What is the nature of the brain mechanisms that give rise to human cognition, and how do these mechanisms evolve? Although it is clear that human cognition, like all organismal traits, must be accounted for by some combination of ancestral and derived brain processes, attempts to decompose human mental processes into functional components whose features have been shaped by the process of natural selection—that is, adaptations—have been highly contested and controversial (Buller, 2005). The controversy centers on the difficulty of establishing whether a particular aspect of cognition or behavior is the result of an adaptation or adaptations, and in what way. Is a given cognitive ability in humans or any other species—for example, the ability to discriminate between different quantities of objects, to navigate spatially, or to learn to speak a language—the product of an adaptation specifically for that ability? Or is it just a specific instantiation of a more general ability, such as associative learning, or the general computational properties of neural networks? Or is it not the result of adaptations at all?
Proposals about functional specialization have long been a source of debate in psychology and the brain sciences. In particular, there is little agreement over whether cognitive processes other than perceptual and motor processes—that is, so-called higher-level processes—are specialized, and if so, how (Mahon and Cantlon, 2011). At stake are both theoretical and empirical issues. Theoretically, although it is clear that the brain is the product of evolutionary processes, including natural selection, we cannot move past this simple truism if we are unable to answer the question of what adaptations it contains, or to distinguish the results of natural selection from the results of other processes. Empirically, a variety of methods have been developed for studying brain specializations, including studies of developmental disorders and brain lesions, brain mapping techniques, experimental psychology tasks, comparative studies of brain anatomy and development, and more recently, studies of gene expression and gene regulation in the brain. However, controversy surrounds virtually all these methods and how they can be used to make inferences about functional specialization (Uttal, 2001). Even when brain researchers agree that specialization in the mature adult brain exists, they often cannot agree whether it is a result of selection specifically for that outcome, or is produced by more general developmental processes (Elman et al., 1996). As a result, there is little or no consensus about the nature of adaptations in the brain or even how to study them, especially for “higher-level” cognitive abilities such as language and reasoning (Mahon and Cantlon, 2011).
Although some of the reasons for this slow progress may be methodological, the impasse may also stem from a lack of biologically plausible models of what adaptations in the brain might be like (Barrett and
Kurzban, 2006). In psychology and neuroscience, it is common to think of brain mechanisms as falling into two categories: specialized and general-purpose. Specialized mechanisms are frequently associated with the idea of cognitive “modules,” which are in turn associated with several kinds of property (Fodor, 1983). Modules are often held to be “innate” in the sense that they develop similarly or identically across individuals, regardless of environmental input (i.e., they are canalized). They are “domain-specific,” that is, tailored to specific tasks or types of information. In addition, they operate autonomously or “automatically,” that is, independently of other systems and processes, including consciousness, and therefore produce the same outcomes regardless of context. Nonmodular processes, on the contrary, are held to be domain-general, developmentally plastic instead of innate, and interactive rather than autonomous. Many psychologists believe that human cognition can be accounted for by some mix of these two types of mechanism. This is sometimes called a “dual systems” view (Stanovich, 2004).
This view, derived from models of perception, equates specialization only with highly local, narrow, and stereotyped processes, and defines general-purpose processes as whatever “modular” processes are not (Fodor, 1983). Empirically, this means that evidence for developmental plasticity, interactivity, or capacity to respond to evolutionarily novel stimuli is typically taken as evidence that a brain region or process is not evolutionarily specialized. Moreover, proximal factors such as plasticity and developmental constraint are sometimes seen as alternatives to explanations invoking selection for particular outcomes (Elman et al., 1996). Biologically speaking, however, these distinctions may be based on false dichotomies. There is no reason why adaptations in the brain (or elsewhere) need to be developmentally canalized as opposed to plastic, isolated from other systems rather than interactive, or tightly locked to specific categories of information regardless of developmental circumstance.
If adaptations in the brain resemble other organismal adaptations—for example, tissue types, limbs, organs, and the molecular machinery of cells—they are likely to be both heterogeneous and hierarchical. Heterogeneity arises from the fact of form-function fit: adaptations have different histories and have evolved to do different things, so they are likely to have diverse properties rather than coming in just two kinds. Hierarchical organization, in turn, is characteristic of systems that evolve via descent with modification. Because new structures evolve from older structures, adaptations frequently share a mix of ancestral and derived features, with relatively ancient features (e.g., properties of neurons in general) shared more widely across organismal structures, and relatively recent ones (e.g., properties of specialized brain regions) more narrowly distributed, in a hierarchically organized fashion (Carroll et al., 2005).
If this is true of adaptations in the brain, it has important implications for current debates about them. Here I outline features of a hierarchical specialization model of brain evolution and show how it may require rethinking some commonly held assumptions about brain adaptations in psychology and the social sciences.
EVOLUTION AND DEVELOPMENT OF BRAIN ARCHITECTURE
If adaptations in the brain exist, they are likely to be built during ontogeny by developmental systems that orchestrate interactions between external inputs (e.g., sensory information), internal inputs (e.g., interactions within and between brain regions), and genetic regulatory machinery to shape phenotypic structure, including the computational properties of developed brain networks. Natural selection acts on these systems based on the phenotypes they produce, and newer developmental systems and mechanisms evolve from older ones via descent with modification. These points have several implications for what specialization means in the context of the brain.
Type and Token Outcomes of Developmental Processes
Because natural selection acts on phenotypes, developmental processes are selected based on the phenotypic outcomes they produce. However, the plastic nature of mammalian brain development means that actual phenotypic outcomes may vary substantially between individuals along some dimensions, while exhibiting similarities along others. For example, the brains of some mammals and birds may contain adaptations for developing cognitive maps of their local environments, but presumably the actual content of those maps varies widely across individuals (Jacobs and Schenk, 2003). Similarly, if human brains contain adaptations for learning language (still a controversial proposal), then the content of the developed phenotypes of linguistic knowledge must vary across individuals in all the ways that human languages vary (Evans and Levinson, 2009). Thus, developmental processes can be described functionally in terms of the type of outcomes they produce (e.g., cognitive maps, linguistic knowledge), although the instantiated tokens in individually developed brains vary in their phenotypic details (e.g., French, Quechua). This is presumably the norm rather than the exception for much of the brain (Buonomano and Merzenich, 1998).
Reaction Norms in the Brain
Plastic developmental systems can produce different phenotypes when placed into different environments. The mapping functions between
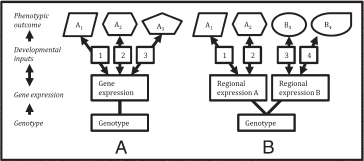
FIGURE 17.1 A reaction norm describes the mapping relationship between genotype, developmental inputs, and phenotypic outcomes (A). Brain regions may exhibit different reaction norms as a result of differential patterns of gene expression (B).
genotype, environment, and phenotype are known as reaction norms (Schlichting and Pigliucci, 1998) (Fig. 17.1). Because human brains contain multiple developmental processes, they are likely to contain different reaction norms for different functional regions and processes. For example, the reaction norms of motor cortex, which is partly organized around coordinated motor routines such as grasping and defense, may differ from those of somatosensory cortex, which is typically organized around body topology (Stepniewska et al., 2011). Moreover, tissue may be induced to adopt different reaction norms depending on the kinds of input it receives, both external (e.g., sensory) and internal (e.g., from other brain regions) (Sur and Rubenstein, 2005). Because reaction norms are the products of inherited developmental machinery, and because that machinery can be modified by selection based on the phenotypic outcomes it produces—that is, the brain structure that develops in response to external and internal inputs during development—reaction norms themselves evolve through processes of descent with modification. Thus, the developmental components of brain specializations may be thought of as a set of reaction norms, and their phenotypic components as the developed neural structures that they produce.
Ontogenetic Tuning and Module Spawning
As is the case for morphological development more generally, brain development is likely to proceed through processes of serial differentiation, subdividing into progressively finer elements whose neural and computational properties are fine-tuned based on the inputs they receive, interacting with whatever developmental processes are locally active (Sur
and Rubenstein, 2005; Rash and Grove, 2006). As development proceeds, brain tissue can become increasingly dedicated to the function that it will serve, with its computational properties becoming progressively tuned to carry out that function. This process is sometimes known as modularization (Karmiloff-Smith, 1992; Meunier et al., 2009). At least two factors must play a role in this modularization process: the inputs that the tissue receives (including patterns of neural firing) and the developmental procedures (i.e., reaction norms) that shape development as a function of inputs. These developmental procedures may include processes that fine-tune the computational properties of tissue based on inputs, such as long-term potentiation, pruning, and cell–cell signaling (Quartz and Sejnowski, 1997; Redies and Puelles, 2001; Hua and Smith, 2004; O’Leary et al., 2007). They may also include “module spawning” processes that give rise to new modules under certain developmental circumstances. For example, an initially undifferentiated region receiving two heterogeneous types of input might bifurcate into two new modules, each becoming progressively tuned to handle one of the two input types (Jacobs, 1997).
Often it is assumed that neural inputs alone play the most important role in ontogenetic differentiation of this kind: that is, that adult cortical organization is largely a function of where inputs are sent, what neural firing patterns they contain, and other properties such as granularity of receptive fields (Quartz and Sejnowski, 1997). However, analogy with morphological specialization elsewhere in animal bodies suggests that contingently activated developmental procedures, themselves potentially stimulated by inputs, may also play a role. Increasing evidence suggests that local patterns of gene expression may influence the developmental reaction norm that an area of tissue adopts, that is, how it self-organizes in response to inputs (Rash and Grove, 2006; O’Leary et al., 2007). If the topological organization of inputs to different brain regions is consistent across generations, then locally contingent developmental procedures can begin to evolve via descent with modification.
Descent with Modification of Reaction Norms
During evolution of the brain, the developmental properties of brain tissue are subject to evolutionary modification based on the effects they have on brain phenotypes. This can be initiated by initial changes in developmental systems (e.g., via mutation), changes in the environment in which they develop, or both. For example, as organisms’ environments change (including changes in the social environment), developmental outcomes that were theoretically part of the brain’s reaction norm, but rarely or never produced, can become more strongly acted upon by selection (Price et al., 2003). For example, if a previously non–language-speaking
species begins to evolve language capacities, developmental processes that were not previously involved in language may come under selection specifically because of their effects in language acquisition, resulting in modification of older adaptations.
Descent with modification results in patterns of specialization that have a hierarchical character (Fig. 17.2). As brain specializations evolve through descent with modification, they inherit ancestral design features—including underlying genomic building blocks and regulatory machinery—that were present before recently derived changes. This means that adaptations usually exhibit a mix of ancestral and derived features, which interact in their contribution to the adaptation’s function. Ancestral features may in turn be shared across homologous specializations within or between taxa, meaning that derived specializations may be tokens of homologous types within the same organism, and across organisms.
For example, the evolution of limb specialization in animals exhibits this hierarchical character. Within taxa, diverse limb types evolve through processes of serial homology, descending from ancestral limb types via processes of diverging specialization. The result is that the distinct limb types of a given species of animal, such as a crustacean, an insect, or a mammal, exhibit many shared design features and shared developmental machinery, but nested within this shared specialization at the level of limbs are divergent specializations for each limb type (Carroll et al., 2005). In this sense, limb specialization is hierarchical. It exhibits substantial evolutionary conservation of developmental machinery, meaning that “new” specializations are composed largely of “old” design features, rearranged and modified. Brain specializations are likely to exhibit these properties as well.
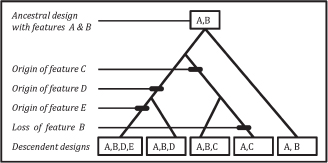
FIGURE 17.2 Descent with modification of organismal traits can lead to hierarchically organized design features. In this example, descendent versions (Lower) of an ancestral design (Upper) exhibit a mix of ancestral and derived features.
Implications for Conventional Models in Psychology
Psychologists typically assume a choice between domain-specific and domain-general mechanisms: a given psychological process must be handled by one or the other, or perhaps a mix of the two. However, if brain specializations contain a mix of general and specialized features within the same adaptation, this has important implications for efforts to empirically test between domain-specific and domain-general accounts in psychology, because the assumption that domain-specific and domain-general aspects of processing represent distinct mechanisms may be false.
As an example, consider the debate over face recognition in cognitive neuroscience. Studies with functional MRI and single-unit recording have shown that humans and other primates possess brain regions that are differentially sensitive to faces, particularly the so-called facial fusiform area (FFA) in the fusiform gyrus (Kanwisher and Yovel, 2006). Impairments to this area can produce deficits in face recognition while leaving other object recognition abilities relatively intact, a condition known as prosopagnosia (Duchaine et al., 2006). Debates have ensued over whether the FFA is an evolved adaptation for face recognition. The domain-specific view holds that it is (Kanwisher and Yovel, 2006). The domain-general or “expertise” view holds that the relevant adaptation is for developing expertise about objects in the local environment, and that faces are simply a type of object that is frequently encountered, leading to ontogenetic specialization of an area highly sensitive to faces without any evolved adaptation for recognizing faces per se (Gauthier and Nelson, 2001). Evidence in favor of this view includes training studies that show that exposure to repeated instances of novel objects can produce cognitive processing signatures similar to those seen with faces (e.g., inversion effects), and activation of the FFA for those stimuli (Gauthier et al., 1999).
Although both positions are cogent, a hierarchical specialization view suggests that they might not be as distinct as the debate suggests. Given the location of the FFA within a larger region known to be active in object recognition more generally, it is likely that face recognition abilities are a specific token within a type category of object recognition procedures, akin to claws as a token of crustacean limbs more generally. Thus, processing signatures characteristic of objects in general are of limited use in testing between the domain-specific and domain-general hypotheses because, like limbs, specialized brain structures are likely to exhibit a combination of specialized and general properties. Moreover, observations suggesting that the FFA becomes progressively tuned to faces during development (Scherf et al., 2007) do not rule out the domain-specific hypothesis, because one would expect module-spawning procedures to use input as part of their ontogenetic differentiation process. The question is whether the ontogenetic specialization of the FFA is something that has been specifically
selected for as a result of its consequences for fitness in ancestral environments—a much more difficult question to answer.
These problems have been viewed as weighing against domain--specific hypotheses, on the assumption that domain-general hypotheses are more parsimonious (i.e., simpler) and therefore more likely to be true. However, analogy with morphological development suggests that this is a problematic assumption. It would be hard to argue that morphological differentiation in animals, for example, proceeds via the simplest possible set of processes, or that parsimony considerations alone would lead us to correctly infer their design. Moreover, the phylogeny and natural history of taxa can shift the burden regarding which account is more parsimonious. Many primates are highly social and can identify individuals in the wild, an ability that likely has fitness benefits (Cheney and Seyfarth, 2007), and social species such as macaques appear to have face recognition areas homologous to those in humans (Rolls, 2000). Thus, the hypothesis that there has been no selection for face recognition in our lineage may be less likely than the hypothesis that there has been.
An implication of the hierarchical specialization view is that signatures of general processing, such as Bayesian updating or statistical learning, may be shared by specialized mechanisms as well. Thus, the common assumption that such signatures weigh against more domain-specific accounts (Elman et al., 1996) should be taken with caution, and other factors should be weighed in mediating between domain-general and domain-specific hypotheses, including phylogeny, natural history, and cognitive form-function analyses akin to those used in functional morphology (Tooby and Cosmides, 1992).
ORIGIN OF NEW BRAIN SPECIALIZATIONS
How do “new” brain specializations—that is, specializations that are derived rather than ancestral in a particular lineage—evolve? If derived brain specializations evolve from ancestral ones via processes of descent with modification, and if these historical processes leave a signature in the design and organization of brain mechanisms, this has implications for the study of human brain architecture and the evolution of so-called uniquely human traits such as language and complex culture.
Varieties of Homology
Homologous traits are traits that descended from a single ancestral trait. Homologies therefore exhibit nested hierarchical relationships that are the signature of phylogenetic processes of descent with modification. Complex brains in humans and other vertebrates likely evolved from
simpler nervous systems through processes of divergent specialization of brain regions and structures, so many (but not all) human brain mechanisms and processes are likely to exhibit relationships of homology (Kaas, 1989; Striedter, 2005).
Several types of homology can be distinguished based on how and when they originate (Fig. 17.3). Orthologous traits are traits in two species that originate from a single ancestral trait in the last shared common ancestor of those species. Paralogous traits, also known as serial homologs, are homologous traits within a single species that have originated through a process of duplication and divergence (Fitch, 1970; Ohno, 1970; Hall, 1995; Koonin, 2005). Outparalogs are traits that arose via duplication and divergence before a speciation event that split two taxa; the descendent taxa will therefore all possess versions of the multiple, paralogous traits. Inparalogs evolved via duplication and divergence within a specific lineage (note that these terms were originally proposed to refer to gene homologies, but are extended here to phenotypic and developmental traits).
Many traits of organisms appear to have arisen through processes of duplication and divergence. Examples include the specialized limb types of vertebrates and invertebrates (Carroll et al., 2005), protein families such as opsins (Dulai et al., 1999), and regulatory gene families such as the Hox cluster (Lemons and McGinnis, 2006). Brain scientists believe that processes
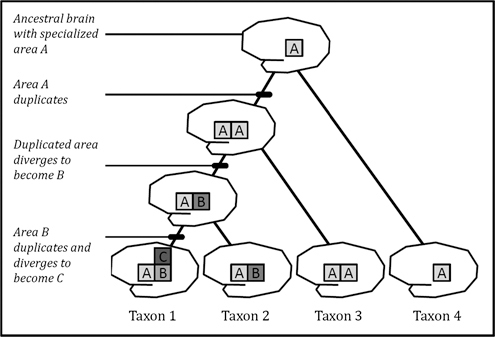
FIGURE 17.3 Varieties of homology. Region A is orthologous across taxa 1 to 4 as a result of shared descent from the ancestral taxon. Regions A and B are paralogs, originating through a duplication event (as are the two copies of A in taxon 3). Regions A and B are outparalogs in taxa 1 and 2, originating through duplication before divergence of the two taxa. Regions B and C are inparalogs in taxon 1.
of duplication and divergence may account for the origin of new brain areas and processes as well (Kaas, 1984, 1989; Striedter, 2005; Marcus, 2006).
Duplication and Divergence in the Brain
There are several possibilities for how new brain structures might evolve through duplication and divergence. One is that an initial change in development, for example, caused by a mutation, duplicates an existing brain area, producing two structures where there had been one. These can then diverge if, for example, one structure retains its initial function while selection then modifies the function of the other (Ohno, 1970; Kaas, 1989). Duplication may also alter selection on both structures, allowing them to carve up what was previously a single functional space, in a process akin to adaptive radiation (Hughes, 1994). Such a process may have driven functional divergence following gene duplication in the evolution of primate digestive enzymes (Zhang et al., 2002b) and color vision (Dulai et al., 1999). It is also possible that divergence could begin without an initial mutation, with an environmental change producing novel phenotypic outcomes, which are then exposed to selection (Price et al., 2003). For example, module-spawning reaction norms might initially bifurcate an area into two as a function of new inputs in the environment (e.g., tools, language), setting the stage for selection to act independently on the two new areas (Krubitzer and Huffman, 2000).
Specialized, category-specific object recognition capacities may have evolved via duplication and divergence from a previously undifferentiated object recognition system. There is evidence for such category-specific capacities in humans and other primates: for example, areas possibly specialized for recognition of faces (Kanwisher and Yovel, 2006), bodies (Downing et al., 2001), places (Epstein and Kanwisher, 1998), and tools (Johnson-Frey, 2004). Such areas could have evolved from a single, primitive, object recognition system in an ancestral mammal, which had not yet been parcellated into specialized regions. In such a scenario, an initial change (i.e., a mutation or environmental change) could have caused developmental subdivision or duplication of this region, allowing selection to favor divergence of the new areas.
Consider a hypothetical scenario for the evolution of a specialized capacity to distinguish between individual conspecifics based on their facial features. In some social species, there may be significant benefits to being able to recognize and distinguish between individual conspecifics (e.g., distinguishing between kin and nonkin, remembering prior cooperative partners), setting the stage for selection to act on variants that might enhance this ability (Cheney and Seyfarth, 2007). One can imagine an ancestral state in which no face-specific ability existed, only
more general object recognition systems that develop expertise through exposure to large samples of within- and between-category variation in objects repeatedly attended to (e.g., predators, conspecifics). Against such a background, any initial change that caused individuals to attend specifically to faces would begin to drive the development of face expertise within the object recognition area, a change that could be favored by selection if it yielded fitness advantages. For example, a mutation or set of mutations that altered perceptual and/or attentional systems to draw more attention to eyes or other facial features (themselves potentially favored for additional reasons, for example, emotion processing) would lead to longer bouts of face input to object systems and in turn greater face expertise. Additionally, any event leading to duplication or bifurcation of the object recognition area—including, perhaps, a module-spawning process triggered by increased face input—could set the stage for further specialization of a dedicated face area via duplication and divergence. In such a scenario, one would expect development of the resulting region to be reliant both on external inputs (i.e., exposure to faces) and mechanisms causing preferential attention to faces during development. There is evidence for attention-orienting mechanisms of this kind in newborn human infants (Johnson et al., 1991) and in other primates (Sugita, 2008).
Similar scenarios could account for the evolution of other specialized capacities from more generalized precursors, including other types of specialized object recognition (e.g., tools, places, body parts) and higher-level skills of language and reasoning as specialized versions of more general primate brain processes. We might expect many new abilities to exhibit relationships of homology to more general-purpose abilities, and relationships of paralogy to their relatives in the duplication and divergence process. If so, this could be evidenced by, among other things, shared network connectivity in the brain, adjacent localization, and shared processing signatures. For example, features of object processing such as inversion effects (i.e., difficulties with recognizing individual objects upside-down) and “holistic” processing effects (i.e., processing of relationships between parts) could be shared partly or fully across distinct object-processing systems (Bukach et al., 2006). More generally, other signatures of neural processing might be widely duplicated across brain mechanisms and regions, for example, Bayesian updating procedures, statistical learning, effects of magnitude such as those described in Weber’s law, and others (Kirkham et al., 2002; Nieder and Miller, 2003; Chater et al., 2006).
Role of Evolutionary Feedback
Over evolutionary time, changes in the brain can beget further evolutionary changes through processes of evolutionary feedback, including
“runaway” or self-catalyzing evolutionary processes (Lehtonen and Kokko, 2012). Changes in one part of the brain can alter how information is routed to or processed by other parts of the brain, potentially altering how natural selection acts on those areas, as in the scenario described earlier in which increased attention to faces might alter selection on object recognition areas. Changes in the brain can also alter the environment itself, setting the stage for further evolutionary change as the new environmental properties in turn alter selection on those same brain regions or others, a process sometimes known as niche construction (Laland et al., 2000). For example, an initial change in the brain that enables slightly more complex communicative abilities—for example, the ability to combine words into more complex utterances in an early protolanguage—changes what is possible for individuals to communicate to each other, potentially leading to further selection when new variants on these communicative skills arise (Jackendoff, 1999). This can lead to a runaway process as brain mechanisms and their behavioral products increase in complexity over evolutionary time.
Similar effects may have obtained throughout human evolution as ancestral hominins developed more sophisticated cultural transmission abilities, leading to environments filled with the products of culture, such as complex languages, tools, and built environments (Richerson and Boyd, 2006). In addition, increasing social complexity may have favored the evolution of new or modified brain mechanisms for social cognition, such as increasingly sophisticated abilities to make inferences about the intentions and mental states of others, known as “mindreading” or “theory of mind” (Saxe, 2006), as well as improved abilities of cooperation and an associated moral sense (Richerson and Boyd, 2006). In all these cases, evolutionary feedback effects could have occurred between brain mechanisms (i.e., evolutionary change of one brain mechanism alters selection on others) and between brain and world (i.e., evolutionary change in the brain alters the species’ environment, and vice-versa).
Word Perception as an Example
A useful example of how such evolutionary change might occur comes from studies of how reading occurs in the brain. Converging evidence from brain mapping, behavioral studies, and cases of brain damage point to the existence of an area in the left fusiform gyrus of the visual cortex that is specialized for the processing of written words. This area, called the visual word form area (VFWA), occupies a similar location across individuals literate in different languages and exhibits processing signatures consistent with specialization for identifying whole written words, such as insensitivity to font and word length (Cohen and Dehaene, 2004; Dehaene, 2009).
What does it mean to say that this area is “specialized” for word recognition? That natural selection has shaped this region specifically because of the fitness benefits of reading seems unlikely, as the oldest human writing systems are no more than a few thousand years old. Instead, it seems more likely that this area becomes ontogenetically specialized for words through a process of increasing expertise (Dehaene and Cohen, 2007; Dehaene, 2009; Anderson, 2010). Indeed, the development of this area shares similarities with development of perceptual expertise more generally, including correlations between practice and developmental speed and experience-specific sensitivity to properties of the stimulus class. However, this is not to say that the region in which the VWFA area develops is evolutionarily general-purpose, nor that the VWFA could develop anywhere in the brain. Instead, the location of the VWFA is remarkably similar across individuals literate in diverse languages, and it develops within an area of the visual cortex, the fusiform gyrus, in which other specialized object recognition capacities, such as face recognition, develop (Dehaene, 2009). This is consistent with a hierarchical specialization view: word recognition is a token, albeit an evolutionarily novel one, of an evolutionarily specialized type of brain mechanism, that is, a category-specific object recognition module. It develops when and where it does, in individuals exposed to written language, because written words activate the reaction norm of a specialized developmental system that spawns category-specific modules upon repeated exposure to a recurring class of objects.
Interestingly, there is evidence that written languages themselves have culturally evolved to satisfy the input conditions of object recognition systems. A recent study (Changizi et al., 2006) found that the distribution of junction types in the written letters of diverse world languages closely overlaps the distribution of such junctions in natural scenes, suggesting that processes of cultural evolution have favored retention of letters that are easily processed by human object recognition systems. This appears to be a case of evolutionary feedback in which the design of perceptual systems influences the cultural evolution of written words, which in turn ontogenetically shape a specialized brain area. It may also represent a case of evolution in progress and could be a useful exemplar of how new brain specializations evolve following an initial event such as the appearance of writing.
Effects of Increasing Brain Size
Humans have much larger brains than our closest primate relatives, even relative to body size (Striedter, 2005). When explaining unique aspects of human intelligence and flexibility, increased brain size is sometimes
presented as an alternative to the idea that humans possess species-specific brain specializations. However, a hierarchical specialization view suggests that these are not necessarily mutually exclusive alternatives. Indeed, increasing brain size may lead to more specialization, not less, and more specialization may be related to greater, not less, flexibility.
Modularity can be defined in multiple ways. In network theory, modularity refers to the relative amount of within-region vs. between-region connectivity in a network, such as a network of neurons: more modularity means less relative connectivity between regions (Meunier et al., 2009). As brains increase in size, there are simple architectural reasons to expect that modularity, in this sense, will increase (Kaas, 1989; Striedter, 2005). As the number of nodes in a network increases, keeping them all connected to every other node becomes more and more difficult for reasons of space, leading to greater modularity. Comparative brain studies suggest that species with larger brains tend to have greater differentiation of the expanded brain areas, for example, cortical regions (Kaas, 1989, 2000; Striedter, 2005).
If increasing brain size and increasing modularity are linked, there are interesting empirical questions about what selective factors have driven the evolution of large brains in humans. One possibility is that the prime mover in brain expansion was selection for increased neural processing power per se. However, if increased brain size forces increased modularity for architectural reasons, this may set the stage for natural selection to favor further specialization of the resulting brain regions. Another possibility is that selection for specialization itself was the prime mover. If the best way to produce new specialized regions is to increase brain size—including, perhaps, duplicating existing brain areas—then selection for specialization could have favored mutations that increased overall brain volume, thereby increasing modularity. These are not mutually exclusive scenarios, and it may be difficult if not impossible to empirically tease them apart.
In psychology, it is common to assume that increasing modularity is associated with decreasing flexibility, and that undifferentiated, general-purpose systems are more flexible than differentiated, modular ones. However, there are reasons to think that the opposite may be true. In computer science, for example, it is generally recognized that modular software designs yield greater flexibility than nonmodular ones: adding a new modular algorithm to an existing system increases the number of functions it can perform while keeping previously existing functions intact, thereby adding flexibility (as well as robustness, i.e., ability of the system to withstand partial loss of function) (Baldwin and Clark, 2000). Similarly, it may be that the greater modularity seen in larger brains may yield greater behavioral flexibility compared with smaller, less modular brains (Kaas,
1989). The reason is that increasing modularity allows a greater number of interacting parts, yielding more and more complex combinatorial repertoires. If modularity and flexibility are positively rather than negatively related, this may have important implications for understanding the evolution of drastically larger brains in the human lineage.
EXPLAINING HUMAN COGNITION
One of psychology’s holy grails is to explain what makes us psychologically unique: different from other apes, primates, mammals, and animals more generally. The facts of descent with modification mean that this will mostly involve modifications to the brain machinery present in the chimpanzee-human common ancestor (CHCA), along with the addition of some truly new, or derived, mechanisms. These changes include modifications to the base pair sequences in our genome (Chimpanzee Sequencing and Analysis Consortium, 2005), modifications to the regulatory machinery that shapes how genes are expressed during development (Khaitovich et al., 2004; Preuss et al., 2004), and changes in the physical and cultural environments in which humans develop, which differ substantially from those of chimpanzees (Richerson and Boyd, 2006).
What We Will Need to Explain
The CHCA was a hominoid ape with a likely brain volume in the range of 300 to 400 cm3 and the large and complex cortex characteristic of ape brains (Kappelman, 1996). Comparisons with modern chimps and bonobos suggest that the CHCA was likely to be a social species with a relatively long lifespan and a sophisticated cognitive toolkit including social learning of tool use, “Machiavellian” social intelligence, and some elements of theory of mind, such as tracking others’ knowledge of food in food competition and sensitivity to intentional communication in contexts such as aggression and reconciliation (Call and Tomasello, 2008; Whiten, 2011). However, although humans and chimps share versions of all of these abilities, most appear to have been substantially elaborated in our lineage, along with some genuinely new abilities not present in chimps.
Modern humans differ from chimps, and probably from the CHCA, in many ways. Humans have spoken languages with complex grammars and arbitrary symbol-meaning mappings (Pinker, 1994). We live and cooperate in larger and more diverse social groups, and are the only species known to have cumulative or “ratcheting” cultural evolution in which the products of culture (e.g., languages, tools, social practices) increase in complexity over generations (Richerson and Boyd, 2006).
Humans are much more rapid social learners than chimps, probably at least in part because of greater sensitivity to others’ goals and mental states (Whiten, 2011). There are likely many other differences as well, such as finer-grained motor capacities (Gibson, 2002) and improved “executive” capacities of impulse control and deliberative weighing of behavioral options (Striedter, 2005). Changes in human brains and human lifeways likely coevolved via a feedback processes, involving multiple changes in brain and behavior (Kaplan et al., 2004).
Changes in Genes, Gene Regulation, and Environments
Derived features of human cognition must eventually be accounted for by changes in genes, gene regulation, and human environments (e.g., cultural, linguistic, and artifactual environments). Although we still have a long way to go before understanding these changes, rapid technological advances—including advances in genome sequencing, expression studies, and the sequencing of archaic DNA—are beginning to yield the raw data that can be used to make inferences about how hominin brain architecture has been modified since the CHCA.
Several candidate genes thought to influence brain size (Evans et al., 2005), brain differentiation (Pollard et al., 2006), and other aspects of nervous system development (Dorus et al., 2004) show evidence of selection in the human lineage, although the functional significance of many of these changes is still unknown and they are the subject of active debate and research (Montgomery et al., 2011). In addition to changes in cortical development, there appears to have been selection for increased white matter in humans (Schoenemann et al., 2005), suggesting that modifications in how brain regions communicate with each other may have played an important role in hominin brain evolution.
If the hierarchical specialization view is correct, we should expect to see selection on genes with different patterns of expression or activity in different parts of the brain. Evidence suggests that some brain areas in humans have expanded differentially with respect to their orthologs in other primates, for example, prefrontal cortex (Rilling and Insel, 1999; Schoenemann et al., 2005; Balsters et al., 2010). Work with other species suggests area-specific gene expression is likely to play an important role in such differential development (Rash and Grove, 2006; O’Leary et al., 2007). Studies of gene expression in the brain have shown substantial differences between humans and chimps (Khaitovich et al., 2004; Preuss et al., 2004), although these studies involve brainwide differences in gene expression measured at the end of life, not ontogenesis. Although detailed studies of regional gene expression in the brain during development must await technological advances, the hierarchical model suggests
several types of changes we might expect to see in humans compared with other primates.
Modified Orthologies
Many derived features of human cognition may be the result of modifications to older brain systems, in the form of modifying the design of those systems per se or modifying how they interface with other and perhaps newer systems. Such modifications are likely to be involved, for example, in the evolution of human language abilities. Although there is debate about exactly how to characterize the uniquely derived features of human linguistic abilities, there is little doubt that these abilities, taken as a whole, are unique among primates and animals more generally (Christiansen and Kirby, 2003). Yet, human abilities to learn, produce, and understand speech appear to mostly or entirely depend on brain regions and processes that have homologs in other primates (Hickok and Poeppel, 2007; Rauschecker and Scott, 2009). This implies that unique aspects of human language may result from derived changes in one or more of these regions, along with, perhaps, changes in how they interface with each other during development and language processing. One example may be the planum temporale, a region associated with language processing that appears to have undergone internal changes in the organization of minicolumns compared with chimpanzees, and specifically in the left hemisphere (Buxhoeveden et al., 2001). Some regions involved in human language processing exhibit substantial laterality (Hickok and Poeppel, 2007), have greater connectivity between them via white matter pathways (Friederici, 2009), and have greater connectivity to other brain areas than do orthologous regions in nonhuman primates (Rilling et al., 2008). This suggests that modifications in how specialized structures interact may play an important role in derived human abilities, in addition to modifications within specialized structures themselves (Balsters et al., 2010). Human language capacities may also rely heavily on interfaces between language areas and other systems, allowing us to, for example, refer to objects in our visual field, talk about things we remember, and use metaphors in the service of reasoning (Jackendoff, 1999; Boroditsky, 2000; Hickok and Poeppel, 2007). Thus, at least some apparently unique aspects of human cognition may result from novel synergies between phylogenetically older mechanisms, enabled by changes in how these mechanisms interact.
Paralogies
Studies of language areas and other cortical regions showing anatomical evidence of microstructural changes within the areas of themselves—
for example, planum temporale (Friederici, 2009) and Brodmann area 10, implicated in executive functioning (Semendeferi et al., 2001; Gilbert et al., 2006)—suggest the possibility of duplication of subunits within those regions. There may be other cases of paralogy at larger scales as well. Some of these might be outparalogies, as in the case of specialized areas in the visual cortex for recognizing faces, bodies, and other kinds of objects. Others might be inparalogies: duplication and divergence events that have occurred within the hominin lineage. One, the VWFA, might be an example of a paralogy in progress. Others might be older.
Consider, for example, areas specialized for tool use. There is considerable evidence for specialized processing of human-made tools in the human brain, involving coordinated links among perceptual, conceptual, and motor systems (Johnson-Frey, 2004). Although studies of tool use in other primates show that homology is clearly involved (Obayashi et al., 2001), specialized regions in temporal cortex and parietal cortex (for tool identification and action knowledge, respectively) may have evolved through processes of differentiation and specialization as use of complex tools became a regular part of the human cognitive and behavioral repertoire from the origins of the genus Homo onward. Tool identification regions in the temporal lobes may be paralogous with other specialized object perception regions, for example, for faces, and tool areas in the parietal lobes may represent tool-specific tokens of adaptations for systematizing gestural knowledge.
Of course, these examples are tentative and await further work. There may be other mechanisms of higher-level cognition that have evolved through duplication and divergence—a possibility suggested by expansion of prefrontal cortex in humans—but there remains controversy over how to characterize specializations in this area. Given the many apparently unique aspects of human cognition, including ratcheting cultural evolution, language, the ability to cooperate in large groups, morality, and a unique elaborated theory of mind, there are likely to be many additional examples of derived specializations in humans that we have not yet discovered. However, we should not necessarily expect all or even most of these to have appeared entirely de novo in our lineage, but rather, to have evolved from older precursors through descent with modification.
CONCLUSIONS
If the model presented here is correct, many widely held views in psychology about the nature of brain specializations may need to be rethought, along with the empirical implications of those views. In particular, many of the perceived tensions between specialized and general-purpose mechanisms may not exist, or at least not in the form envisioned
by “dualist” accounts. In addition, many types of evidence widely thought to adjudicate between domain-specific and domain-general accounts—for example, plasticity, which is often held to weigh against specialization—might not.
From a biological point of view, what makes an aspect of brain structure an adaptation is whether it has been selected for, not whether it has a particular set of features such as canalization, narrow targeting of a particular class of stimuli, or isolation from other systems. If the hierarchical specialization model is correct, some brain networks and processes may be minimally different from others, highly plastic, and depend on human-specific environmental factors to develop—and yet may still be the products of selection. If so, the human brain contains adaptations whose empirical signature is quite different from what many psychologists expect to see for a “module.”
For example, variation in developmental outcomes across individuals, environments, or cultures—typically interpreted by psychologists as evidence against specialized adaptations—might be standard for many brain adaptations, especially in our highly variable and cultural species. Adaptations for language acquisition, if they exist, would be an example: they must produce highly variable outcomes as part of their evolved design, given the many ways in which the world’s languages differ (Evans and Levinson, 2009). Moreover, if new brain specializations evolve through divergent specialization from existing structures, “gene shortage” arguments against the existence of multiple, derived brain specializations in humans—that is, that there are not sufficient genetic and regulatory differences between humans and chimpanzees to account for brain differences—may not hold water (Marcus, 2004).
Although specialist/generalist tradeoffs are likely to be important in shaping brain evolution, they might not always take the form we envision. Many psychologists believe that evolutionary considerations imply a tradeoff between a few generalized processes and many specialized ones, and that the former is more likely because generalized processes yield more flexibility. However, if it turns out that the way evolution creates more flexible brains is by proliferating specialized brain regions that carve up computational problems via specialized division of labor, this widely held assumption may turn out to be wrong.
The hierarchical model presented here poses new challenges for developing and testing hypotheses about evolved specializations in the brain. First, it suggests that the “checklist” of features widely associated with modules does not constitute a checklist for adaptations. Second, it suggests that proximate-level accounts invoking, for example, spatial and temporal interactions between developing brain regions, should not be treated as alternatives to ultimate-level accounts invoking selection; after
all, modification of those interactions is an important way in which developmental outcomes can be selected for. Third, it suggests that domain-general processing signatures may be characteristic of more specialized mechanisms as well.
If these conclusions are true, many current debates about how to interpret data for or against specialization may represent arguments over apples vs. oranges. For example, a particular phenotypic outcome in the brain may be contingent on developmental input, and also the result of a reaction norm selected to produce that phenotype given that input. To properly test evolutionary hypotheses about brain specialization, then, it is important to compare apples against apples and oranges against oranges: to compare hypotheses posed at equivalent levels of the ultimate–proximate continuum of evolutionary causation. Ideally, the most progress will be made when we can compare hypotheses that specify both proximate mechanisms, such as developmental constraints and neural wiring rules, and ultimate reasons for how and why those mechanisms have evolved and been modified in various species, including us, to produce the outcomes we see.