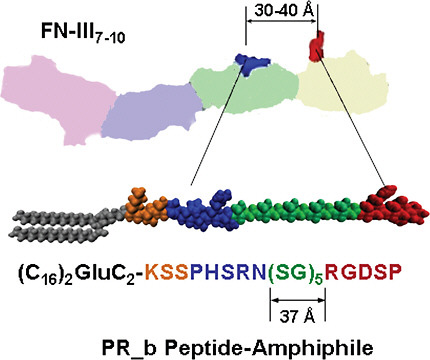
FIGURE 2 The four repeats of the fibronectin (FN) fragment III7–10 are shown: far left repeat for III7 to far right for III10. The synergy site PHSRN is in the III9 repeat. The GRGDS is in the III10. The schematic drawing of the PR_b peptide-amphiphile shows the four building blocks of the peptide headgroup: a KSS spacer, the PHSRN synergy site, a (SG)5 linker, and the RGDSP binding site. When the PR_b peptide-amphiphile is used for the preparation of functionalized liposomes, the hydrophobic tail is part of the membrane, and the peptide headgroup is exposed at the interface. Color figure available online at http://www.nap.edu/catalog.php?record_id=13043.
ing affinity and is critical for specificity to the α5β1 integrin (Aota et al., 1994; Leahy et al., 1996). Although various targeting moieties incorporating both the RGD and PHSRN sequences have been tested, most of these designs did not achieve the cell-adhesion densities supported by native fibronectin over similar time scales (Aucoin et al., 2002; Benoit and Anseth, 2005; Kao, 1999; Kim et al., 2002; Petrie et al., 2006).
Mardilovich and Kokkoli postulated that, for a small peptide to effectively mimic the α5β1 binding site of fibronectin, the primary (RGD) and synergistic (PHSRN) binding sequences must be connected by a linker that approximates both the distance and hydrophobicity/hydrophilicity between the fibronectin sequences, which results in a neutral linker (Mardilovich and Kokkoli, 2004).
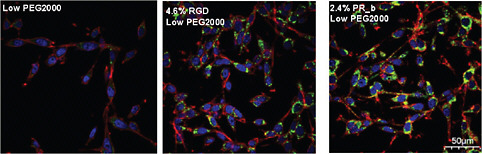
FIGURE 3 Confocal images that show internalization of targeted stealth liposomes to CT26 colon carcinoma cells. The images show the cell membrane, the nucleus, and the drug delivery systems shown between the nuclear region and the cell membrane. Different formulations with low densities of PEG2000 (2–3 percent) were incubated with CT26 at 37°C for 24 hours. The scale bar is 50 μm for all images. Color figure available online at http://www.nap.edu/catalog.php?record_id=13043. Source: Adapted from Garg et al., 2009.
Although previous attempts had been made to match the distance between the RGD and PHSRN sequences, they did not pay particular attention to the hydrophilicity/hydrophobicity of the linker.
Mardilovich and Kokkoli’s efforts culminated in the design of a biomimetic peptide, named PR_b, which is now well established as a close mimic of the α5β1 binding site in fibronectin and is a highly effective and specific targeting peptide (Mardilovich et al., 2006). PR_b has been shown to bind specifically to the α5β1 integrin and to promote cell adhesion more effectively than similar peptides with hydrophobic or hydrophilic linkers, and even more effectively than fibronectin (Craig et al., 2008). When attached to a 16-carbon dialkyl tail to form a peptideamphiphile (Figure 2), PR_b can easily be incorporated into a liposome.
Recently, stealth liposomes functionalized with PR_b were used for the targeted delivery of therapeutics to colon cancer cells (Garg and Kokkoli, 2011; Garg et al., 2009) and prostate cancer cells (Demirgöz et al., 2008). In these studies, PR_b-functionalized stealth liposomes loaded with a chemotherapy agent were more effective than RGD-functionalized stealth liposomes or non-targeted stealth liposomes in terms of cell adhesion, internalization, and cancer-cell toxicity (Figure 3).
CONCLUSION
A wide range of peptide-targeting ligands have been studied. The ones most frequently used in a variety of delivery systems, both liposomal and polymeric, are RGD, TAT, NGR, and bFGF. Installing a targeting ligand onto the surface of a delivery nanovector has been shown to have numerous advantages, such as
increased cellular adhesion, internalization, and targeting. These advantages have now been confirmed by countless researchers.
REFERENCES
Akiyama, S.K., S. Aota, and K.M. Yamada. 1995. Function and receptor specificity of a minimal 20-kilodalton cell adhesive fragment of fibronectin. Cell Adhesion and Communication 3(1): 13–25.
Aota, S., M. Nomizu, and K. Yamada. 1994. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. Journal of Biological Chemistry 269(40): 24756–24761.
Aucoin, L., C.M. Griffith, G. Pleizier, Y. Deslandes, and H. Sheardown. 2002. Interactions of corneal epithelial cells and surfaces modified with cell adhesion peptide combinations. Journal of Biomaterials Science. Polymer Edition 13(4): 447–462.
Benoit, D.S.W., and K.S. Anseth. 2005. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 26(25): 5209–5220.
Breunig, M., S. Bauer, and A. Goepferich. 2008. Polymers and nanoparticles: intelligent tools for intracellular targeting? European Journal of Pharmaceutics and Biopharmaceutics 68(1): 112–128.
Chelberg, M.K., J.B. McCarthy, A.P. Skubitz, L.T. Furcht, and E.C. Tsilibary. 1990. Characterization of a synthetic peptide from type IV collagen that promotes melanoma cell adhesion, spreading, and motility. Journal of Cell Biology 111(1): 261–270.
Craig, J.A., E.L. Rexeisen, A. Mardilovich, K. Shroff, and E. Kokkoli. 2008. Effect of linker and spacer on the design of a fibronectin mimetic peptide evaluated via cell studies and AFM adhesion forces. Langmuir 24(18): 10282–10292.
Demirgöz, D., A. Garg, and E. Kokkoli. 2008. PR_b-targeted PEGylated liposomes for prostate cancer therapy. Langmuir 24: 13518–13524.
Fields, C.G., D.J. Mickelson, S.L. Drake, J.B. McCarthy, and G.B. Fields. 1993. Melanoma cell adhesion and spreading activities of a synthetic 124-residue triple-helical “mini-collagen.” Journal of Biological Chemistry 268(19): 14153–14160.
Garcia, A.J., J.E. Schwarzbauer, and D. Boettiger. 2002. Distinct activation states of alpha5beta1 Integrin show differential binding to RGD and synergy domains of fibronectin. Biochemistry 41(29): 9063–9069.
Garg, A., and E. Kokkoli. 2011. pH-Sensitive PEGylated liposomes functionalized with a fibronectinmimetic peptide show enhanced intracellular delivery to colon cancer cells. Current Pharmaceutical Biotechnology: in press.
Garg, A., A.W. Tisdale, E. Haidari, and E. Kokkoli. 2009. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. International Journal of Pharmaceutics 366: 201–210.
Jiang, H., R.S. Peterson, W. Wang, E. Bartnik, C.B. Knudson, and W. Knudson. 2002. A requirement for the CD44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly, and receptor-mediated endocytosis in COS-7 cells. Journal of Biological Chemistry 277(12): 10531–10538.
Kale, A.A., and V.P. Torchilin. 2007. “Smart” drug carriers: PEGylated TATp-Modified pH-sensitive liposomes. Journal of Liposome Research 17(3–4): 197–203.
Kao, W.J. 1999. Evaluation of protein-modulated macrophage behavior on biomaterials: designing biomimetic materials for cellular engineering. Biomaterials 20(23–24): 2213–2221.
Kim, T.I., J.H. Jang, Y.M. Lee, I.C. Ryu, C.P. Chung, S.B. Han, S.M. Choi, and Y. Ku. 2002. Design and biological activity of synthetic oligopeptides with Pro-His-Ser-Arg-Asn (PHSRN) and Arg-Gly-Asp (RGD) motifs for human osteoblast-like cell (MG-63) adhesion. Biotechnology Letters 24(24): 2029–2033.
Lauer-Fields, J.L., N.B. Malkar, G. Richet, K. Drauz, and G.B. Fields. 2003. Melanoma cell CD44 interaction with the alpha 1(IV)1263-1277 region from basement membrane collagen is modulated by ligand glycosylation. Journal of Biological Chemistry 278(16): 14321–14330.
Leahy, D.J., I. Aukhil, and H.P. Erickson. 1996. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell 84(1): 155–164.
Li, W., F. Nicol, and J. Szoka. 2004. GALA: a designed synthetic pHresponsive amphipathic peptide with applications in drug and gene delivery. Advanced Drug Delivery Reviews 56: 967–985.
Malkar, N.B., J.L. Lauer-Fields, J.A. Borgia, and G.B. Fields. 2002. Modulation of triple-helical stability and subsequent melanoma cellular responses by single-site substitution of fluoroproline derivatives. Biochemistry 41(19): 6054–6064.
Mardilovich, A., and E. Kokkoli. 2004. Biomimetic peptide-amphiphiles for functional biomaterials: the role of GRGDSP and PHSRN. Biomacromolecules 5(3): 950–957.
Mardilovich, A., J.A. Craig, M.Q. McCammon, A. Garg, and E. Kokkoli. 2006. Design of a novel fibronectin-mimetic peptide-amphiphile for functionalized biomaterials. Langmuir 22(7): 3259–3264.
Marty, C., C. Meylan, H. Schott, K. Ballmer-Hofer, and R.A. Schwendener. 2004. Enhanced heparan sulfate proteoglycan-mediated uptake of cell-penetrating peptide-modified liposomes. Cellular and Molecular Life Sciences 61(14): 1785–1794.
Naor, D., S. Nedvetzki, I. Golan, L. Melnik, and Y. Faitelson. 2002. CD44 in cancer. Critical Reviews in Clinical Laboratory Sciences 39(6): 527–579.
Oba, M., S. Fukushima, N. Kanayama, K. Aoyagi, N. Nishiyama, H. Koyama, and K. Kataoka. 2007. Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ3 and αvβ5 integrins. Bioconjugate Chemistry 18(5): 1415–1423.
Pangburn, T.O., M.A. Petersen, B. Waybrant, M.M. Adil, and E. Kokkoli. 2009. Peptide- and aptamer-functionalized nanovectors for targeted delivery of therapeutics. Journal of Biomechanical Engineering 131(7): 074005.
Petrie, T.A., J.R. Capadona, C.D. Reyes, and A.J. Garcia. 2006. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials 27(31): 5459–5470.
Plank, C., W. Zauner, and E. Wagner. 1998. Application of membrane-active peptides for drug and gene delivery across cellular membranes. Advanced Drug Delivery Reviews 34: 21–35.
Rezler, E.M., D.R. Khan, J. Lauer-Fields, M. Cudic, D. Baronas-Lowell, and G.B. Fields. 2007. Targeted drug delivery utilizing protein-like molecular architecture. Journal of the American Chemical Society 129(16): 4961–4972.
Tammi, R., K. Rilla, J.-P. Pienimaki, D.K. MacCallum, M. Hogg, M. Luukkonen, V.C. Hascall, and M. Tammi. 2001. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. Journal of Biological Chemistry 276(37): 35111–35122.
Tirrell, M., E. Kokkoli, and M. Biesalski. 2002. The role of surface science in bioengineered materials. Surface Science 500(1–3): 61–83.
Torchilin, V.P. 2008. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Advanced Drug Delivery Reviews 60(4–5): 548–558.
Torchilin, V.P., T.S. Levchenko, R. Rammohan, N. Volodina, B. Papahadjopoulos-Sternberg, and G.G.M. D’Souza. 2003. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proceedings of the National Academy of Sciences 100(4): 1972–1977.
Tseng, Y.L., J.J. Liu, and R.L. Hong. 2002. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and TAT: a kinetic and efficacy study. Molecular Pharmaceutics 62(4): 864–872.
Tu, R.S., K. Mohanty, and M.V. Tirrell. 2004. Liposomal targeting through peptide-amphiphile functionalization. American Pharmaceutical Review 7(2): 36–41.
Yang, X.B., H.I. Roach, N.M.P. Clarke, S.M. Howdle, R. Quirk, K.M. Shakesheff, and R.O.C. Oreffo. 2001. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone 29(6): 523–531.
Yu, Y.C., P. Berndt, M. Tirrell, and G.B. Fields. 1996. Self-assembling amphiphiles for construction of protein molecular architecture. Journal of the American Chemical Society 118(50): 12515–12520.
Yu, Y.C., V. Roontga, V.A. Daragan, K.H. Mayo, M. Tirrell, and G.B. Fields. 1999. Structure and dynamics of peptide-amphiphiles incorporating triple-helical proteinlike molecular architecture. Biochemistry 38(5): 1659–1668.
Yu, Y.C., M. Tirrell, and G.B. Fields. 1998. Minimal lipidation stabilizes protein-like molecular architecture. Journal of the American Chemical Society 120(39): 9979–9987.










