Disparities in health and health care may be found at each step along the continuum of chronic disease, from primary prevention to disease management. To identify and understand these disparities, a surveillance system must be able to provide data to analyze disparities in incidence and prevalence, morbidity and mortality, functional health outcomes, primary and secondary prevention approaches, risk factors, and healthcare delivery. This system must function not only at the national level but also at the regional, state, and local levels. The system should be effective in monitoring populations defined by race and ethnicity, gender, age, income, education, social and physical environments, and geographic factors such as birthplace and years of residence in the United States.
A contemporary national framework for the surveillance of cardiovascular disease (CVD) and chronic obstructive pulmonary disease (COPD) can drive the development of policies and programs at the local level that help to ensure high-quality effective preventive and therapeutic programs for the entire U.S. population. Much of our knowledge of racial and ethnic disparities has been derived from national population samples, but efforts to eliminate health disparities must occur in collaboration with local and regional healthcare organizations, communities, healthcare institutions, and healthcare providers. Federal databases are the source of much of the information currently available on racial and ethnic health disparities (Sequist and Schneider, 2006). Although the federal government will remain a major source of data on racial and ethnic health and healthcare disparities, linkage to Census data, vital statistics, household surveys, small area data, administrative data, and data from local groups and healthcare organizations should be an integral part of the national surveillance system.
WHY SHOULD HEALTH DISPARITIES BE MEASURED?
In Healthy People 2010, the federal government established two major goals for health promotion and disease prevention: (1) to increase life expectancy and improve quality of life; and (2) to eliminate health disparities (HHS, 2000). Healthy People 2010 made the elimination of health disparities one of the highest priorities of the federal government (Satcher, 2010). Many of these contemporary health disparities in the United States have deep roots in historical economic and political conditions related to racism and unequal access to resources and opportunities for better health spanning generations and across the life course. A recent assessment of the nation’s progress toward meeting the ambitious goals of Healthy People 2010 observed that “although some progress has been made, there is much work to be done toward the Healthy People 2010 targets and both overarching goals” (Sondik et al., 2010). Healthy People 2020 continues the focus on this area with the goal to achieve health equity and to eliminate
disparities. The National Healthcare Disparity Report, first produced in 2003 and published annually thereafter by the Agency for Healthcare Research and Quality (along with the National Healthcare Quality Report), found that even though coronary heart disease- (CHD-) and stroke-related mortality have decreased for all major racial/ethnic groups between 1980 and 2003, the burden of CVD and CVD risk factors remained disproportionately high in segments of the population defined by race, ethnicity, socioeconomic status (SES) and geography (AHRQ, 2006).
The selection and definition of population groups for study is critical to the process of building a framework for national surveillance of health disparities. Margaret Whitehead proposed a conceptual model of health equity and disparities in the early 1990s that offers a framework for examining the determinants of health disparities and provides a useful perspective to guide the development of a contemporary nationwide framework for CVD and COPD surveillance (Whitehead, 1991). Whitehead’s seven determinants of health disparities are: (1) natural biological variation; (2) health-damaging behavior that is freely chosen; (3) the transient health advantage of one group over another when one group is first to adopt health-promoting behavior (as long as other groups have the means to catch up fairly soon); (4) health-damaging behavior in which the degree of choice of lifestyles is severely restricted; (5) exposure to unhealthy, stressful living and working conditions; (6) inadequate access to essential healthcare services and other basic services; and (7) natural selection or health-related social mobility involving the tendency for sick people to move down the social scale. Since Whitehead first outlined these seven determinants of health disparities in 1991, health-damaging behaviors such as smoking and unhealthy diet, which were presumed to be freely chosen, have also been linked to social networks that may strongly influence these behaviors (Christakis and Fowler, 2007, 2008). Therefore, such health behaviors must be considered within their social context, and they cannot be detached from the historical, sociocultural, and economic conditions that promote and constrain behavioral choices.
Surveillance of health disparities is complicated by the need to provide data from several distinct domains whose interaction leads to disparities in health and health care. The task is further challenged by the variability of determinants at the neighborhood, city, county, state, regional, and national levels, as well as between and among population groups and subgroups defined by race and ethnicity. For example, rather than beginning with race and ethnicity as the fundamental categories, health disparities could be tracked according to broad categories, such as social context and physical environment, age, and gender. The more proximate effects of other covariates (e.g., income, educational attainment, employment status and discrimination, health behaviors, the healthcare system, and psychosocial factors) could be assessed within a framework based on social context and physical environment, age, and gender. In this conceptual model (Figure 4-1), health indicators such as CVD and COPD prevalence and incidence, morbidity and mortality, obesity, hypertension, diabetes, and hyperlipidemia would be viewed as products of the interrelationship of the foregoing factors (Schulz et al., 2005).
EVIDENCE OF THE NEED FOR ONGOING SURVEILLANCE OF HEALTH DISPARITIES
Age and Gender
Age and gender are established categories for reporting health and healthcare surveillance data. Concomitant with the decline in death rates attributed to CHD in Americans over the past several decades, life expectancy has increased. Between 1980 and 2003, life expectancy increased by 4.8 years in American men and by 2.7 years in women.
CVD increases with advancing age in both women and men. Across the spectrum of CVD (hypertension, CHD, heart failure, valvular heart disease, peripheral arterial disease, and stroke), there are corresponding age-related increases in CVD morbidity and mortality (Yazdanyar and Newman, 2009). In 2007, the leading causes of death in women as well as men aged 65 and older were diseases of the heart. One in three women aged 65 and older has coronary artery disease, and the underlying disease process, atherosclerosis, begins at an early age in both sexes (NCHS, 2010).
In-hospital mortality related to acute myocardial infarction (AMI) is higher in women than in men, and the long-term prognosis after hospitalization for AMI has been shown to be worse in women than in men (Eastwood and Doering, 2005). Unadjusted mortality and complication rates remain higher in women than in men treated with percutaneous coronary interventions (PCIs). CVD risk scores also increase progressively with advancing age
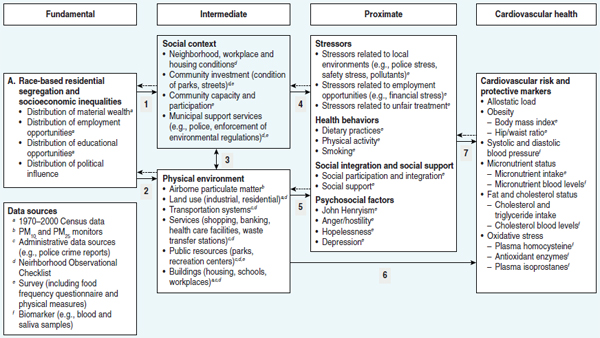
FIGURE 4-1 Conceptual model and data sources for Healthy Environments Partnership: Social and physical environmental factors and disparities in cardiovascular risk.
SOURCE: Schultz (2005).
in both men and women in the absence of diagnosed CVD. The prevalence of subclinical forms of CVD—such as carotid artery atherosclerosis and elevated coronary artery calcium score—have been shown to increase with advancing age (Rich and Mensah, 2009).
Performance of coronary revascularization soon after AMI in the elderly has become very common. Although the use of this effective treatment modality over time has increased considerably in men and women of all ages, age disparities continue (Pagé et al., 2010; Peterson et al., 2004). Because older patients with coronary artery disease often have additional comorbid illness, it is important to determine whether this procedure in older patients will translate into increases in quality of life and long-term survival in a cost-effective manner.
Similar to CVD, the occurrence of many chronic lung diseases increases with advancing age. An exception is asthma, which is more common in childhood (Brown et al., 2008; Mannino et al., 2002). In contrast to CVD, the occurrence of COPD has been increasing in recent decades, with the highest mortality rates observed among older white males (Brown et al., 2008; Lewis et al., 2009; Mannino et al., 2002). Although chronic lung diseases are more common among men, for selected conditions such as COPD the rate of increase has been greater among women. U.S. mortality rates from COPD, increased from 1980 through 2000, with a greater relative increase among females (20.1 to 56.7 per 100,000) compared with males (73 to 82.6 per 100,000). In 2000, the absolute number of deaths from COPD was higher among females compared with males. Between 2000 and 2005, mortality rates for females remained relatively flat, but declined among males (Brown et al., 2008). In addition to the relatively greater increase in mortality among females, women have a higher rate of use of inpatient services (Shaya et al., 2009). This may be partly explained by limited evidence suggesting that women are more susceptible to the adverse effects of cigarette smoke compared to men (Camp et al., 2009; Chatila et al., 2004; Dransfield and Bailey, 2006; Sin et al., 2007).
Race and Ethnicity
Because of the major roles race and (more recently) ethnicity have played in American political and social history, race and ethnic categorization of health and health care has been a distinguishing feature of health surveillance in the United States. As a result of segregation (racial, social, economic, and residential) throughout much of American history, race has served as a proxy for social, cultural, and economic features of populations and subpopulations described by race and ethnicity. The use of race as a social risk marker should be distinguished from the use of race as a biological risk factor. When used as a risk marker, race suggests a collinear association with some other quantifiable variable, such as income or education. By contrast, when used as a risk factor, race implies shared genetic heritage and consequent susceptibility to specific diseases such as sickle cell anemia or cystic fibrosis (Joseph et al., 2006; Osborne and Feit, 1992). When using race or ethnicity in health surveillance, it is important to acknowledge the social context in which these terms are used and to avoid presumptions of socioeconomic and cultural homogeneity or biological and genetic “sameness.” Also important is recognizing that race and ethnicity are not biological or genetic variables that cause differences in health, but they are instead associated with other biological, social, or environmental risk factors that contribute to disparities in health between racial and ethnic groups (Ellison et al., 2007).
Understanding the root causes of health disparities requires surveillance at the population level for incidence and prevalence, predisposing factors, morbidity, mortality, and long-term outcomes. Other important factors are linkage of such data to environmental, residential, geographic, socioeconomic, cultural, and educational domains. Racial and ethnic disparities in CVD and COPD prevention, diagnosis, treatment, and outcomes have been extensively documented (IOM, 2003; Kaiser Family Foundation/American College of Cardiology Foundation, 2002). Prior surveillance data have shown that in comparison with white populations, racial and ethnic minorities generally have higher rates of CVD risk factors, CVD-related morbidity and mortality, poorer health, less adequate health care, and worse outcomes (Roger et al., 2010).
Although the overall occurrence of COPD is higher among non-Hispanic white males compared with other racial and ethnic groups, in recent years the occurrence has been increasing more rapidly among African Americans compared to whites (Brown et al., 2008; Coultas et al., 1994; Keppel et al., 2010; Kirkpatrick and Dransfield, 2009; Mannino et al., 2002). Moreover, relative disparities in mortality rates have increased from 1999 to 2006 for heart disease, from 1990 to 1998 for COPD, and from 1990 to 2006 for chronic lower respiratory disease (Keppel et al., 2010).
For COPD, limited evidence suggests that black men may be more susceptible to the adverse effects of cigarette smoke compared to white men (Chatila et al., 2004; Dransfield et al., 2006). Sarrazin and colleagues (2009) examined mortality rates among African American (n = 7,159) and white (n = 43,820) veterans admitted for a COPD exacerbation from 2003 to 2006. Overall mortality was lower among African Americans (7.1 percent) compared to whites (9.2 percent), with a risk-adjusted mortality ratio of 0.71. Although crude mortality rates from COPD have been higher among African Americans compared with whites, there may be no difference in these deaths after adjustment for age, body mass index, smoking, alcohol use, diabetes, hypertension, education, and sports index (Chamberlain et al., 2009). Among the heterogeneous Hispanic population, limited data are available about chronic lung diseases (Brehm et al., 2008). Mortality from CVD and COPD is lower among black and Hispanic immigrants compared to U.S.-born populations of the same race and ethnic groups, suggesting untoward effects of the American lifestyle (Singh and Hiatt, 2006). The influence of access to health care and quality of care among different racial and ethnic groups is discussed in greater detail in subsequent sections.
Measurement and classification of populations and subpopulations by race and ethnicity for surveillance has become more challenging because of increased immigration from Central and South America as well as Asia and Africa. Changes in the demographic characteristics of the U.S. population have also resulted from increased racial and ethnic admixture due to growth in the number of intermarriages and evolving conventions of racial and ethnic self-identification (Waters, 2000). The Pew Research Center reported that in 2008, a record one in seven of all new U.S. marriages were between individuals of a different race or ethnicity (with significant variation across U.S. regions). The Pew Research Center has produced estimates of future changes in the proportions of racial and ethnic groups. According to those estimates, from 2005 to 2050, the proportion of U.S. whites will decrease from 67 to 47 percent; the Hispanic population will increase from 14 percent of the population to 29 percent; U.S. blacks will remain at 13 percent of the population; and the proportion of Asians will rise from 5 to 9 percent (Passel and Cohen, 2008).
Nativity and Immigration
Growth in the proportion of foreign-born residents and their progeny in the United States has reinforced the importance of examining differences in the health and healthcare of immigrants, especially in regions, states, counties, or neighborhoods with significant proportions of immigrants. Because of their long history of discrimination, residential segregation, unemployment, and poor SES, immigrant populations can have less favorable risk factor awareness, diagnosis, treatment, and control. Immigrants and migrants have had a tendency to move to and live in areas populated by people with similar backgrounds. Residential segregation has held true historically, not only for immigrants but also for African American “migrants” already living in the United States and for many Native Americans. According to the 2000 Census, immigrants have settled most often in California, Florida, Illinois, New Jersey, New York, Pennsylvania, and Texas. Immigrants, particularly those who lack fluency in English, health literacy, and familiarity with the U.S. healthcare system, are at increased risk for some chronic diseases and injuries. Observed health disparities in specific racial and ethnic subgroups may result from shared social, economic, and physical environments as well as race or ethnicity.
The relationship between acculturation and chronic disease indicators is complex and may have a significant effect on observed health disparities. Surveillance systems typically have not focused on collecting and/or combining social, economic, and environmental data when addressing health disparities. Acculturation (or lack thereof) may influence the health of socioeconomically and culturally homogeneous populations, whether native born or foreign born, residing in the same neighborhoods. The effects of acculturation may be subgroup specific, with differing impacts on the burden of disease, risk factors, markers of comorbidities, and outcomes. In a study of participants in the Multi-Ethnic Study of Atherosclerosis, a higher prevalence of carotid plaque (a marker for carotid atherosclerosis) was observed among whites, blacks, and Hispanics who had been in the United States for more generations, as well as in whites with less education and blacks with lower incomes (Lutsey et al., 2008). Among immigrants from diverse ethnic backgrounds, longer length of residence in the United States has been associated with increased odds of obesity, hyperlipidemia, and cigarette smoking, even after adjusting for relevant confounding factors. High levels of acculturation have also been associated with poorer risk factor control or a higher prevalence of chronic disease risk factors. Immigrants who speak their native language at home or have resided briefly in the United States may have reduced risk factor control.
Assessing Hispanic ethnicity and disease or risk factor surveillance is complex because of differing geographic origins and admixture of various subgroups in the United States. The ancestry of Hispanics depends on the country of origin, the region of the country in which they first settle, and the region in which they ultimately reside. Hispanics in California emigrated predominately from Mexico, while Hispanics in New York emigrated largely from Puerto Rico and the Dominican Republic. Although both populations are “Hispanic,” their ancestral origins differ considerably (Lai et al., 2009).
Furthermore, use of the term “black” to categorize persons of African origin may not be optimal in CVD and COPD surveillance. Approximately 6 percent of persons who self-identified as black or African American in the 2000 Census were not born in the United States (CDC, 2005). For example, in New York large subpopulations of people of African origin could be classified into different categories, such as Barbadian, Haitian, Jamaican, Nigerian, Panamanian, Senegalese, Trinidadian, or from other locations in the African Diaspora. The “black” category presents difficulties in surveillance because it encompasses a heterogeneous group, but does not account for variations within the group or among subgroups (Ford and Kelly, 2005).
Geography, Residence, and Environment
In Whitehead’s formulation of health disparities, a distinction is made between damaging behaviors that are freely chosen (modifiable risk factors) and behaviors in which the degree of choice is severely restricted, such as birthplace and residence. Unhealthy living and working conditions and inadequate access to essential health services and other basic services (e.g., screening services) are influenced by environment, region, state, county, and neighborhood. Despite efforts to address health disparities by improving the quality of health care and health services delivered at the population, subpopulation, and individual levels, disparities in the major indicators of
high-quality health and health care persist, and differences in damaging or beneficial health behaviors have been shown to contribute to observed health disparities. These disparities persist in spite of the wide array of interventions available at the individual level, including improving primary and secondary prevention; increasing awareness, treatment, and control of predisposing factors; and increasing access to the latest diagnostic and therapeutic technologies. This persistence of health disparities has focused attention on other possible determinants of health disparities, including geography, residence, and environment (Do et al., 2008).
Substantial evidence shows geographic variation in risk factors, prevalence and incidence, morbidity, and mortality for CHD and stroke. For example, in a report of state-based prevalence estimates of CHD, variations among states by sex, race/ethnicity, and education were observed, with an approximate twofold difference between states with the highest and lowest prevalence rates of CHD (CDC, 2007). High heart disease mortality rates also have been observed in several U.S. regions, such as the “Coronary Valley” of the Ohio-Mississippi River Basin (Pickle and Gillum, 1999), and the “Heart Failure Belt” of the southeastern United States (Mujib et al., 2011).
The classic example of regional variation in CVD mortality is the “Stroke Belt.” This belt is composed of 11 southeastern states where higher rates of stroke mortality have been observed compared to other U.S. regions (Lanska, 1993). The numerous hypotheses for the concentration of CVD and stroke mortality in the Southeast include geographic differences in the distribution of major cerebrovascular disease risk factors (e.g., high blood pressure, diabetes, cigarette smoking, and obesity) and differences in socioeconomic and environmental factors (Liao et al., 2009). However, even though many possible explanations for the Stroke Belt have been considered, the reasons for regional variation in stroke-related mortality have not been definitively established.
A possible explanation for the observed concentration of stroke mortality in the southeastern United States is the higher prevalence of hypertension among Southern-born blacks than in blacks born elsewhere. Geographic heterogeneity of hypertension suggests that differences in the prevalence of hypertension between blacks and whites are not constant, but they may vary depending on which geographic groups are compared. The presence of large variations in black–white differences suggests that race differences are not immutable (i.e., not simply genetic or biological) and may vary substantially by social and environmental context (Byers et al., 1998; Kershaw et al., 2010). Liao and colleagues (2009) observed that “socioeconomic status, hypertension, diabetes, coronary heart disease, and smoking are still the basic crucial contributors to the disparities. Most of these factors are either modifiable or potentially amenable to interventions. Given these findings, public health interventions are essential for progress in reducing the stroke burden in the Stroke Belt region.”
Studies of increased stroke-related mortality in southeastern U.S. residents have generally suggested that stroke risk is primarily linked to residence in the Stroke Belt. Less is known, however, regarding the importance of birth versus residence in the Stroke Belt in native- and foreign-born blacks and whites. In a study of the association between birthplace and mortality from CVD among black and white residents of New York City, similar CVD death rates were observed for white and black men and white and black women born in the Northeast (Fang et al., 1996). Black men born in the South had death rates 30 percent higher than northeastern-born blacks and four times that of Caribbean-born blacks of the same sex and age. Higher rates of CVD mortality among blacks compared with whites may obscure substantial variation among blacks based on birthplace.
Disparities may be influenced by the characteristics of the local community or neighborhoods, which may engender healthy or unhealthy behavioral practices. The perception of neighborhood safety is positively associated with physical exercise, and this association is larger for minority groups than for whites. Neighborhoods also differ in the existence and quality of recreational facilities and open, green spaces. The availability and cost of healthful products in grocery stores also has been shown to vary across residential areas, and the availability of nutritious foods is positively associated with their consumption. In addition, it has been demonstrated that both the tobacco and alcohol industries heavily market their products to poor minority communities (Williams and Jackson, 2005). Furthermore, they are more likely to have jobs in workplaces that expose them to dusts, gases, and fumes, which have been associated with an increased risk for COPD, which disproportionately affects African Americans and Hispanics (Hnizdo et al., 2004).
Williams and Jackson (2005) observed the factors in Box 4-1 in the social environment that can initiate and sustain disparities in health.
BOX 4-1
Social Environment That Can Initiate and Sustain Disparities in Health
“Socioeconomic status, whether measured by income, education, or occupation, is a strong predictor of variations in health … all of the indicators of SES [socioeconomic status] are strongly patterned by race, such that racial differences in SES contribute to racial difference in health. Moreover, the differences in health by SES within each racial group are often larger than the overall racial differences in health. Income also plays a role in understanding racial differences in CHD (coronary heart disease) mortality. For example, death rates from heart disease are two to three times higher among low-income blacks and whites than among their middle-income peers. In addition, for both males and females at every level of income, blacks have higher death rates from CHD than whites. Mortality from heart disease among low- and middle-income black women is 65 percent and 50 percent higher, respectively, than for comparable white women.… Health practices. Another pathway underlying the association between race and chronic diseases is the patterning of health practices by race and socioeconomic status. Dietary behavior, physical activity, tobacco use, and alcohol abuse are important risk factors for chronic diseases including CHD, stroke, and chronic lung disease. Moreover, changes in these health practices over time are patterned by social status. Disadvantaged racial groups and those with low SES are less likely to reduce high-risk behavior or to initiate new health-enhancing practices.… Stress. Exposure to psychosocial stressors may be another pathway linking SES and race to the development of poor health and adverse outcomes once disease has been diagnosed. The subjective experience of discrimination is a neglected stressor that can adversely affect the health of African Americans. Reports of discrimination are positively related to SES among blacks and may contribute to the elevated risk of disease that is sometimes observed among middle-class blacks.… Residential segregation. The persistence of racial differences in health after individual differences in SES are accounted for may reflect the role that residential segregation and neighborhood quality can play in racial disparities in health. Because of segregation, middle-class blacks live in poorer areas than whites of similar economic status, and poor whites live in much better neighborhoods than poor blacks.… Impact on income. Residential segregation is a central mechanism by which racial economic inequality has been created and reinforced in the United States. It is a key determinant of observed racial differences in SES because it determines access to education and employment opportunities. Violence. In addition, segregation creates health-damaging conditions in both the physical and social environments. Because of its restriction of educational and employment opportunities, residential segregation creates areas with high rates of concentrated poverty and small pools of employable and stably employed males.”
SOURCE: Williams and Jackson, 2005.
Socioeconomic Factors
Traditionally, public health data have been stratified primarily by “race,” for many years without the collection and reporting of socioeconomic data. With recent recognition of worsening economic and social inequalities, more attention has been focused on the contribution of socioeconomic factors to health disparities. Multiple socioeconomic factors contribute to health disparities, including income, education, residential segregation, stress, social and physical environment, employment, and many others. Disparities according to income and education have increased for smoking, with low-income persons smoking at higher rates. Diabetes prevalence has increased largely among persons from lower socioeconomic strata (Kanjilal et al., 2006).
Using data from NHANES III (1988–1994), Sharma and colleagues (2004) observed increased CVD risk factor clustering among Americans with low SES, particularly among non-Hispanic blacks. Among persons with high SES, Mexican Americans and non-Hispanic blacks have a higher risk of CVD than non-Hispanic whites. Low educational attainment may also impact mortality rates. In a study examining the relationship of education and race to mortality, Jemal and colleagues (2008) found that “48 [percent] of all deaths among men aged 25–64
(white, black, and Hispanic) and 38 [percent] of all deaths in women would not have occurred in this age range if all segments of the population experienced the death rates of college graduates. However, the total number of deaths associated with low education status was not confined to any single racial or ethnic group.”
Using NHANES data from 2001–2006, Karlamangla and associates (2010) evaluated the association between SES and ethnic disparities in cardiovascular risk. They observed marked inverse socioeconomic gradients with risk in all race/ethnicity groups, except foreign-born Mexican American men. Disparities according to race/ethnicity were seen in some, but not all, socioeconomic strata, with some non-Hispanic blacks and U.S.-born Mexican Americans having higher risk, and some foreign-born Mexican Americans having lower risk.
Low SES is associated with a higher prevalence of risk factors, greater chronic disease burden, and higher expenses for health care, medications, and hospitalization. The sick and poor are at risk of moving even farther down the socioeconomic ladder (Fiscella and Williams, 2004). The reverse is also evident: those at the highest socioeconomic rank are likely to be more educated, have better risk factor profiles, improved health, and better health-related outcomes. With greater access to information, more financial resources, greater access to high-quality health care, and the capacity and capability to benefit from advances in pharmaceuticals and healthcare technology, those who are more advantaged can move further up the socioeconomic ladder, while disadvantaged populations remain mired in unhealthy neighborhoods with the highest burden of CVD and COPD. Improving the national surveillance of SES and its relationship to indicators of risk and health outcomes is a critical step toward reducing health disparities.
PRIORITIES FOR SURVEILLANCE OF HEALTHCARE DISPARITIES
Primary Prevention
Reducing the magnitude of clinically evident CVD and COPD in populations that bear a disproportionate burden of disease is an essential element in the struggle to eliminate health disparities. The principal goals of primary prevention include risk assessment; reduction of risk by control of key pre-disposing factors, including cigarette smoking, elevated cholesterol, elevated blood pressure, obesity, and diabetes; and limitation of progression of subclinical disease.
The prevalence of hypertension in U.S. blacks is among the highest in the world (Roger et al., 2010). Pre-hypertension (blood pressure levels greater than 120/80mmHG, but less than 140/90) is more prevalent in men than women, and more prevalent in African American men aged 20–39 years than comparably aged whites and Mexican Americans. As in other subclinical CVD conditions, primary prevention for individuals with pre-hypertension is recommended through vigorous lifestyle and diet modification, and may also include affordable pharmacologic therapy if shown to improve health outcomes (Greenlund et al., 2004; Pimenta and Oparil, 2010).
Secondary Prevention
Successful therapeutic interventions in patients with CVD—particularly myocardial infarction and stroke—have expanded the population of U.S. individuals who could benefit from the enhanced use of evidence-based secondary interventions. Interventions for secondary prevention include lifestyle modifications and pharmacologic treatments to control smoking, hypertension, hyperlipidemia, and diabetes, as well as coronary revascularization procedures that can relieve symptoms and, in some cases, extend survival. The growing number of older adults with CVD and COPD requires specific surveillance of health disparities, with special attention to monitoring adherence to healthy lifestyle practices and effective treatment regimens and the effect of different treatment approaches on quality of life, recurrence, and long-term prognosis. Standardized surveillance approaches for monitoring the effectiveness of secondary prevention are needed (Willson et al., 2010).
Coronary revascularization procedures such as coronary artery bypass (CABG) and PCI, along with bare-metal and drug-eluting stents, have advanced the management of CHD. Racial and ethnic differences in the receipt of catheterization and coronary revascularization were reported in early studies (Gillum et al., 1997; Kressin and Petersen, 2001); however, more recent investigations suggest a reduction in racial disparities in the use of these
interventions. Brown and colleagues (2008) analyzed the receipt of cardiac catheterization, PCI, and CABG by age, sex, insurance status, and race among black and white patients discharged from U.S. hospitals over a 25-year period beginning in 1979. They found that consistent and significant disparities in the receipt of cardiac catheterization, PCI, and CABG by age, sex, insurance status, and race persisted across the 25 years of study; however, attenuation of these differences were observed from 1979 to 2004 for each subgroup examined. Specifically, although blacks were 27 percent less likely to receive diagnostic cardiac catheterization in 1979, they were only 11 percent less likely to undergo cardiac catheterization in 2004 (Brown et al., 2008). Racial disparities in the use of drug-eluting stents have also been reported (Gaglia et al., 2009; Hannan et al., 2007).
A number of investigations have been conducted in different patient populations to explore potential racial differences in healthcare use and quality of care for persons with COPD. In a Medicaid population of 9,131 patients with COPD and asthma, African Americans had lower overall healthcare use and costs when compared to whites, including physician office visits and outpatient and inpatient services (Shaya et al., 2009). Gordon and coworkers (2002) examined the quality of processes of care for CHF and COPD at Veterans Administration hospitals and found no difference in the quality of care provided to blacks and whites. Tsai and colleagues (2009) examined racial and ethnic differences in processes and outcomes of emergency room care among a cohort of 330 patients with COPD enrolled from 24 emergency departments from 15 states. Compared to whites, African American and Hispanic patients had lower SES and primary care access and more frequent exacerbations, but there were no statistically significant differences in the processes or outcomes of care. Hasnain-Wynia and coworkers (2010) found that a higher proportion of racial and ethnic minorities were cared for at lower performing hospitals. Among patients with severe COPD waiting for lung transplantation, African American patients were less likely to have a transplant and more likely to die (Lederer et al., 2008).
Rates and trends of risk-adjusted hospitalization rates for specific conditions provide population-level evidence on the adequacy of access to primary care, known as ambulatory care sensitive conditions (ACSCs), and effectiveness of various interventions (AHRQ, 2004). The cardiovascular and chronic lung diseases considered to be ACSCs include angina, hypertension, congestive heart failure (CHF), asthma, and COPD (AHRQ, 2004). Variations in risk-adjusted hospitalization rates for ACSCs have been examined to determine racial, ethnic, socioeconomic, and geographic disparities for these conditions (Bindman et al., 2008; Jackson et al., 2011; Laditka and Laditka, 2006; O’Neil et al., 2010). A nationwide sample of community hospital discharge data demonstrated that compared to non-Hispanic whites, African American men (adjusted relative rates of 1.9 and 1.6 for ages 19–64 years and 65+ years, respectively) and Hispanic males (2.6 and 2.3, respectively) and females (1.6 and 2.1, respectively) had higher rates of hospitalizations for COPD, adjusted for disease prevalence (Laditka and Laditka, 2006). On the other hand, an analysis of admission rates in North Carolina among Medicare beneficiaries for ambulatory sensitive conditions, including COPD, found that African Americans had lower admission rates for COPD compared to whites (odds ratio 0.67) (Howard et al., 2007). In Texas, wide variations have been found for hospitalization rates for COPD. The highest rates of hospitalization have been found among rural counties, the elderly, non-Hispanic whites, and women in urban areas (Jackson et al., 2011). African Americans had lower hospitalization rates compared to non-Hispanic whites, and Hispanics had the lowest rates.
CONCLUSION
Untangling the effects of environment, income, education, race, ethnicity, and genetics may lead to the more precise targeting of preventive, diagnostic, and therapeutic interventions. This in turn will contribute to the elimination of health disparities, reduction in the magnitude of chronic disease, and improvements in prognosis and quality of life in those with established disease. However, there is a lack of standardization in the collection of race, ethnicity, and language data at the federal, state, and local levels. This lack of standardization creates difficulty in identifying disparities and appropriately targeting quality improvement efforts. Surveys such as the BRFSS, NHANES, and NHIS routinely collect self-reported multiple race data on individuals, and collect ethnicity data independent of race. However, gaps in the collection of disparity data are evident at various levels. For example, among the sources of data collected by Centers for Medicare & Medicaid Services, only the Consumer Assessment of Health Plans Survey allows multiple race designation of individuals, and only the Medicare Current Beneficiary
Survey, the Consumer Assessment of Health Plans Survey, and the Medicare End Stage Renal Disease Program collect ethnicity data that are independent of race. Methodological issues concerning the use of data to assess racial and ethnic disparities include the validity of the classification of individuals’ race and ethnicity, sample size limitations, the smallest analyzable geographic or institutional unit, and the availability of data on other cultural or socioeconomic characteristics (Sequist and Schneider, 2006).
The principal challenge is to develop systems that more effectively and efficiently link conventional surveillance data to more contextually relevant data (e.g., SES, birthplace, acculturation, geography, language, and insurance). A wide array of factors may interact to determine population health, including biological or genetic factors, health behaviors and lifestyle practices, socioeconomic status, the environment, access to health services, and cultural or linguistic isolation. Appreciation of the heterogeneity of the general population and the many health-related factors that distinguish populations, subpopulations, and groups within subpopulations from each other has grown over time and in importance. Therefore, a critical need remains for standard definitions of CVD and COPD data elements, as well as a need for consensus regarding the operationalization of race and ethnicity, SES, and biological risk factors in the surveillance of CVD and chronic lung disease.
Impressive gains have been achieved in life expectancy for the overall American population, as well as distinct subpopulations defined by race and ethnicity. However, inequities in health status and health systems remain in many neighborhoods, cities, states, and regions. A contemporary and ongoing national framework for the surveillance of CVD and COPD disparities will facilitate the development of actionable policies and programs informed by data gathered at the national, regional, state, and community levels.
For example, at the national and state levels, incidence and prevalence information accompanied by improved data on race/ethnicity and geographic region will enable more effective goal setting for national and state programs and policies aimed at eliminating health disparities. This aggregation and reporting can provide information about where persistent disparities in health and health care exist. Local-level data on health behaviors coupled with local-area data on race/ethnicity, language, nativity, and immigration can aid health plan managers in developing culturally and linguistically appropriate interventions to modify unhealthy behaviors. These data will help providers understand the populations they serve, address disparities, and improve and monitor healthcare quality. A lack of valid race and ethnicity data creates difficulty in identifying disparities and appropriately targeting strategies to address them.
This framework will support efforts to advance the prevention and effective treatment of chronic disease to ensure the highest quality health care for the U.S. population as a whole and for important subgroups in this population. The committee concluded that the national framework for surveillance would be enhanced by the recommendations of the Institute of Medicine, Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement (2009). Therefore the committee supports these recommendations.
REFERENCES
AHRQ (Agency for Healthcare Research and Quality). 2004. Guide to prevention quality indicators. Rockville, MD: Agency for Healthcare Research and Quality.
AHRQ. 2006. National healthcare disparities report. http://www.ahrq.gov/qual/nhdr06/nhdr06.htm (accessed March 8, 2011).
Bindman, A. B., A. Chattopadhyay, and G. M. Auerback. 2008. Interruptions in Medicaid coverage and risk for hospitalization for ambulatory care-sensitive conditions. Annals of Internal Medicine 149(12):854-860.
Brehm, J. M., and J. C. Celedon. 2008. Chronic obstructive pulmonary disease in Hispanics. American Journal of Respiratory Critical Care Medicine 177(5):473-478.
Brown, C. P., L. Ross, I. Lopez, A. Thornton, and G. E. Kiros. 2008. Disparities in the receipt of cardiac revascularization procedures between blacks and whites: An analysis of secular trends. Ethnicity & Disease 18(2 Suppl 2):S2-112-117.
Byers, T., R. Anda, D. McQueen, D. Williamson, A. Mokdad, M. Casper, E. Ford, and J. Marks. 1998. The correspondence between coronary heart disease mortality and risk factor prevalence among states in the United States, 1991-1992. Preventive Medicine 27(3):311-316.
Camp, P. G., D. E. O’Donnell, and D. S. Postma. 2009. Chronic obstructive pulmonary disease in men and women: Myths and reality. Proceedings of the American Thoracic Society 6(6):535-538.
CDC (Centers for Disease Control and Prevention). 2005. Health disparities experienced by black or African Americans—United States. Morbidity Mortality Weekly Report 54(1):1-3.
CDC. 2007. Prevalence of heart disease—United States, 2005. Morbidity Mortality Weekly Report 56(6):113-118.
Chamberlain, A. M., M. B. Schabath, and A. R. Folsom. 2009. Associations of chronic obstructive pulmonary disease with all-cause mortality in blacks and whites: The atherosclerosis risk in communities (ARIC) study. Ethnic Disease 19(3):308-314.
Chatila, W. M., W. A. Wynkoop, G. Vance, and G. J. Criner. 2004. Smoking patterns in African Americans and whites with advanced COPD. Chest 125(1):15-21.
Christakis, N. A., and J. H. Fowler. 2007. The spread of obesity in a large social network over 32 years. New England Journal of Medicine 357(4):370-379.
Christakis, N. A., and J. H. Fowler. 2008. The collective dynamics of smoking in a large social network. New England Journal of Medicine 358(21):2249-2258.
Coultas, D. B., H. Gong, Jr., R. Grad, A. Handler, S. A. McCurdy, R. Player, E. R. Rhoades, J. M. Samet, A. Thomas, and M. Westley. 1994. Respiratory diseases in minorities of the United States. American Journal of Respiratory and Critical Care Medicine 149(3 Pt 2):S93-S131.
Do, D. P., B. K. Finch, R. Basurto-Davila, C. Bird, J. Escarce, and N. Lurie. 2008. Does place explain racial health disparities? Quantifying the contribution of residential context to the black/white health gap in the United States. Social Science & Medicine 67(8):1258-1268.
Dransfield, M. T., and W. C. Bailey. 2006. COPD: Racial disparities in susceptibility, treatment, and outcomes. Clinical Chest Medicine 27(3):463-471, vii.
Eastwood, J.-A., and L. V. Doering. 2005. Gender differences in coronary artery disease. Journal of Cardiovascular Nursing 20(5):340-351.
Ellison, G. T., A. Smart, R. Tutton, S. M. Outram, R. Ashcroft, and P. Martin. 2007. Racial categories in medicine: A failure of evidence-based practice? PLoS Medicine 4(9):e287.
Fang, J., S. Madhavan, and M. H. Alderman. 1996. The association between birthplace and mortality from cardiovascular causes among black and white residents of New York City. New England Journal of Medicine 335(21):1545-1551.
Fiscella, K., and D. R. Williams. 2004. Health disparities based on socioeconomic inequities: Implications for urban health care. Academic Medicine 79(12):1139-1147.
Ford, M. E., and P. A. Kelly. 2005. Conceptualizing and categorizing race and ethnicity in health services research. Health Services Research 40(5p2):1658-1675.
Gaglia, M. A., D. H. Steinberg, T. L. Pinto Slottow, P. K. Roy, L. Bonello, A. DeLabriolle, G. Lemesle, T. Okabe, R. Torguson, K. Kaneshige, Z. Xue, W. O. Suddath, K. M. Kent, L. F. Satler, A. D. Pichard, J. Lindsay, and R. Waksman. 2009. Racial disparities in outcomes following percutaneous coronary intervention with drug-eluting stents. The American Journal of Cardiology 103(5):653-658.
Gillum, R. F., B. S. Gillum, and C. K. Francis. 1997. Coronary revascularization and cardiac catheterization in the United States: Trends in racial differences. Journal of the American College of Cardiology 29(7):1557-1562.
Gordon, H. S., M. L. Johnson, and C. M. Ashton. 2002. Process of care in hispanic, black, and white va beneficiaries. Medical Care 40(9):824-833.
Greenlund, K. J., J. B. Croft, and G. A. Mensah. 2004. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999-2000. Archives of Internal Medicine 164(19):2113-2118.
Hannan, E. L., M. Racz, G. Walford, L. T. Clark, D. R. Holmes, S. B. King III, and S. Sharma. 2007. Differences in utilization of drug-eluting stents by race and payer. The American Journal of Cardiology 100(8):1192-1198.
Hasnain-Wynia, R., R. Kang, M. B. Landrum, C. Vogeli, D. W. Baker, and J. S. Weissman. 2010. Racial and ethnic disparities within and between hospitals for inpatient quality of care: An examination of patient-level hospital quality alliance measures. Journal of Health Care for the Poor and Underserved 21(2):629-648.
HHS (U.S. Department of Health and Human Services). 2000. Healthy people 2010: Understanding and improving health. 2nd ed. Washington, DC: U.S. Department of Health and Human Services.
Hnizdo, E., P. A. Sullivan, K. M. Bang, and G. Wagner. 2004. Airflow obstruction attributable to work in industry and occupation among U.S. race/ethnic groups: A study of NHANES III data. American Journal of Industrial Medicine 46(2):126-135.
Howard, D. L., F. B. Hakeem, C. Njue, T. Carey, and Y. Jallah. 2007. Racially disproportionate admission rates for ambulatory care sensitive conditions in North Carolina. Public Health Reports 122(3):362-372.
IOM (Institute of Medicine). 2003. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press.
IOM. 2009. Race, ethnicity, and language data: Standardization for health care quality improvement. Washington, DC: The National Academies Press.
Jackson, Y., B. Broers, L. Di Pollina, M. D. Dao, S. Durieux-Paillard, E. Escard, N. J. Perron, A. S. Steiner, H. Wolff, and J. M. Gaspoz. 2011. [General internal medicine: Important articles about ambulatory care published in 2010]. Revue Medicale Suisse 7(280):285-288.
Jemal, A., M. J. Thun, E. E. Ward, S. J. Henley, V. E. Cokkinides, and T. E. Murray. 2008. Mortality from leading causes by education and race in the United States, 2001. American Journal of Preventive Medicine 34(1):1-8.e7.
Joseph, C. L., L. K. Williams, D. R. Ownby, J. Saltzgaber, and C. C. Johnson. 2006. Applying epidemiologic concepts of primary, secondary, and tertiary prevention to the elimination of racial disparities in asthma. The Journal of Allergy and Clinical Immunology 117(2):233-240; quiz 241-232.
Kaiser Family Foundation/American College of Cardiology Foundation. 2002. Racial/ethnic differences in cardiac care: The weight of the evidence. Menlo Park, CA: Henry J. Kaiser Family Foundation.
Kanjilal, S., E. W. Gregg, Y. J. Cheng, P. Zhang, D. E. Nelson, G. Mensah, and G. L. Beckles. 2006. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among U.S. adults, 1971-2002. Archives of Internal Medicine 166(21):2348-2355.
Karlamangla, A. S., S. S. Merkin, E. M. Crimmins, and T. E. Seeman. 2010. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001-2006. Annals of Epidemiology 20(8):617-628.
Keppel, K. G., J. N. Pearcy, and M. P. Heron. 2010. Is there progress toward eliminating racial/ethnic disparities in the leading causes of death? Public Health Reports 125(5):689-697.
Kershaw, K. N., A. V. Diez Roux, M. Carnethon, C. Darwin, D. C. Goff, Jr., W. Post, P. J. Schreiner, and K. Watson. 2010. Geographic variation in hypertension prevalence among blacks and whites: The multi-ethnic study of atherosclerosis. American Journal of Hypertension 23(1):46-53.
Kirkpatrick, P., and M. T. Dransfield. 2009. Racial and sex differences in chronic obstructive pulmonary disease susceptibility, diagnosis, and treatment. Current Opinions in Pulmonary Medicine 15(2):100-104.
Kressin, N. R., and L. A. Petersen. 2001. Racial differences in the use of invasive cardiovascular procedures: Review of the literature and prescription for future research. Annals of Internal Medicine 135(5):352-366.
Laditka, J. N., and S. B. Laditka. 2006. Race, ethnicity and hospitalization for six chronic ambulatory care sensitive conditions in the USA. Ethnicity & Health 11(3):247-263.
Lai, C.-Q., K. Tucker, S. Choudhry, L. Parnell, J. Mattei, B. García-Bailo, K. Beckman, E. Burchard, and J. Ordovás. 2009. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Human Genetics 125(2):199-209.
Lanska, D. J. 1993. Geographic distribution of stroke mortality in the United States: 1939-1941 to 1979-1981. Neurology 43(9):1839-1851.
Lederer, S. E. 2008. Putting death in context. Hastings Center Report 38(6):3.
Lewis, D. R., L. X. Clegg, and N. J. Johnson. 2009. Lung disease mortality in the United States: The national longitudinal mortality study. International Journal of Tuberculosis and Lung Disease 13(8):1008-1014.
Liao, Y., K. J. Greenlund, J. B. Croft, N. L. Keenan, and W. H. Giles. 2009. Factors explaining excess stroke prevalence in the US stroke belt. Stroke 40(10):3336-3341.
Lutsey, P. L., A. V. Diez Roux, D. R. Jacobs, Jr., G. L. Burke, J. Harman, S. Shea, and A. R. Folsom. 2008. Associations of acculturation and socioeconomic status with subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. American Journal of Public Health 98(11):1963-1970.
Mannino, D. M., D. M. Homa, L. J. Akinbami, E. S. Ford, and S. C. Redd. 2002. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveillence Summary 51(6):1-16.
Mujib, M., Y. Zhang, M. A. Feller, and A. Ahmed. 2011. Evidence of a “Heart Failure Belt” in the southeastern United States. The American Journal of Cardiology 107(6):935-937.
NCHS (National Center for Health Statistics). 2010. Deaths for 358 selected causes by 5-year age groups, race, and sex: United States, 1999–2007. http://www.cdc.gov/nchs/data/dvs/MortFinal2007_Worktable292F.pdf (accessed November 2, 2010).
O’Neil, S. S., T. Lake, A. Merrill, A. Wilson, D. A. Mann, and L. M. Bartnyska. 2010. Racial disparities in hospitalizations for ambulatory care-sensitive conditions. American Journal of Preventive Medicine 38(4):381-388.
Osborne, N. G., and M. D. Feit. 1992. The use of race in medical research. JAMA 267(2):275-279.
Pagé, M., M. Doucet, M. J. Eisenberg, H. Behlouli, and L. Pilote. 2010. Temporal trends in revascularization and outcomes after acute myocardial infarction among the very elderly. Canadian Medical Association Journal 182(13):1415-1420.
Passel, J., and D. Cohn. 2008. U.S. Population projections: 2005-2050 Washington, DC: Pew Research Center.
Peterson, E. D., K. P. Alexander, D. J. Malenka, E. L. Hannan, G. T. O’Conner, B. D. McCallister, W. S. Weintraub, and F. L. Grover. 2004. Multicenter experience in revascularization of very elderly patients. American Heart Journal 148(3):486-492.
Pickle, L. W., and R. F. Gillum. 1999. Geographic variation in cardiovascular disease mortality in US blacks and whites. Journal of the National Medical Association 91(10):545-556.
Pimenta, E., and S. Oparil. 2010. Prehypertension: Epidemiology, consequences and treatment. Nature Reviews Nephrology 6(1):21-30.
Rich, M. W., and G. A. Mensah, for the PRICE-V Investigators. 2009. Fifth pivotal research in cardiology in the elderly (PRICE-V) symposium: Preventive cardiology in the elderly—executive summary. Part I: Morning session. Preventive Cardiology 12(4):198-204.
Roger, V. L., A. S. Go, D. M. Lloyd-Jones, R. J. Adams, J. D. Berry, T. M. Brown, M. R. Carnethon, S. Dai, G. de Simone, E. S. Ford, C. S. Fox, H. J. Fullerton, C. Gillespie, K. J. Greenlund, S. M. Hailpern, J. A. Heit, P. M. Ho, V. J. Howard, B. M. Kissela, S. J. Kittner, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, D. M. Makuc, G. M. Marcus, A. Marelli, D. B. Matchar, M. M. McDermott, J. B. Meigs, C. S. Moy, D. Mozaffarian, M. E. Mussolino, G. Nichol, N. P. Paynter, W. D. Rosamond, P. D. Sorlie, R. S. Stafford, T. N. Turan, M. B. Turner, N. D. Wong, and J. Wylie-Rosett, on behalf of the American Heart Association Statistics Committee Stroke Statistics Subcommittee,. 2010. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation 123(4):e18-209.
Sarrazin, M. V., K. T. Cannon, G. E. Rosenthal, and L. C. Kaldjian. 2009. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. Journal of the National Medical Association 101(7):656-662.
Satcher, D. 2010. Include a social determinants of health approach to reduce health inequities. Public Health Reports 125(Suppl 4):6-7.
Schulz, A. J., S. Kannan, J. T. Dvonch, B. A. Israel, A. Allen, 3rd, S. A. James, J. S. House, and J. Lepkowski. 2005. Social and physical environments and disparities in risk for cardiovascular disease: The healthy environments partnership conceptual model. Environmental Health Perspectives 113(12):1817-1825.
Sequist, T. D., and E. C. Schneider. 2006. Addressing racial and ethnic disparities in health care: Using federal data to support local programs to eliminate disparities. Health Services Research 41(4 Pt 1):1451-1468.
Sharma, S., A. M. Malarcher, W. H. Giles, and G. Myers. 2004. Racial, ethnic and socioeconomic disparities in the clustering of cardiovascular disease risk factors. Ethnicity & Disease 14(1):43-48.
Shaya, F. T., M. S. Maneval, C. M. Gbarayor, K. Sohn, A. A. Dalal, D. Du, and S. M. Scharf. 2009. Burden of COPD, asthma, and concomitant COPD and asthma among adults: Racial disparities in a Medicaid population. Chest 136(2):405-411.
Sin, D. D., E. Wong, I. Mayers, D. C. Lien, D. Feeny, H. Cheung, W. Q. Gan, and S. F. Man. 2007. Effects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPD. Chest 131(1):156-163.
Singh, G. K., and R. A. Hiatt. 2006. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979-2003. International Journal of Epidemiology 35(4):903-919.
Sondik, E. J., D. T. Huang, R. J. Klein, and D. Satcher. 2010. Progress toward the Healthy People 2010 goals and objectives. Annual Review of Public Health 31(1):271-281.
Tsai, C. L., and C. A. Camargo, Jr. 2009. Racial and ethnic differences in emergency care for acute exacerbation of chronic obstructive pulmonary disease. Academic Emergency Medicine 16(2):108-115.
Waters, M. C. 2000. Immigration, intermarriage, and the challenges of measuring racial/ethnic identities. American Journal of Public Health 90(11):1735-1737.
Whitehead, M. 1991. The concepts and principles of equity and health. Health Promotion International 6(3):217-228.
Williams, D. R., and P. B. Jackson. 2005. Social sources of racial disparities in health. Health Affairs 24(2):325-334.
Willson, M. N., J. J. Neumiller, D. A. Sclar, L. M. Robison, and T. L. Skaer. 2010. Ethnicity/race, use of pharmacotherapy, scope of physician-ordered cholesterol screening, and provision of diet/nutrition or exercise counseling during US office-based visits by patients with hyperlipidemia. American Journal of Cardiovascular Drugs 10(2):105-108.
Yazdanyar, A., and A. B. Newman. 2009. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clinics in Geriatric Medicine 25(4):563-577.














