6
Waste Forms and Disposal Environments
The first charge of the statement of task for this study (see Box 2.1 in Chapter 2) calls on the National Academies to identify and describe “Essential characteristics of waste forms that will govern their performance within relevant disposal systems. This study will focus on disposal systems associated with high-cost waste streams such as high-level tank waste and calcine but include some consideration of low-level and transuranic waste disposal.”
The most essential characteristic of a waste form that governs its performance1 in a disposal system is durability: that is, the resistance of a waste form material to physical and chemical alteration and associated release of contained radioactive and hazardous constituents2 (see Chapter 5). Durability depends partly on intrinsic material properties, as described in Chapter 3, but also on the physical and chemical conditions in the disposal facility into which the waste form has been emplaced. Consequently, the durability of a waste form in a disposal environment can be optimized by matching it with the appropriate physical and chemical conditions that foster long-term stability.
The focus of this chapter is on waste form performance in disposal facilities. Given the emphasis of the study charge on high-cost waste streams, this chapter focuses primarily on waste form performance in disposal facili-
________________________
1 That is, the ability of waste forms to sequester radioactive and hazardous constituents.
2 As noted in previous chapters, radioactive waste can contain both radioactive and hazardous constituents.
ties for spent nuclear fuel (SNF), high-level radioactive waste (HLW), and transuranic (TRU) waste.
6.1 DISPOSAL OF WASTE FORMS
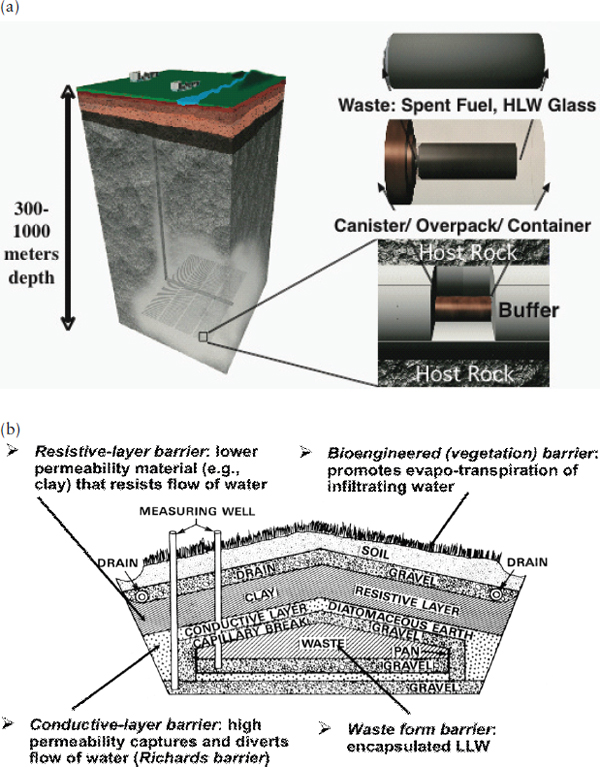
Waste forms containing radioactive waste are intended for disposal in engineered facilities constructed in stable geologic formations. Geologic repositories are designed for the disposal of higher-hazard wastes3 such as SNF, HLW, and TRU waste. These facilities are constructed in geologic formations located hundreds of meters below Earth’s surface. Shallow-land disposal facilities are designed for the disposal of lower-hazard wastes such as low-level radioactive waste (LLW). These facilities are typically excavated into sediments located within 10 meters or so of the Earth’s surface (Figure 6.1).
Given the focus of this report on HLW, the discussion in this chapter focuses on geologic repositories. However, many of the environmental processes that govern waste form performance in geologic repositories would also apply to shallow-land facilities.
Geologic repositories are designed with multiple barriers to isolate waste from the environment (NRC, 2003; OECD-NEA, 2003). They contain both engineered barriers, which include the waste form, disposal canisters, and backfills, if present, and natural barriers such as the host rock. These barriers are intended to work in concert, passively providing different safety functions at different levels of effectiveness and reliability and at different times into the future for long-term isolation of radioactive waste (Figure 6.1). Barrier safety functions can overlap, providing so-called latent safety functions, with each barrier contributing to waste isolation at varying levels and times. Taken collectively, the barriers and their safety functions define the safety concept for the disposal system.
There are two basic isolation strategies to achieve long-term (103-106 years) safety of multiple-barrier disposal facilities (Apted and Ahn, 2010):
- Containment of radionuclides within the engineered and natural barriers of the disposal facility. Containment allows time for radioactive decay, which reduces the hazard of the radioactive component of the waste.
- Attenuation of the concentrations of radionuclides that are released from the disposal facility. This strategy may, for example, rely on
________________________
3 Hazard depends on several factors, including the types and concentrations of radioelements and their mobility in the environment. The classification of radioactive wastes in the United States is based on waste origin rather than hazard. Nevertheless, wastes destined for geologic disposal generally have a higher hazard than wastes disposed of in shallow facilities.
extremely slow waste form dissolution rates; on solubility limits for radionuclides in groundwater contacting the waste forms; on dispersion of radionuclides as they migrate through the barriers in the disposal system; on dilution, which can be volumetric (by mixing contaminated and uncontaminated groundwater) or isotopic (by mixing radioactive and stable isotopes of the same elements; see Section 6.3.8); and on long transport times from the disposal facility to the biosphere.
A number of concepts have been developed both in the United States and internationally for geologic repositories that utilize different barriers and rely on different processes to provide containment and attenuation (e.g., NWTRB, 2009; Witherspoon and Bodvarrson, 2006). A full review of these alternative concepts is beyond the scope of this report. It is important to understand that such concepts are typically tailored to work in concert with the environmental conditions that have been measured and characterized for specific disposal sites.
6.2 GEOLOGIC ENVIRONMENTS
The desirable geologic characteristics of repositories have been well-delineated. According to Bodansky (1984):4
A good repository site is one for which the location and type of rock (a) prevent or limit the flow of water into the repository, (b) provide geochemical conditions favorable for a low rate of corrosion of the waste package and low solubility of radionuclides in the event of entry of water, (c) slow the outward migration of water to the biosphere, (d) retard the motion of major radionuclides so that they move more slowly than the water, and (e) are at low risk of future disruption by earthquake, volcano, erosion, or other natural phenomena. Together, these attributes provide a series of natural barriers.
Decades of research nationally and internationally have created a wealth of world-wide accumulated experience concerning the disposal of radioactive waste in various geologic environments (Table 6.1). Studies have examined the merits of developing disposal facilities in salt, basalt, granite, tuff, clay, shale, and metamorphic rocks. Detailed studies, in some cases supporting planning and construction of disposal facilities, have been executed for clay, tuff, salt, and granite. Some important characteristics of these materials are described in the following sections.
________________________
4 Bodansky (1984) also provides a discussion of geologic environments for disposal facilities.
TABLE 6.1 Geologic Investigations of Potential Sites for Disposal of SNF/HLW
| GEOLOGICAL INVESTIGATIONS | ||
| COUNTRY | GEOLOGIC ENVIRONMENTS CONSIDERED OR INVESTIGATED | INDIGENOUS UNDERGROUND RESEARCH LABORATORY ESTABLISHED |
| United States | Salt, basalt, granite, tuff, clay, and shale | The Explorotory Studies Facility at Yucca Mountain served the function of an underground research laboratory (tuff). |
| Belgium | Clay and shale | Mol (clay)* |
| Canada | Granite and sedimentary rock | Pinawa (granite)* |
| China | Granite | None |
| Finland | Granite, gneiss, grandiorite, and migmalite | Construction of ONKALO underground rock characterization facility in Eurajoki began in 2004 and is continuing (granite). |
| France | Argillite and granite | Bure (argillite) |
| Germany | Sail | Gorleben (salt) |
| Japan | Granite and sedimentary rack | Tono (granite) Mizunami (granite) Horonobe (sedimentary rock) |
| Republic of Korea | Granite | Korea Underground Research Tunnel (granite)** |
| Spain | Granite, clay, and salt | None |
| Sweden | Granite | Äspö (granite) |
| Switzerland | Clay and granite | Mont Terri (clay) and Grimsel (granite) |
| United Kingdom | No decision made. | None |
*In the process of being decommissioned
**At shallow depth only
SOURCE: NWTRB (2009).
6.2.1 Salt
Salt deposits form where water is evaporated, typically over thousands of years. Evaporation of a shallow inland sea or lake can give rise to large deposits composed primarily of the minerals halite (NaCl) and sylvite (KCl) and secondarily of the minerals gypsum (CaSO4(H2O)2), anhydrite (CaSO4), calcite (CaCO3), dolomite (CaMg(CO3)2), and various borate minerals.
Two general types of salt deposits have been investigated for disposal of SNF/HLW and TRU waste: bedded salt deposits and salt domes. Bedded salt deposits consist of thick layers of salt with significant lateral extent. In contrast, salt domes are the result of buoyancy-driven migration of salt into overlying rocks to form large diapir structures. Salt deposits of either type may have associated oil and gas or other mineral resources, the existence of which may increase the probability of human intrusion (by drilling or excavation) into disposal facilities located within those deposits.
The halide minerals5 that constitute the bulk of salt deposits are water soluble and easily deformed under moderate pressure. Permeability in such a rock is essentially non-existent because deformation readily closes pore spaces and seals fractures. Some water is trapped as fluid inclusions in the salt crystals, and pockets of saline brines trapped during formation of the salt deposits often persist for geologic times (i.e., tens to hundreds of millions of years). Such inclusions have been observed, for example, within the Salado Formation at the Waste Isolation Pilot Plant (WIPP) in New Mexico (Stein, 1985). These saline brines are high ionic strength chloride solutions.
A waste form emplaced in a salt deposit well below Earth’s surface will not contact flowing water unless a disruptive geologic event such as a meteorite impact or human intrusion takes place. Groundwater flow is thus not a factor in determining waste form performance in salt. Because salt deposits are easily deformed, however, materials placed within them can physically move over the course of time if they are more or less buoyant than the salt itself. Buoyancy-driven transport of materials can be enhanced at elevated temperatures (e.g., Nunn, 1996; Speight, 1964); brine inclusions in salt will preferentially move toward heat sources (i.e., up the thermal gradient) in the salt. (The brine will preferentially dissolve salt at the higher-temperature wall of the inclusion while simultaneously precipitating salt at the lower-temperature wall of the same inclusion.) Consequently, emplaced materials with significant heat loads can be problematic for disposal in salt.
________________________
5 Halide minerals contain one or more of the halogen anions bromine, chlorine, fluorine, or iodine.
6.2.2 Clay
Clay minerals6 consist of sheets of silicate tetrahedra combined with layers of octahedra containing various metals surrounded by oxygen or hydroxyl ions; they may also contain water and various alkali and alkaline earth elements. Clay minerals are the major weathering products of silicate minerals such as feldspar; they can form in sedimentary deposits and where any silica-rich rock is weathered or altered by relatively low-temperature geologic fluids. Clay deposits can form in situ as a result of weathering of rocks such as volcanic ash or tuff, or accumulated in sedimentary deposits as a result of transport by wind, water, gravity, or ice. Clay deposits that form in situ can have significant lateral extent, whereas sedimentary deposits tend to be more restricted in area.
It is important to note that there are dozens of varieties of clay minerals with considerably varied chemical and physical properties. Some clay minerals, for example, readily exchange cations contained between the silicate sheets in their structures; others may swell significantly when they absorb water. The specific chemical and physical properties of a given clay deposit depend strongly on its mineralogy and the presence of organic matter. Organic matter is often present, sometimes in large quantities, and ensures that any water that is present does not contain dissolved oxygen. In the absence of radiolytic decomposition of water (see Section 6.3.7), its oxidizing potential is minimal.
Owing to the extremely small size of clay grains, clay deposits are readily compacted and have low porosity and extremely low permeability.7 Clay minerals have been proposed both as the host rock for SNF/HLW repositories and for use as buffer/backfill materials. For example, swelling smectite clay has been proposed for use as an engineered buffer/backfill barrier in most planned repositories for SNF/HLW in Europe (NWTRB, 2009). However, not all clay deposits have desirable characteristics for emplacement of radioactive waste forms.
6.2.3 Other Sedimentary Rocks
Sedimentary deposits are produced as a result of transport and deposition of weathered rocks near the Earth’s surface. The transporting agents include water, wind, gravity, and ice. Such deposits are initially unconsolidated, but they may later become consolidated as a result of compaction (if they are buried) and cementation by minerals precipitated from ground-
________________________
6 In geologic and engineering terminology clay also refers to minerals having small grain sizes regardless of their composition. They include clay minerals as well as small grains of quartz and feldspar.
7 Permeability is the ability of a material to transmit fluids (such as water) by advective flow.
water flowing through them. Consolidation typically occurs over thousands to millions of years.
The physical and chemical properties of such deposits can be highly variable and depend both on the source material and the depositional environment. For example, materials deposited by ice (glacial tills) are typically unsorted, unstratified, and highly variable in composition. In contrast, sediments deposited by water (e.g., in a river or beach environment) are typically well sorted, stratified, and may contain a restricted range of mineral compositions (dominantly quartz) and grain sizes. Deposits from wind likewise exhibit restricted grain size distributions and also are dominantly quartz.
Unconsolidated sediments can experience very high groundwater flow rates because of their high permeability. The pore spaces within these sediments will be filled with both air and water above the groundwater table (the unsaturated zone, also referred to as the vadose zone). Below the groundwater table (in the saturated zone), the pore space is filled with water. Oxidizing conditions will prevail in the vadose zone in the absence of significant organic matter or microbial activity. At least locally oxidizing conditions are likely to prevail even in saturated unconsolidated sediments if they are near enough to the surface to be subjected a high influx rate of oxygenated water from the overlying vadose zone.
Thick alluvial deposits, especially in the Great Basin in the western United States, have attracted attention as potential sites for disposal of radioactive waste (Tyler et al., 1996). Disposal of radioactive waste forms in shallow unconsolidated sediments may be suitable for relatively low-activity materials when combined with appropriate supporting engineered barriers (see Figure 6.1b).
Sedimentary rocks with significant organic components present a strongly reducing geochemical environment. An example is oil shale, a fine-grained sedimentary rock that contains a number of solid organic compounds that can be thermally processed to recover oil. The permeability of such a rock may be low, and any groundwater that is present will be devoid of dissolved oxygen. The rock presents substantial reducing potential and may continue to buffer the redox potential of water for a very long time.8
6.2.4 Crystalline Rocks
The continental crust is composed primarily of crystalline igneous and metamorphic rocks containing aluminosilicate minerals such as quartz, feldspar, mica, and minor amounts of other minerals. These rocks are pro-
________________________
8 Such reducing shale formations account for the existence of the natural reactor at Oklo in Gabon (Janeczek, 1999).
duced by the cooling and crystallization of molten magma (igneous rocks) or by solid-state mineral reactions at elevated pressures and temperatures (metamorphic rocks). Extrusive igneous rocks (e.g., basalts), intrusive igneous rocks (e.g., granite), and metamorphic rocks (e.g., gneiss) have been or are being considered as potential environments for the disposal of SNF/HLW (see Table 6.1).
Extrusive igneous rocks form when molten lava is cooled at or near Earth’s surface. Although there is essentially a continuum of silica concentrations in these rocks, ranging from felsic (silica rich) to ultramafic (silica poor), basalt (mafic) is the most abundant extrusive igneous rock on Earth. Fissure eruptions have created vast accumulations of basalt, known as flood basalts; notable examples of flood basalts include the Columbia River basalts in the western United States and the Deccan Traps in India. Fissure eruptions produce layers of basalt in a series of extrusive events. Such sequences of basalt flows may be several kilometers thick and provide a relatively homogeneous geochemical environment for a disposal facility.
Basaltic rocks are variably crystalline, often with glassy components, depending on their cooling history. These rocks commonly contain significant porosity because of the development of gas bubbles prior to solidification, but these void spaces are not well connected and therefore do not result in high permeability. However, basalt flows can contain extensive systems of vertical fractures that arise from contraction during cooling. An extreme example is columnar basalt in which the entire flow layer has fractured into vertical columns a few tens of centimeters in cross section. As sequences of basalt flows originate from a series of discrete eruptive events, horizontal rubble zones and buried soil horizons can also occur. Water can flow rapidly through these fractures, zones, and horizons in what would otherwise be an impermeable rock.
Lavas of felsic composition are more viscous than their mafic counterparts, and they are usually erupted more violently as a result of the explosive decompression of dissolved gases. These eruptions can eject substantial volumes of mineral, rock, and glass fragments (referred to as volcanic ash) that accumulate to form tuff. These fragments may be compacted and fused together to form welded tuff. Tuffs can contain significant porosity because of trapped air bubbles, but increased degrees of welding reduce porosity. The permeability associated with this porosity tends to be low. However, additional permeability can be produced by fracturing as the tuff cools and contracts, and additional fracturing can occur through subsequent tectonic processes. These fractures provide pathways for the flow of groundwater through the tuff, which can result in the formation of a suite of lower-temperature minerals, such as zeolites, with high ion-exchange capabilities.
Groundwater traveling through fractured rocks above the groundwater table contains dissolved oxygen and is therefore oxidizing. Such oxidizing
environments may not be optimal for disposal of waste forms that are susceptible to oxidation, for example, SNF comprised of uranium/plutonium dioxide and some metal alloys. As will be discussed in more detail in Section 6.3.2, uranium, neptunium, plutonium, iodine, selenium, and technetium are more soluble in their oxidized forms.
Intrusive igneous and metamorphic rocks are characterized by interlocking crystals with little or no primary porosity. However, these rocks usually contain fractures due to some combination of cooling and tectonic processes, and these fractures provide pathways for groundwater flow. Groundwater flow rates in these rocks can vary significantly, from merely a few centimeters or less per year to rates exceeding several meters per year.
Groundwater in crystalline rocks below the water table may be oxidizing or reducing depending on the presence and reactivity of redox-active minerals (see Section 6.3.2). For example, water in a granitic rock containing significant magnetite (Fe3O4) and pyrite (FeS2) or other reducing minerals will not contain dissolved oxygen in any appreciable quantities. Under these conditions dissolution rates of some waste forms may be much lower than in the presence of water containing dissolved oxygen. Moreover, the concentrations of actinides and some long-lived fission products will be lower under reducing conditions.
6.3 FACTORS AFFECTING WASTE FORMS IN DISPOSAL ENVIRONMENTS
A disposal environment is defined as the time-dependent physical and chemical conditions in a facility designed for the disposal of radioactive waste. An initially undisturbed geologic environment can be substantially perturbed by the development of a disposal facility. Factors that change the environment include the actual excavation, the addition of building materials (such as steel and concrete) for facility stabilization, and the emplacement of waste. The environment will also be impacted by the presence of engineered barriers such as buffer/backfill, overpacking materials, and canisters.
The disposal environment will change from the construction of underground facilities and emplacement of wastes. These changes continue over timescales of hundreds to thousands of years as physical and chemical conditions evolve and as the various components of the disposal facility—including the emplaced waste forms, other engineered barriers, and natural barriers—interact in a coupled fashion (see Chapter 7). In fact, interactions among these components under evolving environmental conditions of a repository will impact the stability and chemical durability of waste forms, as well as the mobility of any radionuclides released into the near-field
host rock.9 The complex interactions between the host rock of the facility, groundwater, and the waste forms, and engineered barriers make each disposal environment unique.
The stability of a waste form over time and its alteration products in a given repository environment dictate its performance. Waste forms will perform optimally when matched with appropriate physical and chemical conditions that foster long-term stability.
Given sufficient time, waste forms will eventually be contacted by groundwater and will begin to dissolve, releasing radionuclides into and through the near-field environment of the disposal facility, representing the source term for the facility. Degradation can occur through a number of physical and chemical processes, including chemical corrosion through reactions of the waste form with groundwater or physical alteration through buildup of radiation-induced damage or in growth of daughter products.
The following sections provide brief descriptions of some key processes and environmental factors that can affect waste form performance and radionuclide mobility in disposal environments.
6.3.1 Groundwater
The interaction of groundwater with waste forms in the near-field environment of the disposal facility is of paramount importance in determining waste form performance. The most important attributes of groundwater include volume and rate of flow, temperature, pH, presence of dissolved oxygen, ionic strength of dissolved constituents, and presence of inorganic and organic species that complex radioactive constituents or otherwise interact with the waste form in a deleterious fashion. Some of these factors are described in greater detail in subsequent sections.
As precipitation (rain, snow, hail), water contains comparatively few dissolved species, mainly atmospheric gases. Once in contact with soils and rocks, however, the chemical composition of the water will be altered as it passes through and over mineral surfaces. In fact, the total dissolved solids in groundwater are generally governed by the solubility of the mineral phases in the soil and rock through which it flows. The solubilization of major and trace constituents governs the ambient pH of the water and often the redox conditions of the environment.
The quantity of water moving through the vadose zone and into the saturated zone is directly dependent on rainfall, infiltration, and evapora-
________________________
9 As noted in Chapter 1, the near-field environment is generally taken to include the engineered barriers in a disposal system (e.g., waste canisters) as well as the host geologic media in contact with or near these barriers whose properties have been affected by the presence of the repository.
tion rates, which can change markedly over time. Groundwater flows are impacted by changes in recharge and withdrawal rates, water table gradients, and rock porosity and permeability because of changes in strain state and mineral precipitation/dissolution. The location of the groundwater table can change through time as a result of the interplays among these factors. As a consequence, a disposal facility constructed in the vadose zone or saturated zone near the groundwater table can cycle from saturated to unsaturated conditions.
Climate change has the most significant long-term impact on groundwater characteristics. Consider for example the Death Valley drainage basin in eastern California (Lowenstein et al., 1999). At present, Death Valley is a desert with minimal precipitation and a deep groundwater table. Toward the end of the last continental glaciation about 12,000 years ago, however, Death Valley contained several hundred feet of water. The Great Salt Lake in Utah is the remnant of the pluvial lake Bonneville, which existed at the same time and had a much greater areal extent and depth than the current lake. Climate changes that trigger such large variations can occur over the course of a few thousand years or less. There is substantial reason to believe that dramatic changes of the climate will occur in the future, as they have for millennia.
Consequently, a disposal facility in the vadose zone may experience significantly increased water flow rates and perhaps even fully saturated conditions in the future because of climate change. A disposal facility located below the water table would likely experience less variation as long as it remained saturated with water.
6.3.2 Redox Potential (Eh)
Redox potential (Eh) determines the activity of oxygen in geologic formations. It can be buffered by pairs of minerals containing an element, typically iron but also possibly manganese, sulfur, or carbon that can exist in two or more valence states. One mineral of the buffer pair contains the redox-active element in a more reduced valance state, and the other mineral contains the same redox-active element in a more oxidized valance state. Below the near-surface oxidizing zone (>100 m typically), oxygen has been removed by microbially mediated processes, which produces mildly to strongly reducing conditions depending on the mineralogical buffer-pair (Langmuir, 1997).
The prevailing buffered Eh state of a geologic formation can be perturbed by changing the availability of electron donors (reducing agents) or electron acceptors (oxidizing agents). Such changes can arise from numerous processes, for example, from introduction of atmospheric oxygen during construction of a repository or possibly from deep circulation of
oxidizing waters during glacial periods. The mineral buffer-pair will act to restore the Eh to the ambient value unless the redox buffering capacity is exhausted (e.g., because all of the reduced mineral is converted to the oxidized-mineral).
Radioelements that occur in multiple oxidation states can exhibit variable chemical behavior depending on Eh, including carbon, selenium, iodine, technetium, plutonium, neptunium, and uranium. Each of these elements has accessible multiple (two or more) oxidation states under typical environmental conditions, for example iodine (–1 to +7); technetium (0 to +7); and plutonium, neptunium, and uranium (+3 to +6). The stability of phases containing such redox-active elements, and the subsequent transport behavior of such elements, will be strongly affected by redox conditions with disposal systems and host rock formations.
The oxidation state of the element can affect its mobility in the environment. For example:
- Technetium is relatively insoluble under reducing conditions (TcO2s) (Chen et al., 2000) but forms the soluble pertechnetate anion (TcO4–) under oxidizing conditions. In contact with organic complexing agents10 in solution, technetium can exhibit multiple valence states. At the Hanford Site, the presence of organic complexing agents in tank waste was seen to produce soluble technetium species that were not TcO4– (Icenhower et al., 2010).
- Iodine occurs in mobile forms whether conditions are oxidizing (IO3–) or reducing (I–).
- Under conditions typically existing in subsurface environments, uranium, neptunium, and plutonium are relatively insoluble under reducing conditions (as AnO2s, where An = actinide), moderately soluble (UO22+/PuO22+) but susceptible to hydrolysis/mobilization by complexation, or intrinsically soluble (NpO2+/PuO2+) in the presence of oxidizing agents. Hexavalent actinides interact strongly with natural complexing agents such as carbonate or humic materials and can exhibit substantial environmental mobility in this circumstance (Ewing et al., 2010).
- Anionic species are highly mobile and can transport from their point of release from a waste form with the water front.
- Cations with low charge (such as cesium (+1)) are measurably mobile, but less so than anionic species.
________________________
10 Such agents can include naturally occurring species such as citrate or oxalate or man-made reagents like EDTA.
6.3.3 pH
pH is a measure of the dissolved hydrogen ion (H+ or H3O+) activity in a solution. Although there can be natural occurrences of relatively low pH (strongly acidic) and high pH (strongly alkaline) groundwater, these rather unfavorable pH conditions arise from known mineralogical factors and can be avoided through careful site characterization and selection. Many geologic formations are characterized by porewater that is buffered by mineralogical reactions to ambient pH values close to neutrality (Figure 6.2).
In the same way that Eh can be perturbed by events such as excavation of the rock during repository construction or deep circulation of surface water, pH can also be perturbed away from ambient values. For example, pH can be raised to high values by the introduction of cement-based materials into the repository, either as mechanical supports, flow barriers, or as a waste-form matrix. However, pH can also decrease to low values when groundwater contacts corroding engineered barriers (such as corroding waste canisters) in the repository. In the same manner as Eh buffering, the pH-active minerals of the rock formation will act to restore pore water pH to ambient values as long as the buffering potential of the minerals is not exhausted.
The presence of cementitious materials in concretes used in disposal facilities promotes alkaline conditions. This can lead to a significant increase in the dissolution rate of silica-based waste forms, such as borosilicate glass, attributable to the increased solubility of silica at pH > 9. This concern applies not only to conventional Portland-type cements, but also to recently developed so-called “low pH cements” as well slag- and fly ash-based concretes (Savage and Benbow, 2007).
Absent the presence of dissolved CO2, alkaline conditions tend to precipitate polyvalent cations, including actinides, but will have minimal direct effects on cesium, strontium, technetium, or iodine solubility. Under alkaline and CO2- saturated conditions, the solubility of oxidized actinide species can be expected to increase substantially. On the other hand, the presence of phosphate minerals will tend to retard the mobility of actinides, but such retardation will probably be more effective under reducing conditions. High pH can also lead to rather rapid (few days) mineralogical alteration of swelling smectite clay, used as buffer/backfill in many disposal facilities (Figure 6.1a), to non-swelling illite clay (Eberl et al., 1993).
6.3.4 Anions/Salinity
Another important characteristic of groundwater affecting the performance of waste forms and disposal systems in general is the concentration
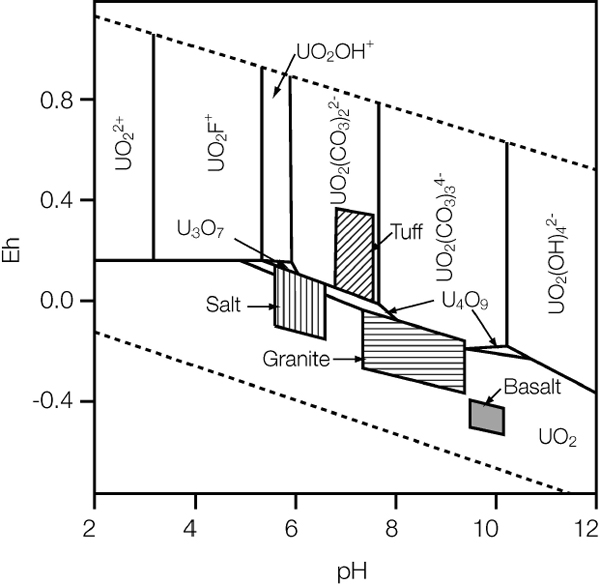
FIGURE 6.2 Stability diagram for UO2 in various groundwaters at 25°C showing the pH-Eh stability fields for aqueous uranium species. The Eh-pH conditions for groundwaters in tuff (Yucca Mountain, Nevada), basalt (Hanford, Washington), granite (Sweden Stripa), and salt (Permian brine groundwater) are represented by the shaded areas. Note that basaltic and granitic groundwaters are reducing whereas tuffaceous groundwaters are oxidizing. Groundwater in shale (not shown) will likely be reducing, depending on its content of organic material, but pH would depend on rock composition and geologic setting.
SOURCE: Data from Jantzen (1992) and Johnson and Shoesmith (1988).
of anionic species.11 These anions are important primarily because they can form stable aqueous complexes with radioelements, thus increasing radioelement solubilities in groundwater and modifying the transport characteristics of such radioelements through the near-field environment. Formation of anion complexes increases with increasing concentration of anions, so that high-salinity groundwaters can be a proxy indicator for anion complexation and its impact on radioelement solubilities.
For potable water, ionic strength is typically low, but highly saline waters are frequently encountered in the subsurface. The chemical properties of the water can be altered significantly by the presence of substantial amounts of dissolved salts.
6.3.5 Complexation
The solubilities of many radioelements can be significantly different in water containing complexing agents. For example, oxidized waters containing significant concentrations of carbonate will tend to support higher concentrations of uranium, neptunium, and plutonium but will tend to precipitate strontium (+2). High-sulfate waters should also lower the solubility of strontium (+2), although sulfate will have only a minimal effect on actinide mobility.
Organic complexing agents, if present, tend to increase the potential mobility of actinides; some may even suppress hydrolysis reactions that tend to precipitate such species. Examples have been reported of radionuclides being transported substantial distances from near-surface, LLW disposal sites through the agency of complexants like EDTA (Cleveland and Rees, 1976). The transport properties of cesium, iodine, and technetium will be minimally impacted by the presence of complexing agents (man-made or natural) unless the complexants also happen to be reducing agents. Humic and fulvic acids12 can strongly complex polyvalent cations. Terrestrial humic materials are often polycyclic aromatic moieties13 and may also reduce polyvalent metal ions, including actinides. Humic matter derived from aquatic sources tends to be more aliphatic14 in nature and is less likely to produce reducing conditions. Many humic materials have colloidal dimensions and are thus capable of transporting complexed metal ions in
________________________
11 Anionic species include chloride, carbonate, sulfate, phosphate, and several other less abundant species.
12 Humic and fulvic acids are the degradation products of naturally occurring complexants resulting most commonly from the decomposition of plant debris. These materials contain a wide variety of carboxylic acid, phenolic, alcoholic, and amino groups.
13 That is, functional groups containing two or more simple aromatic rings that share carbon atoms. The carbon atoms in such rings are unsaturated.
14 Aliphatic compounds contain fully saturated carbon atoms.
surface or subsurface waters. Humic colloids can also interact strongly with mineral surfaces to retard radionuclide mobility.
6.3.6 Colloid Formation
Radionuclides that are chemically sorbed to low-molecular-weight colloidal15 materials have been observed to travel unexpectedly large distances in the subsurface environment. For example, radionuclides have been transported hundreds of meters in the subsurface via such transport methods at the Nevada Test Site and Mayak (Russia) Site (Kersting et al., 1999; Novikov et al., 2006).
Several materials can serve as a source of colloids including inorganic clay mineral degradation products and natural organic compounds such as humic acids. The latter can also serve as complexing agents. These compounds tend to be intrinsically reducing, but they are strong complexants for polyvalent metal ions and capable of transporting metals over substantial distances. Both humic and inorganic colloids can also interact strongly with mineral surfaces to retard radionuclide migration. Humic materials are ubiquitous in surface and near-surface environments and have also been reported to be present at low concentrations in deep groundwater. These complex polyelectrolytes can have substantial impact on cation transport in natural waters (Koppold and Choppin, 1987).
6.3.7 Radioactive Decay and Radiolysis
Radioactive decay and radiolysis may have profound effects on waste form performance in disposal facilities. The decay of radionuclides and formation of daughter products in a waste form can affect its stability. Additionally, radiogenic heating will create thermal, compositional, and radiation-field gradients that can alter thermodynamic equilibria and rates of chemical reactions in the engineered and natural barriers, including the waste form.
The radiolytic decomposition of water16 produces an OH radical, H atom, and a hydrated electron (e–aq) as primary products, all of which are reactive and unstable species, whether oxidizing or reducing. The significance of radiolysis pertaining to waste form performance has been extensively studied (Shoesmith and Sunder, 1992). Radical recombination produces H2O2 (hydrogen peroxide), H2O, and H2. Reactions of the primary radicals or recombination molecules with other ions will produce
________________________
15 In the context of this report, a colloid is a sub-micron particle suspended in a liquid.
16 Radiolysis is the dissociation of water molecules by alpha, gamma, and to a lesser extent beta radiation.
other radicals such as carbonate radical anion (CO3=) that can cause further redox changes.
Radical recombination reactions are most common in the energetic ionization tracks formed by alpha decay, the most common decay mode for heavy elements such as actinides. Most actinide isotopes emit alpha particles with 4-6 million electron volts (MeV) of kinetic energy, sufficient to break tens of thousands of chemical bonds. Hence, radiolysis can be an important process in actinide speciation; peroxide chemistry is an integral feature of this chemistry in systems containing water. Radioactive ion decay recoil represents another pathway for radionuclide release from waste forms.
In the near-field environment of a repository, ambient radiation fields will be substantial for hundreds to thousands of years (Roddy et al., 1986) after emplacement of the waste packages. In general, the waste packages are designed to minimize the potential impact of the radiolysis on the mobility of the wastes. However, it is possible that some percentage of the waste packages thus emplaced could experience a premature failure; consequently, the wastes could be subjected to a groundwater flow that has been impacted by the ambient radiolysis field. Redox active species (iodine, technetium, and actinides) released from the waste form will be most significantly impacted by this effect.
The near-field environment of a repository will be impacted by an ambient radiation field for millennia after emplacement of radioactive materials. Although the most intensely radioactive isotopes decay over a few tens of decades, long-lived radionuclides will cause a radiation field that persists essentially indefinitely. Evidence indicates that radiolysis impacts actinides even in natural uranium deposits (Kubatko et al., 2003). Once a waste form is exposed to groundwater, radiolytically derived species may impact redox active radionuclides such as the actinides, iodine, and technetium (Spahiu et al., 2004).
6.3.8 Natural Isotopic Dilution
Once a radionuclide leaves the waste form and enters the near-field environment of the repository it can be diluted naturally by stable isotopes of the same element. Such dilution can significantly reduce the biological hazard of the radionuclide.17 For example, alluvial deposits in the Atacama desert (Chile) are sufficiently rich in iodine that several iodate
________________________
17 Although intentional dilution is generally considered to be inappropriate as a waste disposal strategy, natural isotopic dilution can serve as an important natural barrier in a disposal system. This further illustrates the importance of matching waste forms to disposal environments.
minerals (which are soluble) precipitate (Burns and Hawthorne, 1993). In such an environment introduction of radioactive iodine may have a negligible impact on the specific activity of the iodine in the environment. Other radioisotopes that can be affected by natural isotopic dilution are carbon-14, chlorine-36, and tritium.
As a specific example, Moeller and Ryan (2004) estimated the potential annual dose to adults from the intake of radioactive iodine-129 released from the proposed repository at Yucca Mountain, Nevada. According to their study, stable iodine-127 would dilute iodine-129 by a factor of more than 2 billion to 1. This natural dilution places an upper bound on the annual effective dose to adults from the release of iodine of about one millionth of the 0.15 mSv standard.
6.3.9 Biological Activity
Biological activity can also impact the transport of radionuclides. Bio-transport mechanisms are likely to be minimized by high thermal gradients and high radiation fields during the first few decades after emplacement of waste, although extremophile microorganisms that are not adversely impacted by radiation are known to occur in nature. There is some information available about the biogeochemistry of some nuclides (iodine and possibly technetium), but comparatively little is known about actinide interactions with biological systems. It is known that cell debris can absorb and transport (as colloids) or retard mobility of some nuclides. Biological interactions with radionuclides in a repository environment are considered to be a frontier area for investigation, as there is so little information available.
6.3.10 Thermal Effects
Ambient temperatures increase with depth in Earth’s crust, typically at a rate of 25-30°C per kilometer. Emplacement of waste forms that contain relatively short-lived radionuclides such as cesium-137 and strontium-90 (as is typical for spent nuclear fuel, for example) in a geologic repository will generate considerable heat from the decay of these isotopes. The impact on the thermal environment of the repository depends, among other factors, on the quantity of waste emplaced, the presence or absence of backfill, the waste configuration in the repository, and exchange of air between areas of the repository. Several of the factors affecting waste forms in disposal environments delineated in the sections above are temperature dependent, as are the complex couplings between such factors.
The boiling point of pure water in hydrologically saturated repository environments is about 220°C at a depth of 300 meters and is about 250°C at a depth of 500 meters. Typical maximum design temperature for satu-
rated zone repositories is conservatively set between 80°C (e.g., SKB, 2006) and 120°C (e.g., Nagra, 2002), so no boiling of water will occur after re-saturation of the repository. For unsaturated sites, groundwater boils at a temperature near 100°C, moderately affected by local atmospheric pressure and salinity. Promoting boiling conditions in an unsaturated site has been proposed to delay the contact of HLW/SNF by groundwater (e.g., DOE, 2008), although the maximum temperature was set below the thermal phase-transition for certain common rock-forming minerals such as silica.
Even below the boiling point of water, elevated temperatures will impact the reactivity of groundwater with a waste form (typically increasing reactivity), modify the solubility and complexation of radionuclides, and change the impact of radiolysis on waste form stability. Increased temperatures will also impact the chemical interactions of groundwater with the minerals present in the rocks, which may alter the salinity and dissolved gas content of the water. Furthermore, significant changes in the thermal regime surrounding a repository will modify the flow of both vapor and groundwater in the vicinity (Webb et al., 2003).
6.4 DISCUSSION
The focus of this chapter is on waste form performance with respect to the disposal of SNF, HLW, and TRU waste in repositories located several hundreds of meters below Earth’s surface. The physical and chemical conditions in such repositories can be highly variable, depending on the rock type and subsurface conditions, especially with respect to groundwater. Furthermore, these conditions will evolve over time as a result of the construction of the facility, emplacement of radiogenic-heating waste forms, and natural events including climate change. Waste forms in such facilities will perform optimally in a repository when they are matched with the appropriate physical and chemical conditions that foster long-term stability. An important implication of this fact is that the suitability of a waste form for disposal depends crucially on the characteristics of the disposal facility into which it will be emplaced. The suitability of a particular waste form material for disposal in a particular repository can be assessed quantitatively, as discussed in the next chapter.
REFERENCES
Apted, M. and J. Ahn. 2010. “Multiple-Barrier Geological Repository Design and Operation Strategies for Safe Disposal of Radioactive Materials,” In Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste, Woodhead Publishing Ltd., Oxford, U.K., 3-28.
Bodansky, D. 1984. Nuclear Energy. Principles, Practices, and Prospects, Springer, New York.
Burns, P. C. and F. C. Hawthorne. 1993. “The Crystal Structure of Dietzeite, Ca2H2O(IO3)2(CrO4), a Heteropolyhedral Framework Mineral,” Can. Miner. 31, 313-319.
Chen, F., P. C. Burns, and R. C. Ewing. 2000. “Near-field Behavior of 99Tc During the Oxidative Alteration of Spent Nuclear Fuel,” J. Nucl. Mat. 278, 225-232.
Cleveland, J. M. and T. F. Rees. 1976. “Investigation of Solubilization of Plutonium and Americium in Soil by Natural Humic Compounds,” Environ. Sci. Technol. 10(8), 802-806.
DOE [U.S. Department of Energy]. 2008. Yucca Mountain Repository License Application: Safety Analysis Report, Available at http://www.nrc.gov/waste/hlw-disposal/yucca-lic-app/yucca-lic-app-safety-report.html.
Eberl, D. D., B. Velde, and T. McCormick. 1993. “Synthesis of Illite-Smectite from Smectite at Earth Surface Temperatures and High pH,” Clay Miner. 28, 49-60.
Ewing, R. C., W. Runde, and T. E. Albrecht-Schmidtt. 2010. “Environmental Impact of the Nuclear Fuel Cycle: Fate of Actinides,” Mat. Res. Soc. Bull. 35, 859-866.
Icenhower, J. P., N. P. Oafoku, J. M. Zachara, and W. J. Martin. 2010. “The Geochemistry of Technetium: A Review of the Behavior of an Artificial Element in the Natural Environment,” Amer. J. Sci. 310(8), 721-752.
Janeczek, J. 1999. “Mineralogy and Geochemistry of Natural Fission Reactors in Gabon,” Rev. Mineral. Geochem. 38, 321-392.
Jantzen, C. M. 1992. “Nuclear Waste Glass Durability: I, Predicting Environmental Response from Thermodynamic (Pourbaix) Diagrams.” J. Amer. Ceram. Soc. 75(9), 2433-2448.
Johnson, L. H. and D. W. Shoesmith. 1988. “Spent Fuel,” In Radioactive Waste Forms for the Future, W. Lutze and R.C. Ewing (Eds.), North Holland, Amsterdam.
Kersting, A. B., D. W. Efurd, D. L. Finnegan, D. J. Rokop, D. K. Smith, and J. L. Thompson. 1999. “Migration of Plutonium in Ground Water at the Nevada Test Site,” Nature 397, 56-59.
Koppold, F. X. and G. R. Choppin. 1987. “Interaction of Actinide Cations with Synthetic Polyelectrolytes,” Radiochimica Acta 42, 29.
Kubatko, K-A. H., K. B. Helean, A. Navrotsky, and P. C. Burns. 2003. “Stability of Peroxide-Containing Uranyl Minerals,” Science 302(5648), 1191-1193.
Langmuir, D. 1997. Aqueous Environmental Geochemistry, Prentice Hall, Upper Saddle River, N.J.
Lowenstein, T. K., L. Jianren, C. Brown, S. M. Roberts, T-L. Ku, S. Luo, and W. Yang. 1999. “200 k.y. Paleoclimate Record from Death Valley Salt Core,” Geology 27, 3-6.
Moeller, D. W. and M. T. Ryan. 2004. “Limitations on Upper Bound Dose to Adults Due to Intake of I129 in Drinking Water and a Total Diet—Implications Relative to the Proposed Yucca Mountain High Level Radioactive Waste Repository,” Health Physics 86(6), 586-589.
Nagra [National Cooperative for the Disposal of Radioactive Waste]. 2002. Project Opalinus Clay: Demonstration of Disposal Feasibility for Spent Fuel, Vitrified High-Level Waste and Long-Lived Intermediate-Level Waste (Entsorgungsnachweis), Technical Report 02-05, Nagra, Wettingen, Switzerland.
Novikov, A. P., S. N. Kalmykov, S. Utsunomiya, R. C. Ewing, F. Horreard, A. Merkulov, S. B. Clark, V. V. Tkachev, and B. F. Myasoedov. 2006. “Colloid Transport of Plutonium in the Far-Field of the Mayak Production Association, Russia,” Science 314, 638-641.
NRC [National Research Council]. 2003. One Step at a Time: The Staged Development of Geological Repositories, National Academies Press, Washington, D.C.
Nunn, J. A. 1996. “Buoyancy-driven Propagation of Isolated Fluid-filled Fractures: Implications for Fluid Transport in Gulf of Mexico Geopressured Sediments,” J. Geophys. Res. 101(B2), 2963-2970.
NWTRB [Nuclear Waste Technical Review Board]. 2009. Survey of National Programs for Managing High-Level Radioactive Waste and Spent Nuclear Fuel: A Report to Congress and the Secretary of Energy, Nuclear Waste Technical Review Board, Arlington, Va.
OECD-NEA [Organisation for Economic Co-Operation and Development-Nuclear Energy Agency]. 2003. Engineered Barrier Systems and the Safety of Deep Geological Repositories: State-of-the-Art Report, OECD-NEA, Paris, France.
Roddy, J. W., H. C. Claiborne, R. C. Ashline, P. J. Johnson, and B. T. Rhyne. 1986. Physical and Chemical Characteristics of Commercial LWR Spent Fuel, ORNL/TM-9591/V1&R1, Oak Ridge National Laboratory, Oak Ridge, Tenn.
Savage, D. and S. Benbow. 2007. Low pH Cements. SKI Report 2007:32, Swedish Nuclear Power Inspectorate (SKI), Stockholm, Sweden.
Schulz, R. K., R. W. Ridky, and E. O’Donnell. 1992. Control of Water Infiltration into Near Surface LLW Disposal Units, NUREG/CR-4918, Volume 6, U.S. Nuclear Regulatory Commission, Washington, D.C.
Shoesmith, D.W. and S. Sunder. 1992. “Prediction of Nuclear Fuel (UO2) Dissolution Under Waste Disposal Conditions,” J. Nucl. Mat. 190, 20-35.
SKB [Swedish Nuclear Fuel and Waste Management Co.]. 2006. Long-Term Safety for KBS-3 Repositories at Forsmark and Laxemar–A First Evaluation. SKB TR-06-09, SKB, Stockholm, Sweden.
Spahiu, K., D. Cui, and M. Lundström. 2004. “The Fate of Radiolytic Oxidants During Spent Fuel Leaching in the Presence of Dissolved Near-Field Hydrogen,” Radiochim. Acta 92, 625-629.
Speight, M. V. 1964. “The Migration of Gas Bubbles in Material Subject to a Temperature Gradient,” J. Nucl. Mat. 13, 207-209.
Stein, C. L. 1985. Preliminary Report on Fluid Inclusions from Halites in the Castile and Lower Salado Formations of the Delaware Basin, Southeastern New Mexico, SAND-83-0451, Sandia National Laboratories, Albuquerque, N.M.
Tyler, S. W., J. B. Chapman, H. Conrad, D. P. Hammermeister, D. O. Blout, J. J. Miller, M. J. Sully, and J. M. Ginanni. 1996. “Soil-Water Flux in the Southern Great Basin, United States: Temporal and Spatial Variations over the last 120,000 years,” Water Resources Res. 32(6), 1481-1499.
Webb, S. W., N. D. Francis, S. D. Dunn. M. T. Itamura, and D. L. James. 2003. “Thermally Induced Natural Convection Effects in Yucca Mountain Drifts,” J. Cont. Hydrol. 62-63, 713-730.
Witherspoon, P. and G. Bodvarsson. 2006. Geological Challenges in Radioactive Waste Isolation: Fourth Worldwide Review, LBNL-59808, Lawrence Berkeley National Laboratory, Berkeley, Calif.