Retinal Prosthetic Systems for Treatment of Blindness
JAMES D. WEILAND AND MARK S. HUMAYUN
Doheny Eye Institute, University of Southern California
Most functions of the human body are controlled by small electrical signals delivered via nerves. Thus, it is no surprise that electrical signals applied to the body from external sources can modulate physiological activity. In fact, reports of such physiological electromodulation date back to the eighteenth century, but scientists of that time lacked the understanding of neurophysiology to understand the basic mechanism behind electricity’s ability to modulate biological activity. Advances in neurobiology, medicine, and engineering have allowed informed design of clinically beneficial implantable neurostimulation devices that are now used for a number of debilitating neurological diseases.
Implantable neural stimulators activate nerve cells. Nerves are a class of cells responsible for processing and communicating information between the brain and other parts of the body (Kandel et al., 1991). Sensory receptors are specialized nerve cells that convert physical stimuli into electrical signals that can be relayed by other nerve cells to the brain. Nerve cells are polarized, meaning an electrical potential can be measured across the cell membrane. Transient changes in membrane potential signal to other cells that an event has occurred. Neural networks composed of connected neurons determine if that event, along with inputs from others cells, requires action by other parts of the nervous system. Diseases or injuries that damage nerve cells, particularly sensory cells, can result in significant disability for the affected individual, including loss of sensory input, diminished capability to process information, or reduced motor function.
How can an electrical signal generated by an implanted device activate a nerve cell? It is not as simple as two wires being connected. Part of the complexity arises from the difference in electrical charge carriers; in metals, electrons carry charge, whereas in the body, ions carry charge. The conversion from electrons to
ions occurs at the electrode, typically a metal or metal oxide, in direct contact with the extracellular fluid. An electrical signal applied to the electrode will cause current flow in the tissue via movement of charged ions, which include ions of sodium, chloride, and potassium, among others. The end effect of this charge movement is to depolarize the nerve cell membrane, after which the natural signaling mechanism of the nerve cell takes place and the brain, and person, observe an electrically elicited sensation or modulated function.
A number of successful neurostimulators are in widespread use. Cochlear implants stimulate the auditory nerve to give hearing to deaf people. Using these implants allows deaf people to hear so well that they can talk on a telephone. Unrelenting pain sensations sometimes result from nerve damage or disease. Implantable devices that stimulate the lower spinal cord are known to decrease or even eliminate the feeling of pain. One of the most dramatic successes of electrical stimulation is in the treatment of Parkinson’s disease. By stimulating a part of the brain called the thalamus, many symptoms of Parkinson’s subside almost immediately. Parkinson’s patients with uncontrollable tremor or rigidity that severely limits motor function show improved coordination within minutes of commencing stimulation.
TREATING BLINDNESS WITH ELECTRICAL STIMULATION
The retina is the light-sensitive, multilayer tissue that lines the interior surface of the back of the eye (Figure 1) (see http://webvision.med.utah.edu/). Photoreceptors are the light-sensing cells of the retina, while the other cells process photoreceptor signals and send information to the brain via the optic nerve. When photoreceptors degenerate due to disease, the retina can no longer respond to light. However, the other nerve cells of the retina remain in sufficient numbers that electrical stimulation of these cells results in the perception of light. Blindness often results from progressive degeneration of the photoreceptors, which are the sensory nerves of the eye. Diseases like retinitis pigmentosa and age-related macular degeneration blind millions (Gehrs et al., 2010; Hartong et al., 2006). At first, symptoms are subtle, including difficulty seeing at night or blurred central vision. Ultimately, these conditions result in blindness. Given that vision is the sense by which people obtain most of the information about their environment, blindness has an extremely detrimental impact on the afflicted.
Electrical stimulation has been proposed as a treatment for blindness for decades, but only recently have systems been implemented that are consistent with clinical deployment. This first documented use of electrical stimulation to create visual perception dates to 1755, when Charles LeRoy discharged a large capacitor through the head of a blind person, who described “flames descending downwards” (Marg, 1991). Over time, as science, medicine, and engineering progressed, feasibility of a permanent implant to stimulate the retina was established.
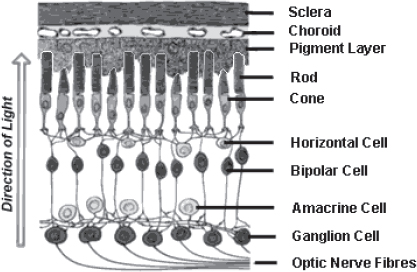
FIGURE 1 Cross section of the retina and other layers of the eye (choroid and sclera). The rods and cones are the photoreceptors that sense light. The other cells process this information and transmit electrical impulses via the optic nerve fibers to the brain. Source: Image from http://www.catalase.com/retina.gif.
RETINAL PROSTHESES: GENERAL DESCRIPTION AND CURRENT CLINICAL SYSTEMS
A retinal prosthesis consists of several components that perform specific functions: a camera to convert photons to digital data, a processing unit to generate stimulus commands based on the image, analog drivers to produce stimulus current, and an array of stimulating electrodes to deliver stimulus current to the retina (Weiland et al., 2005). As shown in Figure 2, the electrode array can be positioned in two locations in the eye, the epiretinal surface and the subretinal space, and these anatomical locations have come to define the two basic approaches that are being pursued for retinal prostheses. An epiretinal implant will rest on the inner limiting membrane of the retina, whereas a subretinal implant would be inserted in the space occupied by photoreceptors in a healthy retina.
Systems have been tested in blind humans in several clinical trials. For brevity, the two main trials that have produced the most significant results will be discussed. Retina Implant, GmbH has developed an externally powered, subretinal microphotodiode array (Figure 3-top). This device has been tested in 12 subjects (Zrenner et al., 2011). The device has 1,500 repeating units on a single silicon chip. Each unit has the following elements: a microphotodiode to sense light,
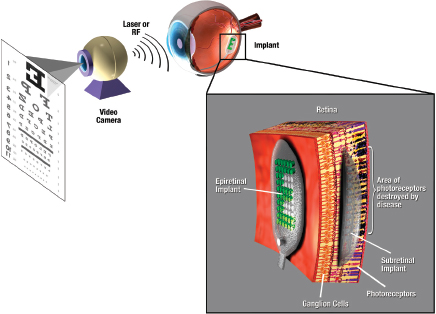
FIGURE 2 Retinal prosthesis concept. An image is captured by a camera and transmitted to an implant. The implant applies a patterned, complex stimulus to the retina via an array of electrodes on the surface of the retina (epiretinal) or underneath the retina (subretinal). Source: Weiland et al., 2005.
digital and analog circuitry to scale a voltage stimulus based on sensed light, and a microelectrode to apply the stimulus to the retina. The voltage stimulus pulse is supplied by a source that is outside the eye. The best test subject was able to read large letters (although it took a long time to do so) and demonstrated visual acuity of approximately 20/1000. The ARGUS II retinal prosthesis (Second Sight Medical Products, Inc.) has been implanted in 30 subjects (Figure 3-bottom). The ARGUS II has 60 electrodes. An external camera unit delivers image information wirelessly to the implant. The best visual acuity result to date is 20/1200 (Humayun et al., 2011). Letter reading was demonstrated in 22 of 30 subjects. Like the subretinal device, reading letters took much longer than reading with natural vision.
These results have generated considerable excitement in ophthalmology, vision research, and biomedical engineering. The possibility of restoring vision captures the imagination of many, and the reports from the human implantees testify to the promise of this approach. In controlled tests, improved mobility is clearly evident, and the subjects report more confidence during ambulation. However,
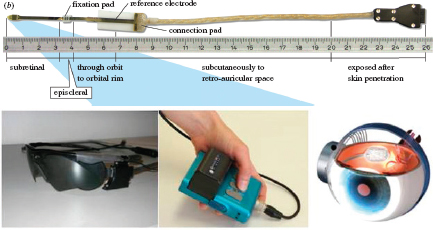
FIGURE 3 (top) A subretinal implant developed by Retina Implant GmbH has been tested in 11 subjects. The microphotodiode array that is implanted in the subretinal space is to the far left, while the remainder of the implant is cabling to allow connection to external test equipment. The epiretinal implant system developed by Second Sight Medical Products, Inc. includes (bottom left) a videocamera mounted in a pair of glasses, (bottom center) a wearable image processing system, and (bottom right) an epiretinal implant with components inside and outside the eye. Stimulus commands generated by the wearable image processing system are transmitted wirelessly to the implant via an inductive coil in the glasses frame. Source: (top) Zrenner et al., 2011. (bottom) Ahuja et al., 2011.
from an objective analysis of the data as a whole, one must conclude that there remains a long road before the claim of “vision restoration” can be considered valid. By all measures, the individuals are still considered blind, even when the devices function well. The best reported visual acuity was 20/1000, whereas the cutoff for legal blindness is 20/200 (normal vision is 20/20). The field of view is limited. The ARGUS II device extends to 20 degrees (which is at the limit for legal blindness), but natural vision has a 180-degree field. The subretinal approach is limited in this regard, with current implants less than 10 degrees.
CHALLENGES FOR ARTIFICIAL VISION
To increase visual acuity with future implants, both technical and biological advances are needed. Simulations of artificial vision project that about 1,000 individual pixels are needed to allow visual function such as reading (at near normal speed) and face recognition. Improvements in electronic packaging (the materials and assembly techniques that protect the circuits from saline) will be
needed to allow 1,000 signals from the circuits to connect to the retina. Low-power integrated circuits must be developed to generate a complex stimulus pattern to evoke form perception. Even if these barriers are overcome, more needs to be understood about how to connect the device to the retina. The subretinal device described above has 1,500 electrodes, but it does not achieve the visual acuity possible if each of these electrodes were acting independently. As electrode arrays become denser with smaller electrodes, better positioning of individual electrodes is needed to increase electrode-retina proximity. This will allow each electrode to activate a small part of the retina, thereby increasing visual acuity. Better stimulation strategies are needed to make the vision appear more natural and less artificial. Currently, perceptions fade within seconds as neural adaptation mechanisms attenuate the artificial input. Research questions should focus on optimizing the stimulus protocols to maximize user performance.
A new approach to artificial vision has potential to address some of the challenges facing electronic retinal prosthesis. The “optogenetic” technique modifies individual neurons to incorporate light-sensitive ion channels into the cell membrane, the most common light-sensitive channel being channelrhodopsin2 (ChR2) (Gradinaru et al., 2010). Ion channels are the means by which ions pass through the cell membrane, and whether channels are open or closed contributes to membrane potential. When light of a specific wavelength is shone on the cell, ChR2 ion channels open, resulting in depolarization of the cell. Bi et al. (2006) first demonstrated that this could be used to modify retinal ganglion cells, showing that light-evoked neural responses were present in a mouse model of retinal degeneration when the mouse retina cells contained ChR2, and others have extended this work. The optogenetic approach has some significant advantages over the bioelectronic approach. By making each cell light sensitive, vision can potentially be restored to near-normal acuity. Also, using light as the activating signal allows the optics of the eye to focus an image on the retina. In other words, the optogenetic technique can come much closer to restoring natural vision, versus artificial vision provided by bioelectronic approaches. However, artificial vision based on optogenetics has its own set of challenges that preclude clinical use. The main issue relates to sensitivity. Currently, modified cells require bright, blue light (460 nm) to be activated, roughly 7 orders of magnitude above the light sensitivity threshold in normally sighted people. It is not clear how such intense light would interact with a diseased retina, with remnant light sensitivity. Also, it is not known if cells can be modified permanently.
SUMMARY
These are interesting times for retinal prostheses. Clinical trials have shown both the promise and limitations of electrical stimulation as a treatment for blindness. Working in this field, one is constantly reminded of the wondrous sense of vision available to most people, how reliant we are on vision, and the devastating
impact of vision loss. Given the complexity of vision, it is clear that prosthetic vision systems will, for the foreseeable future, provide vision that is artificial in appearance and below the resolution needed for complex, visually guided tasks. However, even a slight improvement in vision can have a large impact on quality of life. Restoring the ability to see large objects and detect motion can provide more confidence for someone as they navigate through unfamiliar environments. In addition, the brain has an amazing ability to adapt to new input and the individual, through experience, can use other contextual and sensory information to better understand what they are “seeing” via an artificial vision system. Although important breakthroughs have been achieved in this field, it is still early, and the journey toward restoration of high-acuity vision will involve concerted efforts by scientists, engineers, clinicians, and, most importantly, blind patients.
Financial Disclosures
Dr. Humayun has a financial interest in Second Sight Medical Products, Inc.
REFERENCES
Ahuja, A.K., J.D. Dorn, A. Caspi, M.J. McMahon, G. Dagnelie, L. daCruz, P. Stanga, M.S. Humayun, R.J. Greenberg, Argus II study group. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. British Journal of Ophthalmology 95(12):539–543.
Bi, A., J. Cui, Y.-P. Ma, E. Olshevskaya, M. Pu, A.M. Dizhoor, and Z.-H. Pan. 2006. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50(1):23–33.
Gehrs, K.M., J.R. Jackson, E.N. Brown, R. Allikmets, and G.S. Hageman. 2010. Complement, age-related macular degneration and a vision of the future. Archives of Opthalmology 128(3):349–358.
Gradinaru, V., F. Zhang, C. Ramakrishnan, J. Mattis, R. Prakash, I. Diester, I. Goshen, K.R. Thompson, and K. Deisseroth. 2010. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141(1):154–165.
Hartong, D.T., E.L. Berson, and T.P. Dryja. 2006. Retinitis pigmentosa. The Lancet 368(November):1795–1809.
Humayun, M.S., L. da Cruz, G. Dagnelie, J.A. Sahel, P.E. Stanga, E. Filley, D. Eliott, J.L. Duncan, and R.J. Greenberg. 2011. Interim performance results from the Second Sight Argus II retinal prosthesis study. Investigative Ophthalmology & Visual Science 52:E-Abstract 2594.
Kandel, E.R., J.H. Schwartz, and T.M. Jessel. 1991. Principles of Neural Science, 3rd edition. Norwalk, CT: Appleton & Lange.
Marg, E. 1991. Magnetostimulation of vision: Direct noninvasive stimulation of the retina and the visual brain. Optometry and Vision Science 68(6):427–440.
Weiland, J.D., W. Liu, and M.S. Humayun. 2005. Retinal prosthesis. Annual Review of Biomedical Engineering 7:361–401.
Zrenner, E., K.U. Bartz-Schmidt, H. Benav, D. Besch, A. Bruckmann, V.P. Gabel, F. Gekeler, U. Greppmaier, A. Harscher, S. Kibbel, J. Koch, A. Kusnyerik, T. Peters, K. Stingl, H. Sachs, A. Stett, P. Szurman, B. Wilhelm, and R. Wilke. 2011. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proceedings of the Royal Society of London, Series B: Biological Sciences 278(1711):1489–1497.
This Page is Blank








