Identification and Modulation of
Biophysical Signals That Control
Stem Cell Function and Fate
DAVID V. SCHAFFER
University of California, Berkeley
Stem cells are defined by two hallmark properties: the ability to self-renew, or divide while maintaining themselves in an immature state, and the capacity to differentiate into one or more specialized cell types. By virtue of these properties, stem cells play central roles in the development and maintenance of tissues throughout the body, and researchers in the biomedical field are increasingly exploring their potential in cell replacement therapies for treating human disease or injury. In particular, stem cells can theoretically be harvested, expanded, and differentiated in culture, and implanted for tissue regeneration. Alternatively, it may be possible to modulate endogenous pools of stem cells for tissue repair. To achieve both a deeper understanding of their natural biological functions and the ability to tap into their promise as next-generation therapeutics, fundamental knowledge is needed about how stem cell behavior is controlled and, specifically, about the processes of self-renewal and differentiation.
BACKGROUND
Populations of stem cells reside in specialized regions of developing and adult tissues that continuously present them with regulatory cues, and this repertoire of signals is collectively referred to as the stem cell niche (Scadden 2006). This niche includes small molecules, proteins, and other components of the extracellular matrix (ECM; the solid phase material that enmeshes most cells in the body), small growth factor and morphogen proteins that may be soluble or immobilized to the ECM, and signals from the surface of neighboring cells (Figure 1).
Thanks to many successful efforts in genetics, developmental biology, and cell biology, it is well recognized that biochemical cues in the niche play criti-
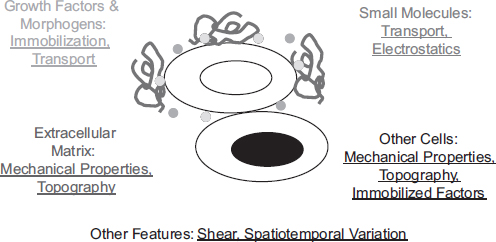
FIGURE 1 Schematic of the stem cell niche. Soluble small molecules, soluble and immobilized growth factor and morphogen proteins, extracellular matrix components, and intercellular components collaborate to regulate stem cell behavior. In addition, numerous physical and engineering principles modulate the manner in which these components present information, including mechanical properties, spatial organization and temporal variation in the presentation of cues, topographical features of the niche on the nano-and microscale, mass transport properties, and electrostatics.
cal roles in regulating stem cell function. However, biology encodes regulatory information to cells not only in the binary absence or presence of a given molecule but also in numerous biophysical aspects of tissues—mechanics, topographical features, electrostatics, biological transport phenomena, and spatiotemporal variation in each of these cues (Figure 1). Thus a major difficulty in studying and manipulating the biophysical properties of the niche is that they are not monogenic but depend on the properties of many molecules and genes.
An emerging theme in stem cell research is to use engineered systems in cell culture—ranging from synthetic materials to microfluidic devices—to systematically vary these biophysical properties and thereby study their effects on stem cells, that is, to provide an “x-axis” in a manner that is not currently possible with genetic approaches. While there are inherent challenges with this paradigm—including establishing the in vivo relevance of findings, as well as integrating engineering and biology approaches to explore the underlying mechanisms—these engineering studies have broadened the field’s view of the stem cell niche (Discher et al. 2009; Keung et al. 2010). Furthermore, because of the complexity of their endogenous niches, stem cells are exceedingly difficult to control in culture; therefore, each biophysical property offers a new opportunity to engineer synthetic systems and materials to control stem cell function for regenerative medicine applications.
MECHANOREGULATION OF STEM CELL FUNCTION
There are many mechanical features and processes of tissues that may regulate cell function, including elasticity, viscosity, strain, and others. Landmark work by Engler and colleagues (2006) demonstrated that the lineage choice of differentiating mesenchymal stem cells (MSCs) is strongly influenced by elastic modulus of the surrounding material—i.e., the linear proportionality constant between its strain and stress—such that cells developed into neuron-like cells on soft hydrogels, myoblasts on intermediate stiffnesses, and osteocytes on harder substrates. Subsequent work showed that neural stem cells (NSCs) preferentially differentiate into neurons when cultured on soft materials and astrocytes on hard materials (Saha et al. 2008). Also, a recent study reported that human embryonic stem cell and induced pluripotent stem cell differentiation into neural lineages, but not self-renewal, is mechanosensitive (Figure 2) (Keung et al. 2012). In addition to differentiation, modulus can influence stem cell self-renewal. For example, it was shown that substrate stiffness strongly affects the ability of muscle stem cells to undergo self-renewal in culture and subsequently their capacity to undergo reimplantation into muscle (Gilbert et al. 2010).
In parallel, the regulation of stem cell behavior by extracellular forces requires mechanisms to convert a mechanical cue into a biochemical signal that drives cell fate decisions. ECM protein structures, cell adhesion receptors, the intracellular network of structural proteins known as the cytoskeleton, and key proteins in the nucleus may all serve as mechanosensors. In addition to the stiffness of the cellular microenvironment, shear flow and cyclic strain have both been implicated in regulating the self-renewal and/or differentiation of several classes of stem cells.
Collectively, the studies described above have established the mechanical properties of the stem cell niche as a prominent regulator of fate choice, and offer the promise that mechanical aspects of synthetic materials can be manipulated to better control stem cell fate choice in culture.
TOPOGRAPHICAL AND SHAPE FEATURES
OF THE STEM CELL NICHE
In addition to providing resident stem cells with a mechanical milieu, niches provide features that can alter the shape of a cell. On the microscale, ECM and neighboring cells can modulate and even constrain the surface area or volume available to, and therefore the shape of, a cell in a manner important for its function. Likewise, on the nanoscale, ECM proteins often assemble into fibers and other structural features that modulate the topographical features that an adherent cell experiences. Advances in lithography and in materials science have enabled investigators to investigate the effects of these features on stem cell behavior (Kolind et al. 2012).
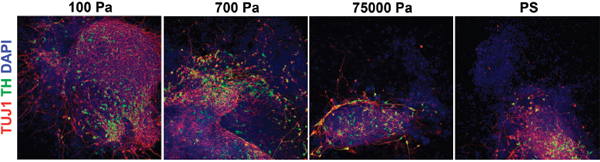
FIGURE 2 Mechanical cues regulate human embryonic stem cell differentiation. Human embryonic stem cells were differentiated into dopaminergic neurons, the cell type that is lost in patients with Parkinson’s disease. During the initial phase of differentiation, cells were cultured on polymeric hydrogels of various elastic moduli. The softer materials, coincidentally with stiffnesses characteristic of the brain, led to significantly higher numbers of dopaminergic neurons. Red is the neuronal marker TUJ1, green is the neuronal marker tyrosine hydroxylase (TH), and blue is the nuclear dye DAPI. Pa = Pascal; PS = polystyrene. Adapted from Keung et al. (2012) with permission of The Royal Society of Chemistry. This figure appears in color in the online posting of this article at http://www.nap.edu/catalog.php?record_id=18185.
In seminal work, microcontact printing was used to pattern adhesive islands of different sizes onto a surface (McBeath et al. 2004). When MSCs were seeded onto these substrates, the investigators observed that large islands that enabled cells to spread subsequently promoted osteogenic (bone cell) differentiation, whereas small islands that did not permit substantial cell spreading instead promoted adipogenic (fat cell) differentiation. There has been progress in both extending this principle to other fate choices and elucidating its underlying mechanisms.
In addition to microenvironmental properties that alter cell shape on the micron scale, topographical cues—such as the organization of the ECM into fibers—offer a cell with features that can modulate its shape at the nanometer scale. In early work in this area, culturing NSCs on microgrooves patterned into polystyrene led to significantly higher extents of neuronal differentiation compared to flat surfaces (Recknor et al. 2006). Another study explored the effects of electrospun fibers of polyethersulfone with different dimensions on the NSC behavior and found that small fibers promoted differentiation into one major central nervous system cell type (oligodendrocytes) while larger fibers increased differentiation into neurons (Christopherson et al. 2009). These studies have yielded insights into mechanisms by which structural features in the niche can regulate cell function, and again offer potential opportunities to design biomimetic culture systems that can better control stem cell behavior.
ELECTRIC FIELDS
The role of electrophysiology in the cardiovascular and nervous systems is well appreciated, and a growing body of work has explored the possibility that electric fields may regulate the function of stem cells from these tissues. In initial work, heart muscle precursors became aligned with the direction of an electric field, exhibited a substantial increase in contractile amplitude, and expressed higher levels of various cardiac protein markers compared to cells that were not electrically stimulated (Radisic et al. 2004). Subsequent research has shown that electric fields promote the maturation and differentiation of skeletal muscle precursors (Serena et al. 2008), neural precursors (Ariza et al. 2010), and embryonic stem cells (Kabiri et al. 2012).
FUTURE DIRECTIONS
The application of the physical sciences and engineering to stem cell research has contributed significantly to the development of culture systems to elucidate the basic effects of a biophysical property on cell regulation. Such investigations will greatly benefit from further technological advances, particularly in the development of novel materials whose properties can be varied spatiotemporally to mimic tissue heterogeneity and development. Furthermore, there are consider-
able additional opportunities for “analysis by synthesis”—engineering systems to emulate and thereby investigate more features of the cellular microenvironment.
Another major need in the field is the development of scalable, safe, and reproducible stem cell culture systems for biomedical translation. Many current culture systems use complex and poorly defined protein mixtures (e.g., serum, matrix) to recreate the complexity of the niche. Basic progress in understanding of key biochemical and biophysical cues can be integrated toward the development of advanced, defined, synthetic culture systems that are in some ways less complicated than current systems containing components derived from animal or human tissue.
Finally, a major challenge in the application of stem cells for tissue engineering and repair is poor cell survival upon implantation into a site of injury or disease, although engineered systems and materials that increasingly integrate biological information to mimic the natural properties of tissue may serve as vehicles that enable cells to better adapt to their new niche after implantation. The integration of biology, physical sciences, and engineering is thus poised to greatly advance stem cell biology and medicine.
REFERENCES
Ariza CA, Fleury AT, Tormos CJ, Petruk V, Chawla S, Oh J, Sakaguchi DS, Mallapragada SK. 2010. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Reviews 6:585–600.
Christopherson GT, Song H, Mao H-Q. 2009. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 30:556–564.
Discher DE, Mooney DJ, Zandstra PW. 2009. Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–1677.
Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689.
Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. 2010. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329:1078–1081.
Kabiri M, Soleimani M, Shabani I, Futrega K, Ghaemi N, Ahvaz HH, Elahi E, Doran MR. 2012. Neural differentiation of mouse embryonic stem cells on conductive nanofiber scaffolds. Biotechnology Letters 34:1357–1365.
Keung AJ, Kumar S, Schaffer DV. 2010. Presentation counts: Microenvironmental regulation of stem cells by biophysical and material cues. Annual Review of Cell and Developmental Biology 26:533–556.
Keung AJ, Asuri P, Kumar S, Schaffer DV. 2012. Soft microenvironments promote the early neuro-genic differentiation but not self-renewal of human pluripotent stem cells. Integrative Biology: Quantitative Biosciences from Nano to Macro 4(9):1049–1058.
Kolind K, Leong KW, Besenbacher F, Foss M. 2012. Guidance of stem cell fate on 2D patterned surfaces. Biomaterials 33:6626–6633.
McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell 6:483–495.
Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. 2004. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America 101:18129–18134.
Recknor JB, Sakaguchi DS, Mallapragada SK. 2006. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials 27:4098–4108.
Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. 2008. Substrate modulus directs neural stem cell behavior. Biophysical Journal 95:4426–4438.
Scadden DT. 2006. The stem-cell niche as an entity of action. Nature 441:1075–1079.
Serena E, Flaibani M, Carnio S, Boldrin L, Vitiello L, De Coppi P, Elvassore N. 2008. Electrophysi-ologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurological Research 30:207–214.








