“Between animal and human medicine there is
no dividing line nor should there be.
The object is different but the experience obtained
constitutes the basis of all medicine.”
—Rudolf Virchow (1958)
IMPROVING FOOD SAFETY THROUGH A
ONE HEALTH APPROACH
The daily activity of producing, preparing, and consuming food directly links our health with the health of the planet in both direct and indirect ways. Over the past century, the distance between “farm” and “fork” has gone global such that the ingredients in a single meal may be obtained from numerous “local” and “global” sources. Food production and distribution for the developed world takes place across vast and complex global networks in increasingly shorter timescales. As consumers, many of us fail to recognize that our local and domestic food supplies are part of an increasingly interconnected, globalized, food production system.
The U.S. food supply comprises thousands of types of foods and food components—many grown and processed outside of the borders of the United States—as illustrated in Figure WO-1, “the well-traveled salad.” The well-traveled salad’s 10 ingredients originate in more than 37 countries. The increasingly global nature of both domestic and local food supplies underscores the need for a comprehensive One Health approach to food safety, as even common and “whole” ingredients may travel across the world before they reach the table. The
![]()
1 The planning committee’s role was limited to planning the workshop. The workshop summary has been prepared by the workshop rapporteurs (with the assistance of Katherine McClure, LeighAnne Olsen, Rebekah Hutton, and Pamela Bertelson of the staff of the IOM’s Forum on Microbial Threats) as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not necessarily endorsed or verified by the Forum or the Institute of Medicine. They should not be construed as reflecting any group consensus.

FIGURE WO-1 The well-traveled salad. To view an interactive version of this infographic on your computer or to download the static version of this image, visit http://iom.edu/Activities/PublicHealth/MicrobialThreats/2011-DEC-13.aspx. To use your smartphone to link directly to the interactive version use the QR code.
health of humans, animals, and crops plays a pivotal role in ensuring the safety of the world’s food supply.
Globalization of the food supply has created conditions favorable for emergence, reemergence, and spread of food-borne pathogens and has compounded the challenge of anticipating, detecting, and effectively responding to food-borne threats to health. In the United States alone, food-borne agents cause approximately 48 million illnesses, 128,000 hospitalizations, and 3,000 deaths each year (Scallan et al., 2011b). This figure likely represents just the tip of the iceberg, because it fails to account for the broad array of food-borne infections that run the gamut from asymptomatic to serious disease with complications such as renal failure and death2 or for the wide-ranging repercussions they can have for consumers, government, and the food industry—both domestically and internationally.
Most food-borne illnesses are preventable. The interconnectedness of individual, regional, and global public health; the health of the planetary environment(s); and billions of food animals and wildlife would suggest the need for a new paradigm—one that shifts away from a reactive to a more anticipatory, proactive approach to food safety. Such a prime example might be captured in a “One Health” approach to food safety—which has been defined as “the collaborative effort of multiple disciplines—working locally, nationally, and globally—to attain optimal health for people, animals and the environment” (AVMA, 2008).3 Were such an approach to be implemented for food safety, it may hold the promise of harnessing and integrating the expertise and resources from across the spectrum of multiple health domains including the human and veterinary medical, and plant pathology, communities with those of the wildlife and aquatic health and ecology communities.
Statement of Task
Such transdisciplinary synergies could reveal important insights into sources, reservoirs, and factors underlying emergence of infectious diseases; trace and disrupt pathways that lead to food contamination; and contribute to creating systems needed to anticipate and prevent adverse health impacts associated with emergence and spread of novel, emerging, or reemerging food-borne diseases. On December 13 and 14, 2011, the Institute of Medicine’s (IOM’s) Forum on Microbial Threats hosted a public workshop that examined the potential of a “One Health” approach to improve the safety of the food supply domestically and
![]()
2 For the purposes of this workshop summary report, food-borne illness refers to a broad group of illnesses that are caused by the consumption of food contaminated with viruses, bacteria, or parasites that are pathogenic in susceptible human hosts (Tauxe et al., 2010). Food-borne illness is also referred to as food-borne disease, food-borne infection, or food poisoning.
3 There are many, many definitions for “One Health.” The definition from the American Veterinary Medical Association (AVMA) is being used for convenience.
globally. Through invited presentations and discussions, workshop participants explored existing knowledge and unanswered questions on the nature and extent of food-borne threats to health, and considered the structure of food systems, the spectrum of food-borne threats, and the particulars of illustrative case studies. Participants also reviewed existing research, policies, and practices to prevent and mitigate food-borne threats and identified opportunities to implement and strengthen practices informed by One Health throughout the global food system.
Organization of the Workshop Summary
This workshop summary was prepared by the rapporteurs for the Forum’s members and includes a collection of individually authored papers and commentary. Sections of the workshop summary not specifically attributed to an individual reflect the views of the rapporteurs and not those of the members of the Forum on Microbial Threats, its sponsors, or the IOM. The contents of the unattributed sections of this summary report provide a context for the reader to appreciate the presentations and discussions that occurred over the 2 days of this workshop.
The summary is organized into sections as a topic-by-topic description of the presentations and discussions that took place at the workshop. Its purpose is to present information from relevant experience, to delineate a range of pivotal issues and their respective challenges, and to offer differing perspectives on the topic as discussed and described by the workshop participants. Manuscripts and reprinted articles submitted by some but not all of the workshop’s participants may be found, in alphabetical order, in Appendix A.
Although this workshop summary provides a description of the individual presentations, it also reflects an important aspect of the Forum’s philosophy. The workshop functions as a dialogue among representatives from different sectors and allows them to present their views about which areas, in their opinion, merit further study. This report only summarizes the statements of participants at the workshop over the course of 2 consecutive days. This workshop summary is not intended to be an exhaustive exploration of the subject matter nor does it represent the findings, conclusions, or recommendations of a consensus committee process.
Recent Food-Borne Outbreaks: The Changing Nature of the “Threat”
Recent, well-publicized, national and international outbreaks4—discussed in greater detail in Box WO-3, “The Changing Nature of the Threat” (found on pages 36-43)—of food-borne illnesses and death illustrate their far-reaching
![]()
4 In public health practice, a food-borne disease outbreak is defined as the occurrence of two or more cases of similar illness resulting from the ingestion of a common food (CDC, 2012).
public health and economic consequences. Today, the ecological context of food encompasses the planet, as food commodities are traded across the globe and the ingredients in a single meal may be obtained from hundreds of sources in dozens of countries. Multistate and multicountry outbreaks of food-borne morbidity and mortality linked to Listeria in cantaloupe; Salmonella spp. in eggs, ground turkey, and ground beef; and Escherichia coli in bean sprouts are but some of the most recent examples of a growing threat to health, trade, and local economies.
Listeria Contamination of Cantaloupe
One of the largest and deadliest multistate outbreaks of listeriosis in the United States occurred in late summer of 2011. The incident marked the first time that Listeria contamination had been linked to whole cantaloupe and one of the few times it had been linked to fresh produce (Figure WO-3-3) (CDC, 2011g). As of November 1, 139 individuals5 had become ill after being infected with the outbreak strain of Listeria; 29 deaths and 1 miscarriage had also been attributed to infection (CDC, 2011f). In response to the Centers for Disease Control and Prevention (CDC) outbreak investigation, the cantaloupe producer, Jensen Farms of Holly, Colorado, announced a voluntary recall of the 300,000 cases of cantaloupes produced between July 29 and September 10 (CDC, 2011f; FDA, 2011c). The recall included 1.5 to 4.5 million melons that were distributed at supermarkets and chain stores in at least 28 states.
Salmonella Enteritidis Contamination of Chicken Eggs
In late 2010, an outbreak of Salmonella Enteritidis infections led to the recall of more than half a billion shell eggs (CDC, 2010). More than 1,900 people in 11 states became ill, and epidemiological investigations traced the source of the outbreak to eggs supplied by two Iowa egg farms: Wright County Egg and Hillandale Farms. Environmental samples confirmed the presence of the outbreak strain on both farms. A contaminated feed mill provided a connection between these two farms, as Wright County Egg used finished feed from this mill to raise the flocks of egg-laying hens that populated all of the Wright County Egg and Hillandale Farms facilities in Iowa (FDA, 2010a). In August 2010, Wright County Egg and Hillandale Farms conducted nationwide voluntary recalls of shell eggs. Recalled eggs had been packaged under a dozen different brand names and distributed to grocery distribution centers, retail grocery stores, and foodservice companies located in 22 states and in Mexico (FDA, 2010a). Salmonella Enteritidis contamination is not limited to large, industrial-scale, egg producers. In October 2011, an outbreak of Salmonella Enteritidis in Minnesota was traced to eggs produced by the Larry Schultz Organic Farm in Owatonna. These eggs
![]()
5 The mean age of all people infected was 78.
were subsequently distributed to restaurants, grocery stores, food wholesalers, and co-ops in Minnesota, Wisconsin, and Michigan and sickened at least six individuals (Food Safety News, 2011).
Salmonella Heidelberg in Ground Turkey
Between March and September 2011, at least 136 persons from 34 states were infected with the outbreak strain of Salmonella Heidelberg (USDA, 2011a). On July 29, the U.S. Department of Agriculture’s (USDA’s) Food Safety and Inspection Service (FSIS) issued a public health alert about the potential association of these illnesses with the consumption of ground turkey (USDA, 2011a). The outbreak strain of Salmonella Heidelberg is resistant to several commonly prescribed antibiotics, such as ciprofloxacin, ceftriaxone, and trimethoprim-sulfamethoxazole. This antibiotic resistance may be associated with an increase in the risk of hospitalization or possible treatment failure in infected individuals (CDC, 2011b). Ill persons range in age from less than 1 year to 90 years old, with a median age of 23 years (CDC, 2011b).
Epidemiological and traceback investigations, as well as in-plant findings, determined a link between disease outbreak and ground turkey products produced by the Springdale Arkansas establishment of Cargill Meat Solutions (USDA, 2011a). On August 3, 2011, Cargill recalled approximately 36 million pounds of fresh and frozen ground turkey products (CDC, 2011b). In addition to the recall, Cargill addressed conditions in the processing facility. The plant where the turkey was processed was completely disassembled, steam-cleaned, treated with an antibacterial wash, and equipped with the most current monitoring and sampling system. Unfortunately, less than a month later, another 185,000 pounds of turkey—produced at the same factory—was recalled with the same strain of Salmonella (CDC, 2011b).
E. coli O104:H4 Contamination of Fenugreek Seeds
Outbreaks of food-borne diseases increasingly span multiple states and countries, and recall efforts can shut down global markets of entire product lines. The outbreak of a rare strain of E. coli O104:H4, first identified in northern Germany in May 2011, resulted in 4,321 outbreak cases, including 3,469 cases of Shiga toxin—producing E. coli and 852 cases of hemolytic-uremic syndrome (HUS), and 53 deaths had been reported in 14 European countries, the United States, and Canada6 when the epidemic was declared to be over at the end of July 2011
![]()
6 The majority of illnesses associated with this outbreak were reported in Germany and France. Cases were also reported in Austria, Canada, the Czech Republic, Denmark, Greece, Luxembourg, the Netherlands, Norway, Poland, Spain, Sweden, Switzerland, the United Kingdom, and the United States. Cases outside of Germany and France are suspected to be travel-related or incidences of secondary spread of infection by those who had recently travelled to the affected area in Germany.
(Buchholz et al., 2011; Burger, 2011; Robert Koch Institute, 2001; WHO, 2011). Confusion over the source of the outbreak caused economic losses and political frictions that transcended national boundaries and continue to this day. The European Union approved U.S.$287 million in emergency aid for European vegetable farmers affected by the crisis—a sum estimated to be a mere fraction of actual losses (Marucheck et al., 2011).
THE GLOBAL FOOD SYSTEM
Globalization of the food supply has served to expand the range of food-borne pathogens as well as to amplify health and economic impacts of a single contamination incident. Production, processing, and distribution of food increasingly takes place across vast and complex networks—each part or pathway of which must be working optimally—without the introduction of contaminants and/ or adulterants that could taint the final product(s).
The U.S. food supply is composed of thousands of types of foods,7 much of it grown and processed elsewhere (Figure WO-2). The increased distance between the sources of production and consumption is a global phenomenon; more than two-thirds of countries are now net importers of food (Buzby et al., 2008). In 2010, the United States imported an estimated 10 to 15 percent of all food consumed by U.S. households, including more than three-quarters of the fresh fruits and vegetables and more than 80 percent of fresh or frozen fish and seafood (FDA, 2011a). Upon arrival, these products—along with domestically produced foodstuffs—are typically distributed across the country from central facilities. The meat prepared and consumed at a typical American table, for example, has traveled 1,000 miles from its farm (or farms) of origin (Chalk, 2004).
Innovations such as refrigeration, transportation (air, sea, and land), and instantaneous communication support food distribution systems that can rapidly transport perishable goods, provide just-in-time restocking of non-perishable items, and take advantage of economies of scale (ERS, 2001; FDA, 2011a). These innovations have also linked U.S. food safety concerns to conditions in the more than 200 countries and territories from which the United States imports food (IOM, 2010b). An estimated 200,000 overseas facilities are registered with the Food and Drug Administration (FDA) to sell foods to the United States, and there are likely substantial variations in the sanitation and hygiene practices at these facilities (Taylor, 2009). Screening processes at the more than 300 U.S. ports of entry identify and reject contaminated or damaged goods; yet, just barely 1 percent of all foods imported into the United States are subjected to border inspections (CRS, 2009). This reality along with the complexity of food distribution
![]()
7 According to the Food Marketing Institute, the average number of items stocked by U.S. grocery stores is approximately 39,000 (FMI, 2010). In the 1950s, U.S. grocery stores stocked an average of 300 food items (Dupont, 2007).
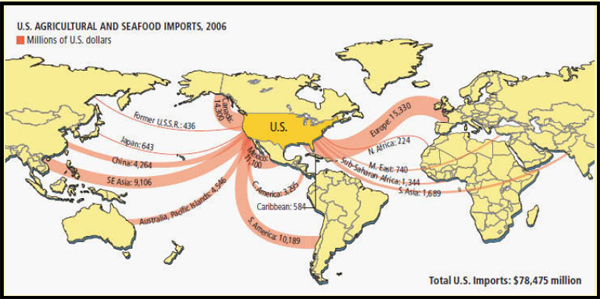
FIGURE WO-2 U.S. agricultural and seafood imports (millions of U.S. dollars).
SOURCE: George Retseck and Lucy Reading-Ikkanda for Scientific American magazine in Fischetti (2007).
makes food extremely vulnerable not just for inadvertent microbial and chemical contamination but also for potential intentional or bioterrorist activities.
Emerging Food-Borne Diseases and the One Health Paradigm
The workshop opened with a keynote presentation by two speakers, Lonnie King of The Ohio State University (Dr. King’s contribution to the workshop summary report may be found in Appendix A, pages 218-225.) and Peter Daszak of EcoHealth Alliance (Dr. Daszak’s contribution to the workshop summary report may be found in Appendix A, pages 130-140.). They discussed the convergence of factors leading to the global emergence of food-borne diseases and defined the principles of One Health, which they characterized as a paradigm for addressing the complex problem posed by these conditions and diseases.
King, referring to the Forum on Microbial Threat’s longstanding “convergence model” of factors influencing infectious disease emergence (IOM, 2003), characterized the spectrum of global threats to food safety and why diseases emerge (illustrated in Figure WO-3) as a “perfect microbial storm.”
King went on to discuss the many factors that influence the complex interactions among host, pathogen, and environment that can lead to the emergence or reemergence of infectious diseases (IOM, 1992, 2003; and illustrated in Figure WO-3). Several environmental factors are of particular relevance in driving emergence and spread of food-borne pathogens, including, but not limited to the following:
• Intensive agricultural practices. In the drive for efficient production, practices such as raising and transporting large livestock herds, flocks of birds, or schools of fish or shellfish in close quarters create ideal conditions for disease emergence and spread (King, 2004).
• Increased interactions between humans, domestic animals, and wildlife. Often caused by habitat destruction, changing land-use patterns, and hunting of animals for food or for the food trade, increased contact between humans, animals, and their associated microbes also increases the potential for pathogen transmission between animal species or between humans and animals (Pike et al., 2010).
• Environmental “commons” such as water. Contamination of common resources distributes and increases both the risk of pathogen emergence and chemical contaminants and can be spread across different farms, regions, states, and nations.
As previously discussed, approximately 48 million cases of food-borne illness occur annually in the United States—1 for every 6 residents (CDC, 2011a; Scallan et al., 2011a). Extrapolating that figure to a global scale, King estimated that at least 1 billion cases of food-borne disease arise annually—a largely silent
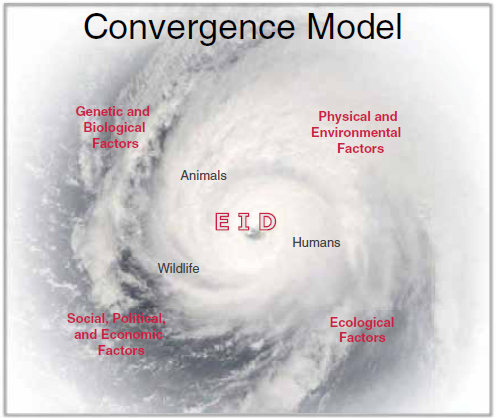
FIGURE WO-3 The convergence model.
SOURCE: King (2011).
“raging epidemic.” Moreover, as Daszak observed, significant emerging viral diseases such as HIV/AIDS8 and severe acute respiratory syndrome (SARS)9 should be characterized as food-borne pathogens, in view of the fact that their introduction into humans and subsequent transmission is intimately linked to the provision of food. These include a large number of viruses that have jumped from wildlife or livestock into humans who hunt for bush meat (HIV/AIDS) or who butcher and process exotic and domesticated animals in wet markets10 (Rasko et al., 2011).
![]()
8 Emergence of HIV and Ebola hemorrhagic fever is likely associated with the butchering and percutaneous and mucous membrane exposure to blood and body fluids of nonhuman primates hunted for food in Sub-Saharan Africa.
9 The SARS outbreak was associated with the trade of a small carnivore, the palm civet, sold for human consumption in Guangdong Province, China. Subsequent investigations found the virus in other wild animals sold in Guangdong’s markets as well as domestic cats. Human infection was the direct result of contact with these animals. The virus was later determined to be of bat origin.
10 A wet market is generally an open food market. The main characteristics of the market have traditionally been associated with a place that sells live animals out in the open. The collection may
Trends Threatening Food Safety
Several workshop presentations addressed the complex and interconnected factors influencing food safety, among them the following key trends introduced by King and Daszak. Several of these trends have been discussed in depth in previous Forum workshop summary reports, including Addressing Foodborne Threats to Health (IOM, 2006), Infectious Disease Movement in a Borderless World (IOM, 2010c), and Antibiotic Resistance: Implications for Global Health and Novel Intervention Strategies (IOM, 2010a).
Growth, migration, and aging of human populations As depicted in Figure WO-4, the overwhelming majority of global population growth is occurring in developing countries. An estimated 1 billion people reside in periurban slums, which, King noted, are home to the fastest-growing human populations; by 2020 their numbers are expected to increase by 50 percent (UN, 2006). These areas are potential hotspots for infectious disease emergence, including water- and food-borne diseases, he observed.
At the same time, human migration from rural to urban settings is just one facet of the more general phenomenon of increased migration—of humans, animals, plants, and diseases, King continued. “More than 1 billion people cross international borders every year, often bringing their food with them,” he stated. Meanwhile, populations in developed countries such as the United States are aging and, therefore, increasingly vulnerable to illness associated with consumption of foods tainted by food-borne pathogens.
Globalization of food trade We live in a world of “collapsed space,” King observed, and it is becoming increasingly smaller, faster, and more interconnected. Vast amounts of food and food products move around the world, as he and several other workshop speakers observed. The global nature of food supply chains is reflected in the United States, he said, where approximately 75 percent of processed food items contain ingredients from another country.11 Upon arrival, these products—along with domestically produced raw and finished foodstuffs—are typically dispersed hundreds or thousands of miles across the country from central distribution or processing facilities. Food distribution networks are designed to rapidly move perishable goods, to provide just-in-time restocking of nonperishable items, and to take advantage of economies of scale (Sobel, 2005). Unfortunately, he added, there is a “disconnect between health and commerce”
![]()
include poultry, fish, reptiles, and pigs. Depending on the region, animals are usually caged and killed for live preparation. Fresh fruits and vegetables are also available. Wet markets generally include butcher shops and fish markets, which are in a separate section from the fruit and vegetable stalls. (University of Hong Kong Social Mapping Project: http://www.wix.com/geog3414/geog3414-wet-market; accessed April 24, 2012).
11 On an annual basis, this country imports more than 75 percent of its fresh fruits and vegetables and more than 80 percent of its seafood (FDA, 2011a).
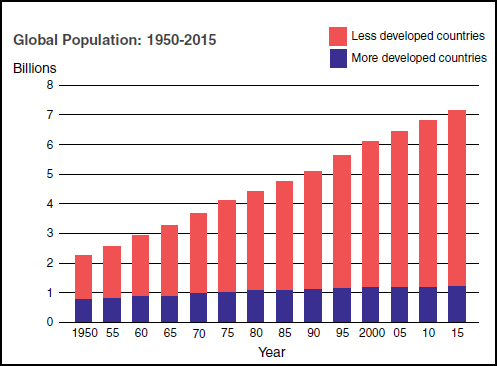
FIGURE WO-4 Trends in global population: 1950-2015.
SOURCE: King (2011).
and, as a result, “real concern about the vulnerability of these remarkable food systems to unintentional natural or even intentional introduction of pathogens and contaminants.”
Increased meat consumption Since 1983, meat consumption has risen steadily in developed countries and steeply in developing countries. As illustrated in Figure WO-5, this exponential growth in the developing world is expected to continue through the next decade. In 2010, nearly 30 billion food animals were produced to help feed the world’s 7 billion people, King reported. If the demand curve for animal protein continues to grow as projected—by more than 50 percent over the next two decades—another 15 billion animals will be needed to feed the world’s estimated population of 9 billion people.
Expansion of the human—animal interface All three trends described above have led to increased contact between humans and animals. Humans migrating from rural areas to urban centers bring their domestic animals such as poultry, swine, and cattle along with them. Eventually, King observed, all agricultural activity will shift toward urban areas. Meanwhile, the expanding human popula-
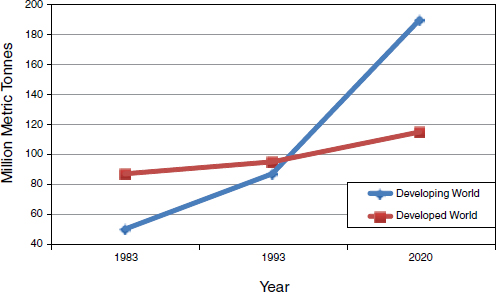
FIGURE WO-5 World meat consumption, 1983-2020.
SOURCE: King (2011).
tion’s demand for meat drives increased contact between hunters and wildlife, as well as the intensification of livestock production. “We have never experienced the intensity and scope of the human—animal interface that we observe today,” he stated. “This is, I think, the great possibility for emerging zoonoses, and certainly food-borne illnesses and rapid changes in our environment.” As illustrated in Figure WO-6 on why diseases emerge, it is essential to understand how pathogen behavior changes in response to environmental upheaval, such as the transition to intensive agriculture, he said.
“What we have now is an incredibly difficult system, a mixture of very intensively farmed production animals in developed countries, with a huge global connectivity,” Daszak added. At the same time, in some parts of the world, and in increasingly remote areas, wildlife continues to be hunted, in increasingly remote areas, he said, “so it really is no surprise that we’re seeing new pathogens that have a higher and higher impact and are emerging at a growing rate.”
Addressing the “Wicked Problem” of Food Safety with a One Health Paradigm
King introduced the concept of the “wicked problem,” as defined in Box WO-1, and explained why the quest for safe food in a globalized environment fits that definition. The term “wicked problems”—referring to problems that arise in complex and interdependent systems and that are difficult or impossible to solve because of incomplete, contradictory, changing, or incomprehensible

FIGURE WO-6 Why diseases emerge.
SOURCE: King (2011).
Wicked problems often arise as organizations face constant or unparalleled change, and in social contexts featuring numerous stakeholders with diverse opinions. The problem of food safety fits this description and displays the following characteristics that define a wicked problem:
• complex and tangled;
• unprecedented and unique, unrelated to past experiences;
• difficult to define and enigmatic;
• having many possible solutions, none of which involves an either/or, yes-or-no choice;
• one for which any solution may generate unexpected consequences;
• threatening; and
• often a symptom of another problem.
SOURCES: Ackoff (2008); King (2011).
requirements—surfaced in the social sciences during the 1960s and was formally defined in the social policy literature a decade later. The concept subsequently has been generalized to other disciplines, such as economics, environmental science, politics, and business (Ackoff, 2008).
Wickedness, he said, does not refer to the difficulty of such problems, but to their inability to be solved by standard approaches. “We have made some really good progress in food safety, without question, but we continue to come back with problem after problem, and new problems emerge,” King observed. He went on to note that it may be time “to think about whether these traditional processes and the way we operate still resolve these difficult and emerging problems.”
Traditional approaches for ensuring food safety are rooted in principles of medical training and education that attempt to define a problem, make a diagnosis, and prescribe a treatment, King explained. A One Health paradigm recognizes the interconnectedness of people, animals, and the environment and emphasizes disease prevention. As discussed in greater detail in King’s contributed manuscript in Appendix A (see pages 218-225), One Health is the collaborative effort of multiple disciplines working locally, nationally, and globally to attain optimal health for people, animals, and our environment. The scale and complexity of food safety issues demand that scientists, researchers, and others move beyond the confines of their own disciplines, professions, and mindsets and explore new organizational modes of team science; a One Health concept embodies this declaration. The scope of One Health is impressive, broad, and growing. Much of the recent focus of One Health has been limited to emerging infectious diseases, yet the concept clearly embraces environmental and ecosystem health, social sciences, ecology, non-infectious and chronic diseases, wildlife, land use, antimicrobial resistance, biodiversity, and much more.
While these components are appreciated within our understanding of the broad dimensions of health, they also add to the complexity of One Health and the difficulty in implementing strategies, building effective coalitions, and mobilizing scientific communities who embrace One Health yet who have been trained and think in much narrower scope and scale. Although there may be disagreement on the exact definition of One Health there is broad consensus that a new framework for preventing food-borne diseases is essential rather than the alternative of constantly responding to them reactively.
The concepts expressed as One Health are not new but are predicated on the discoveries of Louis Pasteur in the late 19th century and were widely accepted before the advent of specialized medicine, King observed. He speculated that these concepts have “re-emerged” as One Health because they place the problem of infectious disease emergence within ecosystems, a relationship championed by the late Nobel Laureate Joshua Lederberg, a founding member of the Forum on Microbial Threats. In his essay “Infectious History,” Lederberg observed that “an axiomatic starting point for progress [against emerging infectious diseases] is the simple recognition that humans, animals, plants, and microbes are cohabi-
tants of this planet. That leads to refined questions that focus on the origin and dynamics of instabilities within this context of cohabitation. These instabilities arise from two main sources loosely definable as ecological and evolutionary” (Lederberg, 2000).
Taking a One Health approach to food safety is an example of changing paradigms, as described by philosopher of science Thomas Kuhn in his seminal work, The Structure of Scientific Revolutions (Kuhn, 1996), according to King. With regard to the science of food safety, we have reached an era when old models are failing, but new models have yet to be created; a time when basic assumptions must be questioned and changed. Table WO-1 lists several key parameters underlying the paradigm shift to One Health.
Such changes need not be led by the scientific community. King observed that, in the case of food safety, the paradigm shift to One Health may be consumer-driven. Indeed, he continued, One Health should be considered in terms of its economic benefits to stakeholders, and its value judged according to evidence of its superiority to current approaches to food safety, or to alternative models. “The evidence has to be based on metrics of reduced costs, reduced or elimination of cases and deaths, [and greater] effectiveness,” he said.
TABLE WO-1 Understanding the One Health Paradigm for Food Safety
|
|
||||
| Dimension | From | To | ||
|
|
||||
| Problem solving | Specific, technical solutions that exist | Managing complex dilemmas and wicked problems | ||
| Perspective | Fragmented and siloed | Systems approach, integrated and holistic | ||
| How work is done | Individual and often isolated | Collaborative and across disciplines and professions | ||
| With whom work is done | Without partners | Partners; government, industry, academe, and public/consumers | ||
| Where work is done | Focus on human illness | Closest to origin of infection or contamination | ||
| What we work on | Single domain | Human, animal, and environmental health domains | ||
| Surveillance and information | Limited to human health and disconnected from other domains | Food, animals, environment, and peoples; shared data | ||
| Time line | Reactive and emphasis on treating disease | Proactive, preventive, and anticipatory | ||
|
|
||||
SOURCE: King (2011).
“In many of its current forms, the concept of One Health is long on visionary scope and maddeningly short on tangible specifics and short term action steps for implementation.”
—Peter Rabinowitz (2010)
Key Challenges and Questions
Many workshop participants, in the discussion that followed the keynote presentations of King and Daszak, focused on the challenges and questions to be addressed in pursuing a One Health approach to preventing food-borne diseases. The following issues, summarized below, were identified by many participants as significant barriers to this goal:
• Public health agencies have yet to adapt to globalization, which demands that they collaborate and cooperate to reduce the burden of food-borne disease.
• Regulation involves negotiating national and regional differences in approaches to food safety.
• The “stovepiped” state of scientific training, research, and funding inhibits interdisciplinary and transdisciplinary research and collaboration.
• There is a need to train medical, veterinary, and public health professionals in One Health precepts.
Many workshop participants suggested that the questions captured in bullet points below might stimulate new ways of thinking about the process of adopting a One Health approach to food safety:
• What are the greatest threats to the global food supply, and which of these threats are most amenable to intervention?
• Despite the “wickedness” of emerging food-borne diseases, can promising “control points” be identified that will increase the likelihood of predicting or preventing potential outbreaks? Can one elucidate ecological rules that govern disease emergence?
• What novel approaches might be taken to increase “upstream” surveillance of food-borne diseases and their associated risk factors?
• What incentives might increase participation by the food industry in such efforts?
• What are the key scientific questions from the One Health perspective that should be pursued but which are not currently given sufficient attention?
• What metrics must be developed to evaluate the effectiveness of interventions based on One Health?
These challenges and questions laid the foundation for ongoing discussions throughout the 2 days of the workshop.
Overview of the Global Food System
Will Hueston, of the University of Minnesota, began his presentation on the global food system with a brief history of human food systems, from the time of hunter-gatherers to today’s complex, interdependent, globalized world in which, he said, “everyone trades food.” (Dr. Hueston’s contribution to the workshop summary report can be found in Appendix A, pages 189-198.) According to Hueston, food systems emerged with the dawn of civilization when agriculture, including the domestication of animals, set the stage for permanent settlements. Inhabitants could grow more crops and raise more animals than necessary to feed those who tended them. This changed human culture; unlike earlier hunter-gatherers, agriculturalists did not need to be in constant motion to find new sources of food. Cultivating grain allowed for drying and storage of some of the harvest for later consumption. Different grain cultures emerged in each of the cradles of civilization—maize in Mexico, rice in China, and wheat and barley in the Middle East. The ability to produce a surplus of grain also set the stage for the development of art, religion, and government.
Hueston observed that, since agriculture began, food systems have constantly evolved, with each change bringing new advantages and challenges and ever-greater diversity and complexity. In the early 1900s people in the United States bought mostly unprocessed foodstuffs from local producers to be prepared and consumed in the home (CAST, 2004). More than a century later, one hamburger from Burger King® can contain ingredients from approximately 200 suppliers located throughout the United States and around the world (Scholl, 2005). And this is just one of the many food choice options available to more than 8 million customers served each day at more than 11,000 Burger King outlets worldwide (Scholl, 2005). Figure WO-7 illustrates both the breadth and the intricacy of current supply chains, through the example of the “inputs” and ingredients for the creation of a classic “megaburger.”
Each of the ingredients listed may come from multiple sources and multiple countries, depending upon the ingredient, time of year, and price of the commodity. Hueston predicted that the future will bring even longer and more complex food supply chains, in part because of the increasingly urbanized global population, and also in response to consumer demand in terms of purchasing power combined with a desire to purchase any kind of food year-round.
Moreover, as illustrated in Figure WO-8, vast—and, in some cases, unknown—numbers of farms and livestock operations, processors, packers, shippers, and retail outlets comprise the current global food system, upon which the U.S. food supply12 increasingly depends. This complex, dynamic web of relationships is prone to the sorts of “wicked problems” described by King.
![]()
12 Altered dietary habits, higher living standards, and lifestyle changes have contributed to changing patterns of food consumption (ERS, 2001, 2005). In a later presentation, David Acheson, of Leavitt Partners, LLC, stated that approximately 15 percent of the food currently consumed in the United
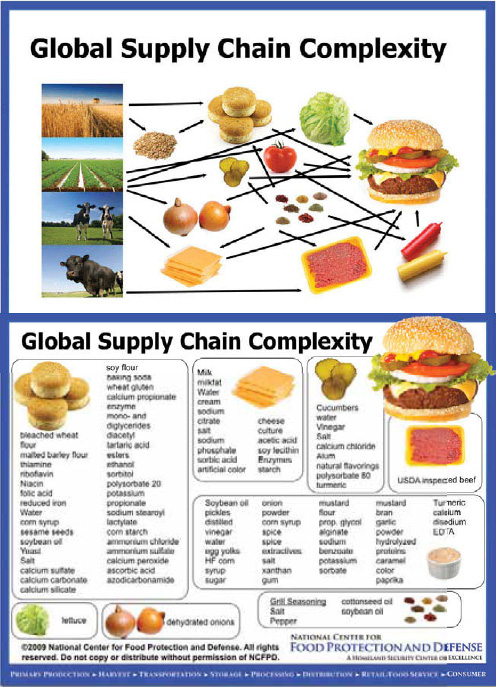
FIGURE WO-7 Global supply chain complexity: Origin and contents of a generic “megaburger.”
SOURCE: Shaun Kennedy, Director, National Center for Food Protection and Defense, University of Minnesota, as cited by Hueston (2011).
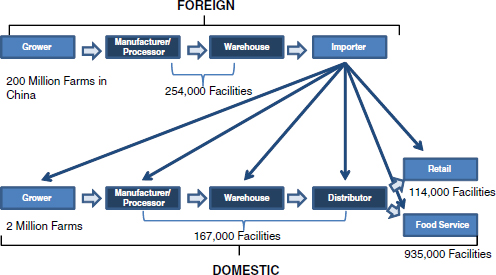
FIGURE WO-8 The global U.S. food supply: Many components.
SOURCE: Acheson (2011).
Hueston insisted that there is no single global food system but rather a multitude of interdependent food systems driven by the diverse needs of different countries and populations. These interconnected systems are also affected by environmental conditions and advancements in technology, he observed. “There is no best system,” he said, “and … every success in improving one food system perturbs the whole system of systems and changes the nature of [global] food safety problems.”
Hueston identified some of the characteristics of this “system of systems” and trends of particular relevance to One Health and the future of food safety:
1. Continuous and dynamic change: Food systems adapt to a host of factors, including trade patterns, population growth, political upheaval, social instability, and advances in technology. The global “system of systems” exhibits properties that are not predictable from its individual subsystems; for example, a small, local perturbation may have a large effect at a global level, or it may have a proportional effect, or none at all.
2. Panarchy: Exponential growth in connectedness and efficiency makes systems less and less resilient, which inevitably leads to collapse. Afterward, systems return to a state of greater resilience, with fewer connections and less efficiency. This model could describe the peril of food
![]()
States is imported; this includes more than 70 percent of seafood and 50 percent of fresh produce sold in this country. Over the past decade, the amount of food importation into the United States grew by more than 10 percent per year.
systems dependent entirely on “just-in-time” supply chains; the more interconnected and efficient they become, the greater their vulnerability to failure at multiple points.
3. Demand-driven economy: “Big-box” stores, multinational fast-food chains, and large processors compete to meet consumer demands—including the demand for safe food—at the lowest possible price. As a result, coalitions of companies are setting standards for food safety ahead of governments and international organizations.
4, Culture clash: Countries and cultures differ in assigning responsibility for food safety. In many developing countries, Hueston observed, “they cook the heck out of everything … [so] there is no microbial food safety threat.” In such cultures, consumers are assumed to be responsible for the safety of their food.
Workshop participants considered another consumer demand trend in subsequent discussion—foods that are locally raised by small (often organic) producers. “There is pressure in a number of states to expand the exclusion of small producers from any and all food safety regulation,” Hueston noted; such exemptions already exist for small producers of meat and poultry. “I applaud the enthusiasm and commitment of the individuals involved, and I am horrified at the lack of knowledge of basic sanitation,” he said. “Public health interventions that have been successful over the years in reducing the likelihood of food-borne illness are now called into question,” Hueston observed. “When we no longer see the problem, then we don’t think the problem exists. It’s the curse of high health status.”
Dr. Robert Tauxe, of the CDC, identified the desire for locally sourced food as arising from a need to know who is responsible and accountable for food safety. (Dr. Tauxe’s contribution to the workshop summary report can be found in Appendix A, pages 307-331.) “I depend entirely on the people who produced it to make sure it’s safe, so I have some comfort at least, if I know who they are,” he said. He urged the food industry to consider satisfying that need by providing information to consumers as to the origins of their products and ingredients. “Maybe that captures some of that market interest and increases the safety of all,” he concluded.
Given these conditions, we must accept that no one system can make food unfailingly safe, and that the problem of food safety cannot be understood in its entirety, Hueston argued. While we need to act to make food safer, we also need to recognize that every action we take perturbs the system, he continued; that will require systems thinking, shared leadership among all stakeholders, and a holistic view of public health and its relationship to the health of ecosystems, economies, and societies.
Hueston also observed that such a multifaceted approach is consistent with the One Health paradigm. He also noted that similar thinking informed the definition of health adopted by the World Health Organization (WHO) at its in-
ception in 1946 as a “state of complete physical, social, and mental well-being, and not merely the absence of disease or infirmity.”13 The WHO and the Food and Agriculture Organization (FAO) of the United Nations (Joint FAO/WHO Food Standards Programme) jointly defined food safety as “all the conditions and measures necessary during production, processing, storage, distribution, and preparation of food to ensure that it is safe, sound, wholesome, and fit for human consumption [sic].”14
“We need to move from finger-pointing to shared leadership,” Hueston asserted. He envisioned a new model of partnership that engages the food industry through a flexible and realistic regulatory system. “Voluntary compliance [with food safety standards], building a trusting relationship between the food industry and public health, has a much higher likelihood of achieving prompt action early in an epidemic and preventing illness and saving lives,” he concluded. “This isn’t something that’s going to be solved by regulation.” Partnership between government and industry, a central theme of workshop discussion, is further considered in the final two sections of this overview.
COMMON FOOD-BORNE PATHOGENS IN THE UNITED STATES
More than 250 pathogens and toxins are known to be transmitted by food, and this list continues to grow steadily, Robert Tauxe reported. Table WO-2 lists food-borne pathogens identified since 1970, which include several nonbacterial organisms.
In the United States, the food-borne pathogens Campylobacter, Clostridium perfringens, E. coli, Listeria monocytogenes, Norovirus, Salmonella spp., and Toxoplasma account for more than 90 percent of all symptomatic food-related illnesses with a known cause. These are briefly discussed in Box WO-2.
![]()
13 The Constitution of the WHO (1946) states that good health is a state of complete physical, social, and mental well-being, and not merely the absence of disease or infirmity. Health is a resource for everyday life, not the object of living, and is a positive concept emphasizing social and personal resources as well as physical capabilities. Health is a fundamental human right, recognized in the Universal Declaration of Human Rights (1948). It is also an essential component of development, vital to a nation’s economic growth and internal stability. Along with the traditional and unequivocal arguments on social justice and the importance of health, it is now accepted that better health outcomes play a crucial role in reducing poverty. There is also increased understanding of how health fits into a wider cross-sectoral, cross-border, and globalized framework. Source: http://www.who.int/trade/glossary/story046/en/index.html.
14 The Codex Alimentarius Procedural Manual states that food hygiene “comprises conditions and measures necessary for the production, processing, storage and distribution of food designed to ensure a safe, sound, wholesome product fit for human consumption” (FAO/WHO, 2001; ftp://ftp.fao.org/docrep/fao/005/Y2200E/Y2200E00.pdf).
|
|
|||
| Bacteria | Viruses | ||
| Bacillus cereus | Astrovirus* | ||
| Brucella spp. | Hepatitis A virus | ||
| Campylobacter spp.* | Norovirus* | ||
| Clostridium botulinum | Rotavirus* | ||
| Clostridium perfringens* | Sapovirus | ||
| E. coli (STEC) O157 | |||
| E. coli (STEC) non-O157* | Parasites | ||
| E. coli other diarrheogenic (not STEC or ETEC)* | Cryptosporidium* | ||
| Enterotoxigenic E. coli (ETEC) | Cyclospora cayetanensis* | ||
| Listeria monocytogenes* | Giardia intestinalis* | ||
| Mycobacterium bovis | Taenia saginata | ||
| Salmonella spp. nontyphoidal | Taenia solium | ||
| Salmonella enterica Serotype Typhi | Toxoplasma gondii* | ||
| Shigella spp. | Trichinella spp. | ||
| Streptococcus | |||
| Streptococcus spp. group A, foodborne | |||
| Vibrio cholerae, toxigenic (O1 and O139*) | |||
| Vibrio vulnificus* | |||
| Vibrio parahaemolyticus* | |||
| Vibrio spp., other | |||
| Yersinia enterocolitica* | |||
|
|
|||
NOTE: Pathogens that have emerged or been recognized as predominantly food-borne in the past 40 years are indicated with an asterisk (*).
SOURCE: CDC (2011h); Tauxe (2002).
Recent Food-Borne Disease Outbreaks: Patterns of Emergence and Lessons Learned
Even in the industrialized world, food-borne illness is a relatively common phenomenon. The true incidence of food-borne illness is unknown because of a combination of factors. A case of food-borne illness is only reported to a health department if a person has become ill, has sought medical care, and has undergone diagnostic testing that has revealed evidence of a pathogen in stool or other specimen. Diagnosed cases are therefore likely to represent only a small fraction of the cases of food-borne illness that actually occur. It is likely that many people do not seek medical attention for symptoms of food-borne illness. Moreover, the diagnosis of some food-borne diseases is difficult, if not impossible, as illustrated by the fact that “unrecognized agents” account for 81 percent of all U.S. food-borne illnesses and hospitalizations and 64 percent of deaths (Mead et al., 1999; Scallan et al., 2011a, 2011b). In developing countries, where food safety presents even greater challenges, food-borne disease is a daily fact of life and a significant cause of death due to diarrheal illness (Mead et al., 1999).
BOX WO-2
The Seven Most Common Food-Borne Pathogens
in the United States
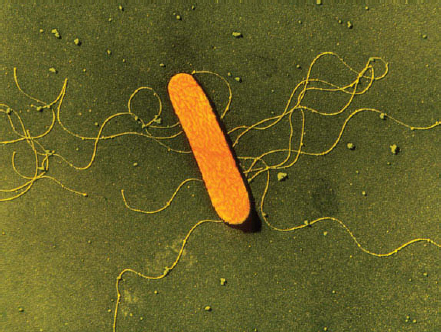
Campylobacter
Campylobacter spp. is one of the most common causes of diarrheal illness—responsible for approximately 850,000 illnesses, 8,500 hospitalizations, and 76 deaths in the United States each year (Scallan et al., 2011b) (Figure WO-2-1). Guillain-Barre syndrome, an acute paralytic illness that may leave chronic deficits, can follow Campylobacter infections. Campylobacter spp. are part of the normal intestinal flora of a wide variety of healthy domestic and wild animalsa and are often found associated with bodies of water such as water troughs and streams. Most cases of campylobacteriosis are associated with eating raw or undercooked poultry meat or from cross-contamination of other foods by these items; outbreaks of Campylobacter-associated disease are also linked to unpasteurized milk or contaminated water.
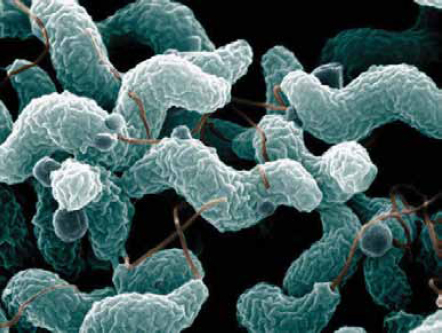
SOURCES: De Wood, Pooley, U.S. Department of Agriculture, Agricultural Research Service, Electron Microscopy Unit.
![]()
a Including cattle, sheep, goats, pigs, chickens, ducks, geese, wild birds, dogs, cats, rodents, and marine mammals.
Clostridium perfringens
Clostridium perfringens is a spore-forming bacterium that produces a toxin estimated to cause nearly a million cases of food-borne illness, 440 hospitalizations, and 26 deaths in the United States each year (Scallan et al., 2011b) (Figure WO-2-2). This organism is found in many “external” environments, as well as in the intestines of humans and animals, and commonly on raw meat and poultry, as well as in gravies and in dried or pre-cooked foods. C. perfringens spores can survive high temperatures. Spores germinate during cooling and storage at temperatures from 68°F to 140°F (20°C to 60°C). If food is served without reheating to kill bacteria, live bacteria may be eaten and cause infection.
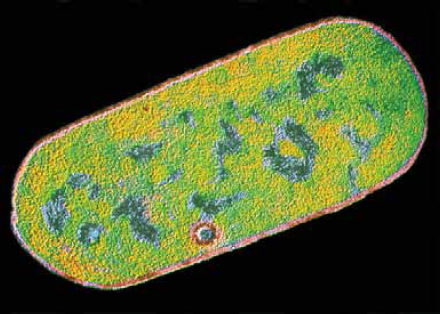
FIGURE WO-2-2 Clostridium perfringens bacterium. Colored TEM. Magnification 43,000x.
SOURCE: CNRI/Science Photo Library.
Escherichia coli
Escherichia coli comprise a large and diverse group of bacteria. Although most strains of E. coli are harmless, others can be pathogenic to humans, including Shiga toxin-producing E. coli (STEC). The most commonly identified STEC in North America is E. coli 0157:H7 (Figure WO-2-3). 0157 was first identified in 1982 in outbreaks of severe bloody diarrhea in North America. STEC live in the guts of ruminant animals, including cattle, goats, sheep, deer, and elk. Other kinds of animals, including pigs and birds, sometimes pick up STEC from the environ-
continued
BOX WO-2 Continued
ment and may spread it (CDC, 2011c). Today almost any food vehicle in contact with ruminant feces is a potential exposure source, including vegetables, sprouts, fruits, meat products, juices, and milk. Drinking, recreational, and bathing waters may be fecally contaminated. Novel transmission routes for outbreaks continue to arise.
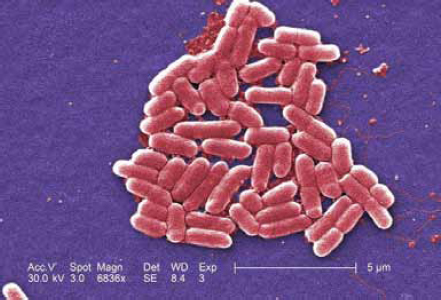
SOURCE: Janice Haney Carr, CDC Public Health Image Library (10068).
Listeria monocytogenes
Listeriosis—a serious infection usually caused by eating food contaminated with the bacterium Listeria monocytogenes—is a relatively rare disease with a high mortality rate (20 to 30 percent) that makes it one of the deadliest food-borne threats (CDC, 2011i; Weinstein, 2011) (Figure WO-2-4). The bacterium is found in soil and water and is carried asymptomatically by numerous animal species. The bacterium has been found in a variety of raw foods, such as uncooked meats and vegetables, as well as in foods that become contaminated after cooking or processing (CDC, 2011i). L. monocytogenes is considered an opportunistic pathogen and causes disease in older adults, pregnant women, newborns, and adults with weakened immune systems (CDC, 2011i). Infections in pregnant women can be devastating to the fetus, resulting in miscarriages, stillbirths, and birth defects. Unlike many other food-borne pathogens, Listeria multiplies in cold environments such as refrigerators (Jemmi and Stephen, 2006). It can quickly spread in damp
buildings, dripping off pipes or ceilings onto food. Once Listeria bacteria get into a food-processing factory, they can live there for years, sometimes contaminating food products (Jemmi and Stephen, 2006).
SOURCE: A.B. Dowsett/Photo Researchers, Inc.
Noroviruses
Noroviruses are the most common source of gastroenteritis outbreaks in the United States, causing nearly 21 million gastrointestinal illnesses annually (Desai et al., 2011) (Figure WO-2-5). Fecaloral spread is the primary mode of transmission. The virus’s abilities to withstand a wide range of temperatures (from freezing to 60°C) and to persist on environmental surfaces and food items contribute to rapid dissemination, particularly via secondary spread (via food handlers or to family members) (Glass et al., 2009). Food can be contaminated at the source (via contaminated water) or during preparation (Glass et al., 2009). Recent evidence suggests the possibility of animal reservoirs, but direct zoonotic transmission appears to be rare. Some noroviruses have been identified in animals—such as pigs and cattle—but none of these strains has yet been
continued
BOX WO-2 Continued
detected in humansb (Glass et al., 2009; Koopmans, 2008). While usually associated with cruise ships, a recent CDC study reports transmission of norovirus among National Basketball Association players and staff during the winter 2010-2011 season (Desai et al., 2011).
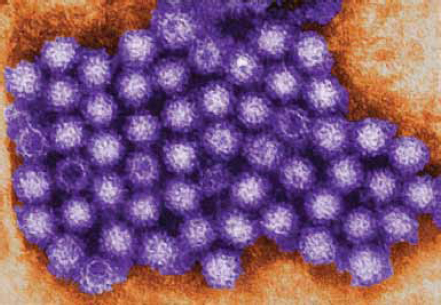
FIGURE WO-2-5 Transmission electron micrograph of norovirus virions.
SOURCE: Charles D. Humphrey/CDC Public Health Image Library (10708).
Salmonella
Salmonella is the leading bacterial cause of food-borne illness in the United States. The CDC estimates that more than 1 million people in the United States contract Salmonella each year, with an average of 19,000 hospitalizations and 380 deaths (Scallan et al., 2011b) (Figure WO-2-6). Salmonella live in the intestines of most livestock and many wild animals. Salmonella infection usually occurs when a person eats food contaminated with the feces of animals or humans carrying the bacteria. Salmonella outbreaks are commonly associated with eggs,
![]()
b Humans are believed to be the only host for human norovirus, but several genogroups (GII and GIV) contain both human and animal strains, raising the possibility of zoonotic transmission.
meat, and poultry, but these bacteria can also contaminate other foods such as fruits and vegetables.c
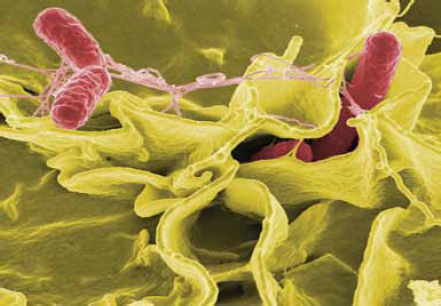
SOURCE: National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Toxoplasma gondii
Toxoplasma gondii is one of the world’s most common parasites (Figure WO-2-7). Although cats are the only known host in which the parasite can com-
![]()
c More recently, the CDC has reported a total of 258 persons infected with the outbreak strain of Salmonella Bareilly (247 persons) or Salmonella Nchanga (11 persons) from 24 states and the District of Columbia. The numbers of ill persons with the outbreak strain of Salmonella Bareilly identified in each state are as follows: Alabama (2), Arkansas (1), California (2), Connecticut (9), District of Columbia (2), Florida (1), Georgia (10), Illinois (23), Louisiana (3), Maryland (24), Massachusetts (27), Mississippi (2), Missouri (4), Nebraska (1), New Jersey (25), New York (39), North Carolina (4), Pennsylvania (20), Rhode Island (6), South Carolina (3), Tennessee (2), Texas (4), Virginia (16), Vermont (1), and Wisconsin (16). Thirty-two ill persons have been hospitalized, and no deaths have been reported. Collaborative investigation efforts of state, local, and federal public health agencies indicate that a frozen raw yellowfin tuna product, known as Nakaochi Scrape, from Moon Marine USA Corporation is the likely source of this outbreak. http://www.cdc.gov/salmonella/bareilly-05-02 (accessed May 3, 2012).
continued
BOX WO-2 Continued
plete its life cycle, this parasite can use almost all warm-blooded vertebrates—including humans—as hosts. T. gondii infections are estimated to cause approximately 87,000 illnesses, 4,400 hospitalizations, and 330 deaths each year in the United States, making it the second leading cause of food-borne mortality in the United States and the third leading cause of food-borne hospitalizations (Scallan et al., 2011 b). The most common sources of Toxoplasma are undercooked meat, animal feces, and transmission from mother to unborn child. While most people infected with Toxoplasma experience no symptoms, unborn children (who contract it from their mothers) and adults with compromised immune systems risk serious side effects. An estimated 22.5 percent of the U.S. population over the age of 12 has been infected with Toxoplasma. For some countries, this figure is as high as 95 percent.
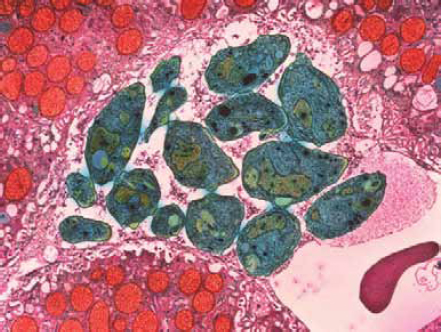
FIGURE WO-2-7 Colored transmission electron micrograph (TEM) of Toxoplasma gondii parasites (green), cause of toxoplasmosis. This unicellular parasite is seen here in liver tissue (pink). Magnification: 12,000x.
SOURCE: Moredum Scientific, Ltd./Photo Researchers, Inc.
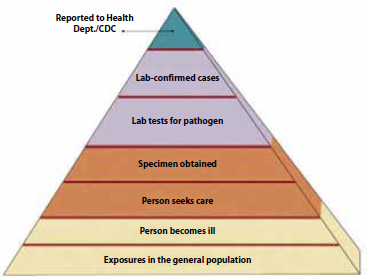
FIGURE WO-9 The true burden of food-borne disease remains unknown.
SOURCE: CRS, 2010. Adapted from CDC, “FoodNet Surveillance—Burden of Illness Pyramid,” http://www.cdc.gov/FoodNet/surveillance_pages/burden_pyramid.htm.
Be that as it may, food-borne disease is a persistent and evolving threat to global health. These diseases occur daily, in all countries—from the least to the most developed—and are caused by consumption of foods and food components contaminated with a variety of microorganisms. According to the CDC, more than 250 different food-borne diseases have been identified (CDC, 2011j). The health impacts15 associated with these diseases can be acute or long term, including episodes of mild to severe diarrheal illness, kidney failure, chronic arthritis, brain or nerve damage, and death (CDC, 2011j). The health burden of these illnesses is substantial,16 but because many cases are often not reported to health officials, the true health impact of food-borne illness is unknown17 (Figure WO-9). Outbreaks of disease also cause billions of dollars in health care—related and industry costs annually (CDC, 2011k).
Beyond the health effects of infection, food-borne illness can also cause substantial economic hardships. Salmonella infections cause approximately 1 million food-borne infections and cost US$365 million in direct medical expenditures annually. The societal cost of a single fatal case of E. coli (STEC) O157 infec-
![]()
15 The most severe cases tend to occur in the very old, in the very young, in those who have compromised immune system function, and in healthy people exposed to a very high dose of an organism (CDC, 2005).
16 Seventy percent of the 2.2 million deaths that occur each year due to acute diarrheal disease are associated with either water- or food-borne contamination (WHO, 2007).
17 The WHO launched an initiative in 2007 to provide better estimates of the global burden of food-borne disease. See http://www.who.int/foodsafety/foodborne_disease/ferg/en/.
tion has been estimated at US$7 million (Frenzen et al., 2005). The USDA estimates costs associated with medical expenses and losses in productivity due to missed work and premature deaths attributed to five major types of food-borne pathogens (Campylobacter, E. coli O157:H7, Shiga toxin-producing strains of E. coli, Listeria monocytogenes, and Salmonella spp.) at US$6.9 billion annually ( Crutchfield and Roberts, 2000).
Several workshop presentations described the unfolding investigation, and analysis, of recent food-borne disease events that have informed a One Health view of food-borne disease emergence. To introduce this topic, Tauxe provided both an overview of domestic trends in food-borne disease and a review of recent progress toward reducing that threat.
Food-Borne Illness Trends in the United States
Tauxe illustrated the consequences of a health threat he called “common, costly, and preventable” with the following statistics (Scallan et al., 2011a, 2011b):
• Each year, an estimated 48 million Americans—1 out of every 6—become sick after eating contaminated food. Of them, 128,000 are hospitalized, and 3,000 die. The domestic burden of disease associated with six major food-borne pathogens is shown in Figure WO-10.
• Approximately 1,200 food-borne outbreaks occur annually in the United States.
• Salmonella infections alone cost the United States US$2.8 billion.
• Preventing a single fatal case of E. coli O157 infection would save an estimated US$7 million.
“Each one of these required a public health response somewhere, and almost all of them were identified in the course of public health investigations of outbreaks,” he observed. Many of these organisms (e.g., Campylobacter, E. coli O157:H7, Salmonella spp., Vibrio spp., and Yersinia enterocolitica) have animal reservoirs and live primarily as commensals or colonists that do not appear to cause illness in nonhuman hosts, he added.
As illustrated in Figure WO-11, between 2003 and 2008, 1,565 outbreaks associated with single foods were reported to the CDC. Both foods of animal origin and produce are important food vehicles in these outbreaks. Tauxe noted that since 2006 food-borne outbreaks have been associated with the following food items not previously identified in the United States as vehicles for food-borne disease. Nearly half of these items were imported, he added, and nearly all of them either consist partly or entirely of plant-based foods, including produce, nuts, seeds, flour, or spices:
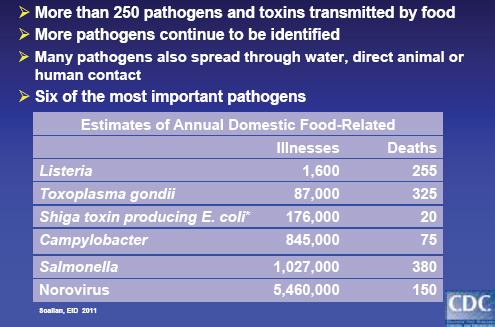
FIGURE WO-10 Many different pathogens and toxins.
SOURCES: Tauxe (2011); from Scallan et al. (2011a, 2011b).
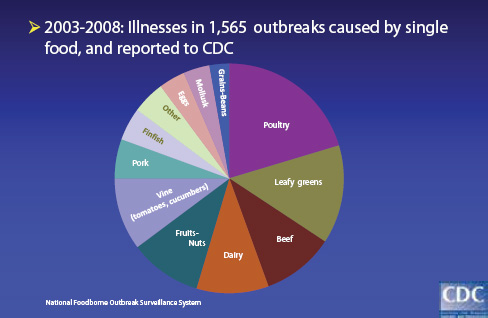
FIGURE WO-11 Foods implicated in outbreaks.
SOURCE: Tauxe (2011).
• bagged spinach
• carrot juice
• peanut butter
• broccoli powder on a snack food
• dry dog food
• frozen pot pies
• canned chili sauce
• hot peppers
• white and black pepper
• raw cookie dough
• hazelnuts
• fenugreek sprouts
• papayas
• pine nuts
In subsequent discussions of food-borne disease trends, workshop participants also considered the threat of food-borne contaminants, such as mycotoxins and aflatoxins,18 which may cause long-term, chronic health problems in both people and animals—in contrast to the acute symptoms of food-borne infections. Such problems are known to exist but are very difficult to study, Tauxe observed. “Mycotoxins, particularly in the developing world, have been a recurrent issue when there’s famine, when there’s food shortage,” he said. “When the only thing left to eat is moldy corn, that’s what you eat.”
Research on the food safety implications of mycotoxins and aflatoxins is a potential arena for One Health, Hueston noted. “The veterinary profession and animal scientists have done a lot more work on [the health effects of these compounds], because it has direct impact on animal production,” he said. Combining their knowledge with the expertise of plant pathologists in a cross-disciplinary, cross-sectoral approach to food safety has “huge potential,” he declared.
Some food-borne infections may also have enduring consequences, Tauxe added. “About 11 percent of the U.S. population has antibodies to toxoplasmosis, which probably means they have cysts in them, and some of those are in their brains,” he stated. “What is that long-term effect? I don’t think we know.”
Many recent disease outbreaks reflect the changing nature of food-borne threats to health. These case studies underscore the vital connections between human, animal, and environmental health, and how changes in ecology or technology can drive the emergence or reemergence of food-borne pathogens by connecting “a potential pathogen with the food chain” (Tauxe et al., 2010). A deeper understanding of the ecology of food-borne pathogens and the root causes of their
![]()
18 Mycotoxins and aflatoxins are naturally occurring toxins produced by fungi, which may be present in moldy grains such as corn or rice, and in peanuts. Aflatoxins are known to cause cancer in some animals, and mycotoxins have been associated with several cancers in humans (e.g., liver cancer, esophageal cancer). Sources: http://www.medterms.com/script/main/art.asp?articlekey=26613; http://www.medterms.com/script/main/art.asp?articlekey=10796.
emergence and spread through the food system will enhance our capabilities to anticipate and prevent future emergence events.
Wake-up Calls: Case Studies of Food-borne Illnesses
Recent incidents of food-borne illness (discussed in greater detail below) that have received widespread attention illustrate the breadth and depth of potential threats from microbial food adulterants. In 1984, cult members in Oregon contaminated local salad bars with Salmonella typhimurium with the intent of influencing an election by incapacitating voters; a limited “trial run” of their plan sickened more than 700 people (Torok et al., 1997). In 1996, a worker in a large Texas medical center laboratory deliberately infected at least 12 coworkers with Shigella dysenteriae by leaving tainted pastries in their break room.
More devastating casualties have resulted from inadvertent food contamination. In 1994, approximately 224,000 people across a widespread area of the United States were infected with Salmonella Enteritidis from ice cream that was contaminated following pasteurization (Sobel et al., 2002). More than 7,000 Japanese children became ill with E. coli O157:H7 in a 1996 outbreak that originated in radish sprouts in school lunches (Sobel et al., 2002). Contaminated clams caused a 1991 outbreak of hepatitis A in China that affected more than 300,000 people and is perhaps the largest known food-borne epidemic (WHO, 2002). Despite the fact that an excellent vaccine for hepatitis A was licensed more than a decade ago, hepatitis A virus contamination of imported vegetables recently resulted in a large epidemic with many hundreds of cases and three deaths in the United States. This resulted from accidental contamination of the foodstuff with the virus; purposeful contamination could be substantially more devastating.
In recent years, special concern has been raised about the safety of fresh fruits and vegetables following several incidents of food-borne illness associated with produce. Fruits and vegetables have been associated with an increasing proportion of outbreaks; however, this trend has probably been influenced by the increased consumption of raw produce and by the advent of better surveillance techniques (Wang and Moran, 2004). In particular, recent outbreaks caused by the coccidian parasite Cyclospora cayetanensis and by hepatitis A virus bear examination as object lessons in the etiology, transmission, surveillance, diagnosis, and control of produce-associated illness.
Large-scale, centralized, food-processing operations followed by broad product distribution pathways create additional vulnerabilities in the food supply (ERS, 2005; Maki, 2009). The “bundling” of large quantities of single ingredients or mixing dozens of ingredients of various origins into a single batch can amplify the effects of a single contamination event. It has been estimated that just one infected beef carcass can lead to the contamination of 8 tons of ground beef; and the origin of a single lot of hamburger processed at one plant can be traced to more than 400 individual animals from six states (Nestle, 2003). These scenarios are reflected in the following real-world incidents of large-scale food contamination below, and in Box WO-3:
BOX WO-3
Recent Food-Borne Outbreaks:
The Changing Nature of the “Threat”
As demonstrated in the case examples below, many recent outbreaks of disease reflect the changing nature of food-borne threats to health. These case studies underscore the vital connections between human, animal, and environmental health, and how changes in ecology or technology can drive the emergence or reemergence of food-borne pathogens by connecting “a potential pathogen with the food chain” (Tauxe et al., 2010). A deeper understanding of the ecology of food-borne pathogens and the root causes of their emergence and spread through the food system will enhance our capabilities to anticipate and prevent future emergence events.
Escherichia coli
Escherichia coli is a large and diverse group of bacteria that are present in the environment and as commensala organisms in a wide range of animals, including humans (Garcia et al., 2010). Most strains of E. coli are harmless. Other strains have acquired characteristics, such as the production of toxins, which make them pathogenic to humansb (CDC, 2011c). Transmission of E. coli occurs when food or water that is contaminated with feces of infected humans or animals is consumed. Contamination of animal products often occurs during the slaughter and processing of animals (Garcia et al., 2010). The use of manure from cattle or other animals as fertilizer for agricultural crops can contaminate produce and irrigation water (Garcia et al., 2010). E. coli can survive for long periods in the environment and can proliferate in vegetables and other foods.
Shiga toxin-producing E. coli (STEC) are particularly notorious food-borne pathogens. STEC infection can cause episodes of mild to severe diarrhea, and 5 to 10 percent of infections develop into hemolytic-uremic syndrome (HUS)—a severe complication marked by profuse bleeding that can lead to kidney failure and death (CDC, 2011c). STEC strain 0157:H7 is estimated to cause 63,000 illnesses, 2,100 hospitalizations, and 20 deaths each year (Scallan et al., 2011b). The principal reservoir for this zoonotic pathogen is the intestinal tract of cattle, but other animals may also serve as reservoirs. 0157:H7 emerged as a significant public health threat in 1982 during two outbreaks of disease that investigators associated with the consumption of undercooked ground meat. A wide variety of foods, including fresh produce, have since served as a vehicle for E. coli0157:H7 outbreaks.c Some
![]()
a Organisms in a mutually symbiotic relationship where both live peacefully together while not being completely dependent on one another.
b Researchers have associated intestinal disease with six different mechanisms or “patho-types": enteropathogenic E. coli (EPEC); enterohemorrhagic E. coli (EHEC, also known as Shiga toxin-producing E. coli [STEC] and formerly referred to as verotoxin-producing E. coli [VTEC]); enterotoxigenic E. coli (ETEC); enteroaggregative E. coli (EAggEC); enteroinvasive E. coli (EIEC); attaching and effacing E. coli (A/EEC).
c Food producers must report the presence of E. coli 0157:H7 to health authorities. There are more than 100 “non-0157” STEC strains, and 6 of these strains cause up to two-thirds of
recent outbreaks include contamination events involving spinach and fenugreek bean sprouts.
E. coli O157:H7 contamination of spinach. In 2006, investigators linked at least 205 illnesses and 5 deaths to the consumption of fresh spinach contaminated with E. coli0157:H7 (Weise and Schmit, 2007). In response to the growing outbreak—which included cases across 26 states and Canada—FDA advised consumers to stop eating all uncooked, fresh spinach, or products containing uncooked spinachd (Calvin, 2007). Epidemiological studies traced the contamination to a single shift at a Natural Selections Foods processing plant in San Juan Batista, California, which had produced 42,000 bags of pre-washed and ready-to-eat baby spinach (Weise and Schmit, 2007). Based on isolates from contaminated produce from sick consumers, investigators matched the outbreak strain to environmental samples from a single field in central California. Organic spinach grown on this 2.8-acre plot was surrounded by an 8,000-acre plot of land primarily dedicated to cattle grazing (Jay et al., 2007). Environmental sampling revealed the presence of the outbreak strain in river water and the feces of cattle and wild pigs less than 1 mile away from the spinach field (Figure WO-3-1) (Bergeret al., 2010; Jay et al., 2007). Because the contamination event occurred before the start of the investigation, the precise means by which the bacteria were transmitted to the spinach field remain unknown (Garcia et al., 2010).
E. coli O104:H4 contamination of fenugreek seeds. In 2011, a rare strain of E. coli O104:H4 caused the second largest and the deadliest outbreak of E. coli-associated disease ever recorded. Between May 21 and July 22, 2011, more than 4,000 people became ill—in 16 countries—and 50 individuals died (Rasko et al., 2011) (Figure WO-3-2). By the time the outbreak ended in early July (2011), there were reports of more than 4,000 illnesses, 800 cases of HUS, and 50 deaths in Germany and 15 other countries (Blaser, 2011).
The outbreak was unusual because of the high proportion of adult patients (˜25 percent) with HUS and the frequent development of neurological symptoms in these patients (Frank et al., 2011a). Research suggests that these clinical characteristics were due to the unique combination of traits carried by the pathogen, which included features typical of enteroaggregativee E. coli and the capacity to produce Shiga toxin (Frank et al., 2011a). This strain also has a distinct set of additional virulence and antibiotic-resistance factors f (Rasko etal., 2011).
continued
![]()
associated illnesses. As of March 2012, these “big six” non-0157 STEC serotypes will also be tested by certain food producers, such as beef producers. Food products contaminated with these bacteria will need to be destroyed or cooked to kill the bacteria (USDA, 2011a).
d The resulting drop in sales and consumer confidence in the fresh spinach industry cost the $3.5 billion dollar industry more than $350 million (Weise and Schmit, 2007).
e Enteroaggregative E. coli infections are common in humans, but no animal reservoir has been described (Rasko et al., 2011).
f The strain produces extended-spectrum beta-lactamase (ESBL) enzymes and other factors that render it resistant to at least a dozen antibiotics in eight different drug classes.
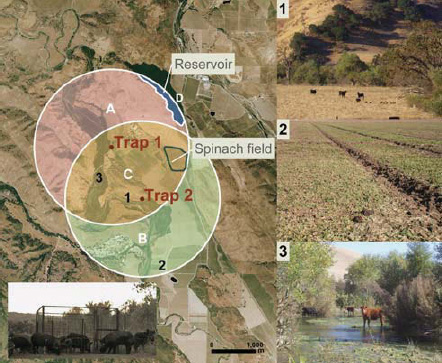
FIGURE WO-3-1 Left: Aerial (˜15 km2) photograph of ranch A showing overlapping circular buffer regions around feral swine trap 1 and trap 2 (San Benito Crop Year 2006; Image Trader, Flagstaff, Arizona). The radius for the buffer (1.8 km) is the circumference of the mean home range for feral swine in mainland California. Estimated density = 4.6 swine/km2 and total area = (A + B + C) - D = 14.8 km2. Areas A, B, and C, combined with counts of individual feral swine from October through November 2006, were used to calculate the average population density. Bottom left: digital infrared photograph of feral swine at trap 1. Right: potential risk factors for E. coli 0157:H7 contamination of spinach at ranch A: (1) feral sow and piglets sharing rangeland with cattle; (2) feral swine feces, tracks, and rooting in a neighboring spinach field; and (3) cattle in surface water.
SOURCE: Jay et al. (2007).
Investigators initially identified fresh produce—including leafy greens, tomatoes, and cucumbers as likely sources of the outbreak (Frank et al., 2011b). Traceback studies of disease clusters in five German provinces that were affected early in the outbreak pointed to sprouts produced by an organic grower in Lower Saxony (Kupferschmidt, 2011). A smaller, second wave of illnesses around the French city of Bordeaux also resulted from the consumption of sprouts, and patient isolates from both outbreaks were identical (EFSA, 2011b). It was later dis covered that sprout seeds associated with both outbreaks had a common origin
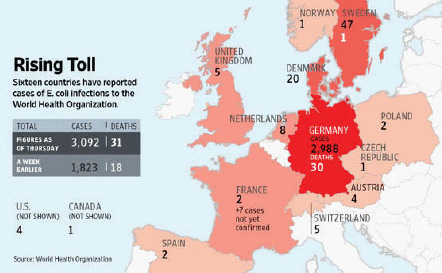
FIGURE WO-3-2 Incidence of HUS. Sixteen countries reported cases of food-borne illness or death associated with the 2011 E. coli O104:H4. The numbers of cases and deaths noted in this figure reflect the outbreak statistics as of June 9, 2011.
SOURCES: Reprinted by permission of the Wall Street Journal. Copyright 2011, Dow Jones & Company, Inc. All Rights Reserved Worldwide.
in a 16.5-ton shipment of fenugreek seeds from Egypt (McKenna, 2011). Upon the shipment’s arrival in Germany in 2009, various distributors in Germany and other European countries subdivided, packaged, repackaged, and widely distributed these seeds as part of thousands of packets of “seed mixes” (McKenna, 2011). Despite extensive recall efforts, the complex chain of packaging and distribution may mean that contaminated seeds could remain on store shelves until their expiration date in 2014 (McKenna, 2011). The pathogen was not isolated from any remaining batches of the suspect seeds,g and questions remain as to the source and reservoir of the contaminating pathogen (EFSA, 2011a).
Listeria monocytogenes
Listeria monocytogenes is a bacterium that is widely distributed in nature. It is commonly found in soil, surface water, plants, and foods and is carried by a variety of animals.h Most infections are acquired by ingestion of contaminated food
continued
![]()
g It is possible that contaminated seeds were no longer in stock when sampling took place, or even if present were contaminated at a level that made isolation of the organism impossible (EFSA, 2011b).
h In addition to humans, at least 42 species of wild and domestic mammals and 17 avian species, including domestic and game fowl, can harbor Listeria. Listeria has also been isolated from crustaceans, fish, oysters, ticks, and flies, http://textbookofbacteriology.net/themicrobialworld/Listeria.html.
BOX WO-3 Continued
or feed, and infected animals can shed the bacterium in feces, milk, and uterine discharges (Jemmi and Stephan, 2006). In humans, Listeria infection can result in the relatively rare but dangerous disease Listeriosis, which has a case fatality rate of approximately 20 percent.i Disease primarily affects the very young or old and pregnant women, but it can also affect healthy individuals (CDC, 2011i). Listeria is well adapted to food-processing and storage environments. It can grow and multiply at low “refrigeration” temperatures and establish persistent infections on food-processing equipment (Ghandhi and Chikindas, 2007).j Listeria is killed by pasteurization and cooking; however, in some ready-to-eat foods contamination may occur after factory cooking but before packaging. Deli meats, hot dogs, unpasteurized milk, and soft cheeses are common sources of Listeria infections (CDC, 2011i).
Listeria contamination of cantaloupe. As discussed earlier in this volume, one of the largest and deadliest multi-state outbreaks of listeriosis in the United States occurred in late summer of 2011. The incident marked the first time that Listeria contamination had been linked to whole cantaloupe and one of the few times it had been linked to fresh produce (Figure WO-3-3) (MMWR, 2011). As of December 2 (2011), 146 individuals had become ill after being infected with the outbreak strain of Listeria; 29 deaths and 1 miscarriage had also been attributed to the infection (CDC, 2011f). In response to the CDC outbreak investigation, the cantaloupe producer, Jensen Farms of Holly, Colorado, announced a voluntary recall of the 300,000 cases of cantaloupes harvested and produced between July 29 and September 10 (CDC, 2011f; FDA, 2011c). The recall included 1.5 to 4.5 million melons that were distributed at supermarkets and chain stores in at least 28 states.
Federal officials found four separate strains of Listeria on contaminated cantaloupes and equipment in the packing shed of the Colorado farm (CDC, 2011g; FDA, 2011b). FDA inspectors cited unsanitary conditions—such as old, corroded, and difficult-to-clean equipment and standing pools of water—and the absence of processing steps to cool the melons before cold storage as likely contributors to contamination (FDA, 2011b, 2011c). The bacterium was not found on fruit or soil in the fields, so questions remain as to the initial source of contamination.
Norovirus
Norovirusesk cause the majority of acute viral gastroenteritis cases worldwide, including an estimated 5.4 million cases, 14,000 hospitalizations, and 149 deaths in the United States annually (Scallan et al., 2011b). Recent improvements to diagnostic techniques have allowed researchers to describe the signifi-
![]()
i Scallan et al. estimate that Listeria monocytogenes causes on average 1,591 episodes of domestically acquired food-borne illnesses, 1,455 hospitalizations, and 255 deaths annually in the United States (Scallan et al., 2011b).
j Listeria monocytogenes may grow in biofilms that protect them against environ mental stress and can be isolated from surfaces after cleaning and disinfection (Ghandi and Chikindas, 2007).
k Also called Calicivirus, Norwalk-like virus, small round structured viruses (SRSVs).
FIGURE WO-3-3 Persons infected with the outbreak-associated strains of Listeria monocytogenes, by state, n= 146 for whom information was reported to CDC on December 2, 2011. A total of 146 persons infected with any of the four outbreak-associated strains of Listeria monocytogenes were reported to CDC from 28 states. The number of infected persons identified in each state was as follows: Alabama (1), Arkansas (1), California (4), Colorado (40), Idaho (2), Illinois (4), Indiana (3), Iowa (1), Kansas (11), Louisiana (2), Maryland (1), Missouri (7), Montana (1), Nebraska (6), Nevada (1), New Mexico (15), New York (2), North Dakota (2), Oklahoma (12), Oregon (1), Pennsylvania (1), South Dakota (1), Texas (18), Utah (1), Virginia (1), West Virginia (1), Wisconsin (2), and Wyoming (4).
SOURCE: Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases (NCEZID); Division of Foodborne, Waterborne, and Environmental Diseases (DFWED)
cant contribution of this highly infectious RNA virus to the burden of food-borne illness—particularly as the cause of numerous outbreaks of food-borne disease in community settings such as nursing homes, hospitals, the military, and cruise ships (Estes et al., 2006; Glass et al., 2009).l Humans are likely to be the primary
![]()
l Among the 232 outbreaks of norovirus illness reported to the CDC from July 1997 to June 2000, 57 percent were food-borne, 16 percent were due to person-to-person spread, and 3 percent were water-borne; in 23 percent of outbreaks, the cause of transmission was not determined. Among these outbreaks, common settings included restaurants and catered meals (36 percent), nursing homes (23 percent), schools (13 percent), and vacation settings or cruise ships (10 percent) (CDC, http://www.cdc.gov/ncidod/dvrd/revb/gastro/norovirus-factsheet.htm).
continued
BOX WO-3 Continued
reservoirm for several norovirus strains, and transmission of the virus between individuals can occur in a variety of ways—via ingestion of contaminated food and water, person-to-person contact, or fecal-oral or aerosol spread (Koopmans and Duizer, 2004). Prevention of infection is difficult because these viruses can persist on environmental surfaces and food items. Comparison of norovirus sequences collected from around the world over the past decade have raised the possibility that pandemic strains of norovirus are spread through foods sold internationally, or through person-to-person contact when travelers carry the virus (Glass et al., 2009; Verhoef et al., 2011).
Norovirus outbreaks and cruise tourism. Organized much like large, floating hotels, cruise ships provide ideal conditions for the introduction and the rapid, global spread of norovirus infection. Thousands of passengers from different geographic areas are transported in close quarters to multiple destinations around the world. Passengers and crew often disembark at multiple ports throughout the cruise where they can sample the local foods and culture (Figure WO-3-4).
Cruise ships account for 10 percent of all reported outbreaks of norovirus in the United States (CDC, 2011l). With the average carrying capacity of a cruise ship now exceeding 2,500 passengers and crew, these outbreaks often affect a large number of people. In 2010, outbreaks of diarrhea and vomiting among passengers and crew on the Celebrity Cruise ship “Mercury” occurred during three consecutive sailings. More than 10 to 22 percent of the passengers and 2 to 4 percent of the crew fell ill during each trip, resulting in a total of 1,058 cases of illness over the course of a month.n These outbreaks also have “off-ship”
![]()
m Within the norovirus genus, there are two branches represented by animal strains, with bovine viruses in GII and murine noroviruses in GV. The GII and GIV genogroups contain both human and animal strains. This raises questions about zoonotic transmission. To date, there is little evidence for direct zoonotic transmission, but because mixing of genes from human viruses (by virus recombination) within a genogroup has been observed, the question arises whether it could also happen in recombination events with animal strains (Koopmans, 2008).
• In 1994, 138,000 gallons of ice cream were contaminated by Salmonella. This “single batch” of ice cream was consumed by individuals in 15 states, where it sickened an estimated 225,000 individuals (Hennessy et al., 1996).
• In 1996, 1,465 persons in 20 states, the District of Columbia, and two Canadian provinces became ill after consuming fresh raspberries that were imported from Guatemala and infected with the parasite Cyclospora cayetanensis (Tauxe, 2002). Following several additional outbreaks in 1997, Guatemalan producers temporarily suspended raspberry exports to the United States, which resulted in more than US$10 million in losses for growers in this region (ERS, 2001).
SOURCE: Marty Cetron, CDC.
community-wide consequences, contributing to disease dissemination at ports of call (IOM, 2010c).
Infection can thus be introduced to the cruise ship environment in a variety of ways: by passengers or crew infected before embarkation; with food items contaminated before loading; by persistently contaminated environmental surfaces; or after ships dock in countries where sanitation might be inadequate—either through contaminated food or water, or via passengers that have been infected while ashore (Hall et al., 2005).
• In 2003, a series of hepatitis A outbreaks resulted in 1,000 cases of illness across multiple states and 3 deaths. The outbreaks were linked to green onions imported from four farms in Mexico where hepatitis A is endemic (FDA, 2003; IOM, 2006). FDA subsequently banned imports from these farms.
• In 2008, 1,450 individuals in 43 states and the District of Columbia became ill from salmonellosis and two patients died after consuming jalapeño and serrano peppers imported from Mexico. Investigations traced the contaminated peppers to one farm in Mexico, but the source of contamination is unknown (Maki, 2009).
• In 2009, Salmonella contamination of peanuts and peanut products led to one of the largest product recalls in U.S. history. More than 714 people in 46 states were sickened in this outbreak and 9 individuals died (Cavallaro et al., 2011). Investigators traced the contamination to a single facility that produced peanuts, peanut butter, and peanut paste. Because more than 200 companies used these foodstuffs as ingredients in a variety of other products,19 the recall extended to more than 3,900 products (Cavallaro et al., 2011).
Recent Efforts to Reduce the Threat of Food-Borne Disease
Tauxe divided the “farm-to-table” continuum into three stages in which risk for food-borne disease can be reduced: production, processing, and final preparation and cooking. Although, as he later noted, most food-borne pathogens are heat-labile and therefore can be inactivated by cooking, an increasing proportion of outbreak-associated foods are uncooked (e.g., produce), requiring attention to earlier stages in their procurement; other foods, including meats, are frequently cooked or served at temperatures insufficient to inactivate pathogens. Since a 1993 outbreak associated with hamburgers purchased from a fast food chain resulted in more than 500 laboratory-confirmed infections with E. coli O157:H7 and at least 4 deaths (CDC, 1993), several interventions have been introduced to reduce the contamination of beef during processing and in the retail and restaurant industries (FSIS, 2002).
Many recent food-borne outbreaks have been identified through PulseNet (CDC, 2011e), the national network for molecular surveillance of bacterial enteric infections, Tauxe explained. Established in 1996, PulseNet connects state health departments, city health departments, and laboratories of the CDC, FDA, and the USDA’s FSIS, all of which collect genetic information on food-borne pathogens from infected people, foods, and animals and submit it electronically to a common database, so that sudden increases in a particular subtype can be flagged and investigated. This network is in turn linked with similar databases created by the Public Health Agency of Canada (http://www.nml-lnm.gc.ca/Pulsenet/index-eng.htm), by U.S. veterinarians,20 and by the food industry. A similar collaborative program coordinated by the CDC, FoodNet, conducts active surveillance for several major food-borne pathogens to measure burden and track trends over time (CDC, 2011b). Figure WO-12, which depicts trends in infections caused
![]()
19 Other products included brownie products, cake and pie products, candy products, cereal products, cookie products, cracker products, prepackaged meals, snack mix products, ice cream, pet food, and topping products (Maki, 2009).
20 USDA VetNet commenced in March 2004. The objectives of USDA VetNet are to determine PFGE (Pulse Field Gel Electrophoresis) patterns of Salmonella isolates submitted to the National Antimicrobial Resistance Monitoring System (NARMS), compare USDA VetNet and PulseNet’s PFGE patterns, and to use the comparative data for surveillance and investigation of food-borne illness outbreaks. Source: http://www.ars.usda.gov/research/publications/publications.htm?seq_no_115=199378.
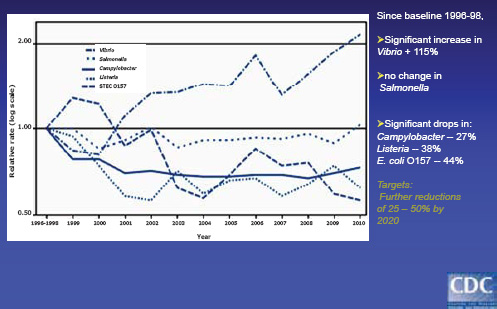
SOURCE: Tauxe (2011).
by some of these pathogens over the past 15 years, reveals significant decreases in Campylobacter, Listeria, and E. coli O157 cases, little change in Salmonella cases, and a significant increase in Vibrio cases.
Tauxe attributed the significant subsequent decrease in E. coli infections to these measures, which he said were achieved through a combination of regulatory, industry, and public health efforts. In a later discussion of the response to the threat of E. coli O157:H7 in meat, speaker Cathie Woteki, Chief Scientist and Under Secretary for Research, Education, and Economics at the USDA, attributed the subsequent decline in such illnesses in part to the introduction of Hazard Analysis and Critical Control Point (HACCP) systems21 and their implementation by the ground beef industry. “HACCP by its nature is a holistic approach to an environment where food is processed,” she said.
Meanwhile, Tauxe observed, few measures have been taken to prevent food-borne infections at the level of production, a stage emphasized by One Health. Returning to the E. coli O157:H7 example, Tauxe noted that several production-stage interventions against food-borne disease—including two vaccines and a
![]()
21 HACCP systems are science-based, systematic protocols for identifying hazards to food safety that arise in the course of processing a specific food, as well as measures for the controlling these hazards. HACCP is intended to assess hazards and establish control systems that focus on prevention rather than relying mainly on end-product testing. Source: http://www.fao.org/DOCREP/005/Y1579E/y1579e03.htm (accessed June 27, 2012).
promising feed additive—had been developed, but not adopted, in the United States. This is not due to a technical impasse, Tauxe stated, but to challenges in gaining regulatory approval—as well as the need for incentives to offset costs to producers—for animal products that benefit human health.
On the other hand, Tauxe recognized farm-based control efforts in other countries that have significantly reduced poultry-associated Campylobacter and Salmonella infections (for example, see the subsequent discussion of Danish efforts to reduce antimicrobial-resistant infections). In Iceland, all chicken flocks are tested for Campylobacter, he reported; those that test positive must be used only to produce frozen meat, considerably reducing potential profits. “The year after [this measure] was introduced, domestic Campylobacter dropped 70 percent in Iceland,” Tauxe said (Tustin et al., 2011). He also described a voluntary program of flock sanitation, hygiene, and vaccination that dramatically reduced Salmonella in egg-layer and broiler breeder flocks in the United Kingdom, and noted that similar steps are being considered in the United States (DEFRA, 2008).
Progress over the past 15 years in reducing the risk of food-borne disease has largely resulted from improvements in post-slaughter or post-harvest practices, Tauxe concluded. “We are very pleased to see that E. coli O157 is essentially half of what it was in the 1990s,” he said, “but contamination often starts before harvest or slaughter. Interventions in food animals exist. They have worked in other countries. Implementing them may depend on getting the incentives right.”
Enterohemorrhagic22 E. coli (EHEC) O104:H4
Reinhard Burger, of the Robert Koch Institute, discussed the largest outbreak of hemolytic-uremic syndrome (HUS) ever reported in the world, caused by a Shiga toxin—producing strain of E. coli, that occurred during the summer of 2011 (Buchholz et al., 2011). (Dr. Burger’s contribution to the workshop summary report can be found in Appendix A, pages 115-130.) “The events this (past) summer in Germany show how rapidly—literally over a weekend—an infectious agent can develop into a major health threat for a whole country,” he said of the outbreak, which was focused in Germany but which also affected several other European countries, the United States, and Canada. The outbreak, which resulted in many severe cases of illness and dozens of deaths, caused fear and changed basic eating habits among consumers, and had enormous economic consequences for farmers, he reported. “It was literally a tragedy for many people,” he concluded. “We should learn from this critical event.”
![]()
22 Enterohemorrhagic strains of E. coli (EHEC) produce compounds known as Shiga toxins because of their similarity to those produced by another enteric pathogen, Shigella dysenteriae Type 1. EHEC is transmitted to humans primarily through consumption of contaminated foods, including raw or undercooked ground meat products, raw milk, and contaminated raw vegetables or greens. Most people with EHEC infections recover within 10 days, but up to 10 percent of patients—especially young children and the elderly—develop HUS, a potentially life-threatening condition (WHO, 2011).
Approximately 4,321 people became infected with EHEC during this outbreak, of which more than 850 developed HUS—a much higher rate of progression to this life-threatening condition than is typical of such infections—and 53 died, Burger reported. Also atypical was the population affected by the disease, which is usually limited almost exclusively to children: 90 percent of the outbreak infections occurred in adults, among whom there was a preponderance of young females, he said.
By the time the local authorities in Hamburg recognized that an outbreak was occurring and alerted Burger and his colleagues, the epidemic had already peaked, he said—a conclusion they reached after conducting a comprehensive range of epidemiological studies. “The first call came on Thursday, May 19,” he recounted. “The next day, the first team went to Hamburg, discussed it. We informed other agencies. On Sunday, we reported to the early-warning response system and gave the first interview that vegetables may be involved. On Tuesday, four days later, we had the first official press conference. On Wednesday, the pathogen was identified” (Frank et al., 2011a, 2011b).
At a press conference that day, the Robert Koch Institute warned against the consumption of raw tomato, cucumber, and salad in northern Germany, based on their early findings, Burger recounted. “The next day, most newspapers wrote ‘from northern Germany.’ Of course, this caused major concern with all the farmers, and the economic consequences were immediately clear.” Later, without consulting Burger or his colleagues, a German Minister of Health in Bremen associated Spanish cucumbers with the source of the outbreak, he said, causing an immediate drop in the sale and import of Spanish cucumbers along with frictions between Spain and Germany. The Spanish farmers who suffered from this mistake were eventually compensated for their losses. These circumstances led Burger to wonder aloud, “How do you communicate the risk—and also the uncertainty—in such exceptional situations? The demand for information was enormous,” he observed. “To inform reliably, to the best of present knowledge, without losing credibility and convincing the people that it’s appropriate, this was a challenge, which one should really be aware of in advance of such crisis situations.”
Cohort studies of groups of people who became ill (such as a team from a Swedish company who stayed a short time in Germany, so it could easily be determined where they stayed and what they ate) helped identify sources of contaminated food items, Burger recalled. Ten such cohorts, comprising 168 people, were found to have eaten at a particular restaurant within the likely time of infection, he explained; the 31 people among them who developed bloody diarrhea or HUS within 14 days of their visit to the restaurant were questioned as to what they had eaten, and the common ingredient in every meal (as identified by the chef) was found to be sprouts (Buchholz et al., 2011).
However, Burger continued, because bean sprouts are often a mixture, the specific type of sprout involved in this outbreak remained unknown.
Then unexpected help came in the form of another outbreak near Bordeaux, France, which involved one type of sprouts, grown from fenugreek (Trigonella foenum-graecum) seeds by individuals in their own homes. Epidemiological investigation linked a single Egyptian supplier of fenugreek seeds to both French and German outbreaks ( Buchholz et al., 2011).
This discovery did not surprise speaker Michael Doyle, of the University of Georgia, who in his prepared remarks showed electron micrographic evidence that fecal bacteria can enter cracks in the hard coats of seeds and flourish inside (Michino et al., 1999; Scallan et al., 2011a). (Dr. Doyle’s contribution to the workshop summary report can be found in Appendix A, pages 140-175.) Many food microbiologists consider sprouts to be one of the most hazardous of foods, he observed. Since 1988, dozens of sprout-associated food-borne disease outbreaks caused by Salmonella and E. coli have been reported, he stated. As a result, FDA has recommended that pregnant women, the elderly, and immuno-compromised women should not consume raw sprouts—and in his opinion, Doyle added, neither should anyone else.
The pathogen responsible for the 2011 outbreak, EHEC O104:H4, was isolated from patients for characterization but has yet to be isolated from sprouts or seeds, Burger reported. It is a rare serotype, previously identified only a few times in humans and never in animals (Bielaszewska et al., 2011). This observation allowed epidemiologists to rule out meat and dairy products as possible vehicles of this outbreak. Unexpectedly, he noted, in addition to expressing Shiga toxin, EHEC O104:H4 was found to be antibiotic resistant because of the expression of ESBL (Rasko et al., 2011). Therefore, it has been hypothesized that this strain acquired its increased virulence from two independent events (Brzuszkiewicz et al., 2011). “It’s obviously a virulence combination of two different E. coli,” Burger concluded. When asked in the subsequent discussion about the possibility that this outbreak resulted from an intentional release of the pathogen, Burger replied that this scenario had been considered but was dismissed as unlikely. Only one researcher was in possession of the outbreak strain prior to this event, he said, and it is doubtful that the strain could have been produced independently.
As illustrated schematically in Figure WO-13, the supplier of the tainted fenugreek seed distributed more than 15,000 kilograms of seed from the same lot (lot number 48088) to companies throughout Europe, which in turn distributed it further, including to people who grow their own sprouts at home, Burger said. Given the enormous interconnectedness of the distribution and supply chain for just one lot of fenugreek seeds it is unlikely that all the contaminated seed has been removed from the supply chain with hundreds of distributors. Furthermore, E. coli can survive on seeds for years, serving as a potential source for future infections. The pathogen can also be shed from infected individuals for more than 6 months, and it may be possible that some infections persist, creating carriers. Secondary infections—of the sort that have already been identified within households, hospitals, and laboratories—may also continue into the future, he
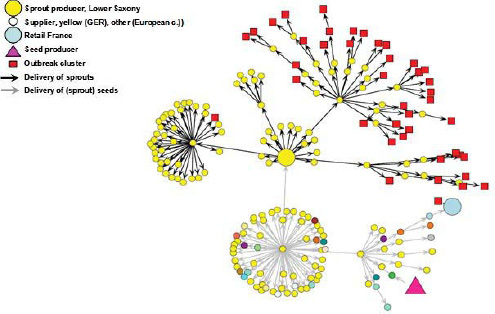
FIGURE WO-13 EHEC outbreak 2011: Investigation of the outbreak along the food chain.
SOURCES: Published by B. Appel, G.-F. Böl, M. Greiner, M. Lahrssen-Wiederholt and A. Hensel, BfR, Federal Institute for Risk Assessment Max-Dohrn-Str. 8-10, 10589 Berlin; Burger (2011); Weiser et al. (2011).
observed. At a press conference at the end of July (2011), Burger declared that the outbreak had ended. However, he added, “there are still one or two cases per month, always secondary cases connected to previous cases.”
Nipah Virus in Malaysia and Bangladesh
The emergence of Nipah virus (NiV) in Malaysia and Bangladesh provides particularly deadly examples of the many routes of zoonotic disease transmission that are associated with the food system. The animal reservoir for this paramyxovirus is fruit bats of the genus Pteropus (Halpin et al., 2011). In the 1990s, the development of large commercial pig farms in Malaysia expanded agricultural lands into the natural habitat of the fruit bat. The resulting increase in interactions between swine and fruit bat populations—including materials contaminated with the saliva or urine from fruit bats—led to an outbreak of disease in swine and humans (Epstein et al., 2006). Although unknown to science before this outbreak, NiV had been circulating in fruit bats for several decades (Epstein et al., 2006).
Exposure of this virus to large numbers of swine facilitated the amplification of NiV in the respiratory tracts of swine and the infection of farm workers
(Epstein et al., 2006). From September 1998 through May 1999, 283 human cases of NiV infection were reported in peninsular Malaysia and Singapore, and most of these patients had come into contact with sick pigs, as illustrated in Figure WO-14 (Chew et al., 2000; Chua, 2003; Luby et al., 2009; Parashar et al., 2000; Paton et al., 1999).
The Malaysian Nipah outbreak ended following the culling of more than 900,000 pigs (Uppal, 2000). This action, plus the loss of market for Malaysian pork in response to the outbreak, decimated the Malaysian swine industry. There have been no new cases of Nipah virus reported in Malaysia or Singapore since the 1998 to 1999 outbreak (Epstein et al., 2006). Between 2001 and 2008, recurrent NiV outbreaks in Bangladesh have caused at least 135 human infections and 98 deaths (Luby et al., 2009).
First recognized as the result of a 1999 outbreak in Malaysia, Nipah virus has since been more frequently associated with Bangladesh and adjacent areas of India, where many outbreaks over the past decade have resulted in more than 250 cases and nearly 200 deaths, according to speaker Steve Luby of the CDC (Luby et al., 2006, 2009). (Dr. Luby’s contribution to the workshop summary report can be found in Appendix A, pages 271-298.) The Malaysian outbreak, which claimed more than 100 lives—about 40 percent of those known to have been infected with the virus—was first traced to direct contact with infected pigs, which in turn were likely infected by bats living in forested areas close to large commercial pig farms (Epstein et al., 2006).
“We think that the bats were eating fruit, including the fruit from trees that had been intentionally planted near the piggeries to provide food for the pigs, as well as for separate agricultural production,” Luby explained. “Partially
FIGURE WO-14 Malaysia Nipah outbreak.
SOURCE: Luby (2011); Adapted from CDC.
eaten saliva-contaminated fruit, as well as bat urine, would be dropped into the piggeries. The pigs became sick. We had a big pig outbreak, and then eventually this went on to people.” Mango and pork production had skyrocketed in Malaysia in the years preceding the outbreak, he said, bringing together bats carrying Nipah virus, pigs, and humans.
Uncovering the story of Nipah virus transmission in Bangladesh proved to be much more complicated than in Malaysia, Luby explained. Beginning with the first reports in early 2005, Nipah cases in Bangladesh tended to be clustered in space and time. A case-control assessment of a broad range of possible exposures shared among the first 12 cases of viral encephalitis (among which all but 1 died) revealed that these people were far more likely to drink raw palm sap than were healthy controls (Luby et al., 2006).
Date palm sap collection, which occurs from late November through March in Bangladesh, involves cutting into and shaving the tree so that the sap, which rises overnight, flows into clay pots hung beneath the cuts, he said; the pots are gathered early in the morning. The collection of date palm sap is illustrated in Figure WO-15. Most of the sap is then cooked into molasses, but some is sold immediately as a drink and is considered to be a delicacy.
One of the fatal cases, in 2005, was the son of a date palm sap collector. His family reported having heard bats in the trees from which they were collecting sap, and they had found bat excrement on some of the collecting pots, Luby said. Several days before the outbreak, the family sent date palm sap to nearby relatives; three people in that household were also among the cases in that outbreak. “As we sorted this out, we said, we’re epidemiologists, so we’re going to put this in an epidemiology journal and we’re going to talk about food-borne transmission of Nipah virus,” Luby recalled—but their conclusion was questioned by micro-biologists, who noted that the virus had never been found in food, and wanted

FIGURE WO-15 Date palm sap collection.
SOURCE: Luby (2011).
the words “Evidence for” prefacing the title, “Foodborne transmission of Nipah virus, Bangladesh” (Luby et al., 2006).
As Nipah recurred in Bangladesh, Luby and his colleagues continued to collect epidemiological evidence linking Nipah infection to date palm sap consumption. “We knew that Pteropus bats occasionally shed Nipah virus RNA in their saliva,” he recounted. “We knew that if you put Nipah virus into fruit juice, it would survive for days at 22 degrees. We knew date palm sap had been implicated in outbreak investigations, and we knew that it was almost impossible to isolate the virus in the sap. By the time we knew of an outbreak, by the time we implicated sap, by the time we occasionally could figure out which tree it came from, this would have been weeks since the transmission event. So, yes, we looked for Nipah virus in sap, and we never found it.” Then, a veterinarian colleague suggested a different approach: using infrared cameras to monitor nocturnal bat activity around sap collection sites (Khan et al., 2011; Rahman et al., 2011). “Sure enough, we could see bats coming in,” Luby said; a typical tree would get 49 bat visits, during which they drank sap an average of 29 times. The experiment to monitor the nightly visitations of bats to drink date palm sap is presented in Figure WO-16.
This discovery prompted Forum member Gerald Keusch, of Boston University, to remark on the growing recognition of the role of bats as carriers of infectious diseases, and to suggest that epidemiological surveillance should be conducted on bats to identify prospective human pathogens. Forum member Fred Sparling, of the University of North Carolina at Chapel Hill, noted that this idea

FIGURE WO-16 How often do bats visit date palm trees to drink their sap?
SOURCE: Luby (2011). Photo by Salah Uddin Khan.
spurred a recent metagenomic analysis of viral sequences present in the feces of North American bats (Donaldson et al., 2010). The authors identified a wide variety of both known and novel viral sequences, suggesting that bats encounter and disseminate a large assortment of viruses capable of infecting many different animals, insects, and plants in nature.
“The molecular evolution of the Nipah virus suggests that it coevolved with the bat over the course of the last 10,000 years,” Luby stated. Bats carry both Nipah and Hendra viruses, apparently asymptomatically. Occasional human infections—which have probably occurred whenever human and bat populations have been in close contact—represent “collateral damage” in the co-evolution of bats and these viruses, he explained; this relationship is only now being recognized due to advances in epidemiological surveillance and global communications.
Transmission pathways associated with Nipah and Hendra viruses are clearly complex and much remains to be understood about them, Luby observed. Their epidemiological investigations in Bangladesh have identified drinking fresh date palm sap as the most frequent pathway of viral transmission from bats to humans (Luby et al., 2009). Other outbreaks arose when bats transmitted the virus to domestic animals (as occurred in Malaysia; in this case, the vehicle was sometimes date palm sap fed to animals). About half of all Nipah infections in Bangladesh resulted from person-to-person transmission, he added; in many of these instances, people who were caring for infected relatives themselves became infected, producing clusters of disease.
Luby observed that since farms in Bangladesh tend to be small, in contrast to the large commercial pig farms where Nipah emerged as a widespread zoonosis in Malaysia, the Nipah outbreaks in Bangladesh have tended to be localized. In the course of investigating transmission to humans through domestic animals, Luby and coworkers discovered that some cattle and goats living near outbreak areas several years later—especially those that were regularly exposed to bats—had antibodies against both Nipah and Hendra viruses. They were also found to have apparent cross-reactivity against another unknown virus of the same (Henipah) family. Bats shed Nipah virus intermittently, he noted. Research is under way to determine factors that influence periodic viral shedding.
Luby and coworkers have also attempted to devise methods to prevent bats from contaminating date palm sap. One method, already used in parts of Bangladesh, involved applying lime to trees around the collection sites, but this did not deter bats from drinking sap, as infrared photographs revealed. A physical barrier proved more successful, he reported; bats did not visit trees with “skirts” made of polythene, or of bamboo and other readily available materials, whereas control trees received thousands of bat visits (Khan et al., 2011). Attempts to get palm sap harvesters to adapt this technology have received mixed results, he observed. “People are willing to try it for a while, particularly on that minority of trees that they are interested in drinking fresh sap out of,” he said. “But we are concerned
about long-term acceptability and uptake, and about how we would roll this out to all producers. These are issues that [we] are still studying.”
The emergence of Nipah virus offers several relevant lessons to One Health and food safety, Luby observed. First, it illustrates that food is produced in the environment, and so shares environmental pathogens, he said. Second, spillovers of virus to humans occur through a confluence of multiple factors in a complex, dynamic system. Third, while Nipah and related viruses have had little impact on the United States to date, their potential for both genetic instability and respiratory transmission bear watching. “It is certainly conceivable that somebody, while incubating the illness, could step on an airplane,” he acknowledged. The discovery of cross-reactive antibodies in domestic animals suggests the existence of additional henipaviruses that may present emerging threats to human health.
Finally, Luby added, “I think the whole process of working on this for several years also illustrates the value of interdisciplinary research and what we call the public health cycle, the idea that we are doing surveillance for serious disease. When we find it, we do outbreak investigations. We work to identify risk factors, to mount interventions, to evaluate those interventions, even when those evaluations are not quite as resoundingly successful as we would like.”
Challenges in Food-Borne Pathogen Detection
One workshop participant noted in discussion, that in the case of both the German EHEC outbreak and the Nipah virus epidemic in Bangladesh, investigators were unable to isolate the pathogen from the suspected food source. This observation led to a discussion of sampling and testing strategies for food-borne pathogens.
“It’s actually not a typical event to identify the organism in the implicated food,” Tauxe stated. There are several reasons for this, he explained: food is transient, food-borne organisms are transient within foods, and many food-borne pathogens are resistant to extraction and culture from food sources. Thus, he said, “over the last decades, one of the most important advances we have been able to make is to get regulatory action to occur, industry action to occur if we have strong epidemiologic implications and a traceback to a particular source, without necessarily requiring that [the causative agent] be isolated from the food. If we [were to] require that, there will be far less protective action.”
“Prompt regulatory action” is an oxymoron, Hueston responded. “Regulatory action requires that you meet an administrative law level of evidence … [whereas] voluntary compliance, building a trusting relationship between the food industry and public health, has a much higher likelihood of achieving prompt action early in an epidemic and preventing illness and saving lives.”
This situation underscores the importance of integrating all kinds of evidence in the course of investigating food-borne disease, Luby argued. “We need to look broadly at the whole story we’re telling in order to reach reasonable scientific
inference,” he said. “I think that’s part of what the United States has done in terms of moving to regulatory action in the absence of microbiological confirmation in source food.”
Given the rapid evolution of molecular technologies and the continued lag in capability of recovery of food-borne pathogens by cultivation, one should expect that molecular and sequence-based data will be increasingly used to link exposures to causative agents and hosts, observed Forum chair David Relman, of Stanford University. The benefits of this approach include improved sensitivity and more information, but there are also drawbacks. While whole-genome sequencing technologies will be able to detect extracted DNA, it will be difficult to pinpoint which organism in a complex mixture of genes and gene fragments revealed by such techniques is the actual causative agent of the epidemic.
“If you just look at the sequence data in isolation, as you suggest, it is actually very hard to interpret,” Luby agreed. “But if we know that we are dealing with an implicated food, if we have anecdotal evidence, if we have epidemiologic evidence … then you are really looking at your microarray data very differently,” he added. This implies a greater need for interdisciplinary collaboration, he continued. “I see it as not just an internal bioinformatics microbiological issue, but more broadly, whether we can tell coherent stories.”
Tauxe noted that the CDC has used molecular subtyping of food-borne pathogens for many years as part of public health surveillance of food-borne diseases to detect and investigate outbreaks that would otherwise be missed. Now, concerns have arisen regarding the transition in the tests that clinical laboratories will use to diagnose these infections from isolating the organism in culture to diagnosis based on detecting antigen or sequence. “When rapid culture-independent diagnostic tests come into play and diagnosis is made on the basis of something that doesn’t yield a culture, we will lose what we have now, unless we can replace that with something that is also sequence-based, that also depends on the same sample, and perhaps something that can even be integrated with clinical diagnosis,” he said. “We’re going to be in a transition period … [during which] we are going to still depend on routine microbiology for our surveillance and our testing of foods and so forth, because we need the specificity of the sub-typing we get from [culturing] the living organism. But … new methods have to be developed that are going to let us move to a probably swifter and probably finer-grained system of surveillance in the future.”
“Food-borne pathogens are ubiquitous,” Hueston observed. “Everybody in this room will be exposed to food-borne pathogens today. If you come up with a fancy enough test and test for a large enough number of pathogens, you’re going to find them in everything.” Moreover, he asserted, “we can’t test our way to food safety.” Instead, he advised, people from a range of disciplines—epidemiologists, food scientists, and business people—who understand the constellation of factors that result in food-borne disease should devise strategies to minimize risk, he said; that would be One Health in action. “The vast majority of the food-borne
outbreaks of which I’m aware have a huge human component,” Hueston added. “We are completely underestimating and missing the opportunity to work effectively with the human component.”
Food-Borne Vehicles and Pathogens: Illustrative Challenges
Workshop presentations describing the roles of plant products as vehicles associated with food-borne disease, viruses as food-borne pathogens, and antimicrobial resistance as a driver of food-borne infections offered insights into many of the critical challenges to be addressed, and opportunities to employ a One Health paradigm.
Plant Foods
As described earlier, this country imports more than 75 percent of its fresh fruits and vegetables, annually (FDA, 2011a). Upon arrival, these products—along with domestically produced foodstuffs—are typically distributed hundreds or thousands of miles across the country from central distribution or processing facilities. Food distribution networks are designed to rapidly transport perishable goods, to provide just-in-time restocking of non-perishable items, and to take advantage of economies of scale (Sobel, 2005).
This system of multiple food “inputs” of diverse—and frequently foreign—origin, quickly dispersed over an elaborate network of processors, distributors, and purveyors to a public with increasingly broad tastes and immense purchasing power, is staggering in its scope, scale, and complexity. It also represents a vehicle for rapid and widespread distribution of food-borne disease, a situation that may delay recognition of an outbreak and impede timely identification of the source (Sobel, 2005). Even more challenging, the U.S. food supply offers countless opportunities for intentional contamination, many of which would be difficult to trace back to their “origin” because of the intricacies of food production and distribution networks.
Both Tauxe and Doyle discussed the nature, scope, and environmental sources of plant food-borne disease. As previously noted by Tauxe, produce and other plant-associated products are important vehicles of food-borne illness in the United States. According to Doyle, about one-third of produce-associated outbreaks of food-borne disease are attributed to leafy greens contaminated with norovirus (discussed in greater detail below) or with EHEC (WHO, 2011). Following leafy greens, melons (mainly cantaloupe) and tomatoes are the next most common outbreak-associated plant food vehicles, he said; the contaminant in these cases is often Salmonella. Doyle also noted the growing importance of spices as a vehicle for food-borne disease. “Seventy-five percent of our spices come from eight countries,” he reported. “These are developing countries, and
if you saw how these things were grown, harvested, dried, and transported you might not eat spices anymore.”
Food-borne pathogens generally make their way onto plant surfaces through direct or indirect contact with animal manure or human feces, Doyle stated. He noted that several different species of food-borne pathogens (e.g., Salmonella, Campylobacter, EHEC, Cryptosporidium, and Listeria) are naturally and harmlessly present in the digestive tracts of animals and shed in their feces, in which pathogens can survive for months to years. A recent study in the United Kingdom found that fresh and stored manure samples from cattle, swine, poultry, and sheep frequently contained EHEC, Salmonella, and/or Campylobacter (Hutchison et al., 2004). In 1997, 5 tons of animal manure were produced for every person living in the United States, he reported.
Plant-derived foods become contaminated with pathogens by one of four primary routes, Doyle explained:
• as a result of wildlife incursion into growing areas;
• through the use of contaminated irrigation or processing water;
• through the use of human or animal feces as soil amendments; or
• from infected humans who handle the food (e.g., food handlers infected with food-borne viruses, as discussed below).
Numerous studies suggest that pathogens can easily travel any of these routes, Doyle observed. A wide range of animal species—including feral pigs and boars, deer, coyotes, rabbits, skunks, rodents, birds, reptiles, and insects—have been found to function as carriers for various food-borne pathogens.
Plants frequently come into contact with pathogens through contaminated water. “You would be surprised how prevalent Salmonella and E. coli O157 can be in environmental water sources,” Doyle observed. Among the results of several studies of both domestic and foreign environmental water sources he presented, Doyle noted that nearly 60 percent of river water tested in Canada, and 80 percent in the state of Georgia, was positive for Salmonella. EHEC, though not found as frequently as Salmonella overall, was nonetheless detected in up to 15 percent of ponds and creeks tested in Brisbane, Australia, he reported. Protozoan parasites, including Cryptosporidium and Giardia, have been found in high concentrations in irrigation water in Mexico and the United States. Doyle observed that global trade in produce means that local water problems can result in food-borne disease anywhere in the world.
Doyle suggested that reducing the fecal shedding of harmful microbes by animals should be a focus of a One Health strategy to improve food safety. This is especially important given the phenomenon of so-called super-shedders: the 10 percent or so of cattle that excrete the majority of E. coli O157:H7, he said. “Those are the ones we have to either vaccinate or use probiotics or a variety of
other practical interventions that can be applied on the farm,” he observed. In addition, animal waste must be managed to improve containment and control of pathogens, according to Doyle, and to ensure that they are inactivated before manure or its by-products are applied to soil.
There are also many pathways by which pathogens can enter the interiors of plant tissues, including fruits and seeds, Tauxe pointed out, either passively or actively. Once internalized, these microbial contaminants can persist or multiply; they cannot, however, be washed off or inactivated by surface treatments. “A bruise on a tomato is a great place for Salmonella to grow,” he said. “So a bruise is actually not just a quality issue; it’s a safety issue.”
Pathogens may be passively internalized during produce processing, Tauxe said. This occurred in 1999, when mangoes imported to the United States from Brazil were treated to kill possible Mediterranean fruit fly by dipping them in hot water, after which they were chilled in a cold-water bath. “The problem was, the cold water was not treated, nor potable nor, in fact, clean,” he stated; rather, it was contaminated with Salmonella Newport, which infected 78 people in 13 states (Penteado et al., 2004; Sivapalasingam et al., 2003). “If you take a hot fruit and put it in a cold bath, the internal spaces contract … and the fruit takes up fluid through the stem scar, the calyx, or other pores, and any bacteria in the water are drawn in,” he explained. “This general phenomenon has been demonstrated for a variety of fruits.”
Active internalization of pathogens into plants can also occur, Tauxe continued. For example, he noted, an electron micrographic study of Salmonella distribution on fresh lettuce leaves shows that the bacterial cells are distributed randomly over the leaf surface during the night, while in daylight, they are concentrated near the stomata, where metabolic products of photosynthesis are released (Kroupitski et al., 2009). Stomata typically close in response to bacterial flagella, he pointed out, but Salmonella and other bacteria, including E. coli O157:H7, can manipulate leaf stomata to open them and get inside the plant tissue as illustrated in Figure WO-17 (Melotto et al., 2006; Saldaña et al., 2011).
The presence of a specific stoma-opening factor—which apparently serves no role in the pathogen’s animal hosts—raises the possibility that some enteric pathogens have a two-host life cycle, involving both plants and animals, Tauxe said. While herbivores are generally considered to be a reservoir for enteric pathogens, plants may be part of the cycle if they are colonized by pathogens excreted by herbivores. “That might make evolutionary sense, because the plant, producing edible materials, is then eaten by the herbivore, and if the bacteria can ride this cycle, then they can move around and they can colonize the next generation of plants and the next generation of herbivores,” he observed. A better understanding of this relationship may present new opportunities to interrupt pathogen transmission “upstream” of human consumption of either plant- or animal-derived food, he concluded.
FIGURE WO-17 Bacteria manipulate leaf stomata and get inside. (A) Scanning electron micrograph showing bacteria on leaf epidermis at 6 h of infection. (B) High magnification of boxed area in (A) showing flagellate bacteria internalized in the stomata. (C-F) Micrographs (60X) of time-course EDL933 infection experiments between 3, 6, 12, and 24 h showing progressive association of bacteria with stomata. (G-J) Same experiment as before employing IFM and anti-0157 antibodies to stain bacteria (green).
SOURCE: © 2011 Saldaña, Sánchez, Xicohtencatl-Cortes, Puente and Girón. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
Food-Borne Viruses
According to speaker Marion Koopmans, of Erasmus University in Rotterdam, Netherlands, there is evidence for food-borne transmission for members of many virus families (Duizer and Koopmans, 2008). (Dr. Koopmans’ contribution to the workshop summary report can be found in Appendix A, pages 225-251.) As shown in Figure WO-18, viruses tend to follow one of three routes to cause food-borne disease:
• through infected food handlers;
• through contamination during production (e.g., of irrigation water); and,
• through zoonotic transmission from an animal reservoir (e.g., a wild animal, consumed as bush meat).

FIGURE WO-18 Grouping of (potential) food-borne viruses.
SOURCE: Microbial Risk Assessment Series: Viruses in Food (WHO, 2008); Tomato image: istock ©Mark Penny; Contaminated water: istock ©Claes Torstensson; Necropsy: FAO Animal Production and Health Commission for Asia and the Pacific.
“The common theme for all of these is really how little we know,” she observed, asserting that the prevalence and burden of food-borne viral disease is vastly underestimated because of the lack of systematic surveillance of viral food-borne outbreaks, and a general lack of knowledge of these viruses within the food sector and of the threat they pose.
Despite evidence that viruses rank among the top causes of diarrheal disease, there is no systematic testing of patients for these viruses, Koopmans stated. Food-borne viral disease outbreaks that are recognized as such represent the “tip of the iceberg,” she asserted. “Not only do we need to have people with [gastrointestinal] illness tested and notified, but they also need to think about the potential for food as a source of their illness,” she said. “We need the [suspected] food tested as well to get conclusive evidence. That hardly ever happens.”
Noroviruses, the most common viral cause of diarrhea, infect 1 in 20 people each year, Koopmans estimated. Infected people—and also infected wild and domestic animals—shed large amounts of virus through the gastrointestinal tract. Because the virus is not effectively removed through sewage treatment systems, it can go on to contaminate seafood and crops that come into contact with recycled water. Koopmans observed that among European shellfish-growing areas that are graded according to their influx of sewage, Grade A areas—those that have a very rare influx of sewage—are virtually nonexistent.
While noroviral disease is relatively common, Koopmans observed, its symptoms are mild compared with hepatitis A, which causes the vast majority of mortality associated with food-borne viral disease (Scallan et al., 2011b). In an effort to better understand patterns of emergence of noroviruses, Koopmans and colleagues established an informal global surveillance network, NoroNet, in 2006.23 The researchers have observed that there are two main types of norovirus outbreaks reported: those that occur seasonally in nursing homes and other health care settings, which are typically caused by a single viral genotype, and all other outbreaks (including those associated with food), which appear to be caused by an evolving mixture of viral genotypes (Kroneman et al., 2008; Siebenga et al., 2010; van Asten et al., 2011). The public health impact of noroviral disease may, in fact, be underestimated. Data collected by NoroNet suggests that recently introduced noroviral variants cause increased outbreak activity and more severe disease, Koopmans reported.
The evident frequency of recombination among food-borne viruses—demonstrated, for example, in the results of multi-year analyses of viruses from shellfish samples—is a reason for concern, Koopmans argued. “Particularly with these sewage-contaminated food-borne outbreaks, we run the risk of generating more diversity through recombination,” she warned. “We should look at this as a warning sign. These are relatively mild viruses, but this is going on all the time.”
Although lack of diagnosis and reporting makes food-borne virus outbreaks difficult to investigate as they unfold, mining of molecular data on viral genotypes permits retrospective detection of clusters of outbreaks linked to common, internationally distributed food sources, Koopmans explained (Verhoef et al., 2011). At the present time, epidemiological and molecular surveillance of food-borne viral outbreaks is insufficient to permit their early detection, she said, but this should be a goal for the future. In the meantime, once an outbreak is detected, these methods may be useful in warning potential consumers of infected food preserved by freezing or drying.
“I feel that food-borne transmission of viruses is very common, but it’s rarely diagnosed,” Koopmans concluded. She urged increased international efforts to exchange molecular and epidemiological information to enable the sequence-based linking of clusters of viral enteric disease, and thereby to track global food-borne outbreaks—outbreaks that threaten to produce more virulent viruses through recombination.
Ultimately, Koopmans added, “I think we need to start moving away from individual surveillance systems for individual pathogens and really think through what the fecal flows and the produce flows are, what smart sampling is, and … use the developing technologies to not just look for a single pathogen, but whatever is around there quantitatively.” For example, she noted, researchers have
![]()
mapped networks of hospitals to determine which experience the highest rates of patient exchanges—an important risk factor for the transmission of drug-resistant microbes; a similar analysis might also reveal risks for food-borne outbreaks, she speculated.
Antimicrobial-Resistant Pathogens
The general phenomenon of antimicrobial resistance (AMR) has been widely discussed, including by this Forum (IOM, 2010a). According to speaker Henrik Wegener, of the Technical University of Denmark, AMR represents another “wicked problem” of the sort previously described by King: a complex and not entirely predictable system of transmission of resistance genes among animals, humans, and the environment. (Dr. Wegener’s contribution to the workshop summary report can be found in Appendix A, pages 331-349.) Food figures prominently in this system, because the use of antimicrobials in low doses as a growth promotant in food animals acts as a chemical driver for AMR, Wegener observed. “There is lifelong exposure of animals and other factors that certainly enhance the selective pressure favoring the emergence of resistance,” he concluded.
This selective pressure operates well beyond the bodies of livestock treated with low-dose antimicrobials, Wegener pointed out. One hundred percent of Vietnamese shrimp farms uses ciprofloxacin. Fluoroquinolone concentrations in sediments and surface waters may reach >4,000μg/kg (Thuy et al., 2011). All kinds of bacteria inhabit these ponds, including those present in the manure of terrestrial animals (such as chickens) that is fed to the shrimp, he reported. “Nobody can know where this leads,” he observed.
Agricultural and aquacultural systems for raising food animals are vertically integrated, Wegener explained. As illustrated in Figure WO-19, “just a few thousand animals in the top of a breeding pyramid become trillions of eggs or trillions of broilers or trillions of slaughter pigs at the bottom of this pyramid,” he said. “That is what has happened with Salmonella Enteritidis, where the unwillingness to sacrifice a few thousand pedigree birds led to millions and millions of human cases. These things have to be addressed from the top down if we want to really control them.”
In Denmark, this was accomplished by instituting serological surveillance of egg producers in 1997, an intervention Wegener described as simple and inexpensive. Flocks found positive for the pathogen are either culled and repopulated, or they are used solely to produce heat-processed eggs. After these practices were instituted, along with a similar program of surveillance and eradication of infected broiler flocks, Denmark experienced a significant decline in human Salmonella infections. However, he noted, these results would not have been so effective if Denmark imported more meat and eggs or breeding poultry and livestock, which may also carry pathogenic bacteria and viruses, including those that are drug-resistant.
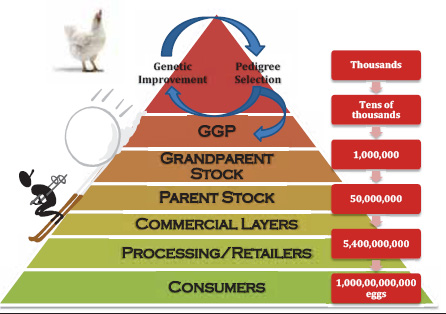
FIGURE WO-19 Pyramids and snowball effects.
SOURCE: Wegener (2011).
Additional contributors to the threat of food-borne AMR pathogens include the use of critical human antibiotics for animal therapy and growth promotion and the overuse of all antimicrobials—encouraged by their easy acquisition by livestock producers, as well as by the significant profit veterinarians receive from selling antimicrobials, Wegener said.
Integrated surveillance for AMR Building on the success of surveillance to reduce the incidence of salmonellosis, and out of concern for the increasing emergence of resistant strains of Salmonella and Enterococci, Denmark instituted an integrated surveillance system for AMR, called DANMAP (DANMAP, 2012; Hammerum et al., 2007), along with a complementary surveillance program for antimicrobial usage (Rodo et al., 2011), called VETSTAT (Stege et al., 2003). DANMAP monitors antimicrobial resistance, through systematic sampling and testing of bacterial isolates, from humans, food, and food animals. It includes human and animal pathogens, as well as indicator bacteria. The results are published annually in a report, which can be found online, according to Wegener. A schematic of surveillance inputs to DANMAP is illustrated in Figure WO-20.
The part of DANMAP that monitors antimicrobial usage in animals is called the VETSTAT program. Started in the year 2000, it monitors the use of prescribed antimicrobials in animals at a very detailed level. According to Wegener, for each record in the database, VETSTAT has information on:
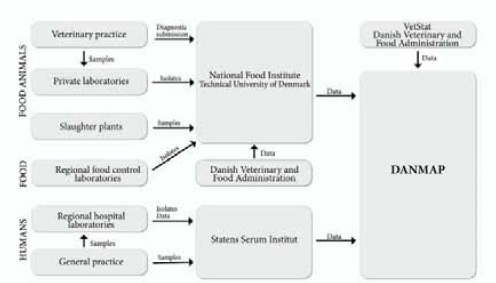
SOURCE: DANMAP, Danish Integrated Antimicrobial Resistance Monitoring and Research Program; National Food Institute, Technical University of Denmark (DTU); Wegener (2011).
• farm ID
• animal species
• age group
• date
• drug ID
• drug quantity
• disease category
• prescriber ID
Antimicrobial usage data collected by VETSTAT also supports Denmark’s “yellow card” system, which identifies high-usage swine producers, warns their veterinarians, and encourages reduction in usage within a 9-month period. “If they don’t do that, they may get a visit from the district veterinarian’s office,” Wegener explained; however, there are no defined consequences for ignoring the “yellow card.” Nevertheless, he said, this measure has been associated with a 20 percent reduction in antibiotic use since it was instituted in 2010. Efforts are under way to implement integrative surveillance of AMR and antimicrobial usage throughout Europe and also at the global level, through the WHO’s Advisory Group on Integrated Surveillance of Antimicrobial Resistance,24 he reported.
Based on his experience with DANMAP and VETSTAT, and in recognition
![]()
24http://www.who.int/foodborne_disease/resistance/agisar/en/.
of the challenges of extending Denmark’s success to the regional and global levels, Wegener suggested that systems for the integrated surveillance of AMR meet the following criteria:
• systematic sampling, harmonized laboratory methods, and good data management;
• detailed information on pathogen sample origin and antimicrobial usage;
• sub-typing-of bacterial isolates and molecular characterization of resistance genes;
• collaboration and coordination among all parties, including data sharing and comparison; and
• establishment of a solid basis for further detailed investigation of specific questions.
Upstream interventions for AMR Wegener described a range of strategies Denmark has implemented to reduce the emergence and spread of AMR through the food chain (Aarestrup et al., 2008; Wegener, 2006). In 1995, the country passed legislation to limit profits to veterinarians on the sale of antimicrobials to 5 percent of their cost, Wegener stated. At the same time, routine prophylaxis—the use of antimicrobials to treat animal flocks without a disease history—was also outlawed. During the year after these changes were made, veterinary antibiotic use in Denmark declined by more than 30 percent, he reported (Wegener, 2006).
Additional voluntary actions further limited antimicrobial use in Danish food animals. In 1999, Danish swine producers voluntarily terminated the use of in-feed fluoroquinolones. Despite the voluntary termination of the use of this class of antibiotics, Wegener reported, usage of the drug slowly increased thereafter. To address this problem, in 2002, veterinarians were barred from administering fluoroquinolones to animals unless no alternative treatment existed. This requirement effectively reduced fluoroquinolone usage and, with it, the frequency of fluoroquinolone-resistant E. coli in humans, he said. Despite the loss of several growth-promoting antimicrobials over the past two decades, Wegener noted that the Danish swine industry has experienced increased productivity over this period (Aarestrup et al., 2010). More recently, Danish swine and cattle producers have voluntarily agreed to eliminate the use of cephalosporins. The effects of this change on both human and animal health have yet to be determined.
The Danish food animals consume less than 20 percent of the amount of antimicrobials used by U.S. producers to yield the same amount of meat, Wegener stated. Organic meat producers in Denmark use a further 10-fold less than conventional ones, at an apparent productivity loss of only 10 percent. “Maybe if you had spent as much science on improving a production system like this, we could have 100 percent productivity, but make do with one-tenth of the antibiotics,” he speculated.
Adopting—or ignoring—the Danish model In the discussion session that followed Wegener’s presentation, Forum member Jeffrey Duchin, of the University of Washington, noted that the documented success of restrictions on the use of antibiotics in poultry and livestock in Denmark (as well as in other countries) has done little to influence U.S. policies. “It makes me a little bit cynical about our ability to take action on other food safety issues, when we … [fail to act on evidence] … that is as far advanced as the data and the research on anti microbial drug resistance,” he confessed. This situation may be changing. In a recent development, a federal court judge in late March 2012 ordered the FDA to take action on its own 35-year-old rule that would stop farmers from mixing popular antibiotics into animal feed, a practice which is widely believed to have led to a surge in dangerous, drug-resistant bacteria (Perrone, 2012).
“I think the lesson from my own country is that you can never have complete evidence of anything, and at some point in time, you should intervene and then learn,” Wegener observed. “For many of the interventions that we have done, we had no evidence that they would work before we intervened. We intervened based on, say, best scientific evidence and common microbiology sense. Then you have a huge experiment with that entire production system, and you evaluate it and you change your program or your policies if you find out that it’s not working as you expected.” These kinds of interventions are more possible in Denmark because the scientific culture is far less polarized than in the United States, he added. “We don’t really see industry and government as being opposites and in opposition to each other. It is more based on a culture of agreeing to a common problem and then trying to agree to a solution and then moving along.”
Although Wegener stated that these decisions were made primarily to satisfy risk-averse Danish consumers, Hueston observed that Denmark “made a decision decades ago to focus on their export market, as they should.” Daszak added that Denmark’s policies had allowed it to gain a market-share advantage—a strategy that would not be as effective in the United States, which does not export the majority of the meat it produces.
Within the United States, much of the discussion of the use of antibiotics in animals has been limited to two alternatives—bans or unrestricted use—despite the fact that a myriad of options exist for managing the associated risk, Hueston asserted. “There is a need for antibiotics, but we don’t need to misuse and overuse,” Wegener agreed. “I would just like to see concerted movements toward trying to find out how low we can go,” he continued, and he encouraged Europe and the United States to agree to a strategy of reduction in the use of antibiotics that does not compromise productivity—and to document their progress so as to influence future policy in the rapidly expanding markets of Southeast Asia, China, and Africa.
Approaches to Food-Borne Disease Surveillance, Detection, and Response
A central tenet of a One Health approach is its focus “upstream” of disease outbreaks, ideally in order to prevent them from occurring in the first place. When that is not possible, however, the same perspective may enable researchers to anticipate disease emergence and to detect outbreaks as early as possible. Workshop participants discussed several such efforts, including computer-generated predictions of “hot spots” for disease emergence, the development of digital epidemiology methods for early outbreak detection, the use of sequence information to identify novel pathogens, and the implementation of test-and-hold strategies to detect food-borne contamination before it reaches consumers.
Predicting Food-Borne Disease Emergence
Nipah and avian influenza outbreaks Although neither the timing nor the pathogen type involved in food-borne disease events can be accurately predicted, it is increasingly possible to identify likely outbreak scenarios that can be used to target surveillance efforts for specific food-borne diseases, as well as for food-borne disease in general, Daszak observed. Returning to the emergence of Nipah virus in Malaysia, Daszak described how he and his colleagues used a One Health approach to analyze the livestock production system and surrounding ecosystem in order to understand how the outbreak happened. After unraveling the story of the virus-carrying bats messily eating mangoes among the pigsties, the researchers attempted to determine why the outbreak occurred when and where it did.
Nipah virus has probably been circulating in bats for millions of years, Daszak said, so why did it suddenly emerge on this particular pig farm in 1999? To answer this question, the researchers constructed a mathematical model to simulate transmission dynamics of the virus within the index farm’s pig population, based on evidence that the virus was introduced repeatedly to pigs there over a 2-year period prior to the human outbreak (Pulliam et al., 2012). The result suggests that Nipah emerged in Malaysia when the intensive farming of pigs reached a certain threshold, with “conditions that allowed the virus to keep ticking over,” he explained. In this model, it was assumed that the index farm was not biosecure, he continued, and that bats repeatedly visited, allowing the virus to be reintroduced multiple times into the pig population. The particular, highly compartmentalized structure of the farm created the perfect condition for Nipah to exist.
“Using computer models allows you to re-create epidemics and, to some extent, predict issues around future emergence of that pathogen,” Daszak observed. Based on their findings, the investigators advised the Malaysian government on ways to reduce the risk of future human Nipah outbreaks associated with pig farms. One is to avoid raising pigs near fruit trees of species that are particularly attractive to bats, which include mango, durian, and papaya, he said; the other is
to optimize farm size. “What happened with the outbreak was that the virus got into a really big, highly structured farm and persisted. Then, when people started dying … everybody started to sell the pigs. They sold them to the fattening farms … ready to send on to Singapore. That’s when the outbreak expanded,” he explained, because in these small farms more people were exposed to each infected animal. Under those circumstances, he concluded, “as your farm size decreases, the risk of getting infected actually increases.”
Taking a similar approach, Daszak and colleagues modeled the transmission dynamics for highly pathogenic avian influenza (HPAI) to identify potential hot spots for outbreaks. Previous studies suggested that the practice of double-cropping rice, which attracts HPAI-carrying ducks to the fields to feed throughout the year, increases the potential for viral crossover into pigs and, eventually, to humans (Martin et al., 2011). Daszak and coworkers attempted to build on this work to understand the role of farm size and connectivity as risk factors for avian influenza transmission. Using an outbreak simulation model, as depicted in the cartoon in Figure WO-21, they found that HPAI viral introductions to small “backyard” farms posed a relatively high risk for human infection; however, as farm size increases, outbreaks that do occur last longer.
“The worst-case scenario for avian influenza to persist is when you have a mixture of backyard and large-scale farming,” Daszak observed. Although very
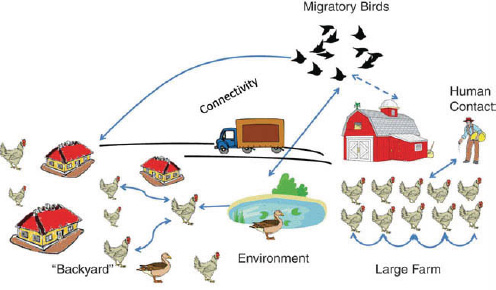
FIGURE WO-21 A schematic representation of how farm size can affect risk of avian influenza emergence. Highly pathogenic avian influenza has trouble persisting on large farms, where it is rapidly noticed and birds culled. Mathematical models show that the virus persists much better when both large and small farms co-exist.
SOURCE: Image provided courtesy of L. Mendiola and P.R. Hosseini, EcoHealth Alliance.
large farms tend to be more biosecure than backyard operations, the virus seems able to circulate between large- and small-sized farms, persisting longer than if only one farm system existed. Thus, he concluded, a mixture of backyard farms with less-than-secure large farms raises the risk for human transmission of HPAI.
Identifying food-borne disease hotspots Daszak and colleagues developed a database of every infectious disease that has emerged over the past five decades in order to develop more generalized rules for predicting where infectious diseases are likely to emerge (Jones et al., 2008). He reported that a significant proportion of these diseases are driven by food-borne transmission, and that most were caused by bacteria; however, the number of viral emerging diseases, particularly zoonoses, has increased in recent years. The main drivers associated with food-borne diseases are increasing technology and industry, travel, commerce, and human susceptibility, he said.
“When we know all the drivers of food-borne infections, we can map those out spatially, analyze the presence or absence of prior outbreaks, and try and get at the map of where food-borne pathogens will emerge in the future,” Daszak continued. As illustrated in Figure WO-22, hotspots for food-borne pathogen emergence are concentrated in the tropics, including the increasingly population-dense areas of South and Southeast Asia, and also in parts of Europe and North America. When an additional driver of infectious disease emergence, land-use change—a proxy for broad-scale deforestation and agricultural development—is incorporated into this analysis, “parts of Latin America light up and parts of Southeast Asia become less important, where land-use change has already had its impact,” he observed. Further, if travel and trade out of hotspot areas are taken into consideration, he said, “what you see is an incredible risk from the rapidly developing areas of the planet, where there’s a lot of export, a lot of import. If you follow the trade routes, it all points to the developed countries that import these products.” This knowledge can help focus surveillance efforts, he added.
The EcoHealth Alliance, through a collaboration with the university of California, Davis, and the U.S. Agency for International Development (USAID) called Deep Forest,25 is analyzing the effects of deforestation and agricultural development in three regions: the Brazilian Amazon near Manaus, the Bwindi Impenetrable Forest in Uganda, and the Maliau “Lost” Basin and Kinabatangan River in Borneo. Within these areas, each of which encompasses a gradient ranging from primary forest to rural farmland to urban landscape, researchers will attempt—through a combination of metagenomic techniques and interviews
![]()
25 Launched in 2011, Project Deep Forest builds on EcoHealth Alliance scientists’ work, which shows that deforestation threatens global health by leading to the emergence and spread of new diseases. Initially the project focuses on the tropical forests of Brazil, Uganda, and Malaysia, identifying health threats to people and wildlife in the communities closest to these forests, and working to prevent their possible spread to nearby urban areas and ultimately around the world. http://www.ecohealthalliance.org/writable/publications/annual_gala_invitation_2012.pdf (accessed April 3, 2012).
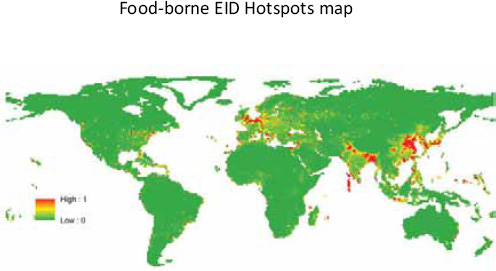
Relative risk of food-borne EID events, based on Jones et al. (2008). Human population density and human population growth are the most important variables.
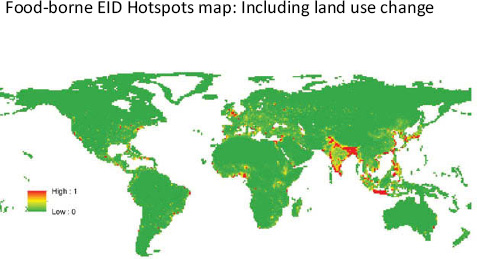
Relative risk of food-borne EID events, additional drivers included the change in area of pasture and crop between 1900 – 2000 (modified from Jones et al., 2008).
Human population density, mammal diversity and the land use change were the most important variables.
FIGURE WO-22 Hotspots for food-borne pathogen emergence.
SOURCE: Jones et al. (2008).
of residents about their interactions with wildlife—to measure both the number of unknown pathogens of interest carried by representative wildlife species and the amount of human-animal contact, Daszak said. Using that information, the researchers hope to answer the following questions:
• How many viruses are out there?
• What animals carry them?
• What is the risk of them emerging?
• How can we stop them from emerging?
• Can we use the health angle to reduce deforestation?
The ultimate goal of the Deep Forest project is to elucidate precisely how agricultural change drives disease emergence, Daszak stated. “Once we have done that, we can start to work with local people and say [to them], ‘you’re at risk of a new, emerging disease. There are ways you can change behavior … that are cheap, cost-effective, and will save your health, and you can still make money,’” he concluded.
Wildlife and Food-Borne Disease
Wildlife are known to transmit a variety of food-borne diseases to humans through multiple routes, the most direct of which is through human consumption of wild animals, speaker William Karesh, also of EcoHealth Alliance, observed. (Dr. Karesh’s contribution to the workshop summary report can be found in Appendix A, pages 207-217.) While this description conjures images of hunters eating wild prey—a focus of Karesh’s work in wildlife conservation and ecology—he also reminded the audience that nearly half of all seafood consumed is wild caught and therefore fits the description of “wildlife.” Seafood has been characterized as an important source of emerging food-borne diseases (Broglia and Kapel, 2011; Nawa et al., 2005).
Karesh and his colleagues have long engaged hunters around the globe to participate in the surveillance of wildlife for infectious diseases. Before wildlife-associated emerging infectious diseases such as SARS, monkeypox, or avian influenza commanded headlines, EcoHealth Alliance was examining connections between wildlife and infectious diseases in such settings as logging camps and bush meat markets, he said. People in logging camps, with populations numbering into the thousands, are essentially hunter-gatherers who must obtain all of their food from the surrounding forest. Bush meat markets, a common food supply system for much of the world, yield approximately 1 billion kilograms of meat per year in central Africa alone, according to Karesh. The mass culling of wildlife is not a sustainable system for providing food, he insisted; therefore, strategies to replace these practices could simultaneously reduce the risk of food-borne disease and conserve wildlife.
Using the case of the emergence of monkeypox26 in the United States as an example, Karesh noted that food insecurity in one part of a world linked by global trade can prefigure outbreaks of infectious diseases in places where food is plentiful. Thus, he concluded, we should be concerned about food safety everywhere and investigate food-borne diseases wherever they occur. In Africa, where EcoHealth Alliance has engaged with hunters and their communities to assist in the surveillance of emerging infectious disesases, the organization has also worked to educate people about food safety. “You work with the hunters and tell them what’s safe to hunt and what’s not safe to hunt, what should they bring back to the village, what not to bring back, to cook your food and wash your hands,” he explained. “You work with the suppliers and the consumers.”
These efforts may have paid off, as there have been no human cases of Ebola hemorrhagic fever in northern Congo since 2005, Karesh stated, despite the fact that the disease continues to circulate in wildlife in that region. He attributed this success to the development of an “honest, multi-stakeholder dialogue” as a foundation for intervention. Karesh described additional EcoHealth Alliance projects that reflect this same approach and alignment with the One Health paradigm. These include collaboration with the CDC to identify pathogens present in bush meat imported into the United States in order to begin to estimate associated risks for infectious disease.
A similar effort is needed to estimate the volume of illegal wildlife (e.g., exotic pets) imported into the United States, and their associated risks, he continued (Karesh et al., 2005). “There is no financial support for CDC or USDA or FDA to really do inspections,” Karesh observed. “It’s very hard to do a risk analysis when you don’t have any data to do the analysis on, but we do see this stuff coming in every day. It’s probably a threat to livestock. It’s probably a threat to human health. It’s certainly a threat to wildlife because it’s depleting wildlife resources. Probably one of the biggest threats to wildlife conservation left in the world today, [along with] habitat destruction … [is] the illegal wildlife trade.” In the meantime, EcoHealth Alliance works to educate consumers about how to pick a safe pet, through their PetWatch website.27
EcoHealth Alliance also participates in a project sponsored by USAID known as PREDICT (USAID, 2009), which seeks to improve monitoring of infectious disease emergence in wildlife. Its strategies include a range of efforts to better target infectious disease surveillance, such as geospatial risk modeling (Jones et al., 2008), determining routes of transmission, identifying animal species most likely to transmit infectious diseases, and conducting Internet surveillance for outbreak cues. PREDICT is also building surveillance capacity in hotspots for disease emergence to increase the possibility of early detection and effective containment.
![]()
26 Humans initially acquired monkeypox when Africans consumed infected rats, having nothing else to eat, Karesh stated. Monkeypox was introduced into the United States in 2003, in a shipment of small mammals from Ghana intended for sale as exotic pets (CDC, 2008).
As Karesh noted, all of these strategies are applicable to food-borne diseases, as a subset of emerging infectious diseases (Cardoen et al., 2009).
Detecting Viral Outbreaks
Speaker Nathan Wolfe, of the Global Virus Forecasting Initiative (GVFI) and Stanford University, invoked John Snow’s map of the 1854 London cholera epidemic (UCLA Department of Epidemiology, 2012), the “ghost map” reproduced in Figure WO-23, which led Snow to conclude that cholera was a water-borne disease, and thereby to the means to stop its deadly spread. (Dr. Wolfe’s contribution to the workshop summary report can be found in Appendix A, pages 349-362.) Among computer scientists, that map is known as the first geographic information system, and despite the technological advances that have occurred
FIGURE WO-23 John Snow’s map of the 1854 cholera epidemic in London.
SOURCE: Cheffins (1854).
since then, epidemiologists continue to investigate outbreaks much as Snow did, Wolfe observed. Therefore, he continued, when considering novel ways to detect food-borne diseases, “it’s worth our asking ourselves, if John Snow was in the audience today, how would he be thinking about these problems?”
Wolfe proceeded to describe several innovative approaches to detecting the emergence of infectious diseases, focusing on food-borne viral outbreaks. Viruses frequently jump from wild animals to domesticated animals and human hosts, but only by degrees, and often unsuccessfully—as illustrated in Figure WO-24—a phenomenon he referred to as “viral chatter.” Only occasionally do viruses become exclusively adapted to a human host, he said, but until recently, these relatively rare events have commanded researchers’ attention and have prevented them from understanding—and therefore predicting—how infectious diseases emerge. Consequently, for the past decade, GVFI has focused on human populations most exposed to wild animals, such as people living in central Africa, where HIV is thought to have emerged—as a food-borne illness, he asserted. “This is definitely a virus which was associated with the hunting and butchering of chimpanzees and the contact of food handlers with these sorts of viruses,” he explained. “Whenever we are going to have an interface with this sort of diversity
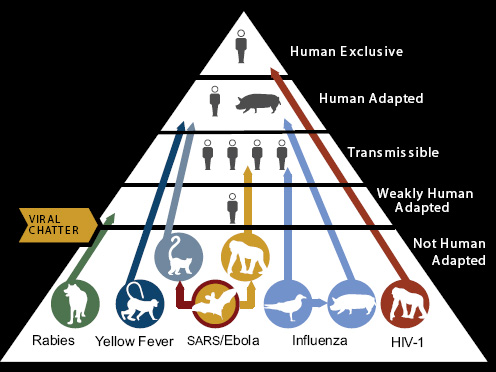
FIGURE WO-24 Human viruses have animal origins.
SOURCES: Wolfe (2011); adapted from Wolfe et al. (2007).
of animal viruses, we are going to have the opportunity for these new viruses to enter into human populations and spread.”
In order to monitor the transition of viruses from animals into humans at the interface between their populations, Wolfe said that GVFI works with partners to establish collection sites and collaborations in many parts of the world; this includes the creation of laboratories, as well as training programs to staff these facilities, throughout central Africa and parts of Asia. “The basic idea is to move pathogen discovery from … ivory tower laboratories to places where we have this biological diversity,” he explained. For example, he said, they have enlisted volunteers who have high levels of contact with the blood and body fluids of wild animals, such as hunters and workers in wet markets, to spot filter paper with samples of their own blood, as well as blood from the animals with which they come into contact.
GVFI has amassed a large collection of these specimens from humans and more than 140 animal species, Wolfe reported. “In the last twelve years we’ve assembled some of the most comprehensive sample sets of human [>120,000] and animal [>60,000] blood spot collections in the world,” he stated. These resources allow the researchers to monitor the flow of viruses into the human population, and even to witness the moment at which an outbreak is born, he said; for example, they were able to detect the crossing of simian foamy viruses (SFVs) from gorillas and mandrills into the humans who hunt and butcher them (Wolfe et al., 2004). Daszak also made note of these retroviruses, observing that they “could be the next HIV, coming in through the food system just like HIV did.”
Wolfe and colleagues conducted similar analyses of human T-lymphotropic viruses (HTLVs) types 1 and 2, which originated independently and are related to simian T-lymphotropic viruses STLV-1 and STLV-2, respectively (Wolfe et al., 2005). HTLV-1 and HTLV-2 are pandemic viruses that infect between 5 million and 20 million people worldwide, causing severe disease in a percentage of those individuals, he reported. They found that central Africans who had contact with the blood and body fluids of non-human primates (e.g., through hunting, butchering, and pets) were infected with a wide variety of HTLVs, including two previously unknown retroviruses (HTLV-3 and HTLV-4). In addition to revealing new levels of HTLV diversity and suggesting that human exposure to non-human primates contributes to HTLV emergence, these findings also indicate that cross-species transmission is not the rate-limiting step in pandemic retrovirus emergence; rather, they show that it may be possible to predict and prevent disease emergence by surveillance of populations exposed to animal reservoirs, as well as through interventions to reduce human exposure to non-human primates.
GVFI has also been involved in a collaborative effort in the Democratic Republic of the Congo (DRC) to study the transmission of human monkeypox virus (Rimoin et al., 2010). Thirty years after mass smallpox vaccination campaigns ceased, rendering the population increasingly immunologically naïve to orthopoxviruses including monkeypox virus, the incidence of monkeypox
infection has dramatically increased in rural areas of the DRC. Using filter paper—based blood samples from people exposed to wildlife through hunting or meat-handling, as previously described, GVFI researchers will be able to determine whether monkeypox virus emerged in a similar fashion to SFV and the HTLVs.
In addition to their retrospective analyses of food-borne disease emergence, GVFI investigators are exploring the detection of outbreaks in “real time” through the application of digital epidemiology. The global expansion of cellular phone usage and social networks is producing a wealth of data that can be mined for both content and location, Wolfe observed; this is the basis for GVFI’s EpidemicIQ project, which queries a variety of open-source and proprietary data feeds (e.g., blogs, Twitter feeds) to detect patterns of illness in their content and combine this with geolocation. These methods are capable of detecting outbreaks well before they are announced, he continued; they can also permit estimation of the outbreak’s impact and identification of risk factors associated with infection.
Digital epidemiology transcends the division between epidemiological and microbiological investigation of food-borne outbreaks, Wolfe observed. This approach has the potential to detect infectious outbreaks early, based on sequence data that represent both host susceptibility and the genetic diversity of various populations of microorganisms, and on indirect information gleaned from data sources, in order to prevent or limit their spread.
Microbe Hunting
Advances in technology are rapidly expanding the viral sequence database. According to speaker W. Ian Lipkin, of Columbia University, the vast majority of the estimated 1 million viruses carried by vertebrate animals have yet to be identified (Morse, 1993). (Dr. Lipkin’s contribution to the workshop summary report can be found in Appendix A, pages 251-271.) Thus, a major challenge facing today’s microbe hunters is the need to discriminate among this vast array of potential pathogens—in Lipkin’s case, viruses—to identify the causative agent of a specific outbreak. Researchers can take a number of different approaches to pathogen identification Lipkin noted; these are summarized in Figure WO-25 (Lipkin, 2010).
Classical methods involve culturing the pathogen, a critical step in fulfilling Koch’s postulates,28 while molecular techniques for detecting pathogen sequences—which have provided a wealth of information—frequently lead to the discovery of microbes that cannot be propagated in vitro, or for which no animal model systems have been developed, he observed. Lipkin presented numerous
![]()
28 Koch’s postulates, which establish the causal relationship between pathogen and disease, stipulate that the pathogen is present in every case of the disease, specific for that disease, and can be propagated in culture and capable of replicating the original disease upon inoculation into a naïve host (Koch, 1891).
FIGURE WO-25 A staged strategy for pathogen discovery.
SOURCE: Microbiology and Molecular Biology Reviews, 2010, 363-377, doi: 10.1128/MMBR.00007-10 and reproduced with permission from the American Society for Microbiology.
illustrative examples, gleaned from his own experience, of the application of a range of molecular methods for pathogen discovery, described in Box WO-4, to the investigation of infectious outbreaks that appear to be caused by novel infectious agents.
In one case, when Lipkin and colleagues used MassTag PCR (see Box WO-4) to investigate an outbreak of influenza-like illness in New York State, they discovered a novel rhinovirus was the likely cause of this outbreak (Lamson et al., 2006). Follow-on studies implicated these viruses not only in influenza-like illnesses but also in asthma, pediatric pneumonia, and otitis media. He noted that a similar study implicated rhinoviruses and enteroviruses as the cause of influenzalike illness during the summer months in New York City at the time that H1N1 was circulating (Tokarz et al., 2011); another determined that high case fatality
BOX WO-4
Molecular Methods for Pathogen Discovery
Singleplex Assays
The most common singleplex assays employed in clinical microbiology and microbial surveillance are polymerase chain reaction (PCR) assays, wherein DNA strand replication results in either the cleavage or release of a fluorescence-labeled oligonucleotide probe bound to a sequence between the forward and reverse primers. Nested PCR, in which two amplification reactions are pursued sequentially with either one (hemi-nested) or two (fully nested) primers located 3’ with respect to the original primer set, may be more sensitive than fluorescent reporter dye singleplex assays. However, because the original reaction vessels must be opened to add reagents for the second, nested reaction, the risk for contamination is high, even in laboratories with scrupulous experimental hygiene.
Multiplex Assays
Signs and symptoms of disease are rarely suggestive of a single agent, particularly early in the course of an illness. Multiplex assays may be helpful in such situations because they may be used to entertain many hypotheses simultaneously. The number of candidates considered ranges from 10 to 100 with multiplex PCR, to thousands with microarrays, to the entire tree of life with unbiased high-throughput sequencing. In multiplex assays many genetic targets compete for assay components (e.g., nucleotides, polymerases, and dyes), in some instances with variable efficiencies. Thus, multiplex assays tend to be less sensitive than singleplex assays.
Multiplex PCR Assays
Gel-based multiplex PCR assays, wherein products are distinguished by mass, can detect as many as 10 distinct targets. Two platforms that combine PCR and mass spectroscopy (MS) for the sensitive, simultaneous detection of several targets have been established. The Ibis T5000 biosensor system uses matrix-assisted laser desorptionionization MS to directly measure the molecular weights of PCR products obtained in an experimental sample and to compare them with a database of known or predicted product weights. MassTag PCR uses atmospheric pressure chemical ionization MS to detect molecular weight reporter tags attached to PCR primers. Syndrome-specific MassTag PCR panels have been established for the detection of viruses, bacteria, fungi, and parasites associated with acute
associated with H1N1 in Argentina was not caused by a more virulent influenza virus, but by co-infection with Streptococcus pneumoniae (Palacios et al., 2009). These findings illustrate the potential pitfalls in prematurely narrowing an outbreak investigation to one pathogen, or even a single class of pathogens, he observed.
Sequencing technologies to identify potential pathogens are fast, inexpensive,
respiratory diseases, diarrheas, encephalitides/meningitides, and hemorrhagic fevers. The Bio-Plex (also known as Luminex) platform employs flow cytometry to detect PCR amplification products bound to matching oligonucleotides on fluorescent beads. Assay panels that allow the detection of up to 50 genetic targets simultaneously have been developed.
Microarrays
Microarray technology runs the gamut from assays that comprise hundreds to those comprising millions of probes. Probes can be designed to discriminate differences in sequence that allow virus speciation or to detect thousands of agents across the tree of life. Arrays comprising longer probes (e.g., >60 nt) are more tolerant of sequence mismatches and may detect agents that have only modest similarity to those already known. Two longer probe array platforms are in common use: the GreeneChip and the Virochip. Although they differ in design, both employ random amplification strategies to allow an unbiased detection of microbial targets.
Unbiased High-Throughput Sequencing
The power of unbiased high-throughput sequencing has enabled unique advances in microbial surveillance and discovery. Applications include metagenomic characterization of environmental and clinical samples, rapid and comprehensive sequence analysis of microbial strains and isolates, and pathogen discovery. Unlike cPCR or array methods, whereby investigators are limited by known sequence information and must choose the pathogens to be considered in an experiment, high-throughput sequencing can be unbiased and allow an opportunity to inventory the entire tree of life.
After amplification and sequencing, raw sequence reads are clustered into non-redundant sequence sets. Unique sequence reads are assembled into contiguous sequences, which are then compared to databases using programs that examine homology at the nucleotide and amino acid levels using all six potential reading frames. However, because a truly novel pathogen might elude this level of analysis, researchers are exploring ways in which insights into the identity of agents may be determined by features such as nucleotide composition or predicted secondary or tertiary structures.
SOURCE: Excerpted from Microbiology and Molecular Biology Reviews, 2010, 363-377, doi: 10.1128/MMBR.00007-10 and reproduced with permission from the American Society for Microbiology.
and likely to become increasingly so, which begs the question of how best to analyze the volumes of data these methods generate, Lipkin stated. “The traditional methods that people use are alignment-based strategies,” he explained. “You look for similarities between what you’ve found and what is known at the nucleotide level and at the protein level.” Other approaches to investigating virus-like
sequences not identifiable through alignment include nucleotide composition and order analysis—methods Lipkin likened to cryptography—to identify sequences as belonging to a specific viral genus or family or to infer the infected host species. For example, he said, viral sequences obtained from fecal material may originate from humans, animals or plants consumed by humans, or even from plant material eaten by an animal that the human later consumed. Sometimes, however, putative outbreaks turn out to be caused by non-infectious agents, Lipkin reported. One such event occurred among workers at a pork processing plant who developed severe peripheral neuropathy (Holzbauer et al., 2010). All of the affected workers, whose task it was to extract pig brains with high-pressure air hoses, were not protected from exposure to brain tissue by facemasks or skin covering, he said. However, because the disease did not spread beyond these workers, it seemed unlikely to be infectious; eventually, it was confirmed that the workers were suffering from a previously described autoimmune reaction, not an infectious disease, he reported.
Another episode of pathogen “de-discovery,” described in detail in a recent review by Lipkin, involved evidence that contributed to discrediting a proposed causal relationship between the measles, mumps, and rubella (MMR) vaccine and the development of autism (Hornig et al., 2008). According to the discredited model, measles virus present in the vaccine was hypothesized to alter the permeability of the intestinal lumen to neuroactive molecules that passed, via the circulatory system, to the brain; however, Lipkin and coworkers found little evidence for the presence of measles virus RNA in the intestinal lumen of children with autism and gastrointestinal disturbances (case) or gastrointestinal disturbances alone (control), and no differences between case and control groups. They did discover intriguing differences between children with autism and gastrointestinal dysfunction, and children with gastrointestinal dysfunction alone, in levels of enzymes that break down complex sugars and of transporters that carry simple sugars from the lumen of the intestine into the systemic circulation. These differences were associated with differences in the microflora in the intestines of the two groups of children that may prove to be clinically significant.
Lipkin also provided a brief review of a number of novel viruses his group has recently identified:
• The first filovirus found to be endemic to Europe (Negredo et al., 2011). The virus is an intermediate between the Marburg and Ebola viruses, which suggests that many more filoviruses have yet to be discovered. The virus’s host is a widely distributed bat species, Miniopterus schreibersii.
• Canine hepatitis virus, also present in horses, the closest relative to human hepatitis C virus, which infects more than 200 million people worldwide (Kapoor et al., 2011).
• An influenza virus that infected and killed ringed seals in Alaska (Nolen, 2011) and New England (NOAA, 2011a) in 2011. The H3N8 influenza virus had previously been isolated from dogs and birds, and was appar-
ently transmitted from marine birds to seals, Lipkin said (NOAA, 2011b). It has the potential to jump to domestic animals and perhaps, even to humans, he added.
One Health Approaches to Food System Biosecurity
The number of food-borne disease outbreaks is increasing in frequency, yet the myriad approaches to food safety used around the world make it difficult to implement a unified, risk-based approach to managing and controlling these hazards (Coker et al., 2011; Karesh et al., 2005). Food and agricultural systems have become so complex and extensive in size that food safety hazards have the potential to cause extensive and far-reaching damage to human and animal health. The changing nature of food-borne pathogens further compounds efforts to keep pace with this “wicked problem.” The complexity of maintaining food system biosecurity also makes it a natural place to apply a One Health approach, which focuses on upstream factors such as animal health and ecological disturbances (Figure WO-26).
The global food system depends on the ability to safely trade food-related goods and services. Food and water are major pathways for the introduction and spread of emerging and reemerging infectious diseases (World Bank, 2010). Ensuring their health is a means of maintaining a safe and adequate food supply, but it requires expertise from multiple disciplines in order to comprehensively evaluate current approaches to managing and preventing disease outbreaks. The complex nature of the global food supply chain thus increases the importance of a cross-disciplinary approach to examining food-borne zoonoses (e.g., Salmonella, E. coli, etc.) linked to livestock production (Coker et al., 2011). Disease outbreaks in animals and contamination events erode confidence in international trade while simultaneously exerting economic consequences associated with reporting adverse health events linked to a food item.
While globalization has increased the need for efficient, effective, coordinated, and comprehensive responses to zoonotic diseases, food-borne disease outbreaks, and detrimental changes to the environment, stakeholders in relevant sectors have continued to operate in relative isolation without considering the obvious links that One Health underscores (Figure WO-27). Rapid disease transmission across borders and between humans and animals has ramifications for health, international trade, international development, and the global economy (WHO, 2011). The past outbreaks of H5N1 and H1N1 influenza viruses were important events that helped focus international attention on One Health. The lessons learned from these epidemics have applications for One Health to improve food safety, including the need for integrated microbial surveillance across health domains, readily sharing information and data including the private sector, and building the capacity and infrastructures for both public and animal health.
Several workshop speakers discussed the important implications of a One
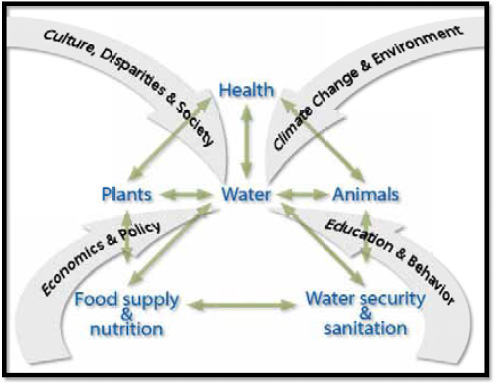
SOURCE: University of California Global Health Institute (http://www.ucghi.universityofcalifornia.edu/images/one-health-chart.png).
Health paradigm for food system biosecurity. Their presentations focused on the essential role of surveillance in understanding the relationships between food-borne diseases and ecosystems, and in using that knowledge to anticipate, detect, and respond to risk in a range of different contexts. Such complex efforts demand the involvement and coordination of multiple stakeholders—a challenge that is only beginning to be met, and one that ultimately will require organizational and institutional changes.
One Health in Australia: The Biosecurity Continuum
While acknowledging that common factors drive the emergence of infectious diseases, including those that are food-borne, speaker Martyn Jeggo, director of the Commonwealth Scientific and Industrial Research Organisation (CSIRO)29 Austra-
![]()
29 CSIRO is Australia’s national science agency.
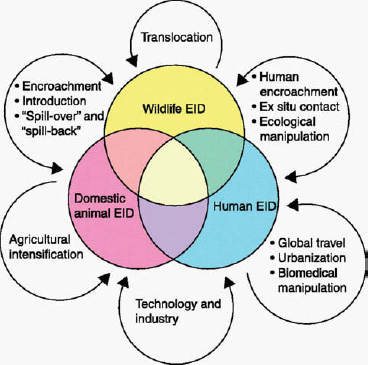
FIGURE WO-27 The “host—parasite” continuum. The host—parasite ecological continuum (in this context “parasites” include viruses and parasitic prokaryotes). Most emerging diseases exist within a host and parasite continuum between wildlife, domestic animal, and human populations. Few diseases affect exclusively any one group, and the complex relations between host populations set the scene for disease emergence. Examples of emerging infectious diseases that overlap these categories are canine distemper (domestic animals to wildlife), Lyme disease (wildlife to humans), cat scratch fever (domestic animals to humans), and rabies (all three categories). Arrows denote some of the key factors driving disease emergence.
SOURCE: From Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2000. Emerging infectious diseases of wildlife—Threats to biodiversity and human health. Science 287(21):443-449. Reprinted with permission from AAAS. http://www.sciencemag.org/content/287/5452/443.full.html.
lian Animal Health Laboratory, noted that food safety tends to be managed quite differently from infectious disease control in Australia (and indeed throughout the word), and that these differences are likely to persist. (Dr. Jeggo’s contribution to the workshop summary report can be found in Appendix A, pages 198-206.) “From an infectious disease point of view we’re concerned with the effect of the disease on the host [which may be an animal or plant], whereas from a food safety point of view we’re primarily concerned with the impact [of adulterated food] on humans,” he noted. Nevertheless, Jeggo continued, Australia has embraced the One Health
paradigm in its emphasis on biosecurity, which he defined as “the protection of the economy, the environment, social amenity, and human health from the negative impacts associated with the entry, establishment, or spread of animal or plant pests and diseases, or invasive plant and animal species.”
Australia divides its efforts to manage biosecurity risk among preborder, border, and postborder activities, Jeggo explained. Preborder activities include epidemiological intelligence, risk analysis, and efforts to address offshore risks. Postborder activities include surveillance, detection, and response to biosecurity threats that have not been excluded by measures such as inspection and quarantine (Commonwealth of Australia, 2008). Although more than half of Australia’s investment in biosecurity currently supports activities at its border, he said, “we actually need to focus a lot more on our postborder activities.” Moreover, while compartmentalizing biosecurity activities relative to the border has been convenient, “we now recognize that if we’re going to be effective, it needs to be managed as a continuum,” Jeggo noted.
Australia’s federal government is primarily responsible for preborder and border biosecurity, whereas the states and territories implement postborder activities. Recently, in response to resource limitations, states and territories have sought support from the federal government and from industry in order to strengthen postborder biosecurity efforts, Jeggo said.
“We do have a very extensive National Animal Health Surveillance System, but we need to improve it,” he observed. “We have a very strong relationship with industry, and industry recognizes that [disease] poses a risk to trade and … local production. We’ve now got a strong dialogue going on, with industry prepared to seriously invest in this area.” However, he added, partnership with industry “comes with the underlying understanding that industry will also want to be involved in at least influencing the decision-making process.”
Australia recognizes that the One Health approach is essential to managing both food safety and infectious disease risks, Jeggo concluded. “It is clear to all of us that if we work together across that continuum of wildlife, animal health, and human health, we should deliver better outcomes,” he said, but he noted that actual evidence for that conclusion is lacking—and that it is necessary to support further efforts.
Jeggo also suggested that intergovernmental organizations that in the past “paid lip service to One Health” should continue to undertake organizational changes necessary to implementing interdisciplinary approaches to food safety. “We need to create divisions, departments, institutes of One Health where we can actually get a genuine partnership going on, [and] where resource allocation will drive the cultural changes that we need,” he insisted. “Organizational change [will] drive what we really want to achieve, and that is a genuine One Health approach.”
One Health in Canada: Integrated Surveillance
As in Australia, responsibility for food safety in Canada is divided among federal and provincial or territorial agencies, according to speaker Rainer Engelhardt, of the Public Health Agency of Canada. (Dr. Engelhardt’s contribution to the workshop summary report can be found in Appendix A, pages 176-188.) Following an international expert consultation on One Health held in Winnipeg in 2009 (Public Health Agency of Canada, 2009), Canada “took up the gauntlet of One Health,” he said, developing a strategic framework for ongoing efforts toward risk identification, assessment, and avoidance.
“Our current approach in the country is to look at One Health in the food safety context in the multiple dimensions—how to optimize health programs, targeting science and research, more integrated surveillance, enhancing food safety epidemiology, risk assessment, inspection and regulation,” Engelhardt explained. To be effective will require collaboration among a broad range of agencies at the federal and provincial/territorial levels, as well as partnership with other countries and with non-governmental agencies, such as the WHO, he added.
Addressing AMR (an issue previously discussed in the subsection titled Antimicrobial-Resistant Pathogens) is a focus of Canada’s One Health approach to food safety, and surveillance is the keystone of these efforts, Engelhardt said. Canada has established two primary complementary surveillance systems, which he described in detail: the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) and the National Integrated Enteric Pathogen Surveillance Program (C-EnterNet). Information from these sources is further integrated with data provided by PulseNet (CDC, 2011d), by the Global Public Health Information Network (Public Health Agency of Canada, 2004), and by parties to the International Health Regulations (WHO, 2008).
CIPARS (Public Health Agency of Canada, 2007) is modeled after D ANMAP (DANMAP, 2012) and also after NARMS (FDA, 2012), Engelhardt said. He explained that CIPARS monitors trends in antimicrobial use, as well as antimicrobial resistance in selected bacteria (particularly Salmonella and E. coli among food-borne pathogens), at major points along the “farm-to-fork” continuum. CIPARS is intended to enable the timely national and international dissemination of surveillance data, and its accurate comparison to similar data collected by other countries, he said. He also noted that in 2005, information from CIPARS linking the use of the antimicrobial ceftiofur in poultry to the development of resistance in humans and animals led to a voluntary ban on the use of the drug by the poultry industry.
Engelhardt described C-EnterNet, which was modeled to some extent on the CDC’s FoodNet surveillance system (CDC, 2011c), as “an integrated program designed to monitor human infectious enteric illness in order to inform food and water safety policy.” Through surveillance, C-EnterNet (currently a pilot program run at only two sentinel sites in Ontario and British Columbia but slated to be expanded soon to additional sites in Canada) detects changes in trends of the human
enteric disease incidence and pathogen exposure levels from food, animals, and water. These data sets are then analyzed to determine the proportion of cases due to water, food, or animal contact and thereby to identify statistically significant risk factors for enteric illness. For example, C-EnterNet analyses revealed that nearly one-third of reported cases of enteric disease (involving both food-borne and non-food-borne pathogens) were travel-related; such information has been used to develop advice for both travelers and physicians, he said.
“It’s important to have the CIPARS system and the C-EnterNet system work congruently,” Engelhardt observed. “The CIPARS side brings into play information on antimicrobial use and relevant elements of animal husbandry and management … [while] C-EnterNet looks at the inputs from the social/cultural and natural environments … [and] economic and trade considerations.” Together, they provide Canada with a national structure for integrated surveillance, he concluded.
Echoing remarks by Karesh and Jeggo, Engelhardt noted that present applications of the One Health paradigm to the complex problem of ensuring a safe food supply are implemented piecemeal, and their integration constitutes a work in progress. The programs and strategies described by these three speakers focus mainly on specific environmental interfaces critical to addressing food-borne disease, but as Engelhardt observed, “as far as the full operationalization of One Health is concerned, we’re not there yet. I think we see how to do it, but we’re not yet fully committed, especially institutionally to implementing the concept.”
One Health in the United States
Publication of Upton Sinclair’s The Jungle (1906) led to the passage of the first food safety law in the United States in the early 1900s; today, 15 federal agencies are responsible for executing the more than 30 laws that direct food inspection in the United States (GAO, 2004, 2005). A One Health approach to food safety emphasizes the sharing of relevant information among disparate organizations, unifying organizational mandates among human, animal, and envi ronmental health professionals, and integrating local national and international surveillance networks. Although there has been limited interaction between human and veterinary health professionals, the implementation of a One Health approach could have numerous applications in the prevention of food-borne illness. Many scientific, regulatory, and surveillance organizations have begun to adopt a One Health approach to their programs,30 but in many cases they have faced barriers to implementation (Atlas et al., 2010; Karesh et al., 2005; World Bank, 2010). Currently, there is no single robust system in
![]()
30 For example, the U.S. Secretary of Agriculture is working to manage interagency cooperation in the area of One Health by creating the USDA One Health Multiagency Coordination Group (USDA, 2011b).
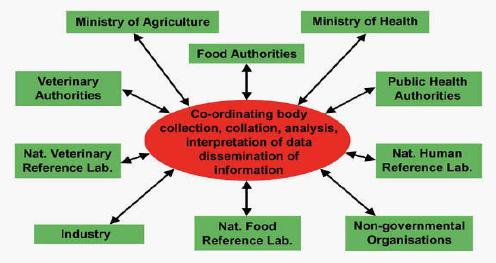
FIGURE WO-28 Schematic presentation of the collection, collation, analysis, and interpretation of surveillance data and the subsequent dissemination of information to all the major stakeholders in food safety. There is currently no single organization responsible for coordinating surveillance data.
SOURCE: Adapted from Wong et al. (2004).
place that embraces this approach,31 as illustrated in Figure WO-28 (Atlas et al., 2010, emphasis added).
Given the resource limitations that most governments face, adopting an efficient system that eliminates redundancy, maximizes benefits to public health, and reduces health risks would allow resources to be allocated in a way that provides the greatest benefit to the public. Yet, no single multilateral organization or government agency has a mandate to pursue policies or collect data related to disease spread based on a One Health approach (Karesh et al., 2005). Nearly all of the outbreaks discussed in this workshop are preventable when measures are taken to prevent, detect, and remove contaminants. Through collaboration of producers, processors, retailers, and consumers, interventions and systemic changes at multiple points along the food safety spectrum can dramatically reduce occurrences of food-borne illness (Taylor, 2002; Wegener, 2006).
![]()
31 The NARMS is a shared project among FDA, the USDA, and the CDC that is a good example of sharing information across agencies. In addition, the CDC has had an integrated strategy in place in a system to monitor West Nile that includes animals, mosquitoes, and people that has been successful, albeit not in food safety. The National Biosurveillance Advisory Committee issued a report to the Director of the CDC titled Improving the Nation’s Ability to Detect and Respond to 21st Century Urgent Health Threats that recommends the need to have a more integrated surveillance strategy for the United States that includes animal populations and food (http://www.cdc.gov/about/advisory/pdf/NBASFinalReport_April2011.pdf).
FDA, One Health, and the U.S. Food Safety Modernization Act The January 2011 passage of the FDA Food Safety Modernization Act (FSMA)32 has increased FDA’s role in food safety regulation, prevention of contamination, and import oversight, as well as its power to issue recalls; however, funding of these mandates remains uncertain (Stewart and Gostin, 2011). With its emphasis on prevention, rather than reaction, and its risk-based framework for inspections and regulation, the FSMA aligns FDA’s food safety practices with core public health tenets, as well as with recent IOM recommendations (IOM, 2010b).
Michael Taylor, FDA’s deputy commissioner for foods, described how the FSMA reflects the principles of One Health in his keynote address to the workshop. Calling the One Health perspective “indispensable to the goal of preventing food-borne illness,” he stressed that One Health is central to FDA’s overall approach to improving food safety, not just to implementation of the FSMA. For example, he said, the Office of Foods, which he directs, was created to integrate the work of FDA’s Center for Food Safety and Applied Nutrition (CFSAN) and its Center for Veterinary Medicine (CVM). “The regulatory activities of CVM cut across to the human food safety side, whether it’s dealing with the residues of animal drugs and animal feed additives in edible tissue of animals, [or with] the antimicrobial resistance issue … [or ] with the issue of food animal shedding of pathogens,” he explained. Resource allocation and budgeting for both Centers are integrated and guided by risk-based decision making aimed at “getting the most public health bang for the buck,” he said.
The Office of Foods has also established a Science and Research Steering Committee, consisting of science and laboratory directors of CFSAN and CVM, as well as research directors from FDA’s Office of Regulatory Affairs, which encourages integrated food safety research and methods development, Taylor continued. Experts from these agencies are also mounting a combined effort to implement key elements of the FSMA, which he called “a remarkable public policy breakthrough.”
FDA’s food safety program did not arise from an overarching vision, but instead consisted of a set of statutory provisions that had evolved in response to crises that arose over the course of the past century, Taylor explained. With the FMSA, Congress recognized the advantages of developing an integrated, whole-system approach to food safety—a view consistent with One Health, he observed. Specifically, the FMSA
• mandates an examination of the entire food system, from farm to table;
• emphasizes evidence-based risk reduction;
• includes both human food and animal feed; and
• recognizes the significant role and the inherent risks of international trade with regard to food safety.
![]()
32 FDA Food Safety Modernization Act, Public Law 111-353, 124 Stat. 3885.
Four illustrative issues In order to illustrate the influence of One Health in guiding FDA food safety policy, Taylor described the agency’s approach to ensuring the safety of produce, eggs, and pet foods, as well as their efforts to reduce the risk of AMR.
As directed by the FSMA, FDA is in the process of establishing regulatory standards for growing practices on the farm to deal with the problem of the microbial contamination of produce and resulting food-borne illness, Taylor stated; these standards will address issues such as the microbial quality of water and the means to protect water supplies from contamination, and they will define the responsibility of the grower to prevent food contamination. However, there is also a need to encourage primary prevention of pathogens entering the food system, he added; to that end, FDA partners with the USDA, which in turn collaborates with the livestock industry, to develop on-farm practices and interventions to reduce pathogen loads in animals that could contaminate produce.
In 2009, FDA issued an egg safety rule intended to reduce the transmission of Salmonella enteritidis to eggs from infected laying hens (FDA, 2009). Salmonella infections are often spread to chickens in such facilities by rodents or birds, he noted. The rule stipulates that laying hens be separated from other animals that could potentially transmit infection. Applying this rule systematically and comprehensively will reduce the burden of salmonellosis in this country, he asserted.
The 2007 contamination of pet foods with melamine (FDA, 2010b), which caused more than 100 pet deaths amongst nearly 500 cases of kidney failure (Associated press, 2007) in the United States, catalyzed political action for pet food safety, Taylor observed. The agency responded by proposing rules governing the safety of pet food; recent incidents of human illness caused by Salmonella-contaminated pet food treats have also been taken into account in these proposed FDA rules, he said.
No issue captures the importance of understanding the link between the health of animals and humans as does the threat of AMR, Taylor observed. In 2010, FDA released a draft guidance document discussing the significant public health challenge posed by AMR and describing FDA’s proposed strategy for addressing this issue, which includes phasing out antibiotic use for food animal production, feed efficiency, and growth promotion, as well as requiring veterinary supervision of the use of medically significant antibiotics. Several major food retailers and fast food chains have already made the decision not to buy meat from animals treated with medically significant antibiotics, he noted.
FDA’s voluntary antibiotic phase-out strategy was informed by discussion with drug companies, the veterinary community, and the animal production industry, Taylor said. “We don’t take the regulatory options off the table, but we are embarked in a very active dialogue with key elements of that community to pursue this phase-out strategy,” he said, and that includes identifying and evaluating the remaining valid prevention or treatment uses of antibiotics in food-producing animals.
Taylor’s description of FDA’s approach to addressing AMR sparked considerable discussion among workshop participants, who had already considered Denmark’s approach to that issue (see the subsection titled Antimicrobial-Resistant Pathogens). When asked what progress FDA had made toward assessing antimicrobial usage by animal type and geographic region, which would provide information necessary to establishing a baseline and monitoring responses to the proposed phase-out, Taylor acknowledged, “When it comes to really understanding in detail the patterns of usage by animal, by amounts, by region, we don’t have that information.” Moreover, he said, it is unclear whether FDA has the authority to collect such information. However, while such data are essential to science-based interventions, he stated his belief that they were not needed in order to support the more judicious uses of antimicrobials.
In the discussion that followed Taylor’s remarks, King asked Taylor if he thought that U.S. government agencies, such as FDA, the USDA, and the Environmental Protection Agency, might coordinate their policies and regulatory activi ties under a One Health framework. Taylor expressed doubt that the lack of a conceptual framework was keeping these agencies from working together; he was more inclined to attribute “classic institutional organizational behavior issues” such as the creation of specialized “silos” of expertise and “turf” that must be funded and defended. However, he also noted that FDA was taking several steps to encourage collaboration, such as research under way with the CDC on ways to identify specific foods and pathogen-food combinations as causes of food-borne disease outbreaks.
The FMSA stipulates that the CDC’s conduct of food chain surveillance should fulfill the needs of consumers, FDA and other state and local regulatory agencies, and the food industry, Taylor continued. In addition, he contended, such surveillance should be designed not only to generate data, but also to derive the greatest possible value from the data collected. Pursuing this goal as a collaborative effort is currently difficult, because the CDC and FDA budgets are separately funded, he added. “Ideally,” he observed, “on cross-cutting subjects like surveillance you’d actually have an integrated budget initiative approach,” but unfortunately, the appropriations process does not encourage it.
Keusch asked whether, given that more than 40 years of discussion and recommendations to reduce AMR had not produced significant regulation, there could be any reason for optimism on this issue. Nevertheless, he suggested that the possibility of creating partnerships with industry in which antimicrobial usage could be monitored and evaluated, and among which data on AMR were shared, might offer a glimmer of hope. Taylor agreed, and he observed that such partnerships would expand access to data collected by various companies on the distribution of microbial pathogens throughout the food system. Under the FSMA, FDA will examine privately conducted food safety audits of companies seeking accreditation in food safety, he added. “That’s an enormous body of information that could be very valuable to us,” he said, “but only if we have
an information system that permits us to put it together and analyze it and take advantage of it.”
In response to a question from Duchin, Taylor stated that FDA has not set a quantitative goal for reducing the non-therapeutic use of antimicrobials in animals. Rather, he said, FDA’s strategy represents a shift from the uncontrolled use of antimicrobials to controlled and monitored use, and the consequent monitoring of impact. The FSMA directs FDA to identify the most significant food-borne hazards across the food supply, and to implement measures to minimize those hazards, he further explained. “There will no doubt be some opportunities to set perhaps some quantitative benchmarks as performance standards,” he said. For example, he noted, FDA has long regarded Salmonella in ready-to-eat foods as an adulterant; in that case, he said, “the performance standard is we don’t want any.” But in many cases, the specific practices and verification tests needed to minimize risk from a given food-borne pathogen remain to be determined.
Another concept in need of definition is the “non-therapeutic” or “preventive” use of antimicrobials, as several workshop participants observed. In a forthcoming guidance statement, FDA defines as “medically important” those antimicrobials that are targeted to specific pathogens and are demonstrated to have prevented disease as having legitimate preventive use, Taylor explained; once this guidance is released, FDA will establish practices based on this definition and informed by dialogue with industry. Similarly, FDA has compiled a list of antimicrobials it considers to be “medically important,” but he noted that the list needs to be revised and updated (FDA, 2010b, 2011b).
Research agenda The overarching challenge in improving food safety through One Health is to bring interdisciplinary science to bear to implement interventions of proven benefit, Taylor observed. That cannot be achieved without cutting across organizational lines within FDA and among federal agencies, and forming both interstate and international partnerships, as well as interdisciplinary collaborations, he concluded.
Significant scientific questions remain to be answered before the benefits of many potential interventions to improve food safety can be evaluated, Taylor noted. For example, standards for the use of raw manure on crops, and for the microbial quality of irrigation water, cannot be set without detailed knowledge of pathogen survival under various environmental conditions; FDA is engaged with the USDA and other government agencies, with the food industry, and with academic researchers to gain the understanding necessary to set evidence-based standards, he said.
Methods development is another crucial area of food safety research, Taylor continued. The increasing role of verification testing among food processors and purveyors, and the enhanced role of microbial testing as performed by FDA, is driving demand for fast and reliable diagnostic methods, he said. While there is no FDA approval process for the use of testing technologies by the food indus-
try, he claimed that there is less a need for formal guidance in this area than there is for harmonization—and modernization—of testing methods used in all sectors.
Taylor noted that a key partner in the dissemination and implementation of research and regulation to improve food safety is the USDA’s Cooperative Extension System.33 For example, he said, FDA has formed a produce safety alliance in partnership with the USDA and the National Association of State Departments of Agriculture with the goal of educating and guiding small growers and food producers in best practices for food safety. The use of the Cooperative Service and its array of educational and technical resources will be critical to implementing the FSMA throughout the community of food growers, and particularly among smaller operations, he observed.
USDA, One Health, and Food Safety Research Speaker Cathie Woteki directs four agencies within the USDA that participate in food safety research: the Agricultural Research Service, the Economic Research Service, the National Agricultural Statistics Service, and the National Institute of Food and Agriculture, an extramural agency that supports research and education programs and extension. (Dr. Woteki’s contribution to the workshop summary report can be found in Appendix A, pages 362-368.) She noted that three additional USDA agencies have food safety responsibility: the Animal and Plant Health Inspection Service, the FSIS, and the Forest Service.
Woteki’s presentation focused on the importance of food safety research and the USDA’s contribution to the field, which is increasingly aligned with One Health. She characterized the USDA as an organization where expertise in animal health and science, human food safety and nutrition, wildlife ecology, plant and crop science, and economics come together in one place: fertile ground for establishing a One Health approach, which has evolved out of the department’s efforts to plan for pandemic influenza. “This comprehensive approach is going to improve global capabilities to detect, prevent, prepare for, and respond to emerging diseases, pandemic threats, and other issues in the human, animal, and ecosystem interface,” she stated. “By applying the One Health principles, it’s our hope at USDA to encourage a synergy of ideas, reduce our program redundancy, and apply this holistic approach ultimately to improving global health, whether it’s human health, animal health, or the health of the environment.”
“Research is often a silent partner in food safety,” Woteki observed. She noted that while outbreaks raise public consciousness about the importance of food safety and outbreak investigation, research programs are crucial to the identification of novel food-borne threats. At the USDA, she continued, “we monitor the food illness epidemiological data to identify emerging threats. We work closely
![]()
33 Each U.S. state and territory has a state office of cooperative extension at its land-grant university and a network of local or regional offices. These offices are staffed by one or more experts who provide useful, practical, and research-based information to agricultural producers, small business owners, and the general public (http://www.csrees.usda.gov/Extension/).
with our research partners to develop tests and new intervention approaches that work in a regulatory setting, as well as to develop intervention strategies to reduce risk throughout the food chain.” Woteki highlighted several such contributions, including the following:
• Agricultural Research Service research on high-priority national and international food-borne pathogens and contaminants, together with pathogen sources and reservoirs, detection methods, and post-harvest processing.
• A collaborative effort between the USDA and the CDC to develop a national swine influenza virus (SIV) surveillance pilot program to better understand the epidemiology of SIV infections and to improve diagnostic tests, preventive management, and vaccines for swine and humans ( Sivapalasingam et al., 2003). This program was instrumental in implementing surveillance during the 2009 H1N1 influenza pandemic, Woteki noted.
• The funding of extramural research and education through the National Institute of Food and Agriculture (NIFA), including a joint program with FDA in 2009 to solicit research focused on integrating food system signals (e.g., clusters of illnesses reported by government authorities or problems identified through routine testing) with innovative technologies (e.g., geospatial analysis) to detect product contamination. NIFA also recently awarded a very large integrated grant to facilitate research on norovirus (see previous subsection titled “Food-Borne Viruses”), Woteki reported.
Two formidable challenges threaten the continued advancement of food safety research in the United States, according to Woteki. First, limited public funding, which tends to support basic and “public goods” research (as compared with private-sector research, which favors product development), constrains not only the improvement of food safety but also the overall productivity of the food system (Heisey et al., 2011), she argued. Second, she noted that the production of agricultural scientists in disciplines relevant to food safety has been flat for many years. “There are, according to the private sector, very good jobs that are going vacant because we’re not producing the well-trained scientists to fill them,” she said.
One Health in Practice:
Regulations, Research, and Industrial Applications
Several speakers attested to the influence of the One Health paradigm in shaping regulations, research agendas, and industry practices to improve food safety. Each of the presentations summarized below identified ways in which government agencies, food companies, and sectors of the food industry have looked across the food chain to identify opportunities to minimize risk of food-borne disease. However, as many workshop participants noted, most of this activity
has occurred within industries and agencies, leaving vast untapped potential for transdisciplinary, transagency, and trans-sectoral collaboration.
The Role of Industry
Globalization of the international food supply has brought an increasing variety of foods to the global marketplace as well as reduced food costs; it has also led to recent food-borne outbreaks covered by the media (see Box WO-3). The trade-off has been an increased risk for food-borne illnesses (IOM, 2006), as well as increased bureaucracy among the agencies responsible for monitoring food safety and responding to outbreaks. Ensuring that all parts of the global food supply system function properly is critical to keeping the food supply safe.
Ultimately, consumer safety is the responsibility of industry. Technological advances have dramatically improved food safety; however, they do not necessarily represent advancement in prevention. Risk management is complicated not only by the numerous points where contamination may occur, but also by the diversity of food supply chains. Food producers face multiple risks. Whether microbiological or chemical in nature, these risks can be the result of poor sanitation, contaminated water, purposeful adulteration of products for economic gain (e.g., melamine in milk powder), non-adherence to best practices, or even intentional contamination. Unlike the United Kingdom, Canada, and many other countries, the United States uses a “risk-by-risk” approach to food safety rather than a comprehensive and unified preventive system (IOM, 2009).
The private sector, working synergistically with the public sector, must be able to develop and establish food safety protocols even in the absence of a specific law or regulation. A number of companies have recognized the value of going above and beyond mandated regulations in order to bolster consumer confidence. The cost of recalls and the damage that association with a food-borne outbreak can do to an industry are strong incentives for private-sector regulatory compliance.
It is in every country’s best interest that regulatory agencies collaborate with industry and incentivize improvements to food safety systems. Countries also have their own “brand and reputation” to preserve. When countries are linked to food-borne illness, it is extremely difficult to rebuild consumer confidence. By collaborating, it is likely that industry compliance would increase, and regulatory agencies would be able to decrease inspections. Increased collaboration would also allow regulators to make better risk-management decisions (IOM, 2009).
Industry Response to Food-Borne Disease Risks:
Costco’s Approach to Food Safety
Food represents a significant proportion of sales by Costco Wholesale Corporation, the third-largest retailer in the United States and the eighth-largest in the world, according to speaker Craig Wilson, the company’s vice president and
general merchandising manager of quality assurance and food safety. Costco has a comprehensive program of microbial food testing, he said, that is guided by the premise that prevention beats the alternatives.
Every food product sold by Costco must conform to microbiological Quality Assurance/Quality Control (QA/QC) criteria, Wilson stated, including total aerobic plate count and measures of coliform bacteria, E. coli species, EHECs, Salmonella, and Listeria. Until each lot of a given food product passes these and other tests, including X-ray analysis for metals and other solid contaminants, it is withheld from distribution. This test-and-hold policy has reduced the number of recalls for several products, including bagged ready-to-eat salads and meat, he observed.
Costco also investigates food safety practices at its food suppliers. For the past decade, every supplier’s facility has been required to have a HACCP system in place. In addition, Costco insists that suppliers prove that they know the origins of every ingredient they use. Why go to these lengths? “We want to ensure that the processes are validated,” Wilson explained. “We want to document due diligence … [and] to minimize recalls,” he continued. “We never want you to go into a Costco and even think about food safety.”
While he agreed with Hueston’s earlier assessment that testing in and of itself does not ensure food safety, Wilson stated that testing is an important way to gauge process control by food suppliers, as well as to determine whether interventions taken to improve food safety are effective. “We do a lot of food safety audits,” he said; these involve not only checking the microbial specifications of the product, but also determining how well the suppliers themselves perform these tests and keep their records, and how they perform in a mock recall of their product. “We want to inspect what we expect,” he concluded.
Despite these measures, Costco has inevitably experienced recalls. Food safety will never be absolute, Wilson said; however, the numbers of recalls can be continually driven down. He noted a number of factors limiting improvement in food safety, including the fact that a proven intervention, food irradiation, has yet to gain public acceptance; nevertheless, he expressed certainty that “its time is coming” (see further discussion of food irradiation in the subsection entitled Industry’s Performance). Similarly, in an effort to address the issue of antimicrobial resistance, Costco also offers—and expects to expand—a range of antibiotic-free meat products.
Industry Perspective: Cargill’s Approach to Ensuring the Safety of the Global Food System
Mike Robach, of Cargill Incorporated, offered the perspective of an international food company with interests that span the entire food system. (Mr. Robach’s contribution to the workshop summary report can be found in Appendix A, pages 298-307.) The company’s 1,200 facilities in 66 countries are united by a set of core operating principles, including prevention-based, third
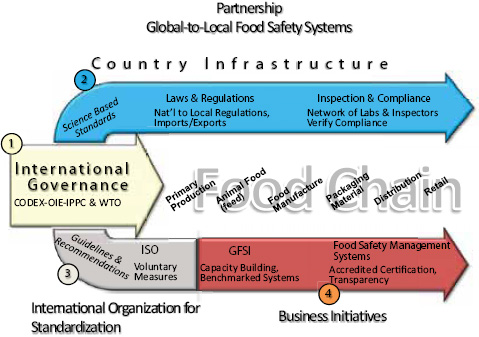
FIGURE WO-29 Global-to-local food safety systems.
SOURCE: Cargill; Robach (2011).
party—accredited food safety standards, which he described as “critical to our success.” This approach integrates with a broad vision of partnership in managing food safety that incorporates international governance and standardization, national governance, and business initiatives, depicted in Figure WO-29.
As discussed by Robach, the key elements of safety systems across the food chain include
1. international governance, including the Codex Alimentarius (Codex; www.codexalimentarius.net/web/index_en.jsp), the World Organisation for Animal Health (www.oie.int/), International Plant Protection Convention (www.ippc.int/), and the World Trade Organization (www.wto.org/);
2. country infrastructure (including laws, regulations, and their enforcement at all levels of governance) founded on science-based standards;
3. guidelines and recommendations issued by the International Organization for Standardization, which include voluntary standards and implementation procedures for food safety accreditation, audits, and management systems; and
4. business initiatives, including the Global Food Safety Initiative, a multi-stakeholder group that has developed guidance and benchmarks for food safety systems based on Codex.
According to Robach, Cargill’s food safety policy is based on the Codex Alimentarius (often known simply as the Codex), a collection of international food standards maintained since 1961 by a commission comprised of members of the FAO and the WHO (Joint FAO/WHO Food Standards Programme, 2006). Codex specifies a comprehensive program of food safety policies and procedures, including management responsibility, resource management, hazard analysis, traceability, and validation. Among the many programs Cargill has developed to meet these standards, Robach described two illustrative examples: the management of purchased materials and measures taken to prevent cross-contamination of foods.
Cargill expects the more than 400 external manufacturers that produce products on the company’s behalf to meet the same food safety standards as its own manufacturing plants, Robach stated. To evaluate the suitability and compliance of external manufacturers, Cargill uses a risk assessment model that scores the risk inherent to the materials being supplied, as well as the capability of the supplier to manage that risk. The company carefully scrutinizes and works intensively with the small percentage of suppliers judged to be high risk, he said.
Most Cargill facilities employ environmental monitoring in order to prevent cross-contamination of foods, Robach reported; in facilities where contamination with Salmonella and Listeria are deemed likeliest to occur, there are specific control programs in place for those pathogens, he added. He noted that a decision tree, used in every facility to support accurate risk assessment, encourages Cargill’s employees to think beyond the manufacturing process to the rest of the food chain. “The more preventative measures we can have in place around the world, the more assurance we’re going to have of an abundant, safe food supply,” he stated. “It builds confidence in food safety, enhances global trade. It enhances food security.”
In subsequent discussion, several participants took up the more difficult challenge of imposing food safety standards on small-scale suppliers in developing countries, where, for example, shrimp might be raised in high concentrations of antibiotics or toxic chemicals. In an attempt to avoid or ameliorate such problems, Cargill partners with major customers who buy products from these markets to build better capacity and educate growers and suppliers about food safety, Robach said.
The One Health approach at Cargill hinges on global partnerships, Robach observed. “We work closely not only with our supply chain and our competitors in the industry, but also with our customers and with the regulatory agencies,” he said, adding that the company also works closely and shares information with the CDC. “Working with academia, consumer groups, government, and industry is the path forward,” he continued. “We’ve got to work together.”
Nevertheless, Robach concluded, “business shoulders the responsibility for safe food. I know a lot of times government thinks they have the responsibility. They don’t. We do. It’s our product. It’s our brand. They’re our customers. We
want to work together, and we want to work collaboratively. But at the end of the day, we’re the ones who have the responsibility, and we accept that.”
Industry Perspective: Fresh Produce
Although most food-borne illness is in theory preventable, the especially vulnerable fresh produce sector does not yet have the tools to eliminate such risk, according to speaker David Gombas, of the United Fresh Produce Association. An estimated 1 billion servings of fresh produce—a category that comprises more than 300 different foods—are consumed in the United States each day, he noted. These foods originate from more than 100,000 farms in the United States and many times that number of foreign farms, he continued, with the largest operations contributing the majority of fresh produce sold.
The produce industry’s primary food safety tool is prevention, Gombas stated. There is no “kill step” that effectively removes all pathogens from produce while preserving its “fresh” status. “While we are very good at getting rid of 90 to 99 percent of the contamination that could be on fresh produce, there is always going to be some residual number of organisms that are able to hide away,” he said. “Therefore, we strive at every point in the supply chain to prevent contamination from occurring, and we’re not always successful.”
When prevention fails and a produce-associated outbreak of food-borne disease occurs, it is frequently difficult to discern its cause—and therefore to avoid a recurrence, Gombas observed. For example, he noted, all Listeria cases so far have been linked to processing, yet the pathogen’s primary habitat is in the field. He also questioned as speculative the interpretation of recent investigations of salmonellosis linked to hot peppers and papayas and of E.coli O157:H7 linked to strawberries, and in particular that of the 2006 outbreak of E. coli O157:H7 linked to bagged spinach, which had previously been described by King, Tauxe, and Doyle. “At the end of the day we really don’t know what happened in this incident, and in many of the other incidents we don’t know what the vector was either,” he concluded.
According to Gombas, the most likely sources and vectors of produce contamination, as identified in FDA guidance (FDA, 1998) are
• water (in all its forms),
• workers,
• surface contact (e.g., equipment, containers, utensils),
• animals (domestic and wild),
• soil amendments,
• prior land use,
• adjacent land activities, and
• cross-contamination.
These factors are well known and carefully considered by the produce industry, he asserted. Improving on this general approach will require identifying the actual risk factors at each stage of produce growing and processing. In particular, he noted, risk factors for preharvest produce contamination—those associated with water, animals, soil amendments, and land use—are not well understood. He demonstrated this point with a lengthy series of unanswered questions about the actual and comparative risks of various agricultural practices (Can manure be safely composted? Can some crops be safely amended with manure? Which wild animals pose the greatest risks as vectors of food-borne disease? What precautions should be taken if animal droppings are found in a field?). Much is known about risk factors for food-borne disease, he concluded, but very little is known about what is actually safe.
Recognition of these risk factors has inspired an escalation in food safety standards based on fear, rather than on science, which consume resources that might be better spent to improve the overall healthfulness of food, Gombas suggested. “You’ve got a limited number of dollars to spend on the quality control and food safety of these fresh produce items,” he observed. “There are consequences to the escalating food safety standards. There are also consequences on conflicting [food safety] audit standards, conflicting training messages, industry and consumer confusion.”
The answer to this dilemma is research to determine the actual risks associated with every step of food processing, Gombas argued. “We need the research based on real-world conditions … [because] produce is grown in a completely uncontrolled environment,” he said. “We need to be able to understand what those environmental [risk] factors are and what influence they have on the survival of the pathogen. We need to know what’s really happening, not what could be made to happen [in the laboratory]. And it has to be solution-directed research. We don’t need more basic research on potential pathogens, potential risk factors because we’ve got plenty enough right now that we don’t have answers for.”
David Acheson, of Levitt Partners, LLC, agreed that specific measurements of risk factors are necessary to improving food safety. For example, he noted, it was once assumed that a person would need to consume approximately 1 million Salmonella bacteria to become ill—until precise measurements were made, which reduced the “dose” to only 25 organisms. “Funding agencies need to change their metric and put the money where the tough questions are, and not on the easy lab stuff,” he declared.
Fletcher responded by pointing out several factors that make field-based research difficult. Detecting tiny amounts of naturally occurring pathogens in the field—amounts that could nevertheless pose a health threat—is currently impossible, she noted. Researchers are typically constrained (both legally and financially) from inoculating virulent bacteria in the field, she added, and attenuated strains may not accurately reflect pathogen behavior. “How can we do the experiments in a way that is meaningful?” she asked.
Forum member David Rizzo, of the University of California, Davis, wondered who would fund and perform such field experiments. Applied work is not favored by granting agencies, he observed, and field experiments tend to be performed by agricultural extension agents, whose positions have been cut in the name of deficit reduction. Gombas replied that his organization had advocated for a program to fund such research jointly with industry as part of the 2008 Farm Bill, but that it is only now beginning to live up to its promise. “With the 2012 Farm Bill coming along very soon, we’re hoping to put some fixes in place that will get the money to those individuals that can do the work that we need to have done,” he said.
Food Safety Trends: Implications and Possibilities for the Future
In a presentation titled “How Well Are Food Companies Addressing Microbiological Safety Issues?” Acheson disputed the common perception—based on increasing numbers of reported outbreaks and product recalls—that food is becoming less and less safe. On the contrary, he insisted: recalls are good, because they show that the food safety system is working.
That system has been strengthened by several recent improvements over the past two decades, including the ability to link food with disease and to detect lower levels of chemical adulterants, Acheson reported. In addition, greater fidelity of epidemiology, aided by improvements in genetic testing, enable quicker and more accurate outbreak investigations.
Nevertheless, the increasing importation of food presents obvious challenges to maintaining a safe domestic food supply—a task rendered even more difficult by shifting expectations among consumers, Acheson observed. Concerns regarding the intentional adulteration of food, whether it is done for profit or as an act of terrorism, are well-founded, he acknowledged. However, he observed, those worries often accompany the unrealistic expectation that all foods available to Americans will be unfailingly safe; when outbreaks inevitably occur, consumers blame food producers, causing damage to their businesses and brands. He also noted that American consumers increasingly want to buy local and unprocessed food, free of chemical pesticides and fertilizers.
The news media profoundly influences how the U.S. consumer views food safety and offers unmatched potential to educate the public about food-borne illness, Acheson said. Unfortunately, he added, both the corporate news media and social media outlets respond rapidly (and sometimes hastily) to food-borne disease events and are vulnerable to bias, selective reporting, and a tendency to seek blame.
In an attempt to answer the question he posed in his presentation’s title, Acheson reviewed trends in annual numbers of food recalls and Warning Letters34
![]()
34 When it is consistent with the public protection responsibilities of the agency and depending on the nature of the violation, it is FDA’s practice to give individuals and firms an opportunity to take voluntary and prompt corrective action before it initiates an enforcement action. Warning Letters are
issued by FDA, as well as in rates of laboratory-confirmed infections with important microbial pathogens. There was a “massive” increase in FDA-reported recalls of contaminated foods in 2009-2010—many of them due to Salmonella—and the same trend is likely for 2011, he reported. However, he contended, most recalls are triggered by testing and process control analysis by the food industry detects contaminants, and thus before outbreaks occur. Similarly, in 2010 the number of warning letters issued by FDA nearly doubled as compared with previous years, he said; he believes this reflects both increased enforcement and vigilance by that agency, as well as a lower bar for issuing such letters. Finally, many microbial pathogens (Vibrio spp. and Salmonella excepted) have been associated with decreasing numbers of food-borne outbreaks in the United States over the past 15 years—despite increased capacity to detect and investigate food-borne illness, and an increasingly vulnerable population (due to aging and compromised immunity). All three trends suggest that the food industry is doing a good job of controlling food-borne pathogens, he concluded.
Industry leaders are pursuing a range of strategies to continue to improve food safety, Acheson said; these measures include better tracking of the materials they use and the products they sell, and the use of process controls such as good manufacturing practices, judicious testing, and system monitoring. Unfortunately, he noted, these advancements are not yet feasible for many smaller companies that, collectively, play a significant role in the U.S. food supply. Further improvement in the overall safety of the U.S. food supply is also limited by consumer aversion to technological solutions such as irradiation, he observed; conference participants pointed out additional drawbacks to food irradiation, including cost (in the case of leafy greens, according to Gombas) and aesthetics (in the case of ground beef, which—according to Robach—has been said to [smell] like a wet dog).
Strategic Partnership with Industry
Although the ability to link food with disease continues to increase, capacity to respond to such information remains limited, King observed, leading him to wonder whether industry could help bridge this widening gap by leading adoption of the One Health paradigm. Robach provided an example of such leadership: a recent voluntary recall of ground turkey, prompted by Cargill’s discovery that its product was contaminated with Salmonella. “It was through a series of pieces of
![]()
issued to achieve voluntary compliance and to establish prior notice. The use of Warning Letters and the prior notice policy are based on the expectation that most individuals and firms will voluntarily comply with the law. The agency position is that Warning Letters are issued only for violations of regulatory significance. Significant violations are those violations that may lead to enforcement action if not promptly and adequately corrected. A Warning Letter is the agency’s principal means of achieving prompt voluntary compliance with the Federal Food, Drug, and Cosmetic Act (the Act). For more information please see FDA, Regulatory Procedures Manual 4-1; Warning Letters. http://www.fda.gov/ICECI/ComplianceManuals/RegulatoryProceduresManual/ucm176870.htm (accessed April 5, 2012).
information that we collected rather serendipitously from different sources combined with information that we had internally that led us to the conclusion that our product was likely associated with some illnesses that were being reported,” he said; Cargill initiated the recall without prompting from the USDA.
This incident illustrates the need for better and clearer lines of communication between the public health community and industry, Robach observed. For example, he said, combining the CDC’s preliminary epidemiological information with industry’s knowledge of supply chains could reveal potential sources or vectors associated with food-borne disease clusters early in their investigation. He stressed that two-way communication—now a relative rarity—is essential to such strategic partnerships.
“There’s a tendency from the regulator side to want it all wrapped up in a nice little bow, and then take it to the food industry and say, ‘We’ve got you,’” Acheson observed. “That’s not the way forward because we all know from our experiences in the public sector that taking these disparate facts and connecting the dots takes a lot of footwork and … dollars.” Instead, he encouraged regulators to establish a trust-based relationship with industry in order to collaborate in solving food-borne disease problems.
Gombas’ long list of unanswered research questions toward defining “what is safe?” for produce reminded Tauxe of similar questions posed by ground beef producers following the previously described 1993 outbreak of E. coli O157:H7 (see the earlier section Food-Borne Disease Trends in the United States). At that time, key safety questions were addressed by business leaders, who set aside competition to develop practices that could benefit the industry as a whole, he recalled. “Theirs was actually a very practical approach, not an enormous, high-level research approach,” he observed. Nevertheless, he added, their efforts produced a substantial reduction in E. coli O157:H7 infections without devastating the ground beef industry. Could the produce industry adopt that model, Tauxe wondered?
Such efforts are under way, Gombas said; they include partnering with FDA to develop guidance statements for produce growing and processing, along with programs to support grower adoption of recommended practices. “The industry has gotten together in many of these cases and has established what the risk factors are and what are the best mitigations we know today,” he concluded.
The Future of One Health
As the workshop drew to a close, King presented a summary of strategic actions identified by individual workshop participants that could advance the cause of improving food safety with One Health beyond mere awareness of its promise, and into action. These steps include the following:
• presenting a sufficiently compelling case for the One Health paradigm that is expressed in training and education programs;
• conducting outcomes research to demonstrate the economic advantages of One Health;
• embracing One Health as an opportunity for organizational change, directed toward cross-disciplinary education and collaboration; and
• designing research prototypes for proof-of-concept validation of One Health principles as applied to food safety in the developing world, and also to public-private partnerships between government and the food industry.
Finally, King emphasized the importance of a unified effort to advance the One Health paradigm. As the breadth of workshop presentations demonstrated, many stakeholders in the global food system have recognized the promise of One Health and are exploring its strategic adaptation; however, he continued, these activities are largely independent of and isolated from each other. “There’s already a concern that these different pieces of One Health are already competing and going their different ways,” he observed, and, in so doing, undermining One Health principles of cooperation and collaboration. “Somehow before we get too far along there needs to be a unification of these efforts, and to rethink this in a way that will be effective and worthwhile,” he concluded.
As discussed earlier in this chapter, prevention is the chief means for achieving food safety, but preventing food-borne outbreaks will require a much broader approach than currently exists (Karesh et al., 2005). A risk-based food safety approach is the underpinning of a strong food safety system that is able to prioritize risks and allocate limited resources where they will be the most effective. Moving away from many of the current practices to a system that allows agencies, the private sector, and other third parties to share responsibility for maintaining a safe food supply will help to eliminate regulatory gaps as well as reduce resource burden (IOM, 2010b; Stewart and Gostin, 2011).
A key component of prevention will be the ability to use data to anticipate where outbreaks are likely to occur. Shifting to a proactive food safety approach will require governments to implement research-based interventions through regulation and education that will produce the greatest reduction in disease burden at the lowest cost. Such transformations will require a substantial “sea change” in philosophy—moving away from a top-down approach to public health and toward cooperative, interdisciplinary strategies for disease prevention; this is the essence of the One Health principles.
Aarestrup, F. M., H. C. Wegener, and P. Collignon. 2008. Resistance in bacteria of the food chain: Epidemiology and control strategies. Expert Review of Anti-Infective Therapy 6(5):733-750.
Aarestrup, F. M., V. F. Jensen, H. D. Emborg, E. Jacobsen, and H. C. Wegener. 2010. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. American Journal of Veterinary Research 71(7):726-733.
Acheson, D. 2011. How well are food companies addressing microbiological safety issues? Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Ackoff, R. 2008. Systems, messes, and interactive planning portions of Chapters 1 and 2 of redesigning the future. New York/London: Wiley.
Associated Press. 2007. 104 deaths reported in pet food recall. New York Times, March 28.
Atlas, R., C. Rubin, S. Maloy, P. Daszak, R. Colwell, and B. Hyde. 2010. One Health—attaining optimal health for people, animals, and the environment. Microbe 5(9):383-389.
AVMA (American Veterinary Medical Association). 2008. One Health Initiative Task Force. One Health: A new professional imperative. July 15. http://www.avma.org/onehealth/onehealth_final.pdf (accessed June 27, 2012).
Berger C. N., S. V. Sodha, R. K. Shaw, P. M. Griffin, D. Pink, P. Hand and G. Frankel, 2010. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environmental Microbiology 12:2385-2397.
Bielaszewska, M., A. Mellmann, W. Zhang, R. Kock, A. Fruth, A. Bauwens, G. Peters, and H. Karch. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infectious Diseases 11(9):671-676.
Blaser, M. J. 2011. Deconstructing a lethal foodborne epidemic. Editorial. New England Journal of Medicine 365:1835-1836.
Broglia, A., and C. Kapel. 2011. Changing dietary habits in a changing world: Emerging drivers for the transmission of foodborne parasitic zoonoses. Veterinary Parasitology 182(1):2-13.
Brzuszkiewicz, E., A. Thurmer, J. Schuldes, A. Leimbach, H. Liesegang, F. D. Meyer, J. Boelter, H. Petersen, G. Gottschalk, and R. Daniel. 2011. Genome sequence analyses of two isolates from the recent Escherichia coli outbreak in Germany reveal the emergence of a new pathotype: Entero-aggregative-haemorrhagic Escherichia coli (EAHEC). Archives of Microbiology 193(12):883-891.
Buchholz, U., H. Bernard, D. Werber, M. M. Bohmer, C. Remschmidt, H. Wilking, Y. Delere, M. an der Heiden, C. Adlhoch, J. Dreesman, J. Ehlers, S. Ethelberg, M. Faber, C. Frank, G. Fricke, M. Greiner, M. Hohle, S. Ivarsson, U. Jark, M. Kirchner, J. Koch, G. Krause, P. Luber, B. Rosner, K. Stark, and M. Kuhne. 2011. German outbreak of Escherichia coli O104:H4 associated with sprouts. New England Journal of Medicine 365(19):1763-1770.
Burger, R. 2011. EHEC O104:H4 in Germany 2011: Large outbreak of bloody diarrhea and haemolytic uremic syndrome by shigatoxin-producing E. coli via contaminated food. Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Buzby, J. C., L. J. Unnevehr, and D. Roberts. 2008. Food safety and imports: An analysis of FDA food-related import refusal reports. EIB-39, U.S. Department of Agriculture, Economic Research Service.
Calvin, L. 2007. Outbreak linked to spinach forces reassessment of food safety practices. AmberWaves, June.
Cardoen, S., X. Van Huffel, D. Berkvens, S. Quoilin, G. Ducoffre, C. Saegerman, N. Speybroeck, H. Imberechts, L. Herman, R. Ducatelle, and K. Dierick. 2009. Evidence-based semiquantita-tive methodology for prioritization of foodborne zoonoses. Foodborne Pathogens and Disease 6(9):1083-1096.
CAST (Council of Agricultural Science and Technology). 2004. Intervention strategies for the microbiological safety of foods of animal origin. Issue Paper 25.
Cavallaro et al., 2011. Salmonella Typhimurium infections associated with peanut products. New England Journal of Medicine 365:601-610.
CDC (Centers for Disease Control and Prevention). 1993. Update: Multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States, 1992-1993. Morbidity and Mortality Weekly Report 42(14):258-263.
———. 2005. Foodborne illness—technical information. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/foodborneinfections_t.htm. (accessed September 12, 2011).
———. 2008. Questions and answers about monkeypox. http://www.cdc.gov/ncidod/monkeypox/qa.htm (accessed January 21, 2012).
———. 2010. Investigation Update: Multistate Outbreak of Human Salmonella Enteritidis Infections Associated with Shell Eggs. http://www.cdc.gov/salmonella/enteritidis/ (accessed November 23, 2011).
———. 2011a. CDC estimates of foodborne illness in the United States. http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed February 9, 2012).
———. 2011b. FoodNet—Foodborne Diseases Active Surveillance Network. http://www.cdc.gov/foodnet/index.htm (accessed February 15, 2012).
———. 2011c. FoodNet—Foodborne Diseases Active Surveillance Network. http://www.cdc.gov/foodnet/ (accessed January 23, 2012).
———. 2011d. Pulsenet. http://www.cdc.gov/pulsenet/ (accessed January 17, 2012).
———. 2011e. Pulsenet & foodborne disease outbreak detection. http://www.cdc.gov/features/dsPulseNetFoodborneIllness/ (accessed February 15, 2012).
———. 2011f. Vital signs: Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996-2010. Morbidity and Mortality Weekly Report 60(22):749-755.
———. 2011g. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August-September 2011. Morbidity and Mortality Weekly Report 60(39):1357-1358.
———. 2011h. Pathogens causing US foodborne illness, hospitalization, and death, 2000-2008. http://www.cdc.gov/foodborneburden/PDFs/pathogens-complete-list.pdf.
———. 2011i. Listeriosis. http://www.cdc.gov/listeria/.
———. 2011j. Foodborne illness: General information. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/foodborneinfections_g.htm#happensbody (accessed November 14, 2011).
———. 2011k. National Center for Emerging and Zoonotic Infectious Diseases. http://www.cdc/gov/ncezid (accessed September 14, 2011).
———. 2011l. Norovirus: Technical fact sheet. http://www.cdc.gov/ncidod/dvrd/revb/gastro/noro (accessed September 6, 2011).
———. 2012. What is a foodborne disease outbreak and why do they occur? http://www.cdc.gov/foodsafety/facts.html#whatisanoutbreak (accessed March 12, 2012).
Chalk P, 2004. Hitting America’s soft underbelly: The potential threat of deliberate biological attacks against the U.S. agricultural and food industry. Rand National Defense Institute (prepared for the Office of the Secretary of Defense).
Cheffins, C. F. 1854. Lith, Southhampton Buildings, London, England, 1854. In Snow, J. 1855. On the mode of communication of cholera, 2nd ed. London: John Churchill.
Chew, M. H., P. M. Arguin, D. K. Shay, K. T. Goh, P. E. Rollin, W. J. Shieh, S. R. Zaki, P. A. Rota, A. E. Ling, T. G. Ksiazek, S. K. Chew, and L. J. Anderson. 2000. Risk factors for Nipah virus infection among abattoir workers in Singapore. Journal of Infectious Diseases 181(5):1760-1763.
Chua, K. B. 2003. Nipah virus outbreak in Malaysia. Journal of Clinical Virology 26(3):265-275.
Coker, R., J. Rushton, S. Mounier-Jack, E. Karimuribo, P. Latumba, D. Kambarage, D. U. Pfeiffer, K. Stark, and M. Rweyemamu.2011. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infectious Diseases 11:326-331.
Commonwealth of Australia. 2008. One biosecurity: A working partnership. http://www.daff.gov.au/__data/assets/pdf_file/0010/931609/report-single.pdf (accessed June 27, 2012).
CRS (Congressional Research Service). 2009. U.S. food and agricultural imports: Safeguards and selected issues. http://www.nationalaglawcenter.org/assets/crs/RL34198.pdf (accessed November 14, 2011).
———. 2010. Food safety: Foodborne illness and selected recalls of FDA-regulated foods. http://www.nationalaglaawcenter.org/assets/crs/R40916.pdf (accessed November 10, 2011).
Crutchfield, S. R., S. Roberts. 2000. Food safety efforts accelerate in the 1990s. Food Review 23(3):44-49.
DANMAP (Danish Integrated Antimicrobial Resistance Monitoring and Research Programme). 2012. About DANMAP. http://www.danmap.org/About%20Danmap.aspx (accessed January 17, 2012).
DEFRA (Department for Environment, Food and Rural Affairs). 2008. UK National Control Programme for Salmonella in chickens (Gallus gallus) reared for meat (broilers). http://www.defra.gov.uk/foodfarm/farmanimal/diseases/ (accessed May 2, 2012).
Desai, R., C. Yen, M. Wikswo, N. A. Gregoricus, J. E. Provo, U. D. Parashar, and A. J. Hall. 2011. Transmission of norovirus among NBA players and staff, Winter 2010-2011. Report Brief. Clinical Infectious Diseases. http://www.oxfordjournals.org/our_journals/cid/prpaper.pdf (accessed November 9, 2011).
Donaldson, E. F., A. N. Haskew, J. E. Gates, J. Huynh, C. J. Moore, and M. B. Frieman. 2010. Metagenomic analysis of the viromes of three North American bat species: Viral diversity among different bat species that share a common habitat. Journal of Virology 84(24):13004-13018.
Duizer, E., and M. Koopmans. 2008. Emerging food-borne viral diseases. In Food-borne viruses: Progress and challenges, edited by M. P. G. Koopmans, D. O. Cliver, and A. Bosch. Washington, DC: ASM Press. Pp. 117-145.
Dupont, H. L. 2007. The growing threat of foodborne bacterial enteropathogens of animal origin. Clinical Infectious Diseases 45:1353-1361.
EFSA. 2011a. Scientific report of the EFSA: Shiga toxin-producing E. Coli (STEC) O104:H4 2011 outbreaks in Europe: Taking Stock. EFSA.
EFSA. 2011b. Technical report: Tracing seeds, in particular fenugreek (trigonella foenum-graecum) seeds, in relation to the shiga toxin-producing E. Coli (STEC) O104:H4 2011 outbreaks in Germany and France. EFSA.
Epstein, J. H., H. E. Field, S. Luby, J. R. Pulliam, and P. Daszak. 2006. Nipah virus: Impact, origins, and causes of emergence. Current Infectious Disease Reports 8(1):59-65.
ERS (Economic Research Service). 2001. Changing structure of global food consumption and trade, edited by Anita Regmi. Market and Trade Economics Division, Economic Research Service, U.S. Department of Agriculture, Agriculture and Trade Report. WRS-01-1.
———. 2005. New directions in global food markets, edited by Anita Regmi and Mark Gehlhar, AIB-794, USDA/ERS, February 2005. http://www.ers.usda.gov/publications/aib794/.
Estes, M. K., B. V. Verkataram Prasad, and R. L. Atmar. 2006. Noroviruses everywhere: Has something changed? Current Opinion in Infectious Diseases 19:467-474.
FAO/WHO. 2001. Codex Alimetarius Commission. Procedural manual, 12th ed. ftp://ftp.fao.org/docrep/fao/005/Y2200E/Y2200E00.pdf (accessed July 2, 2012).
FDA (Food and Drug Administration). 1998. Guide to minimize microbial food safety hazards for fresh fruits and vegetables. Washington, DC: FDA.
———. 2003. Import alert #25-20: Detention without physical examination of green onions (scallions) from specific firms in Mexico. http://www.fda.gov/ora/fiars/ora_import_ia2520.html) (accessed November 30, 2011).
———. 2009. Prevention of Salmonella enteritidis in shell eggs during production, storage, and transportation. http://www.fda.gov/Food/FoodSafety/Product-SpecificInformation/EggSafety/EggSafetyActionPlan/ucm170746.htm (accessed March 30, 2012).
———. 2010a. Frequently asked questions and answers: FDA’s investigation into the Salmonella Enteritidis outbreak involving the recall of shell eggs. http://www.fda.gov/Food/NewsEvents/WhatsNewinFood/ucm223723.htm (accessed November 23, 2011).
———. 2010b. Melamine pet food recall of 2007. http://www.fda.gov/animalveterinary/safetyhealth/recallswithdrawals/ucm129575.htm (accessed March 2, 2012).
———. 2011a. Pathway to global product safety and quality. FDA.
———. 2011b. Withdrawal of notices of opportunity for a hearing; penicillin and tetracycline used in animal feed. http://www.gpo.gov/fdsys/pkg/FR-2011-12-22/html/2011-32775.htm (accessed March 30, 2012).
———. 2011c. Information on the recalled Jensen Farms whole cantaloupe. http://www.fda.gov/Food/FoodSafety/CORENetwork/ucm272372.htm (accessed November 14, 2011).
———. 2012. National antimicrobial resistance monitoring system. http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/default.htm (accessed January 17, 2012).
Fischetti, M. 2007. Is your food contaminated? Scientific American (September):112-117.
FMI (Food Marketing Institute). 2010. Supermarket facts—industry overview 2010. http://www.fmi.org/facts_figs/?fuseaction=superfact (accessed September 22, 2011).
Food Safety News. 2011. Six ill in Minnesota Salmonella egg outbreak—organic eggs recalled. October 20, 2011. http://www.foodsafetynews.com/2011/10/six-ill-in-minnesota-salmonella-egg-outbreak/ (accessed November 23, 2011).
Frank, C., M. S. Faber, M. Askar, H. Bernard, A. Fruth, A. Gilsdorf, M. Hohle, H. Karch, G. Krause, R. Prager, A. Spode, K. Stark, and D. Werber. 2011a. Large and ongoing outbreak of haemolytic uraemic syndrome, Germany, May 2011. Euro Surveillance 16(21).
Frank, C., D. Werber, J. P. Cramer, M. Askar, M. Faber, M. an der Heiden, H. Bernard, A. Fruth, R. Prager, A. Spode, M. Wadl, A. Zoufaly, S. Jordan, M. J. Kemper, P. Follin, L. Muller, L. A. King, B. Rosner, U. Buchholz, K. Stark, and G. Krause. 2011b. Epidemic profile of shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. New England Journal of Medicine 365(19):1771-1780.
Frenzen, P. D., A. Drake, and F. J. Angulo. 2005. Economic cost of illness due to Escherichia coli O157 infections in the United States. Journal of Food Protection 68:2623-2630.
FSIS (Food Safety and Inspection Service). 2002. New measures to address E. coli O157:H7 contamination. http://www.fsis.usda.gov/oa/background/ec0902.htm (accessed March 13, 2012).
Garcia, A., J. G. Fox, and T. E. Besser. 2010. Zoonotic enterohemorrhagic Eschericia coli: A One Health perspective. ILAR Journal 51(3):221-232.
GAO. 2004. Federal food safety and security system: Fundamental restructuring is needed to address fragmentation and overlap, GAO-04-588T (Washington DC: March 30).
GAO. 2005. Overseeing the U.S. food supply: Steps should be taken to reduce overlapping inspections and related activities. GAO-05-549T (Washington DC: May 17).
Ghandhi, M., and M. L. Chikindas. 2007. Listeria: A foodborne pathogen that knows how to survive. International Journal of Food Microbiology 113:1-15.
Glass, R. I., U. D. Parashar, and M. K. Estes. 2009. Norovirus Gastroenteritis. New England Journal of Medicine 361(18):1776-1785.
Hall, A. J., J. Vinjé, B. Lopman, G. W. Park, C. Yen, N. Gregoricus, and U. Parashar. 2005. Updated Norovirus Outbreak Management and Disease Prevention Guidelines 60(3):1-15.
Halpin, K., A. D. Hyatt, R. Fogarty, D. Middleton, J. Bingham, J. H. Epstein, S. A. Rahman, T. Hughes, C. Smith, H. E. Field, and P. Daszak. 2011. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: A comprehensive experimental study of virus transmission. American Journal of Tropical Medicine and Hygiene 85:946-951.
Hammerum, A. M., O. E. Heuer, H. D. Emborg, L. Bagger-Skjot, V. F. Jensen, A. M. Rogues, R. L. Skov, Y. Agerso, C. T. Brandt, A. M. Seyfarth, A. Muller, K. Hovgaard, J. Ajufo, F. Bager, F. M. Aarestrup, N. Frimodt-Moller, H. C. Wegener, and D. L. Monnet. 2007. Danish integrated antimicrobial resistance monitoring and research program. Emerging Infectious Diseases 13(11):1632-1639.
Heisey, P., S. L. Wang, and K. Fuglie. 2011. Public agriculture research spending and future U.S. agricultural productivity growth: Scenarios for 2010-2050. Economic Brief No. EB-17. http://www.ers.usda.gov/Publications/EB17/ (accessed April 30, 2012).
Hennessy, T. W., C. W. Hedberg, L. Slutsker, K. E. White, J. M. Besser-Wiek, M. E. Moen, J. Feldman, W. W. Coleman, L. M. Edmonson, K. L. MacDonald, and M. T. Osterholm. 1996. A national outbreak of Salmonella enteritidis infections from ice cream. New England Journal of Medicine 334:1281-1286.
Holzbauer, S. M., A. S. DeVries, J. J. Sejvar, C. H. Lees, J. Adjemian, J. H. McQuiston, C. Medus, C. A. Lexau, J. R. Harris, S. E. Recuenco, E. D. Belay, J. F. Howell, B. F. Buss, M. Hornig, J. D. Gibbins, S. E. Brueck, K. E. Smith, R. N. Danila, W. I. Lipkin, D. H. Lachance, P. J. Dyck, and R. Lynfield. 2010. Epidemiologic investigation of immunemediated polyradiculoneuropathy among abattoir workers exposed to porcine brain. PLoS One 5(3):e9782.
Hornig, M., T. Briese, T. Buie, M. L. Bauman, G. Lauwers, U. Siemetzki, K. Hummel, P. A. Rota, W. J. Bellini, J. J. O’Leary, O. Sheils, E. Alden, L. Pickering, and W. I. Lipkin. 2008. Lack of association between measles virus vaccine and autism with enteropathy: A case-control study. PLoS One 3(9):e3140.
Hueston, W. 2011. Overview of the global food system: Changes over time/space and lessons for the future. Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Hutchison, M. L., L. D. Walters, S. M. Avery, B. A. Synge, and A. Moore. 2004. Levels of zoonotic agents in British livestock manures. Letters in Applied Microbiology 39(2):207-214.
IOM (Institute of Medicine). 1992. Emerging infections: Microbial threats to health in the United States. Washington DC: National Academy Press.
———. 2003. Microbial threats to health: Emergence, detection, and response. Washington, DC: The National Academies Press.
———. 2006. Addressing foodborne threats to health: Policies, practices, and global coordination. Washington, DC: The National Academies Press.
———. 2009. Managing food safety practices from farm to table. Washington, DC: The National Academies Press.
———. 2010a. Antibiotic resistance: Implications for global health and novel intervention strategies. Washington, DC: The National Academies Press.
———. 2010b. Enhancing food safety: The role of the Food and Drug Administration. Washington, DC: The National Academies Press.
———. 2010c. Infectious disease movement in a borderless world. Washington, DC: The National Academies Press.
Jay, M. T., M. Colley, D. Carychao, G. W. Wiscomb, R. A. Sweitzer, L. Crawford-Miksza, J. A. Farrar, D. K. Lau, J. O’Connell, A. Millington, R. V. Asmundson, E. R. Atwill, and R. E. Mandrell. 2007. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerging Infectious Diseases 13(12):1908-1911.
Jemmi, T., and R. Stephan. 2006. Listeria monocytogenes: Food-borne pathogen and hygiene indicator. Revue Scientifique et Technique de l’OIE 25(2):571-580.
Joint FAO/WHO Food Standards Programme. 2006. Understanding the Codex Alimentarius. Rome: WHO and FAO.
Jones, K. E., N. G. Patel, M. A. Levy, A. Storeygard, D. Balk, J. L. Gittleman, and P. Daszak. 2008. Global trends in emerging infectious diseases. Nature 451(7181):990-993.
Kapoor, A., P. Simmonds, G. Gerold, N. Qaisar, K. Jain, J. A. Henriquez, C. Firth, D. L. Hirschberg, C. M. Rice, S. Shields, and W. I. Lipkin. 2011. Characterization of a canine homolog of hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America 108(28):11608-11613.
Karesh, W. B., R. A. Cook, E. L. Bennett, and J. Newcomb. 2005. Wildlife trade and global disease emergence. Emerging Infectious Diseases 11(7):1000-1002.
Khan, M. S., J. Hossain, E. S. Gurley, N. Nahar, R. Sultana, and S. P. Luby. 2011. Use of infrared camera to understand bats’ access to date palm sap: Implications for preventing Nipah virus transmission. Ecohealth 7(4):517-525.
King, L. J. 2004. Emerging zoonoes and pathogens of public health concern. Revue Scientifique et Technique de l’OIE 23(2):429-433.
King, L. 2011. What is One Health and why is it relevant to food safety? Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Koch, R. 1891. Ueber bakteriologische forschung, verhandl. Des x. Interna. Med. Congr., Berlin 1890. Berlin: August Hirschwald.
Koopmans, M. 2008. Progress in understanding norovirus epidemiology. Current Opinion in Infectious Diseases 21:544-552.
Koopmans, M., and E. Duizer. 2004, Foodborne viruses: An emerging problem. International Journal of Food Microbiology 90:23-41.
Kroneman, A., L. Verhoef, J. Harris, H. Vennema, E. Duizer, Y. van Duynhoven, J. Gray, M. Iturriza, B. Bottiger, G. Falkenhorst, C. Johnsen, C. H. von Bonsdorff, L. Maunula, M. Kuusi, P. Pothier, A. Gallay, E. Schreier, M. Hohne, J. Koch, G. Szucs, G. Reuter, K. Krisztalovics, M. Lynch, P. McKeown, B. Foley, S. Coughlan, F. M. Ruggeri, I. Di Bartolo, K. Vainio, E. Isakbaeva, M. Poljsak-Prijatelj, A. H. Grom, J. Z. Mijovski, A. Bosch, J. Buesa, A. S. Fauquier, G. Hernandez-Pezzi, K. O. Hedlund, and M. Koopmans. 2008. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the foodborne viruses in Europe network from 1 July 2001 to 30 June 2006. Journal of Clinical Microbiology 46(9):2959-2965.
Kroupitski, Y., D. Golberg, E. Belausov, R. Pinto, D. Swartzberg, D. Granot, and S. Sela. 2009. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Applied and Environmental Microbiology 75(19):6076-6086.
Kuhn, T. S. 1996. The structure of scientific revolutions, 3rd ed. Chicago: University of Chicago Press.
Kupferschmidt, K. 2011. As E. coli outbreak recedes, new questions come to the fore. Science 33:27.
Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St. George, T. Briese, and W. I. Lipkin. 2006. Masstag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. Journal of Infectious Diseases 194(10):1398-1402.
Lederberg, J. 2000. Infectious history. Science 288(5464):287-293.
Lipkin, W. I. 2010. Microbe hunting. Microbiology and Molecular Biology Reviews 74(3):363-377.
Luby, S. 2011. Nipah virus in Bangladesh. Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Luby, S. P., M. Rahman, M. J. Hossain, L. S. Blum, M. M. Husain, E. Gurley, R. Khan, B. N. Ahmed, S. Rahman, N. Nahar, E. Kenah, J. A. Comer, and T. G. Ksiazek. 2006. Foodborne transmission of Nipah virus, Bangladesh. Emerging Infectious Diseases 12(12):1888-1894.
Luby, S. P., E. S. Gurley, and M. J. Hossain. 2009. Transmission of human infection with Nipah virus. Clinical Infectious Diseases 49(11):1743-1748.
Maki, D. G. 2009. Coming to grips with foodborne infection—peanut butter, peppers, and nationwide Salmonella outbreaks. New England Journal of Medicine 360(10):949-953.
Martin, V., D. U. Pfeiffer, X. Zhou, X. Xiao, D. J. Prosser, F. Guo, and M. Gilbert. 2011. Spatial distribution and risk factors of highly pathogenic avian influenza (HPAI) H5N1 in China. PLoS Pathogens 7(3):e1001308.
Marucheck, A., N. Greis, C. Mena, L. Cai. 2011. Product safety and security in the global supply chain: Issues, challenges, and research opportunities. Journal of Operations Management 29:707-720.
McKenna, M. 2011. E. coli: A risk for 3 more years from who knows where. http://www.wired.com/wiredscience/2011/07/e-coli-3-years/ (accessed October 22, 2011).
Mead, P. S., L. S. V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe, 1999. Food-related illness and death in the United States. Emerging Infectious Diseases 5(5):607-625.
Melotto, M., W. Underwood, J. Koczan, K. Nomura, and S. Y. He. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126(5):969-980.
Michino, H., K. Araki, S. Minami, S. Takaya, N. Sakai, M. Miyazaki, A. Ono, and H. Yanagawa. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. American Journal of Epidemiology 150(8):787-796.
MMWR. 2011. Multistate outbreak of Listeriosis associated with Jensen Farms cantaloupes—United States, August-September 2011.
Morse, S. S. 1993. Examining the origins of emerging viruses. In Emerging viruses, edited by S. S. Morse. New York: Oxford University Press.
Nawa, Y., C. Hatz, and J. Blum. 2005. Sushi delights and parasites: The risk of fishborne and food-borne parasitic zoonoses in Asia. Clinical Infectious Diseases 41(9):1297-1303.
Negredo, A., G. Palacios, S. Vazquez-Moron, F. Gonzalez, H. Dopazo, F. Molero, J. Juste, J. Quetglas, N. Savji, M. de la Cruz Martinez, J. E. Herrera, M. Pizarro, S. K. Hutchison, J. E. Echevarria, W. I. Lipkin, and A. Tenorio. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathogens 7(10):e1002304.
Nestle, M. 2003. Safe food—bacteria, biotechnology, and bioterrorism. London: University of California Press.
NOAA (National Oceanic and Atmospheric Administration). 2011a. NOAA declares string of seal deaths in New England an unusual mortality event. http://www.noaanews.noaa.gov/stories2011/20111104_ume.html (accessed February 27, 2012).
NOAA. 2011b. Science team identifies influenza virus subtype that infected five dead seals. http://www.nero.noaa.gov/nero/hotnews/NR1134/ (accessed March 30, 2012).
Nolen, R. S. 2011. Questions—and cases—mount in seal disease outbreak. JAVMA News (December 15). http://www.avma.org/onlnews/javma/dec11/111215m.asp (accessed February 27, 2012).
Palacios, G., M. Hornig, D. Cisterna, N. Savji, A. V. Bussetti, V. Kapoor, J. Hui, R. Tokarz, T. Briese, E. Baumeister, and W. I. Lipkin. 2009. Streptococcus pneumoniae co-infection is correlated with the severity of H1N1 pandemic influenza. PLoS One 4(12):e8540.
Parashar, U. D., L. M. Sunn, F. Ong, A. W. Mounts, M. T. Arif, T. G. Ksiazek, M. A. Kamaluddin, A. N. Mustafa, H. Kaur, L. M. Ding, G. Othman, H. M. Radzi, P. T. Kitsutani, P. C. Stockton, J. Arokiasamy, H. E. Gary Jr., and L. J. Anderson. 2000. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. Journal of Infectious Diseases 181(5):1755-1759.
Paton, N. I., Y. S. Leo, S. R. Zaki, A. P. Auchus, K. E. Lee, A. E. Ling, S. K. Chew, B. Ang, P. E. Rollin, T. Umapathi, I. Sng, C. C. Lee, E. Lim, and T. G. Ksiazek. 1999. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 354(9186):1253-1256.
Penteado, A. L., B. S. Eblen, and A. J. Miller. 2004. Evidence of salmonella internalization into fresh mangos during simulated postharvest insect dis-infestation procedures. Journal of Food Protection 67(1):181-184.
Perrone, M. 2012. Court orders FDA action on antibiotic use on farms. Associated Press. http://www.msnbc.msn.com/id/46835476/ (accessed April 3, 2012).
Pike, B. L., K. E. Saylors, J. N. Fair, M. LeBreton, U. Tamoufe, C. F. Djoko, A. W. Rimoin, N. D. Wolfe. 2010. The origination and prevention of pandemics. Emerging Infections 50(12):1636-1640.
Public Health Agency of Canada. 2004. Global Public Health Intelligence Network (GPHIN). http://www.phac-aspc.gc.ca/media/nr-rp/2004/2004_gphin-rmispbk-eng.php (accessed January 17, 2012).
———. 2007. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS). http://www.phac-aspc.gc.ca/cipars-picra/index-eng.php (accessed January 17, 2012).
———. 2009. Report of the expert consultation. Paper read at One World One Health: From Ideas to Action, March 16-19, 2009, Winnipeg.
Pulliam, J. R. C., J. H. Epstein, J. Dushoff, S. A. Rahman, M. Bunning, A. A. Jamaluddin, A. D. Hyatt, H. E. Field, A. P. Dobson, and P. Daszak; the Henipavirus Ecology Research Group (HERG). 2012. Agricultural intensification, priming for persistence and the emergence of Nipah virus: A lethal bat-borne zoonosis. Journal of the Royal Society Interface 9(66):89-101.
Rahman, M. A., M. J. Hossain, S. Sultana, N. Homaira, S. U. Khan, M. Rahman, E. S. Gurley, P. E. Rollin, M. K. Lo, J. A. Comer, L. Lowe, P. A. Rota, T. G. Ksiazek, E. Kenah, Y. Sharker, and S. P. Luby. 2011. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Diseases 12(1):65-72.
Rasko, D. A., D. R. Webster, J. W. Sahl, A. Bashir, N. Boisen, F. Scheutz, E. E. Paxinos, R. Sebra, C. S. Chin, D. Iliopoulos, A. Klammer, P. Peluso, L. Lee, A. O. Kislyuk, J. Bullard, A. Kasarskis, S. Wang, J. Eid, D. Rank, J. C. Redman, S. R. Steyert, J. Frimodt-Moller, C. Struve, A. M. Petersen, K. A. Krogfelt, J. P. Nataro, E. E. Schadt, and M. K. Waldor. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. New England Journal of Medicine 365(8):709-717.
Rimoin, A. W., P. M. Mulembakani, S. C. Johnston, J. O. Lloyd Smith, N. K. Kisalu, T. L. Kinkela, S. Blumberg, H. A. Thomassen, B. L. Pike, J. N. Fair, N. D. Wolfe, R. L. Shongo, B. S. Graham, P. Formenty, E. Okitolonda, L. E. Hensley, H. Meyer, L. L. Wright, and J. J. Muyembe. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proceedings of the National Academy of Sciences of the United States of America 107(37):16262-16267.
Robach, M. 2011. Food-borne pathogen control programs. Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Robert Koch Institute. 2011. Report: Final presentation and evaluation of epidemiological findings in the EHEC O104:H4 outbreak, Germany 2011. Berlin: Robert Koch Institute. http://edoc.rki.de/documents/rki_ab/reQHS31jDrGxc/PDF/23NXL3JomOyAA.pdf (accessed June 27, 2012).
Rodo, X., J. Ballester, D. Cayan, M. E. Melish, Y. Nakamura, R. Uehara, and J. C. Burns. 2011. Association of Kawasaki disease with tropospheric wind patterns. Scientific Reports 1:152.
Saldaña, Z., E. Sánchez, J. Xicohtencatl-Cortes, J. L. Puente, and J. A. Girón. 2011. Surface structures involved in plant stomata and leaf colonization by shiga-toxigenic Escherichia coli O157:H7. Frontiers in Microbiology 2.
Scallan, E., P. M. Griffin, F. J. Angulo, R. V. Tauxe, and R. M. Hoekstra. 2011a. Foodborne illness acquired in the United States—unspecified agents. Emerging Infectious Diseases 17(1):16-22. Scallan, E., R. M. Hoekstra, F. J. Angulo, R. V. Tauxe, M. A. Widdowson, S. L. Roy, J. L. Jones, and P. M. Griffin. 2011b. Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Diseases 17(1):7-15.
Scholl, 2005. Powerpoint presentation presented to Forum on Microbial Threats. June 28, Washington, DC.
Siebenga, J. J., P. Lemey, S. L. Kosakovsky Pond, A. Rambaut, H. Vennema, and M. Koopmans. 2010. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathogens 6(5):e1000884.
Sinclair, U. 1906. The jungle. New York: Doubleday, Page, and Company.
Sivapalasingam, S., E. Barrett, A. Kimura, S. Van Duyne, W. De Witt, M. Ying, A. Frisch, Q. Phan, E. Gould, P. Shillam, V. Reddy, T. Cooper, M. Hoekstra, C. Higgins, J. P. Sanders, R. V. Tauxe, and L. Slutsker. 2003. A multistate outbreak of Salmonella enterica serotype newport infection linked to mango consumption: Impact of water-dip disinfestation technology. Clinical Infectious Diseases 37(12):1585-1590.
Sobel, J., 2005. Food and beverage sabotage. In Encyclopedia of Bioterrorism Defense, R. F. Pilch and R. A. Zilinskas, eds. New York: Wiley-Liss, Inc. pp. 215-220.
Sobel, J., A. S. Khan, and D. L. Swerdlow. 2002. Threat of a biological terrorist attack on the US food supply: The CDC perspective. Lancet 359:874-80 and reference #9 cited therein.
Stege, H., F. Bager, E. Jacobsen, and A. Thougaard. 2003. VETSTAT—the Danish system for surveillance of the veterinary use of drugs for production animals. Preventive Veterinary Medicine 57(3):105-115.
Stevens, L. 2011. Germany now says sprouts are to blame for outbreak. 2011. Wall Street Journal, June 11.
Stewart, K., and L. O. Gostin. 2011. Food and Drug Administration regulation of food safety. Journal of the American Medical Association 306(1):88-89.
Tauxe, R. V. 2002. Emerging foodborne pathogens. International Journal of Food Microbiology 78:31-41.
Tauxe, R. 2011. Emerging pathogens in food—trends and changes over the past 20 years. Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Tauxe, R. V., M. P. Doyle, T. Kuchenmuller, J. Schlundt, and C. E. Stein. 2010. Evolving public health approaches to the global challenge of foodborne infections. International Journal of Food Microbiology 139(Suppl 1):S16-S28.
Taylor, M. R. 2002. Reforming food safety: A model for the future. Resources for the Future Issue Brief 02-02.
Taylor, M. R. 2009. Statement by Michael R. Taylor, J.D. Senior Advisor to the Commissioner, Food and Drug Administration, U.S. Department of Health and Human Services on Full Committee Hearing on Food Safety before the Committee on Agriculture, U.S. House of Representatives. http://www.hhs.gov/asl/testify/2009/07/t20090716a.html (accessed April 30, 2012).
Thuy, H. T. T., L. P. Nga, and T. T. C. Loan. 2011. Antibiotic contaminants in coastal wetlands from Vietnamese shrimp farming. Environmental Science and Pollution Research International 18:835-841.
Tokarz, R., V. Kapoor, W. Wu, J. Lurio, K. Jain, F. Mostashari, T. Briese, and W. I. Lipkin. 2011. Longitudinal molecular microbial analysis of influenza-like illness in New York City, May 2009 through May 2010. Virology Journal 9(8):288.
Torok, T., et al. 1997. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. Journal of the American Medical Association 278(5):389-395.
Tustin, J., K. Laberg, P. Michel, J. Reiersen, S. Dadadóttir, H. Briem, H. Hardardóttir, K. Kristinsson, E. Gunnarsson, V. Fridriksdóttir, and F. Georgsson. 2011. A national epidemic of campylo-bacteriosis in Iceland, lessons learned. Zoonoses and Public Health 58(6):440-447.
UCLA (University of California, Los Angeles) Department of Epidemiology. 2012. John Snow. http://www.ph.ucla.edu/epi/snow.html (accessed February 26, 2012).
UN (United Nations). 2006. New UN-habitat report says urban dwellers badly off. http://www.unhabitat.org/content.asp?cid=3177&catid=5&typeid=6&subMenuId=0 (accessed February 5, 2012).
Uppal, P. K. 2000. Emergence of Nipah virus in Malaysia. Annals of the New York Academy of Sciences 916:354-357.
USAID (U.S. Agency for International Development). 2009. USAID launches emerging pandemic threats program. http://www.usaid.gov/press/releases/2009/pr091021_1.html (accessed January 21, 2012).
USDA (U.S. Department of Agriculture). 2011a. Arkansas firm recalls ground turkey products due to possible Salmonella contamination. http://www.fsis.usda.gov/News_&_Events/Recall_060_2011_Release/index.asp (accessed July 2, 2012).
———. 2011b. Veterinary Services 2015 One Health. Animal and Plant Health Inspection Service. http://www.aphis.usda.gov/about_aphis/programs_offices/veterinary_services/downloads/vs (accessed July 2, 2012).
van Asten, L., J. Siebenga, C. van den Wijngaard, R. Verheij, H. van Vliet, M. Kretzschmar, H. Boshuizen, W. van Pelt, and M. Koopmans. 2011. Unspecified gastroenteritis illness and deaths in the elderly associated with norovirus epidemics. Epidemiology 22(3):336-343.
Verhoef, L., R. D. Kouyos, H. Vennema, A. Kroneman, J. Siebenga, W. van Pelt, and M. Koopmans. 2011. An integrated approach to identifying international foodborne norovirus outbreaks. Emerging Infectious Diseases 17(3):412-418.
Wang, M. J., G. J. Moran. 2004. Update on emerging infections: News from the Centers for Disease Control and Prevention. Annals of Emergency Medicine 43(5):660-663.
Wegener, H. C. 2006. Risk management for the limitation of antibiotic resistance—experience of Denmark. International Journal of Medical Microbiology 296(Suppl 41):11-13.
Wegener, H. 2011. Antibiotic resistance: Linking human and animal health. Presentation given at the December 13-14, 2011, public workshop Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Weinstein, K. B. 2011. Listeria monocytogenes. MedScape Reference. http://emedicine.medscape.com/article/220684-overview#a0199 (accessed November 14, 2011).
Weise, E., and J. Schmit. 2007. Spinach recall: 5 faces. 5 agonizing deaths. 1 year later. USA Today. http://www.usatoday.com/money/industries/food/ (accessed September 20, 2011).
Weiser, A., Adolphs, J., Appel, B., Greiner. M., Berlin 2011, BfR-Wissenschaft issue 3/2012, ISBN 3-938 163-90-9.
WHO (World Health Organization). 2002. Food safety and foodborne illness. Fact sheet #237.
———. 2007. Food safety and foodborne illness. Fact Sheet N 237. March 2007.
———. 2008. What are the international health regulations? http://www.who.int/features/qa/39/en/index.html (accessed January 17, 2012).
———. 2011. Enterohaemorrhagic escherichia coli (EHEC). http://www.who.int/mediacentre/factsheets/fs125/en/ (accessed January 27, 2012).
Wolfe, N. 2011. Novel approaches for detecting food-borne outbreaks. Presentation given at the December 13-14, 2011, public workshop, Improving Food Safety Through One Health, Forum on Microbial Threats, Institute of Medicine, Washington, DC.
Wolfe, N. D., W. M. Switzer, J. K. Carr, V. B. Bhullar, V. Shanmugam, U. Tamoufe, A. T. Prosser, J. N. Torimiro, A. Wright, E. Mpoudi-Ngole, F. E. McCutchan, D. L. Birx, T. M. Folks, D. S.
Burke, and W. Heneine. 2004. Naturally acquired simian retrovirus infections in Central African hunters. Lancet 363(9413):932-937.
Wolfe, N. D., W. Heneine, J. K. Carr, A. D. Garcia, V. Shanmugam, U. Tamoufe, J. N. Torimiro, A. T. Prosser, M. Lebreton, E. Mpoudi-Ngole, F. E. McCutchan, D. L. Birx, T. M. Folks, D. S. Burke, and W. M. Switzer. 2005. Emergence of unique primate T-lymphotropic viruses among Central African bushmeat hunters. Proceedings of the National Academy of Sciences of the United States of America 102(22):7994-7999.
Wolfe, N., C. Panosian Dunavan, and J. Diamond, 2007. Origins of human infectious diseases. Nature 447:279-283.
Wong, L. F., J. K. Anderson, B. Norrung, and H. C. Wegener. 2004. Food contamination and food-borne disease surveillance at national level. In Second FAO/WHO Global Forum of Food Safety Regulations, 12-14. Bangkok, Thailand. Rome, Italy: Food and Agriculture Organization of the United Nations. http://www.fao.org/docrep/meeting/008/y5871e/y5871e0n.htm (accessed September 19, 2011).
World Bank. 2010. People, pathogens, and our planet—volume 1: Towards a One Health approach for controlling zoonotic diseases. Washington DC: The World Bank.


















































































































